Abstract
Electronic cigarettes (e-cigarettes) are nicotine delivery devices advertised as a healthier alternative to conventional tobacco products, but their rapid rise in popularity outpaces research on potential health consequences. Since conventional tobacco use is a risk factor for osteoporosis, this study examines whether exposure to electronic liquid (e-liquid) used in e-cigarettes affects bone-forming osteoblasts. Human MG-63 and Saos-2 osteoblast-like cells were treated for 48 h with 0.004%−4.0% dilutions of commercially available e-liquids of various flavors with or without nicotine. Changes in cell viability and key osteoblast markers, RUNX2 and Col1a1, were assessed. With all e-liquids tested, cell viability decreased in a dose-dependent manner, which was least pronounced in flavorless e-liquids, most pronounced in cinnamon-flavored e-liquids, and occurred independently of nicotine. Col1a1, but not RUNX2, mRNA expression was upregulated in response to coffee-flavored and fruit-flavored e-liquids. Cells treated with a non-cytotoxic concentration of fruit-flavored Mango Blast e-liquid with or without nicotine showed significantly increased collagen type I protein expression compared to culture medium only. We conclude that the degree of osteotoxicity is flavor-dependent and occurs independently of nicotine and that flavored e-liquids reveal collagen type I as a potential target in osteoblasts. This study elucidates potential consequences of e-cigarette use in bone.
Keywords: Electronic cigarette liquid, MG-63 osteoblast-like cells, Saos-2 osteoblast-like cells, Col1a1, RUNX2, cytotoxicity
SHORT ABSTRACT
This study investigates the effects of electronic cigarette liquids on human MG-63 and Saos-2 osteoblast-like cells exposed to dilutions of e-liquids with or without nicotine. All e-liquids decreased cell viability in a dose-dependent manner, which was exacerbated by flavorings but independent of nicotine. Cinnamon-flavored e-liquids were the most osteotoxic. Coffee- and fruit-flavored e-liquids increased collagen type I revealing this matrix protein as a target for e-liquid osteotoxicity and demonstrating further need for research on bone health consequences of e-cigarette use.
1. Introduction
Electronic cigarettes (e-cigarettes) are nicotine-delivery devices that are rapidly gaining worldwide popularity as a combustion-free alternative to conventional cigarettes. Emerging on the Chinese market in 2004 and in the USA in 2007, e-cigarettes are now a multi-billion dollar industry (U.S. Department of Health and Human Services. Centers for Disease Control and Prevention, 2016). A standard e-cigarette features a battery, heating element, and an electronic liquid (e-liquid) chamber. The e-liquid, which typically contains a mixture of nicotine, propylene glycol, vegetable glycerin, and flavoring agents, is vaporized into an aerosol when the e-cigarette battery warms the heating element. The aerosol is then inhaled by the user in a process known as vaping.
While e-cigarettes are advertised as a healthier alternative to tobacco, their rapid rise in popularity outpaces research on potential health consequences associated with their use. In the USA alone, over two million middle and high school students report using e-cigarettes, making e-cigarettes more popular than tobacco products in this age group (CDC & Prevention, 2017). Furthermore, the appeal to teenagers fuels growing concerns over health risks associated with e-cigarette use. A recent systemic review of case reports of e-cigarette users summarizes adverse health effects ascribed to e-cigarette use, including respiratory, gastrointestinal, and cardiovascular complications (Hua & Talbot, 2016). Another important, although under investigated, area of e-cigarette research is their potential impact on the skeletal system. Childhood and adolescence are critical times for optimal bone growth and development. Approximately 90% of bone mass is accrued by early adulthood at around 18 years of age (Bachrach, 2001). Hence, it is possible that young e-cigarette users are impairing their bone development, which may increase their risk of developing osteoporosis later in life. Osteoporosis, characterized by reduced bone mineral density and deterioration of the bone microarchitecture, is the leading cause of bone fractures (NIH Consensus Development Panel on Osteoporosis Prevention, 2001). Furthermore, osteoporosis is the most common metabolic bone disease in humans; thus, understanding risk factors associated with this disease is germane.
The effects of e-cigarette use on bone health are unknown; however, conventional tobacco cigarette use is linked to the pathogenesis of osteoporosis. Epidemiological studies demonstrate smoking conventional tobacco leads to reduced bone mineral density and increased risk for osteoporotic fractures (Kanis et al., 2005; Yoon, Maalouf, & Sakhaee, 2012). There are two proposed mechanisms by which smoking tobacco leads to reduced bone mineral density. First, tobacco smoke exposure can indirectly alter bone function by increasing parathyroid hormone release, increasing cortisol production, or reducing vitamin D metabolism (Abate, Vanni, Pantalone, & Salini, 2013; Yoon et al., 2012). Second, tobacco smoke can directly act on bone by targeting the proliferation, differentiation, and matrix deposition of bone-forming cells called osteoblasts (Ko et al., 2015). In either case, disturbance in the normal pattern of bone remodeling can contribute to the development of osteoporosis.
Importantly, although e-liquids do not contain all the known carcinogens found in tobacco smoke, many contain nicotine. In vivo and in vitro studies report alterations in bone metabolism in response to nicotine concentrations comparable to that found in saliva (0.6 μM to 10 mM) or blood (0.03 μM to 0.5 μM) of tobacco consumers (Benowitz, 1988; Russell, Jarvis, Iyer, & Feyerabend, 1980). Several in vitro studies report a biphasic effect of nicotine exposure on normal or tumor-derived osteoblasts with low concentrations stimulating proliferation and gene upregulation, and high concentrations eliciting the opposite effect (Marinucci, Bodo, Balloni, Locci, & Baroni, 2014; Rothem, Rothem, Soudry, Dahan, & Eliakim, 2009). The mRNA expression of collagen type I (Col1a1), the main organic component of bone extracellular matrix, is upregulated in MG-63 osteoblast-like cells exposed for 24 hours to 0.1 μM to 100 μM nicotine but downregulated upon exposure to 10 mM nicotine (Rothem et al., 2009). More recently, Marinucci et al., 2014 reported that several other key osteoblast genes, including the critical mediator of the osteoblast phenotype runt-related transcription factor 2 (RUNX2), are upregulated or repressed in normal human osteoblasts cultured in the presence of 0.1–10 μM nicotine (Marinucci et al., 2014). RUNX2 is a transcription factor essential for the development, maturation and maintenance of osteoblasts (Ducy et al., 1999).
Besides nicotine, flavoring agents in e-liquids could negatively alter osteoblast proliferation, differentiation, or matrix deposition. Several surveys indicate that the primary reason for increased e-cigarette use in youth is the wide variety of flavorings available (Dai & Hao, 2016; Patel et al., 2016; Villanti et al., 2017). There are over 8,000 e-liquids flavors on the market ranging from “Peanut Butter and Jelly Sandwich” and “Mango Blast” to Tobacco flavors (Zhu et al., 2014). In 2016, the US Federal Drug Administration began regulating e-cigarettes under tobacco products. However, unlike conventional tobacco products where flavorings (except menthol) are no longer permitted, e-liquids may still contain flavoring agents. Furthermore, many of these flavoring agents are categorized as “generally recognized as safe” by the Flavor Extracts Manufacturers Association (FEMA) for ingestion; however, the classification does not pertain to inhalation. To this point, several in vitro studies using human, rat or mouse cells demonstrate that some flavoring agents in e-liquids are cytotoxic (Bahl et al., 2012; Behar et al., 2014; Behar, Wang, & Talbot, 2017; Farsalinos et al., 2013; Lerner et al., 2015; Otreba, Kosmider, Knysak, Warncke, & Sobczak, 2018; Rowell et al., 2017). Cinnamon-flavored e-liquids are particularly cytotoxic in rat cardiomyoblasts and human CALU3 airway cells (Farsalinos et al., 2013; Rowell et al., 2017). Another recent study using primary human oropharyngeal mucosal cultures found fruity-flavored e-liquids to be overly cytotoxic and more so than tobacco-flavored e-liquids (Welz et al., 2016). Notable is that the cytotoxic effect of flavored e-liquids can occur independently of the presence of nicotine, suggesting that flavoring agents alone can induce cellular damage (Bahl et al., 2012; Kaur, Muthumalage, & Rahman, 2018; Rowell et al., 2017). Taken together, these studies provide a compelling rationale for the current research, which investigates the impact of in vitro exposure to flavored e-liquids on osteoblasts.
Because smoking conventional cigarettes is a risk factor for osteoporosis, we hypothesize that vaping impairs bone by targeting bone-forming osteoblasts. We used human MG-63 and Saos-2 osteoblast-like cell lines in this study. Cells were exposed to a variety of flavored unvaped e-liquids, with or without nicotine, from four commercially available brands and assessed for changes in cell viability, RUNX2 and Col1a1 mRNA expression, and collagen type I protein expression. Several recent in vitro studies found comparable results between aerosolized and unvaped e-liquids on cell viability, justifying the use of unvaped e-liquid exposures in this study as a first pass screening method to assess osteotoxicity (Behar et al., 2017; Rowell et al., 2017). This study aims to increase awareness of possible bone-related health risks associated with e-cigarette use.
2. Materials and methods
2.1. Cell culture
The human osteosarcoma cell lines Saos-2 and MG-63 were purchased from American Type Culture Collection (ATCC, Manassas, VA) and maintained using established culture conditions (Arbon, Christensen, Harvey, & Heggland, 2012; Coonse, Coonts, Morrison, & Heggland, 2007; Ha, Burwell, Goodwin, Noeker, & Heggland, 2016; Smith et al., 2009). Briefly, Saos-2 cells were cultured in McCoy’s 5A medium and MG-63 cells in Eagles MEM medium, each supplemented with 10% FBS (Atlanta Biologicals, Lawrenceville, GA), 2 mM L-glutamine, 100 IU/ml penicillin, and 100 μg/ml streptomycin (Sigma–Aldrich, St. Louis, MO). Cells were cultured at 37 °C in air containing 5% CO2. For routine maintenance, medium was changed every 3–4 days and cells were subcultured weekly.
2.2. Sources of e-liquids.
Twenty-three commercially available e-liquids from four different brands were purchased for this study. Vapor Emporium and Lotus brands were bought from retail shops in Nampa, ID. Mister-E-liquid (https://www.mister-e-liquid.com/) and Vape Dudes (https://www.vapedudes.com) were purchased online. Each e-liquid arrived packaged in a sealed bottle that was labeled by the manufacturer as containing 0 mg/ml nicotine (used as a nicotine-free control) or 24 mg/ml nicotine. Refer to Table 1 for information on e-liquid flavors and propylene glycol/vegetable glycerin (PG/VG) ratios for each e-liquid. PG and VG are humectants that keep flavorings and nicotine in suspension and facilitate vaporization when heated. Note the PG/VG ratio for Lotus Brand and Vapor Emporium were not included on the label.
TABLE 1: E-liquid Product Information and Cytotoxicity.
EC50 values for e-liquids from Vapor Emporium, Lotus, Mister-E-liquid, and Vape Dudes brands. For each e-liquid, its brand, name, PG/VG ratio, and stock concentrations of nicotine are identified. MG-63 and Saos-2 cells were treated for 48 h with e-liquid containing a final nicotine concentration of 0.001, 0.01, 0.1, 0.5, or 1.0 mg/ml or an equivalent volume of e-liquid without nicotine at 0.004% 0.04%, 0.4%, 2.0% and 4.0%, respectively. Each EC50 value (expressed as % volume of e-liquid) was calculated using a linear model of the compiled cell viability data, with or without nicotine, for each cell line.
| Flavor | Brand | Name | PG/VG | Stock Concentration Nicotine (mg/ml) | MG-63 EC50 (% volume) | Saos-2 EC50 (% volume) |
|---|---|---|---|---|---|---|
| Flavorless | Vapor Emporium | Flavorless | Not Reported | 0 | 3.10 | 3.10 |
| 24 | 2.38 | 3.26 | ||||
| Mister E-liquid | Clear | 50/50 | 0 | 6.19 | 3.27 | |
| 24 | 4.71 | 2.38 | ||||
| Vape Dudes | Flavorless | 50/50 | 0 | 5.40 | 3.92 | |
| 24 | 7.30 | 3.14 | ||||
| Watermelon | Lotus | Sweet Melon | Not Reported | 0 | 2.68 | 2.12 |
| 24 | 2.01 | 1.62 | ||||
| Mister E-liquid | Watermelon | 50/50 | 0 | 3.47 | 3.32 | |
| 24 | 2.93 | 3.21 | ||||
| Vape Dudes | Watermelon Drip | 50/50 | 0 | 2.71 | 2.63 | |
| 24 | 2.13 | 2.04 | ||||
| Mango | Lotus | Mango Blast | Not Reported | 0 | 2.41 | 2.10 |
| 24 | 2.76 | 2.18 | ||||
| Mixed Fruits | Lotus | XXX Berry | Not Reported | 0 | 1.93 | 1.64 |
| 24 | 2.07 | 1.63 | ||||
| Mister E-liquid | Heartbreaker | 50/50 | 0 | 2.57 | 2.08 | |
| 24 | 2.74 | 2.64 | ||||
| Vape Dudes | Possum Sauce | 50/50 | 0 | 2.73 | 2.60 | |
| 24 | 2.51 | 2.28 | ||||
| Coffee | Lotus | Irish Latte | Not Reported | 0 | 3.20 | 2.49 |
| 24 | 2.30 | 1.98 | ||||
| Mister E-liquid | G.T.F.O. | 50/50 | 0 | 2.40 | 2.36 | |
| 24 | 1.65 | 1.50 | ||||
| Vape Dudes | Irish Coffee | 50/50 | 0 | 6.67 | 2.96 | |
| 24 | 5.92 | 2.93 | ||||
| Apple Pie | Mister E-liquid | Gran-E’s Apple Pie | 50/50 | 0 | 2.11 | 1.78 |
| 24 | 1.77 | 1.01 | ||||
| Vape Dudes | Apple Pie | 50/50 | 0 | 3.02 | 2.62 | |
| 24 | 2.63 | 2.41 | ||||
| Menthol & Watermelon | Vape Dudes | Watermelon ICE | 50/50 | 0 | 2.12 | 2.38 |
| 24 | 2.00 | 1.68 | ||||
| Menthol | Lotus | Menthol | Not Reported | 0 | 1.75 | 1.99 |
| 24 | 1.50 | 2.21 | ||||
| Mister E-liquid | Mister E’s Menthol | 50/50 | 0 | 2.15 | 2.32 | |
| 24 | 1.62 | 1.43 | ||||
| Vape Dudes | ICE ICE | 50/50 | 0 | 1.86 | 2.12 | |
| 24 | 1.93 | 2.19 | ||||
| Hot Cinnamon | Lotus | Fireball | Not Reported | 0 | 1.23 | <0.004 |
| 24 | 0.97 | <0.004 | ||||
| Mister E-liquid | Napalm | 50/50 | 0 | 1.21 | <0.004 | |
| 24 | 0.54 | <0.004 | ||||
| Vape Dudes | Cinn Candy | 50/50 | 0 | 1.76 | <0.004 | |
| 24 | 1.56 | <0.004 | ||||
| Menthol & Cinnamon | Vape Dudes | FIRE & ICE | 50/50 | 0 | 1.66 | 2.02 |
| 24 | 0.97 | 0.01 |
2.3. Cell treatment
Cells were plated at different densities depending on the assay. The culture medium was changed after 24 h and treatment was initiated in Opti-MEM medium, which is serum-free and phenol-red free (Invitrogen, Carlsbad, CA). Sterile filtered e-liquid treatments were prepared by diluting unvaped e-liquid in Opti-MEM medium. Cells were treated for 48 h with e-liquid containing a final nicotine concentration of 0.001, 0.01, 0.1, 0.5, or 1.0 mg/ml or an equivalent volume of e-liquid without nicotine at 0.004%, 0.04%, 0.4%, 2.0% and 4.0%, respectively. Volumes of diluted e-liquid were selected based on previously reported cell culture conditions as well as nicotine concentrations that were comparable to human blood and saliva of tobacco users (Bahl et al., 2012; Behar et al., 2017; Benowitz, 1988; Rowell et al., 2017; Russell et al., 1980). Since scientific analyses of e-liquids report up to 10% inaccuracy in the actual nicotine concentrations compared to what is indicated on the manufacturer’s label (Davis, Dang, Kim, & Talbot, 2015), cells were also treated with 0.001, 0.01, 0.1, 0.5, or 1.0 mg/ml nicotine purchased from Sigma-Aldrich (catalog # 612596) for 48 h. All experiments included an additional control whereby cells were treated with Opti-MEM serum-free medium only.
2.4. Cell viability assay
Cells were plated at a density of 8 × 104 cells/well in a 96- well culture plate. After 48 h treatment, cells were washed with phosphate buffered saline (PSB) and incubated at 37°C with 10 μg/ml MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium-bromide; ATCC, Manassas, VA) for 4 h. The conversion of tetrazolium salt MTT to a colored formazan by mitochondrial dehydrogenase was used to assess cell viability. After the supernatant was removed, 100 μl of DMSO was added to each well and absorbance was read at 570 nm.
2.5. Immunofluorescence detection of collagen type I protein
MG-63 cells were plated at 6 × 104 cells/well in Poly-D-Lysine/Laminin 8-well culture chamber slides (BD BioSciences, Bedford, MA). After treatment, cells were washed with EMEM serum-free medium, fixed with 3.7% formaldehyde, rinsed with PBS, and permeabilized with methanol before being blocked for 1 hour with 2% BSA + 0.1% Triton X in PBS. Cells were incubated with a primary antibody to collagen type I (AbCam, Cambridge, MA) for 90 minutes, washed twice with 0.1% Triton X in PBS, and then followed by a 1-hour incubation with a secondary antibody conjugated to Alexa Fluor 488. Cells were washed three times with 0.1% Triton X in PBS. All incubations were done at 37°C. Collagen type I was visualized using a Nikon Epifluorescence microscope and digital images were captured using ImagePro software by media Cybergenetics (Silver Spring, MD) using the same exposure time and filter setting for all images.
2.6. RNA isolation and quantitative real-time PCR (qRT-PCR) analysis
MG-63 cells were plated at a density of 6 × 105 cells/well in six-well culture plate. After treatment, cells were washed twice with PBS and total RNA was extracted using the E.Z.N.A.® Total RNA Kit (Omega Bio-Tek, Norcross, GA). RNA concentrations and purity were measured by ultraviolet absorbance, and quality was assessed on an agarose bleach gel. RNA was reverse-transcribed using the Applied Biosystems High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Carlsbad, CA). Gene-specific primers (listed below) were used for qRT-PCR, which was performed using Roche FastStart Essential DNA Green Master reaction mix on a LightCycler® 96 thermocycler (Roche, Indianapolis, IN).
| Primer seauence (5’ to 3’) for aRT-PCR | Temp (°C) |
| RUNX2: TAT GGC ACT TCG TCA GGA TCC | 64°C |
| AAT AGC GTG CTG CCA TTC G | |
| Collal: AAC ATG ACC AAA AAC CAA AAG TG | 63°C |
| CAT TGT TTC CTG TGT CTT CTG G | |
| GAPDH: CTC TGC TCC TCC TGT TCG AC | 53°C |
| TTA AAA GCA GCC CTG GTG AC |
2.7. Statistical analysis
For all experiments, the mean ± SEM values represent at least three independent experiments. MTT data were analyzed using the Dunnett’s test for comparisons between e-liquid treatments and the medium-only control. For multiple comparisons to assess the influence of the cell line or nicotine, we used a two-way ANOVA followed by a Tukey post-hoc test. Each EC50 value (expressed as % volume of e-liquid) was calculated using a linear model of the compiled cell viability data, with or without nicotine, for each cell line. Relative mRNA levels were estimated using the ΔΔCq method normalized to GAPDH, and data were presented as fold-change compared to culture medium only control. ΔCq values were used for statistical testing using the Bonferroni post-hoc test. Collagen type I immunofluorescence staining was quantified using Image J software (National Institute of Health, Bethesda, MD), and the amount of staining was expressed as percent of the total area of the captured image. A p-value of < 0.05 was considered statistically significant. All statistical analyses were carried out using the software program SigmaPlot 13.0.
3. Results
3.1. The degree of osteotoxicity occurs independently of nicotine and is flavor-dependent.
A variety of flavored and flavorless e-liquids from four different brands were selected for cytotoxicity screening. In order to assess the effect of nicotine, a nicotine-free matched control was used. Each experiment also included a culture medium only control. Table 1 summarizes the results of cytotoxicity screening of 23 nicotine-containing e-liquids and their matching nicotine-free e-liquids with EC50 values for both MG-63 and Saos-2 osteoblast-like cell lines.
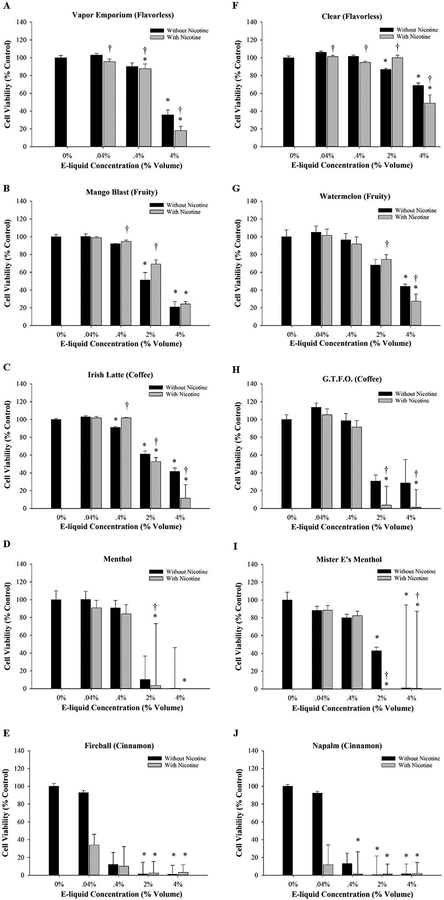
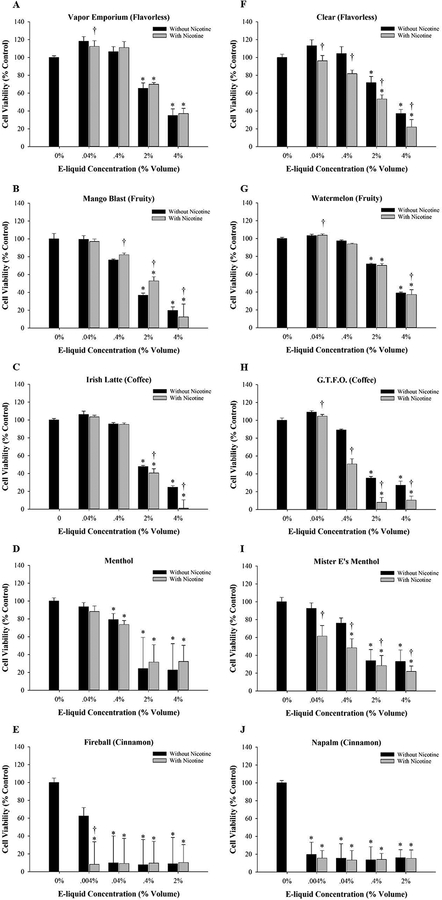
Dose-response MTT experiments using MG-63 and Saos-2 cells exposed to selected flavorless, fruity, coffee, menthol, and cinnamon flavored e-liquids are shown in Figures 1 and 2. An important finding from these experiments was that a dose-dependent decrease in viability was detected after 48 h exposure to all e-liquids tested in both cell lines compared to culture medium only. Several of the e-liquids were highly cytotoxic at 2% volume or higher, contributing to data variability (Fig. 1D, E, I, J and 2E and J). However, there were no consistent differences between e-liquid treatments with or without nicotine, suggesting that the changes in viability occurred independently of nicotine.
Figure 1:
The effect of e-liquids on cell viability. MG-63 cells were treated for 48 h with e-liquid containing a final nicotine concentration of 0.01, 0.1, 0.5, or 1.0 mg/ml or an equivalent volume of e-liquid without nicotine at 0.04%, 0.4%, 2.0% and 4.0%, respectively. Cell viability was determined using the MTT assay. Results are expressed as percent cell viability. Each bar represents the mean ± SEM of at least 3 independent experiments. * denotes significant difference from culture medium only control (p <0.05). † denotes significant difference from the matched control without nicotine (p <0.05). The e-liquids tested were (A) Vapor Emporium brand, (B-E) Lotus brand, (F-J) Mister-E-Liquid brand.
Figure 2:
The effect of e-liquids on cell viability. Saos-2 cells were treated for 48 h with e-liquid containing a final nicotine concentration of 0.001, 0.01, 0.1, 0.5, or 1.0 mg/ml or an equivalent volume of e-liquid without nicotine at 0.004% 0.04%, 0.4%, 2.0% or 4.0%, respectively. Cell viability was determined using the MTT assay. Results are expressed as percent cell viability. Each bar represents the mean ± SEM of at least 3 independent experiments.* denotes a significant difference from culture medium only control (p <0.05). † denotes significant difference from the matched control without nicotine (p <0.05). The e-liquids tested were (A) Vapor Emporium brand, (B-E) Lotus brand, (F-J) Mister-E-Liquid brand.
Another key finding from these cell viability experiments was that flavored e-liquids caused a more pronounced reduction in viability compared to e-liquids without flavorings (Fig. 1 and 2). Consistent among the brands tested, the least cytotoxic e-liquids were the flavorless e-liquids (Fig. 1A, F and 2A, F). With regards to the flavored e-liquids, the degree of osteotoxicity varied. The least cytotoxic flavored e-liquids were coffee (Fig. 1C, H and 2C, H) and fruity (Fig. 1B, G and 2B, G), followed by menthol (Fig. 1D, I and 2D, I). The most cytotoxic e-liquids were cinnamon-flavored e-liquids Fireball (Fig. 1E and2E) and Napalm (Fig. 1J and2J). These results were confirmed by treating cells with known nicotine concentrations diluted to 0.001–1.0 mg/ml. Consistent with the e-liquid nicotine-containing treatments, there were no significant changes in viability in MG-63 or Saos-2 cells exposed to nicotine purchased from Sigma-Aldrich at all concentrations tested (data not shown).
Table 2 depicts EC50 values for MG-63 and Saos-2 cells treated with e-liquids with or without nicotine and grouped as flavorless, coffee, fruity, menthol, or cinnamon. The grouping of e-liquids further illustrates that flavorless e-liquids were the least cytotoxic and cinnamon-flavored e-liquids were the most cytotoxic. In addition, there were no significant differences between e-liquids with or without nicotine in any flavor category in either cell line. Thus, this confirms the cytotoxicity occurs independently of nicotine. Both cell lines responded similarly to the e-liquids with the only differences being that Saos-2 cells were more sensitive to the flavorless and cinnamon-flavors compared to MG-63 cells. Because both osteoblast-like cell lines responded similarly to the e-liquid treatments, MG-63 cells were used in subsequent experiments. Based on EC50 values, solutions of 0.4% coffee-flavored and fruity-flavored e-liquids either with or without 0.1 mg/ml nicotine were chosen for use in subsequent experiments to avoid excessive cell death. Cinnamon-flavored e-liquids were not used due to their high cytotoxicity.
TABLE 2: Average EC50 Values by Flavor Categories.
Compiled EC50 values (% volume of e-liquid) by flavor categories. “Flavorless” e-liquids include Flavorless (Vapor Emporium), Clear (Mister-E-Liquid), and Flavorless (Vape Dudes). “Coffee” e-liquids include Irish Latte (Lotus), G.T.F.O. (Mister-E-Liquid), and Irish Coffee (Vape Dudes). “Fruity” e-liquids include Sweet Melon (Lotus), Watermelon (Mister-E-Liquid), Watermelon Drip (Vape Dudes), Mango Blast (Lotus), XXX Berry (Lotus), Heartbreaker (Mister-E-Liquid), and Possum Sauce (Vape Dudes). “Menthol” e-liquids include Menthol (Lotus), Mister E’s Menthol (Mister-E-Liquid), and ICE ICE (Vape Dudes). “Cinnamon” e-liquids includes Fireball (Lotus), Napalm (Mister-E-Liquid), and Cinn Candy (Vape Dudes). * denotes significant difference when compared to the flavorless category within a cell line (p <0.05). † denotes significant difference when compared to same e-liquid treatment in other cell line (p <0.05).
| Cell Line | Nicotine | Flavorless | Fruity | Coffee | Menthol | Cinnamon |
|---|---|---|---|---|---|---|
| MG-63 | − | 4.90±0.13 | *2.64±0.05 | 4.09±0.22 | *1.92±0.04 | *1.40±0.09 |
| + | 4.80±0.21 | *2.45±0.06 | *3.29±0.29 | *1.68±0.05 | *1.02±0.20 | |
| Saos-2 | − | †3.08±0.06 | 2.36±0.06 | 2.60±0.05 | *2.14±0.03 | †*<0.004 |
| + | †2.99±0.06 | 2.23±0.10 | *2.13±0.14 | *1.94±0.09 | †*<0.004 |
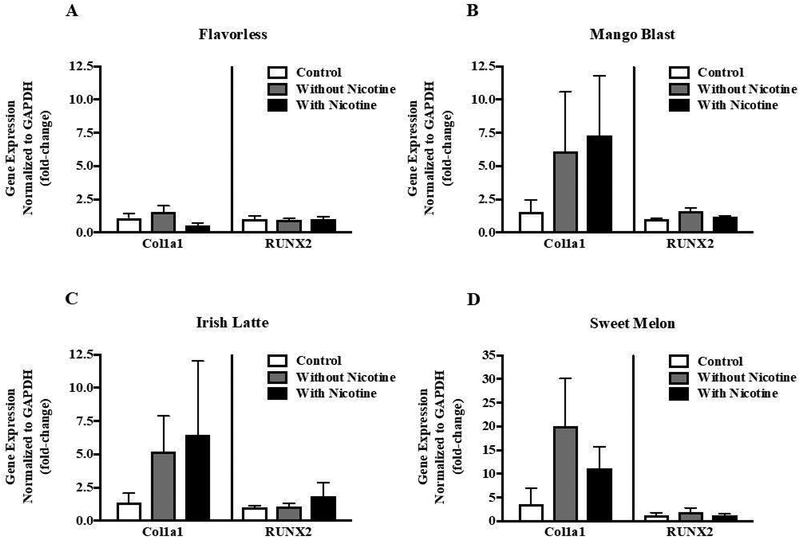
3.2. Col1a1 mRNA expression increases in response to flavored e-liquid exposure.
We were interested in whether flavored e-liquids with or without nicotine altered mRNA expression of the key osteoblast genes RUNX2 and Col1a1 in MG-63 cells. Based on the results described above, we specifically chose fruity and coffee flavors, which were not overly cytotoxic. Cells treated with 0.4% flavored Lotus e-liquids, with or without 0.1 mg/ml nicotine, showed no detectable changes in RUNX2 expression, while there was an increase in Col1a1 expression compared to culture medium control (Fig. 3A–D). Mango Blast and Irish Latte flavors induced an approximate 5-fold increase in Col1a1 expression (Fig. 3B and 3C), whereas Sweet Melon elicited a 10–15 fold increase (Fig. 3D). In contrast, treatment with flavorless e-liquid had no impact on Col1a1 expression (Fig. 3A). There were no consistent differences between the nicotine-free and nicotine-containing treatments. These results suggest that the flavorings in e-liquids alone may specifically target Col1a1 in MG-63 cells although at the mRNA level these trends were not statistically significant.
Figure 3:
The effect of e-liquids on mRNA expression. MG-63 cells were treated for 48 h with culture medium only, 0.4% e-liquid treatment without nicotine or 0.4% e-liquid treatment containing 0.1 mg/ml nicotine. The e-liquids used were (A) Vapor Emporium Flavorless, Lotus brand (B) Mango Blast, (C) Irish Latte, and (D) Sweet Melon. Col1a1 and RUNX2 mRNA expression was measured by qRT-PCR and normalized to GAPDH. Each bar represents the mean ± SEM of at least 3 independent experiments.
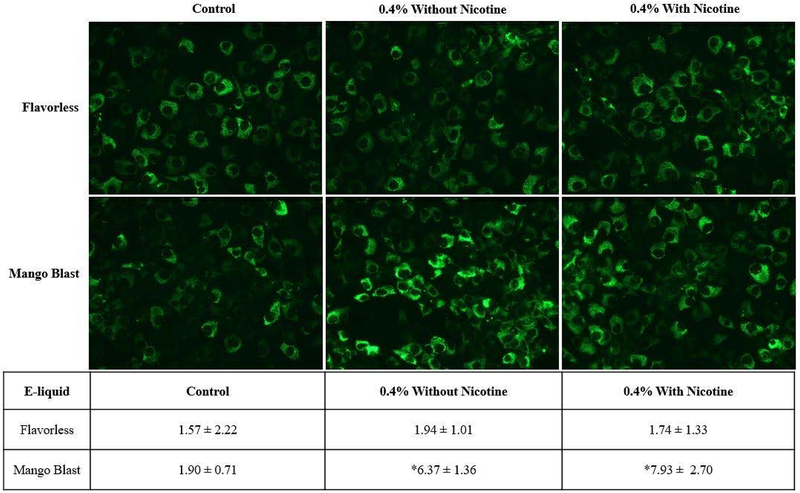
3.3. Collagen type I protein expression increases upon exposure to Mango Blast.
Next, we explored whether the trend in mRNA expression would be reflected in collagen type I protein expression. Using the same concentration of e-liquid as for the mRNA experiments, MG-63 cells were treated for 48 h with Mango Blast or Flavorless e-liquid and analyzed for collagen type I protein expression using immunofluorescence. Consistent with Figure 1B, treatment with 0.4% Mango Blast with or without nicotine resulted in no observable change in cell number (Fig. 4A). The Mango Blast e-liquid treatments, with or without nicotine, significantly increased collagen type I protein expression compared to cells treated with culture medium only (Fig. 4B). Consistent with the mRNA results, there were no significant changes in collagen type I expression in MG-63 cells exposed to flavorless e-liquid with or without nicotine.
Figure 4:
Immunofluorescence detection of cytosolic collagen type I protein. MG-63 cells were treated for 48 h with Lotus brand Mango Blast or Mister-E-liquid Clear (Flavorless). A panel of images representative of one experiment with culture medium control, 0.4% e-liquid treatment without nicotine, and 0.4% e-liquid treatment containing 0.1mg/ml nicotine. Images were analyzed and quantified as the percent area of the image within the intensity threshold using ImageJ software. The values presented are the mean ± SEM of 3 independent experiments. * denotes significant difference from culture medium only control (p <0.05).
4. Discussion
Nicotine delivery devices known as electronic cigarettes (e-cigarettes) are rapidly increasing in worldwide popularity among both adults and teenagers. Although advertised as a safer alternative to combustible tobacco, potential adverse health effects related to e-cigarette use remain under investigated. Extensive research, both in vitro and in vivo, demonstrates the detrimental impact of conventional cigarette smoke on the skeletomuscular system, in part by disrupting bone formation by osteoblasts (Ajiro, Tokuhashi, Matsuzaki, Nakajima, & Ogawa, 2010; El-Zawawy, Gill, Wright, & Sandell, 2006; Giorgetti et al., 2010; Liu et al., 2003; Marinucci et al., 2014; Vo et al., 2011). The end result can lead to decreased bone mineral density, which increases the risk for the development of osteoporosis (Abate et al., 2013). Since conventional tobacco products are reported to impair normal bone formation, an understanding of how e-cigarettes may affect bone is essential to characterizing the overall health consequences of e-cigarette use. This study focuses on the impact of flavorings and nicotine found in e-liquids on human tumor-derived osteoblast-like cells and evaluates osteotoxicity and alterations of key osteoblast markers, collagen type I and runt-related transcription factor 2 (RUNX2). The two cell lines used in this study, MG-63 and Saos-2, are well characterized and exhibit similar, though not identical, phenotypes to normal human osteoblasts (Czekanska, Stoddart, Richards, & Hayes, 2012; Pautke et al., 2004). It is important to note that recent publications indicate that unvaped e-liquid treatments accurately predict toxicity of corresponding aerosols, justifying the use of unvaped e-liquid treatments as a first screening model for e-cigarette in vitro studies (Behar et al., 2017; Rowell et al., 2017).
In this study, we use nicotine concentrations comparable to those found in blood and saliva of tobacco users that can range from 0.03 μM to 10 mM (Benowitz, 1988; Russell et al., 1980). Here we report a dose-dependent decrease in cell viability in Saos-2 and MG-63 osteoblast cell lines exposed to 23 different e-liquids without nicotine and with 0.01–1.0 mg/ml (6.2 μM-6.2 mM) nicotine compared to cells treated with culture medium only. Interestingly, we report no significant differences between e-liquid treatments with or without nicotine when grouped by flavor categories, suggesting the decrease in viability occurs independently of nicotine. Furthermore, other researchers using a direct unvaped exposure method, with comparable nicotine concentrations and culture conditions, report e-liquids to induce cytotoxicity irrespective of the presence of nicotine in human gingival fibroblasts and oropharyngeal mucosa cells, human embryonic stem cells, adult pulmonary fibroblasts, and mouse neural stem cells. In addition, two studies using e-liquid vapor extracts report cytotoxicity occurring independently of nicotine in myocardial and air-way related cells (Farsalinos et al., 2013; Leslie et al., 2017). Hence, several studies to date that use different cell types and screen of a wide variety of e-liquid brands and flavors report cytotoxicity differences related to flavorings rather than nicotine alone.
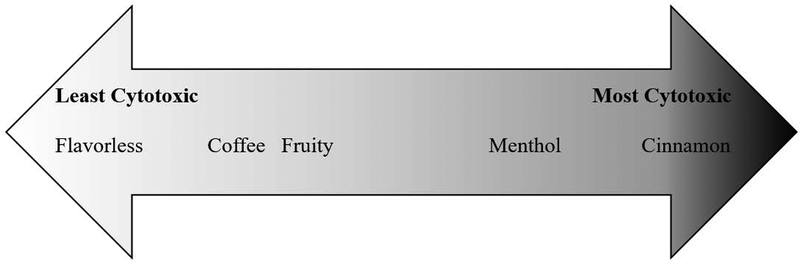
The current research demonstrates a spectrum of osteotoxicity that is flavor-dependent and consistent among the brands tested. Key to this conclusion is that treatments with unflavored e-liquids are the least cytotoxic, thereby implying that flavoring agents are a primary contributor to cytotoxicity. The observed trend from least to greatest osteotoxicity is as follows: unflavored, coffee and fruity, menthol, and cinnamon (Fig. 5). This trend is consistent between the two cell-lines, although Saos-2 is more sensitive, especially to the cinnamon-flavored e-liquids. These findings are similar to others that report flavored e-liquids to have varying degrees of cytotoxicity. For example, fruity flavors, in particular strawberry, show greater cytotoxicity among a variety of flavors tested in air-way cells exposed to vaped extracts (Leslie et al., 2017). Using a similar experimental design to the current study, oropharyngeal mucosal cells treated with 10–25% volume of unvaped e-liquids for 24 hours show fruity flavors to be more cytotoxic than tobacco flavors (Welz et al., 2016). Another study reports that menthol, strawberry and coffee flavors are overly cytotoxic to H292 human bronchial epithelial cells using an air-liquid interface exposure method (Leigh, Lawton, Hershberger, & Goniewicz, 2016). A trend consistently reported, in a variety of cell types, is that cinnamon flavors tend to be the most cytotoxic using both unvaped and vaped exposure methods (Lerner et al., 2015; Bahl et al., 2012; Behar et al., 2017).
Figure 5:
Diagram depicting osteotoxicity gradient. The position on the diagram represents the relative osteotoxicity between flavor categories, as defined in Table 2. Osteotoxicity is shown from least to greatest as read from left to right. For each of the brands tested, the flavorless e-liquid is the least cytotoxic; fruit-flavored and coffee-flavored e-liquids are mildly cytotoxic; menthol-flavored e-liquids are more cytotoxic than the aforementioned; and cinnamon-flavored e-liquids are the most cytotoxic.
Mounting evidence demonstrates that e-liquid flavorings alone can induce adverse cellular effects and points to the need to investigate the chemicals used as flavoring agents. For example, exposure to the e-liquid chemicals vanillin and chocolate 2,5-dimethylpyrazine leads to cell death via cystic fibrosis transmembrane conductance regulator through PKA activation in airway epithelial cells (Sherwood & Boitano, 2016). In relation to our study, Behar et al., 2014 identified chemicals in cinnamon-flavored e-liquids with cinnamaldehyde being the dominant flavoring chemical (Behar et al., 2014). In support of the current study, a recent report demonstrates cinnamaldehyde exposure to be the most cytotoxic among the flavoring chemicals tested in human monocytic cell lines (Muthumalage et al., 2017). Furthermore, treatment with noncytotoxic concentrations of cinnamaldehyde leads to a pro-inflammatory response in human lung epithelial cells and fibroblasts (Gerloff et al., 2017) and results in cytoskeletal alterations and genotoxicity in human pulmonary fibroblasts (Behar et al., 2016). Interestingly, cinnamaldehyde also is commonly found in fruit-flavored and sweet-flavored e-liquids (Behar et al., 2016). Hence, the widespread use of cinnamaldehyde in e-liquids warrants further mechanistic studies on its toxic action, including in bone.
The expression of RUNX2 is essential for the development, maturation and maintenance of osteoblasts (Ducy et al., 1999). Here we report no detectable change in RUNX2 expression in MG-63 cells exposed to any of the coffee or fruity e-liquids tested with or without nicotine when compared to culture medium only. One possible explanation for these results is the concentration of nicotine used. For example, Kim et al., report a decrease in RUNX2 expression in alveolar bone marrow-derived mesenchymal stem cells when exposed to 2 mM nicotine, a higher concentration than the 0.62 mM used in this study (Kim et al., 2012). Another study reports RUNX2 mRNA to be repressed in human osteoblasts cultured with 0.1–10 μM nicotine, but only after chronic continuous exposure (Marinucci et al., 2014). Another variable to consider is that RUNX2 expression varies depending on the state of osteoblast maturation and mineralization in culture (Prideaux et al., 2014). MG-63 cells are less differentiated (pre-osteoblastic phenotype) compared to Saos-2 cells (Czekanska et al., 2012). Hence, an interesting follow up study would be to examine RUNX2 mRNA in Saos-2 cells exposed to e-liquids.
In contrast to RUNX2, Col1a1 mRNA expression is upregulated in MG-63 cells treated with highlighted coffee and fruity e-liquids with or without nicotine, but not in the flavorless e-liquid, when compared to culture medium only. Upon further analysis using Lotus brand Mango Blast, with or without nicotine, there is also a significant increase in collagen type I protein expression. Others report a biphasic effect of nicotine on collagen type I mRNA levels in MG-63 cells, with increasing expression observed at nicotine concentrations less than 100 μM and decreasing expression at concentrations of 1mM and higher (Rothem et al., 2009). The nicotine-containing treatment in our collagen type I RNA and protein experiments had a concentration of 620 μM (0.1mg/ml), which falls in between these previous reports, although it is below the 1mM concentration reported to result in down regulation. Consistent with the biphasic nature of nicotine, when human oral fibroblasts are exposed to unvaped e-liquid containing 6.2 mM nicotine for 24 hours there is a decrease in collagen type I protein (Sancilio et al., 2017). In addition to nicotine, the flavorings alone may alter osteoblast gene expression. For example, Col1a1 mRNA expression was found to be increased in adult osteopenic ovariectomized mice fed a diet of dried mango but not in mice fed dried grape or apricot (Rendina et al., 2013). This study implies natural dried mango has chemical properties that could modulate osteoblast functionality. It remains to be determined whether chemicals used to create artificial mango flavors, like those used in e-liquids, could induce the same responses in osteoblasts. Collectively, this study supports further investigation into the cellular mechanisms by which e-liquids alter osteoblast gene expression, such as the induction of oxidative stress by e-liquid exposure (Bitzer et al., 2018; Lerner et al., 2015; Muthumalage et al., 2017).
There are several challenges to e-cigarette research and studying e-liquid cytotoxicity (Orr, 2014). The lack of manufacturing standards and content labeling on e-liquid bottles creates obstacles for toxicological evaluations. The vast number of e-cigarette models and e-liquids available on the market compound the issue. Another challenge is the lack of standardized in vitro testing that is physiologically relevant to the vaping experience (Lerner et al., 2015; Neilson et al., 2015; Romagna et al., 2013). Thus, using unvaped e-liquids allows for fast screening and provides a way to compare studies from different laboratories and identify cytotoxic e-liquids that warrant further chemical and biological characterization. Standardization in the manufacturing of e-cigarette products, consistent and reliable disclosure of chemical content in e-liquids, and stringent testing procedures are needed for robust toxicological assessments and chemical analyses of e-liquids.
We conclude that the degree of osteotoxicity is flavor-dependent and occurs independently of nicotine and that flavored e-liquids reveal collagen type I as a potential target in osteoblasts. This study provides insight into the potential impact of e-cigarette use on bone health and points to the need for further studies to assess the impact of e-liquid flavorings in bone.
Acknowledgements
This publication was made possible by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under Grant #P20GM103408.
References
- Abate M, Vanni D, Pantalone A, & Salini V (2013). Cigarette smoking and musculoskeletal disorders. Muscles, Ligaments and Tendons Journal, 3(2), 63–69. doi: 10.11138/mltj/2013.3.2.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajiro Y, Tokuhashi Y, Matsuzaki H, Nakajima S, & Ogawa T (2010). Impact of passive smoking on the bones of rats. Orthopedics, 33(2), 90–95. doi: 10.3928/01477447-20100104-14 [DOI] [PubMed] [Google Scholar]
- Arbon KS, Christensen CM, Harvey WA, & Heggland SJ (2012). Cadmium exposure activates the ERK signaling pathway leading to altered osteoblast gene expression and apoptotic death in Saos-2 cells. Food and Chemical Toxicology, 50(2), 198–205. doi: 10.1016/j.fct.2011.10.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachrach LK (2001). Acquisition of optimal bone mass in childhood and adolescence. Trends in Endocrinology and Metabolism, 12(1), 22–28. [DOI] [PubMed] [Google Scholar]
- Bahl V, Lin S, Xu N, Davis B, Wang YH, & Talbot P (2012). Comparison of electronic cigarette refill fluid cytotoxicity using embryonic and adult models. Reproductive Toxicology, 34(4), 529–537. doi: 10.1016/j.reprotox.2012.08.001 [DOI] [PubMed] [Google Scholar]
- Behar RZ, Davis B, Wang Y, Bahl V, Lin S, & Talbot P (2014). Identification of toxicants in cinnamon-flavored electronic cigarette refill fluids. Toxicology In Vitro, 28(2), 198–208. [DOI] [PubMed] [Google Scholar]
- Behar RZ, Luo W, Lin SC, Wang Y, Valle J, Pankow JF, & Talbot P (2016). Distribution, quantification and toxicity of cinnamaldehyde in electronic cigarette refill fluids and aerosols. Tobacco Control, 25(Suppl 2), ii94–ii102. doi: 10.1136/tobaccocontrol-2016-053224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behar RZ, Wang Y, & Talbot P (2017). Comparing the cytotoxicity of electronic cigarette fluids, aerosols and solvents. Tobacco Control. doi: 10.1136/tobaccocontrol-2016-053472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL (1988). Drug therapy. Pharmacologic aspects of cigarette smoking and nicotine addiction. New England Journal of Medicine, 319(20), 1318–1330. doi: 10.1056/nejm198811173192005 [DOI] [PubMed] [Google Scholar]
- Bitzer ZT, Goel R, Reilly SM, Elias RJ, Silakov A, Foulds J, … Richie JP Jr. (2018). Effect of flavoring chemicals on free radical formation in electronic cigarette aerosols. Free Radical Biology and Medicine, 120, 72–79. doi: 10.1016/j.freeradbiomed.2018.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC, & Prevention, C. f. D. C. a. (2017). Tobacco use among middle and high school students – United States, 2011–2016. Morbidity and Mortality Weekly Report, 66(23), 597–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coonse KG, Coonts AJ, Morrison EV, & Heggland SJ (2007). Cadmium induces apoptosis in the human osteoblast-like cell line Saos-2. Journal of Toxicology and Environmental Health A, 70(7), 575–581. doi: 10.1080/15287390600882663 [DOI] [PubMed] [Google Scholar]
- Czekanska EM, Stoddart MJ, Richards RG, & Hayes JS (2012). In search of an osteoblast cell model for in vitro research. European Cells and Materials, 24, 1–17. [DOI] [PubMed] [Google Scholar]
- Dai H, & Hao J (2016). Flavored Electronic Cigarette Use and Smoking Among Youth. Pediatrics, 138(6). doi: 10.1542/peds.2016-2513 [DOI] [PubMed] [Google Scholar]
- Davis B, Dang M, Kim J, & Talbot P (2015). Nicotine concentrations in electronic cigarette refill and do-it-yourself fluids. Nicotine and Tobacco Research, 17(2), 134–141. doi: 10.1093/ntr/ntu080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducy P, Starbuck M, Priemel M, Shen J, Pinero G, Geoffroy V, … Karsenty G (1999). A Cbfa1-dependent genetic pathway controls bone formation beyond embryonic development. Genes and Development, 13(8), 1025–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Zawawy HB, Gill CS, Wright RW, & Sandell LJ (2006). Smoking delays chondrogenesis in a mouse model of closed tibial fracture healing. Journal of Orthopaedic Research, 24(12), 2150–2158. doi: 10.1002/jor.20263 [DOI] [PubMed] [Google Scholar]
- Farsalinos KE, Romagna G, Allifranchini E, Ripamonti E, Bocchietto E, Todeschi S, … Voudris V (2013). Comparison of the cytotoxic potential of cigarette smoke and electronic cigarette vapour extract on cultured myocardial cells. International Journal of Environmental Research and Public Health, 10(10), 5146–5162. doi: 10.3390/ijerph10105146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerloff J, Sundar IK, Freter R, Sekera ER, Friedman AE, Robinson R, … Rahman I (2017). Inflammatory Response and Barrier Dysfunction by Different e-Cigarette Flavoring Chemicals Identified by Gas Chromatography-Mass Spectrometry in e-Liquids and e-Vapors on Human Lung Epithelial Cells and Fibroblasts. Applied In Vitro Toxicology, 3(1), 28–40. doi: 10.1089/aivt.2016.0030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgetti AP, Cesar Neto JB, Ruiz KG, Casati MZ, Sallum EA, & Nociti FH Jr. (2010). Cigarette smoke inhalation modulates gene expression in sites of bone healing: a study in rats. Oral Surgery, Oral Medicine, Oral Pathology and Oral Radiology Endodontology, 110(4), 447–452. doi: 10.1016/j.tripleo.2010.02.029 [DOI] [PubMed] [Google Scholar]
- Ha TT, Burwell ST, Goodwin ML, Noeker JA, & Heggland SJ (2016). Pleiotropic roles of Ca(+2)/calmodulin-dependent in regulating cadmium-induced toxicity in human osteoblast-like cell lines. Toxicology Letters, 260, 18–27. doi: 10.1016/j.toxlet.2016.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua M, & Talbot P (2016). Potential health effects of electronic cigarettes: A systematic review of case reports. Preventive Medicine Report, 4, 169–178. doi: 10.1016/j.pmedr.2016.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanis JA, Johnell O, Oden A, Johansson H, De Laet C, Eisman JA, … Tenenhouse A (2005). Smoking and fracture risk: a meta-analysis. Osteoporosis International, 16(2), 155–162. doi: 10.1007/s00198-004-1640-3 [DOI] [PubMed] [Google Scholar]
- Kaur G, Muthumalage T, & Rahman I (2018). Mechanisms of toxicity and biomarkers of flavoring and flavor enhancing chemicals in emerging tobacco and non-tobacco products. Toxicology Letters, 288, 143–155. doi: 10.1016/j.toxlet.2018.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BS, Kim SJ, Kim HJ, Lee SJ, Park YJ, Lee J, & You HK (2012). Effects of nicotine on proliferation and osteoblast differentiation in human alveolar bone marrow-derived mesenchymal stem cells. Life Sciences, 90(3–4), 109–115. doi: 10.1016/j.lfs.2011.10.019 [DOI] [PubMed] [Google Scholar]
- Ko CH, Chan RL, Siu WS, Shum WT, Leung PC, Zhang L, & Cho CH (2015). Deteriorating effect on bone metabolism and microstructure by passive cigarette smoking through dual actions on osteoblast and osteoclast. Calcified Tissue International, 96(5), 389–400. doi: 10.1007/s00223-015-9966-8 [DOI] [PubMed] [Google Scholar]
- Leigh NJ, Lawton RI, Hershberger PA, & Goniewicz ML (2016). Flavourings significantly affect inhalation toxicity of aerosol generated from electronic nicotine delivery systems (ENDS). Tobacco Control, 25(Suppl 2), ii81–ii87. doi: 10.1136/tobaccocontrol-2016-053205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner CA, Sundar IK, Yao H, Gerloff J, Ossip DJ, McIntosh S, … Rahman I (2015). Vapors produced by electronic cigarettes and e-juices with flavorings induce toxicity, oxidative stress, and inflammatory response in lung epithelial cells and in mouse lung. PLoS One, 10(2), e0116732. doi: 10.1371/journal.pone.0116732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie LJ, Vasanthi Bathrinarayanan P, Jackson P, Mabiala Ma Muanda JA, Pallett R, Stillman CJP, & Marshall LJ (2017). A comparative study of electronic cigarette vapor extracts on airway-related cell lines in vitro. Inhalation Toxicology, 29(3), 126–136. doi: 10.1080/08958378.2017.1318193 [DOI] [PubMed] [Google Scholar]
- Liu X, Kohyama T, Kobayashi T, Abe S, Kim HJ, Reed EC, & Rennard SI (2003). Cigarette smoke extract inhibits chemotaxis and collagen gel contraction mediated by human bone marrow osteoprogenitor cells and osteoblast-like cells. Osteoporosis International, 14(3), 235–242. doi: 10.1007/s00198-002-1350-7 [DOI] [PubMed] [Google Scholar]
- Marinucci L, Bodo M, Balloni S, Locci P, & Baroni T (2014). Sub-toxic nicotine concentrations affect extracellular matrix and growth factor signaling gene expressions in human osteoblasts. Journal of Cellular Physiology, 229(12), 2038–2048. doi: 10.1002/jcp.24661 [DOI] [PubMed] [Google Scholar]
- Muthumalage T, Prinz M, Ansah KO, Gerloff J, Sundar IK, & Rahman I (2017). Inflammatory and Oxidative Responses Induced by Exposure to Commonly Used e-Cigarette Flavoring Chemicals and Flavored e-Liquids without Nicotine. Frontiers in Physiology, 8, 1130. doi: 10.3389/fphys.2017.01130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilson L, Mankus C, Thorne D, Jackson G, DeBay J, & Meredith C (2015). Development of an in vitro cytotoxicity model for aerosol exposure using 3D reconstructed human airway tissue; application for assessment of e-cigarette aerosol. Toxicology In Vitro, 29(7), 1952–1962. doi: 10.1016/j.tiv.2015.05.018 [DOI] [PubMed] [Google Scholar]
- NIH Consensus Development Panel on Osteoporosis Prevention, D., and Therapy. (2001). Osteoporosis prevention, diagnosis, and therapy. Journal of the American Medical Association, 285(6), 785–795. [DOI] [PubMed] [Google Scholar]
- Orr MS (2014). Electronic cigarettes in the USA: a summary of available toxicology data and suggestions for the future. Tobacco Control, 23 Suppl 2, ii18–22. doi: 10.1136/tobaccocontrol-2013-051474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otreba M, Kosmider L, Knysak J, Warncke JD, & Sobczak A (2018). E-cigarettes: voltage- and concentration-dependent loss in human lung adenocarcinoma viability. Journal of Applied Toxicology, 38(8), 1135–1143. doi: 10.1002/jat.3625 [DOI] [PubMed] [Google Scholar]
- Patel D, Davis KC, Cox S, Bradfield B, King BA, Shafer P, … Bunnell R (2016). Reasons for current E-cigarette use among U.S. adults. Preventive Medicine, 93, 14–20. doi: 10.1016/j.ypmed.2016.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pautke C, Schieker M, Tischer T, Kolk A, Neth P, Mutschler W, & Milz S (2004). Characterization of osteosarcoma cell lines MG-63, Saos-2 and U-2 OS in comparison to human osteoblasts. Anticancer Research, 24(6), 3743–3748. [PubMed] [Google Scholar]
- Prideaux M, Wijenayaka AR, Kumarasinghe DD, Ormsby RT, Evdokiou A, Findlay DM, & Atkins GJ (2014). SaOS2 Osteosarcoma cells as an in vitro model for studying the transition of human osteoblasts to osteocytes. Calcified Tissue International, 95(2), 183–193. doi: 10.1007/s00223-014-9879-y [DOI] [PubMed] [Google Scholar]
- Rendina E, Hembree KD, Davis MR, Marlow D, Clarke SL, Halloran BP, … Smith BJ (2013). Dried plum’s unique capacity to reverse bone loss and alter bone metabolism in postmenopausal osteoporosis model. PLoS One, 8(3), e60569. doi: 10.1371/journal.pone.0060569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagna G, Allifranchini E, Bocchietto E, Todeschi S, Esposito M, & Farsalinos KE (2013). Cytotoxicity evaluation of electronic cigarette vapor extract on cultured mammalian fibroblasts (ClearStream-LIFE): comparison with tobacco cigarette smoke extract. Inhalation Toxicology, 25(6), 354–361. doi: 10.3109/08958378.2013.793439 [DOI] [PubMed] [Google Scholar]
- Rothem DE, Rothem L, Soudry M, Dahan A, & Eliakim R (2009). Nicotine modulates bone metabolism-associated gene expression in osteoblast cells. Journal of Bone and Mineral Metabolism, 27(5), 555–561. doi: 10.1007/s00774-009-0075-5 [DOI] [PubMed] [Google Scholar]
- Rowell TR, Reeber SL, Lee SL, Harris RA, Nethery RC, Herring AH, … Tarran R (2017). Flavored e-cigarette liquids reduce proliferation and viability in the CALU3 airway epithelial cell line. American Journal of Physiology-Lung Cellular and Molecular Physiology, 313(1), L52–l66. doi: 10.1152/ajplung.00392.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell MA, Jarvis M, Iyer R, & Feyerabend C (1980). Relation of nicotine yield of cigarettes to blood nicotine concentrations in smokers. British Medical Journal, 280(6219), 972–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancilio S, Gallorini M, Cataldi A, Sancillo L, Rana RA, & di Giacomo V (2017). Modifications in Human Oral Fibroblast Ultrastructure, Collagen Production, and Lysosomal Compartment in Response to Electronic Cigarette Fluids. Journal of Periodontology, 88(7), 673–680. doi: 10.1902/jop.2017.160629 [DOI] [PubMed] [Google Scholar]
- Sherwood CL, & Boitano S (2016). Airway epithelial cell exposure to distinct e-cigarette liquid flavorings reveals toxicity thresholds and activation of CFTR by the chocolate flavoring 2,5-dimethypyrazine. Respiratory Research, 17(1), 57. doi: 10.1186/s12931-016-0369-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SS, Reyes JR, Arbon KS, Harvey WA, Hunt LM, & Heggland SJ (2009). Cadmium-induced decrease in RUNX2 mRNA expression and recovery by the antioxidant N-acetylcysteine (NAC) in the human osteoblast-like cell line, Saos-2. Toxicology In Vitro, 23(1), 60–66. doi: 10.1016/j.tiv.2008.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. Centers for Disease Control and Prevention, N. C. f. C. D. P. a. H. P., Office on Smoking and Health. (2016). E-Cigarette Use Among Youth and Young Adults: A Report of the Surgeon General—Executive Summary. Atlanta, GA: U.S. Department of Health and Human Services. [Google Scholar]
- Villanti AC, Johnson AL, Ambrose BK, Cummings KM, Stanton CA, Rose SW, … Hyland A (2017). Flavored Tobacco Product Use in Youth and Adults: Findings From the First Wave of the PATH Study (2013–2014). American Journal of Preventive Medicine. doi: 10.1016/j.amepre.2017.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vo N, Wang D, Sowa G, Witt W, Ngo K, Coelho P, … Kang J (2011). Differential effects of nicotine and tobacco smoke condensate on human annulus fibrosus cell metabolism. Journal of Orthopaedic Research, 29(10), 1585–1591. doi: 10.1002/jor.21417 [DOI] [PubMed] [Google Scholar]
- Welz C, Canis M, Schwenk-Zieger S, Becker S, Stucke V, Ihler F, & Baumeister P (2016). Cytotoxic and Genotoxic Effects of Electronic Cigarette Liquids on Human Mucosal Tissue Cultures of the Oropharynx. Journal of Environmental Pathology, Toxicology and Oncology, 35(4), 343–354. doi: 10.1615/JEnvironPatholToxicolOncol.2016016652 [DOI] [PubMed] [Google Scholar]
- Yoon V, Maalouf NM, & Sakhaee K (2012). The effects of smoking on bone metabolism. Osteoporosis International, 23(8), 2081–2092. doi: 10.1007/s00198-012-1940-y [DOI] [PubMed] [Google Scholar]
- Zhu SH, Sun JY, Bonnevie E, Cummins SE, Gamst A, Yin L, & Lee M (2014). Four hundred and sixty brands of e-cigarettes and counting: implications for product regulation. Tobacco Control, 23 Suppl 3, iii3–9. doi: 10.1136/tobaccocontrol-2014-051670 [DOI] [PMC free article] [PubMed] [Google Scholar]