Abstract
Background:
This study aimed to compare the efficacy and safety of oral tranexamic acid (TXA) with intravenous (IV) TXA in reducing perioperative blood loss in total-knee arthroplasty (TKA) and total-hip arthroplasty (THA).
Methods:
PubMed, Web of Science, Embase, and Cochrane Library were fully searched for relevant studies. Studies comparing the efficacy and safety of oral TXA with IV TXA in TKA and THA were included in this research. Odds ratio (OR) or risk difference (RD) was applied to compare dichotomous variables, while mean difference (MD) was used to compare continues variables.
Results:
A total of 7 studies (5 randomized controlled trials and 2 retrospective studies) were included into this study. As for patients undergoing TKA or THA, there were no obvious differences between oral TXA group and IV TXA group in hemoglobin (Hb) drop (MD = 0.06, 95% confidence interval [CI] = −0.01 to 0.13, P = .09), transfusion rate (OR = 0.78, 95% CI = 0.54–1.13, P = .19), total blood loss (MD = 16.31, 95% CI = −69.85 to 102.46, P = .71), total Hb loss (MD = 5.18, 95% CI = −12.65 to 23.02, P = .57), length of hospital stay (MD = -0.06, 95% CI = −0.30 to 0.18, P = .63), drain out (MD = 21.04, 95% CI = −15.81 to 57.88, P = .26), incidence of deep vein deep vein thrombosis (RD = 0.00, 95% CI = −0.01 to 0.01, P = .82) or pulmonary embolism (RD = 0.00, 95% CI = −0.01 to 0.01, P = .91). The sample size of this study was small and several included studies were with relatively low quality.
Conclusion:
Oral TXA is equivalent to IV TXA in reducing perioperative blood loss and should be recommended in TKA and THA. More high-quality studies are needed to elucidate this issue.
Keywords: administration route, arthroplasty, blood loss, complications, tranexamic acid
1. Introduction
Patients with osteoarthritis, rheumatoid arthritis, or fractures of knee or hip often have to receive total-knee arthroplasty (TKA) and total-hip arthroplasty (THA).[1–6] Despite of satisfactory results of TKA and THA, patients must face the risks of postoperative anemia and transfusion for the perioperative blood loss, which is supposed to increase the mobility and costs.[7,8] Therefore, reducing the blood loss and minimizing the risk of transfusions in TKA and THA have drew great attentions of orthopedic surgeons.[9–11]
Tranexamic acid (TXA), an antifibrinolytic agent, is conducive to reducing the perioperative blood loss and the risk of transfusions in TKA and THA.[12–14] TXA can be administered via intravenous (IV), topical, or oral routes.[15–17] Previous studies have suggested that IV TXA and topical TXA had comparable efficacy in TKA and THA.[10,17,18] However, the dispute on the comparison between oral TXA and IV TXA regarding reducing perioperative blood loss still remains.[19–25] Irwin et al retrospectively analyzed 3000 patients and observed less hemoglobin (Hb) drop in IV TXA group compared to oral TXA group,[21] similar results were found in Kayupov et al study.[20] Nevertheless, other studies showed there was no statistical difference between oral TXA group and IV TXA group in Hb drop.[22,25] Hence, this systematic review and meta-analysis aimed to compare the efficacy and safety of oral TXA with IV TXA in reducing perioperative blood loss in TKA and THA.
2. Materials and methods
This study was conducted according to preferred reporting items for systematic reviews and meta-analyses (PRISMA).[26] The registration number is review registry 621 (https://www.researchregistry.com/).
2.1. Literature search and selection
PubMed, Web of Science, Embase, and Cochrane Library were fully searched up to April 19, 2018. The literature search strategy was as follows: (“intravenous” AND “oral”) AND (“tranexamic acid” OR “TXA”) AND (“total-knee arthroplasty” OR “total-knee replacement” OR “total-hip replacement” OR “total-hip arthroplasty”). There was no restriction on the language. The references of retrieved articles were also checked to avoid missing relevant studies. Literature search was completed by 2 authors independently.
2.2. Inclusion criteria and exclusion criteria
The included studies should meet following PICOS criteria (patients, intervention, comparator, outcome, and study design): Patients: patients undergoing TKA or THA; Intervention: oral TXA for TKA or THA; Comparator: IV TXA for TKA or THA; Outcomes: Hb drop, transfusion rate, total blood loss, total Hb loss, drain out, length of hospital stay, incidence of deep vein thrombosis (DVT), and pulmonary embolism (PE); Study design: randomized controlled trial (RCT) or retrospective study (RST). Reviews, comments, duplications, cell experiments, or animal experiments were excluded from this study.
2.3. Data extraction and quality assessment
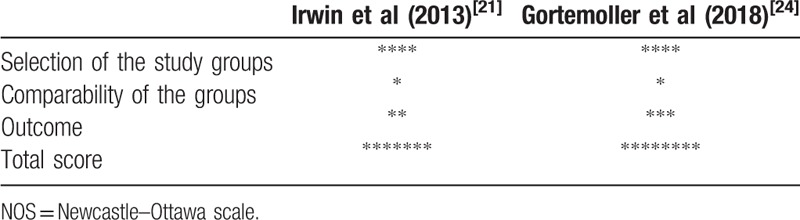
Data extraction and quality assessment were completed by 2 authors independently. Any disagreement would be solved by discussing with the 3rd author. The following information was extracted: the 1st author, study design, sample size, gender, age, body mass index (BMI), transfusion rate, dosage of TXA, surgery, anesthesia, pneumatic tourniquet, and clinical outcomes. The main clinical outcomes included Hb drop, transfusion rate, total blood loss, total Hb loss, drain out, length of hospital stay, incidence of DVT and PE. Cochrane Collaboration's “risk of bias” was used to assess the quality of RCTs, and “risk of bias” was classified into three grades: “low risk, middle risk, and high risk.”[27] Newcastle–Ottawa scale (NOS) was utilized to evaluate the study quality of RST.[28] In NOS scale, 3 domains, including selection, comparability, and outcome were assessed, with 4 categories in the selection domain, 1 category in the comparability domain, and 3 categories in the outcome domain. The total possible score was 9 points. The quality of studies with NOS > 5 was considered high.
2.4. Statistical analysis
All analyses were performed by Review Manager 5.3 (The Cochrane Collaboration, Copenhagen, Denmark) and STATA 12.0 software (Stata, College Station, TX). Odds ratio (OR) or risk difference (RD) with corresponding 95% confidence interval (CI) was applied to compare dichotomous variables, such as gender, transfusion rate, and so on. Mean difference (MD) was used to compare continues variables, including length of hospital stay, total blood loss, Hb drop, and so on. Subgroup analyses for Hb drop and incidence of transfusion rate were performed. Hb drop and incidence of transfusion rate in each group were calculated using STATA 12.0. The inter-study heterogeneity was assessed by I2 statistic. I2 ≤50% indicated there was no obvious heterogeneity among studies, and a fixed-effect model should be used. If not, a random-effect model should be applied. Publication bias was detected by funnel plots. All P values were 2 sides and the difference was considered to be significant when P values lowered than .05.
3. Results
3.1. Literature search and selection
As shown in Figure 1, a total of 167 articles were initially retrieved. After the removal of duplications, 98 remaining articles were further evaluated. Seventy-nine articles were directly excluded by scanning titles or abstracts. The full-texts of remaining 10 studies were carefully assessed and 3 articles were excluded for the following reasons: 1 was irrelevant to this topic and 2 reported the data from duplicated patients. Ultimately, 7 articles were included into this study.[19–25]
Figure 1.
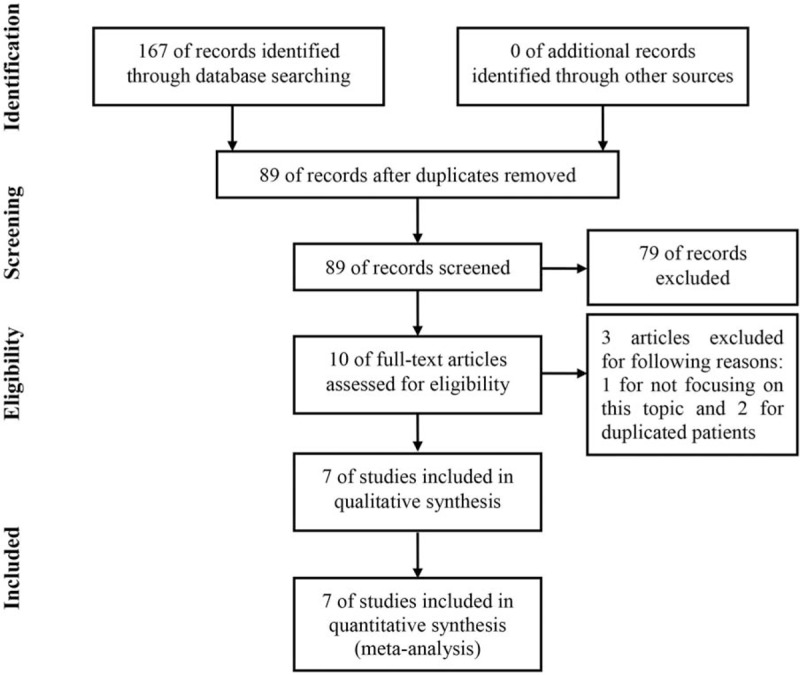
Flow chart of literature search.
3.2. Characteristics of included studies
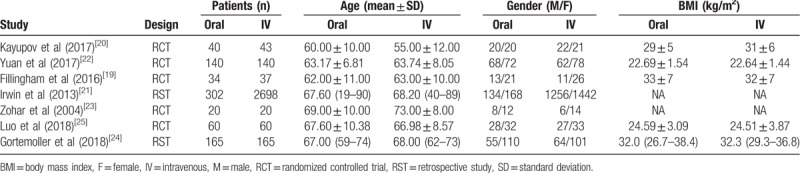
As shown in Table 1, a total of 7 studies containing 3924 patients were analyzed in this study.[19–25] All included studies were published in English. There were 5 RCTs[19,20,22,23,25] and 2 RSTs.[21,24] The mean age of patients ranged from 60 to 69 years in oral TXA group and 55 to 73 years in IV TXA group. Besides, 5 studies reported the BMI, which ranged from 22.69 to 33 kg/m2 in oral TXA group and 22.64 to 32.3 kg/m2 in IV TXA group.
Table 1.
The demographics of included studies.
The details of surgeries are summarized in Table 2. The transfusion trigger was Hb < 7.0 g/dL in 4 studies[19–21,25] and Hb < 8.0 g/dL in 1 study,[22] besides, 1 study regarded hematocrit < 28% as the transfusion trigger[23] and 1 study did not reported the transfusion trigger.[24] The dosage of TXA varied a lot in different studies. With respect to surgeries, 2 studies focused on THA,[20,25] 3 studies focused on TKA,[19,22,23] and 2 studies focused on both THA and TKA.[21,24] Spinal-epidural anesthesia, general anesthesia, adductor canal block, or spinal anesthesia were used. Pneumatic tourniquet was used in 5 studies.[19,21–24] The quality of included studies was evaluated by Cochrane Collaboration's “risk of bias” for RCTs (Table 3) and NOS for RSTs (Table 4). All RSTs included were with high quality; however, the quality of included RCTs was middle and even poor.
Table 2.
The details of surgeries in include studies.
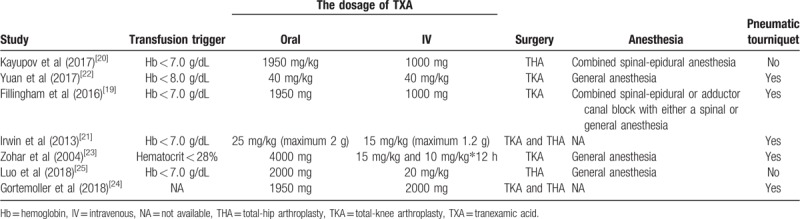
Table 3.
The quality assessment of included RCTs based on Cochrane Collaboration's “risk of bias.”.
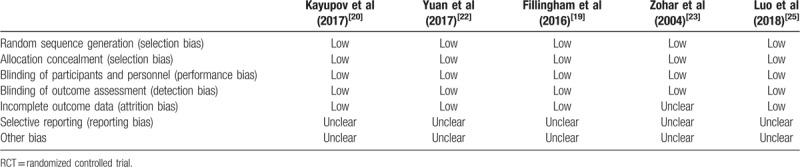
Table 4.
The quality assessment of included retrospective studies based on NOS.
3.3. Meta-analysis for Hb drop
As show in Figure 2, the mean Hb drop was 2.85 (95% CI = 2.48–3.22) g/dL in oral TXA group and 2.81 (95% CI = 2.44–3.19) g/dL in IV TXA group. As shown in Figure 3, a fixed effect model was used because of mild heterogeneity (I2 = 39%), and no significant difference was observed (MD = 0.06, 95% CI = −0.01 to 0.13, P = .09). In the subgroup analysis, no obvious difference was observed between 2 groups when only analyzing RCTs (MD = −0.01, 95% CI = −0.10 to 0.08, P = .81; I2 = 0%). However, significantly more Hb drop was detected in oral TXA group compared to IV TXA group in the subgroup analysis of RSTs (MD = 0.18, 95% CI = 0.06–0.29, P < .01; I2 = 20%).
Figure 2.
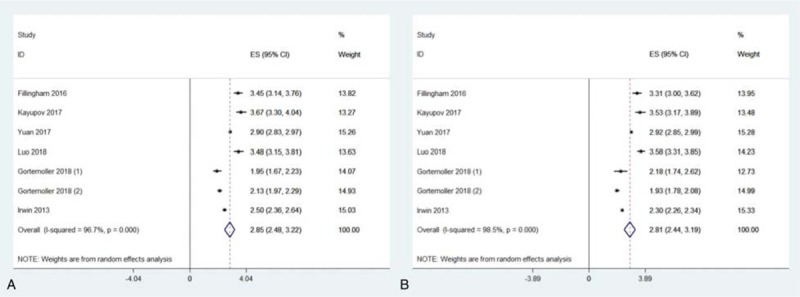
Meta-analyses for hemoglobin drop in oral tranexamic acid (TXA) and intravenous TXA group.
Figure 3.
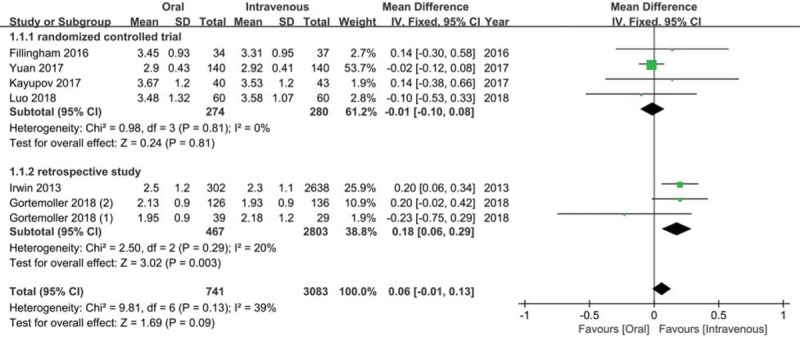
Meta-analysis for the comparison of hemoglobin drop between oral tranexamic acid (TXA) and intravenous TXA group.
3.4. Meta-analysis for transfusion rate
As shown in Figure 4, the overall transfusion rate was 0.051 (95% CI = 0.036–0.067, I2 = 37.9%) in oral TXA group and 0.060 (95% CI = 0.032–0.088; I2 = 72.9%) in IV TXA group. There was no distinct difference between 2 groups regarding transfusion rate (OR = 0.78, 95% CI = 0.54–1.13, P = .19; I2 = 0%) (Fig. 5). In the subgroup analysis, comparable transfusion rate between 2 groups was detected both for RCTs (OR = 1.10, 95% CI = 0.62–1.95, P = .74; I2 = 0%) and RSTs (OR = 0.63, 95% CI = 0.38–1.03, P = .07; I2 = 22%).
Figure 4.
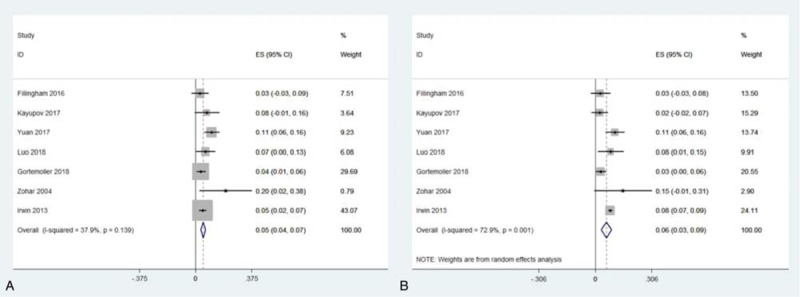
Meta-analyses for transfusion rate in oral tranexamic acid (TXA) and intravenous TXA group.
Figure 5.
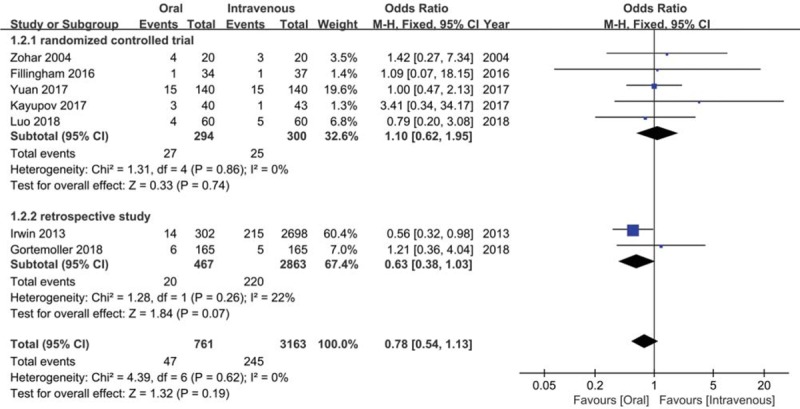
Meta-analysis for the comparison of transfusion rate between oral tranexamic acid (TXA) and intravenous TXA group.
3.5. Meta-analysis for total blood loss
As showed in Figure 6A, a fixed-effect model was used for insignificant heterogeneity (I2 = 0%). The results presented that patients in 2 groups had comparative total blood loss (MD = 16.31, 95% CI = −69.85 to 102.46, P = .71).
Figure 6.
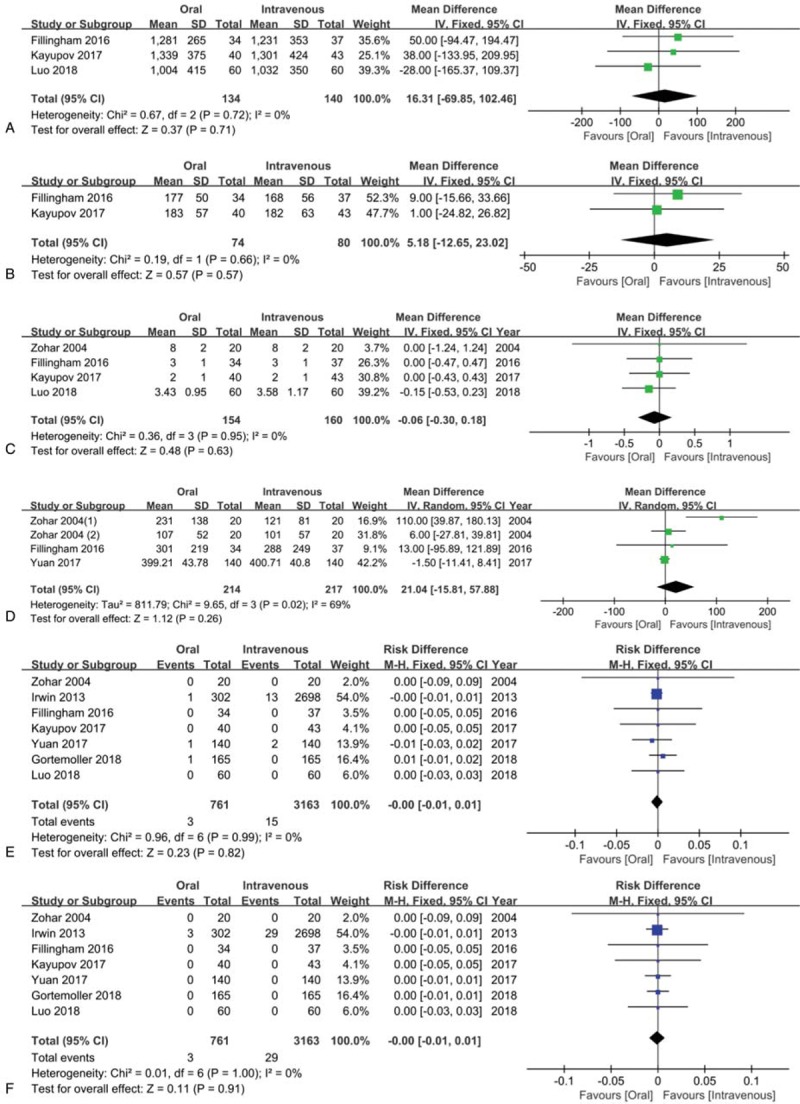
Meta-analyses for the comparisons of total blood loss (A), total hemoglobin loss (B), length of hospital stay (C), drain out (D), incidence of deep vein thrombosis (E) and PE (F).
3.6. Meta-analysis for total Hb loss
There was no apparent difference between oral and IV TXA groups in total Hb loss (MD = 5.18, 95% CI = −12.65 to 23.02, P = .57; I2 = 0%) (Fig. 6B).
3.7. Meta-analysis for length of hospital stay
There was no obvious difference between 2 groups regarding length of hospital stay (MD = −0.06, 95% CI = −0.30 to 0.18, P = .63; I2 = 0%) (Fig. 6C).
3.8. Meta-analysis for drain out
As shown in Figure 6D, the meta-analysis showed there was no obvious difference between 2 groups (MD = 21.04, 95% CI = −15.81 to 57.88, P = .26; I2 = 69%).
3.9. Meta-analysis for the incidence of DVT
The incidence of PE was 0.4% (3/761) and 0.5% (15/3163) in oral TXA group and IV TXA group, respectively. No significant difference was observed between 2 groups (RD = 0.00, 95% CI = −0.01 to 0.01, P = .82; I2 = 0%) (Fig. 6E).
3.10. Meta-analysis for the incidence of PE
The incidence of PE was 0.4% (3/761) and 0.9% (29/3163) in oral TXA group and IV TXA group, respectively. No statistical difference was detected between 2 groups in the incidence of PE (RD = 0.00, 95% CI = −0.01 to 0.01, P = .91; I2 = 0%) (Fig. 6F).
3.11. Publication bias
All funnel plots were symmetrical, and there was no obvious publication bias among included studies in Hb drop, transfusion rate, total blood loss, total Hb loss, length of hospital stay, drain out, incidence of DVT, or PE (Fig. 7).
Figure 7.
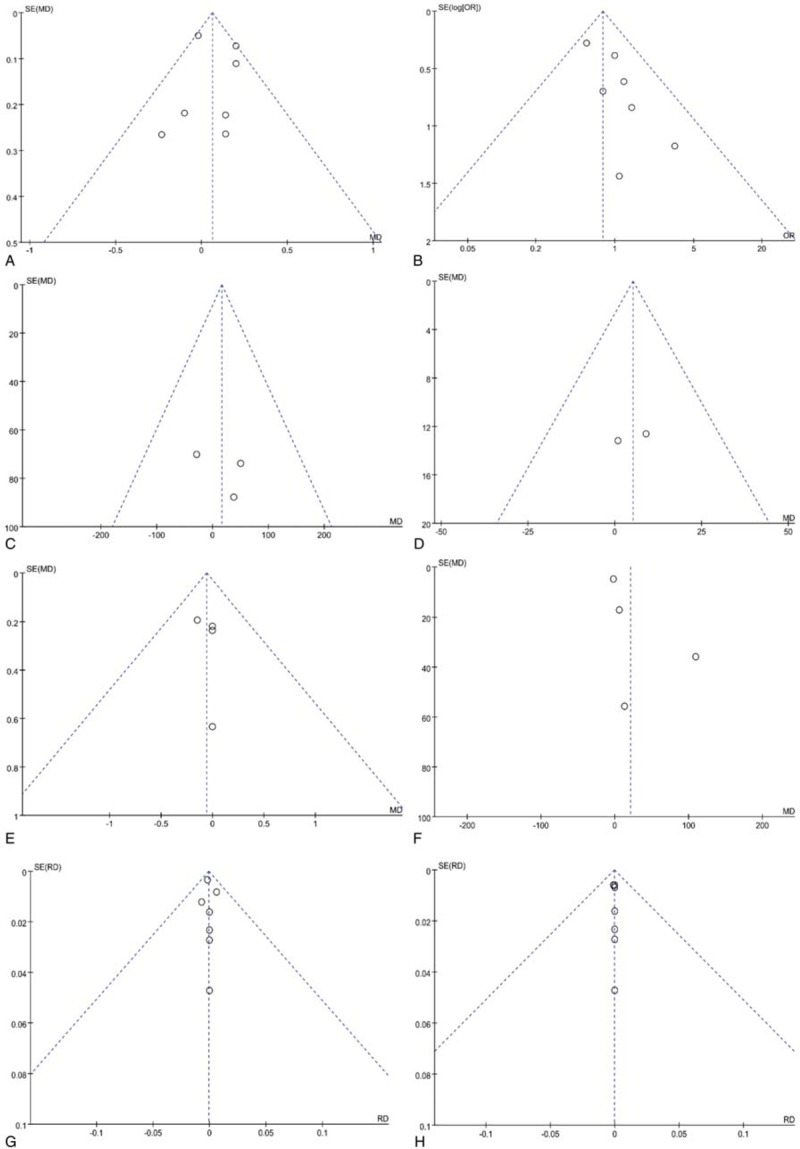
Funnel plots for hemoglobin drop (A), transfusion rate (B), total blood loss (C), total hemoglobin loss (D), length of hospital stay (E), drain out (F), incidence of deep vein thrombosis (G) and PE (H).
4. Discussion
In our study, there were no obvious differences between oral TXA group and IV TXA group in Hb drop, transfusion rate, total blood loss, total Hb loss, length of hospital stay, drain out, or the incidence of DVT or PE in TKA and THA.
The Hb drop was the primary outcome in our study because it reflected the blood loss and has been regarded as the trigger for transfusion.[19,23,25] In our study, the average Hb drop was 2.81 g/dL in IV TXA group, which was similar to previous studies and indicated IV TXA had satisfactory efficacy in reducing blood loss in TKA and THA.[20,22–24] Other than IV TXA group, limited studies concerned on the effect of oral TXA in total joint arthroplasty. The mean Hb drop was 2.85 g/dL in oral TXA group in our study, which manifested that oral TXA had favorable effects on blood sparing. In an RCT conducted by Luo et al, 3.48 g/dL Hb drop was found in oral TXA group, which was larger than us.[25] Similarly, 3.0 g/dL Hb drop was detected in oral TXA group in Lee et al investigation.[29] This difference might be explained that their studies only focused on the patients undergoing THA, which generally caused more blood loss than TKA.[29] More importantly, we obtained comparable effects in Hb drop between oral TXA and IV TXA, which implied that oral TXA was equivalent to IV TXA in decreasing blood loss in TKA and THA. Several RCTs have also found alike effects between oral TXA and IV TXA in TKA and THA.[22,25] However, Irwin et al retrospectively evaluated 3000 patients and discovered oral TXA group (2.5 g/dL) had more Hb drop than IV TXA group (2.3 g/dL). It should be noted many factors might affect their results on account of the retrospective design.[21] Similarly, Hao et al performed a meta-analysis and also found IV TXA provided superior Hb drop in TKA or THA, but this difference might attribute to the high weight of Irwin et al study.[30] Therefore, to eliminate the influence of RSTs, we performed the subgroup analysis containing RCTs, and found oral TXA was equivalent to IV TXA in reducing blood loss in TKA and THA.
Transfusion rate was also compared between 2 groups in our study. Transfusion rate was 5.1% in oral TXA group and 6.0% in IV TXA group, which was similar to previous studies.[23–25] Our findings also suggested that oral TXA and IV TXA both could significantly reduce the risk of transfusions compared to control group reported in other studies.[15,22] Furthermore, there was no distinct difference regarding transfusion rate between oral TXA group and IV TXA group, which indicated these 2 administrations both could provide satisfactory blood-sparing efficacy in TKA and THA.
Complications were also major concerns for surgeons operating TKA or THA. The incidence of DVT was 0.4% in oral TXA group and 0.5% in IV TXA group. And 0.4% patients in oral TXA group and 0.9% patients in IV TXA group suffered from PE. Similar to previous studies,[23,25] there were no significant differences between 2 administrations regarding the incidences of DVT and PE in our study. Therefore, our work demonstrated oral TXA did not increase the risks of the occurrence of DVT or PE compared to IV TXA in TKA and THA.
Although the analysis for TXA costs was not performed in our study, 1 major advantage of oral TXA over IV TXA was less cost. Yuan et al conducted an RCT and observed less TXA cost in oral administration compared to IV administration in TKA (1936.6 vs 6062.4 ¥, P < .01).[22] Similarly, less TXA cost in oral TXA group was detected compared to IV TXA group (480 vs 3329.28 ¥, P < .01) in Luo et al study focusing on THA.[25] All these findings showed, compared to IV TXA in TKA and THA, oral TXA could decease the economic burdens of healthcare expenditures and patients’ families.
Previous meta-analyses have compared the efficacy and safety between oral TXA and IV TXA in TKA and THA.[30,31] However, some highlights deserved to be mentioned in our study. First, 7 studies were included into this meta-analysis; therefore, larger sample size of our study could provide more forceful evidence. Second, subgroup analyses of Hb drop and transfusion rate were performed to eliminate the influence of Irwin et al study, which was an RST but with a large sample size.[21] Third, we observed comparable efficacy of 2 administrations in decreasing Hb drop, which was in contradiction with Hao et al meta-analysis where IV TXA was superior to oral TXA in Hb drop.[30] Fourth, Hb drop, incidence of DVT, and PE in each group were firstly calculated using STATA 12.0 in our study. Fifth, we performed the comparison of drain out between oral TXA group and IV TXA group, which offered new evidence on this topic. Nevertheless, several limitations should be considered when interrupting our results. First, the dosage and administration time of TXA varied a lot among included studies, which might affect the stringency of conclusions. Second, transfusion volume and TXA cost were not compared between 2 administrations for insufficient published data.
5. Conclusion
Oral TXA is equivalent to IV TXA in reducing perioperative blood loss in TKA and THA. Oral TXA should be advocated in TKA and THA in light of less cost and more convenience. Future studies should focus on the optimal dose of oral TXA in TKA and THA.
Acknowledgment
The authors thank the authors of the included studies.
Author contributions
Data curation: Xuanhuang Chen.
Formal analysis: Xuanhuang Chen, Feng Zheng, Xianwei Wu.
Methodology: Feng Zheng, Zugao Zheng, Xianwei Wu.
Resources: Xuanhuang Chen, Xianwei Wu.
Software: Xuanhuang Chen, Zugao Zheng, Changfu Wu.
Validation: Xuanhuang Chen, Changfu Wu.
Writing – original draft: Xuanhuang Chen, Feng Zheng, Changfu Wu.
Writing – review & editing: Xuanhuang Chen, Zugao Zheng.
Footnotes
Abbreviations: BMI = body mass index, CI = confidence interval, DVT = deep vein thrombosis, Hb = hemoglobin, IV = intravenous, MD = mean difference, NOS = Newcastle–Ottawa scale, OR = odds ratio, PE = pulmonary embolism, PRISMA = preferred reporting items for systematic reviews and meta-analyses, RCT = randomized controlled trial, RD = risk difference, RST = retrospective study, THA = total-hip arthroplasty, TKA = total-knee arthroplasty, TXA = tranexamic acid.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Courpied JP, Caton JH. Total Hip Arthroplasty, state of the art for the 21st century. Int Orthop 2011;35:149–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Franceschini R, et al. Joint replacement in osteoarthritis: state of the art. Semin Arthritis Rheum 2005;34Suppl 2:73–7. [DOI] [PubMed] [Google Scholar]
- [3].Yan D, Yang J, Pei F. Total knee arthroplasty treatment of rheumatoid arthritis with severe versus moderate flexion contracture. J Orthop Surg Res 2013;8:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Oladeji LO, Dreger TK, Pratte EL, et al. Total knee arthroplasty versus osteochondral allograft: prevalence and risk factors following tibial plateau fractures. J Knee Surg 2019;32:380–6. [DOI] [PubMed] [Google Scholar]
- [5].Cherubino P. Total hip arthroplasty. Orthopedics 2012;35:1039–41. [DOI] [PubMed] [Google Scholar]
- [6].Lee YK, Kim KC, Ha YC, et al. Combined anterior and posterior approach in total hip arthroplasty for Crowe IV dysplasia or ankylosed hips. J Arthroplasty 2015;30:797–802. [DOI] [PubMed] [Google Scholar]
- [7].Albinarrate A, López-Picado A, Oiartzabal I, et al. Assessment of the introduction of a blood management program in orthopaedic surgery. Rev Esp Anestesiol Reanim 2015;62:443–9. [DOI] [PubMed] [Google Scholar]
- [8].Jans Ø, Bandholm T, Kurbegovic S, et al. Postoperative anemia and early functional outcomes after fast-track hip arthroplasty: a prospective cohort study. Transfusion 2016;56:917–25. [DOI] [PubMed] [Google Scholar]
- [9].Gonzalez-Porras JR, Colado E, Conde MP, et al. An individualized pre-operative blood saving protocol can increase pre-operative haemoglobin levels and reduce the need for transfusion in elective total hip or knee arthroplasty. Transfus Med 2009;19:35–42. [DOI] [PubMed] [Google Scholar]
- [10].Xie J, Hu Q, Huang Q, et al. Comparison of intravenous versus topical tranexamic acid in primary total hip and knee arthroplasty: an updated meta-analysis. Thromb Res 2017;153:28–36. [DOI] [PubMed] [Google Scholar]
- [11].Yoshihara H, Yoneoka D. Predictors of allogeneic blood transfusion in total hip and knee arthroplasty in the United States, 2000–2009. J Arthroplasty 2014;29:1736–40. [DOI] [PubMed] [Google Scholar]
- [12].Lin PC, Hsu CH, Chen WS, et al. Does tranexamic acid save blood in minimally invasive total knee arthroplasty? Clin Orthop Relat Res 2011;469:1995–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].MacGillivray RG, Tarabichi SB, Hawari MF, et al. Tranexamic acid to reduce blood loss after bilateral total knee arthroplasty: a prospective, randomized double blind study. J Arthroplasty 2011;26:24–8. [DOI] [PubMed] [Google Scholar]
- [14].Wind TC, Barfield WR, Moskal JT. The effect of tranexamic acid on transfusion rate in primary total hip arthroplasty. J Arthroplasty 2014;29:387–9. [DOI] [PubMed] [Google Scholar]
- [15].Perreault RE, Fournier CA, Mattingly DA, et al. Oral tranexamic acid reduces transfusions in total knee arthroplasty. J Arthroplasty 2017;32:2990–4. [DOI] [PubMed] [Google Scholar]
- [16].Zhang LK, Ma JX, Kuang MJ, et al. The efficacy of tranexamic acid using oral administration in total knee arthroplasty: a systematic review and meta-analysis. J Orthop Surg Res 2017;12:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wang Z, Shen X. The efficacy of combined intra-articular and intravenous tranexamic acid for blood loss in primary total knee arthroplasty: a meta-analysis. Medicine (Baltimore) 2017;96:e8123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Georgiev GP, Tanchev PP, Zheleva Z, et al. Comparison of topical and intravenous administration of tranexamic acid for blood loss control during total joint replacement: Review of literature. J Orthop Translat 2018;13:7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Fillingham YA, Kayupov E, Plummer DR, et al. The James A. Rand Young Investigator's Award: a randomized controlled trial of oral and intravenous tranexamic acid in total knee arthroplasty: the same efficacy at lower cost? J Arthroplasty 2016;31Suppl:26–30. [DOI] [PubMed] [Google Scholar]
- [20].Kayupov E, Fillingham YA, Okroj K, et al. Oral and intravenous tranexamic acid are equivalent at reducing blood loss following total hip arthroplasty: a randomized controlled trial. J Bone Joint Surg Am 2017;99:373–8. [DOI] [PubMed] [Google Scholar]
- [21].Irwin A, Khan SK, Jameson SS, et al. Oral versus intravenous tranexamic acid in enhanced-recovery primary total hip and knee replacement: results of 3000 procedures. Bone Joint J 2013;95-b:1556–61. [DOI] [PubMed] [Google Scholar]
- [22].Yuan X, Li B, Wang Q, et al. Comparison of 3 routes of administration of tranexamic acid on primary unilateral total knee arthroplasty: a prospective, randomized, controlled study. J Arthroplasty 2017;32:2738–43. [DOI] [PubMed] [Google Scholar]
- [23].Zohar E, Ellis M, Ifrach N, et al. The postoperative blood-sparing efficacy of oral versus intravenous tranexamic acid after total knee replacement. Anesth Analg 2004;99:1679–83. [DOI] [PubMed] [Google Scholar]
- [24].Gortemoller MA, Allen B, Forsyth R, et al. Comparison of oral and intravenous tranexamic acid for prevention of perioperative blood loss in total knee and total hip arthroplasty. Ann Pharmacother 2018;52:246–50. [DOI] [PubMed] [Google Scholar]
- [25].Luo ZY, Wang HY, Wang D, et al. Oral vs intravenous vs topical tranexamic acid in primary hip arthroplasty: a prospective, randomized, double-blind, controlled study. J Arthroplasty 2018;33:786–93. [DOI] [PubMed] [Google Scholar]
- [26].Moher D, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Higgins J, GS Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 [updated February 2008]. The Cochrane Collaboration, 2008 [Google Scholar]
- [28].Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [29].Lee QJ, Chang WY, Wong YC. Blood-sparing efficacy of oral tranexamic acid in primary total hip arthroplasty. J Arthroplasty 2017;32:139–42. [DOI] [PubMed] [Google Scholar]
- [30].Hao LQ, Wang QW, Chen WQ, et al. Comparison of oral and intravenous tranexamic acid in total knee and hip arthroplasty: a systematic review and meta-analysis. Int J Surg 2017;47:52–3. [DOI] [PubMed] [Google Scholar]
- [31].Zhang LK, Ma JX, Kuang MJ, et al. Comparison of oral versus intravenous application of tranexamic acid in total knee and hip arthroplasty: a systematic review and meta-analysis. Int J Surg 2017;45:77–84. [DOI] [PubMed] [Google Scholar]


