Abstract
Background:
Growing evidence suggests that interpregnancy weight change (IPWC) is a risk factor for perinatal outcomes, since it may increase the probability of gestational complications including gestational diabetes or cesarean delivery. Additionally, IPWC may affect neonatal outcomes increasing the prevalence of newborns small for gestational age or preterm birth. However, the association between IPWC and perinatal outcomes has not systematically synthesized thus far. This study protocol aims to provide a clear, transparent and standardized procedure for systematically reviewing the association between IPWC and perinatal outcomes.
Methods and analysis:
This systematic review and meta-analyses protocol is based on the preferred reporting items for systematic review and meta-analysis protocols and the Cochrane Collaboration Handbook. MEDLINE, EMBASE, the Cochrane Library, and Web of Science will be systematically searched from their inception. No limits will be defined by study design, as such different tools to assess risk of bias will be used:
-
(1)
a critical appraisal checklist for analytical cross-sectional studies;
-
(2)
the Newcastle–Ottawa quality assessment scale for longitudinal studies (including case–control and cohort studies); and
-
(3)
Cochrane Collaboration's tool for clinical trials.
Odd ratios and their corresponding 95% confidence intervals will be reported to evaluate associations between IPWC and perinatal outcomes.
Results:
The results of this study will be published in a peer-reviewed journal.
Conclusion:
This systematic review and meta-analysis will systematically synthesize the evidence regarding the association between IPWC and perinatal outcomes. Data will be extracted from published articles and findings will be published in peer-reviewed journals. Ethical approval and informed consent will not be required due to the nature of the study.
Systematic review registration:
PROSPERO CRD42018100449.
Keywords: body mass index, body weight, gestational outcomes, intergestational, interpregnancy, maternal outcomes and neonatal outcomes, perinatal outcomes
1. Introduction
Approximately 30% of women of reproductive age are overweight or obese.[1,2] prepregnancy body mass index (BMI) has been associated with adverse perinatal outcomes, such as gestational diabetes, hypertensive disorders, higher cesarean section rates, postpartum hemorrhage, low birth weight, macrosomia, death, and stillbirth.[3–6] However, there is little or weak evidence regarding the association of changes in pre-pregnancy BMI or body weight between pregnancies and its association with some perinatal outcomes.
Interpregnancy weight change (IPWC) has been defined as the difference in weight between consecutive pregnancies, taking as reference either weight at the first prenatal visit[7,8] or at delivery.[9] Additionally, IPWC may be reported in different ways:
-
(1)
by units of increase or decrease in BMI[7];
-
(2)
BMI changes based on the World Health Organization (WHO) classification[10];
- (3)
-
(4)
percentage weight change.[13]
Several studies have reported an association between IPWC and several maternal and newborn perinatal complications.[7,14,15] An increase in IPWC has been related with a higher incidence of gestational diabetes,[7,16,17] preeclampsia,[7] higher rates of cesarean section,[7,15,18] or a higher prevalence of large for gestational age (LGA),[7,8] while a decrease in IPWC may result in a higher incidence of newborns small for gestational age (SGA).[15]
Several factors have been related with IPWC. For instance, gestational weight gain influences postpartum weight retention[19,20] which is associated with IPWC.[21] Moreover, parity is positively related with IPWC, so each pregnancy increases IPWC.[22] Also sociodemographic factors such as educational level[23] or ethnic group[24] have been related to IPWC; although a Finnish study did not find differences in IPWC across different ethnic origins but by different geographic locations.[25]
There are no international recommendations on IPWC. Although many studies have reported their results using different classification criteria the risk for preeclampsia,[26,27] hypertensive disorders,[15,26] gestational diabetes,[26,28] or cesarean delivery[9,26] has been found to increase when IPWC rises 1 to 2 kg/m2 or more. Likewise, the risk of neonatal complications such as respiratory distress[29] or SGA[30] increases with a decrease of 1 kg/m2 or more between pregnancies.
2. Objectives
This systematic review and meta-analysis aimed to provide a clear, standardized and transparent methodology for conducting a systematic review and meta-analysis aimed to assess the association between IPWC and perinatal outcomes.
3. Methods
This systematic review and meta-analysis protocol is guided by the preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P).[31] The systematic review and meta-analysis will be guided by The meta-analysis of observational studies in epidemiology statement,[32] the PRISMA,[33] and the Cochrane Collaboration Handbook.[34] This protocol has been registered in PROSPERO (Registration number: CRD42018100449).
3.1. Eligibility criteria
3.1.1. Types of studies
Published studies examining the relationship between IPWC and perinatal outcomes. The exposure will be IPWC, as units of pre-pregnancy BMI, percentage pre-pregnancy BMI or weight change, weight change in kilograms or pounds, weight percentage of change or the WHO classification criteria changes at the start of the pregnancy. The study design will be observational (including cohort, case–control, and cross-sectional) or clinical trial studies without language restriction.
3.1.2. Types of participants
The participants included will be mothers with at least a previous pregnancy and their offspring. No restrictions regarding race/ethnicity, sex, economic status, and education will be applied.
3.1.3. Types of outcome measures
Maternal outcomes will be: type of delivery, gestational diabetes mellitus, hypertensive disorders, preterm delivery, and stillbirth; and newborn outcomes will be: Apgar score, birth weight, LGA, or SGA.
3.1.4. Exclusion criteria
Studies that included twin pregnancies or higher-order multiples will be excluded.
3.2. Information sources
An electronic search will be conducted in MEDLINE (via PubMed), Web of Science, Cochrane Library, and EMBASE (via Scopus), from their inception.
3.3. Search strategy
The search will be conducted using Boolean operators and the following keywords: “interpregnancy,” “interdelivery,” “between pregnancies,” “successive pregnancies,” “consecutive pregnancies,” “weight change,” “gestational weight gain,” “maternal weight,” “pregnancy weight gain,” “body mass index,” and “BMI.” The search strategy for PubMed is shown in Table 1. The references of previous systematic reviews and of the selected studies will be reviewed to identify additional articles. Study records will be organized using the COEVIDENCE Reference Manager.
Table 1.
Search strategy for the MEDILINE database.

3.4. Data collection
3.4.1. Study selection
Titles and abstracts of the retrieved articles will be independently reviewed by 2 researchers (JAM-H and DPP-C) to identify studies for this systematic review and meta-analysis. If studies do not meet the inclusion criteria, they will be excluded (Fig. 1). If the abstract does not provide enough information, the study will be selected for full-text evaluation. Two reviewers will review the included and excluded studies to verify the reasons for each decision. If consensus is not reached, a third researcher will be consulted (VM-V).
Figure 1.
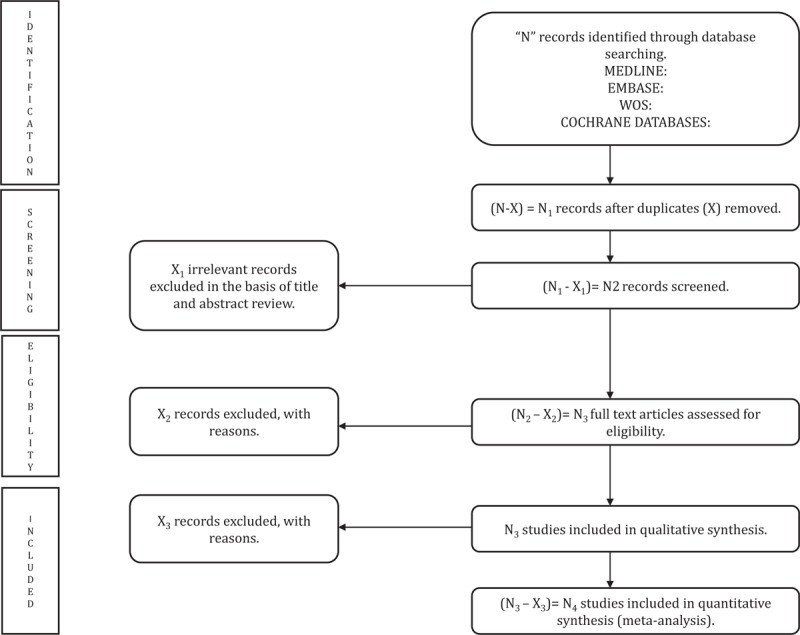
Preferred reporting items for systematic reviews and meta-analyses flow diagram of identification, screening, eligibility, and inclusion of studies.
3.4.2. Data extraction and management
The following data will be extracted from the selected studies:
-
(1)
study data: author, year of publication, country, study design, sample size of mothers and children;
-
(2)
characteristics of participants: mother's age, birth date,
-
(3)
maternal outcomes: type of delivery, gestational diabetes mellitus, hypertensive disorders, preterm delivery or stillbirth;
-
(4)
newborn outcomes: Apgar score, birth weight, LGA, or SGA; and
-
(5)
adjustment variables.
3.5. Assessment of risk of bias
Reviewers will be blinded to the authors, title, and year of publication of the studies and quality will be independently assessed by 2 authors (JAM-H and DPP-C). Standardized checklists will be used:
-
(1)
the Critical Appraisal Checklist for Analytical Cross Sectional Studies from The Joanna Briggs Institute will be used to assess the quality of cross-sectional studies.[35] This checklist has 8 components evaluated as “Yes,” “No,” “Unclear,” and “Not applicable.” The results of this checklist will show possible bias in the design, conduct, and analysis of each study.
Moreover,
-
(2)
the Newcastle–Ottawa Quality assessment scale will be used to assess the quality of longitudinal studies, including case–control and cohort studies.[36] This scale consists of 8 items grouped in 3 categories: (a) selection; (b) comparability; and (c) exposure in case–control studies or outcome in cohort studies. Each study can obtain 1 star for each item in the (a) selection and (c) exposure categories, and a maximum of 2 stars in the (b) comparability category.
Finally,
-
(3)
the Cochrane Collaboration's tool will be used for clinical trials.[37] This tool evaluates 7 domains: sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and “other issues.” Each item will be classified based on criteria for judging the risk of bias as: “+,” low risk of bias; “−,” high risk of bias; and “?,” unclear risk of bias.
3.6. Data analysis
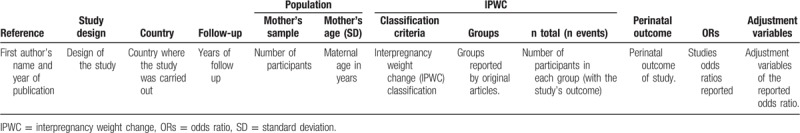
The main characteristics of included studies and relevant information according to the aim of this systematic review and meta-analysis will be summarized in Table 2, in which the study's characteristics, population description, and relevant issues related to perinatal outcomes will be included. The reviewers will determine if meta-analysis is feasible after data extraction. At least 5 studies will be sufficient to conduct meta-analysis, if there will enough studies a narrative synthesis will be conducted. After summarizing data, a meta-analysis will be conducted using STATA V.14 software to compute pooled effect size (ES) estimates with 95% CI.
Table 2.
Characteristics of studies included in the systematic review and meta-analysis.
Adjusted and unadjusted results reported by original articles will be compared. The “no weight change” category will be used as the reference category, compared with the loss or increase weight categories reported by original articles, although they reported different classifications of IPWC. The heterogeneity of results across studies will be evaluated by using the I2 statistic that could be considered as: not important (0% to 40%); moderate (30% to 60%); substantial (50% to 90%), and considerable (75% to 100%), the corresponding P-values will be also considered.[38] The pooled estimate of ES and 95% CI; will be computed using the Mantel–Haenszel fixed effects when I2 lower than 50%[39] and the DerSimonian and Laird random-effects methods[40] will be used with when the I2 is higher than 50%.
3.6.1. Subgroup analyses and meta-regression
Subgroup analyses and meta-regression across variables that could cause heterogeneity will be conducted:
-
(1)
study design (cross-sectional, cohort, or clinical trial studies);
-
(2)
country;
-
(3)
mother's characteristics: age, pre-pregnancy BMI;
-
(4)
IPWC classification;
-
(5)
type of postpartum intervention to manage weight such as: physical activity, diet, or another lifestyle change; and
-
(6)
quality assessment.
3.6.2. Sensitivity analysis
Included studies will be removed one by one from the pooled estimates in order to test whether the results could have been influenced by a single study.
3.7. Publication bias
Additionally, publication bias will be assessed using a funnel plot, according to the method proposed by Egger.[41]
Finally, a systematic review with descriptive analysis will be conducted if a meta-analysis is not possible due to a lack of quantitative information.
3.8. Evidence evaluation
Grading of recommendations, assessment, development, and evaluation (GRADE) system will be used to assess the quality of evidence of outcomes according to 4 categories: high, moderate, low, and very low. GRADE considers the elements of quality, consistency, directness, and ES.[42]
The results of these quality evaluations will be compared and discrepancies will be discussed. A third researcher will be asked when an agreement cannot be reached (VM-V).
4. Discussion
A recent meta-analysis evaluated the association between interpregnancy BMI change and pregnancy outcomes, although it included a few studies for each perinatal outcome, it excluded studies that provided IPWC data other than change in pre-pregnancy BMI in kg/m2.[43] Furthermore, this meta-analysis excluded some perinatal outcomes because there was a lack of relevant data or low-quality studies. Additionally, more studies regarding this association have been published recently.[28,44] The previous meta-analysis reported that gaining weight between pregnancies increases the risk of developing gestational diabetes mellitus, cesarean section, and LGA, as well as reducing rates of SGA.[43] However, they excluded important perinatal outcomes such as preeclampsia and preterm birth. Therefore, a systematic review and, if it is possible, a meta-analysis will be conducted to highlight the influence of IPWC in perinatal outcomes. For this purpose, the protocol of this systematic review provides a clear way for extraction and synthesizing the relevant information.
Thus, if the association between higher IPWC and the increase in adverse outcomes is confirmed in the proposed study, it could support the necessity of implementing lifestyle-based interventions during the interpregnancy period, to manage IPWC in order to avoid postpartum weight retention as an important risk factor for long-term maternal obesity.[45,46] Among the strategies that have been suggested to prevent higher IPWC, breastfeeding promotion,[47] and structured diet and physical activity programs[48] are the most noteworthy.
Because of the design variability in the included studies, 3 tools for quality assessment will be used:
-
(1)
a Critical Appraisal Checklist for Analytical Cross Sectional Studies from The Joanna Briggs Institute for cross-sectional studies[35];
-
(2)
the Newcastle–Ottawa quality assessment scale for longitudinal studies (including case–control and cohort studies)[36]; and
-
(3)
Cochrane Collaboration's tool for clinical trials.[37]
Furthermore, possible sources of heterogeneity, such as study design, geographical location, IPWC classification, and sample characteristics (maternal age) will be considered in this study. Moreover, random-effect meta-regressions will be used to evaluate whether these variables affect heterogeneity.[49] Sensitivity and subgroup analyses will be conducted to determine sources of heterogeneity. For these reasons, there are few exclusion criteria, as such; additional analysis will be conducted in order to highlight the relevance of particular characteristics in the findings.
Potential limitations are inherent to systematic reviews and meta-analyses: publication bias, information bias, poor statically analyses, poor methodological quality, and inadequate reporting of methods and findings of the included studies. Additionally, the lack of homogeneous classification criteria of IPWC may limit comparability. These limitations should be taken into account in order to properly summarize and analyzes the information available in the manuscripts included.
In conclusion, due to the lack of evidence about the relationship between IPWC and perinatal outcomes, it is important to conduct a systematic review and meta-analysis.
Acknowledgments
The authors would like to thank the researchers from the Health and Social Research Center for their support during the preparation of this protocol.
Author contributions
JAM-H, CB-M, and DPP-C designed the protocol and conceived the idea for the systematic review and meta-analyses. VM-V contributed to the study design and he is the principal investigator and guarantor. CB-M, GS-M, and RP-L reviewed and edited the article and contributed to select the quality assessment tools. Finally, VM-V gave statistical support and approved the final version of the manuscript.
Conceptualization: Jose Alberto Martínez-Hortelano, Diana Patricia Pozuelo-Carrascosa.
Data curation: Jose Alberto Martínez-Hortelano, Diana Patricia Pozuelo-Carrascosa.
Formal analysis: Jose Alberto Martínez-Hortelano, Diana Patricia Pozuelo-Carrascosa.
Funding acquisition: Diana Patricia Pozuelo-Carrascosa.
Investigation: Jose Alberto Martínez-Hortelano, Diana Patricia Pozuelo-Carrascosa.
Methodology: Jose Alberto Martínez-Hortelano, Carlos Berlanga-Macías, Diana Patricia Pozuelo-Carrascosa.
Project administration: Vicente Martínez-Vizcaíno.
Resources: Gema Sanabria-Martínez.
Software: Jose Alberto Martínez-Hortelano, Vicente Martínez-Vizcaíno.
Supervision: Gema Sanabria-Martínez, Raquel Poyatos-León, Vicente Martínez-Vizcaíno.
Validation: Carlos Berlanga-Macías, Vicente Martínez-Vizcaíno.
Visualization: Vicente Martínez-Vizcaíno.
Writing – original draft: Jose Alberto Martínez-Hortelano.
Writing – review and editing: Carlos Berlanga-Macías, Diana Patricia Pozuelo-Carrascosa, Gema Sanabria-Martínez, Raquel Poyatos-León, Vicente Martínez-Vizcaíno.
Footnotes
Abbreviations: BMI = body mass index, ES = effect size, GRADE = grading of recommendations, assessment, development, and evaluation, IPWC = interpregnancy weight change, LGA = large for gestational age, SGA = small for gestational age, WHO = World Health Organization.
This study did not receive any funding. Diana Patricia Pozuelo-Carrascosa and Carlos Berlanga-Macías are supported by a grant from the Spanish Ministry of Education, Culture, and Sport (FPU14/01370) and (FPU16/02380), respectively. Mr. Jose Alberto Martínez-Hortelano is supported by a grant from the University of Castilla-La Mancha (6A2400/NL65671).
The authors report no conflicts of interest.
References
- [1].Ferrari N, Mallmann P, Brockmeier K, et al. Secular trends in pregnancy weight gain in German women and their influences on foetal outcome: a hospital-based study. BMC Pregnancy Childbirth 2014;14:228–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Johnson JL, Farr SL, Dietz PM, et al. Trends in gestational weight gain: the pregnancy risk assessment monitoring system, 2000–2009. Am J Obstet Gynecol 2015;212:806e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Doherty DA, Magann EF, Francis J, et al. Pre-pregnancy body mass index and pregnancy outcomes. Int J Gynaecol Obstet 2006;95:242–7. [DOI] [PubMed] [Google Scholar]
- [4].Dawson SI, Smith WC, Watson MS, et al. A cohort study of reproductive risk factors, weight and weight change and the development of diabetes mellitus. Diabetes Obes Metab 2003;5:244–50. [DOI] [PubMed] [Google Scholar]
- [5].Kristensen J, Vestergaard M, Wisborg K, et al. Pre-pregnancy weight and the risk of stillbirth and neonatal death. BJOG 2005;112:403–8. [DOI] [PubMed] [Google Scholar]
- [6].Frederick IO, Williams MA, Sales AE, et al. Pre-pregnancy body mass index, gestational weight gain, and other maternal characteristics in relation to infant birth weight. Matern Child Health J 2008;12:557–67. [DOI] [PubMed] [Google Scholar]
- [7].Villamor E, Cnattingius S. Interpregnancy weight change and risk of adverse pregnancy outcomes: a population-based study. Lancet 2006;368:1164–70. [DOI] [PubMed] [Google Scholar]
- [8].Wallace JM, Bhattacharya S, Campbell DM, et al. Inter-pregnancy weight change impacts placental weight and is associated with the risk of adverse pregnancy outcomes in the second pregnancy. BMC Pregnancy Childbirth 2014;14:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Dude AM, Lane-Cordova AD, Grobman WA. Interdelivery weight gain and risk of cesarean delivery following a prior vaginal delivery. Am J Obstet Gynecol 2017;217:373e1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hoff GL, Cai J, Okah FA, et al. Pre-pregnancy overweight status between successive pregnancies and pregnancy outcomes. J Womens Health (Larchmt) 2009;18:1413–7. [DOI] [PubMed] [Google Scholar]
- [11].Bao W, Yeung E, Tobias DK, et al. Long-term risk of type 2 diabetes mellitus in relation to BMI and weight change among women with a history of gestational diabetes mellitus: a prospective cohort study. Diabetologia 2015;58:1212–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Paramsothy P, Lin YS, Kernic MA, et al. Interpregnancy weight gain and cesarean delivery risk in women with a history of gestational diabetes. Obstet Gynecol 2009;113:817–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Pole JD, Dodds LA. Maternal outcomes associated with weight change between pregnancies. Can J Public Health 1999;90:233–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Jain AP, Gavard JA, Rice JJ, et al. The impact of interpregnancy weight change on birthweight in obese women. Am J Obstet Gynecol 2013;208:205.e1–7. [DOI] [PubMed] [Google Scholar]
- [15].Wallace JM, Bhattacharya S, Campbell DM, et al. Inter-pregnancy weight change and the risk of recurrent pregnancy complications. PLoS One 2016;11:e0154812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ehrlich SF, Hedderson MM, Feng J, et al. Change in body mass index between pregnancies and the risk of gestational diabetes in a second pregnancy. Obstet Gynecol 2011;117:1323–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bogaerts A, Van den Bergh BR, Ameye L, et al. Interpregnancy weight change and risk for adverse perinatal outcome. Obstet Gynecol 2013;122:999–1009. [DOI] [PubMed] [Google Scholar]
- [18].Getahun D, Kaminsky LM, Elsasser DA, et al. Changes in prepregnancy body mass index between pregnancies and risk of primary cesarean delivery. Am J Obstet Gynecol 2007;197:376.e1–7. [DOI] [PubMed] [Google Scholar]
- [19].Whelan E, Armson BA, Ashley-Martin J, et al. Gestational weight gain and interpregnancy weight change in adolescent mothers. J Pediatr Adolesc Gynecol 2017;30:356–61. [DOI] [PubMed] [Google Scholar]
- [20].Rong K, Yu K, Han X, et al. Pre-pregnancy BMI, gestational weight gain and postpartum weight retention: a meta-analysis of observational studies. Public Health Nutr 2015;18:2172–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hutcheon JA, Chapinal N, Bodnar LM, et al. The INTERGROWTH-21st gestational weight gain standard and interpregnancy weight increase: a population-based study of successive pregnancies. Obesity 2017;25:1122–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Iversen DS, Kesmodel US, Ovesen PG. Associations between parity and maternal BMI in a population-based cohort study. Acta Obstet Gynecol Scand 2018;97:694–700. [DOI] [PubMed] [Google Scholar]
- [23].Holowko N, Chaparro MP, Nilsson K, et al. Social inequality in pre-pregnancy BMI and gestational weight gain in the first and second pregnancy among women in Sweden. J Epidemiol Community Health 2015;69:1154–61. [DOI] [PubMed] [Google Scholar]
- [24].Sackoff JE, Yunzal-Butler C. Racial/ethnic differences in impact of gestational weight gain on interconception weight change. Matern Child Health J 2015;19:1348–53. [DOI] [PubMed] [Google Scholar]
- [25].Bastola K, Koponen P, Härkänen T, et al. Pre-pregnancy body mass index and inter-pregnancy weight change among women of Russian, Somali and Kurdish origin and the general Finnish population. Scand J Public Health 2017;45:314–21. [DOI] [PubMed] [Google Scholar]
- [26].Cnattingius S, Villamor E. Weight change between successive pregnancies and risks of stillbirth and infant mortality: a nationwide cohort study. Lancet 2016;387:558–65. [DOI] [PubMed] [Google Scholar]
- [27].Mostello D, Chang JJ, Allen J, et al. Recurrent preeclampsia the effect of weight change between pregnancies. Obstet Gynecol 2010;116:667–72. [DOI] [PubMed] [Google Scholar]
- [28].Sorbye LM, Skjaerven R, Klungsoyr K, et al. Gestational diabetes mellitus and interpregnancy weight change: a population-based cohort study. PLoS Med 2017;14:e1002367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Knight-Agarwal CR, Williams LT, Davis D, et al. Association of BMI and interpregnancy BMI change with birth outcomes in an Australian obstetric population: a retrospective cohort study. BMJ Open 2016;6:e010667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Cheng CJ, Bommarito K, Noguchi A, et al. Body mass index change between pregnancies and small for gestational age births. Obstet Gynecol 2004;104:286–92. [DOI] [PubMed] [Google Scholar]
- [31].Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- [33].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010;8:336–41. [DOI] [PubMed] [Google Scholar]
- [34].Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions version 5.1. 0. The Cochrane Collaboration; 2011. p. 5. [Google Scholar]
- [35].Moola S, Munn Z, Tufanaru C. Chapter 7: Systematic Reviews of Etiology and Risk: Appendix 5 Critical Appraisal Checklist for Analytical Cross-sectional studies. In: Aromataris E, Munn Z. (Editors), Joanna Briggs Institute Reviewer's Manual, 2017. [Online]. Available at: https://wiki.joannabriggs.org/display/MANUAL/Appendix+5+Critical+appraisal+checklist+for+analytical+cross-sectional+studies Accessed 25 September 2018. [Google Scholar]
- [36].Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm Accessed September 19, 2018. [Google Scholar]
- [37].Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Deeks JJ, Higgins JP, Altman DG. Analysing Data and Undertaking Meta-Analyses, in Cochrane Handbook for Systematic Reviews of Interventions: Cochrane book series, 243–296. [Google Scholar]
- [39].Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. JNCI J Natl Cancer Inst 1959;22:719–48. [PubMed] [Google Scholar]
- [40].DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- [41].Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Balshem H, Helfand M, Schünemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 2011;64:401–6. [DOI] [PubMed] [Google Scholar]
- [43].Oteng-Ntim E, Mononen S, Sawicki O, et al. Interpregnancy weight change and adverse pregnancy outcomes: a systematic review and meta-analysis. BJOG-AN Int J Obstet Gynaecol 2018;125(2 SI):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Crosby DA, Walsh JM, Segurado R, et al. Interpregnancy weight changes and impact on pregnancy outcome in a cohort of women with a macrosomic first delivery: a prospective longitudinal study. BMJ Open 2017;7:e016193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Linné Y, Dye L, Barkeling B, et al. Long-term weight development in women: a 15-year follow-up of the effects of pregnancy. Obes Res 2004;12:1166–78. [DOI] [PubMed] [Google Scholar]
- [46].Gore SA, Brown DM, West DS. The role of postpartum weight retention in obesity among women: a review of the evidence. Ann Behav Med 2003;26:149–59. [DOI] [PubMed] [Google Scholar]
- [47].Baker JL, Gamborg M, Heitmann BL, et al. Breastfeeding reduces postpartum weight retention. Am J Clin Nutr 2008;88:1543–51. [DOI] [PubMed] [Google Scholar]
- [48].O’Toole ML, Sawicki MA, Artal R. Structured diet and physical activity prevent postpartum weight retention. J Women's Heal 2003;12:991–8. [DOI] [PubMed] [Google Scholar]
- [49].Thompson SG, Sharp SJ. Explaining heterogeneity in meta-analysis: a comparison of methods. Stat Med 1999;18:2693–708. [DOI] [PubMed] [Google Scholar]


