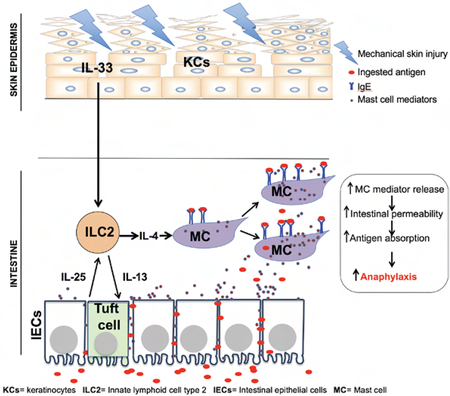
SUMMARY.
Mast cell (MC) mediator release following crosslinking of surface-bound IgE antibody by ingested antigen underlies food allergy. However, IgE antibodies are not uniformly associated with food allergy, and intestinal MC load is an important determinant. Atopic dermatitis (AD), characterized by pruritis and cutaneous sensitization to allergens including foods is strongly associated with food allergy. Tape stripping mouse skin, a surrogate for scratching, caused expansion and activation of small intestinal mast cells (MCs), increased intestinal permeability, and promoted food anaphylaxis in sensitized mice. Tape stripping caused keratinocytes to systemically release interleukin-33 (IL-33) which synergized with intestinal tuft cell-derived IL-25 to drive the expansion and activation of intestinal type-2 innate lymphoid cells (ILC2s). These provided IL-4 that targeted MCs to expand in the intestine. Duodenal MCs were expanded in AD. In addition to promoting cutaneous sensitization to foods, scratching may promote food anaphylaxis in AD by expanding and activating intestinal MCs.
Keywords: Skin, Gut, Mast cells, ILC2s, innate immunity, food allergy
Graphical Abstract
eTOC blurb
Atopic dermatitis is a pruritic inflammatory skin disease highly associated with food allergy. Leyva-Castillo and colleagues demonstrate that a skin-to-gut crosstalk initiated by mechanical skin injury promotes food anaphylaxis by increasing mast cells in the gut.
INTRODUCTION
Food allergy affects 6% of children and 3% of adults in the US, and is on the rise(Sicherer and Sampson, 2009). The life-threatening symptom of food allergy is anaphylaxis. The physiopathology of anaphylaxis involves mast cell (MC) degranulation and release of mediators and cytokines following recognition of antigen by IgE antibodies bound to high affinity IgE receptors (FcεRI) on MCs(Galli and Tsai, 2012). Not all individuals who have food-specific IgE antibodies react upon oral challenge, and food-specific IgE serum concentrations do not necessarily predict the severity of food allergy(Fleischer et al., 2011). Thus, additional factors other than IgE antibody are required for food anaphylaxis. An important such factor is intestinal MC load, which controls intestinal permeability(Lee et al., 2013), and thereby systemic absorption of antigen which is essential for food anaphylaxis(Strait et al., 2011). Importantly, gut MC expansion is associated with increased susceptibility to oral anaphylaxis(Vaali et al., 2012), and intestinal MC load modulates the severity of oral anaphylaxis(Ahrens et al., 2012). Cutaneous exposure to food allergens in infants and mice predisposes to IgE-mediated food allergy (Bartnikas et al., 2013; Lack et al., 2003), implicating the skin as an important route of sensitization. There is a high prevalence of IgE antibodies to foods in atopic dermatitis (AD), a chronic pruritic inflammatory skin disease affecting ~15% of US children(Spergel, 2010). The association between IgE sensitization to food and food anaphylaxis is substantially higher in patients with AD than in the population at large(Salo et al., 2014). Intestinal IgE+ cell numbers are increased in patients with AD(Caffarelli et al., 2001; Kalimo et al., 1988), but the nature of these IgE+ cells is ill defined. Furthermore, intestinal permeability is increased in AD patients(Pike et al., 1986). Tape stripping the skin of mice is a surrogate for scratching. Intestinal MC expansion is observed following application of saline to tape stripped skin(Bartnikas et al., 2013), suggesting a link between mechanical skin injury and intestinal MC expansion. In the present study we demonstrate that tape stripping mouse skin causes selective MC expansion in the small intestine (SI), increases intestinal permeability and promotes food anaphylaxis. IL-33 released by keratinocytes synergized with IL-25 released by intestinal tuft cells to expand intestinal ILC2s and increase their expression of Il4 and/or Il13. ILC2-derived IL-4 and IL-13 targeted MCs to cause their expansion in the SI. We demonstrated that duodenal MCs are expanded in children with AD, independent of overt food allergy. Our observations document a skin-to-gut crosstalk in which mechanical skin injury promotes food anaphylaxis by driving intestinal MC expansion, in addition to facilitating sensitization to food allergens. Thus, our results are directly relevant to food allergy in patients with AD.
RESULTS
Mechanical skin injury elicits expansion of intestinal mast cells.
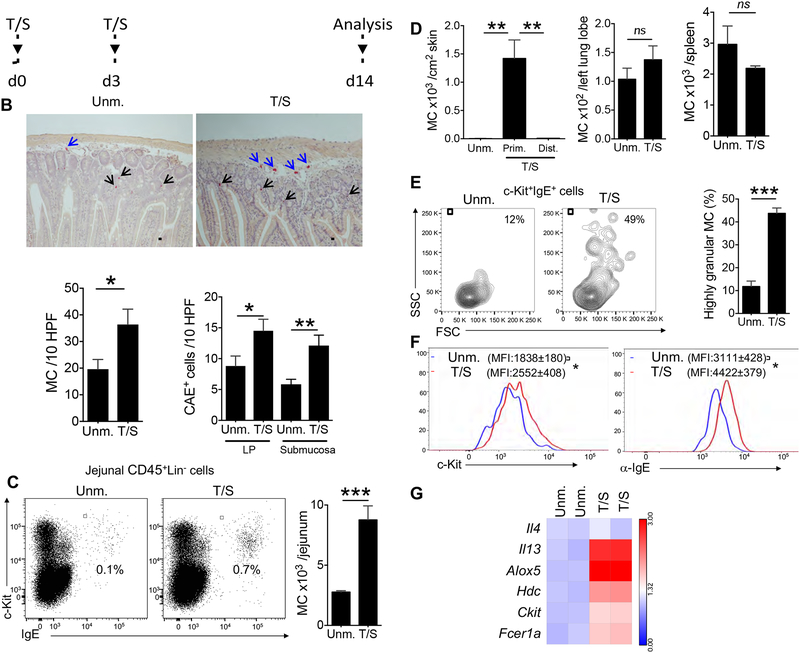
To mimic mechanical skin injury caused by scratching the shaved back skin of BALB/c mice was tape stripped six times on Day 0 and three times on Day 3, and the mice were studied on Day 14 (Fig. 1A). Tape stripping resulted in a 2-fold increase in chloroacetate esterase positive (CAE+) MCs in the jejunum, including MCs in the submucosa, as well as the lamina propria (LP) (Fig. 1B). Immunohistochemical staining demonstrated that tape stripping the skin caused an increase in mMCPT1+ MCs and mMCPT6+ MCs in the jejunum (Fig. S1A). The increase in MC expansion in the jejunal LP of tape stripped mice was confirmed by flow cytometric analysis of CD45+Lin− c-kit+IgE+ cells (Fig. 1C). Tape stripping also caused MC expansion in the duodenum, but not in the colon (Fig. S1B). The total number of CD45+ cells in the SI did not in tape stripped mice (Fig. S1C). MCs expanded at the site of tape stripping, but not in distant skin sites, lungs, or spleen (Fig. 1D).
Figure 1. Tape stripping mouse skin causes expansion of mast cells (MC) in the small intestine.
A. Experimental protocol. BALB/C mouse skin was tape stripped (T/S) 6 times on Day 0 and 3 times on Day 3 using Tegaderm; mice were analyzed on Day 14. B. Representative CAE staining of jejunal sections, with scale bars representing 100 μM (top), and quantitation of MC numbers per 10 HPF in jejunum (bottom left) and jejunal LP and submucosa (bottom right) of T/S mice and unmanipulated (Unm.) controls. Results are derived from 2 independent experiments each with 3 to 5 mice/group. C. Representative flow cytometry analysis of c-kit+IgE+ MCs in the CD45+Lin− fraction of jejunal LP cells from T/S mice and Unm. controls (left), and absolute numbers of jejunal CD45+Lin−c-kit+IgE+ MCs (right). Results are derived from 2 independent experiments each with 4 mice/group. D. Numbers of CD45+Lin−c-kit+IgE+ MCs in primary (Prim.) skin sites subjected to tape stripping, distant (Dist.) skin site (left) lungs (middle) and spleen (right) of T/S mice and Unm. controls. Data is representative of 2 independent experiments each with 3 to 4 mice/group. E. Representative flow cytometry analysis of the granularity (side scatter) and size (forward scatter) of CD45+Lin−c-kit+IgE+ MCs (left) and percentage of SSChighc-kit+IgE+ MCs (right) in the jejunum of T/S mice and Unm. controls. Results are derived from 2 independent experiments each with 4 mice/group. F. Representative flow cytometry analysis of surface expression of c-KIT or CD117 (left) and FcεR1 detected as IgE binding (right) by CD45+Lin−c-kit+IgE+ MCs from the lamina propria (LP) of T/S mice and Unm. controls. Results are representative of 2 independent experiments each with 4 mice/group. G. qPCR analysis of mRNA expression of select genes by CD45+Lin−c-kit+IgE+ MCs sorted from the LP of T/S mice and Unm. controls. expressed relative to the mean of Unm. controls. The lanes correspond to the groups from 2 different experiments in which MCs were pooled from 4–5 mice/group. Columns and bars in B-E represent SEM. ** = p<0.01, *** = p< 0.001, ns: not significant Please see also Figure S1.
Tape stripping the skin caused an increase in the granularity of jejunal c-Kit+IgE+ MCs (Fig. 1E) upregulated their surface expression of c-Kit and IgE binding (Fig. 1F). as well as their expression of Il13, Hdc and Alox5, Kit and Fcer1a, but not Il4,(Fig. 1G). Il5 and Il9 mRNAs were not detectable in jejunal MCs. These results indicate that tape stripping causes selective expansion of mucosal and submucosal MCs in the SI with an increase in MC granularity, maturation and potential ability to produce IL-13, histamine and leukotrienes.
Expansion of intestinal MCs following mechanical skin injury was not specific to the BALB/c strain, as it was observed in C57BL/6 mice (Fig. S1D, E). Furthermore, it was independent of the mouse housing facility. A comparable ~ 2-fold increase in jejunal MCs was observed following tape stripping the skin of BALB/c mice obtained from Taconic Biosciences (Fig. S1E). Importantly, tape stripping elicited an increase in jejunal MCs in germ-free BALB/c mice (Fig. S1E), demonstrating its independence from the microbiota. Because of the differences in baseline numbers of intestinal MCs between mice of different strains and mice housed in different facilities, we subsequently expressed the numbers of intestinal MCs in tape stripped mice relative to their numbers in genetically matched unmanipulated controls.
Tape stripping increases intestinal permeability and promotes oral anaphylaxis.
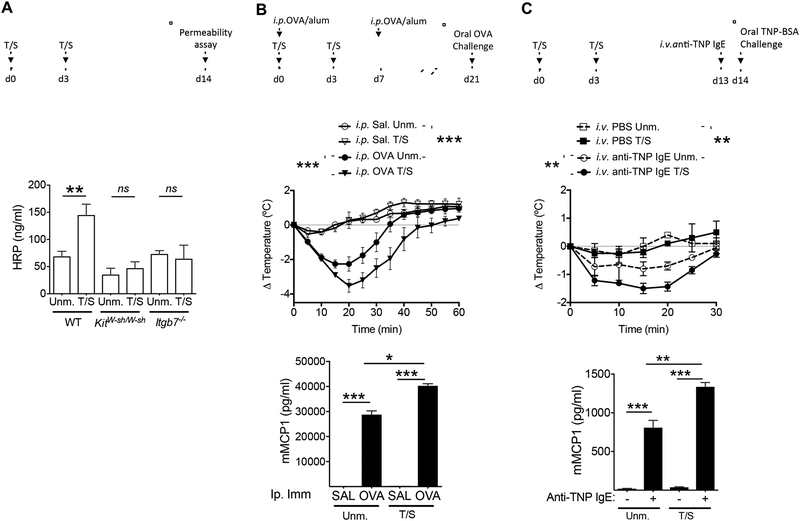
MC numbers in mouse intestine correlate with intestinal permeability(Ahrens et al., 2012). Tape stripping mouse skin caused an increase in intestinal permeability as evidenced by an increase in serum horseradish peroxidase (HRP) concentration following oral gavage of HRP (Fig. 2A). The increase in intestinal permeability was dependent on MCs, and in particular on intestinal MCs, as it was not observed in KitW-sh/W-sh mice, which globally lack MCs, or Itgb7−/− mice, which are selectively deficient in intestinal MCs(Gurish et al., 2001) (Fig 2A).
Figure 2. Tape stripping of the skin increases intestinal permeability and promotes anaphylaxis to oral antigen challenge.
A. Effect of tape stripping (T/S) the skin on intestinal permeability. Upper panel: Experimental protocol. Lower panel: Serum HRP concentrations in T/S and Unm. WT, KitW-sh/W-sh and Itgb7−/− mice. Results are derived from 3 independent experiments each with 4 to 5 mice/group. B. Effect of tape stripping the skin on food anaphylaxis in i.p. immunized T/S and Unm. WT mice. Upper panel: Experimental protocol. Middle panel: Change in core body temperature following oral OVA challenge. Lower panel: serum mMCPT1 concentrations. Data are representative of 2 independent experiments, each with 4 to 5 mice/group. C. Effect of tape stripping the skin on food anaphylaxis in T/S and Unm. mice passively sensitized with IgE anti-TNP. Upper panel: Experimental protocol. Middle panel: change in core body temperature following oral challenge with TNP-BSA. Lower panel: serum mMCPT1 concentrations. Data are representative of 2 independent experiments each with 4 to 5 mice/group. Columns and bars in A, and symbols and bars in B and C represent SEM. ** = p<0.01, *** = p< 0.001, ns: not significant. Please see also Figure S2.
We investigated whether the expansion, increased granularity and activation of intestinal MCs elicited by mechanical skin injury was associated with enhancement of oral anaphylaxis. To that end, mice were tape stripped and i.p. immunized with OVA or saline in alum then challenged orally with OVA (Fig. 2B). As expected, mice i.p. immunized with OVA, but not saline, demonstrated a drop in body temperature and an increase in serum mMCPT1 concentrations following oral OVA challenge (Fig. 2B). Tape stripping of the skin enhanced oral anaphylaxis in mice i.p. immunized with OVA, as evidenced by a greater drop in core body temperature, and higher serum mMCPT1 concentrations following oral OVA challenge, compared to non-tape stripped controls (Fig. 2B). Neither non-tape stripped nor tape stripped i.p. immunized Itgb7−/ mice exhibited a drop in body temperature following oral OVA challenge (data not shown), demonstrating the critical role of intestinal MCs in food anaphylaxis. MC expansion following tape stripping was comparable in mice immunized with OVA and in controls injected with saline (Fig. S2A). Tape stripping had no detectable effect on the IgE anti-OVA response to i.p. immunization (Fig. S2B). These results indicate that that the more severe oral anaphylaxis in tape-stripped mice was not due to a greater IgE antibody response to OVA.
To circumvent other potential effects of tape stripping on the response to OVA immunization, we examined whether tape stripping of the skin enhances IgE-mediated passive food anaphylaxis. Mice were tape stripped and passively sensitized on by i.v. administration of IgE anti-TNP, or saline as control, then challenged orally with TNP-BSA (Fig. 2C). Mice sensitized with IgE anti-TNP, but not unsensitized controls, demonstrated a drop in body temperature and an increase in serum mMCPT1 concentrations following oral TNP-BSA challenge (Fig. 2C). Anaphylaxis to oral antigen challenge following passive sensitization was more severe in tape stripped mice compared to unmanipulated controls, as evidenced by a greater drop in core body temperature, and higher serum concentrations of MCTP1 (Fig. 2C). These results suggest that expansion of intestinal MCs following mechanical skin injury increases intestinal absorption of antigen and promotes oral anaphylaxis.
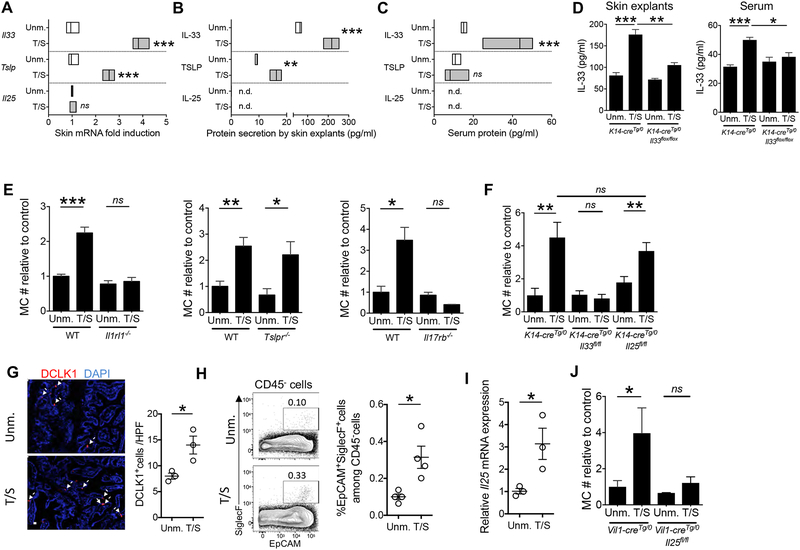
Keratinocyte-derived IL-33 and intestinal cell-derived IL-25 are essential for intestinal MC expansion elicited by mechanical skin injury.
Keratinocytes upregulate the expression and/or release of the cytokines IL-33, TSLP and IL-25 in response to injury (Leyva-Castillo et al., 2013; Oyoshi et al., 2010). Receptors for these cytokines are expressed by ILC2s, MCs, and subsets of T cells(Saenz et al., 2008), rendering epithelial cytokines potential candidates for communicating signals from injured skin to the intestine. mRNA expression of Il33 and Tslp, but not Il25 increased in mouse skin 6 hrs after tape stripping (Fig. 3A). Explants of tape stripped mouse skin released more IL-33 and TSLP than explants from unmanipulated skin, but no detectable amounts of IL-25 (Fig. 3B). Importantly, there was a ~ 3 fold rise in the serum concentration of IL-33, but not TSLP, one hour after tape stripping the skin (Fig. 3C). IL-25 was not detected in the serum. IL-33 release by skin explants, and the increase in IL-33 serum concentrations following tape stripping were abolished in K14-cre Tg/0Il33flox/flox mice, which lack IL-33 specifically in keratinocytes (Fig. 3D). These results suggest that mechanical injury to mouse skin results in the systemic release of the epithelial cytokine IL-33 from keratinocytes. In addition to keratinocytes, the K14 transgene is expressed in mouse thymic epithelial cells (TECs) and oral epithelium(Li et al., 2001). We cannot rule out a potential contribution of these tissues in our model.
Figure 3. Keratinocyte-derived IL-33 and IEC-derived IL-25, are necessary for intestinal MC expansion elicited by tape stripping the skin.
A. Il33, Tslp and Il25 mRNA expression in skin. Values represent fold induction in tape stripped skin relative to unmanipulated (Unm.) skin. Data are representative of 2 independent experiments each with 3 mice/group. B. IL-33, TSLP and IL-25 concentrations in the supernatants of skin explants from T/S and Unm. skin C. Serum concentrations of IL-33, TSLP and IL-25 in mice one hour after tape stripping the skin and in Unm. controls. Data representative of 2 independent experiments each with 3 mice/group. D. IL-33 concentrations in the supernatants of skin explants (left) and serum (right) of T/S and UnmK14-creTg/0Il33flox/flox mice and K14-creTg/0 controls. E. Jejunal MC numbers (#) in T/S and Unm. Il1rl1−/−, Tslpr−/−, and Il17rb−/− mice and genetically matched WT controls relative to the mean of unmanipulated controls. Results are derived from 2 independent experiments with 3 to 5 mice/group. F. Jejunal MC numbers (#) in T/S and Unm. K14- creTg/0Il33flox/flox, K14-creTg/0Il25flox/flox and K14-creTg/0 mice relative to the mean of the unmanipulated K14-creTg/0 controls. Results are derived from 2 independent experiments with 3 to 5 mice/group. G. Representative immunofluorescence staining of jejunal sections for DCLK1 in red and DAPI in blue (left) and quantitation of DCLK1+ tuft cells per HPF (right) in T/S WT mice and Unm. controls. Results are derived from 2 independent experiments each with respectively 1 and 2 mice/group. H. Representative flow cytometry analysis (left) and quantitation of the percentage (right) of SiglecF+EPCAM+ cells gating on CD45− cells from the jejunal epithelial layer. Results are derived from 2 independent experiments, each with 2 mice/group, I. Il25 mRNA expression in intestinal epithelial cells from T/S WT mice and Unm. controls. Values represent fold induction relative to the mean of unmanipulated mice. Results are derived from 2 independent experiments each with respectively 1 and 2 mice/group. J. Jejunal MC numbers (#) in T/S and Unm. Vil1-creTg/0Il25flox/flox mice and Vil1-creTg/0 controls relative to unmanipulated Vil1-creTg/0 controls. Results are derived from 2 independent experiments with 2 to 3 mice/group. Circles in G-I represent individual mice. Floating bars in A-C, columns and bars in D-F and J, and horizontal lines and bars in G-I represent mean and SEM. * = p < 0.05, ** = p <0.01, *** = p< 0.001, ns: not significant. Please see also Figure S3.
Expansion of intestinal MCs was abolished in Il1rl1−/− mice that lack the IL-33R, and in Il17rb−/− mice that lack the high affinity ligand binding chain of the IL-25R, but was preserved in Tslpr−/− mice (Fig. 3E), indicating that IL-33 and IL-25, but not TSLP, play essential and non-redundant roles in intestinal MC expansion elicited by mechanical skin injury. Expansion of intestinal MCs was abolished in tape stripped K14-creTg/0Il33flox/flox mice (Fig. 3F). In contrast, it was preserved in K14-creTg/0Il25flox/flox mice, (Fig. 3F). These results demonstrate that keratinocytes are the source of the IL-33, but not the IL-25, essential for intestinal MC expansion elicited by mechanical skin injury.
Tuft cells constitutively express IL-25 and are the only identified source of IL-25 in the intestine(von Moltke et al., 2016). They are characterized by intracellular expression of Doublecortin Like Kinase 1 (DCLK1) and surface expression of EpCAM and the C-type lectin receptor SiglecF(von Moltke et al., 2016). Immunofluorescence analysis of the jejunum revealed that tape stripping caused a ~2 fold increase in the number of DCLK1+ tuft cells (Fig. 3G). This was confirmed by flow cytometry analysis of CD45−EpCAM+SiglecF+ tuft cells in jejunal IECs (Fig. 3H). Tape stripping also caused a ~ 3 fold increase in Il25 mRNA expression in jejunal IECs (Fig. 3I). Upregulation of Il25 mRNA expression following tape stripping of the skin was selective to IECs, as it was not observed in lung epithelial cells or skin epidermal layer (Fig. S3). Importantly, expansion of jejunal MCs following tape stripping was abolished in Vil1-cre Tg/0Il25flox/flox mice, which lack IL-25 in their IECs(von Moltke et al., 2016)(Fig. 3J). These results demonstrate that tape stripping of the skin causes tuft cell expansion and increased Il25 expression by IECs, and that IEC-derived IL-25 is essential for intestinal MC expansion elicited by mechanical skin injury.
ILC2s are essential for intestinal MC expansion elicited by tape stripping the skin.
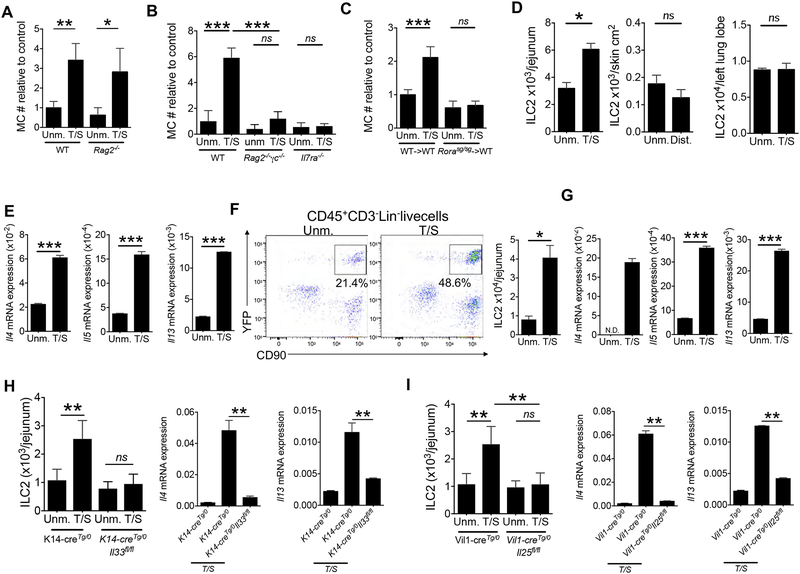
ILC2s and adaptive Th2 cells are targets for IL-33 and IL25(Saenz et al., 2008). Tape stripping of the skin caused intestinal MC expansion in Rag2−/− mice, which are deficient in mature T and B cells, but sufficient in ILCs (Fig. 4A). In contrast, it caused no expansion of intestinal MCs in Rag2−/−γc−/− mice, which in addition lack ILCs (Fig. 4B). Tape stripping also failed to cause intestinal MC expansion in Il7ra−/− mice, which lack ILCs and are deficient in T and B cells (Fig. 4B) These results indicate that ILCs, but not mature T or B cells are important for the expansion of intestinal MCs elicited by mechanical skin injury.
Figure 4. ILC2s are necessary for intestinal MC expansion elicited by tape stripping the skin.
A, B. Jejunal MC numbers in tape stripped (T/S) and unmanipulated (Unm.) Rag2−/−, Rag2−/−γc−/− and Il7ra−/− mice (B) and genetically matched WT controls relative to the mean value of the unmanipulated control group. Results are derived from 2 independent experiments with 2 to 5 mice/group. C. Jejunal MC numbers (#) in T/S and Unm. Rorasg/sg->WT and WT ->WT chimeras, relative to the mean value of the unmanipulated WT->WT chimera group Results are derived from 2 independent experiments each with 2 to 3 mice/group. D. ILC2 numbers in the jejunum (left) distant skin (middle) and lungs (right) in T/S BALB/c mice and Unm. controls. Data pooled from 2 independent experiments each with 3–4 mice/group. E. Cytokine mRNA expression, relative to B2m in sorted CD45+Lin−CD90+ ILCs from the jejunum of T/S mice and Unm. controls. Data pooled from 2 independent experiments each with 3–4 mice/group. F. Representative flow cytometry analysis (left) and quantitation (right) of CD90+YFP+ ILC2s gating on CD45+Lin− live cells from the jejunum of T/S and Unm. Roracre/creROSAYFP mice. G. Cytokine mRNA expression, relative to B2m, in Lin−YPF+ cells (ILC2s) sorted from the jejunum of T/S and Unm. Roracre/creROSAYFP mice. Data pooled from 2 independent experiments each with 3–4 mice/group. H, I. ILC2s numbers (left) and Il4 and Il13 mRNA expression, relative to B2m, in sorted CD45+Lin−CD90+ ILCs (right) from the jejunum of T/S and Unm. K14-creTg/0Il33flox/flox mice, and K14-creTg/0 controls (H) and T/S and Unm. Vilin1-cre I25flox/flox mice and vilin1-cre controls (I). Results are derived from 2 independent experiments with 3 to 5 mice/group. Columns and bars represent mean and SEM. * = p < 0.05, ** = p <0.01, *** = p< 0.001, ns: not significant. Please see also Figure S5 and S6.
ILC2s, but not ILC1s or ILC3s, express IL-25R and IL-33R(Spits et al., 2013). We directly examined the role of ILC2s in intestinal MC expansion following tape stripping of the skin. The transcription factor RORα is expressed selectively in ILC2s, but not ILC1s or ILC3s(Spits et al., 2013). It is also expressed in cerebellar Purkinje cells and a subset of Th2 cells. Rorasg/sg mice which carry a deletion that introduces a frameshift in the exon that encodes the ligand-binding domain of RORα (Dussault et al., 1998) and have no detectable ILC2s. However, they have neurologic abnormalities and a severely reduced life span. We therefore examined Rorasg/sg->WT bone marrow radiation chimeras, which are healthy and have a normal life span(Wong et al., 2012). GATA-3 is a transcription factor expressed in ILC2s, but not ILC1s or ILC3s(Spits et al., 2013), allowing their identification by flow cytometry analysis as CD45+Lin−CD90+CD3−GATA-3+ cells (Fig. S4A). Intestinal ILC2s were virtually undetectable in Rorasg/sg->WT chimeras, but their numbers in control WT->WT chimeras were comparable to those in WT mice (Fig. S4B). Tape stripping caused no expansion of intestinal MCs in Rorasg/sg->WT chimeras, but caused intestinal MC expansion in WT->WT chimeras comparable in magnitude to that in WT mice (Fig. 4C). Since T cells are dispensable for intestinal MC expansion after tape stripping, these results indicate that ILC2s are critical for this expansion.
Keratinocyte-derived IL-33 and IEC-derived IL-25 drive the expansion and activation of intestinal ILC2s following mechanical skin injury.
Tape stripping the skin caused a ~2 fold increase in the numbers of CD45+Lin−CD90+CD3−GATA-3+ ILC2s in the LP of the jejunum of WT mice (Fig 4D). ILC2 expansion following mechanical skin injury was selective to the jejunum, because ILC2 numbers did not increase in distant skin sites, lungs, blood, skin draining lymph nodes, spleen or visceral adipose tissue of tape stripped mice (Fig. 4D and Fig. S4C). We observed a 2-fold increase in ILC2s in tape stripped skin (Fig. S4D). Treatment with the sphingosine-1-phosphate (S1P) analogue FTY720 did not abolish the intestinal MC expansion induced by tape stripping (Fig. S4E), suggesting that migration of ILC2s from tape stripped skin is not required in our model.
There was an increase in Ki67+ intestinal ILC2s at Day 10, but not Day 7 of our tape stripping protocol (Fig. S4F), suggesting that ILC2 are proliferating locally. IL-33 promotes the egress of ILC2 progenitors from the bone marrow (BM)(Stier et al., 2018). There was a decrease in Lin−Sca1+IL-25R+CD25+CD127+ ILC2 progenitors (ILC2p) in the BM at day 7 but not day Day10 of our tape stripping protocol (Fig S4G), but there was no increased expression of the gut-homing receptors CCR9 or integrin beta7 by these ILC2ps (data not shown). ILC2p mobilization from the BM may contribute to the ILC2 expansion in our model.
Activated ILC2s express type 2 cytokines(McKenzie et al., 2014; Spits et al., 2013). Tape stripping the skin upregulated expression of Il4, Il5 and Il13 mRNA in CD45+Lin−CD90+CD3− ILCs sorted from the jejunal LP (Fig 4E), but not in ILCs sorted from distant skin (Fig. S4H). Il4, Il5 and Il13 mRNA was not detectable in CD45+Lin−CD90+CD3− ILCs sorted from the lungs. Il9 mRNA was undetectable in jejunal ILCs (data not shown). In addition, tape stripping did not increase lungs or visceral adipose tissue ILC2 numbers or their expression of IL-5 or IL-13 on Day 7, 10 or 14 (Fig. S4I and J and data not shown). Because CD45+Lin−CD90+CD3− cells include ILCs other than ILC2s, we examined Roracre/creROSA26YFP mice in which ILC2s are identified as CD45+Lin−CD90+CD3−YFP+ cells. Tape stripping the skin caused an increase in the numbers of CD45+Lin−CD90+YFP+ jejunal ILC2s in Roracre/creROSA26YFP mice (Fig. 4F), and upregulated Il4, Il5 and Il13 mRNA expression in YFP+ ILC2s sorted from the jejunum of these mice (Fig 4G). Il4, Il5 and Il13 mRNAs were not detectable in CD45+Lin−CD90+CD3−YFP− cells from the jejunum of tape stripped Roracre/creROSA26YFP mice (data not shown). Serum IL-4 and IL-13 concentrations were increased two fold on day 4 after tape stripping when jejunal expansion and activation are not yet observed, but returned to normal on day 7, before jejunal ILC2s were expanded and activated (Fig. S4K and data not shown).
Jejunal ILC2 expansion and increased expression of Il4 and Il13 by ILCs were abolished in tape-stripped K14-creTg/0Il33flox/flox mice and Vil1- creTg/0Il25flox/flox mice (Fig. 4H and I), demonstrating that keratinocyte-derived IL-33 and IEC- derived IL-25 play non-redundant roles in the expansion and activation of jejunal ILC2s elicited by mechanical skin injury.
IL-33 and IL-25 act directly on ILC2s to cause their expansion and activation following mechanical skin injury.
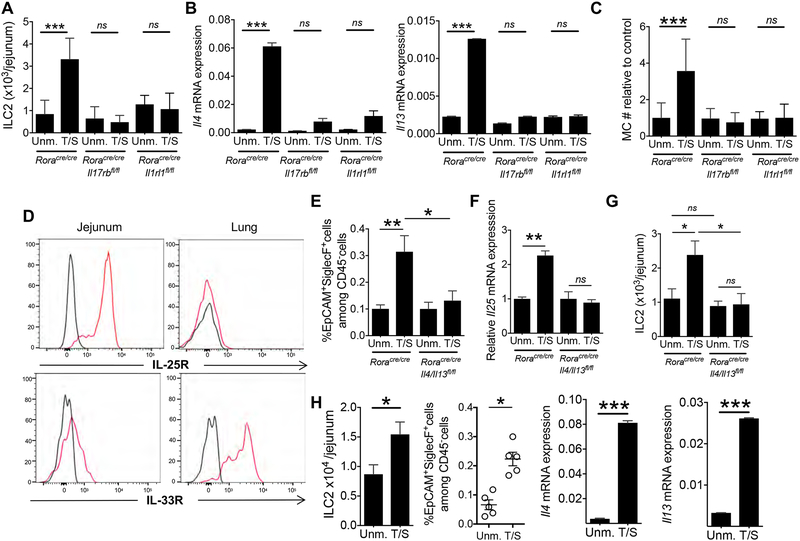
IL-33 and IL-25 act directly on ILC2s to cause proliferation and expression of type 2 cytokines(Spits et al., 2013). We investigated whether IL-33 and IL-25 act directly on ILC2s to cause their expansion in the intestine of tape stripped mice.. Tape stripping the skin caused a ~ 3 fold expansion of jejunal ILC2s and an increase in the expression of Il4 and Il13 mRNAs by jejunal ILCs in Roracre/cre mice, but not in Roracre/creIl17rbflox/flox mice and Roracre/creIl1rl1flox/flox mice which respectively lack IL-25R and IL-33R in Rora expressing cells. (Fig. 5A and B). Furthermore, tape stripping caused expansion of jejunal MCs in Roracre/cre mice, but not Roracre/creIl17rbflox/flox or Roracre/creIl1rl1flox/flox mice (Fig. 5C). These results demonstrate that keratinocyte-derived IL-33 and IEC-derived IL-25 act directly and non-redundantly on intestinal ILC2s to cause their expansion and activation and to promote intestinal MC expansion following mechanical skin injury. YFP expression was detected in less than 2% of Beta III tubulin+ intestinal neurons and was undetectable in intestinal MCs of Roracre/creROSA26YFP mice (Fig S5), demonstrating that Rora driven cre-mediated ablation of gene expression is not operative in these cells.
Figure 5. IL-33 and IL-25 directly target ILC2s to cause their expansion and to activate a feed forward ILC2 and tuft cells loop in the intestine of tape stripped mice.
A-C. ILC2 numbers (A) Il4 and Il13 mRNA expression relative to B2m, in sorted CD45+Lin−CD90+ ILCs and MC numbers in the jejunum of T/S and Unm. Roracre/creIl17rbflox/flox, Roracre/creIl1rl1flox/flox and Roracre/cre mice relative to Unm. Roracre/cre controls (C). Results are derived from 3 independent experiments with 2 to 4 mice/group. D. Representative flow cytometry analysis of IL-25R (left) and IL-33R (right) expression by jejunal and lung CD45+Lin− CD90+YFP+ ILC2s from Roracre/creROSAYFP mice. Black lines represent isotype control. Similar results were obtained in two other independent experiments. E-F. Quantitation of the percentage of SiglecF+EPCAM+ cells gating on CD45− cells from the epithelial layer (E), Il25 mRNA expression in IECs (F) and ILC2s numbers (G) in the jejunum of T/S and Unm. Roracre/creIl4/13flox/flox mice and Roracre/cre controls. Results are derived from 3 independent experiments with 2 to 4 mice/group. H. ILC2s numbers (left), percentage SiglecF+EPCAM+ cells gating on CD45- cells from the jejunal epithelial layer (center) and Il4 and Il13 mRNA expression relative to B2m, in sorted CD45+Lin−CD90+ ILCs (right) in the jejunum of T/S and Unm. Rag2−/− mice. Results are derived from 2 independent experiments with 3 to 4 mice/group. Columns and bars represent mean and SEM. * = p < 0.05, *** = p< 0.001, ns: not significant. Please see also Figure S5.
Flow cytometry analysis demonstrated that IL-25R is expressed robustly on jejunal ILC2s, and weakly on lung ILC2s, whereas IL-33R was expressed robustly on lung ILC2s and less strongly on intestinal ILC2s (Fig. 5D). The strong expression of IL-25R on jejunal ILC2s, together with the selective increase in Il25 mRNA in IECs following tape stripping of the skin, likely contribute to the selective expansion of ILC2s and MCs in the jejunum following mechanical skin injury.
A feed forward loop drives the expansion and activation of intestinal ILC2s and tuft cells following mechanical skin injury.
Recent reports demonstrate the existence of a feed forward loop involving intestinal ILC2s and tuft cells in which IL-13 derived from ILC2s promotes the expansion of intestinal tuft cells and their expression of IL-25, which drives ILC2 expansion and activation(von Moltke et al., 2016). We used Roracre/creIl4/13flox/flox and Rag2−/− mice to investigate whether expansion of intestinal tuft cells following tape stripping of the skin is dependent on ILC2 derived IL-13 and/or IL-4. Tape stripping of the skin caused expansion of EpCAM+SiglecF+ jejunal tuft cells, increased IL-25 mRNA expression by IECs and expansion of ILC2s in Roracre/cre mice, but not Roracre/creIl4/13flox/flox mice (Fig. 5E–G). Expansion of jejunal tuft cells as well as expansion of intestinal ILC2s and their upregulation of ll4 and Il13 expression following tape stripping of the skin were preserved in Rag2−/− mice (Fig. 5H). Together with the dependence of intestinal ILC2 expansion and activation on IEC derived IL-25, (Fig. 4I), these results suggest that following mechanical skin injury IL-13 and/or IL-4 produced by ILC2s in response to keratinocyte derived IL-33, activate a feed forward loop in which expansion and upregulation of IL-25 by tuft cells drives further the expansion and activation of small intestinal ILC2s. Injection of rIL-33 caused an increase in the number of jejunal tuft cells, ILC2s and MCs in WT mice that was abolished or markedly less in Il17rb−/− mice (Fig. S6A–C), whereas injection of rIL-25 caused a comparable increase in the number of jejunal MCs in WT mice and Il1rl1−/− mice, but no increase in Il17rb−/− mice (Fig. S6D). These findings support the notion that keratinocyte derived IL-33 initiates the intestinal ILC2-tuft cell feed forward loop that results in intestinal MC expansion in our model.
IL-4 and IL-13 produced by ILC2s target MCs to cause MC expansion following mechanical skin injury.
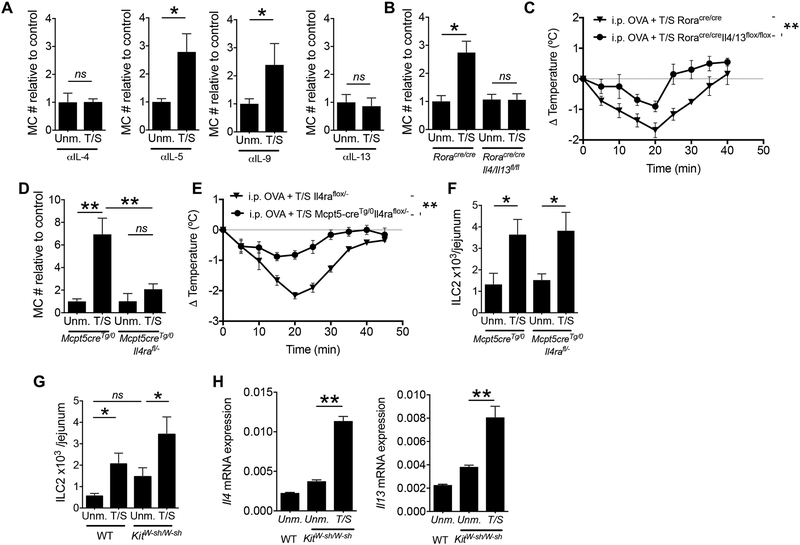
IL-4, IL-5, IL-9 and IL-13 promote the proliferation of bone marrow derived MCs in vitro(Okayama and Kawakami, 2006), while IL-4 and IL9 can drive the expansion of MCs in vivo(Burton et al., 2013). Intestinal MC expansion elicited by tape stripping the skin of WT mice was abolished by administration of neutralizing mAb to IL-4 and IL-13, but not IL-5 and IL-9 (Fig. 6A), indicating that IL-4 and IL-13 play essential and non-redundant roles in small intestinal MC expansion following mechanical skin injury.
Figure 6. ILC2-derived IL-4 and IL-13 directly target MCs to cause their expansion in the intestine of tape stripped mice.
A. Jejunal MC numbers (#) in T/S and Unm. WT mice treated with neutralizing anti-IL-4, anti-IL-5, anti-IL-9 or anti-IL-13 mAbs. MC values are relative to the mean value of the unmanipulated control group. Results are derived from 3 independent experiments with 2 to 4 mice/group. B. MC numbers relative to Unm. RoraCre/Cre controls in the jejunum of T/S and Unm. Roracre/creIl4/13flox/flox and Roracre/cre mice. Results are derived from 3 independent experiments with 2 to 4 mice/group. C. Change in core body temperature following oral OVA challenge in T/S Roracre/creIl4/13flox/flox mice and Roracre/cre controls i.p. sensitized with OVA. Results are derived from 2 independent experiments with 3 to 5 mice/group. D, E. MC numbers relative to Unm. Mcpt5-creTg/0 (D) and ILC2s numbers (E), in the jejunum of T/S and Unm. Mcpt5-creTg/0Il4raflox/- mice and Mcpt5-creTg/0 mice. Results are derived from 2 independent experiments with 3 to 5 mice/group. F. Change in core body temperature following oral OVA challenge in T/S Mcpt5-creTg/0Il4raflox/- mice and Mcpt5-creTg/0 controls i.p. sensitized with OVA. Results are derived from 2 independent experiments with 3 to 5 mice/group. G, H. ILC2 numbers (G) and Il4 and Il13 mRNA expression in sorted CD45+Lin−CD90+ ILCs (H) in the jejunum of T/S and Unm. KitW-sh/W-sh mice and genetically matched controls. Results are derived from 2 independent experiments with 3 to 5 mice/group. Columns and bars represent mean and SEM. * =p < 0.05, ** =p <0.01, ns: not significant.
To investigate whether ILC2s are the source of IL-4 and IL-13 important for intestinal MC expansion elicited by tape stripping the skin, we used Roracre/creIl4/13flox/flox mice. Tape stripping caused no intestinal MC expansion in these mice but caused a ~2 fold expansion of intestinal MCs in Roracre/cre controls (Fig. 6B). The lack of intestinal MC expansion in Roracre/creIl4/13flox/flox mice was accompanied by attenuation of anaphylaxis in response to oral OVA challenge following i.p. sensitization (Fig. 6C). Given that intestinal MC expansion after tape stripping is maintained in Rag2−/− mice, these results suggest that ILC2-derived IL-4 or/and IL-13 are essential for intestinal MC expansion elicited by mechanical skin injury.
To examine whether IL-4 and/or IL-13 target MCs directly to mediate their expansion in the intestine of tape stripped mice, we used Mcpt5-creTg/0Il4raflox/- mice, which selectively lack IL-4Rα, and thus both IL-4R and IL-13R, in MCs. Tape stripping the skin caused no significant intestinal MC expansion in Mcpt5-creTg/0Il4raflox/- mice but caused robust intestinal MC expansion in Mcpt5-cre Tg/0 controls (Fig. 6D). Together with our previous findings, this result strongly suggests that IL-4 and/or IL-13 derived from intestinal ILC2s directly target intestinal MCs to cause their expansion. The lack of intestinal MC expansion in Mcpt5-creTg/0Il4raflox/- mice was accompanied by a decrease in food anaphylaxis in response to oral OVA challenge following i.p. sensitization (Fig. 6E).
MCs release molecules that can drive the expansion and activation of ILC2s(Shimokawa et al., 2017). Intestinal ILC2 expansion following tape stripping of the skin was comparable in Mcpt5-cre Tg/0Il4raflox/- mice and Mcpt5-cre Tg/0 controls (Fig. 6F). Importantly intestinal ILC2 expansion and increased expression of Il4 and Il13 mRNAs following tape stripping of the skin was comparable in MC deficient KitW-sh/W-sh mice and WT controls (Fig. 6G and H). These results demonstrate that intestinal ILC2 expansion and activation following mechanical skin injury is independent of MCs.
Duodenal mast cells are increased in patients with AD.
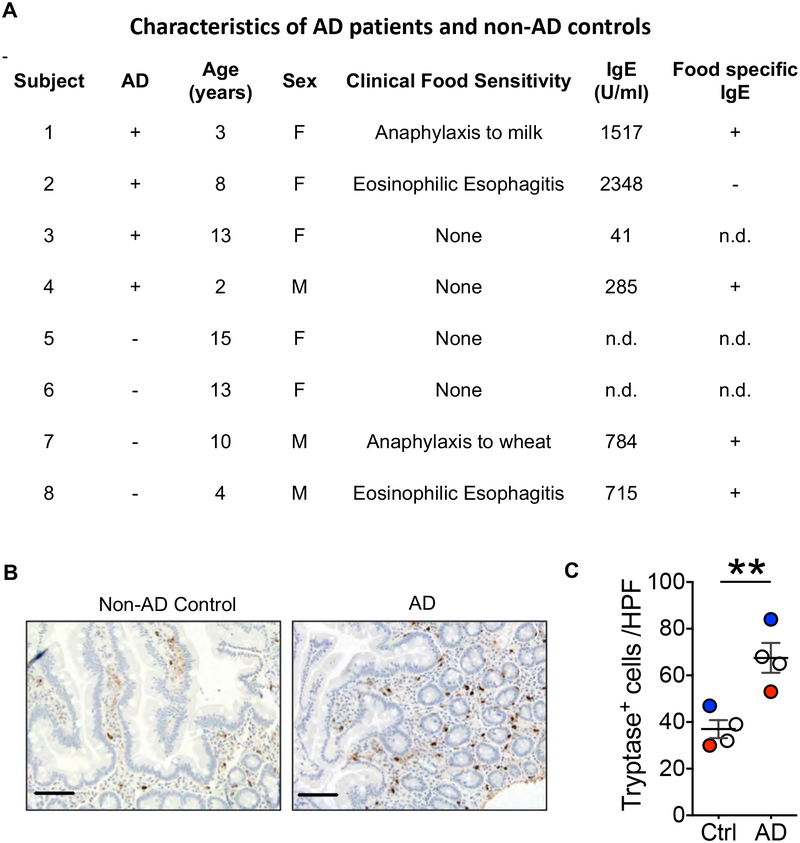
We next investigated whether intestinal MCs are increased in patients with AD. We examined MCs in archived duodenal biopsies from four children with active AD, and four non-AD age-matched children. All patients underwent endoscopy with biopsy of the esophagus, stomach and duodenum because of a history of recurrent upper abdominal pain and/or vomiting. History of food sensitivity, IgE serum concentrations and IgE antibodies to food allergens in the patients are shown in Fig. 7A. Two AD and two non- AD patients and two controls had no history of sensitivity to foods. One AD and one non-AD patients had a history of food anaphylaxis and IgE antibodies to the offending allergens, but were on an avoidance diet, and had no food allergy symptoms at the time of the study. One AD patient and one non-AD patient have been diagnosed clinically with eosinophilic esophagitis (EoE) and had IgE antibodies to multiple food allergens. MCs were identified in tissue sections by staining for the MC specific enzyme tryptase. The number of tryptase positive MCs in the LP of the duodenal mucosa was higher in AD compared to non-AD controls (Fig. 7B, C). Duodenal biopsies did not include the submucosa. Except for the increased MC numbers in the AD patients, and the confirmation of the clinical diagnosis of EoE in one AD patient and one non-AD patient by the presence of more than 15 eosinophils per HPF in the esophagus LP, no other abnormalities were evident in the patients’ biopsies. IgE can promote MC proliferation(Oettgen and Burton, 2015). The increased number of MCs in the duodenum of AD patients could not be simply due to higher serum IgE concentrations, because two of the four AD patients had lower serum IgE than the two non-AD patients on whom serum IgE concentrations was determined, one AD patient had a serum IgE in the normal range, and the mean serum IgE concentrations was not significantly different between the AD and non-AD patients: mean±SEM 1048±540 IU/ml for AD patients, n=4, versus 750±35 IU/ml for non-AD patients, n=2 (p= 0.73, unpaired t test). These results suggest that AD is associated with intestinal MC expansion independent of the presence of food allergy or EoE, and that this expansion is not simply the result of elevated serum IgE.
Figure 7. Duodenal mast cells are increased in patients with AD.
A. Characteristics of AD patients and non-AD controls, n.d.: not done. B. Representative immunohistochemistry staining of duodenal sections with anti-tryptase mAb, with scale bars representing 100 μm (left), and numbers of tryptase+ cells per HPF in the duodenum (right) of 4 children with active AD and scratching, and 4 children with no AD and no scratching. Circles represent individual patients Open circle: no history of food sensitivity, red circle: anaphylaxis to food, blue circle: eosinophilic esophagitis. Horizontal lines and bars in C represent mean and SEM. ** = p <0.01.
DISCUSSION
We unraveled the mechanisms of a crosstalk between skin and gut in which mechanical skin injury elicits expansion of intestinal MCs, and thereby promotes IgE-mediated food anaphylaxis.
Tape stripping of the skin caused selective expansion of both mucosal and submucosal MCs in the jejunum, independently of the microbiota. This suggests that danger signals released in response to mechanical skin injury activate a skin-gut cross talk that culminates in the expansion of intestinal MCs. Expansion of intestinal MCs was accompanied by phenotypic changes that included increased MC granularity, maturation, with increased c-Kit and FcεRI expression, and activation, with increased expression of genes that encode for IL-13 and enzymes involved in histamine and leukotriene synthesis.
MCs regulate homeostatic intestinal epithelial barrier function(Groschwitz et al., 2009). Tape stripping the skin increased intestinal permeability. This was dependent on intestinal MC, as no increase in permeability was observed in tape stripped Igtb7−/− mice that lack these cells. More importantly, tape stripping the skin concurrently with i.p. immunization with OVA, potentiated anaphylaxis to oral antigen challenge. This was not due to an effect on the IgE antibody response to OVA, or other aspects of the immune response to OVA immunization, because tape stripping also potentiated anaphylaxis to oral antigen challenge in mice that were passively sensitized with IgE antibodies. The finding that intestinal MC expansion following mechanical skin injury was associated with increased severity of anaphylaxis to oral challenge is consistent with previous reports that intestinal MC numbers control the severity of oral anaphylaxis(Ahrens et al., 2012). MC mediated intestinal permeability predisposes to oral antigen hypersensitivity(Forbes et al., 2008). Thus, increased intestinal permeability associated with MC expansion likely contributed to the increased susceptibility to oral anaphylaxis caused by mechanical skin injury.
Tape stripping the skin caused increased local expression of the epithelial cytokine genes Il33 and Tslp, but not Il25, and release of IL-33 and TSLP; however only IL-33 was released systemically into the blood. Examination of K14-creTg/0Il33flox/flox mice revealed that keratinocytes were the source of the increased IL-33 released by skin explants and increased serum IL-33 in tape stripped mice. Experiments using receptor deficient mice demonstrated that both IL-33 and IL-25 are required for intestinal MC expansion following mechanical skin injury. Studies in mice with selective deficiency of IL-33 or IL-25 in keratinocytes demonstrated that keratinocyte derived IL-33 was important for elicitation of intestinal MC expansion by mechanical skin injury. In contrast, keratinocyte derived IL-25 played no evident role, consistent with the failure to detect IL-25 in the blood. Intestinal MC expansion following tape stripping was abolished in Vil1-cre Tg/0Il25flox/flox mice, which lack IL-25 in their IECs, demonstrating that tuft cells, the only IECs known produce IL-25, are the relevant source of IL-25 in our model. The finding that tape stripping caused an increase in the numbers of intestinal tuft cells and upregulated Il25 mRNA expression by IECs suggests that mechanical skin injury causes proliferation and activation of tuft cells.
ILC2s were essential mediators of skin to gut crosstalk in our model. This was evidenced by the observation that lack of ILC2s in Rorasg/sg->WT BM chimeras abrogated the ability of tape stripping the skin to elicit intestinal MC expansion. Tape stripping caused expansion of intestinal ILC2s, and their activation as evidenced by marked upregulation of expression of the type 2 cytokines Il4, I5 and Il13. rIL-33 and rIL-25 synergize to cause ILC2 expansion in vivo and ILC2 proliferation and cytokine secretion in vitro(Salimi et al., 2013). Expansion and activation of intestinal ILC2 following tape stripping was abrogated in Roracre/creIl17rbflox/flox mice and Roracre/creIl1rl1flox/flox mice that respectively lack IL-33R or IL-25R in ILC2s. This demonstrates that IL-33 and IL-25 play non-redundant roles in our model, suggesting that in vivo synergy between these two cytokines is necessary to optimally drive intestinal ILC2 expansion and activation in our model. Importantly, intestinal MC expansion after tape stripping was also abrogated in Roracre/creIl17rbflox/flox mice and Roracre/creIl1rl1flox/flox mice, indicating that expansion and activation of intestinal ILC2s is required for intestinal MC expansion. The selective upregulation of Il25 expression in IECs, and the high expression of IL-25R by intestinal ILC2s, but in lung or skin ILC2s(Ricardo-Gonzalez et al., 2018) may underlie the selective expansion and activation of ILC2s in the small intestine following tape stripping. IL-33 acts on MCs to promote food anaphylaxis(Galand et al., 2016). IL-33 action on MCs cannot account by itself for the MC expansion and activation in tape stripped mice as these are strictly dependent on IL-4. However, IL-33 could act in synergy with IL-4 on MCs in our model.
We provide evidence that following mechanical skin injury intestinal ILC2s and tuft cells participate in a feed forward loop that amplifies their expansion and activation. We propose that following tape stripping the skin, increased serum IL-33 activates intestinal ILC2s to produce IL-13 and IL-4, which drive the expansion of tuft cells and upregulate their production of IL-25, which amplifies ILC2 production of IL-13 and IL-4, in a feed forward loop. ILC2 derived IL-4 then drives intestinal MC expansion and activation. Such a feed forward loop has been demonstrated in intestinal helminth infection(Howitt et al., 2016; von Moltke et al., 2016), and may have evolved to protect the intestine from helminths that infect it directly or via the skin as a portal of entry, as in the case of hookworms, and to provide concomitant immunity. In line with this hypothesis, oral administration of sodium succinate, a metabolite from protists and helminths, promotes expansion of intestinal tuft cells ILC2s and intestinal mast cell(Schneider et al., 2018). ILC2 derived IL-4 and/or IL-13 drove intestinal MC expansion in our model by directly targeting MCs, as intestinal MC expansion following tape stripping was abolished in Roracre/creIl4/13flox/flox mice and Mcpt5-cre Tg/0Il4raflox/- mice.
MCs play a protective role against microbes and helminths(Mukai et al., 2017). The crosstalk that we describe between mechanically injured skin and intestine may have evolved as a system to alert the gut to react to agents breaching the skin barrier. Importantly, intestinal MC expansion elicited by mechanical skin injury is likely relevant to food anaphylaxis in patients with AD. We tape stripped ~ 10% of the mouse body surface area in our model. A similar or greater percentage of body area is commonly affected and subject to scratching in patients with AD(Sugarman et al., 2003). Skin and serum IL-33 concentrations are increased in our model and in patients with AD(Galand et al., 2016; Savinko et al., 2012), and Il33 mRNA expression increases in the skin of healthy individuals following scratching(Galand et al., 2016). We observed intestinal MC expansion in patients with AD independent of food allergy history, serum IgE or the presence of EOE. Moreover, increased intestinal permeability has been documented in patients with AD(Caffarelli et al., 2001; Kalimo et al., 1988). Taken together our findings suggest that increased intestinal MCs and permeability elicited by mechanical skin injury inflicted by scratching may play an important role in promoting food anaphylaxis in patients with AD. Interventions that inhibit scratching may be useful in dampening the severity of food allergy in these patients by decreasing their intestinal MC load.
STAR METHODS
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Raif S Geha (raif.geha@childrens.harvard.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Mice.
Wild-type (WT) BALB/c, C57BL/6 and B6.SJL mice were purchased from Charles River Laboratories. BALB/c, Rag2-deficient mice, Rag2-gamma chain-deficient mice were obtained from Taconic. ST2-deficient Il1rl1−/− mice obtained from Dr. Andrew N.J. McKenzie(Townsend et al., 2000); IL-25R-deficient Il17rb−/− mice obtained from Amgen(Rickel et al., 2008); IL-25R-floxed Il17rbfl/fl mice and TSLPR-deficient mice(Al-Shami et al., 2005) were obtained from Dr. Steve F. Ziegler; Il4Rα-floxed mice were obtained from Dr. Frank Brombacher on BALB/c background(Herbert et al., 2004). Mcpt5-cre mice(Scholten et al., 2008), obtained from Dr. Talal Chatila, were derived for >6 generations on BALB/c background. Integrin beta7 Itgb7−/−, K14-creTg/0, Vil1-cre Tg/0, Il7R -deficient, RORα-deficient mice, on C57Bl/6 background and IL-4Rα-deficient mice, on a Balb/c background, were obtained from Jax. Integrin beta7 Itgn7−/− were derived for >6 generations on BALB/c background in the laboratory. ST2-floxed Il1rl1fl/fl mice and IL-33-floxed mice obtained from Dr. Richard T Lee; IL-25-floxed mice obtained from Dr. Chen Dong(Angkasekwinai et al., 2010); and Rora-cre mice obtained from Dr. Dennis O’Leary(Chou et al., 2013), on a C57Bl/6 background were crossed once with mice on BALB/c background. All mice were housed in a specific pathogen-free environment and fed an OVA-free diet. All procedures were performed in accordance with the Animal Care and Use Committee of Boston Children’s Hospital.
Human Subjects.
Excess of duodenal biopsy samples from children endoscoped because of abdominal pain, were collected during routine clinical care. Subjects were divided in 2 groups: active AD and scratching and subjects with no AD and no scratching based on history and clinical examination recorded by their gastroenterologist (E.H.). Samples were submitted in a blinded fashion to the pathologist (J.G.).
METHOD DETAILS
Mechanical skin injury, EC sensitization and oral antigen challenge.
Mechanical skin injury consists in two cycles of tape stripping, one at day 0 and one at day 3 (scheme in Fig. 1A). For the first cycle, 6 to 8 week-old female mice were anesthetized; their back skin was shaved and tape-stripped with a film dressing (TegadermTM, 3M) 6 times. For the other cycle, mice were tape-stripped 3 times. Finally, mice were sacrificed at different time points.
Passive sensitization.
6 to 8 week-old female mice were injected intravenously with 10 μg of anti-trinitrophenyl (TNP) IgE monoclonal antibody (mAb). The following day, mice were challenged intragastrically with 12.5mg of TNP conjugated bovine serum albumin (TNP-BSA). Temperature changes were measured every 5 min following OVA challenge using the DAS-6001 Smart Probe and IPTT-300 transponders (Bio Medic Data Systems) injected subcutaneously.
Intraperitoneal sensitization.
6 to 8 week-old female mice were injected intraperitoneally (i.p.) with 50 μg of OVA and 1 mg of alum at day 0 and day 7. At day 14, mice were challenged intragastrically with 100 mg of OVA. Temperature changes were measured as described above.
Histology.
For the chloroacetate esterase (CAE) staining, 1 cm pieces of mouse jejunum were fixed in 4% paraformaldehyde overnight at 4°C and embedded in paraffin. Paraffin sections were stained with CAE. CAE+ cells were counted by an investigator who was blinded to the experimental groups. For the tryptase staining, duodenal biopsy samples were stained with an anti-tryptase antibody. Ten HPFs in areas of high density MCs were evaluated blinded per the criteria used in evaluating EOE. For DCLK1 staining, the whole SI were treated as previously described(von Moltke et al., 2016), rolled and frozen in OCT. 10μM slices were first incubated with rat serum then stained with purified mAb anti-DCLK1 (ab31704) from Abcam, and with secondary Ab anti-rabbit IgG. Slices were fixed with a mounting medium containing DAPI (Prolon Gold) from ThermoFisher Scientific.
Cell suspension preparation and flow cytometry analysis.
Cell isolation from the jejunum was performed as previously described(Galand et al., 2016). Briefly, the jejuna were harvested, flushed with PBS supplemented with 2% fetal calf serum, opened longitudinally and cut in 1 cm long pieces. Then, intestinal pieces were incubated in HBSS without Ca2+ and Mg2+ supplemented with 10 mM EDTA, 10 mM HEPES, 0.5% fetal calf serum, and 1.5 mM DTE, for 20 minutes at 37°C twice. Intestinal pieces were digested in HBSS with Ca2+ and Mg2+, 20% fetal calf serum, 100 U/mL collagenase VIII (Sigma-Aldrich), and 5 μg/mL DNase (Sigma-Aldrich) for 60 min at 37°C. Immune cells were purified with a 40% Percoll gradient (GE Healthcare).
Cell isolation from the skin was performed as previously described(Leisten et al., 2013). One-centimer squares of back skin were harvested; the layer of fat under the dermis was scrapped. Lower left lobes of lung were harvested. Skin remaining tissue and lung lobes were chopped finely and incubated for 60 and 30 min, respectively, at 37°C in complete RPMI media complemented with 100U Liberase DL (Roche) and 5 μg/mL DNase (Sigma-Aldrich). Red blood cells of the lung suspensions were lysed with ACK buffer. The cell suspensions were washed, incubated with TruStain FcX (anti-CD16/32 antibody; 10 μg/ml; Biolegend) and stained with the eF506 viability dye from eBioscience to exclude dead cells and the following murine fluorochrome labeled-monoclonal antibodies: B220 (RA3–6B2), CD3 (17A2), CD4 (GK1.5), CD11c (N418), CD19 (1D3), CD45 (30F11), CD90.2 (53–2.1), c- Kit (ACK2), Gr1 (RB6–8C5), IL-13Rα1 (13MOKA), ST2 (RMST2–2) from eBioscience, CD11b (M1/70), F4/80 (BM8), FcεRI (MAR-1), IgE (RME-1) and IL-17RB (9B10) from Biolegend. BV605 Streptavidin from Biolegend was used to detect biotinylated antibodies. For transcription factor staining, cells were fixed and permeabilized (eBiosicence fix/perm kit) and stained in permeabilization solution with GATA-3 (TWAJ) and Rorc (B2D) antibodies from eBioscience. For cytokine staining, cells were stimulated with Ionomycin (0.5ug/ml; Sigma), Phorbol 12,13- dibutyrate (1ug/ml; Sigma), Brefeldin A (eBioscience), Monensin (eBioscience) in complete RPMI for 3 hours before surface staining. Then, cells were fixed and permeabilized (BD Biosciences Cytofix/Cytoperm) and stained in permeabilization solution with IL-4 (11B11), IL-5 (TRFK5), IL-9 (RM9A4) antibodies from Biolegend and IL-13 (eBio13A) antibodies from eBioscience. Cells were analyzed by flow cytometry using an LSRFortessa machine (BD Biosciences). The data was analyzed with FlowJo software.
Intestinal permeability.
Intestinal permeability was determined as previously(Mathias et al., 2011). Briefly, mice were gavaged at day 14 with 400 μl of a 1 mg/ml horseradish peroxidase solution (HRP, MW 44 kDa; Sigma). Blood was obtained 4h later and the concentration of HRP was measured in the serum using the TMB substrate system.
Il33, Il25 and Tslp mRNA expression and its release upon tape stripping.
The back skin of anesthetized mice was shaved and subjected to tape stripping six times with a film dressing (TegadermTM, 3M). Six hours later RNA was extracted from the skin with Total RNA isolation kit (Ambion). Intestinal epithelial cells were isolated from the duodenum as previously described(Gerbe et al., 2016). cDNA was prepared with iscript cDNA synthesis kit (Biorad). Quantitative real-time PCR was done with the Taqman gene expression assay, universal PCR master mix and ABI prism 7300 sequence detection system (Applied Biosystems). Il25, Il33 and tslp mRNA fold induction was calculated using delta-delta ct with normalization to the internal control β2microglobulin. An arbitrary unit of 1 was assigned to the mean value of unmanipulated skin samples or intestinal epithelial cells from unmanipulated mice. For measuring IL-33, IL-25 and TSLP release, patches of ~1cm2 skin were excised from unmanipulated back or immediately post-tape stripping. Subcutaneous fat was removed, and the patches were cultured for 1 hr in complete RPMI. IL-25, IL-33 and TSLP in the supernatant and the sera, harvested 1 hr after tape stripping, was measured using Quantikine ELISA kit (R&D Systems) for IL-33 and Ready-set-go ELISA kit (eBioscience) for IL-25 and TSLP.
QUANTIFICATION AND STATISTICAL ANALYSIS
All experiments were performed using randomly assigned mice without investigator blinding. Results of allergen challenge studies were analyzed using 2-way ANOVA. Other results were analyzed by one-way ANOVA or non-parametric t tests using Prism 7 (GraphPad). A p value of less than 0.05 was considered significant
Supplementary Material
4. highlights.
Mechanical skin injury promotes intestinal mast cell expansion
Intestinal mast cell expansion requires skin-derived IL-33 and gut-derived IL-25.
Intestinal mast cell expansion requires ILC2 activation by IL-33 and IL-25.
ILC2-derived IL-4 and IL-13 directly cause intestinal mast cell expansion
ACKNOWLEDGEMENTS.
We thank Dr. T. Chatila for his gift of Mcpt5-cre Tg/0 mice, Dr. Andrew NJ McKenzie for his gift of Il1rl1−/− and Il17rb−/− mice; Dr. Dennis O’Leary for his gift of Roracre/cre mice, Dr. Nicolas Severe for help in immunofluorescence imaging and Drs. T. Chatila, H. Oettgen and J. Chou for reading the manuscript and useful discussions. This work was supported by NIH grant AI113294–01A1 and U19 AI117673. J.M.L.C. is supported by NIAID T32 training grant (5T32AI007512–32).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATION OF INTERESTS.
The authors declare no competing interests.
REFERENCES
- Ahrens R, Osterfeld H, Wu D, Chen CY, Arumugam M, Groschwitz K, Strait R, Wang YH, Finkelman FD, and Hogan SP (2012). Intestinal mast cell levels control severity of oral antigen-induced anaphylaxis in mice. Am J Pathol 180, 1535–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Shami A, Spolski R, Kelly J, Keane-Myers A, and Leonard WJ (2005). A role for TSLP in the development of inflammation in an asthma model. J Exp Med 202, 829–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angkasekwinai P, Chang SH, Thapa M, Watarai H, and Dong C (2010). Regulation of IL-9 expression by IL-25 signaling. Nat Immunol 11, 250–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartnikas LM, Gurish MF, Burton OT, Leisten S, Janssen E, Oettgen HC, Beaupre J, Lewis CN, Austen KF, Schulte S, et al. (2013). Epicutaneous sensitization results in IgE-dependent intestinal mast cell expansion and food-induced anaphylaxis. J Allergy Clin Immunol 131, 451–460 e451–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton OT, Darling AR, Zhou JS, Noval-Rivas M, Jones TG, Gurish MF, Chatila TA, and Oettgen HC (2013). Direct effects of IL-4 on mast cells drive their intestinal expansion and increase susceptibility to anaphylaxis in a murine model of food allergy. In Mucosal Immunology, pp. 740–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caffarelli C, Cavagni G, Romanini E, Caruana P, and de Angelis G (2001). Duodenal IgE-positive cells and elimination diet responsiveness in children with atopic dermatitis. Ann Allergy Asthma Immunol 86, 665–670. [DOI] [PubMed] [Google Scholar]
- Chou SJ, Babot Z, Leingartner A, Studer M, Nakagawa Y, and O’Leary DD (2013). Geniculocortical input drives genetic distinctions between primary and higher-order visual areas. Science 340, 1239–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dussault I, Fawcett D, Matthyssen A, Bader JA, and Giguere V (1998). Orphan nuclear receptor ROR alpha-deficient mice display the cerebellar defects of staggerer. Mech Dev 70, 147–153. [DOI] [PubMed] [Google Scholar]
- Fleischer DM, Bock SA, Spears GC, Wilson CG, Miyazawa NK, Gleason MC, Gyorkos EA, Murphy JR, Atkins D, and Leung DY (2011). Oral food challenges in children with a diagnosis of food allergy. J Pediatr 158, 578–583 e571. [DOI] [PubMed] [Google Scholar]
- Forbes EE, Groschwitz K, Abonia JP, Brandt EB, Cohen E, Blanchard C, Ahrens R, Seidu L, McKenzie A, Strait R, et al. (2008). IL-9- and mast cell-mediated intestinal permeability predisposes to oral antigen hypersensitivity. J Exp Med 205, 897–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galand C, Leyva-Castillo JM, Yoon J, Han A, Lee MS, McKenzie ANJ, Stassen M, Oyoshi MK, Finkelman FD, and Geha RS (2016). IL-33 promotes food anaphylaxis in epicutaneously sensitized mice by targeting mast cells. J Allergy Clin Immunol 138, 1356–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli SJ, and Tsai M (2012). IgE and mast cells in allergic disease. Nat Med 18, 693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groschwitz KR, Ahrens R, Osterfeld H, Gurish MF, Han X, Abrink M, Finkelman FD, Pejler G, and Hogan SP (2009). Mast cells regulate homeostatic intestinal epithelial migration and barrier function by a chymase/Mcpt4-dependent mechanism. Proc Natl Acad Sci U S A 106, 22381–22386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurish MF, Tao H, Abonia JP, Arya A, Friend DS, Parker CM, and Austen KF (2001). Intestinal mast cell progenitors require CD49dbeta7 (alpha4beta7 integrin) for tissue-specific homing. J Exp Med 194, 1243–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert DR, Holscher C, Mohrs M, Arendse B, Schwegmann A, Radwanska M, Leeto M, Kirsch R, Hall P, Mossmann H, et al. (2004). Alternative macrophage activation is essential for survival during schistosomiasis and downmodulates T helper 1 responses and immunopathology. Immunity 20, 623–635. [DOI] [PubMed] [Google Scholar]
- Howitt MR, Lavoie S, Michaud M, Blum AM, Tran SV, Weinstock JV, Gallini CA, Redding K, Margolskee RF, Osborne LC, et al. (2016). Tuft cells, taste-chemosensory cells, orchestrate parasite type 2 immunity in the gut. Science 351, 1329–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalimo K, Lammintausta K, Klemi P, Leino R, Panula P, and Kalimo H (1988). Mast cells and IgE in intestinal mucosa in adult atopic dermatitis patients. Br J Dermatol 119, 579–585. [DOI] [PubMed] [Google Scholar]
- Lack G, Fox D, Northstone K, and Golding J (2003). Factors associated with the development of peanut allergy in childhood. N Engl J Med 348, 977–985. [DOI] [PubMed] [Google Scholar]
- Lee H, Park JH, Park DI, Kim HJ, Cho YK, Sohn CI, Jeon WK, Kim BI, and Chae SW (2013). Mucosal mast cell count is associated with intestinal permeability in patients with diarrhea predominant irritable bowel syndrome. Journal of neurogastroenterology and motility 19, 244–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyva-Castillo JM, Hener P, Jiang H, and Li M (2013). TSLP produced by keratinocytes promotes allergen sensitization through skin and thereby triggers atopic march in mice. J Invest Dermatol 133, 154–163. [DOI] [PubMed] [Google Scholar]
- Li M, Chiba H, Warot X, Messaddeq N, Gerard C, Chambon P, and Metzger D (2001). RXR-alpha ablation in skin keratinocytes results in alopecia and epidermal alterations. Development 128, 675–688. [DOI] [PubMed] [Google Scholar]
- Mathias CB, Hobson SA, Garcia-Lloret M, Lawson G, Poddighe D, Freyschmidt EJ, Xing W, Gurish MF, Chatila TA, and Oettgen HC (2011). IgE-mediated systemic anaphylaxis and impaired tolerance to food antigens in mice with enhanced IL-4 receptor signaling. J Allergy Clin Immunol 127, 795–805 e791–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie AN, Spits H, and Eberl G (2014). Innate lymphoid cells in inflammation and immunity. Immunity 41, 366–374. [DOI] [PubMed] [Google Scholar]
- Mukai K, Karasuyama H, Kabashima K, Kubo M, and Galli SJ (2017). Differences in the Importance of Mast Cells, Basophils, IgE, and IgG versus That of CD4(+) T Cells and ILC2 Cells in Primary and Secondary Immunity to Strongyloides venezuelensis. Infect Immun 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oettgen HC, and Burton OT (2015). IgE and Mast Cells: The Endogenous Adjuvant. Adv Immunol 127, 203–256. [DOI] [PubMed] [Google Scholar]
- Okayama Y, and Kawakami T (2006). Development, migration, and survival of mast cells. Immunol Res 34, 97–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyoshi MK, Larson RP, Ziegler SF, and Geha RS (2010). Mechanical injury polarizes skin dendritic cells to elicit a T(H)2 response by inducing cutaneous thymic stromal lymphopoietin expression. J Allergy Clin Immunol 126, 976–984, 984 e971–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike MG, Heddle RJ, Boulton P, Turner MW, and Atherton DJ (1986). Increased intestinal permeability in atopic eczema. J Invest Dermatol 86, 101–104. [DOI] [PubMed] [Google Scholar]
- Ricardo-Gonzalez RR, Van Dyken SJ, Schneider C, Lee J, Nussbaum JC, Liang HE, Vaka D, Eckalbar WL, Molofsky AB, Erle DJ, and Locksley RM (2018). Tissue signals imprint ILC2 identity with anticipatory function. Nat Immunol 19, 1093–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickel EA, Siegel LA, Yoon B-RP, Rottman JB, Kugler DG, Swart DA, Anders PM, Tocker JE, Comeau MR, and Budelsky AL (2008). Identification of functional roles for both IL-17RB and IL-17RA in mediating IL-25-induced activities. In The Journal of Immunology, pp. 4299–4310. [DOI] [PubMed] [Google Scholar]
- Saenz SA, Taylor BC, and Artis D (2008). Welcome to the neighborhood: epithelial cell-derived cytokines license innate and adaptive immune responses at mucosal sites. Immunological reviews 226, 172–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salimi M, Barlow JL, Saunders SP, Xue L, Gutowska-Owsiak D, Wang X, Huang LC, Johnson D, Scanlon ST, McKenzie AN, et al. (2013). A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis. J Exp Med 210, 2939–2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salo PM, Arbes SJ Jr., Jaramillo R, Calatroni A, Weir CH, Sever ML, Hoppin JA, Rose KM, Liu AH, Gergen PJ, et al. (2014). Prevalence of allergic sensitization in the United States: results from the National Health and Nutrition Examination Survey (NHANES) 2005–2006. J Allergy Clin Immunol 134, 350–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savinko T, Matikainen S, Saarialho-Kere U, Lehto M, Wang G, Lehtimaki S, Karisola P, Reunala T, Wolff H, Lauerma A, and Alenius H (2012). IL-33 and ST2 in atopic dermatitis: expression profiles and modulation by triggering factors. J Invest Dermatol 132, 1392–1400. [DOI] [PubMed] [Google Scholar]
- Schneider C, O’Leary CE, von Moltke J, Liang HE, Ang QY, Turnbaugh PJ, Radhakrishnan S, Pellizzon M, Ma A, and Locksley RM (2018). A Metabolite-Triggered Tuft Cell-ILC2 Circuit Drives Small Intestinal Remodeling. Cell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholten J, Hartmann K, Gerbaulet A, Krieg T, Muller W, Testa G, and Roers A (2008). Mast cell-specific Cre/loxP-mediated recombination in vivo. Transgenic Res 17, 307–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimokawa C, Kanaya T, Hachisuka M, Ishiwata K, Hisaeda H, Kurashima Y, Kiyono H, Yoshimoto T, Kaisho T, and Ohno H (2017). Mast Cells Are Crucial for Induction of Group 2 Innate Lymphoid Cells and Clearance of Helminth Infections. Immunity 46, 863–874 e864. [DOI] [PubMed] [Google Scholar]
- Sicherer SH, and Sampson HA (2009). Food allergy: recent advances in pathophysiology and treatment. Annu Rev Med 60, 261–277. [DOI] [PubMed] [Google Scholar]
- Spergel JM (2010). From atopic dermatitis to asthma: the atopic march. Ann Allergy Asthma Immunol 105, 99–106; quiz 107–109, 117. [DOI] [PubMed] [Google Scholar]
- Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, Koyasu S, Locksley RM, McKenzie AN, Mebius RE, et al. (2013). Innate lymphoid cells--a proposal for uniform nomenclature. Nat Rev Immunol 13, 145–149. [DOI] [PubMed] [Google Scholar]
- Stier MT, Zhang J, Goleniewska K, Cephus JY, Rusznak M, Wu L, Van Kaer L, Zhou B, Newcomb DC, and Peebles RS Jr. (2018). IL-33 promotes the egress of group 2 innate lymphoid cells from the bone marrow. J Exp Med 215, 263–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strait RT, Mahler A, Hogan S, Khodoun M, Shibuya A, and Finkelman FD (2011). Ingested allergens must be absorbed systemically to induce systemic anaphylaxis. J Allergy Clin Immunol 127, 982–989 e981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugarman JL, Fluhr JW, Fowler AJ, Bruckner T, Diepgen TL, and Williams ML (2003). The objective severity assessment of atopic dermatitis score: an objective measure using permeability barrier function and stratum corneum hydration with computer-assisted estimates for extent of disease. Arch Dermatol 139, 1417–1422. [DOI] [PubMed] [Google Scholar]
- Vaali K, Lappalainen J, Lin AH, Mayranpaa MI, Kovanen PT, Berstad A, and Eklund KK (2012). Imatinib mesylate alleviates diarrhea in a mouse model of intestinal allergy. Neurogastroenterol Motil 24, e325–335. [DOI] [PubMed] [Google Scholar]
- von Moltke J, Ji M, Liang HE, and Locksley RM (2016). Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature 529, 221–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SH, Walker JA, Jolin HE, Drynan LF, Hams E, Camelo A, Barlow JL, Neill DR, Panova V, Koch U, et al. (2012). Transcription factor RORalpha is critical for nuocyte development. Nat Immunol 13, 229–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.