Abstract
The aim of the present study was to identify risk factors for intestinal failure (IF) in infants who received surgery for necrotizing enterocolitis (NEC).
A retrospective multicenter case-series study was conducted in a sample of 91 infants admitted to Children's Hospital of Chongqing Medical University between January 2010 and December 2017. The occurrence of IF was defined as the dependence on parenteral nutrition for ≥90 days. Logistic regression was used to investigate the predictors of IF.
Of 179 patients reviewed, excluding those with intestinal malformation and inadequate information, 91 were included in the study, and of these cases, 32 (35.2%) developed IF. Controlling for other factors, multivariate analysis showed that birth weight (OR = 0.999; 95% CI, 0.998–1.000; P = .010), the length of the bowel resected (OR = 1.109; 95% CI, 1.048–1.173; P = .000), and the percentage of small bowel resected (OR = 1.305; 95% CI, 1.133–1.504; P = .000) were factors that increased the chances of IF occurrence.
Our data demonstrated that variables characteristic of severe NEC, including lower birth weight, greater extent of bowel resection, and larger percentage of small bowel resection were associated with the incidence of IF.
Keywords: bowel resection, enteral autonomy, intestinal failure (IF), necrotizing enterocolitis (NEC), short bowel syndrome (SBS)
1. Introduction
Intestinal failure (IF) describes a chronic condition with reduced nutrient, electrolyte, and fluid absorption. Namely, IF is defined by the requirement for parenteral nutrition (PN) for at least 90 days.[1–3] Multidisciplinary care for patients with IF is highly resource-intensive, including prolonged hospital stays, multiple readmissions, and the requirement for multiple surgical procedures. While the care of patients with IF has strikingly improved, the mortality and morbidity rates associated with this condition remain substantial.[4,5]
IF during infancy frequently results from surgical or congenital conditions that lead to acquired short bowel syndrome (SBS), in which there is reduced mucosal surface area. Among infants with low birth weight (<1500 g), short-bowel syndrome is commonly caused by necrotizing enterocolitis (NEC). According to research, IF in premature infancy is high, with the incidence ranging from 13% to 45%.[6,7] The consequences of IF in infants are severe, including high mortality, PN–associated liver disease, multiple micronutrient deficiencies, recurrent central venous catheter infections, chronic diarrhea, osteopenia, and poor growth.[8]
To achieve full enteral nutrition, it is important to identify the population. The identification of high risk patients for IF may allow for heightened vigilance and corresponding interventions, which would possibly improve clinical outcomes. The risk factors of IF, including low birth weight, bowel resection (>45 cm), resection of the ileocecal valve, and a reduced colonic remnant have previously been determined in small, retrospective, single-center case series.[9–11] Limited data also support an association between IF and postoperative nutritional management, including early enteral feeding and the use of breast milk and elemental formulas. Due to the variability in the criteria of IF, patient populations, duration of patient follow-up, characteristics of patient management, and the interpretation of published data should be approached with caution. In addition, the potential relationship between operation features and particular pediatric conditions needs to be elucidated. We sought to investigate the risk factors for IF in infants undergoing surgery for NEC by retrospectively reviewing data on infants with NEC and a history of PN in a multicenter study.
2. Methods
2.1. Patients
We conducted a multicenter retrospective cohort study from January 2010 to October 2017 in a collaborative multidisciplinary program among 3 newborn intensive care units (NICUs) at Yongchuan Hospital, Children's Hospital of Chongqing Medical University and Jinan Maternity and Child Care Hospital. The current research protocol was approved by the Institutional Review Board (IRB) of the Chongqing Medical University and the institutional review board at other sites. Entry criteria for this study were infants with a diagnosis of NEC who had undergone a surgical procedure during the study period. NEC was defined according to the criteria originally proposed by Bell et al and subsequently modified by Walsh and Kliegman.[12] Exclusion criteria included infants conceived through in vitro fertilization (IVF), infants with acute pulmonary bacterial infection, gastrointestinal anomalies (aproctia, intestinal atresia, congenital intestinal malrotation, congenital omphalocele, yolk tube malformation, or Hirschsprung disease) and infants who died during acute NEC. Additionally, to minimize severity differences in the study population, patients managed in the respiratory support unit for more than 30 days were excluded. Infants who were lost to follow up before reaching 90 days could not be classified as developing or not developing IF and were therefore excluded from the analysis. All infants with NEC were followed up in the hospital's outpatient clinic until at least 3 months of corrected gestational age. One hundred seventy-nine patients underwent at least one operation for NEC and met the inclusion criteria for enrollment. The study flow chart is shown in Fig. 1.
Figure 1.
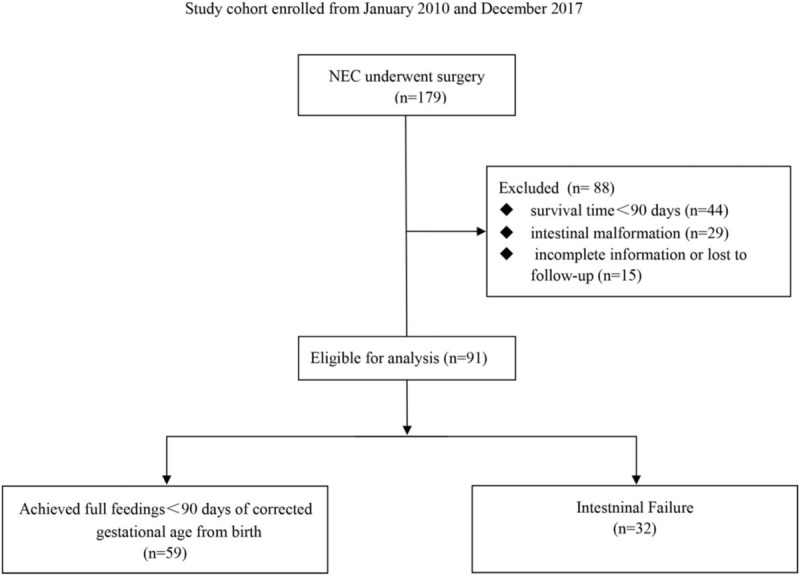
Study flow chart. NEC = necrotizing enterocolitis.
2.2. Surgical procedure, postoperative management and follow up
All the patients were subjected to the same medical program, including feeding regimen, fluid resuscitation, PN support, and perioperative broad-spectrum antibiotics as directed at the discretion of the clinicians. The decision for surgical intervention, including either placement of a peritoneal drain or exploratory laparotomy, with or without intestinal resection for all the patients, as well as the resection range and location was made according to the preference and experience of the surgical team on duty. Abdominal exploration was performed through a transverse abdominal incision. All explicity necrotic intestines were resected, and intestinal stomas or end-to-end intestinal anastomoses were created in the location; otherwise, only abdominal drainage was placed. Patients were followed daily throughout their hospitalization. All the patients were subjected to the same postoperative program, including fluid resuscitation, PN support, and perioperative broad-spectrum antibiotics if necessary. Complications and readmissions were recorded by the surgical team.
2.3. Data collection
To assess the risk factors for IF in infants, comprehensive factors available in clinical practice were selected from a large set of demographic, clinical, and laboratory variables. Two clinicians independently evaluated the abstracted data. We recorded variables, such as demographic data (age, sex, weight, maternal, prenatal, and intrapartum data, medication history for both mother and child), physical examination findings, radiographic findings, laboratory data (blood culture results), clinical management (ventilation status, fluid/hemodynamic status, feeding intolerance, apneic/bradycardic episodes, oxygen desaturation, etc). We also included all surgical features considered to be the surgical predictors of IF, including preoperative and intraoperative variables (the American Society of Anesthesiologists [ASA] physical status classification, operative time, starting time, duration of the operation, anesthesia, intraoperative events, volume of blood lost, histopathology of resected bowel segment, etc).
Data collected during follow up included the mode of feeding (parenteral or enteral), method (bolus or continuous), type of enteral nutrition (breast milk, formula, or a combination), milk-taking amount, and weight gain of the infant. IF was defined as dependence on PN for more than 90 days to maintain protein-energy, fluid, electrolyte, or micronutrient balance. Full enteral feeding was defined as receiving at least 100 kcal/kg/day from enteral nutrition with maintenance of acceptable growth variables (mean weight gain of >15 g/day over 7 days) and discharge to home after discontinuing PN.
3. Statistics
Statistical analysis was conducted using SPSS 20.0 (IBM, Armonk, NY, USA) software. Categorical variables were summarized as frequencies with percentages and tested using the chi-squared test or Fisher's exact test, as appropriate. Continuous data were shown as the mean ± standard deviation (SD) when normally distributed, and as medians (interquartile ranges, IQR) for nonnormally distributed data, which were tested with the Mann–Whitney U test or the Wilcoxon rank-sum test, respectively. To elucidate the risk factors for IF, variables with a P value ≤ .01 in the bivariate analysis or that were deemed clinically relevant were included in multiple regression forward stepwise analysis models (risk ratio [RR], 95% confidence interval [CI]). Statistical significance was accepted at a two-tailed P value < .05.
4. Results
4.1. Demographic data
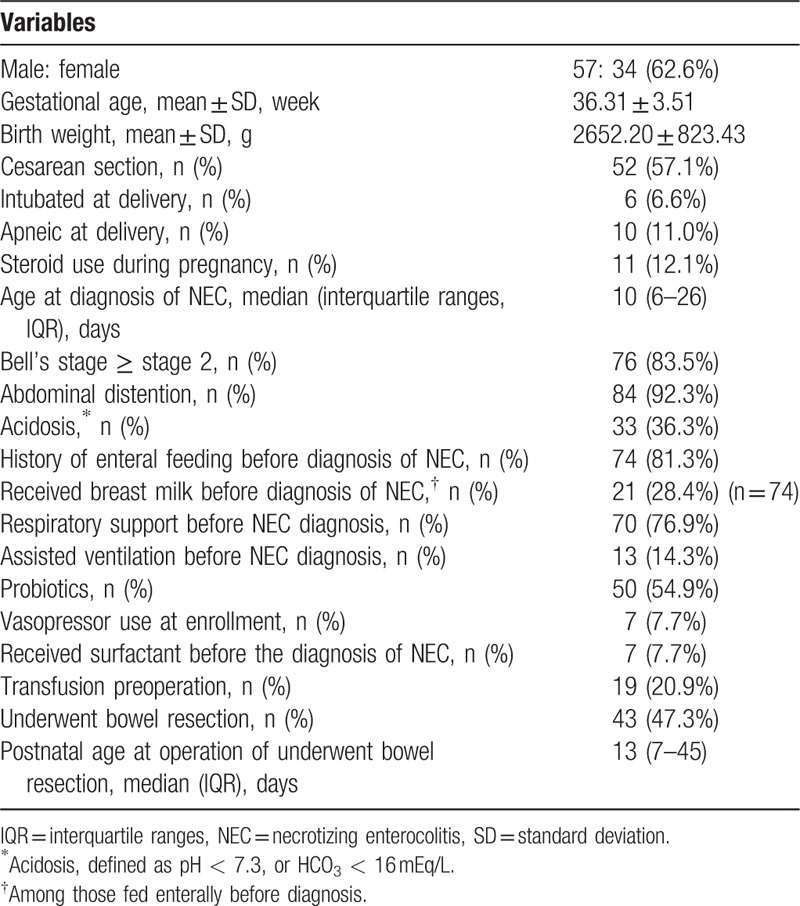
Between January 2010 and October 2017, there were 179 preterm infants with NEC following surgical management eligible for analysis. Among them, 88 cases were excluded due to incomplete information (n = 59) or intestinal malformation (n = 29). Of the remaining 91 cases, 48 patients underwent laparotomy intervention with simple drainage, and 43 underwent bowel resection followed by intestinal anastomosis or ostomy after a median time period of 13 days (range: 7–45). Baseline demographic and clinical characteristics of the infants are shown in Table 1. Seventy-six infants (83.5%) were assessed with Bell's stage 2 disease. The median age at diagnosis was 10 days, and 62% of the infants (n = 57) were male. The average gestational age (GA) and birth weight of the patients were 36.31 ± 3.51 weeks and 2652.20 ± 823.43 g, respectively. Of the infants included in the study, 57.1% were born by cesarean section, and 6.6% were intubated at delivery. Mothers of 11 infants (12.1%) used steroids during pregnancy. At diagnosis of NEC, the most common findings were abdominal distention (92.3%) and acidosis in 33 (36.3%) neonates. Only 7 infants (7.7%) received surfactant before the diagnosis of NEC, and 50 cases (54.9%) received probiotics. The need for respiratory support was 76.9%; 13 infants (14.3%) required assisted ventilation and 7 infants (7.7%) needed hemodynamic support by fluid expansion with the administration of vasoactive amines, reflecting the severity of NEC. Most of the included infants received enteral feeding before the diagnosis of NEC, and more than a quarter of those enterally fed received breast milk (28.4%). Of the 91 surgical NEC infants eligible for analysis, 32 (35.2%) had development of IF and 59 (64.8%) achieved full parenteral feedings before 90 days.
Table 1.
Baseline demographics and clinical characteristics for eligible Cohort (n = 91).
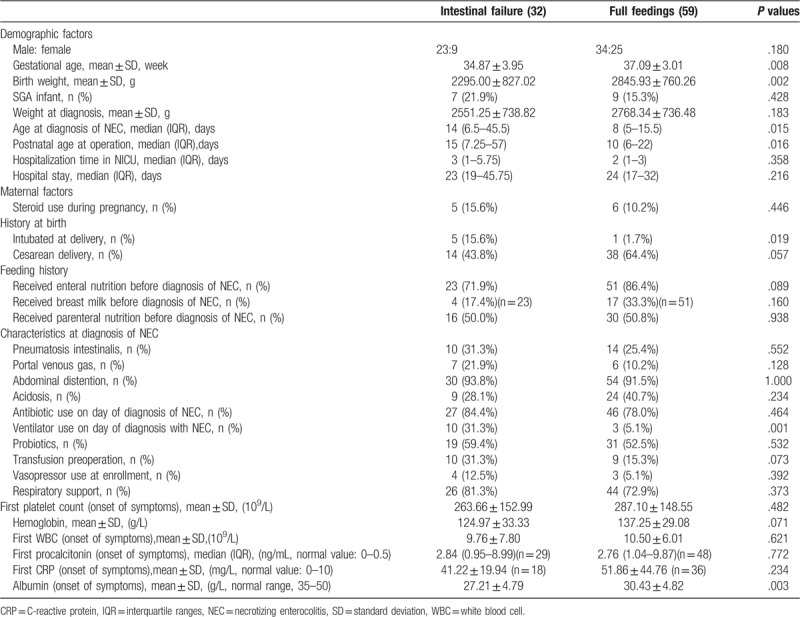
A comparison of the baseline characteristics and clinical status between patients who had development of IF and patients who achieved full feedings within 90 days is reported in Table 2. Infants who had development of IF were more likely to be severe, younger at birth by an average of almost 2 weeks (P = .008) and to have lower birth weight by more than 500 g (P = .002). The need for intubation at delivery (P = .019) and ventilator use on the day of diagnosis with NEC (P = .001) were significantly different, suggesting a greater severity of NEC at diagnosis in those with development of IF. The postnatal age at operation (P = .016) and diagnosis age of NEC (P = .015) in the subjects achieving full feedings before 90 days was significantly younger than those with IF. As expected, the mean hospitalization time in the NICU (P = .358) was longer in the IF group, although the difference was not significant d. For the basic laboratory measures analyzed, a significant difference in albumin was found between the two groups within the hospital (P = .003), which showed that infants who developed IF had a poor nutritional status. In addition, the degree of anemia was more serious in the IF group (P = .071), which had a higher proportion of neonates with transfusion preoperation (P = .073). No significant differences were found in inflammatory markers (leukocyte, C-reactive protein, and procalcitonin) during onset of symptoms between the two groups.
Table 2.
Baseline demographics of eligible patient and preoperative variables (chi-square test and Student's t-test).
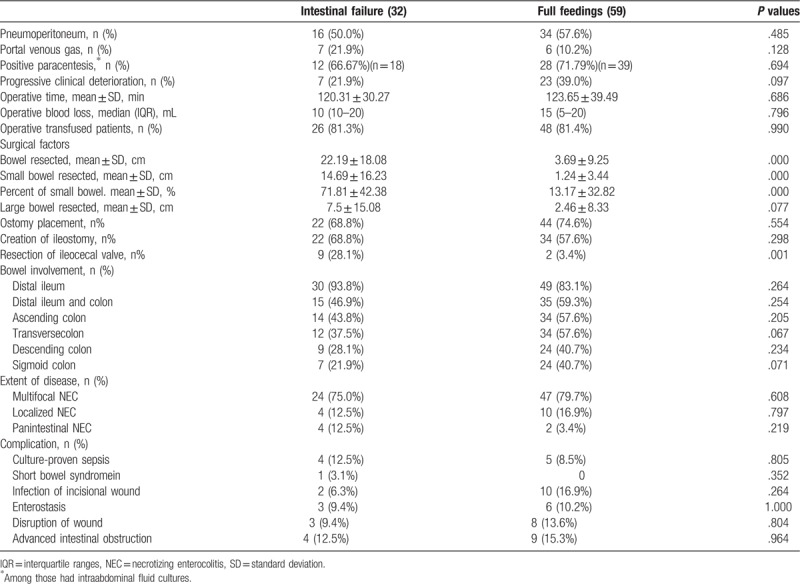
4.2. Surgical feature
The surgical features were further assessed based on patients who had development of IF and patients who achieved full feedings within 90 days (Table 3). A higher percentage of small bowel resection cases and pan-intestinal NEC were observed in the IF group (71.81 ± 42.38% vs 13.17 ± 32.82%, P = .000). The amount of small intestine resected was significantly larger (14.69 ± 16.23 cm vs 1.24 ± 3.44 cm, P = .000) in the infants that developed IF compared to those that did not. The median resected bowel length was 15 cm (10–34.25) for IF and 0 cm (0–0) for infants achieving full feedings. Not surprisingly, patients with pan-intestinal involvement and multifocal disease were more likely to develop IF; although no statistical significance was attained. The cause of IF was primarily attributed to intestinal stricture and SBS in 32 infants. There was no difference between the 2 groups with respect to other surgical factors, including large bowel excision (P = .077), bowel involvement, the extent of bowel complications, operative time, operative blood loss, and operative transfusion. The imaging performance, progressive clinical deterioration, and positive paracentesis reflected the severity of disease. According to common ideas, it seemed that statistically significant differences would be discovered between the two groups; however, no one element mirrored IF development. The ileum was the most common location affected for the infants involved (n = 79, 86.8%), and resection of the ileocecal valve was performed in a higher percentage (P = .001) in the IF group than in those achieving total enteral nutrition, but the creation of an ileostomy was not significantly associated with development of IF. In fact, among 66 subjects who underwent diverting ostomy placement, 22 of 56 (39.3%) patients with an ileostomy had IF development. Among these patients, 34 (60.7%) underwent takedown within 90 days of study entry. No deaths occurred after stoma closure.
Table 3.
Surgical features of patients who undergone surgery (chi-square test and Student's t-test).
4.3. Multivariate analysis
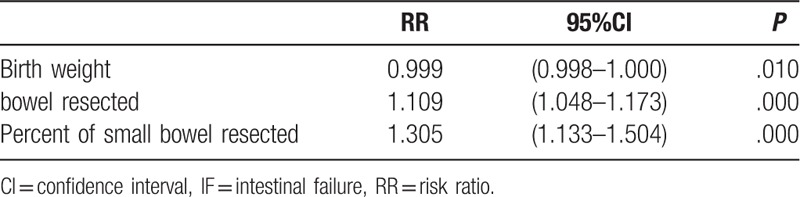
Multivariable logistic regression analysis was performed to adjust for potential confounding factors in order to exclude the effects of correlation between factors, and a forward stepwise regression was adopted to introduce the variables into the analysis. Three risk factors for the development of IF were identified (Table 4). As shown in Table 4, the longer the intestine resected, the more likely the patient developed IF (OR = 1.109; 95% CI, 1.048–1.173; P = .000). Similar results were discovered in the percentage of small bowel resected (OR = 1.305; 95% CI, 1.133–1.504; P = .000). Birth weight (OR = 0.999; 95% CI, 0.998–1.000; P = .010) was also significantly associated with IF.
Table 4.
Multivariate models for the development of IF.
5. Discussion
Risk factors might provide clues about the etiology of IF and, thus, may be important and necessary for clinical measurement and the improvement of follow up care. In this multicenter retrospective cohort of infants with surgical NEC, we found that prematurity and more severe NEC variables were important risk factors for the development of IF, as noted by the significant relationships between IF and birth weight, intubation at delivery, gestational age, ventilator use, albumin, etc. Furthermore, infants undergoing intestinal section for NEC had a considerably higher risk for the development of IF compared with those not requiring intestinal section. A novel discovery in this study was that later NEC onset was associated with IF in the univariate statistical analysis but did not remain a significant predictor in the final multivariate model.
To our knowledge, few studies have directly probed into the incidence of IF among infants undergoing surgical therapy for NEC. In this research, of the 91 infants undergoing surgery and surviving ≥90 days, 32 infants (35.2%) remained dependent on PN more than 90 days after surgery, which was defined as IF. A high incidence of IF (42%) was also reported in patients with NEC undergoing laparotomy or peritoneal drainage.[13] In contrast, an IF incidence of 23% was reported in a study of 175 neonates undergoing any gastrointestinal surgery. The base characteristic differing among these reports was the mean gestational age, which might account for the lower incidence of IF in the latter research.[14]
Although some select maternal and infant clinical characteristics were unable to predict the occurrence of IF, we found several factors to be associated with the development of IF in the univariate analysis, which was not surprising. A novel finding in the present study was that later diagnosis of NEC was associated with IF development in the univariate statistical analysis, which implied that the etiology of NEC for early or later onset may have existential discrepancy.[15] The results from a two-center analysis recently reported that late onset of NEC in the full-term infant is associated with increased mortality.[16] Motta et al reported that many associated illness and anomalies differed greatly regarding time to onset of NEC in the term and preterm infant.[17] Consistent with this, our data indicated an older postnatal age at operation for the IF population. Previous results suggested early-onset NEC might represent misclassified spontaneous intestinal perforation (SIP).[18] In addition, studies have reported poorer hospital and neurodevelopmental outcomes in patients with surgical NEC compared with SIP.[19] Furthermore, later onset NEC patients tend to present with pneumatosis intestinalis or necrosis of their intestines. Patients with pneumatosis might have more extensive intestinal involvement.[20] Despite these differences, the difficulty in correctly identifying one disease state over the other at the time of presentation needs to be acknowledged, allowing us the potential to optimize our surgical and therapeutic approach to this group of patients.
Enteral nutrition before the diagnosis of NEC was marginally associated with subsequent IF in an unadjusted analysis, suggesting that enteral nutrition can be a risk factor for IF development. The lack of a statistically significant association of the enteral nutrition before diagnosis of NEC with IF in this cohort may be due to the small proportion of children and the possibility of confounders (differences in institutional practices and severity of illness, among other factors). This finding should be considered in the context of other known risk factors for IF development in preterm infants. In contrast, NEC among infants who have been enterally fed may be harmful, especially if there is peritoneal contamination by enteral feeds, which was supported with a previous finding that enteral feeding before diagnosis of NEC was associated with subsequent IF.[21,22] Prior available evidence has suggested that not only mode (enteral vs PN) but also the use of breast milk or elemental formulas had influenced outcomes in IF.[23,24] In this research, PN before the diagnosis of NEC and the use of breast milk were not associated with the development of IF. In our analysis, there may have been other variables related to enteral feeding that we were unable to control, such as duration of PN before NEC diagnosis. Randomized controlled clinical trials of different types of enteral feeding in SBS are needed to fully address this question. Currently, human milk should still be considered as the primary type of enteral nutrition for infants with IF for the inherent advantages over commercially prepared formulas, including the presence of secretory IgA, amino acids, numerous growth factors, and other components.[23,25]
Due the absorptive ability of the jejunum and ileum, more proximal bowel resection may indicate a greater need for PN.[26] Among the surgical factors analyzed in the present study, we further confirmed that the percentage of small bowel resection and ileocecal valve resection were most predictive factors for the development of IF and, therefore, should be utilized to risk stratify children with IF. The resection of the ileocecal valve might predispose patients to SBS and, subsequently, to more episodes of IF.[6,27] There are compelling statistical and clinical reasons that ICV could somewhat compensate the amount of bowel resected. Previous data suggested that a threshold value of 41 cm or greater appears to be most predictive of the ability to wean from PN support.[28] In the present study, postnatal age at operation was greater in the patients who suffered from IF. Our study did not allow us to investigate whether later operation was inherently associated with severe damage or if a delay in diagnosis contributed to worse outcomes. Furthermore, jejunostomy and ileostomy might be potential predictors of IF. A reduction in the absorption area may underlie our observed association between IF and small-bowel resection or the creation of a jejunostomy. Another report suggested that most of the patients achieved enteral autonomy in a median time of 1 to 2 years if the remaining small intestine exceeded 50%.[1] Our previous data indicated that pan-intestinal involvement indicated more severe disease; whereas colon involvement indicated milder disease.[29] It is possible that we did not identify this association with IF development due to the small number of patients studied and/or high rate of morbidities associated with prematurity.
The limitations of our study are primarily related to the retrospective nature, patient selection and categorization with inherent risk of selection bias. Misclassification bias was possible since NEC is likely an umbrella diagnosis representing a common pathway for a variety of pathophysiologic conditions with clinical features similar to NEC, such as SIP, Hirschsprung's associated enterocolitis, and milk protein allergy. Although, the pathological reports, operative notes, and radiographic images were reviewed for these infants to meet the recruitment criteria. There have likely been many practice changes over the long time period, and outcomes from many patients within both the surgery and the neonatology divisions were included, leading to different care practices between study patients. In addition, there was a high proportion of treatment withdrawal in the developing circumstance, making it difficult to interpret the survival data.
6. Conclusion
In summary, the present study offers clues about the etiology of IF in specific patient populations, particularly with respect to the degree of prematurity and illness severity. However, none of these factors seem easily amenable to preoperative or postoperative modification. We acknowledge that these results are based on a homogenous group of patients. It will be necessary to conduct quality investigations in the future to help design clinical trials for new therapies to either prevent IF or optimize the treatment of NEC.
Acknowledgments
We thank Prof. Xianqing Jin for providing technical assistance and for insightful discussions during the preparation of the manuscript. We thank Dr Xiaoyong Zhang at the Wistar Institute, USA, for helping with the linguistic revision of the manuscript.
Author contributions
Huan Wang, Chun Deng designed, analyzed the data, and evaluated the manuscript. Chunbao Guo performed the statistic measurement and analyzed the data. Chunbao Guo analyzed the data and wrote the paper.
Data curation: Huan Wang, Yan Wang, Chun Deng, Lei Li, Chunbao Guo.
Methodology: Huan Wang, Chun Deng.
Resources: Huan Wang, Chun Deng.
Writing – original draft: Chunbao Guo.
Footnotes
Abbreviations: ASA = American Society of Anesthesiologists, CI = confidence interval, CRP = C-reactive protein, GA = gestational age, IF = intestinal failure, IQR = interquartile ranges, IRB = Institutional Review Board, IVF = in vitro fertilization, NEC = necrotizing enterocolitis, PN = parenteral nutrition, RR = risk ratio, SD = standard deviation, WBC = white blood cell.
This research was supported by National Natural Science Foundation of China (No: 30973440, 30770950), and the key project of the Chongqing Natural Science Foundation (CSTC, 2008BA0021, cstc2012jjA0155).
The authors report no conflicts of interest.
References
- [1].Belza C, Fitzgerald K, de Silva N, et al. Predicting intestinal adaptation in pediatric intestinal failure: a Retrospective Cohort Study. Ann Surg 2017;269:988–93. [DOI] [PubMed] [Google Scholar]
- [2].Squires RH, Duggan C, Teitelbaum DH, et al. Pediatric Intestinal Failure Consortium. Natural history of pediatric intestinal failure: initial report from the Pediatric Intestinal Failure Consortium. J Pediatr 2012;161:723–8.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Duro D, Kalish LA, Johnston P, et al. Risk factors for intestinal failure in infants with necrotizing enterocolitis: a Glaser Pediatric Research Network study. J Pediatr 2010;157:203–8.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Nucci A, Burns RC, Armah T, et al. Interdisciplinary management of pediatric intestinal failure: a 10-year review of rehabilitation and transplantation. J Gastrointest Surg 2008;12:429–35. [DOI] [PubMed] [Google Scholar]
- [5].Javid PJ, Oron AP, Duggan C, et al. Pediatric Intestinal Failure Consortium. The extent of intestinal failure-associated liver disease in patients referred for intestinal rehabilitation is associated with increased mortality: an analysis of the pediatric intestinal failure consortium database. J Pediatr Surg 2017;53:1399–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Spencer AU, Neaga A, West B, et al. Pediatric short bowel syndrome: redefining predictors of success. Ann Surg 2005;242:403–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Petty JK, Ziegler MM. Operative strategies for necrotizing enterocolitis: the prevention and treatment of short-bowel syndrome. Semin Pediatr Surg 2005;14:191–8. [DOI] [PubMed] [Google Scholar]
- [8].Duro D, Kamin D, Duggan C. Overview of pediatric short bowel syndrome. J Pediatr Gastroenterol Nutr 2008;47Suppl 1:S33–6. [DOI] [PubMed] [Google Scholar]
- [9].Sparks EA, Khan FA, Fisher JG, et al. Necrotizing enterocolitis is associated with earlier achievement of enteral autonomy in children with short bowel syndrome. J Pediatr Surg 2016;51:92–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Fallon EM, Mitchell PD, Nehra D, et al. Neonates with short bowel syndrome: an optimistic future for parenteral nutrition independence. JAMA Surg 2014;149:663–70. [DOI] [PubMed] [Google Scholar]
- [11].Fullerton BS, Sparks EA, Hall AM, et al. Enteral autonomy, cirrhosis, and long term transplant-free survival in pediatric intestinal failure patients. J Pediatr Surg 2016;51:96–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin North Am 1986;33:179–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Moss RL, Dimmitt RA, Barnhart DC, et al. Laparotomy versus peritoneal drainage for necrotizing enterocolitis and perforation. N Engl J Med 2006;354:2225–34. [DOI] [PubMed] [Google Scholar]
- [14].Wales PW, de Silva N, Kim J, et al. Neonatal short bowel syndrome: population-based estimates of incidence and mortality rates. J Pediatr Surg 2004;39:690–5. [DOI] [PubMed] [Google Scholar]
- [15].Gordon PV, Clark R, Swanson JR, et al. Can a national dataset generate a nomogram for necrotizing enterocolitis onset? J Perinatol 2014;34:732–5. [DOI] [PubMed] [Google Scholar]
- [16].Short SS, Papillon S, Berel D, et al. Late onset of necrotizing enterocolitis in the full-term infant is associated with increased mortality: results from a two-center analysis. J Pediatr Surg 2014;49:950–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Motta C, Scott W, Mahony L, et al. The association of congenital heart disease with necrotizing enterocolitis in preterm infants: a birth cohort study. J Perinatol 2015;35:949–53. [DOI] [PubMed] [Google Scholar]
- [18].Singh R, Shah B, Allred EN, et al. The antecedents and correlates of necrotizing enterocolitis and spontaneous intestinal perforation among infants born before the 28th week of gestation. J Neonatal Perinatal Med 2016;9:159–70. [DOI] [PubMed] [Google Scholar]
- [19].Wadhawan R, Oh W, Hintz SR, et al. Neurodevelopmental outcomes of extremely low birth weight infants with spontaneous intestinal perforation or surgical necrotizing enterocolitis. J Perinatol 2014;34:64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Munaco AJ, Veenstra MA, Brownie E, et al. Timing of optimal surgical intervention for neonates with necrotizing enterocolitis. Am Surg 2015;81:438–43. [PubMed] [Google Scholar]
- [21].Moss RL, Kalish LA, Duggan C, et al. Clinical parameters do not adequately predict outcome in necrotizing enterocolitis: a multi-institutional study. J Perinatol 2008;28:665–74. [DOI] [PubMed] [Google Scholar]
- [22].Tyson JE, Kennedy KA. Trophic feedings for parenterally fed infants. Cochrane Database Syst Rev 2005;CD000504. [DOI] [PubMed] [Google Scholar]
- [23].Ching YA, Gura K, Modi B, et al. Pediatric intestinal failure: nutrition, pharmacologic, and surgical approaches. Nutr Clin Pract 2007;22:653–63. [DOI] [PubMed] [Google Scholar]
- [24].Duggan CP, Jaksic T. Pediatric intestinal failure. N Engl J Med 2017;377:666–75. [DOI] [PubMed] [Google Scholar]
- [25].Hylander MA, Strobino DM, Dhanireddy R. Human milk feedings and infection among very low birth weight infants. Pediatrics 1998;102:E38. [DOI] [PubMed] [Google Scholar]
- [26].Khan FA, Mitchell PD, Fisher JG, et al. Magnitude of surgical burden associated with pediatric intestinal failure: a multicenter cohort analysis. J Pediatr Surg 2014;49:1795–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Thakur A, Chiu C, Quiros-Tejeira RE, et al. Morbidity and mortality of short-bowel syndrome in infants with abdominal wall defects. Am Surg 2002;68:75–9. [PubMed] [Google Scholar]
- [28].Khan FA, Squires RH, Litman HJ, et al. Predictors of enteral autonomy in children with intestinal failure: a Multicenter Cohort Study. J Pediatr 2015;167: 29-34.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Li X, Li L, Wang Y, et al. Postoperative characteristics of infants who developed necrotizing enterocolitis with different postnatal ages. Medicine (Baltimore) 2017;96:e7774. [DOI] [PMC free article] [PubMed] [Google Scholar]


