Abstract
Rationale:
At present, data regarding refractory pneumothorax treated with video-assisted thoracic surgery (VATS) in combination with extracorporeal membrane oxygenation (ECMO) in critically ill patients with H7N9 pneumonia have never been reported.
Patient concerns:
A laboratory-confirmed case of human infection with avian influenza A (H7N9) virus was treated in our hospital. Acute respiratory distress syndrome (ARDS) developed and the patient was oxygenated via veno-venous ECMO due to the failure of mechanical ventilation. Unfortunately, a right refractory pneumothorax occurred. Despite treatment with pleural drainage and select bronchial occlusion, the patient still failed to improve.
Diagnosis:
Fatal H7N9 pneumonia complicated with severe ARDS, pulmonary bullae, and refractory pneumothorax.
Interventions:
Successful combination of ECMO with VATS of pulmonary bullae resection was performed and pneumothorax was cured.
Outcomes:
One week after the operation, ECMO was removed. However, the patient finally developed multiorgan failure (MOF) complicated by refractory hypoxemia due to progressive lung fibrosis and died 36 days after admission.
Lessons:
Although the patient died of MOF triggered by severe lung fibrosis at last, the successful treatment of refractory pneumothorax by combination of ECMO with VATS is encouraging. Thus, when refractory pneumothorax in a patient with severe pulmonary dysfunction fails to improve through routine therapy, the treatment of pneumothorax by VATS based on ECMO support can be considered as a feasible selection.
Keywords: extracorporeal membrane oxygenation (ECMO), H7N9, pneumothorax, pulmonary bullae resection, video-assisted thoracic surgery
1. Introduction
Up to now, data regarding critically ill patients with H7N9 pneumonia and severe respiratory failure treated with extracorporeal membrane oxygenation (ECMO) have been mentioned previously.[1,2] However, reports of pulmonary bullae resection with ECMO support in human disease caused by novel influenza A (H7N9) virus have never been described. We herein report a critically ill patient with avian influenza A (H7N9), who was complicated with severe acute respiratory distress syndrome (ARDS), pulmonary bullae, and refractory pneumothorax. In this case, pneumothorax was ultimately cured by the successful combination of ECMO with video-assisted thoracic surgery (VATS) of pulmonary bullae resection, which was first reported in laboratory-confirmed cases of human infection with avian influenza A (H7N9) virus. Patient has provided informed consent for publication of the case.
2. Case report
A 28-year-old male with a 3-day history of hyperpyrexia, hemosputum, and shortness of breath was admitted to our hospital. A chest computed tomography (CT) scan revealed multiple ground-glass opacities and consolidation in both lungs (Fig. 1). The test of nasopharyngeal aspirate obtained upon admission was positive for influenza A (H7N9) virus by reverse transcription PCR (RT-PCR). The patient was treated with oseltamivir (150 mg twice daily) on the first day of hospitalization. However, ARDS progressively developed and veno-venous (VV) ECMO (17 Fr cannula in the right internal jugular vein for inflow, 21 Fr cannula in the left femoral vein for outflow) was required on hospital day 5 because of the failure of invasive mechanical ventilation. Due to the emergence of a right pneumothorax on hospital day 10 and a left pneumothorax on hospital day 15, he was treated with bilateral pleural drainage. Unfortunately, the right pneumothorax had a persistent air leak all the time. Due to refractory pneumothorax, select bronchial occlusion (SBO) was performed for several times; however, the patient still failed to improve. Ultimately, VATS was performed based on ECMO support on hospital day 20. The main findings under thoracoscope showed diffuse pulmonary consolidation with a loss of lung elasticity in the right lung (Fig. 2A) and 2 giant pulmonary bullae measuring 3 cm × 4 cm × 4 cm and 4 cm × 4 cm × 6 cm, respectively, in the lateral segment of right middle lobe (Fig. 2B, green arrows), one of which exhibited an obvious crevasse (Fig. 2C, green arrow). Then, successful pulmonary bullae resection in the right middle lobe was performed later on (Fig. 3, green arrows). The pathological findings of the resected lung tissue showed fibroproliferative changes along with diffuse alveolar damage (Fig. 4). The air leakage ceased after the operation. The bronchoalveolar lavage fluid (BALF) was negative for influenza A (N9) virus on hospital day 22, but the specimen remained positive for influenza A (H7) virus until 25 days after hospitalization. During the period, several bacterial isolates were cultured from BALF and antimicrobial therapy was administered at the same time.
Figure 1.
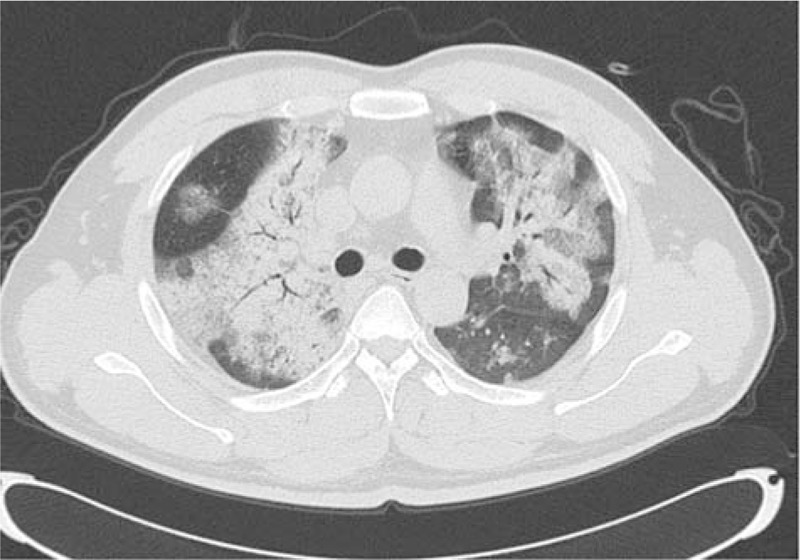
Chest computerized tomography (CT) scan showing multiple ground-glass opacities and consolidation in both lungs.
Figure 2.
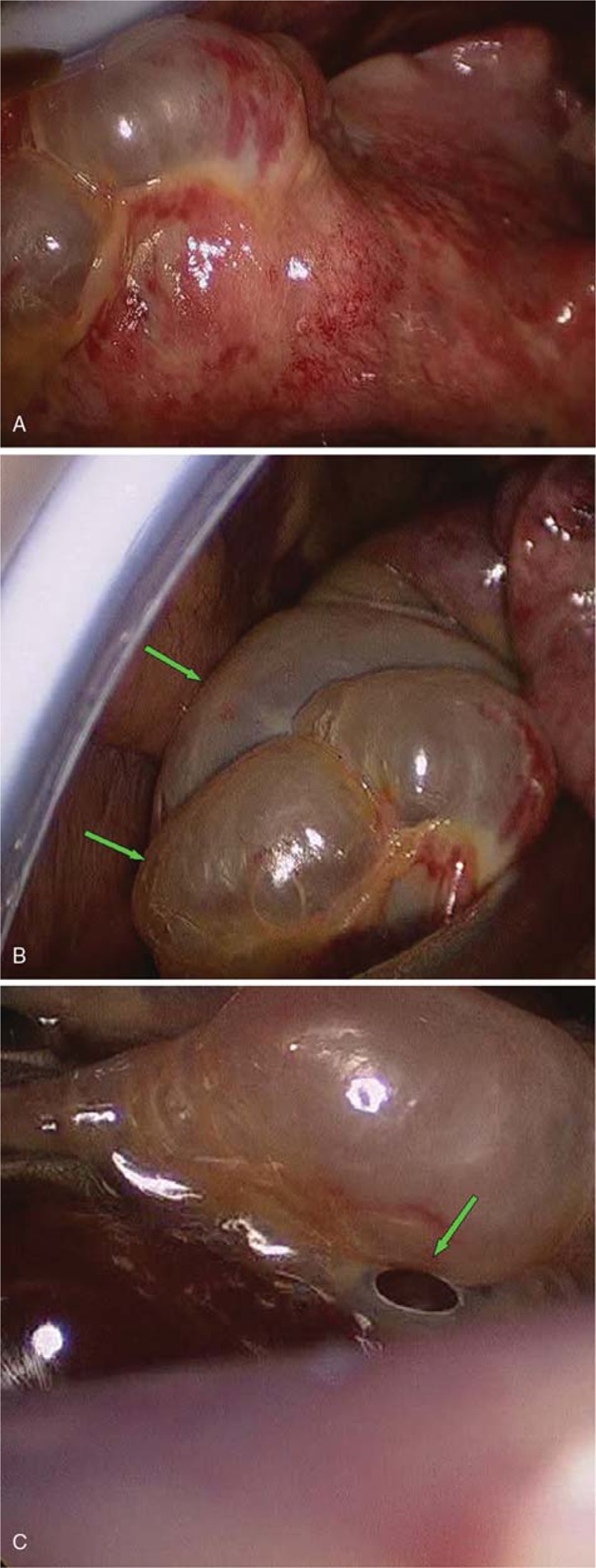
The findings of the right lung under video-assisted thoracic surgery (VATS). (A) The lung exhibited diffuse pulmonary consolidation with a loss of lung elasticity; (B) 2 giant pulmonary bullae measuring 3 cm × 4 cm × 4 cm and 4 cm × 4 cm × 6 cm (green arrows) were observed in the lateral segment of right middle lobe; and (C) one of the bullae exhibited an obvious crevasse (green arrow). Chest X-ray revealing multiple infiltrates in both lungs.
Figure 3.
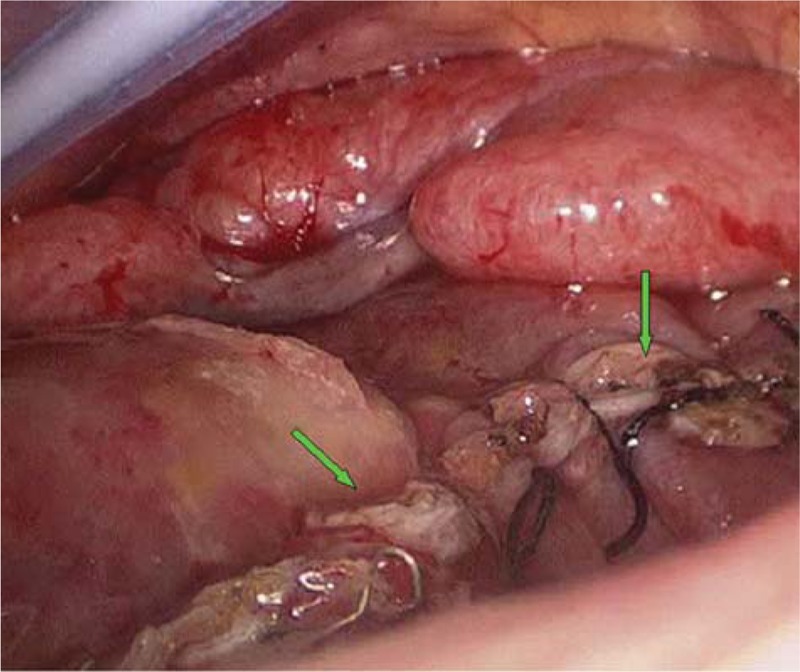
Successful pulmonary bullae resection in the right middle lobe was performed (green arrows).
Figure 4.
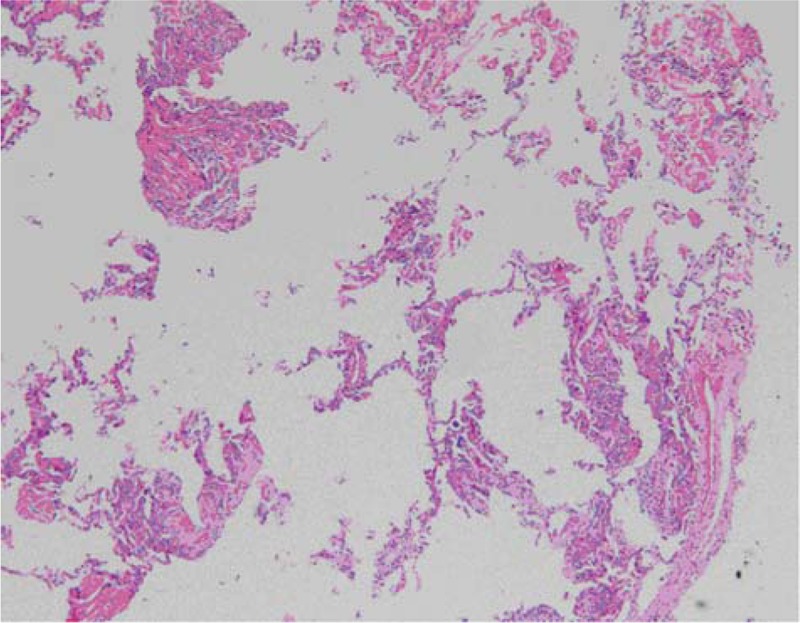
The pathological findings of the resected lung tissue showed fibroproliferative changes in addition to diffuse alveolar damage (hematoxylin and eosin staining, ×50).
ECMO was removed on hospital day 27 because of a certain amount of improvement in oxygenation and suspicious thrombosis in right internal jugular vein. However, 3 days after the removal of ECMO, there was a rapid decline in lung compliance over the subsequent 6 days. No significant new co-infection was confirmed at that time. The patient's condition deteriorated progressively and finally he developed multiorgan failure (MOF), which was considered to be complicated by refractory hypoxemia due to progressive lung fibrosis. He died 36 days after admission and a limited postmortem biopsy of lung tissue revealed diffuse pulmonary fibrosis, which was in consistent with the clinical manifestation of refractory hypoxemia.
3. Discussion
The complication of ARDS from critical illness with avian influenza A (H7N9) may develop rapidly within a brief time period and a high-level pressure ventilation support is often required. However, the necessary of mechanical ventilation with high airway pressures and oxygen concentrations with the addition of serious virus infection and co-infection with bacterial isolates can all contribute to severe lung injury and a decline of lung compliance,[3–5] which may develop the risk of the complication of pneumothorax. Notably, the emergence of pneumothorax can further exacerbate the existing hyoxemia and apparently increase the mortality rate of patients with severe H7N9 pneumonia and ARDS.[6] In the present case, H7N9 virus infection was progressively deteriorated and the ARDS developed rapidly. Then ECMO was required on hospital day 5 (the 8th day after symptom onset). But a right pneumothorax occurred and had a persistent air leak, despite the performance of pleural drainage and SBO. Ultimately, successful combination of ECMO with VATS of pulmonary bullae resection was performed on hospital day 20. The air leakage ceased after the operation. There were no serious intraoperative and postoperative complications. ECMO was stopped 7 days after surgical operation (the 27th day after admission). But 3 days after removal of the cannulae, the patient's condition was aggravated again with a rapid decline in lung compliance. Finally, he developed MOF due to refractory hypoxemia and died on hospital day 36. A postmortem biopsy of lung tissue revealed severe pulmonary interstitial fibrosis which was considered to be the fatal cause due mainly to a lengthy course of H7N9 virus infection.[3]
As pneumothorax was considered to be closely related to the prognosis of critically ill patients with H7N9 infection and ARDS,[6] early recognition of this complication and timely intervention may be useful to improve outcome. Despite the patient in our report finally died of very sever pulmonary fibrosis,[3] the successful treatment of refractory pneumothorax by combination of ECMO with VATS still has a great clinical significance, not only for patients with H7N9. That is to say, when refractory pneumothorax in a patient with severe pulmonary dysfunction fails to improve through routine therapy such as pleural drainage, SBO, and so on, combination of ECMO with VATS can be considered as a feasible selection.
Acknowledgments
We thank Bing Sun, Xuyan Li, and Chunyan Zhang, Being Institute of Respiratory Medicine; Department of Respiratory and Critical Care Medicine, Beijing Chaoyang Hospital, Capital Medical University; Beijing Key Laboratory of Respiratory and Pulmonary Circulation, for the guidance of the ECMO technique.
Author contributions
Conceptualization: Jinbao Huang, Heng Weng.
Data curation: Jinbao Huang, Shuxing Chen, Changqing Lan, Qinghua Lin.
Methodology: Shuxing Chen, Heng Weng.
Writing – original draft: Jinbao Huang, Hongyan Li, Shuxing Chen.
Writing – review and editing: Heng Weng.
Footnotes
Abbreviations: ARDS = acute respiratory distress syndrome; BALF = bronchoalveolar lavage fluid; CT = computed tomography; ECMO = extracorporeal membrane oxygenation; MOF = multiorgan failure; RT-PCR = reverse transcription polymerase chain reaction; SBO = select bronchial occlusion; VATS = video-assisted thoracic surgery; VV = via veno-venous.
JH, HL, and SC equally contributed to this work.
The work was sponsored by the fund of the Key Clinical Specialty Discipline Construction Program of Fujian, P.R.C. (2018-145) and the Clinical Medicine Center Construction Program of Fuzhou, Fujian, P.R.C. (2018080305).
The authors have no conflict of interest to disclose.
References
- [1].Gao HN, Lu HZ, Cao B, et al. Clinical Findings in 111 Cases of Influenza A (H7N9) Virus Infection. N Engl J Med 2013;368:2277–85. [DOI] [PubMed] [Google Scholar]
- [2].Liu C, Li J, Sun W, et al. Extracorporeal membrane oxygenation as rescue therapy for H7N9 influenza-associated acute respiratory distress syndrome. Chin Med J (Engl) 2014;127:1798. [PubMed] [Google Scholar]
- [3].Huang JB, Li HY, Liu JF, et al. Histopathological findings in a critically ill patient with avian influenza A (H7N9). J Thorac Dis 2015;7:E672–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Poh CL, Yan TD. Clinical alert: extracorporeal membrane oxygenation support in management of severe respiratory failure secondary to swine-origin influenza A (H1N1) virus. J Thorac Dis 2010;2:6–8. [PMC free article] [PubMed] [Google Scholar]
- [5].Guo Q, Huang JA, Zhao D, et al. Pathological changes in a patient with acute respiratory distress syndrome and H7N9 influenza virus infection (letter). Crit Care 2014;18:666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zhang YK, Li J, Yang JP, et al. Lung ultrasonography for the diagnosis of 11 patients with acute respiratory distress syndrome due to bird flu H7N9 infection. Virol J 2015;12:176. [DOI] [PMC free article] [PubMed] [Google Scholar]


