Abstract
Background:
androgen receptor variant 7 (AR-V7) has been suggested as potential marker for treatment selection in men with metastatic castration-resistant prostate cancer (mCRPC). The aim of the present review is to critically analyze: frequency of the AR-V7 expression in mCRPC cases—impact of AR-V7 expression on abiraterone, enzalutamide, and taxane therapy.
Methods:
we searched in the Medline and Cochrane Library database from the literature of the past 10 years. We critically evaluated the level of evidence according to the European Association of Urology (EAU) guidelines.
Results:
12 clinical trials were selected. The determination of AR-V7 in peripheral blood using circulating tumor cells mRNA seems to be the preferred method. At baseline, the mean percentage of cases with AR-V7 positivity was 18.3% (range 17.8%–28.8%). All data on mCRPC submitted to enzalutamide or abiraterone reported a significantly (P <.05) lower clinical progression-free survival (CPFS) and overall survival (OS) in AR-V7+ than AR-V7− cases (CPFS hazard ratio [HR]: 2.3; 95% CI 1.1–4.9; OS HR: 3.0; 95% CI 1.4–6.3). In mCRPC cases submitted to chemotherapies data are not homogeneous and some studies showed no association between CPFS or OS and AR-V7 status (OS HR 1.6; 95% CI 0.6–4.4; P = .40).
Conclusions:
the suggestion is that taxane therapy is more efficacious than abiraterone or enzalutamide for men with AR-V7+ CRPC. On the contrary, clinical outcomes did not seem to differ significantly on the basis of the type of therapy used among AR-V7− cases.
Keywords: abiraterone, androgen receptor, castration resistant, enzalutamide, prostate neoplasm, taxane
1. Introduction: the clinical point
Prostate cancer (PC) is an androgen-dependent disease and androgen receptor (AR) is one of the main molecular targets for systemic therapy. After an initial response to first-line androgen deprivation therapies (ADT), nearly all cases with advanced PC progress to a castration-resistant prostate cancer (CRPC). In CRPC, however, AR continues to be a primary molecular driver, as evidenced by significant response to novel hormonal therapies, abiraterone, and enzalutamide.[1] Mechanisms of resistance to systemic therapies in PC are also driven by AR aberrations or overexpression, AR gene amplification, and mutations, AR variants (AR-Vs).[2]
AR-Vs are truncated AR proteins without the AR ligand-binding domain, allowing for constitutive AR signaling in the absence of androgens. A structural rearrangement in AR gene and alternative AR mRNA splicing are 2 possible mechanisms for the development of AR-Vs in CRPC.[3] Since their discovery in 2004, the role of AR-Vs has remained enigmatic.[4] Their possible clinical utility was demonstrated in 2014 by Antonarakis et al[5] who noted an association between androgen receptor variant 7 (AR-V7) in circulating tumor cells (CTC) and outcomes in PC cases treated with second-generation androgen signaling inhibitors.
Multiple AR-Vs have been characterized (Fig. 1). To date, AR-V7 has been studied in greatest detail and it has been suggested as potentially clinical marker for treatment selection in men with CRPC.[6] Prior studies have determined that AR-Vs can be detected at the RNA and protein levels in tissue samples. Although AR-Vs can be detected in untreated PC and in benign prostate tissue, their levels are lower and cannot lead to robust evaluation by RNA in situ hybridization or immunohistochemistry. AR-V7 expression is significantly higher in CRPC, due to AR gene amplification and induction by ADT.[7]
Figure 1.
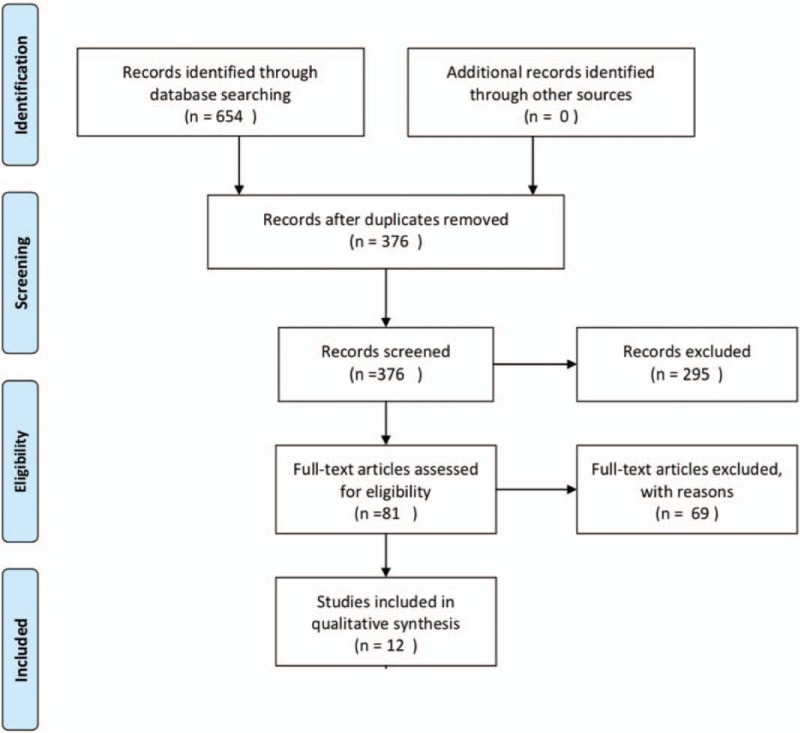
PRISMA diagram for study selection.
Other studies have utilized CTC, plasma exosomes and also whole blood samples for the detection of AR-V7 in men with metastatic castration-resistant prostate cancer (mCRPC).[8] There are 2 clinically available CTC-based AR-V7 tests: one uses a polymerase chain reaction (PCR) in a capture-based CTC system and the second applies an immunofluorescent protein assay.[4] Each of these AR-V7 testing has its limitations and a significant proportion of CRPC cases are CTC negative. The St.Gallen PC conference reached a consensus against the use of AR-V7 in routine practice for mCRPC.[3] However, a recent clinical audit by the Johns Hopkins revealed that the determination of AR-V7 status influenced clinical decision making in a significant proportion of CRPC patients.[9] The results for an AR-V7- test did not change the clinical practice of the providers, whether almost two-thirds (62%) of AR-V7+ tests resulted in a change in management.[9] Patients with an AR-V7− result were preferentially treated with abiraterone or enzalutamide whereas after an AR-V7+ result, most cases shifted from abiraterone or enzalutamide to taxane chemotherapy (Fig. 2).
Figure 2.
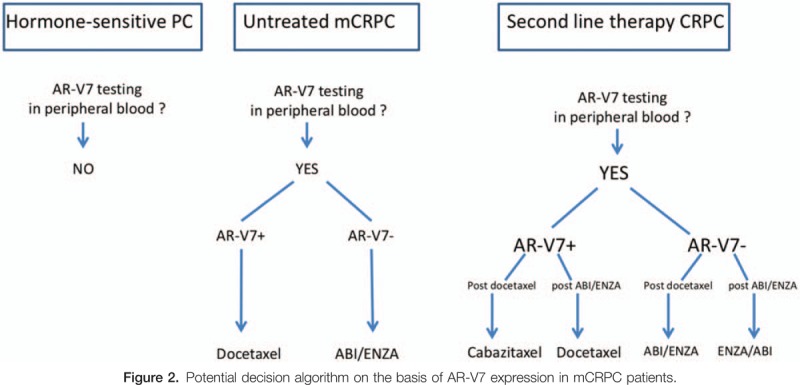
Potential decision algorithm on the basis of AR-V7 expression in mCRPC patients.
The optimal sequencing of therapeutic agents in mCRPC remains a major challenge. The analysis of AR-V7 as biomarker of therapeutic agents’ resistance may help in this treatment decision and it may have a clinical and economic benefit. The National Comprehensive Cancer Network PC guidelines now suggest that AR-V7 testing can be used to define therapy selection in mCRPC, but this suggestion is not traduced as a recommended test.
2. Aim and methods
The aim of the present review is to critically analyze and compare the current evidence on the AR-V7 determination as prognostic clinical indicator of therapeutic response in mCRPC cases. In particular, we analyzed—frequency of the AR-V7 expression in mCRPC cases—impact of AR-V7 expression on abiraterone and enzalutamide therapy—impact of AR-V7 expression on taxane chemotherapy.
2.1. Evidence acquisition
We focused our analysis only on AR-V7 and not on all AR-Vs testing. We searched in the Medline and Cochrane Library database (primary fields: castration-resistant prostate neoplasm, AND AR-V7 determination; secondary fields: abiraterone, enzalutamide, chemotherapy) without language restriction from the literature of the past 10 years, according to PRISMA guidelines (Fig. 1). Original and review articles were included and critically evaluated. Additional references were identified from reference lists of these articles. We have not included abstracts and reports from meetings. We analyzed data only from clinical trials (retrospective or prospective) focusing their attention on the clinical role of AR-V7 in PC.
For all studies, we critically evaluated the level of evidence according to the European Association of Urology (EAU) guidelines.[10]
3. Results
3.1. Search results
The database searches initially yielded 654 journal article references. Of these 573 were subsequently removed due to either duplication or a failure to meet the selection criteria. Full-text articles were then re-evaluated and critically analyzed for the remaining 81 journal references. Of these, 69 did not meet the inclusion criteria. The remaining 12 studies were considered for our critical review (Table 1 ).
Table 1.
Main data from the 12 studies considered in the review.
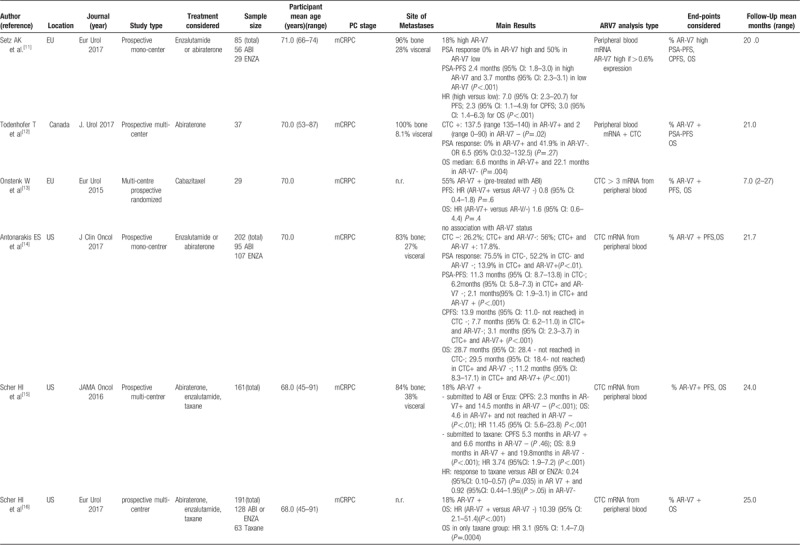
Table 1 (Continued).
Main data from the 12 studies considered in the review.
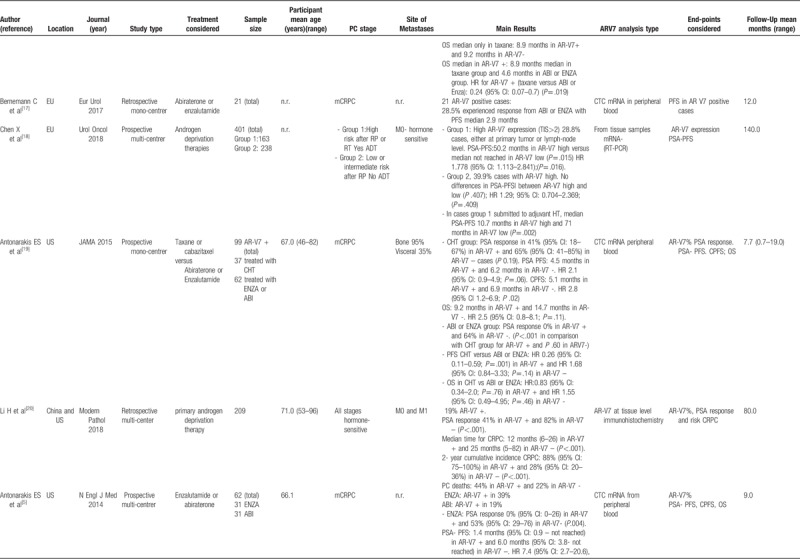
Table 1 (Continued).
Main data from the 12 studies considered in the review.
3.1.1. Study location and type
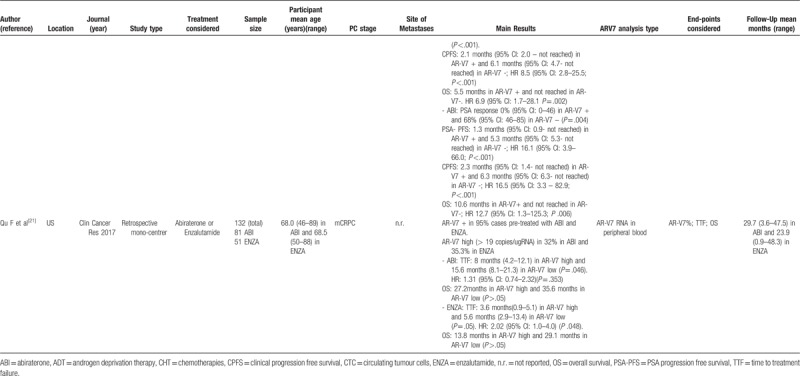
The 12 studies[5,11–21] entered into the review, 4 were conducted in Europe,[11,13,17,18] 7 in US,[5,14–16,19–21] 1 in Canada[12] e 1 in China [20 either in China or US]. One study was multicenter randomized,[13] 8 were prospective mono or multicentric[5,11,12,14–16,18,19] and 3 were retrospective mono or multicentric studies.[17,20,21] Moreover, 5 studies comparatively considered the different treatments for PC [new hormone therapies versus chemotherapy: 15,16,19; abiraterone vs enzalutamide: 5,21; and 7 studies analyzed only 1 treatment[11–14,17,18,20] (Table 1 ).
3.1.2. Study sample sizes and cancer treatment
The sample size strongly varied from 21 to 401 cases analyzed in a single study. The total sample size of the 12 studies was 1629 (Table 1 ).
New hormone therapies such as Abiraterone or enzalutamide were used in 9 studies[5,11,12,14–17,19,21] with a total sample of 809 cases. Chemotherapies (taxane or cabazitaxel) were used in 4 studies[13,15,16,19] with a total sample of 210 cases.
3.1.3. Participant age
The range of mean age across the studies was very similar and it varied from 66.0 to 71.0 years. Also, the mean age of each treatment was similar with 68.5 years (SD ± 2.1) for new hormone therapies and 68.2 years (SD ± 1.7) for chemotherapies group (Table 1 ).
3.1.4. Cancer staging and follow-up
Ten out of 12 studies included only mCRPC cases (total cases: 957). Two studies considered hormone sensitive PC (total cases: 610); in particular one[18] considered M0 hormone sensitive cases stratified in terms of high risk versus low or intermediate risk submitted to androgen deprivation therapies and one[19] included all stages (M0-M1) (Table 1 ). Mean follow-up ranges from 7 to 140 months
3.1.5. AR-V7 analysis
Two studies[11,21] analyzed AR-V7 mRNA in peripheral blood considering high versus low expression. Eight studies[5,12,13–17,19] evaluated AR-V7 positivity in CTC mRNA from peripheral blood. Two studies[18,20] analyzed AR-V7 at tissue level by RT-PCR[18] or by immunohistochemistry[20] (Table 1 ).
3.2. Incidence of AR-V7 positivity in the studies
All studies[5,11–21] reported the level of AR-V7 positivity in the population analyzed. Independently to the method used, at baseline, before the beginning of specific treatments for CRPC, the mean percentage of cases (treated with androgen deprivation therapies for hormone-sensitive PC) with AR-V7 positivity was 18.3% (range 17.8%–28.8%).[5,11,14–20] A higher percentage of AR-V7 positivity was found by Onstenk et al[13] (55.0%) and by Qu et al[21] (95.0%), but their populations were already treated with abiraterone or enzalutamide.
3.2.1. Critical analysis and level of evidences
Data have been analyzed by several prospective multi or mono-center studies. From these data we can expect that, at baseline, a population with mCRPC, before the beginning of specific treatments (abiraterone, enzalutamide or chemotherapies) will show a 18% positivity for AR-V7 (level of evidence 2a). This percentage is very homogeneous among the studies and it is not influenced by the method used for AR-V7 analysis. Unfortunately, studies did not specify the type and the number of androgen deprivation therapies used in the hormone-sensitive phase or the Gleason score of these PC cases.
On the contrary in a population of mCRPC already treated with abiraterone or enzalutamide, we can expect higher percentage (>50%) of AR-V7 positivity (level of evidence 1b).
3.3. Impact of AR-V7 expression on prostate-specific antigen (PSA) response among the different treatment groups
The impact of AR-V7 positivity on PSA response (defined as PSA reduction >50%) was specifically evaluated in 6 studies.[5,11,12,14,19,20]
Li et al[20] retrospectively analyzed 209 cases with hormone-sensitive PC submitted to primary androgen deprivation therapy (M0 and M1 cases). PSA response during the follow-up was significantly (P <.001) influenced by AR-V7 status with lower percentage of response in AR-V7 positive cases (AR-V7+: 41%; AR-V7−: 82%).
In mCRPC cases submitted to abiraterone or enzalutamide,[5,11,12,14,19] homogeneously an AR-V7 positivity was associated with 0% of PSA responses versus a mean of 54.8% (range: 41.9%–64.0%) in AR-V7 negative cases (P <.001).
A prospective comparative study on mCRPC submitted to chemotherapies versus abiraterone or enzalutamide[19] showed that no significant differences (P = .19) in PSA response between AR-V7 positive (41.0%) and negative (65.0%) cases was reported in chemotherapies treated cases.
3.3.1. Critical analysis and level of evidences
The analysis of PSA response in hormone-sensitive PC cases was performed by only 1 retrospective study without stratification of cases on the basis of the lines of ADT used (level of evidence 2b). Different prospective studies well confirmed that mCRPC AR-V7 positive cases do not show a PSA response if treated either with abiraterone or enzalutamide (level of evidence 2a). Generally, stratified or comparative data between enzalutamide and abiraterone are not presented. On the contrary, a prospective comparative study showed that a significant percentage of AR-V7 positive cases show a PSA response if treated with chemotherapies (level of evidence 2b).
In conclusion, in the AR-V7+ mCRPC patients, PSA response, even if commonly considered as a secondary outcome compared with clinical progression or overall survival (OS), was found to be significantly influenced by Abiraterone or Enzalutamide therapy, while showed no response correlation in the group treated with taxane therapy. Data regarding this aspect cannot, however, be considered definitive in order to asses PSA response as a downstream target of the treatment regimen implemented.
3.4. Impact of AR-V7 expression on survival parameters among the different treatment groups
The impact of AR-V7 expression on PSA progression-free survival (PSA-PFS) was specifically examined in 7 studies.[5,11–14,18,19] All studies on mCRPC treated with abiraterone or enzalutamide[5,11,12,14,19] showed significantly (P <.05) lower PSA-PFS in AR-V7 positive (mean 1.8 months, range 1.3–2.4 months) than in AR-V7 negative (mean 5.3 months, range 3.7–6.2 months) (hazard ratio [HR] 7.4;95% CI 2.7–20.6) cases. On the contrary, in mCRPC cases submitted to chemotherapies,[13,19] PSA-PFS did not significantly vary according to AR-V7 status (HR0.8; 95% CI 0.4–1.8; P = .60). Also, in hormones sensitive cases,[18] PSA –PFS did not significantly vary according to AR-V7 (HR 1.29; 95% CI 0.7–2.3; P = .409).
The impact of AR-V7 expression on clinical progression-free survival (CPFS) and OS was evaluated in 9 studies.[5,11–16,19,21] All data on mCRPC submitted to enzalutamide or abiraterone[5,11–16,19,21] reported a significantly (P <.05) lower CPFS and OS in AR-V7 positive than in AR-V7 negative cases (mean CPFS: AR-V7+ 2.9 months; AR-V7− 8.3 months; mean OS: AR-V7+ 7.6 months; AR-V7− 22.1 months) (CPFS HR: 2.3;95% CI 1.1–4.9; OS HR: 3.0; 95% CI 1.4–6.3). In mCRPC cases submitted to chemotherapies,[13,15,16,19] data are not homogeneous. Onstenk et al[13] and Antonarakis et al[19] showed no association among CPFS or OS and AR-V7 status (OS HR 1.6; 95% CI 0.6–4.4; P = .40[13] and OS HR 2.5; 95% CI 0.8–8.1; P = .11[19]). Sher et al[15,16] on the contrary reported significantly (P <.05) lower CPFS and OS in AR-V7+ cases submitted to taxane (mean OS 8.9 months in AR-V7+ and 19.8 months in AR-V7−; HR 3.7; 95% CI 1.9–7.1; P <.001).
3.4.1. Critical analysis and level of evidences
Several prospective mono or multi-center studies confirmed a significant association between AR-V7 expression and survival parameters in mCRPC cases submitted to abiraterone or enzalutamide. These studies affirmed that abiraterone or enzalutamide treatments are associated with a significantly lower PSA-PFS, CPFS, and OS if an AR-V7 positive expression is detected (level of evidence 2a). Only 2 studies[5,21] comparatively analyzed abiraterone and enzalutamide treatment, showing no significant differences.
Data on CRPC cases submitted to chemotherapies are less homogeneous (level of evidence 2b) and the association between AR-V7 expression and survival parameters such as PSA-PFS, CPFS, and OS is no significant or less evident. Antonarakis et al[19] comparatively analyzed chemotherapies and enzalutamide or abiraterone showing no differences (OS HR between chemotherapies and abiraterone or enzalutamide: 0.83; 95% CI 0.34–2.0 in AR-V7+ and 1.55; 95% CI 0.49–4.95 in AR-V7−; P = .46)
3.5. Critical conclusions
Although there are multiple available therapies for men with mCRPC, there are currently no molecular biomarkers to help guide optimal treatment choices in these patients. The recent literature shows an important interest on the determination of AR-V7 expression in PC cases, as prognostic parameter and as indicator for the medical treatment choice.
The determination of AR-V7 in peripheral blood using CTC mRNA seems to be the preferred method.
The clinical usefulness of AR-V7 in hormone-sensitive PC cases is not demonstrated: data are mainly retrospective or limited in no homogeneous populations. It is not possible to hypothesize a role for AR-V7 expression in hormone-sensitive PC as prognostic marker for the treatment choice.
On the contrary in mCRPC treated with abiraterone or enzalutamide, it is well demonstrated that AR-V7+ patients have inferior clinical outcomes compared with AR-V7 negative cases, with respect to PSA response, PSA-PFS, CPFS, and OS. The AR-V7 status has the same impact on abiraterone or enzalutamide response and in some experiences, no AR-V7 positive case had an appreciable clinical benefit from enzalutamide or abiraterone. The impact of this biomarker is less relevant in patients selected for chemotherapies and its expression is not associated with a significant resistance to taxane. The suggestion is that taxane therapy is more efficacious than abiraterone or enzalutamide for men with AR-V7 positive CRPC. On the contrary, clinical outcomes did not seem to differ significantly on the basis of the type of therapy used among AR-V7 negative cases.
All these trials showed relevant limitations. For example, those trials focusing on Chemotherapy treatment did not consider whether patients received any and which treatment before or how long after treatment patients were diagnosed with mCRPC. We, therefore, cannot obtain final conclusion on a definitive role of AR-V7 in the prediction of response or in the selection of mCRPC cases for different treatments.
Author contributions
Conceptualization: Alessandro Sciarra, Ida Silvestri, Martina Maggi.
Data curation: stefano Salciccia, Susanna Catterino, Viviana Frantellizzi, Magnus Von heland, Francesco Del Giudice.
Formal analysis: Alessandro Gentilucci, Antonio gatto, Gian Piero Ricciuti.
Supervision: Francesco Del Giudice.
Validation: Susanna Scarpa.
Writing – review & editing: Susanna Scarpa, Francesco Del Giudice, Alessandro Sciarra.
Footnotes
Abbreviations: ADT = androgen deprivation therapies, AR = androgen receptor, AR-V7 = androgen receptor variant 7, CPFS = clinical progression free survival, CRPC = castration resistant prostate cancer, HR = hazard ratio, mCRPC = metastatic castration resistant prostate cancer, OS = overall survival, PC = prostate cancer, PCR = polymerase chain reaction, PSA = prostate specific antigen.
The manuscript does not directly contain clinical studies or patient data but is a critical review on already published clinical trials.
The authors declare that they have no conflict of interest
References
- [1].Luo J, Attard G, Balk SP, et al. Role of androgen receptor variants in prostate cancer: report from the 2017 mission androgen receptor variants meeting. Eur Urol 2018;73:715–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Takeuchi T, Okuno Y, Hattori-Kato M, et al. Detection of AR-V7 mRNA in whole blood may not predict the effectiveness of novel endocrine drugs for castration resistant prostate cancer. Res Urol 2016;8:21–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Gillessen S, Attard G, Beer TM. Management of patients with advanced prostate cancer: the report of the advanced prostate cancer consensus conference. Eur Urol 2018;73:178–211. [DOI] [PubMed] [Google Scholar]
- [4].Crumbaker M, Savdie R, Joshua AM. Refining the assessment and implications of AR-V7 in castrate-resistant prostate cancer. Eur Urolo 2018;73:736–7. [DOI] [PubMed] [Google Scholar]
- [5].Antonarakis ES, Lu C, Wang H. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med 2014;371:1028–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Luo J. Development of AR-V7 as a putative treatment selection marker for metastatic castration resistant prostate cancer. Asian J Androl 2016;18:580–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Henzler C, Li Y, Yang R. Truncation and constitutive activation of the androgen receptor by diverse genomic rearrangements in prostate cancer. Nat Commun 2016;7:13668–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Del Re M, Biasco E, Crucitta S. The detection of androgen receptor splice variant 7 in plasma-derived exosomal RNA strongly predict resistance to hormonal therapy in metastatic prostate cancer patients. Eur Urol 2017;71:680–7. [DOI] [PubMed] [Google Scholar]
- [9].Markowski MC, Silberstein JL, Eshleman JR, et al. Clinical utility of CLIA-grade AR-V7 testing in patients with metastatic castration resistant prostate cancer. JCO Precision Oncol 2017;1:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Heidenreich A, Bellmunt J, Bolla M, et al. EAU guidelines on prostate cancer. Part 1: screening, diagnosis and treatment of clinically localized disease. Eur Urol 2011;59:61–71. [DOI] [PubMed] [Google Scholar]
- [11].Seits AK, Thoene S, Bietenbeck A, et al. AR-V7 in peripheral blood of patients with castration resistant prostate cancer: association with treatment-specific outcome under abiraterone and enzalutamide. Eur Urol 2017;72:828–34. [DOI] [PubMed] [Google Scholar]
- [12].Todenhofer T, Azad A, Stewart C, et al. Ar-V7 transcripts in whole blood RNA of patients with metastatic castration resistant prostate cancer correlate with response to abiraterone acetate. J Urol 2017;197:135–42. [DOI] [PubMed] [Google Scholar]
- [13].Onstenk W, Sieuwerts AM, Kraan J, et al. Efficacy of cabazitaxel in castration resistant prostate cancer is independent of the presence of AR-V7 in circulating tumor cells. Eur Urol 2015;68:939–45. [DOI] [PubMed] [Google Scholar]
- [14].Antonarakis ES, Lu C, Luber B, et al. Clinical significance of androgen receptor splice variant-7 mRNA detection in circulating tumor cells of men with metastatic castration resistant prostate cancer treated with first and second line abiraterone and enzalutamide. J Clinical Oncol 2017;35:2149–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Scher HI, Lu D, Schreiber NA, et al. Association of AR-V7 on circulating tumor cells as a treatment specific biomarker with outcomes and survival in castration resistant prostate cancer. JAMA 2016;2:1441–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Scher HJ, Graf RP, Schreiber NA, et al. Nuclear-specific AR-V7 protein localization is necessary to guide treatment selection in metastatic castration resistant prostate cancer. Eur Urol 2017;71:874–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bernemann C, Schnoeller TJ, Luedeke M, et al. Expression of AR-V7 in circulating tumor cells does not preclude response to next generation androgen deprivation therapy in patients with castration resistant prostate cancer. Eur Urol 2017;71:1–3. [DOI] [PubMed] [Google Scholar]
- [18].Chen X, Bernemann C, Tolkach Y, et al. Overexpression of nuclear AR-V7 in primary prostate cancer is an independent negative prognostic marker in men with high risk disease receiving adjuvant therapy. Urol Oncol 2018;36:19–31. [DOI] [PubMed] [Google Scholar]
- [19].Antonarakis ES, Lu C, Luber B, et al. Androgen receptor splice variant 7 and efficacy of taxane chemotherapy in patients with metastatic castration resistant prostate cancer. JAMA Oncol 2015;1:582–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Li H, Wang Z, Xiao W, et al. androgen receptor splice variant 7 positive prostate cancer: a novel molecula subtype with markedly worse androgen deprivation therapy outcomes in newly diagnosed patients. Modern Pathol 2018;31:198–208. [DOI] [PubMed] [Google Scholar]
- [21].Qu F, Xie W, Nakabayashi M, et al. Association of AR-V7 and prostate specific antigen RNA levels in blood with efficacies of abiraterone acetate and enzalutamide treatment in men with prostate cancer. Clin Cancer res 2017;23:726–34. [DOI] [PMC free article] [PubMed] [Google Scholar]


