Abstract
Adult stem cell-based therapy has been regarded as a promising treatment for tissue ischemia because of its ability to promote new blood vessel formation. Bone marrow-derived mesenchymal stem cells are the most used angiogenic cells for therapeutic neovascularization, yet the side effects and low efficacy have limited their clinical application. Adipose stromal vascular fraction (SVF) is an easily accessible, heterogeneous cell system comprised of endothelial, stromal, and hematopoietic cell lineages, which has been shown to spontaneously form robust, patent, and functional vasculatures in vivo. However, the characteristics of each cell population and their specific roles in neovascularization remain an area of ongoing investigation. In this review, we summarize the functional capabilities of various SVF constituents during the process of neovascularization and attempt to analyze whether the cross-talk between these constituents generates a synergetic effect, thus contributing to the development of new potential therapeutic strategies to promote neovascularization.
Keywords: stromal vascular fraction, neovascularization, endothelial progenitor cells, endothelial colony forming cells, adipose-derived stem cells, myeloid angiogenic cells
Subject codes selected from the following list: Cell Therapy
Introduction
Adult stem cell-based therapy has become one of the most promising methods to promote collateral vessel formation for ischemia diseases1, 2. It is generally recognized that there are two processes during the formation of a vascular network: angiogenesis and vasculogenesis3. Angiogenesis refers to the development of new vessels by budding from an existing vascular network in an adult4. On the other hand, vasculogenesis is defined as the in situ blood vessel formation by hemangioblasts or vascular stem/progenitor cells and is mostly used to describe vasculature formation in the embryonic period5. However, the formation of stable vessels is a complex process involving both angiogenesis and vasculogenesis and requires orchestrating interactions between endothelial cells (ECs), mural cells, and the surrounding environment5. Thus, the term “neovascularization” is used to indicate these two processes, which represent different aspects of the complex process of vascular network formation6, 7.
Postnatal neovascularization is supposed to be initiated by recruitment and differentiation of vascular precursors, which may be derived from the bone marrow, vascular endothelium, or other mature tissues8–12. For a long time, bone marrow-derived mesenchymal stem cells (BM-MSCs) have been the most extensively used stem cells for therapeutic neovascularization. They are multipotent progenitors regarded as having the capability to differentiate into vascular cells and promote neovascularization2, 13, 14. However, the side effects and possible morbidity of the bone marrow harvest procedure and the relatively low content of BM-MSCs in the bone marrow aspirate have limited their clinical application15.
Adult adipose tissue provides an alternative source of accessible autologous adult stem cells with a high content of endothelial progenitor cells (EPCs) and multipotent mesenchymal stem cells (MSCs)16. Adipose tissue is a highly vascularized tissue in the body, and the remodeling of existing vessels may also play an important role in the physiological functions of adipose tissue17. In 2001, Zuk et al. first described a population of fibroblast-like cells in the stromal vascular fraction (SVF) of adipose tissue, which could differentiate into adipogenic, myogenic, chondrogenic, and osteogenic cells in vitro18. Since then, numerous studies have shown that SVF contains a biologically and clinically interesting heterogeneous cellular population, which can self-assemble into complex vascular networks by supplying ECs or secreting cytokines19, 20. SVF is known to contain ECs, smooth muscle cells, mural cells, fibroblasts, macrophages, and MSCs/other stem cell phenotypes21. Among these cells, most of the attention has been focused on the characteristics and function of adipose-derived mesenchymal/stem cells, which were usually called adipose-derived stem cells (ADSCs) or adipose tissue-derived MSCs (AT-MSCs), in the process of neovascularization. Nevertheless, due to the heterogeneity of SVF and the lack of consensus surface markers to distinguish the definitive cell population, some studies achieved different and even contradictory conclusions in research on SVF induced neovascularization. For instance, whether ADSCs can differentiate into ECs and be directly involved in neovascularization or ADSC transplantation is just “cell-based cytokine therapy” for ischemic disease is still controversial20, 22–25. In addition, although ADSCs have shown efficacy in regenerative and reconstructive medicine, SVF was considered to have superiority compared to ADSC treatment alone26. In a study of a rat cavernous nerve injury model, the SVF treatment group showed statistically better results, with a higher smooth muscle/collagen ratio and more endothelial cell content compared to the ADSC group27. The better therapeutic outcomes observed with SVF treatment are considered to be related to the synergistic promoting effect of heterogeneous cellular composition27–29. How to make the most of SVF in therapeutic neovascularization needs additional investigation.
To clarify the specific mechanisms of neovascularization induced by SVF, the diversity of the cell population isolated by different laboratories and alterations to cell components during in vitro culture must be taken into consideration. This paper will focus on the role of each subpopulation during the SVF neovascularization process and attempt to analyze whether cross-talk between constituents generates a synergetic effect for neovascularization.
Cell subpopulations of SVF and their dynamic changes
It is important to recognize that SVF is a heterogeneous, versatile cellular system, and the degree of heterogeneity is dependent on a variety of factors, such as the adipose tissue isolation site, the digestion protocol, and the patient’s own pathological status. To date, there is no consensus definition to distinguish the specific proportions of these constituents to one another30, 31. In fact, because the previously used markers overlap some cell populations, it is theorized that the composition of the SVF is somewhat like a mosaic. What is clear is that the SVF is a dynamic population of cells with a potentially significant clinical utility; a hallmark characteristic of SVF cells is their ability to self-assemble into a hierarchical, branched, perfused vasculature in vivo21.
Many researchers have proposed different classifications according to different surface markers. Among these markers, the most commonly used in previous studies are CD31 and CD34. CD31 is a classic surface marker for ECs and their progenitors, and CD34 is expressed by both ECs and hematopoietic stem/progenitor cells32. Klar et al. divided the SVF cells into a CD34+/CD31+ cell population and a CD34+/CD31− cell population by flow cytometry sorting33. They observed that the CD31+ population comprised 25% of all cells in the SVF; this subset showed a specific endothelial phenotype, could express CD31 and vascular endothelial growth factor receptor 2 (VEGFR2), and could uptake fluorescent labeled acetylated low-density lipoprotein. On the contrary, the CD31− cell population from human adipose tissue showed stromal stem cell properties, expressed surface markers typical of MSCs, and was positive for CD90 and vimentin33. In addition to endothelial and stromal cell lineages, hematopoietic cells are also a major component of SVF. Morris et al. reported that myeloid cells accounted for about 22% of SVF cells of mice34.
Notably, culturing SVF cells for even one passage would profoundly alter their cellular composition. Nunes et al. examined the differences in the proportions of these cells in fresh versus cultured human SVF isolates35. They found that about 33% of freshly isolated SVF cells were comprised of CD31+ ECs, and that culturing SVF cells significantly reduced this number to about 10% of the total population. Similar trends were also observed in CD14+ monocytes/macrophages and c-Kit+ progenitors35. Harada et al. revealed that, when the freshly isolated SVF cells were cultured in Dulbecco’s modified eagle medium, these cells expressed significantly lower levels of CD31, CD34, and CD45 but a higher level of CD90 even in early passages36. There are two possible reasons for these alterations: 1) most of the hematopoietic cells cannot adhere to the flask during in vitro culture and were removed during the course of passaging; 2) an unsuitable culture medium for an endothelial lineage resulted in the decrease of CD31+ ECs. Szoke et al. reported that cell surface markers, such as CD34 and CD90, quickly disappeared in the adipose tissue derived CD31+ cell population while CD34 and THY1 (CD90) mRNA remained for a period of time even after cell surface expression disappeared, suggesting that the culture conditions might induce a phenotype change37.
The International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT) issued a joint statement that hematopoietic, endothelial, and stromal cells are the main subpopulations of the nucleated cells in SVF32. In this paper, we will review the characteristics of these cell lineages and discuss the cross-talk between them in the process of neovascularization. According to previous studies, the cell lineages isolated from SVF and other tissues, including peripheral blood, vessel wall, and bone marrow, share almost identical characteristics38–42. Thus, we also refer to the research results of these cell populations isolated from different tissues in addition to SVF. The commonly used cell positive and negative surface markers and characteristics are listed in Table 1.
Table 1.
The main subpopulations of SVF and their characteristics
| Classification | Function in neovascularization | Nomenclature | Origin/species | Cell surface marker | Ref |
|---|---|---|---|---|---|
| Endothelial lineage | 1) Serving as “building
blocks” for vessel formation 2) Producing ECM protein to separate the ECs from the surrounding environment |
EC | Mouse, aorta, wound, tumor | CD31+/VEGFR2+/CD34+/VE-Cadherin+/CD45−/Ter119− | 11, 19 |
| ECFC | Human, adipose tissue, and peripheral blood | CD31+/vWF+/VE-cadherin+/CD146+/CD45−/CD14−/CD90− | 38, 47 | ||
| HPP-ECFC LPP-ECFC |
Human, umbilical cord blood, umbilical vein, and aortic ECs | CD31+/CD141+/CD105+/ CD146+/CD144+/vWF+/Flk-1+/CD45−/CD14− | 49, 51 | ||
| EC-SP | Mouse, large and peripheral vessels | VE-cadherin+/ Flk-1+/ Sca-1+/CD133+/CD31+/ CD157+CD200+/CD45−/c-kit-/PDGFR-β-/Hoechstlo | 10, 109 | ||
| EVP | Mouse, aorta, wound, tumor | CD34+/VE-Cadherin+/CD45-/CD31lo/VEGFR2lo | 11 | ||
| OEC | Human, blood | CD31+/VEGFR2+/Tie-2+/Ve-cadherin+/VEGFR2+/vWF+/CD36+/CD146+/CD105+ | 70, 110, 111 | ||
| Stromal lineage | 1) Cooperating with ECs to
stabilize newly forming endothelial networks 2) Improving neovascularization through secretion of angiogenic growth factors |
ADSC | Human and rat, adipose tissue | CD34+/CD10+/CD13+ /CD90+/NG2+CD140a+/CD140b+/calponin+/α-SMA+/CD31−/CD45−/CD144- | 32, 67, 112 |
| ADSC | Human, adipose tissue | CD13+/CD29+/CD44+/CD73+/CD90+/CD105+/CD166+/CD10−/CD14−/CD24−/CD31−/ CD34−/ CD36−/CD38−/ CD45−/CD49d−/CD117−/CD133− | 113 | ||
| ADSC | Mouse, adipose tissue | Sca-1+/CD44+/c-kit−/Lin−/CD34−/CD45−/CD11b−/CD31− | 22 | ||
| Pericyte | Human, adipose tissue, muscle, pancreas, placenta | CD44+/CD73+/CD90+/ CD105+/CD146+/CD34−/CD45−/CD56− | 65 | ||
| Hematopoietic lineage | Inducing endothelial tube formation in vitro and vascular repair in vivo by secreting pro-angiogenic factors | MAC | Human, peripheral blood | CD45+/CD14+/CD68+/MSR1+/MRC1+/CD163+/CD146−/ CD133−/Tie2- | 41, 47 |
| Early EPC | Human, blood | CD45+/CD14+ | 70 | ||
| Macrophage | Human, adipose tissue | CD45+, CD14 +, CD34+, CD206+ | 42 | ||
| Mouse, adipose tissue | CD11b+ and F4/80+ | 19 |
Abbreviation: ADSC, adipose-derived stem cell; α-SMA, α-smooth muscle actin; EC, endothelial cell; ECFC, endothelial colony-forming cell; ECM, extracellular matrix; EC-SP, EC-side population; EPC, endothelial progenitor cell; EVP, endovascular progenitor; HPP-ECFC, high proliferative potential-endothelial colony-forming cell; LPP-ECFC, low proliferative potential-endothelial colony-forming cell; MAC, myeloid angiogenic cell; MSR1, macrophage scavenger receptor 1; MRC1, mannose receptor C type 1; OEC, outgrowth endothelial cell; PDGFR-β, platelet-derived growth factor receptor-β; Sca-1, stem cell antigen-1; VEGFR2, vascular endothelial growth factor receptor 2; vWF, von Willebrand factor.
Endothelial lineage
ECs play a vital role in many physiological activities, including neovascularization, hemostasis, and immune responses43. Many studies have indicated that ECs are capable of forming vascular networks spontaneously in vitro or within a previously avascular tissue3. Koh et al. reported that ECs from freshly isolated SVF implantation can rapidly rebuild a vascular network at the site of implantation19. They also found that depleting ECs from freshly isolated SVF and the cultured SVF cells failed to effectively create a vascular network19.
Patel et al. indicated that endovascular progenitors had self-renewal and colony-forming capacity but differentiated ECs did not11. These progenitor cells exist in many tissues and can form new vascular networks when transferred to a new environment. Some other investigators also believed that neovascularization required vascular progenitor cell homing or recruitment, proliferation, and differentiation into ECs to form a primitive vascular network5, 44, 45. In 1997, Asahara et al. reported the identification of EPCs from peripheral blood, which contributes to the formation of a new vascular network45. However, because there is no specific definition to date, a wide variety of cell populations are named EPCs. It has been demonstrated that the circulating putative EPCs reported in the literature contained two distinct subtypes: 1) myeloid angiogenic cells (MACs) or early outgrowth cells, which stem from the monocyte/macrophage lineage with no/low proliferative activity and 2) endothelial colony-forming cells (ECFCs) or late outgrowth cells, which have a high proliferative capacity41, 46–49. ECFCs represent an EC type with potent intrinsic angiogenic capacity, which are capable of forming a vascular network de novo and contributing to the repair of injured vascular endothelium46, 50. However, the frequency of peripheral blood ECFC colonies was estimated as about only 0.017 per million blood mononuclear cells in healthy adults46. This low content of ECFCs in adult peripheral blood has limited its clinical application, thus an alternative sustainable source of ECs needs to be found. Besides the adult circulating blood, progenitor cells with EC potential were also found in the vascular wall of several organs10, 51, 52. Ingram et al. isolated a hierarchy of ECFC population from the aorta and umbilical vein, which had a similar colony-forming ability to peripheral blood-derived ECFCs51. These results suggest that the vasculature might be a possible source of resident ECFCs53.
Considering the cell surface marker expression pattern of adipose tissue, CD31+ ECs are almost identical to the ECFCs isolated from peripheral blood19; it is postulated that the vasculature of adipose tissue could serve as a dependable source of ECFCs. Lin et al. obtained 109 homogeneous ECFCs from 1 g of white adipose tissue after 30 days of in vitro culture; these cells were consistent with adult blood-derived ECFCs in surface markers and colony-forming ability38. There are several advantages of adipose tissue derived ECFCs (AT-ECFCs). First, adipose tissue is a steady source of ECFCs with easy availability. Second, AT-ECFCs have remarkable expansion potential in vitro and highly pure ECFCs can be obtained after several passages. Third, AT-ECFCs have a stable phenotype similar to blood-derived ECFCs. Fourth, culture expanded ECFCs have a robust vasculogenic potential in vivo and in vitro38. These characteristics make adipose tissue a more practical source of ECFCs than peripheral blood.
Although cell surface markers of various ECFC populations (high proliferation versus no proliferation) were not distinguishable from one another or from ECs, Ingram et al. suggested that the high proliferative potential-endothelial colony-forming cells (HPP-ECFCs), low proliferative potential-endothelial colony-forming cells (LPP-ECFCs), and mature ECs may represent different stages of ECFC differentiation49, 51. They found that HPP-ECFCs could generate all other subsequent stages of endothelial progenitors, while LPP-ECFCs do not form secondary colonies but rather mature EC clusters49, 51. Patel et al. also reported an entirely novel endothelial hierarchy including endovascular progenitor (EVP), transit amplifying cell, and a definitive differentiated cell population in normal endothelium; only EVP cells had self-renewal and colony-forming capacity11. Thus it is concluded that ECs isolated from SVF might be composed of a hierarchical distribution of ECFCs and mature ECs, which jointly provide “building blocks” during the process of SVF induced neovascularization. The degree of neovascularization may be dependent, in part, on the amount of resident ECFCs with proliferative potential or circulating ECFCs that can be recruited into the tissue54.
Stromal/mesenchymal cell lineage
ADSCs are defined as the adherent cell population of SVF; they remain their phenotype and plasticity toward the stromal lineage even after being cultured for several passages32. ADSCs are currently accepted to be positive for stromal markers (such as CD13, CD73, and CD90) and retain these markers in culture, but negative for hematopoietic markers (such as CD11b and CD45)32. CD34 is generally considered to be expressed by hematopoietic and endothelial progenitors; nevertheless, it is transiently expressed on ADSCs up to 8–12 passages in culture32. In many ways, ADSCs are similar to BM-MSCs, such as morphology, immune phenotype, colony frequency, and differentiation capacity, which has led some investigators to regard these two populations as identical39, 40, 55. However, numerous features have distinguished ADSCs and BM-MSCs, and the difference between them may be related to the different microenvironment where these cells reside32. ADSCs were reported to exhibit higher proliferative capacity and better efficacy in angio-inductive ability than BM-MSCs56. Besides, the frequency of MSCs within SVF is reported to be at least 500-fold higher than in bone marrow mononuclear cells57. These results imply that ADSCs from SVF may be a more suitable candidate than BM-MSCs for cell therapy of ischemic disease.
It is generally accepted that ADSCs may be obtained in high numbers from SVF by removing CD31+ cells from adherent cells58. ADSCs have been reported to differentiate into several cell types in vitro, including adipocytes, chondrocytes, and osteoblasts18. In 2004, Planat-Benard et al. first suggested that ADSCs might differentiate into ECs and promote neovascularization20. They reported that the SVF cells cultured in vitro represent a homogeneous CD34+/CD13+ cell population, which could differentiate into ECs and express endothelial surface markers, such as CD31 and von Willebrand factor (vWF). After that, many studies reported the capacity of ADSCs to express endothelial surface markers and their ability to self-assemble into vascular networks24, 59–62.
However, some other studies were unable to prove the differentiation capacity of ADSCs toward an endothelial lineage22, 25. Antonyshyn et al. reported that ADSCs without CD45+ leukocytes and CD31+ ECs have limited endothelial differentiation by well-established biochemical stimuli25. Although ADSCs are isolated from SVF, they are definitely not a homogeneous population32. It is a significant challenge to completely sort out the endothelial lineage from ADSCs due to the absence of definitive surface markers with specificity to both of these cell lineages25. Most importantly, the difference of cell components of SVF between different studies due to different passages, protocols, and adipose tissue sites, can result in various outcomes. One potential reason for the different conclusions is possible contamination of ECs or ECFCs. Some experiments did not deplete the CD31+ cell population completely from the cultured cells; if a small amount of HPP-ECFCs are contained in the cultured cells, they can expand rapidly in suitable environments and become the dominant cell population, which will give a false impression that ADSCs differentiate into ECs. Thus it remains controversial whether ADSCs can actually differentiate into mature ECs.
Nakagami et al. reported that ADSCs could significantly improve neovascularization in the mouse ischemic hind limb model mainly through secretion of angiogenic growth factors rather than differentiating into ECs directly22. Rehman et al. also proved that ADSCs could enhance perfusion in the ischemic hind limb through secretion of multiple angiogenic and antiapoptotic growth factors when delivered to ischemic tissue. In an in vitro experiment, they found that vascular endothelial growth factor (VEGF) secretion increased five-fold when ADSCs were cultured in hypoxic conditions, and the conditioned media obtained from hypoxic ADSCs could significantly promote EC proliferation63. In addition to VEGF, ADSCs also secrete significant amounts of angiogenesis-related mediators, such as hepatocyte growth factor (HGF) and transforming growth factor-β (TGF-β)63, 64.
There is another opinion suggesting that ADSC populations may be identical to pericytes65, 66. Traktuev et al. demonstrated that CD34+ ADSCs are indeed resident pericytes that interacted with ECs mutually and stabilized the vascular structure67. Crisan et al. identified a population of CD146+/NG2+/PDGFRβ+/ALP+/CD34−/CD45−/vWF−/CD144− cells as pericytes in several tissues and described links between MSCs and pericytes. These perivascular cells express the classic MSC markers but not endothelial markers, and also exhibit adipogenic, osteogenic, and chondrogenic differentiation potentials65.
Hematopoietic lineage
It has been demonstrated that adipose SVF contains a significant proportion of hematopoietic cells, which also play critical roles in SVF’s ability to self-assemble into mature vasculature19, 33, 34. Eto et al. reported that the resident hematopoietic cells make up about 10% of the total cells in human adipose tissue69. Adipose tissue-resident hematopoietic cells were composed predominantly of macrophages (about 60%) and lymphocytes (about 40%), mixing with a small amount of helper T cells and NK cells, but no B cells42. Currently, the cells from the myeloid lineage which are able to offer angiogenic support for neovascularization in adults are called “MACs”7. According to the genotypic, proteomic, and immunophenotypic perspectives, MACs are considered to be monocytes in nature70.
Urbich et al. reported that both CD14+ and CD1−mononuclear cells could express endothelial surface markers and delivery of these MAC-like cells improved the neovascularization in a hind limb ischemia mouse model71. Thus, it may be postulated that MACs can differentiate into ECs. However, Medina et al. reported that MACs did not directly incorporate into the vascular network and differentiate into ECs but remained remote from the vasculature and retained their original myeloid phenotype72. It was also found that MAC conditioned media could enhance angiogenesis in an in vitro tubulogenesis assay, which demonstrated that MACs exhibit pro-angiogenic ability through the release of cytokines and chemokines, such as interleukin 8 (IL-8), monocyte chemotactic protein-1 (MCP1), and matrix metallopeptidase 9 (MMP9)72. Endothelial markers on monocytes, such as CD31, VEGFR2, and Tie2, possibly originate from platelet microparticles which are uptaken by the mononuclear cells73.
Adipose tissue macrophages, which are derived from blood monocytes and remain resident as tissue macrophages, are regarded to be involved in angiogenesis regulation74–76. Macrophage subsets are divided into two major groups: M1 (classically activated) and M2 (alternatively activated) macrophages. M1 macrophages can promote inflammation and secrete several kinds of pro-inflammatory cytokines, while M2 macrophages regulate inflammation and contribute to angiogenesis, arteriogenesis, and wound healing76. It has been demonstrated that resident macrophages expressing M2 markers were the predominant adipose tissue macrophage subtype, and there are few M1 macrophages detected in adipose tissue42, 77. Coincidentally, MACs isolated from peripheral blood also display typical M2 markers, such as CD204, CD206, IL-10, and CD163, suggesting that MACs exhibit an M2 macrophage phenotype41, 78. Thus it can be deduced that adipose tissue-resident macrophages might play the role of MACs in the process of SVF induced neovascularization.
Lymphocytes of SVF cells may also be involved in the process of neovascularization. It was reported that Th-1 cells could promote the tumor-associated blood vessel formation directly and Th-2 cells could secrete IL-4 to stimulate M2-like activation of macrophages79. Weirather et al. found that therapeutic Treg cell activation was also related with an M2-like monocyte differentiation after myocardial infarction80. In addition, ADSC transplantation was reported to create a suitable microenvironment in local ischemia for tissue regeneration by stimulating M2 macrophage polarization in the mouse ischemic model81. The cross-talk between T cells, ADSCs, and resident M2 macrophages of SVF may provide a favorable environment for neovascularization in the ischemic area.
Synergistic effects of SVF subpopulations
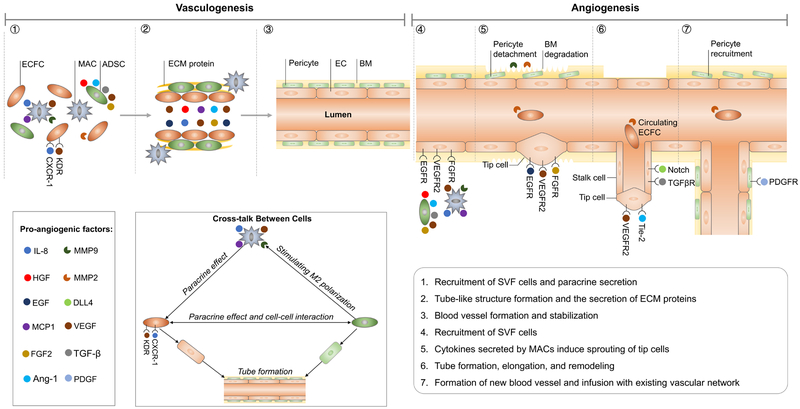
Although both fresh and cultured SVF cells can promote neovascularization, the vessel density of vasculature formed by cultured SVF is lower than fresh SVF. Additionally, the fresh SVF can generate more small capillary-like vessels than the cultured SVF35. Harada et al. also reported that transplantation of freshly isolated SVF cells in mice could significantly improve the capillary density in transplanted ischemic limbs, whereas these effects were not observed in the cultured SVF cell group36. The cells of cultured SVF were found mainly in the perivascular position, while the cells of fresh SVF were detected in both endothelial and perivascular regions. Further investigation found that the number of CD31+ cells contained in the cultured SVF cell population was much less than in fresh SVF cells35. A different proportion of cell components, especially ECs, may be the reason for this discrepancy. Nevertheless, ECs alone are also considered to be insufficient to form a mature vasculature without supporting cells82, 83. The support cells within the SVF, such as perivascular cells, MSCs, and macrophages, may be important42. It was also reported that the freshly isolated cells transcribed a host of genes related to angiogenesis with the production of extracellular matrix (ECM); therefore, they also secreted significantly higher levels of angiogenic factors than cultured cells in the in vitro study36, 84. Based on the above results, the interrelations between each subpopulation of SVF contribute to the process of neovascularization (the possible mechanism is represented in Figure 1).
Figure 1:
- ECFCs can proliferate and differentiate into ECs and supply “building blocks” for tube network formation.
- ADSCs promote ECFC proliferation differentiation by secreting VEGF, HGF, placental growth factor (PGF), FGF-2, TGF-β, and angiopoietin-1 and through cell-cell contact.
- ADSCs and ECs co-produce ECM proteins; ADSCs can differentiate into pericytes, both of which stabilize the newly formed vessel structure.
- MACs enhance angiogenesis tubulogenesis assay through the release of IL-8, MCP1, MMP9, and VEGF2; correspondingly, ECFCs showed significantly higher expressions of the receptors for VEGF (KDR) and IL-8 (CXCR-1).
- Cytokines may induce the resident or circulating ECFCs to aggregate in the ischemic area and promote neovascularization; the angiogenic factor gradient could induce EC tip cell proliferation and migration.
Synergistic effects of ECs/ECFCs and ADSCs
Molecular interactions between ADSCs and ECs, including secreted factors and cell-cell interactions, are appealing because ADSCs co-cultured with ECs can promote vascular formation67, 85–89. To determine the molecular interactions between them in vitro, there are two major methods: direct co-culturing or culturing ECs and ADSCs in each other’s conditioned medium, respectively. Rehman et al. reported that conditioned medium obtained from ADSCs in a hypoxic environment significantly promoted EC proliferation and reduced apoptosis63. Traktuev et al. reported that ADSC conditioned medium did not stimulate the proliferation of ECs but supported endothelial survival, while EC conditioned medium exhibited a significant chemotaxis and mitogenic effect on ADSCs67. Other investigations revealed that ADSC conditioned medium had a positive effect on the proliferation of the EC lineage but the ADSC conditioned medium alone did not result in fully branched networks, which indicated that direct cell-cell contact was also necessary for vascular formation85–87. Interestingly, the phenotype of the ADSCs was also found to be changed in the co-culture system. It was revealed that, in a co-culture system, the ADSCs around the endothelial cord-like structures expressed a high level of α-smooth muscle actin (α-SMA) and eventually stabilized the structures to represent physiological microvasculature85, 87. Lin et al. indicated that, when ADSCs were directly co-cultured with AT-ECFCs or circulating ECFCs, the expression of smooth muscle specific marker, smooth muscle myosin heavy chain (SM-MHC), was upregulated significantly38.
In vivo experiments also proved that ADSCs combined with EC lineage implantation could result in more dense and robust vessel formation37. Traktuev et al. reported that the combination of ECFC and ADSC implantation yielded a higher neovascularization capacity than either ECFC or ADSC implantation alone90. They demonstrated that the majority of CD34+ ADSCs from freshly isolated SVF adopted a supportive role similar to pericytes, which associate with the EC lineage and stabilize the vascular network67. Lin et al. proved that human AT-ECFC co-implantation with ADSCs into immune-deficient mice could quickly form an extensive vascular network. The endothelium of the newly formed vessel was lined with AT-ECFCs, while the perivascular region was occupied by ADSCs38. If ADSCs and ECFCs are appropriately combined, the reciprocal interaction between them might promote neovascularization in a synergistic manner in vivo.
To investigate EC and ADSC interactions in the co-culture system, the angiogenic proteins were determined. The protein expression of supernatants from ECFCs, ADSCs mono-culture, ECFCs cultured in ADSC conditioned medium, and ADSC-ECFC co-culture system were determined after four days of incubation. Compared with ECFC mono-culture, ADSCs expressed much higher levels of different angiogenesis-related proteins, including both pro-angiogenic factors and angiogenesis inhibitors, which further indicates that ECs alone are not sufficient to form a tube-like structure. ADSCs might regulate neovascularization by the interplay of pro- and anti-angiogenic/regulatory proteins. Comparing the results of ECFCs cultured with supernatant from ADSCs and the ECFC-ADSC co-culture system, there was a were more than two-fold increase of several pro-angiogenic factors in the co-culture system, including platelet-derived endothelial cell growth factor (PD-ECGF), fibroblast growth factor (FGF-2), MMP-9, angiopoietin-2 (Ang-2), and pentraxin-3. These angiogenic proteins seemed to be important for neovascularization because ADSC conditioned medium alone failed to induce ECFCs to form a vessel structure86.
The different gene expression levels were also evaluated via quantitative reverse transcription polymerase chain reaction in a co-culture system to investigate molecular interactions of ECs and ADSCs. When ECs were cultured alone, there was no significant change in angiogenesis-related gene expression except vWF, which increased two-fold after one week. The expression levels of genes encoding for CD31, VE-cadherin, VEGFR2, and vWF were upregulated in the co-culture system; however, platelet-derived growth factor (PDGF) and Tie-2 expression remained stable. Both CD31 and VE-cadherin are cellular adhesion molecules which are needed for cells to attach to each other to form networks91, 92. It is considered that the upregulation of CD31, VE-cadherin, VEGFR2, and vWF will contribute to prevent uncontrolled vessel growth. The expression of PDGF and Tie-2 might increase after long-term co-culture because they are mainly involved in vessel stabilization86.
In the ADSC and EC co-culture system, it was reported that ECM production increased, which was also considered to be related to vessel formation87. Collagen IV was not detected in ADSC mono-culture, and a low level was present in EC mono-culture. However, ECs expressed a significantly higher level of collagen IV in co-culture. In addition, perlecan I and fibronectin expression also significantly increased in a co-culture system compared to ADSC and EC mono-culture. The accumulation of this protein was distributed in proximity to the EC-cord structures. Interestingly, laminin was also found to gather around EC vascular structures despite no significant change of protein expression87. Taking together, Collagen IV, fibronectin, perlecan I, and laminin are regarded to be associated with vessel formation, because they are components of the basement membrane and promote the proliferation of SVF ECs into monolayer to form a tube-like structure93. According to these findings, the close interaction of ADSC-EC plays an important role in ECM production and therefore on vessel formation.
Synergistic effects of EC/ECFCs and MACs
Although ECFCs are widely regarded as “building blocks” and are directly involved by forming the endothelial lining of vasculature, these cells alone have yet to be used clinically41, 53. Koh et al. described the role of SVF macrophages on vascular assembly, noting that macrophages were required for proper vascular structural organization. To support this supposition, the depletion of CD11b+ and F4/80+ macrophages from SVF yielded vessels that were blunted and disconnected19. Medina et al. found that MACs significantly increased EC tubulogenesis approximately two-fold when MACs were co-cultured with ECs; however, MACs did not incorporate into the vessel lumen and often remained completely isolated from the vasculature41. Fantin et al. reported that the cytokine or growth factor concentration gradient released by MACs could stimulate tip cell protrusion and vessel sprouting, while the resident macrophages also promoted the fusion of tip cells to add to new circuits within the existing vascular94.
It has been proven that a mixed implantation of MACs and ECFCs could result in superior neovascularization in vivo compared to either cell alone95. MACs cannot differentiate into ECs but indirectly augmented proliferation, migration, and the tube forming capability of ECFCs in a paracrine manner by secretion of various cytokines71, 96–98. MACs secreted large amounts of VEGF and IL-8, and correspondingly, ECFCs showed significantly higher expressions of a receptor of VEGF (KDR) and IL-8 (CXCR-1)95. Both of these cytokines are known to promote endothelial proliferation, tube formation, and migration. Furthermore, when the two kinds of cells were mixed and incubated together, the migrating distance of both types of cells was significantly increased beyond that of any single cell alone; however, the migration could also be significantly inhibited by the neutralizing antibodies of VEGF and IL-895. It was reported that MAC-derived IL-8 could transactivate VEGFR2 of mature ECs independently of extracellular VEGF41. In addition, MACs also contribute to neovascularization by secretion of cytokines and MMP-9, whereas ECFCs secrete MMP-299. The co-culture increased the active form of MMP-9 and the invasion depth of MACs, which was not observed when MACs were cultured alone. MMP-2 can mediate the activation of MMP-9 and enhance matrix degradation100.
The prospect of SVF in stem cell therapy for ischemic disease
The majority of preclinical and clinical trials of human cell therapies for ischemic disease using autologous BM-MSCs have displayed modest regenerative or reparative capability101. Compared to bone marrow cells, SVF cells seem to be a more attractive cell population because human adipose tissue can provide many more cells suitable for autologous implantation and it is an easily accessible and dispensable tissue102. Moreover, SVF comprises abundant endothelial and mesenchymal progenitors in addition to hematopoietic cells; the mixed SVF cell population by a certain percentage is considered highly angiogenic compared to other stem cells alone103.
At present, it is not clear how to mix these heterogeneous cells to get the best result in stem cell therapy. To maximize the regenerative potential of SVF cells, investigators have made some attempts to transplant stem cells within their physiological niche. Hamdi et al. implanted an SVF cell sheet into cardiac infarct mice and got greater post-infarct survival than intra-myocardial injections of the same cells104. It was also reported that implantation of a three-dimensional (3D) graft with SVF cells or intact microvascular segments from adipose tissue could promote the development of coronary microvasculature after acute myocardial infarction105, 106. In summary, the relative ease of isolation and the synergic effects of different cell subpopulations makes SVF a clinically relevant, promising cell source for stem cell therapy of ischemic disease. However, the variety of cell components between different studies and passages makes it hard to elucidate the mechanism underlying neovascularization. How to mix different cell types in an ideal ratio to result in the optimal vascular network and how to organize these cells still needs to be investigated. To solve this problem, there might be three methods: 1) using freshly isolated SVF cells; 2) providing an appropriate growth environment for many cell subpopulations to avoid the loss of EC and the hematopoietic cell lineage; and 3) mixing these cells in specific proportions or arranging them in a 3D location according to different ischemic environments.
Besides differentiating into vascular cells, the other possible fates of the SVF cells when in the ischemic environment also should be noted. For one thing, it is postulated that some of the SVF cells, such as ADSCs, will differentiate into adipocytes because they are generally regarded as adipocyte precursors107. However, adipogenesis by ADSC differentiation is regulated by the specific microenvironment, such as ECM107, 108. Whether the ischemic microenvironment can provide a suitable adipogenic niche for ADSCs still needs further investigation. Also, does ADSC therapy promote oncogenesis? To date, no tumor formation was reported in human recipients of ADSCs15. Nevertheless, long-term follow-up and more clinical experience are needed to confirm the safety of ADSC therapy.
Supplementary Material
Highlights.
Adipose stromal vascular fraction (SVF) is a heterogeneous, versatile cellular system; cellular composition profoundly alters after being cultured. Fresh SVF was considered to have superiority compared to cultured SVF during the process of neovascularization.
Endothelial colony-forming cells (ECFCs) isolated from SVF can proliferate and differentiate into endothelial cells and supply “building blocks” for tube network formation.
Adipose-derived stem cells (ADSCs) from SVF promote ECFC proliferation and differentiation by secreting proangiogenic factors; ADSCs can differentiate into pericytes to stabilize the newly formed vessel structure.
The hematopoietic cells of SVF may provide a favorable environment for neovascularization in ischemic areas through the release of IL-8, MCP1, MMP9, and VEGF. Correspondingly, ECFCs showed significantly higher expressions of the receptors for VEGF (KDR) and IL-8 (CXCR-1).
Acknowledgments
We thank Suzanne Danley for editing the manuscript. Y.S., X.C.Z. and M.P. conceived and designed the study. Y.S. and C.S. completed the draft including Figure and Table. All authors read and approved the final article.
Sources of Funding
This work was partially supported by Research Grants from the Musculoskeletal Transplant Foundation (MTF) and the National Institutes of Health (1R01AR067747-01A1) to M.P. and the Science and Technology Program of Yangzhou City, China (YZ2016063) to X.C.Z.
Nonstandard Abbreviations and Acronyms
- ADSC
adipose-derived stem cell
- AT-MSC
adipose tissue-derived mesenchymal stem cell
- BM-MSC
bone marrow-derived mesenchymal stem cell
- ECM
extracellular matrix
- EC
endothelial cell
- ECFC
endothelial colony-forming cell
- EVP
endovascular progenitor
- EPC
endothelial progenitor cell
- HGF
hepatocyte growth factor
- HPP-ECFC
high proliferative potential-endothelial colony-forming cell
- IL-8
interleukin 8
- LPP-ECFC
low proliferative potential-endothelial colony forming cell
- MCP1
monocyte chemotactic protein-1
- MMP
matrix metallopeptidase
- SVF
stromal vascular fraction
- TGF-β
transforming growth factor-β
- VEGF
vascular endothelial growth factor
- VEGFR2
vascular endothelial growth factor receptor 2
- vWF
von Willebrand factor
Footnotes
Disclosure
None.
TOC category - basic study
TOC subcategory - Arteriosclerosis, Thrombosis, and Vascular Biology
Reference:
- 1.Hou L, Kim JJ, Woo YJ, Huang NF. Stem cell-based therapies to promote angiogenesis in ischemic cardiovascular disease. Am J Physiol Heart Circ Physiol. 2016;310:H455–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silva GV, Litovsky S, Assad JA, Sousa AL, Martin BJ, Vela D, Coulter SC, Lin J, Ober J, Vaughn WK, Branco RV, Oliveira EM, He R, Geng YJ, Willerson JT, Perin EC. Mesenchymal stem cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a canine chronic ischemia model. Circulation. 2005;111:150–156 [DOI] [PubMed] [Google Scholar]
- 3.Rouwkema J, Khademhosseini A. Vascularization and angiogenesis in tissue engineering: Beyond creating static networks. Trends Biotechnol. 2016;34:733–745 [DOI] [PubMed] [Google Scholar]
- 4.Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–660 [DOI] [PubMed] [Google Scholar]
- 5.Eguchi M, Masuda H, Asahara T. Endothelial progenitor cells for postnatal vasculogenesis. Clin Exp Nephrol. 2007;11:18–25 [DOI] [PubMed] [Google Scholar]
- 6.Szoke K, Brinchmann JE. Concise review: Therapeutic potential of adipose tissue-derived angiogenic cells. Stem Cells Transl Med. 2012;1:658–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castro PR, Barbosa AS, Pereira JM, Ranfley H, Felipetto M, Gonçalves CAX, Paiva IR, Berg BB, Barcelos LS. Cellular and molecular heterogeneity associated with vessel formation processes. BioMed Research International. 2018;2018:1–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferguson JE 3rd, Kelley RW, Patterson C. Mechanisms of endothelial differentiation in embryonic vasculogenesis. Arterioscler Thromb Vasc Biol. 2005;25:2246–2254 [DOI] [PubMed] [Google Scholar]
- 9.Fang S, Wei J, Pentinmikko N, Leinonen H, Salven P. Generation of functional blood vessels from a single c-kit+ adult vascular endothelial stem cell. PLoS Biol. 2012;10:e1001407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wakabayashi T, Naito H, Suehiro JI, Lin Y, Kawaji H, Iba T, Kouno T, Ishikawa-Kato S, Furuno M, Takara K, Muramatsu F, Weizhen J, Kidoya H, Ishihara K, Hayashizaki Y, Nishida K, Yoder MC, Takakura N. Cd157 marks tissue-resident endothelial stem cells with homeostatic and regenerative properties. Cell Stem Cell. 2018;22:384–397 e386 [DOI] [PubMed] [Google Scholar]
- 11.Patel J, Seppanen EJ, Rodero MP, Wong HY, Donovan P, Neufeld Z, Fisk NM, Francois M, Khosrotehrani K. Functional definition of progenitors versus mature endothelial cells reveals key soxf-dependent differentiation process. Circulation. 2017;135:786–805 [DOI] [PubMed] [Google Scholar]
- 12.Kovacic JC, Moore J, Herbert A, Ma D, Boehm M, Graham RM. Endothelial progenitor cells, angioblasts, and angiogenesis--old terms reconsidered from a current perspective. Trends Cardiovasc Med. 2008;18:45–51 [DOI] [PubMed] [Google Scholar]
- 13.Oswald J, Boxberger S, Jorgensen B, Feldmann S, Ehninger G, Bornhauser M, Werner C. Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells. 2004;22:377–384 [DOI] [PubMed] [Google Scholar]
- 14.Janeczek Portalska K, Leferink A, Groen N, Fernandes H, Moroni L, van Blitterswijk C, de Boer J. Endothelial differentiation of mesenchymal stromal cells. PLoS One. 2012;7:e46842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strioga M, Viswanathan S, Darinskas A, Slaby O, Michalek J. Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem Cells Dev. 2012;21:2724–2752 [DOI] [PubMed] [Google Scholar]
- 16.Zimmerlin L, Donnenberg VS, Pfeifer ME, Meyer EM, Peault B, Rubin JP, Donnenberg AD. Stromal vascular progenitors in adult human adipose tissue. Cytometry A. 2010;77:22–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cao Y. Angiogenesis modulates adipogenesis and obesity. J Clin Invest. 2007;117:2362–2368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001;7:211–228 [DOI] [PubMed] [Google Scholar]
- 19.Koh YJ, Koh BI, Kim H, Joo HJ, Jin HK, Jeon J, Choi C, Lee DH, Chung JH, Cho CH, Park WS, Ryu JK, Suh JK, Koh GY. Stromal vascular fraction from adipose tissue forms profound vascular network through the dynamic reassembly of blood endothelial cells. Arterioscler Thromb Vasc Biol. 2011;31:1141–1150 [DOI] [PubMed] [Google Scholar]
- 20.Planat-Benard V, Silvestre JS, Cousin B, Andre M, Nibbelink M, Tamarat R, Clergue M, Manneville C, Saillan-Barreau C, Duriez M, Tedgui A, Levy B, Penicaud L, Casteilla L. Plasticity of human adipose lineage cells toward endothelial cells: Physiological and therapeutic perspectives. Circulation. 2004;109:656–663 [DOI] [PubMed] [Google Scholar]
- 21.Ramakrishnan VM, Boyd NL. The adipose stromal vascular fraction as a complex cellular source for tissue engineering applications. Tissue Eng Part B Rev. 2018;24:289–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakagami H, Maeda K, Morishita R, Iguchi S, Nishikawa T, Takami Y, Kikuchi Y, Saito Y, Tamai K, Ogihara T, Kaneda Y. Novel autologous cell therapy in ischemic limb disease through growth factor secretion by cultured adipose tissue-derived stromal cells. Arterioscler Thromb Vasc Biol. 2005;25:2542–2547 [DOI] [PubMed] [Google Scholar]
- 23.Miranville A, Heeschen C, Sengenes C, Curat CA, Busse R, Bouloumie A. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation. 2004;110:349–355 [DOI] [PubMed] [Google Scholar]
- 24.Cao Y, Sun Z, Liao L, Meng Y, Han Q, Zhao RC. Human adipose tissue-derived stem cells differentiate into endothelial cells in vitro and improve postnatal neovascularization in vivo. Biochem Biophys Res Commun. 2005;332:370–379 [DOI] [PubMed] [Google Scholar]
- 25.Antonyshyn JA, McFadden MJ, Gramolini AO, Hofer SOP, Santerre JP. Limited endothelial plasticity of mesenchymal stem cells revealed by quantitative phenotypic comparisons to representative endothelial cell controls. Stem Cells Transl Med. 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bora P, Majumdar AS. Adipose tissue-derived stromal vascular fraction in regenerative medicine: A brief review on biology and translation. Stem Cell Res Ther. 2017;8:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.You D, Jang MJ, Kim BH, Song G, Lee C, Suh N, Jeong IG, Ahn TY, Kim CS. Comparative study of autologous stromal vascular fraction and adipose-derived stem cells for erectile function recovery in a rat model of cavernous nerve injury. Stem Cells Transl Med. 2015;4:351–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Dijk A, Naaijkens BA, Jurgens WJ, Nalliah K, Sairras S, van der Pijl RJ, Vo K, Vonk AB, van Rossum AC, Paulus WJ, van Milligen FJ, Niessen HW. Reduction of infarct size by intravenous injection of uncultured adipose derived stromal cells in a rat model is dependent on the time point of application. Stem Cell Res. 2011;7:219–229 [DOI] [PubMed] [Google Scholar]
- 29.Semon JA, Zhang X, Pandey AC, Alandete SM, Maness C, Zhang S, Scruggs BA, Strong AL, Sharkey SA, Beuttler MM, Gimble JM, Bunnell BA. Administration of murine stromal vascular fraction ameliorates chronic experimental autoimmune encephalomyelitis. Stem Cells Transl Med. 2013;2:789–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gimble JM, Bunnell BA, Chiu ES, Guilak F. Concise review: Adipose-derived stromal vascular fraction cells and stem cells: Let’s not get lost in translation. Stem Cells. 2011;29:749–754 [DOI] [PubMed] [Google Scholar]
- 31.Cousin B, Caspar-Bauguil S, Planat-Benard V, Laharrague P, Penicaud L, Casteilla L. [adipose tissue: A subtle and complex cell system]. J Soc Biol. 2006;200:51–57 [DOI] [PubMed] [Google Scholar]
- 32.Bourin P, Bunnell BA, Casteilla L, Dominici M, Katz AJ, March KL, Redl H, Rubin JP, Yoshimura K, Gimble JM. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the international federation for adipose therapeutics and science (ifats) and the international society for cellular therapy (isct). Cytotherapy. 2013;15:641–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klar AS, Güven S, Zimoch J, Zapiórkowska NA, Biedermann T, Böttcher-Haberzeth S, Meuli-Simmen C, Martin I, Scherberich A, Reichmann E, Meuli M. Characterization of vasculogenic potential of human adipose-derived endothelial cells in a three-dimensional vascularized skin substitute. Pediatric Surgery International. 2015;32:17–27 [DOI] [PubMed] [Google Scholar]
- 34.Morris ME, Beare JE, Reed RM, Dale JR, LeBlanc AJ, Kaufman CL, Zheng H, Ng CK, Williams SK, Hoying JB. Systemically delivered adipose stromal vascular fraction cells disseminate to peripheral artery walls and reduce vasomotor tone through a cd11b+ cell-dependent mechanism. Stem Cells Transl Med. 2015;4:369–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nunes SS, Maijub JG, Krishnan L, Ramakrishnan VM, Clayton LR, Williams SK, Hoying JB, Boyd NL. Generation of a functional liver tissue mimic using adipose stromal vascular fraction cell-derived vasculatures. Sci Rep. 2013;3:2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harada Y, Yamamoto Y, Tsujimoto S, Matsugami H, Yoshida A, Hisatome I. Transplantation of freshly isolated adipose tissue-derived regenerative cells enhances angiogenesis in a murine model of hind limb ischemia. Biomed Res. 2013;34:23–29 [DOI] [PubMed] [Google Scholar]
- 37.Szoke K, Beckstrom KJ, Brinchmann JE. Human adipose tissue as a source of cells with angiogenic potential. Cell Transplant. 2012;21:235–250 [DOI] [PubMed] [Google Scholar]
- 38.Lin RZ, Moreno-Luna R, Munoz-Hernandez R, Li D, Jaminet SC, Greene AK, Melero-Martin JM. Human white adipose tissue vasculature contains endothelial colony-forming cells with robust in vivo vasculogenic potential. Angiogenesis. 2013;16:735–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Izadpanah R, Trygg C, Patel B, Kriedt C, Dufour J, Gimble JM, Bunnell BA. Biologic properties of mesenchymal stem cells derived from bone marrow and adipose tissue. J Cell Biochem. 2006;99:1285–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kern S, Eichler H, Stoeve J, Kluter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294–1301 [DOI] [PubMed] [Google Scholar]
- 41.Medina RJ, O’Neill CL, O’Doherty TM, Knott H, Guduric-Fuchs J, Gardiner TA, Stitt AW. Myeloid angiogenic cells act as alternative m2 macrophages and modulate angiogenesis through interleukin-8. Mol Med. 2011;17:1045–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eto H, Ishimine H, Kinoshita K, Watanabe-Susaki K, Kato H, Doi K, Kuno S, Kurisaki A, Yoshimura K. Characterization of human adipose tissue-resident hematopoietic cell populations reveals a novel macrophage subpopulation with cd34 expression and mesenchymal multipotency. Stem Cells Dev. 2013;22:985–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sturtzel C. Endothelial cells. Adv Exp Med Biol. 2017;1003:71–91 [DOI] [PubMed] [Google Scholar]
- 44.Zhang L, Issa Bhaloo S, Chen T, Zhou B, Xu Q. Role of resident stem cells in vessel formation and arteriosclerosis. Circ Res. 2018;122:1608–1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967 [DOI] [PubMed] [Google Scholar]
- 46.Yoder MC, Mead LE, Prater D, Krier TR, Mroueh KN, Li F, Krasich R, Temm CJ, Prchal JT, Ingram DA. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801–1809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Medina RJ, Barber CL, Sabatier F, Dignat-George F, Melero-Martin JM, Khosrotehrani K, Ohneda O, Randi AM, Chan JKY, Yamaguchi T, Van Hinsbergh VWM, Yoder MC, Stitt AW. Endothelial progenitors: A consensus statement on nomenclature. Stem Cells Transl Med. 2017;6:1316–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Prasain N, Meador JL, Yoder MC. Phenotypic and functional characterization of endothelial colony forming cells derived from human umbilical cord blood. J Vis Exp. 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ingram DA, Mead LE, Tanaka H, Meade V, Fenoglio A, Mortell K, Pollok K, Ferkowicz MJ, Gilley D, Yoder MC. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104:2752–2760 [DOI] [PubMed] [Google Scholar]
- 50.Tasev D, Koolwijk P, van Hinsbergh VW. Therapeutic potential of human-derived endothelial colony-forming cells in animal models. Tissue Eng Part B Rev. 2016;22:371–382 [DOI] [PubMed] [Google Scholar]
- 51.Ingram DA, Mead LE, Moore DB, Woodard W, Fenoglio A, Yoder MC. Vessel wall-derived endothelial cells rapidly proliferate because they contain a complete hierarchy of endothelial progenitor cells. Blood. 2005;105:2783–2786 [DOI] [PubMed] [Google Scholar]
- 52.Torsney E, Xu Q. Resident vascular progenitor cells. Journal of Molecular and Cellular Cardiology. 2011;50:304–311 [DOI] [PubMed] [Google Scholar]
- 53.Yoder MC. Is endothelium the origin of endothelial progenitor cells? Arterioscler Thromb Vasc Biol. 2010;30:1094–1103 [DOI] [PubMed] [Google Scholar]
- 54.Basile DP, Yoder MC. Circulating and tissue resident endothelial progenitor cells. J Cell Physiol. 2014;229:10–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.De Ugarte DA, Morizono K, Elbarbary A, Alfonso Z, Zuk PA, Zhu M, Dragoo JL, Ashjian P, Thomas B, Benhaim P, Chen I, Fraser J, Hedrick MH. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs. 2003;174:101–109 [DOI] [PubMed] [Google Scholar]
- 56.Im GI. Bone marrow-derived stem/stromal cells and adipose tissue-derived stem/stromal cells: Their comparative efficacies and synergistic effects. J Biomed Mater Res A. 2017;105:2640–2648 [DOI] [PubMed] [Google Scholar]
- 57.Fraser JK, Wulur I, Alfonso Z, Hedrick MH. Fat tissue: An underappreciated source of stem cells for biotechnology. Trends Biotechnol. 2006;24:150–154 [DOI] [PubMed] [Google Scholar]
- 58.Boquest AC, Shahdadfar A, Fronsdal K, Sigurjonsson O, Tunheim SH, Collas P, Brinchmann JE. Isolation and transcription profiling of purified uncultured human stromal stem cells: Alteration of gene expression after in vitro cell culture. Mol Biol Cell. 2005;16:1131–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Khan S, Villalobos MA, Choron RL, Chang S, Brown SA, Carpenter JP, Tulenko TN, Zhang P. Fibroblast growth factor and vascular endothelial growth factor play a critical role in endotheliogenesis from human adipose-derived stem cells. J Vasc Surg. 2017;65:1483–1492 [DOI] [PubMed] [Google Scholar]
- 60.Fischer LJ, McIlhenny S, Tulenko T, Golesorkhi N, Zhang P, Larson R, Lombardi J, Shapiro I, DiMuzio PJ. Endothelial differentiation of adipose-derived stem cells: Effects of endothelial cell growth supplement and shear force. J Surg Res. 2009;152:157–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shi Z, Neoh KG, Kang ET, Poh CK, Wang W. Enhanced endothelial differentiation of adipose-derived stem cells by substrate nanotopography. J Tissue Eng Regen Med. 2014;8:50–58 [DOI] [PubMed] [Google Scholar]
- 62.Qiu X, Zhang Y, Zhao X, Zhang S, Wu J, Guo H, Hu Y. Enhancement of endothelial differentiation of adipose derived mesenchymal stem cells by a three-dimensional culture system of microwell. Biomaterials. 2015;53:600–608 [DOI] [PubMed] [Google Scholar]
- 63.Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, Pell CL, Johnstone BH, Considine RV, March KL. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109:1292–1298 [DOI] [PubMed] [Google Scholar]
- 64.Zhu XY, Zhang XZ, Xu L, Zhong XY, Ding Q, Chen YX. Transplantation of adipose-derived stem cells overexpressing hhgf into cardiac tissue. Biochem Biophys Res Commun. 2009;379:1084–1090 [DOI] [PubMed] [Google Scholar]
- 65.Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, Traas J, Schugar R, Deasy BM, Badylak S, Buhring HJ, Giacobino JP, Lazzari L, Huard J, Peault B. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313 [DOI] [PubMed] [Google Scholar]
- 66.Covas DT, Panepucci RA, Fontes AM, Silva WA Jr., Orellana MD, Freitas MC, Neder L, Santos AR, Peres LC, Jamur MC, Zago MA. Multipotent mesenchymal stromal cells obtained from diverse human tissues share functional properties and gene-expression profile with cd146+ perivascular cells and fibroblasts. Exp Hematol. 2008;36:642–654 [DOI] [PubMed] [Google Scholar]
- 67.Traktuev DO, Merfeld-Clauss S, Li J, Kolonin M, Arap W, Pasqualini R, Johnstone BH, March KL. A population of multipotent cd34-positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circ Res. 2008;102:77–85 [DOI] [PubMed] [Google Scholar]
- 68.da Silva Meirelles L, Caplan AI, Nardi NB. In search of the in vivo identity of mesenchymal stem cells. Stem Cells. 2008;26:2287–2299 [DOI] [PubMed] [Google Scholar]
- 69.Eto H, Suga H, Matsumoto D, Inoue K, Aoi N, Kato H, Araki J, Yoshimura K. Characterization of structure and cellular components of aspirated and excised adipose tissue. Plast Reconstr Surg. 2009;124:1087–1097 [DOI] [PubMed] [Google Scholar]
- 70.Medina RJ, O’Neill CL, Sweeney M, Guduric-Fuchs J, Gardiner TA, Simpson DA, Stitt AW. Molecular analysis of endothelial progenitor cell (epc) subtypes reveals two distinct cell populations with different identities. BMC Med Genomics. 2010;3:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Urbich C, Heeschen C, Aicher A, Dernbach E, Zeiher AM, Dimmeler S. Relevance of monocytic features for neovascularization capacity of circulating endothelial progenitor cells. Circulation. 2003;108:2511–2516 [DOI] [PubMed] [Google Scholar]
- 72.Medina RJ, O’Neill CL, O’Doherty TM, Knott H, Guduric-Fuchs J, Gardiner TA, Stitt AW. Myeloid angiogenic cells act as alternative m2 macrophages and modulate angiogenesis through interleukin-8. Mol Med. 2011;17:1045–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Prokopi M, Pula G, Mayr U, Devue C, Gallagher J, Xiao Q, Boulanger CM, Westwood N, Urbich C, Willeit J, Steiner M, Breuss J, Xu Q, Kiechl S, Mayr M. Proteomic analysis reveals presence of platelet microparticles in endothelial progenitor cell cultures. Blood. 2009;114:723–732 [DOI] [PubMed] [Google Scholar]
- 74.Lumeng CN, DelProposto JB, Westcott DJ, Saltiel AR. Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes. 2008;57:3239–3246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964 [DOI] [PubMed] [Google Scholar]
- 76.David Dong ZM, Aplin AC, Nicosia RF. Regulation of angiogenesis by macrophages, dendritic cells, and circulating myelomonocytic cells. Curr Pharm Des. 2009;15:365–379 [DOI] [PubMed] [Google Scholar]
- 77.Morris DL, Oatmen KE, Wang T, DelProposto JL, Lumeng CN. Cx3cr1 deficiency does not influence trafficking of adipose tissue macrophages in mice with diet-induced obesity. Obesity (Silver Spring). 2012;20:1189–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35 [DOI] [PubMed] [Google Scholar]
- 79.De Palma M, Biziato D, Petrova TV. Microenvironmental regulation of tumour angiogenesis. Nat Rev Cancer. 2017;17:457–474 [DOI] [PubMed] [Google Scholar]
- 80.Weirather J, Hofmann UD, Beyersdorf N, Ramos GC, Vogel B, Frey A, Ertl G, Kerkau T, Frantz S. Foxp3+ cd4+ t cells improve healing after myocardial infarction by modulating monocyte/macrophage differentiation. Circ Res. 2014;115:55–67 [DOI] [PubMed] [Google Scholar]
- 81.Hao C, Shintani S, Shimizu Y, Kondo K, Ishii M, Wu H, Murohara T. Therapeutic angiogenesis by autologous adipose-derived regenerative cells: Comparison with bone marrow mononuclear cells. Am J Physiol Heart Circ Physiol. 2014;307:H869–879 [DOI] [PubMed] [Google Scholar]
- 82.Boyd NL, Nunes SS, Krishnan L, Jokinen JD, Ramakrishnan VM, Bugg AR, Hoying JB. Dissecting the role of human embryonic stem cell-derived mesenchymal cells in human umbilical vein endothelial cell network stabilization in three-dimensional environments. Tissue Eng Part A. 2013;19:211–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Koike N, Fukumura D, Gralla O, Au P, Schechner JS, Jain RK. Tissue engineering: Creation of long-lasting blood vessels. Nature. 2004;428:138–139 [DOI] [PubMed] [Google Scholar]
- 84.Katz AJ, Tholpady A, Tholpady SS, Shang H, Ogle RC. Cell surface and transcriptional characterization of human adipose-derived adherent stromal (hadas) cells. Stem Cells. 2005;23:412–423 [DOI] [PubMed] [Google Scholar]
- 85.Verseijden F, Posthumus-van Sluijs SJ, Pavljasevic P, Hofer SO, van Osch GJ, Farrell E. Adult human bone marrow- and adipose tissue-derived stromal cells support the formation of prevascular-like structures from endothelial cells in vitro. Tissue Eng Part A. 2010;16:101–114 [DOI] [PubMed] [Google Scholar]
- 86.Rohringer S, Hofbauer P, Schneider KH, Husa AM, Feichtinger G, Peterbauer-Scherb A, Redl H, Holnthoner W. Mechanisms of vasculogenesis in 3d fibrin matrices mediated by the interaction of adipose-derived stem cells and endothelial cells. Angiogenesis. 2014;17:921–933 [DOI] [PubMed] [Google Scholar]
- 87.Merfeld-Clauss S, Gollahalli N, March KL, Traktuev DO. Adipose tissue progenitor cells directly interact with endothelial cells to induce vascular network formation. Tissue Eng Part A. 2010;16:2953–2966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kachgal S, Putnam AJ. Mesenchymal stem cells from adipose and bone marrow promote angiogenesis via distinct cytokine and protease expression mechanisms. Angiogenesis. 2011;14:47–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Holnthoner W, Hohenegger K, Husa AM, Muehleder S, Meinl A, Peterbauer-Scherb A, Redl H. Adipose-derived stem cells induce vascular tube formation of outgrowth endothelial cells in a fibrin matrix. J Tissue Eng Regen Med. 2015;9:127–136 [DOI] [PubMed] [Google Scholar]
- 90.Traktuev DO, Prater DN, Merfeld-Clauss S, Sanjeevaiah AR, Saadatzadeh MR, Murphy M, Johnstone BH, Ingram DA, March KL. Robust functional vascular network formation in vivo by cooperation of adipose progenitor and endothelial cells. Circ Res. 2009;104:1410–1420 [DOI] [PubMed] [Google Scholar]
- 91.DeLisser HM, Christofidou-Solomidou M, Strieter RM, Burdick MD, Robinson CS, Wexler RS, Kerr JS, Garlanda C, Merwin JR, Madri JA, Albelda SM. Involvement of endothelial pecam-1/cd31 in angiogenesis. Am J Pathol. 1997;151:671–677 [PMC free article] [PubMed] [Google Scholar]
- 92.Bach TL, Barsigian C, Chalupowicz DG, Busler D, Yaen CH, Grant DS, Martinez J. Ve-cadherin mediates endothelial cell capillary tube formation in fibrin and collagen gels. Exp Cell Res. 1998;238:324–334 [DOI] [PubMed] [Google Scholar]
- 93.Laurie GW, Leblond CP, Martin GR. Localization of type iv collagen, laminin, heparan sulfate proteoglycan, and fibronectin to the basal lamina of basement membranes. J Cell Biol. 1982;95:340–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fantin A, Vieira JM, Gestri G, Denti L, Schwarz Q, Prykhozhij S, Peri F, Wilson SW, Ruhrberg C. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of vegf-mediated endothelial tip cell induction. Blood. 2010;116:829–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yoon CH, Hur J, Park KW, Kim JH, Lee CS, Oh IY, Kim TY, Cho HJ, Kang HJ, Chae IH, Yang HK, Oh BH, Park YB, Kim HS. Synergistic neovascularization by mixed transplantation of early endothelial progenitor cells and late outgrowth endothelial cells: The role of angiogenic cytokines and matrix metalloproteinases. Circulation. 2005;112:1618–1627 [DOI] [PubMed] [Google Scholar]
- 96.Pearson JD. Endothelial progenitor cells--an evolving story. Microvasc Res. 2010;79:162–168 [DOI] [PubMed] [Google Scholar]
- 97.Chambers SE, O’Neill CL, O’Doherty TM, Medina RJ, Stitt AW. The role of immune-related myeloid cells in angiogenesis. Immunobiology. 2013;218:1370–1375 [DOI] [PubMed] [Google Scholar]
- 98.Urbich C, Aicher A, Heeschen C, Dernbach E, Hofmann WK, Zeiher AM, Dimmeler S. Soluble factors released by endothelial progenitor cells promote migration of endothelial cells and cardiac resident progenitor cells. J Mol Cell Cardiol. 2005;39:733–742 [DOI] [PubMed] [Google Scholar]
- 99.Yoon CH, Hur J, Park KW, Kim JH, Lee CS, Oh IY, Kim TY, Cho HJ, Kang HJ, Chae IH, Yang HK, Oh BH, Park YB, Kim HS. Synergistic neovascularization by mixed transplantation of early endothelial progenitor cells and late outgrowth endothelial cells - the role of angiogenic cytokines and matrix metalloproteinases. Circulation. 2005;112:1618–1627 [DOI] [PubMed] [Google Scholar]
- 100.Fridman R, Toth M, Pena D, Mobashery S. Activation of progelatinase b (mmp-9) by gelatinase a (mmp-2). Cancer Res. 1995;55:2548–2555 [PubMed] [Google Scholar]
- 101.Tongers J, Roncalli JG, Losordo DW. Therapeutic angiogenesis for critical limb ischemia: Microvascular therapies coming of age. Circulation. 2008;118:9–16 [DOI] [PubMed] [Google Scholar]
- 102.Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100:1249–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Laschke MW, Menger MD. Prevascularization in tissue engineering: Current concepts and future directions. Biotechnology Advances. 2016;34:112–121 [DOI] [PubMed] [Google Scholar]
- 104.Hamdi H, Planat-Benard V, Bel A, Puymirat E, Geha R, Pidial L, Nematalla H, Bellamy V, Bouaziz P, Peyrard S, Casteilla L, Bruneval P, Hagege AA, Agbulut O, Menasche P. Epicardial adipose stem cell sheets results in greater post-infarction survival than intramyocardial injections. Cardiovasc Res. 2011;91:483–491 [DOI] [PubMed] [Google Scholar]
- 105.Shepherd BR, Hoying JB, Williams SK. Microvascular transplantation after acute myocardial infarction. Tissue Eng. 2007;13:2871–2879 [DOI] [PubMed] [Google Scholar]
- 106.Leblanc AJ, Touroo JS, Hoying JB, Williams SK. Adipose stromal vascular fraction cell construct sustains coronary microvascular function after acute myocardial infarction. Am J Physiol Heart Circ Physiol. 2012;302:H973–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Panina YA, Yakimov AS, Komleva YK, Morgun AV, Lopatina OL, Malinovskaya NA, Shuvaev AN, Salmin VV, Taranushenko TE, Salmina AB. Plasticity of adipose tissue-derived stem cells and regulation of angiogenesis. Front Physiol. 2018;9:1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lee J, Abdeen AA, Tang X, Saif TA, Kilian KA. Matrix directed adipogenesis and neurogenesis of mesenchymal stem cells derived from adipose tissue and bone marrow. Acta Biomater. 2016;42:46–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Naito H, Kidoya H, Sakimoto S, Wakabayashi T, Takakura N. Identification and characterization of a resident vascular stem/progenitor cell population in preexisting blood vessels. EMBO J. 2012;31:842–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gulati R, Jevremovic D, Peterson TE, Chatterjee S, Shah V, Vile RG, Simari RD. Diverse origin and function of cells with endothelial phenotype obtained from adult human blood. Circ Res. 2003;93:1023–1025 [DOI] [PubMed] [Google Scholar]
- 111.Lin Y, Weisdorf DJ, Solovey A, Hebbel RP. Origins of circulating endothelial cells and endothelial outgrowth from blood. J Clin Invest. 2000;105:71–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.El-Badawy A, Amer M, Abdelbaset R, Sherif SN, Abo-Elela M, Ghallab YH, Abdelhamid H, Ismail Y, El-Badri N. Adipose stem cells display higher regenerative capacities and more adaptable electro-kinetic properties compared to bone marrow-derived mesenchymal stromal cells. Sci Rep. 2016;6:37801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wagner W, Wein F, Seckinger A, Frankhauser M, Wirkner U, Krause U, Blake J, Schwager C, Eckstein V, Ansorge W, Ho AD. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp Hematol. 2005;33:1402–1416 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.