Abstract
The phagocytosis of photoreceptor outer segments (POSs) by the retinal pigment epithelium (RPE) is essential for retinal homeostasis. Defects in this process can be caused by mutations in the photoreceptor cells, the RPE cells, or both cell types. This function can be experimentally investigated by performing an in vitro phagocytosis assay, in which cultured RPE cells are challenged with isolated POSs, and subsequently tested for their ability to degrade the POSs. A significant advantage of this approach is that mutant phenotypes can be attributed either to the photoreceptor or the RPE cells, by experimenting with different permutations of mutant and control photoreceptor and RPE cells. In this chapter, we detail the method for a double-immunofluorescence assay for analysis of the binding, ingestion, and subsequent degradation of isolated mouse POSs by cultured mouse primary RPE cells.
Keywords: Phagocytosis, Retinal pigment epithelium, Photoreceptor outer segments, Phagosome degradation, Retinal homeostasis
1. Introduction
The retinal pigment epithelium (RPE) is situated between the light-sensitive photoreceptor cells, and the fenestrated choriocapillaris [1]. This single monolayer of cells performs numerous functions that are essential for the health of the retina, including the phagocytosis of photoreceptor outer segment (POS) disk membranes [2]. This phagocytosis allows for the catabolic phase of the renewal of the disk membranes [3], and requires intimate contact between the RPE and POSs [4]. The apical surface of the RPE is decorated with proteins, including integrin alpha V beta 5 and Mer, receptors that have been shown to participate in the binding and internalization of the tips of the POSs by RPE cells, respectively [5, 6]. Once internalized, the POS phagosomes travel on molecular motors bound to cytoskeletal elements, including actin filaments and microtubules [7, 8], which mediate their fusion with degradative organelles such as endosomes and lysosomes [9, 10].
Phagocytosis of the POS disk membranes shed by photoreceptor cells occurs on a daily cycle [11]. In the mouse retina, a single RPE cell serves up to 200 photoreceptor cells [12]. This process represents a heavy metabolic load on the RPE, which it must sustain throughout the lifetime of the animal. Defects or inefficiencies in this process can lead to the generation of indigestible material that aggregates as lipofuscin and sub-RPE deposits, leading to RPE pathogenesis and age-related visual impairment, including age-related macular degeneration (AMD) [13–19]. A primary step in understanding the basis of impaired POS phagocytosis is to identify whether it is due to defects in the photoreceptor cells or in the RPE cells. Defects in the RPE cells could impair ingestion [20, 21] or degradation [8, 16]. Likewise, defects in the photoreceptor cells could have the same result, by making the POS disks less palatable [22].
In this chapter, we provide a detailed description of a pulsechase assay that can be used to analyze POS phagocytosis, using purified mouse POSs and primary cultures of mouse RPE cells. With this assay, and employing different permutations of mutant and control photoreceptor and RPE cells, it is possible to isolate the cause of the mutant phenotype.
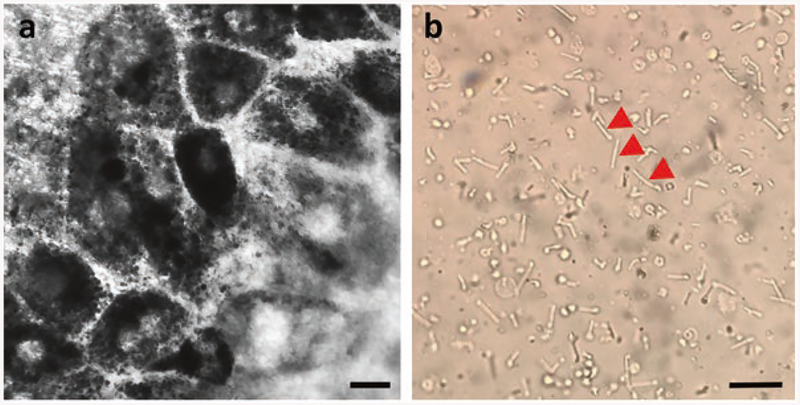
Mouse RPE cells are isolated from eyecups and plated on Transwell inserts to establish primary cultures [7, 23] (Fig. 1a). The RPE cultures are then pulsed with POSs (Fig. 1b) purified from mouse retinas for a defined length of time. Following the pulse, excess and unbound POSs are removed by washing, and the cells are either fixed immediately, or given a chase period to internalize bound POSs and degrade ingested phagosomes. Dualimmunofluorescence labeling is then used to distinguish between surface-bound and internalized POSs [24] (Fig. 2). In this assay, we identify POS phagosomes by immunolabeling for opsin, which is abundant in the POS disk membranes, but is not expressed by the RPE. The opsin immunofluorescence signal can be used to quantify the POSs, and a comparison of the signal after the pulse and following the chase provides a measure of the kinetics of POS binding and degradation by the RPE.
Fig. 1.
Isolation of RPE, and purification of POSs from murine eyes. (a) Primary RPE cells cultured on Transwell inserts for 7 days display RPE-like morphology and pigmentation. (b) POSs purified from mouse retinas have a rod-like structure. A few of the POSs are indicated by red arrows. Scale bars in a and b: 10 μm
Fig. 2.
Dual-labeling of opsin following a pulse-chase phagocytosis assay. Primary mouse RPE cells were incubated with mouse POSs for 20 min, and fixed immediately (pulse, a–c), or allowed a chase period for 120 min and then fixed (chase, d–f). Opsin labeling was performed prior to (a, d) and following cell permeabilization (b, e). In the merged panels (c, f), POSs bound to the surface of RPE cells appear yellow due to their exposure to the two different fluorophore-conjugated antibodies (green and red), whereas internalized POSs only appear red. After the pulse, POSs can be seen on both the outside and inside of the RPE cells, while after the chase, the majority of the POSs are on the inside. The reduced number of POSs in the chase compared with the pulse is due to the degradation of POSs by the RPE cells. Scale bar in a: 10 μm (all panels in figure are of the same magnification)
2. Materials
2.1. Preparation of Primary Mouse RPE Cultures
2.1.1. Solution and Reagents
Medium A: Dulbecco’s Modified Eagle Medium (DMEM) with 4.5 g/l glucose, 1× nonessential amino acids (NEAA), and 1 mM sodium pyruvate.
Medium B: Medium A with 10% fetal bovine serum (FBS), and 1× Pen/Strep.
Medium C: Medium B with 20 mM HEPES.
2% dispase solution made in Medium A.
2.1.2. Equipment and Supply
Small curved scissors.
Upright dissection microscope.
Microdissection forceps.
Transwell inserts (polyester membrane; 6.5-mm Diameter; 0.4-μm pore size).
2.2. Purification of Mouse Photoreceptor Outer Segments
2.2.1. Solutions and Reagents
Isoflurane.
OptiPrep density gradient medium.
Ringer solution: 130 mM NaCl, 3.6 mM KCl, 2.4 mM MgCl2, 1.2 mM CaCl2, 10 mM HEPES, and 0.02 mM EDTA; pH to 7.4 with KOH.
2.2.2. Equipment and Supply
Glass pestle and homogenizer.
Scalpel with surgical blade.
Curved forceps.
Peristaltic pump.
Centrifuge with swinging-bucket rotor.
2.3. Phagocytosis Assay
2.3.1. Solutions and Reagents
Dulbecco’s phosphate-buffered saline with 0.9 mM calcium and 0.49 mM magnesium (DPBS-CM).
Fixative: 4% electron microscopy-grade formaldehyde (methanol-free) in DPBS-CM.
Blocking buffer: 1% bovine serum albumin (BSA) in DPBS-CM.
Opsin antibody (e.g., mouse monoclonal 4D2).
Alexa Fluor-conjugated secondary antibodies (two different fluorophores).
Fluoro-Gel mounting medium with 4′, 6-diamidino-2-phenylindole (DAPI).
3. Methods
3.1. Isolationof Primary Mouse RPE
Sacrifice mouse pups between the age of postnatal day (P) 10–15 by cervical dislocation.
Remove the eyes and collect them in a 15-ml Falcon tube containing Medium A (1 ml per eye) (see Note 1).
Wash the eyes by inverting the tube three times.
Remove the medium from the Falcon tube, and pour the eyes into a new Falcon tube containing 2% dispase solution (1 ml per 2 eyes) (see Note 2).
Incubate the eyes in the dispase solution in a 37 °C water bath for 45 min, inverting the tube several times every 15 min.
Remove the dispase solution completely, and wash the eyes two times in Medium C (1 ml per 2 eyes).
Pour the eyes into a 10-cm petri dish containing 10 ml Medium C, and place the dish under an upright dissection microscope.
Using microdissection forceps, remove the anterior portions of the eyes, including the lens and the iris. This can be accomplished by using the forceps to pull apart the cornea.
Transfer the resulting eyecups into a new 15-ml Falcon tube containing Medium A (1 ml per 2 eyes), and incubate in a 37 °C water bath for 20 min (see Note 3).
Pour the solution containing the eyecups into a new 10-cm petri dish, and using an upright dissection microscope inside a biosafety cabinet, gently remove the retina. The RPE should remain attached to Bruch’s membrane (see Note 4).
Using microdissection forceps, gently scrape sheets of RPE from Bruch’s membrane (see Note 5). Collect the sheets of RPE with a P200 pipette, and transfer them to a tube containing 10 ml Medium B.
Centrifuge the cell suspension at 150 × g for 1 min at room temperature (RT).
Wash the cells three times with 1 ml Medium B.
Resuspend the cells in 1 ml Medium B, and triturate gently with a P200 pipette to break the RPE sheets into small clusters consisting of 5–10 cells.
Centrifuge the cell suspension at 150 × g for 3 min at RT.
Remove the supernatant, resuspend the cells in Medium B, and plate the cells on Transwell inserts fitted in a 24-well cluster plate. For each insert, plate the cells in the inner compartment of the insert at a concentration of 50,000 cells.cm−2 in 100 μl Medium B. Add 600 μl Medium B to the outer compartment of the insert.
Culture the cells in a humidified incubator at 37 °C with 5% CO2 for 3–7 days, changing the medium every 2 days (Fig. 1a).
3.2. Isolation of Mouse Photoreceptor Outer Segments
Dark adapt mice overnight.
- Prepare OptiPrep gradients in 4-ml polycarbonate Sorvall tubes (see Note 6).
- 750 μl of 15% OptiPrep solution (dilute the OptiPrep stock in Ringer solution for all steps).
- 750 μl of 10% OptiPrep solution.
- 750 μl of 8% OptiPrep solution.
Sacrifice the mice, under dim red light, by cervical dislocation, following a brief exposure to isoflurane.
Use curved forceps to pull the eyeball out of the eye socket without completely detaching it. With your other hand, use a scalpel with a surgical blade to make an incision across the eye. Make the incision with a single motion of the blade. Ideally, the lens will come out of the eye, attached to the blade. If the lens does not come out on its own, use a fine forceps to gently remove it from the eye. Pull the curved forceps in an upward direction to squeeze the retina out of the eye.
Collect the retinas in a glass homogenizer containing 750 μl Ringer solution, and place on ice.
Homogenize the retinas gently with a glass pestle to release the POSs, and centrifuge the suspension at 100 × g for 1 min at RT to pellet large debris.
Collect the supernatant, and load 750 μl SLOWLY onto a single gradient. This is best done with a blunt-end pipette tip.
Centrifuge the gradients at 12,000 × g for 20 min at 4 °C (see Note 7).
Collect the purified POSs from each gradient. They appear as a band at the 10–15% interphase of the gradient. When first exposed to light, the band appears orange, due to the unbleached state of the visual pigment.
Dilute the POSs with Ringer solution at a 1:3 (v/v) ratio.
Centrifuge the suspension at 10,000 × g for 10 min at 4 °C.
Resuspend the POSs in DMEM, and determine the density of the POSs by counting them on a hemocytometer (see Note 8) (Fig. 1b).
3.3. Phagocytosis Assay
Add FBS (10% final concentration) to the POSs suspended in DMEM and mix gently by pipetting.
Incubate mouse primary RPE cells cultured on Transwell inserts with POSs (1–5 × 107 POSs/ml) in 100-μl volume of DMEM with FBS at 37 °C for 20 min.
After the incubation period, remove the POS suspension from the Transwell inserts, and wash the cells three times with DPBS-CM to remove unbound POSs (see Note 9).
Fix some of the Transwell inserts with 4% formaldehyde in DPBS-CM for 10 min at RT (see Note 10). These will be the “pulse” inserts. For the other inserts, add fresh Medium B to the inner and outer compartments, and return to the 37 °C incubator for 2 h. These will be the “chase” inserts.
After fixation of the pulse inserts is completed, wash the cells three times with DPBS-CM, and place the inserts in the refrigerator until the chase period has been completed.
Following fixation, incubate the cells with blocking buffer for 30 min at RT.
Add mouse anti-opsin (4D2) diluted in blocking buffer for 10 min (see Note 11).
Wash three times for 5 min each with blocking buffer.
Add Alexa Fluor 488 nm-conjugated goat anti-mouse diluted in blocking buffer for 30 min (Fig. 2a, d). The Transwell inserts will need to be kept in the dark for this and all subsequent steps.
Wash three times for 5 min each with blocking buffer.
Permeabilize the cells with 50% ethanol in DPBS-CM for 5 min at RT.
Wash three times for 5 min each with blocking buffer.
Add 4D2 mouse anti-opsin diluted in blocking buffer for 60 min.
Wash three times for 5 min each with blocking buffer.
Add Alexa Fluor 594 nm-conjugated goat anti-mouse diluted in blocking buffer for 60 min (Fig. 2b, e).
Wash three times for 5 min each with DPBS-CM.
Remove the Transwell insert from the 24-well cluster plate, and use a clean surgical blade to excise the membrane of the insert.
Mount the membrane of an insert onto a microscopy slide using Fluoro-Gel with DAPI (see Note 12). Place a clean glass coverslip on top of the membrane, and seal with nail polish.
Image the POSs on a confocal microscope using a 60× objective. Capture at least ten randomly selected fields of view from a single membrane.
Quantify the number of opsin-positive particles, with at least 1-μm diameter, in both the green and red channel using ImageJ software (see Note 13).
4. Notes
The eyes of P10–15 mouse pups are not completely open. To remove the eyes, lift the eyelid with forceps and cut across it to fully expose the eye.
Always prepare this enzyme solution fresh, and keep it on ice until needed.
This step helps to detach the retina from the RPE layer.
If the animals are older than P15, the RPE will remain attached to the retina instead of Bruch’s membrane, and is more difficult to isolate.
Perform the RPE isolation with a dissection microscope that has a white surface as this helps with the visualization of the pigmented RPE.
Prepare the OptiPrep gradients using a peristaltic pump. If a pump is not available, use a P1000 pipette and add the solutions SLOWLY to ensure a sharp interface between the different densities.
Use a swinging-bucket rotor with the following settings: Acceleration = 4, Deceleration = 0.
The yield is typically 1–1.5 × 106 POSs per animal.
Submerge the insert horizontally in a beaker containing DPBS-CM, using forceps to handle the insert.
It is important to ensure that the formaldehyde fixative is methanol-free. Traces of methanol in the fixative can permeabilize the cell membrane before the first round of immunolabeling, so that external opsin labeling cannot be distinguished from internal opsin labeling.
This short incubation period for the primary antibody ensures that no primary antibodies enter the cell prior to the completion of the first round of immunolabeling.
The membrane should be mounted onto the slide such that the cells are facing the glass coverslip.
Particles that appear in both channels will appear as yellow (green + red), and represent POSs bound to the surface of the RPE cells (Fig. 2c, f). Particles that appear only in the red channel represent internalized POSs (i.e., phagosomes).
Acknowledgments
This study was supported by NIH grants R01EY13408 and P30EY00331 (DSW), and F31EY026805 (RAH).
References
- 1.Strauss O (2005) The retinal pigment epithelium in visual function. Physiol Rev 85:845–881 [DOI] [PubMed] [Google Scholar]
- 2.Young RW, Bok D (1969) Participation of the retinal pigment epithelium in the rod outer segment renewal process. J Cell Biol 42:392–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Young RW (1967) The renewal of photoreceptor cell outer segments. J Cell Biol 33:61–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams DS, Fisher SK (1987) Prevention of the shedding of rod outer segment disks by detachment from the retinal pigment epithelium. Invest Ophthalmol Vis Sci 28:184–187 [PubMed] [Google Scholar]
- 5.Lin H, Clegg DO (1998) Integrin alpha v beta 5 participates in the binding of photoreceptor rod outer segments during phagocytosis by cultured human retinal pigment epithelium. Invest Ophthalmol Vis Sci 39:1703–1712 [PubMed] [Google Scholar]
- 6.D’Cruz PM, Yasumura D, Weir J et al. (2000) Mutation of the receptor tyrosine kinase gene Mertk in the retinal dystrophic RCS rat. Hum Mol Genet 9:645–651 [DOI] [PubMed] [Google Scholar]
- 7.Gibbs D, Kitamoto J, Williams DS (2003) Abnormal phagocytosis by retinal pigmented epithelium that lacks myosin VIIa, the usher syndrome 1b protein. Proc Natl Acad Sci U S A 100:6481–6486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang M, Esteve-Rudd J, Lopes VS et al. (2015) Microtubule motors transport phagosomes in the RPE, and lack of klc1 leads to AMD-like pathogenesis. J Cell Biol 210:595–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bosch E, Horwitz J, Bok D (1993) Phagocytosis of outer segments by retinal pigment epithelium: phagosome-lysosome interaction. J Histochem Cytochem 41:253–263 [DOI] [PubMed] [Google Scholar]
- 10.Wavre-Shapton ST, Meschede IP, Seabra MC et al. (2014) Phagosome maturation during endosome interaction revealed by partial rhodopsin processing in retinal pigment epithelium. J Cell Sci 127:3852–3861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.LaVail MM (1976) Rod outer segment disk shedding in rat retina: relationship to cyclic lighting. Science 194:1071–1074 [DOI] [PubMed] [Google Scholar]
- 12.Volland S, Esteve-Rudd J, Hoo J et al. (2015) A comparison of some organizational characteristics of the mouse central retina and the human macula. PLoS One 10:e0125631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hogan MJ (1972) Role of the retinal pigment epithelium in macular disease. Trans Am Acad Ophthalmol Otolaryngol 76:64–80 [PubMed] [Google Scholar]
- 14.Feeney L (1973) The phagolysosomal system of the pigment epithelium. A key to retinal disease. Investig Ophthalmol 12:635–638 [PubMed] [Google Scholar]
- 15.Brunk UT, Terman A (2002) Lipofuscin: mechanisms of age-related accumulation and influence on cell function. Free Radic Biol Med 33:611–619 [DOI] [PubMed] [Google Scholar]
- 16.Rakoczy PE, Zhang D, Robertson T et al. (2002) Progressive age-related changes similar to age-related macular degeneration in a transgenic mouse model. Am J Pathol 161:1515–1524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sparrow JR, Boulton M (2005) RPE lipofuscin and its role in retinal pathobiology. Exp Eye Res 80:595–606 [DOI] [PubMed] [Google Scholar]
- 18.Bowes Rickman C, Farsiu S, Toth CA et al. (2013) Dry age-related macular degeneration: mechanisms, therapeutic targets, and imaging. Invest Ophthalmol Vis Sci 54:ORSF68–ORSF80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wavre-Shapton ST, Tolmachova T, Lopes da Silva M et al. (2013) Conditional ablation of the choroideremia gene causes age-related changes in mouse retinal pigment epithelium. PLoS One 8:e57769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duncan JL, LaVail MM, Yasumura D et al. (2003) An RCS-like retinal dystrophy phenotype in Mer knockout mice. Invest Ophthalmol Vis Sci 44:826–838 [DOI] [PubMed] [Google Scholar]
- 21.Nandrot EF, Kim Y, Brodie SE et al. (2004) Loss of synchronized retinal phagocytosis and age-related blindness in mice lacking alpha v beta 5 integrin. J Exp Med 200:1539–1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Radu RA, Hu J, Yuan Q et al. (2011) Complement system dysregulation and inflammation in the retinal pigment epithelium of a mouse model for Stargardt macular degeneration. J Biol Chem 286:18593–18601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gibbs D, Williams DS (2003) Isolation and culture of primary mouse retinal pigmented epithlelial cells. Adv Exp Med Biol 533:347–352 [DOI] [PubMed] [Google Scholar]
- 24.Esteve-Rudd J, Lopes VS, Jiang M et al. (2014) In vivo and in vitro monitoring of phagosome [DOI] [PubMed] [Google Scholar]