Abstract
Objective:
Arterial stiffness index (ASI) is independently associated with blood pressure and coronary artery disease (CAD) epidemiologically. However, it is unknown whether these associations represent causal relationships. Here, we assess whether genetic predisposition to increased ASI is associated with elevated blood pressure and CAD risk.
Approach and Results:
We first performed a large-scale epidemiologic association of finger photoplethysmography-derived ASI in the UK Biobank, finding significant associations with systolic blood pressure (SBP; Beta 0.55mmHg, [95% CI, 0.45–0.65], P=5.77×10−24, N=137,858), diastolic blood pressure (DBP; Beta 1.05mmHg, [95% CI, 0.99–1.11], P=7.27×10−272, N=137,862), and incident CAD (HR 1.08 [95% CI, 1.04–1.11], P=1.5×10−6; N=3,692 cases, 126,615 controls) in multivariable models. We then performed an ASI genome-wide association analysis (GWAS) in 131,686 participants from the UK Biobank. Across participants not in the ASI GWAS, a 6-variant ASI polygenic risk score was calculated. Each SD increase in genetic ASI was associated with SBP (Beta 4.63mmHg [95% CI, 2.1–7.2]; P=3.37×10−4; N=208,897), and DBP (Beta 2.61mmHg [95% CI, 1.2–4.0]; P=2.85×10−4; N=208,897); however, no association was observed with incident CAD (HR 1.12 [95% CI, 0.55–2.3]; P=0.75; N=223,061; 7,534 cases). The lack of CAD association observed was replicated among 184,305 participants (60,810 cases) from the Coronary Artery Disease Genetics Consortium (OR 0.56 [95% CI, 0.26–1.24]; P=0.15).
Conclusions:
Our data support the conclusion that finger photoplethysmography-derived ASI is an independent, genetically causal risk factor for blood pressure, but do not support the notion that ASI is a suitable surrogate for CAD risk.
Keywords: Arterial Stiffness, Blood Pressure, Coronary Artery Disease, Genetic Epidemiology, Mendelian Randomization, Population Genetics
Subject codes: Genetic, Association Studies
Introduction:
Arterial stiffness, as measured via various non-invasive measures, has been repeatedly associated with cardiovascular disease risk in multiple epidemiological studies1–9. Increased vascular resistance and diminished viscoelasticity are key features of vascular aging which were previously associated with systolic hypertension5, coronary artery disease (CAD)2,4,7, and all-cause mortality10. Arterial stiffness may be influenced by variations in collagen, elastin, smooth muscle tone, and endothelial dysfunction, in addition to other factors11–17. Carotid-femoral (aortic) pulse wave velocity is the ‘gold-standard’ approach for assessing arterial stiffness. Arterial stiffness index (ASI) measurement using finger infrared analysis is a scalable, non-invasive approach to assess ASI and is correlated with carotid-femoral (aortic) pulse wave velocity18–20.
While arterial stiffness measures are associated with cardiovascular diseases1–8, whether the associations are causal is not clear. For example, non-causal risk factors, such as high-density lipoprotein cholesterol for CAD, are good risk predictors but are disappointing therapeutic targets21–26. Lifestyle factors are separately linked to arterial stiffness and cardiovascular diseases, potentially confounding the observed relationships27. Furthermore, reverse causality could lead to a statistically robust but non-causal relationship. For example, individuals with increased arterial stiffness might develop cardiovascular disease because of reduced exercise28.
Some propose that ASI should be considered a non-invasive surrogate end point for cardiovascular events largely based on robust epidemiological associations29–31,2,32–38. Understanding whether ASI causally mediates CAD, independent of blood pressure, may help determine whether ASI is a suitable surrogate end point for CAD separate from its utility as a risk predictor. Mendelian randomization uses human genetics for causal inference by leveraging the random assortment of genetic variants during meiosis at conception, which diminishes susceptibility to confounding or reverse causality39. Here, we used Mendelian randomization to determine whether a genetic predisposition to increased ASI is associated with elevated blood pressure and increased risk for incident CAD.
Methods:
Anonymized individual-level data are available by application from the UK Biobank (https://www.ukbiobank.ac.uk). Data from all supporting analyses are included in the present paper.
UK Biobank study participants and phenotypes
Individual-level genomic data and longitudinal phenotypic data from the UK Biobank, a large-scale population-based dataset consisting of genotype and phenotype data in approximately 500,000 volunteer participants collected from 2007–2017, was used.
Clinical disease definitions are detailed in Supplementary Table I. In summary, the main outcome, CAD, was defined by billing codes for heart attack, angina pectoris, unstable angina, myocardial infarction, coronary atherosclerosis, coronary artery revascularization, and other acute, subacute, and chronic forms of ischemic heart disease, or with self-reported angina, heart attack/myocardial infarction, coronary angioplasty +/− stent, or coronary artery bypass graft (CABG) surgery. We also assessed systolic and diastolic blood pressures, and adjusted for blood pressure medications by adding 15 and 10 mmHg to systolic and diastolic blood pressures, respectively40,41.
Arterial stiffness index measurement
ASI was previously measured in the UK Biobank using the PulseTrace PCA2 (CareFusion, San Diego, CA), which uses finger photoplethysmography (PPG) over a 10- to 15-second timeframe to obtain the pulse waveform from an infrared sensor clipped to the end of the index finger. ASI (in m/s) was calculated by dividing standing height by the time between forward and the reflected waves of the pulse waveform. ASI by this approach was previously correlated with aortic pulse wave velocity, which is regarded as the gold standard18. ASI was inverse-rank normalized for analysis (with mean = 0, SD = 1).
Genotyping and imputation
Genome-wide genotyping was previously performed in the UK Biobank using two genotyping arrays sharing 95% of marker content: Applied Biosystems UK BiLEVE Axiom Array (807,411 markers in 49,950 participants) and Applied Biosystems UK Biobank Axiom Array (825,927 markers in 438,427 participants) both by Affymetrix (Santa Clara, CA)42. Variants used in the present analysis include those also imputed using the Haplotype Reference Consortium reference panel of up to 39M SNPs43,44.
Quality control and variant annotation
Poor quality variants and genotypes were filtered as previously described42. We further filtered out individuals from both genetic and epidemiological analyses using the following genetic criteria: non-white or not of British ancestry, gender mismatch between reported and genotypic genders, sex chromosome aneuploidy, or one from each pair of 1st or 2nd degree relatives (Supplementary Table II). Non-consenting individuals with prevalent peripheral arterial disease, aortic valve disease, or CAD were excluded, as were extreme outliers for any of the arterial pulse wave phenotypes listed in Supplementary Table III. Extreme outliers were determined by adjusting the traditional box and whisker upper and lower bounds and accounting for skewness in the phenotypic data identified using the Robustbase package in R (setting range=3) (https://cran.r-project.org/web/packages/robustbase/robustbase.pdf). We restricted samples to those of white, British genetic ancestry for two strictly analytical reasons: 1) to minimize spurious associations from differences in population allele frequencies in the GWAS analysis45, 2) to minimize confounding by population stratification in the 2-sample MR analyses46 given the replication cohort (CARDIOGRAMplusC4D) is primarily European.
After filtering samples, variants were further filtered by the following criteria: not in Hardy-Weinberg Equilibrium (P<1×10−10), low imputation quality (INFO score < 0.3), call rate < 95%, and minor allele frequency < 0.05% (minor allele count < 66).
Variant consequences were annotated using with Ensembl’s Variant Effect Predictor (VEP), ascribing the most severe consequence and associated gene among the canonical transcripts present for each variant47. The Hail v0.1 software (https://hail.is) was used to perform quality control and variant annotation48.
Epidemiological association analyses with arterial stiffness index
Epidemiological association of ASI with blood pressure phenotypes and incident CAD was performed using linear regression and Cox proportional hazards model, respectively, in R (version 3.3, R Foundation, Vienna, Austria). For CAD, adjustment was performed for age, sex, ever smoking status, heart rate at pulse wave analysis, prevalent hypertension, prevalent hypercholesterolemia, prevalent diabetes, alcohol intake (self-reported alcohol intake of at least once per month), exercise (self-reported exercise of at least 3x per week), and vegetable intake (self-reported intake of at least 6 tablespoons of vegetable intake per day). The same adjustment variables were used for SBP and DBP, except prevalent hypertension was not included as a covariate.
Analyses were performed using a Cox proportional hazards model for incident CAD, and linear regression for the blood pressure traits. The threshold for significance for the three primary phenotypes was assigned as alpha = 0.05/3 tests = 0.017.
Genome-wide association analysis of arterial stiffness
A genome-wide association of ASI was performed using individual-level data from 131,686 individuals of European descent from the UK Biobank, collected from 2007 to 2017. Each variant was individually associated with ASI in an additive linear regression model and adjusted for sex, age, smoking status, genotyping array type, and the first ten principal components of ancestry49. Only variants with minor allele frequency > 0.05% (minor allele count > 66) were considered. P < 5×10−8 was considered to be significant. The Hail software version 0.1 (https://hail.is) was used for genome-wide association analysis48.
Further evaluation of non-coding regions surround the top loci were performed using the Hi-C Unifying Genomic Integrator50 web browser (https://yunliweb.its.unc.edu/hugin/). This web browser was used to query whether the top variants at the top 5 loci had any chromatin contacts with nearby genes or with enhancers of aorta tissue.
Mendelian randomization
An additive genetic risk score (GRS) was calculated as were m is the number of SNPs, ln(ORi) is the weight for SNPi from the discovery sample, SNPij is the number of alleles (i.e., 0, 1, or 2) for SNPi in person j in the validation sample. Six independent variants (linkage disequilibrium r2 < 0.25 within 500kb windows) demonstrating at least suggestive association with ASI (P<5×10−7) were included in the GRS. The raw GRS was calculated for each individual using PLINK51, inverse-rank normalized, then re-scaled such that one unit increase in the GRS was equivalent to a one standard deviation (SD) increase in ASI.
To confirm that the GRS for ASI was a strong instrument for ASI, an F-statistic for the instrument was calculated in the UK Biobank. An F-statistic is a measure of the significance of an instrument (the GRS) for prediction of the exposure (ASI), controlling for additional covariates (age, sex, ever smoked, 10 principal components of ancestry, and genotyping array type). An F-statistic greater than 10 is evidence of a strong instrument. Furthermore, sensitivity analyses were performed to evaluate for associations between the ASI GRS and potential environmental confounders including sex, ever smoking status, diet (alcohol intake, vegetable intake), and exercise frequency among individuals not in the ASI genome-wide association analyses.
A linear regression model was used to associate the ASI GRS with systolic and diastolic blood pressures. A Cox proportional hazards model was used to associate ASI GRS with incident CAD. For CAD, adjustment was performed for age, sex, ever smoking status, heart rate at blood pressure measurement, prevalent hypertension, prevalent hypercholesterolemia, prevalent diabetes, alcohol intake (self-reported alcohol intake of at least once per month), exercise (self-reported exercise of at least 3x per week), and vegetable intake (self-reported intake of at least 6 tablespoons of vegetable intake per day), where indicated. The same adjustment variables were used for SBP and DBP, except for prevalent hypertension.
2-Sample Mendelian randomization with coronary artery disease
To address potential power limitations from the lack of association between ASI and CAD, we also pursued 2-sample Mendelian randomization using variant-level summary statistics from prior genome-wide association analyses of CAD from several independent case-control studies, specifically 184,305 individuals from the Coronary Artery Disease Genetics Consortium (CARDIOGRAMplusC4D)52. The effect estimates and standard errors for the six GRS variants for ASI (from UK Biobank) and for CAD (from CARDIOGramplusC4D) were used to perform robust, penalized inverse variance weighted (IVW) 2-sample Mendelian randomization using the MendelianRandomization package in R53,54. IVW 2-sample Mendelian randomization uses a weighted linear regression of the ratio of the SNP effects on the outcomes to the SNP effects on the risk factor, without using an intercept term. The threshold for significance was defined as alpha = 0.05.
Additionally, analyses were performed to evaluate the reverse association, of CAD causally impacting ASI. 77 known, independent, genome-wide significant CAD locus variants were identified across several published sources52,55–57 (Supplementary Table IX). These 77 CAD locus variants were used as an instrument in 2-sample Mendelian randomization to evaluate whether CAD causally affects ASI.
Results:
Baseline characteristics
A total of 131,686 individuals in the UK Biobank had ASI measured, genotype data available, and passed quality control (Supplementary Table II). Among these individuals, median age was 59 (IQR 51–63) years, 53.8% were female, 4.6% had diabetes, 27.1% had hypertension, and 12.9% had hypercholesterolemia. Median SBP was 139 (IQR 127–153) mmHg, median DBP was 82 (IQR 75–89) mmHg. 44.1% of individuals were prior or current smokers, and 10.1% of individuals were on antihypertensive medications (Table I). The median ASI was 9 (IQR 7–11) m/s (Supplementary Table III).
Table 1:
Baseline characteristics of analyzed participants with arterial stiffness index and genotypes
| Category | Phenotype† | |
|---|---|---|
| Demographic phenotypes | Age (Median; Q1-Q3 (N)) | 59; 51–63 (131,686) |
| Sex (% Female) | 70,847 (53.8%) | |
|
Prevalent Disease (Cases/Controls) |
Prevalent Diabetes | 6019/125667 (4.6%) |
| Prevalent Hypertension | 35639/96047 (27.1%) | |
| Prevalent Hypercholesterolemia | 17056/114630 (12.9%) | |
| Prevalent Atrial Fibrillation or Atrial Flutter | 1830/129856 (1.4%) | |
| Prevalent Heart Failure | 305/131381 (0.23%) | |
|
Blood Pressure (Median; Q1-Q3 (N)) |
SBP | 139; 127–153 (131,084) |
| DBP | 82; 75–89 (131,086) | |
|
Lifestyle factors & Medications N (%) |
Previous or Current Smoker | 57,974 (44.1%) |
| Antihypertensive Medication | 13,296 (10.1%) |
these values reflect the 131,686 samples with all pulse wave analysis phenotypes and genotype data present used in the genome-wide association analysis; sample outliers for quantitative phenotypes were removed as described in the methods.
SBP=systolic blood pressure, DBP=diastolic blood pressure.
Epidemiological associations of ASI
Univariate association of cardiovascular risk factors with ASI showed the following associations with at least nominal significance (P<0.05): for age (0.024 SD/year, P<1×10−300), sex (0.40 SD higher in males, P<1×10−300), blood pressure medication (0.34 SD, P=1.4×10−317), hypertension (0.21 SD, P=1.4×10−269), hypercholesterolemia (0.20 SD, P=4.1×10−137), diabetes (0.20 SD, P=9.1×10−54), ever smoking (0.18 SD, P=3.0×10−250), exercise ≥3x/wk (−0.16 SD, P=2.9×10−66), alcohol intake ≥1x/mo (0.05 SD, P=3.3×10−20), and ≥6 tablespoons vegetable intake per day (−0.063 SD, P=3.1×10−4) (Supplementary Table IV).
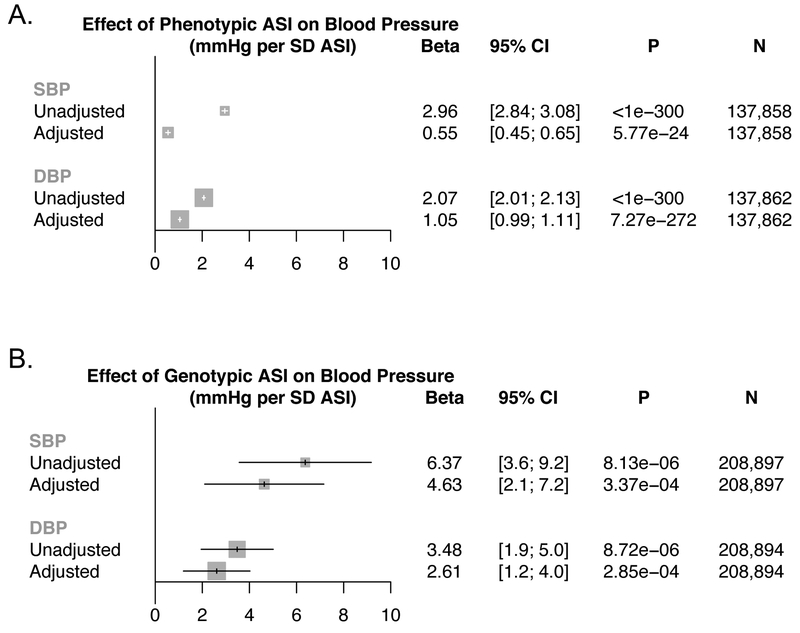
For the associations of ASI with SBP and DBP, both univariable and multivariable, adjusting for age, sex, smoking status, prevalent hypercholesterolemia, prevalent diabetes, vegetable intake, alcohol intake, and exercise, analyses showed consistently strong associations (Figure 1A). Each SD of ASI was associated with elevated SBP by 0.55 mmHg ([95% CI, 0.45–0.65], P=5.77×10−24) and DBP by 1.05 mmHg ([95% CI, 0.99–1.11], P=7.27×10−272).
Figure 1: Epidemiologic and genetic associations of arterial stiffness index with blood pressure.
Association between (A) phenotypic ASI, and, (B) genotypic ASI (ie: the ASI GRS), with systolic and diastolic blood pressures in the UK Biobank. Results are presented as both unadjusted and, separately, adjusted by age, sex, smoking status, prevalent hypercholesterolemia, prevalent diabetes, heart rate, vegetable intake, alcohol intake, and exercise frequency. Effect estimates represent mmHg increase in blood pressure resulting from (A) 1 SD increase in ASI phenotype, and (B) 1 SD increase in genetically-mediated ASI from the ASI GRS.
ASI = Arterial stiffness index, DBP = diastolic blood pressure, GRS = genetic risk score, SBP = systolic blood pressure, SD = standard deviation
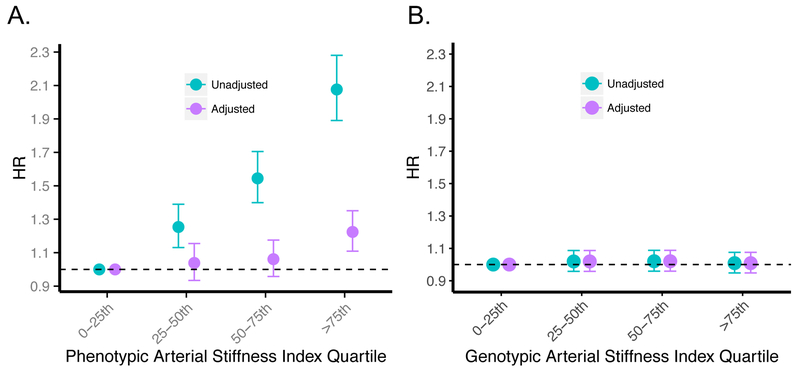
ASI was also significantly independently associated with incident CAD, adjusting for age, sex, ever smoking status, heart rate, prevalent hypertension, prevalent hypercholesterolemia, prevalent diabetes, vegetable intake, alcohol intake, and exercise (HR 1.08 per SD ASI [95% CI, 1.04–1.11], P=7.67×10−6) (Figure 2A).
Figure 2: Epidemiologic and genetic associations of arterial stiffness index with incident coronary artery disease.
Association between (A) phenotypic ASI, and, (B) the ASI GRS, with incident coronary artery disease in the UK Biobank. Results are presented as both unadjusted (cyan) and adjusted (purple) by age, sex, smoking status, prevalent hypertension, prevalent hypercholesterolemia, prevalent diabetes, heart rate, vegetable intake, alcohol intake, and exercise frequency. For the ASI GRS instrument, analysis was performed excluding individuals used in the ASI genome-wide association study. Hazard ratios represent increased risk of incident CAD resulting from (A) 1 SD increase in ASI phenotype, and (B) 1 SD increase in genetically-mediated ASI from the ASI GRS. Sample sizes for (A) the phenotypic association are 3,692 cases, 126,615 controls, and for (B) the genotypic association are 7,534 cases, 215,527 controls.
ASI = Arterial stiffness index, CAD = coronary artery disease, GRS = genetic risk score, HR = hazard ratio, SD = standard deviation.
Genome-wide association analysis of ASI
A genome-wide association analysis of ASI was performed among 131,686 individuals and 13,995,214 variants in the UK Biobank. A quantile-quantile plot of the genome-wide association statistics did not show substantial genomic inflation (λ = 1.05) (Supplementary Figure I). Two genome-wide significant loci were identified (P<5×10−8), the top variants of which were in second intron of TEX41 (rs1006923, −0.025 SD, P=3.7×10−10, minor allele frequency (MAF)=0.32), and first intron of FOXO1 (rs7331212, −0.024 SD, P=9.3×10−9, MAF=0.26). Three additional suggestive loci (P<5×10−7) were also identified, of which the top variants are intronic variants in COL4A2 (rs872588, −0.020 SD, P=2.3×10−7, MAF=0.42), RNF126 (rs1009628, −0.027 SD, P=1.2×10−7, MAF=0.15), and TCF20 (rs55906806, −0.024 SD, P=2.4×10−7, MAF=0.20). Through chromatin conformational changes50, intronic variants at TEX41 and COL4A2 may influence gene expression at nearby enhancers Supplementary Results, Supplementary Figure II). Interrogation of disruptive protein-coding variants yielded moderate association for HFE p.Cys282Tyr (MAF 0.076), the most common variant implicated in hereditary hemochromatosis (Supplementary Results, Supplementary Table V).
Mendelian randomization in the UK Biobank
Six independent and at least suggestive (P<5×10−7) variants were used towards an ASI genetic risk score (GRS) (Supplementary Table VI). The raw ASI GRS was associated with a 0.85 SD increase in ASI (SE: 0.072; P=8.0×10−32). The F-statistic of the GRS was 123 (recommended F-statistic > 10), suggesting high instrument strength. The GRS was re-scaled such that each unit reflected one SD in ASI for comparison with the phenotypic associations (Supplementary Figure III). Sensitivity analysis was performed to evaluate for pleiotropic associations between the ASI GRS and potential environmental confounders including sex, ever smoking status, diet (alcohol intake, vegetable intake), and exercise frequency. No significant associations between the ASI GRS and environmental confounders were observed (Supplementary Table VII).
A 1-SD increase in genetically-mediated ASI was significantly associated with elevated SBP (Beta 4.63 mmHg [95% CI, 2.1–7.2]; P=3.37×10−4), and DBP (Beta 2.61 mmHg [95% CI, 1.2–4.0]; P=2.85×10−4), independent of cardiometabolic risk factors (age, sex, and smoking status, prevalent hypercholesterolemia, prevalent diabetes, heart rate, vegetable intake, alcohol intake, and exercise frequency) (Figure 1B, Supplementary Table VIII).
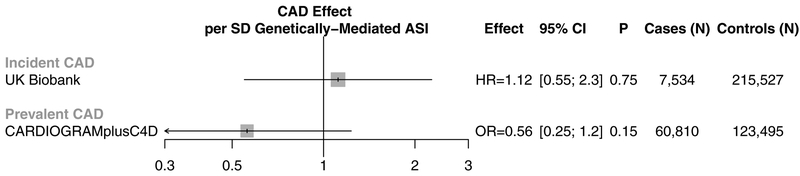
The ASI GRS, however, was not associated with incident CAD in UK Biobank in an unadjusted model (HR 1.3 [95% CI, 0.64–2.6]; P=0.47) or an adjusted model including age, sex, smoking status, prevalent hypertension, prevalent hypercholesterolemia, prevalent diabetes, heart rate, vegetable intake, alcohol intake, and exercise frequency as covariates (HR 1.12 [95% CI, 0.55–2.3]; P=0.75) (Figure 2B, Supplementary Table IX). The association of each of the 6 ASI genetic risk score variants with incident CAD in the UKBB are provided in Supplementary Table X.
2-Sample Mendelian randomization with coronary artery disease
To address potential power limitations impeding association of ASI GRS with incident CAD in the UK Biobank, we also pursued 2-sample Mendelian randomization between ASI and prevalent CAD using variant-level summary statistics from 184,305 separate individuals in the Coronary Artery Disease Genetics Consortium (CARDIOGRAMplusC4D)52. Robust, penalized inverse-variance weighted 2-sample Mendelian randomization similarly did not demonstrate an association between genetically-elevated ASI and CAD (OR 0.56 [95% CI, 0.26–1.24], P=0.15) (Figure 3, Supplementary Figure IV). Furthermore, the six variants showing suggestive association with ASI did not demonstrate a significant positive association with CAD across several different 2-sample Mendelian randomization methods, with no evidence of unmeasured horizontal pleiotropy58 (MR-Egger intercept P=0.53) (Supplementary Table XI).
Figure 3: One- and two-sample Mendelian randomization analyses of arterial stiffness index with coronary artery disease.
Association between the ASI GRS and incident CAD in the UK Biobank, as well as prevalent CAD in the CARDIOGRAMplusC4D consortium. Incident CAD results were derived using individual-level data from the UK Biobank and adjusting by cardiometabolic risk factors (age, sex, smoking status, prevalent hypertension, prevalent hypercholesterolemia, prevalent diabetes, heart rate, vegetable intake, alcohol intake, and exercise frequency). Prevalent CAD results were derived from summary-level genome-wide association data from the CARDIOGRAMplusC4D consortium using robust, penalized inverse-variance weighted 2-sample Mendelian randomization.
ASI = Arterial stiffness index, CAD = coronary artery disease, GRS = genetic risk score, HR = hazard ratio, OR = odds ratio
We also developed an expanded ASI polygenic score using 321 independent variants (P<1×10−4, LD r2< 0.25) to capture additional genetic variation of ASI. The expanded ASI polygenic score explained 3.3% of ASI variance conferring >80% power to detect the CAD effect estimate observed in epidemiologic analyses (i.e., OR=1.08) with alpha = 0.05. With this approach, we again confirmed no significant association in inverse-variance weighted 2-sample Mendelian randomization (OR 0.95 [95% CI, 0.89–1.02], P=0.13).
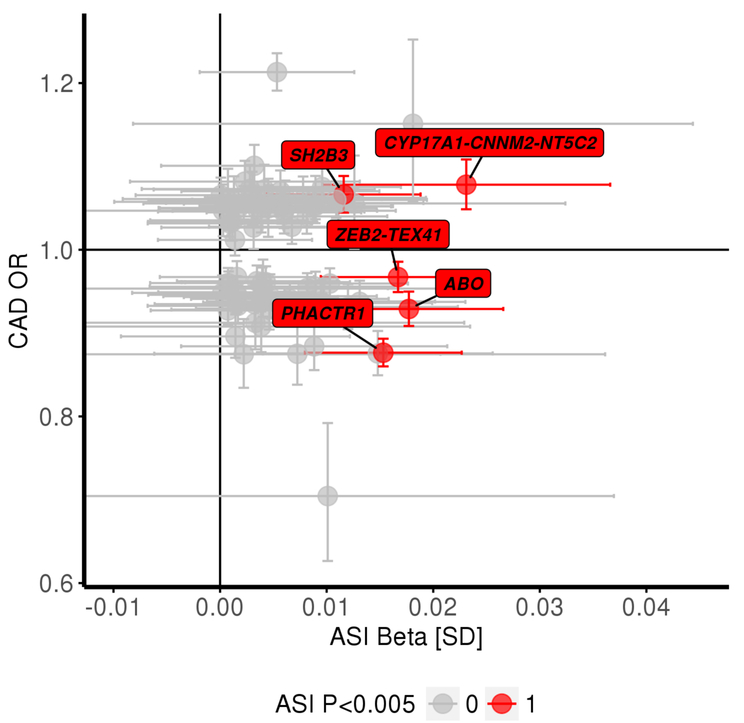
77 genome-wide significant CAD loci from prior GWAS52,56,57 were identified, and CAD risk effect estimates prior studies and ASI effect estimates from this study were catalogued (Figure 4). While 3 of 77 previously-associated CAD loci showed evidence of association with ASI (P<0.05/77=6.5×10−4), effect directions were inconsistent between ASI and CAD. For example, the variant rs9349379-A, an intronic variant in PHACTR1, was associated with increased ASI (0.015 SD, P=4.5×10−5) but decreased risk for CAD (OR= 0.87, P=1.8×10−42). Similarly, ASI-raising alleles at the ZEB2-TEX41 and ABO loci decrease CAD risk, while ASI-raising alleles at CYP17A1-CNNM2-NT4C2 and SH2B3 increase CAD risk. Detailed variant-level summary statistics for these 77 CAD locus variants are provided in Supplementary Tables XII-XIII. These 77 CAD locus variants were also used as an instrument in 2-sample Mendelian randomization for a putative reverse association – whether a genetic susceptibility to CAD increases ASI. No significant associations were observed across various 2-sample Mendelian randomization methods for the reverse association (Supplementary Table XIV).
Figure 4: Comparison of variant level-effects with arterial stiffness index and with coronary artery disease shows inconsistency.
Variant-level effect estimates (from CARDIOGRAMplusC4D) from variants at 77 independent known CAD loci, were compared to their ASI associations. Highlighted are 5 out of the 77 variants with at least suggestive significance with ASI (P<0.005), showing that ASI-raising alleles have inconsistent effects on CAD risk. The variant-level summary statistics for these 77 variants across are detailed in Supplementary Tables XII-XIII.
ASI = arterial stiffness index, CAD = coronary artery disease
Discussion:
We performed the largest genome-wide association analysis to-date of a measure of vascular aging, ASI, in 131,686 individuals, and leveraged these observations to perform causal inference analyses with blood pressure and risk of CAD in up to 407,366 separate individuals. In our genome-wide association analyses, we discover the first genome-wide variants associated with ASI. We replicate the epidemiologic associations of ASI with blood pressure and CAD, and find that genetic analyses do indeed support a causal relationship between ASI and blood pressure. However, our genetic analyses do not support a causal relationship between ASI and CAD.
These results permit several conclusions. First, we observe strong epidemiologic and genetic association between ASI and blood pressure. Prior studies have evaluated the relationship between arterial stiffness and cardiovascular disease outcomes2,4,7. Notably, a previous study in the Young Finns cohort previously demonstrated the longitudinal relation between childhood arterial stiffness and adult-age blood pressure59. Here, our data indicate that non-invasive PPG, employed by a finger probe or by commercially-available wearable monitors that measure heart rate60, may be used to impute continuous blood pressure, and that changes will track with blood pressure changes. However, given independent clinical effects and imperfect correlation, ASI measurement may complement blood pressure assessments. Second, there is a long-standing debate whether ASI precedes elevated blood pressure or vice versa61. Compared to its phenotypic effect, the effect conferred by genetically-elevated ASI is 8.4-fold higher for SBP (4.63 mmHg for ASI GRS versus 0.55 mmHg for ASI phenotype) and 2.5-fold higher for DBP (2.61 mmHg for ASI GRS versus 1.05 mmHg for ASI phenotype), potentially representing the effects of life-long exposure to elevated arterial stiffness on blood pressure. This supports the notion that arterial stiffness may predate the onset of elevated blood pressure indicating that ASI may identify individuals at heightened risk for future blood pressure elevations.
Third, our epidemiological and genetic analyses indicate that ASI is an independent, non-causal risk factor for CAD. Arterial stiffness may be a parallel disrupted pathway in the setting of CAD, as opposed to an upstream causal mediating factor. Thus, while ASI monitoring may still serve as a good proxy for blood pressure, therapeutic modulation of finger PPG-derived ASI in isolation may not have a meaningful impact on CAD outcomes. Similarly, a recent study of twins showed that while carotid-femoral pulse wave velocity was heritable, it did not associate with 5-year progression of carotid intima media thickness62. The lack of significance between genetically-elevated ASI and CAD is also consistent with prior mixed results in experimental models. Fragmentation of elastin fibers and deposition of collagen fibers are features of vascular aging implicated in arterial stiffness63. However, murine models lacking elastin do not have endothelial damage, thrombosis, or inflammation which typically occur with atherosclerosis64.
The observation that genetic ASI is strongly associated with blood pressure but not CAD raises the possibility of diverse vascular phenomena contributing to finger PPG-derived ASI with inconsistent CAD effects. We found that while few variants associated with CAD show apparent association with ASI, our data indicate that ASI may not be mediating the apparent CAD risk. We observed generally inconsistent genetic effects between ASI and CAD risk. In particular, an intronic variant within PHACTR1 (rs9349379-A), which was recently shown to influence endothelin-1 expression in the vasculature, is associated with decreased risk for CAD65, increased blood pressure66, and increased ASI. For this variant, the divergent directionalities of effect on CAD and blood pressure may be due to the differential expression of EDNRA versus EDNRB in the coronary arteries compared to peripheral vasculature65. Additionally, genetic variants disrupting nitric oxide signaling at the NOS3 and GUCY13 loci influence both blood pressure and risk of CAD67–69. Notably, in our study, risk variants at these loci were not strongly associated with ASI. Extensive prior experimental work linked nitric oxide signaling and endothelin-1 with endothelial function and vascular tone70–74. Our data suggests that increased risk of CAD through these pathways is unlikely to be through changes in finger PPG-derived ASI but potentially through alternative vascular mechanisms.
While our study has several strengths, some limitations should be considered. First, we note that the conclusions arrived here are specific to finger PPG-derived ASI and do not reflect large artery stiffness as derived from the gold-standard carotid-femoral pulse wave velocity estimations. Finger PPG-derived ASI and the gold standard carotid-femoral (aortic) pulse wave velocity estimations are significantly correlated (r=0.58–0.65)18–19. A study in 461 subjects using machine learning methods on finger PPG measurements successfully classified up to 87.5% of individuals as high versus low arterial stiffness as separately determined by aortic PWV measurements20. Furthermore, previous Bland-Altman analyses suggests the 95% CI for differences in Z-scores between aortic PWV and finger PPG-derived ASI are in close agreement (CI < 2 SD)18. However, given strong yet imperfect correlation, finger PPG likely captures not only large artery stiffness but also various other vascular phenomena. As such our findings may not extend to large artery stiffness alone. Second, lack of ASI genetic risk score association with CAD may be due to limited statistical power. Our replication of the lack of association using 2-sample Mendelian randomization including with an expanded polygenic score, combined with our analysis showing inconsistent effects of individual variants between CAD and ASI suggests that this is less likely. Thirdly, our imputation of untreated blood pressure among those with prescribed hypertensives assumes a homogenous blood pressure effect across the population. Without prescription data in the UK Biobank, we are unable to account for different medication regimens and adherence. However, our approach to account for medications41 mirrors prior blood pressure genetic analyses40. Furthermore, our additional sensitivity analyses accounting for antihypertensive effects further confirm the genetic relationship of ASI with blood pressure. Lastly, it should be noted that these analyses were performed in European populations to minimize confounding from population stratification and to permit 2-sample Mendelian randomization with the largest (primarily European) CAD GWAS dataset. Replication of these findings in other ethnicities is warranted.
Conclusion:
A genetic predisposition to higher ASI was associated with increased blood pressure, but not increased risk of CAD. Our data support the conclusion that finger photoplethysmography-derived ASI is an independent, genetically causal risk factor for blood pressure and an independent, non-causal risk factor for CAD.
Supplementary Material
Graphical Abstract: Epidemiologic and genetic associations of arterial stiffness with blood pressure and coronary artery disease.
Association between phenotypic ASI, and separately, genotypic ASI (from the ASI GRS), with systolic and diastolic blood pressures, as well as with incident and prevalent CAD. Blood pressure results are adjusted by age, sex, smoking status, prevalent hypercholesterolemia, prevalent diabetes, heart rate, vegetable intake, alcohol intake, and exercise frequency. Blood pressure effect estimates represent mmHg increase in blood pressure resulting from 1 SD increase in ASI phenotype, and separately, 1 SD increase in genetically-mediated ASI from the ASI GRS. Incident CAD associations in the UK Biobank, as well as prevalent CAD associations using the CARDIOGRAMplusC4D consortium, with phenotypic ASI and the ASI GRS are provided. Incident CAD results were derived using individual-level data from the UK Biobank and adjusting by cardiometabolic risk factors (age, sex, smoking status, prevalent hypertension, prevalent hypercholesterolemia, prevalent diabetes, heart rate, vegetable intake, alcohol intake, and exercise frequency). Prevalent CAD results were derived from summary-level genome-wide association data from the CARDIOGRAMplusC4D consortium using robust, penalized inverse-variance weighted 2-sample Mendelian randomization.
ASI = Arterial stiffness index, CAD = coronary artery disease, DBP = diastolic blood pressure, GRS = genetic risk score, SBP = systolic blood pressure, SD = standard deviation
Highlights:
Our epidemiological analyses show a significant, independent association of finger photoplethysmography-derived ASI with elevated systolic and diastolic blood pressure, and with elevated CAD risk. This, combined with prior reports of arterial stiffness epidemiological analyses, motivates our further Mendelian randomization analyses.
We performed the first large-scale genome-wide association analysis of ASI, identifying two significant loci (P<5×10–8) at TEX41-ZEB2 and FOXO1, and three suggestive loci (P<5×10–7) at COL4A2-COL4A1, RNF126, and TCF20.
Each SD increase in genetically-elevated ASI is independently associated with 5 mmHg higher systolic blood pressure and 3 mmHg higher diastolic blood pressure.
However, a genetic predisposition to higher ASI was not associated with incident CAD in the UK Biobank (P=0.75) or with prevalent CAD in CARDIOGRAMplusC4D (P=0.15). This data, from a total of ~410,000 individuals, suggests finger photoplethysmography-derived ASI is not a suitable surrogate for CAD risk.
Acknowledgements:
The authors would like to acknowledge and thank the participants and staff of the UK Biobank and of the CARDIOGramplusC4D consortium cohorts.
Funding:
S.M.Z. is supported by the National Institutes of Health’s Medical Scientist Training Program at the Yale School of Medicine and the Paul and Daisy Soros Fellowship. P.N. is supported by a Hassenfeld Scholar Award from the Massachusetts General Hospital, and K08 HL140203 from the National Heart, Lung, and Blood Institute.
Abbreviations:
- ASI
arterial stiffness index
- CAD
coronary artery disease
- CARDIOGRAMplusC4D
Coronary Artery Disease Genetics Consortium
- DBP
diastolic blood pressure
- GRS
genetic risk score
- PPG
photoplethysmography
- SBP
systolic blood pressure
Footnotes
Disclosures:
P.N. reports investigator-initiated grants from Amgen and Boston Scientific, and consulting income from Apple. The remaining authors have nothing to disclose.
References:
- 1.Ikonomidis I, Ntai K, Kadoglou NP, et al. The evaluation of pulse wave velocity using Arteriograph and Complior apparatus across multiple cohorts of cardiovascular-related diseases. Int J Cardiol. 2013;168(5):4890–4892. [DOI] [PubMed] [Google Scholar]
- 2.Mattace-Raso FU, van der Cammen TJ, Hofman A, et al. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation. 2006;113(5):657–663. [DOI] [PubMed] [Google Scholar]
- 3.Ben-Shlomo Y, Spears M, Boustred C, et al. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol. 2014;63(7):636–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ikonomidis I, Makavos G, Lekakis J. Arterial stiffness and coronary artery disease. Curr Opin Cardiol. 2015;30(4):422–431. [DOI] [PubMed] [Google Scholar]
- 5.Franklin SS, Gustin Wt, Wong ND, et al. Hemodynamic patterns of age-related changes in blood pressure. The Framingham Heart Study. Circulation. 1997;96(1):308–315. [DOI] [PubMed] [Google Scholar]
- 6.Chirinos JA, Kips JG, Jacobs DR Jr., et al. Arterial wave reflections and incident cardiovascular events and heart failure: MESA (Multiethnic Study of Atherosclerosis). J Am Coll Cardiol. 2012;60(21):2170–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sutton-Tyrrell K, Najjar SS, Boudreau RM, et al. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation. 2005;111(25):3384–3390. [DOI] [PubMed] [Google Scholar]
- 8.Tsao CW, Lyass A, Larson MG, et al. Relation of Central Arterial Stiffness to Incident Heart Failure in the Community. J Am Heart Assoc. 2015;4(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Said MA, Eppinga RN, Lipsic E, Verweij N, van der Harst P. Relationship of Arterial Stiffness Index and Pulse Pressure With Cardiovascular Disease and Mortality. J Am Heart Assoc. 2018;7(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55(13):1318–1327. [DOI] [PubMed] [Google Scholar]
- 11.Bank AJ, Kaiser DR. Smooth muscle relaxation: effects on arterial compliance, distensibility, elastic modulus, and pulse wave velocity. Hypertension. 1998;32(2):356–359. [DOI] [PubMed] [Google Scholar]
- 12.Anderson TJ. Arterial stiffness or endothelial dysfunction as a surrogate marker of vascular risk. Can J Cardiol. 2006;22 Suppl B:72B–80B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ikonomidis I, Lekakis J, Papadopoulos C, et al. Incremental value of pulse wave velocity in the determination of coronary microcirculatory dysfunction in never-treated patients with essential hypertension. Am J Hypertens. 2008;21(7):806–813. [DOI] [PubMed] [Google Scholar]
- 14.McNulty M, Mahmud A, Spiers P, Feely J. Collagen type-I degradation is related to arterial stiffness in hypertensive and normotensive subjects. J Hum Hypertens. 2006;20(11):867–873. [DOI] [PubMed] [Google Scholar]
- 15.Nigam A, Mitchell GF, Lambert J, Tardif JC. Relation between conduit vessel stiffness (assessed by tonometry) and endothelial function (assessed by flow-mediated dilatation) in patients with and without coronary heart disease. Am J Cardiol. 2003;92(4):395–399. [DOI] [PubMed] [Google Scholar]
- 16.Wagenseil JE, Mecham RP. Elastin in large artery stiffness and hypertension. J Cardiovasc Transl Res. 2012;5(3):264–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25(5):932–943. [DOI] [PubMed] [Google Scholar]
- 18.Woodman RJ, Kingwell BA, Beilin LJ, Hamilton SE, Dart AM, Watts GF. Assessment of central and peripheral arterial stiffness: studies indicating the need to use a combination of techniques. Am J Hypertens. 2005;18(2 Pt 1):249–260. [DOI] [PubMed] [Google Scholar]
- 19.Millasseau SC, Kelly RP, Ritter JM, Chowienczyk PJ. Determination of age-related increases in large artery stiffness by digital pulse contour analysis. Clin Sci (Lond). 2002;103(4):371–377. [DOI] [PubMed] [Google Scholar]
- 20.Alty SR, Angarita-Jaimes N, Millasseau SC, Chowienczyk PJ. Predicting arterial stiffness from the digital volume pulse waveform. IEEE Trans Biomed Eng. 2007;54(12):2268–2275. [DOI] [PubMed] [Google Scholar]
- 21.Brousseau ME, Schaefer EJ, Wolfe ML, et al. Effects of an inhibitor of cholesteryl ester transfer protein on HDL cholesterol. N Engl J Med. 2004;350(15):1505–1515. [DOI] [PubMed] [Google Scholar]
- 22.Emerging Risk Factors C, Di Angelantonio E, Sarwar N, et al. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302(18):1993–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Group HTC, Landray MJ, Haynes R, et al. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med. 2014;371(3):203–212. [DOI] [PubMed] [Google Scholar]
- 24.Investigators A-H, Boden WE, Probstfield JL, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365(24):2255–2267. [DOI] [PubMed] [Google Scholar]
- 25.Schwartz GG, Olsson AG, Abt M, et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med. 2012;367(22):2089–2099. [DOI] [PubMed] [Google Scholar]
- 26.Voight BF, Peloso GM, Orho-Melander M, et al. Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet. 2012;380(9841):572–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanaka H, Safar ME. Influence of lifestyle modification on arterial stiffness and wave reflections. Am J Hypertens. 2005;18(1):137–144. [DOI] [PubMed] [Google Scholar]
- 28.Sacre JW, Jennings GL, Kingwell BA. Exercise and dietary influences on arterial stiffness in cardiometabolic disease. Hypertension. 2014;63(5):888–893. [DOI] [PubMed] [Google Scholar]
- 29.Laurent S, Briet M, Boutouyrie P. Arterial stiffness as surrogate end point: needed clinical trials. Hypertension. 2012;60(2):518–522. [DOI] [PubMed] [Google Scholar]
- 30.Laurent S Arterial stiffness: intermediate or surrogate endpoint for cardiovascular events? Eur Heart J. 2005;26(12):1152–1154. [DOI] [PubMed] [Google Scholar]
- 31.Cohn JN, Quyyumi AA, Hollenberg NK, Jamerson KA. Surrogate markers for cardiovascular disease: functional markers. Circulation. 2004;109(25 Suppl 1):IV31–46. [DOI] [PubMed] [Google Scholar]
- 32.Blacher J, Guerin AP, Pannier B, Marchais SJ, Safar ME, London GM. Impact of aortic stiffness on survival in end-stage renal disease. Circulation. 1999;99(18):2434–2439. [DOI] [PubMed] [Google Scholar]
- 33.Laurent S, Boutouyrie P, Asmar R, et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37(5):1236–1241. [DOI] [PubMed] [Google Scholar]
- 34.London GM, Blacher J, Pannier B, Guerin AP, Marchais SJ, Safar ME. Arterial wave reflections and survival in end-stage renal failure. Hypertension. 2001;38(3):434–438. [DOI] [PubMed] [Google Scholar]
- 35.Mitchell GF, Moye LA, Braunwald E, et al. Sphygmomanometrically determined pulse pressure is a powerful independent predictor of recurrent events after myocardial infarction in patients with impaired left ventricular function. SAVE investigators. Survival and Ventricular Enlargement. Circulation. 1997;96(12):4254–4260. [DOI] [PubMed] [Google Scholar]
- 36.Safar ME. Systolic blood pressure, pulse pressure and arterial stiffness as cardiovascular risk factors. Curr Opin Nephrol Hypertens. 2001;10(2):257–261. [DOI] [PubMed] [Google Scholar]
- 37.van Popele NM, Grobbee DE, Bots ML, et al. Association between arterial stiffness and atherosclerosis: the Rotterdam Study. Stroke. 2001;32(2):454–460. [DOI] [PubMed] [Google Scholar]
- 38.Weber T, Auer J, O’Rourke MF, et al. Arterial stiffness, wave reflections, and the risk of coronary artery disease. Circulation. 2004;109(2):184–189. [DOI] [PubMed] [Google Scholar]
- 39.Smith GD, Ebrahim S. ‘Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol. 2003;32(1):1–22. [DOI] [PubMed] [Google Scholar]
- 40.Warren HR, Evangelou E, Cabrera CP, et al. Genome-wide association analysis identifies novel blood pressure loci and offers biological insights into cardiovascular risk. Nat Genet. 2017;49(3):403–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tobin MD, Sheehan NA, Scurrah KJ, Burton PR. Adjusting for treatment effects in studies of quantitative traits: antihypertensive therapy and systolic blood pressure. Stat Med. 2005;24(19):2911–2935. [DOI] [PubMed] [Google Scholar]
- 42.Bycroft C, Freeman C, Petkova D, et al. The UK Biobank resource with deep phenotyping and genomic data. Nature. 2018;562(7726):203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McCarthy S, Das S, Kretzschmar W, et al. A reference panel of 64,976 haplotypes for genotype imputation. Nat Genet. 2016;48(10):1279–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bycroft C, Freeman C, Petkova D, et al. Genome-wide genetic data on ~500,000 UK Biobank participants. bioRxiv. 2017. [Google Scholar]
- 45.Haworth S, Mitchell R, Corbin L, et al. Apparent latent structure within the UK Biobank sample has implications for epidemiological analysis. Nat Commun. 2019;10(1):333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Davies NM, Holmes MV, Davey Smith G. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ. 2018;362:k601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McLaren W, Pritchard B, Rios D, Chen Y, Flicek P, Cunningham F. Deriving the consequences of genomic variants with the Ensembl API and SNP Effect Predictor. Bioinformatics. 2010;26(16):2069–2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ganna A, Genovese G, Howrigan DP, et al. Ultra-rare disruptive and damaging mutations influence educational attainment in the general population. Nat Neurosci. 2016;19(12):1563–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoggart CJ, Parra EJ, Shriver MD, et al. Control of confounding of genetic associations in stratified populations. Am J Hum Genet. 2003;72(6):1492–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martin JS, Xu Z, Reiner AP, et al. HUGIn: Hi-C Unifying Genomic Interrogator. Bioinformatics. 2017;33(23):3793–3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Purcell S, Neale B, Todd-Brown K, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nikpay M, Goel A, Won HH, et al. A comprehensive 1,000 Genomes-based genome-wide association meta-analysis of coronary artery disease. Nat Genet. 2015;47(10):1121–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yavorska OO, Burgess S. MendelianRandomization: an R package for performing Mendelian randomization analyses using summarized data. Int J Epidemiol. 2017;46(6):1734–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Burgess S, Bowden J, Dudbridge F, Thompson SG. Robust instrumental variable methods using multiple candidate instruments with application to Mendelian randomization. ArXiv e-prints. 2016. https://ui.adsabs.harvard.edu/#abs/2016arXiv160603729B. Accessed June 01, 2016.
- 55.Webb TR, Erdmann J, Stirrups KE, et al. Systematic Evaluation of Pleiotropy Identifies 6 Further Loci Associated With Coronary Artery Disease. J Am Coll Cardiol. 2017;69(7):823–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Klarin D, Zhu QM, Emdin CA, et al. Genetic analysis in UK Biobank links insulin resistance and transendothelial migration pathways to coronary artery disease. Nat Genet. 2017;49(9):1392–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Consortium CAD, Deloukas P, Kanoni S, et al. Large-scale association analysis identifies new risk loci for coronary artery disease. Nat Genet. 2013;45(1):25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44(2):512–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aatola H, Koivistoinen T, Tuominen H, et al. Influence of Child and Adult Elevated Blood Pressure on Adult Arterial Stiffness: The Cardiovascular Risk in Young Finns Study. Hypertension. 2017;70(3):531–536. [DOI] [PubMed] [Google Scholar]
- 60.Georgiou K, Larentzakis AV, Khamis NN, Alsuhaibani GI, Alaska YA, Giallafos EJ. Can Wearable Devices Accurately Measure Heart Rate Variability? A Systematic Review. Folia Med (Plovdiv). 2018;60(1):7–20. [DOI] [PubMed] [Google Scholar]
- 61.Mitchell GF. Arterial stiffness and hypertension: chicken or egg? Hypertension. 2014;64(2):210–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cecelja M, Jiang B, Keehn L, et al. Arterial stiffening is a heritable trait associated with arterial dilation but not wall thickening: a longitudinal study in the twins UK cohort. Eur Heart J. 2018;39(24):2282–2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xu J, Shi GP. Vascular wall extracellular matrix proteins and vascular diseases. Biochim Biophys Acta. 2014;1842(11):2106–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li DY, Brooke B, Davis EC, et al. Elastin is an essential determinant of arterial morphogenesis. Nature. 1998;393(6682):276–280. [DOI] [PubMed] [Google Scholar]
- 65.Gupta RM, Hadaya J, Trehan A, et al. A Genetic Variant Associated with Five Vascular Diseases Is a Distal Regulator of Endothelin-1Gene Expression. Cell. 2017;170(3):522–533 e515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Surendran P, Drenos F, Young R, et al. Trans-ancestry meta-analyses identify rare and common variants associated with blood pressure and hypertension. Nat Genet. 2016;48(10):1151–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Emdin CA, Khera AV, Klarin D, et al. Phenotypic Consequences of a Genetic Predisposition to Enhanced Nitric Oxide Signaling. Circulation. 2018;137(3):222–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kessler T, Wobst J, Wolf B, et al. Functional Characterization of the GUCY1A3 Coronary Artery Disease Risk Locus. Circulation. 2017;136(5):476–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Erdmann J, Stark K, Esslinger UB, et al. Dysfunctional nitric oxide signalling increases risk of myocardial infarction. Nature. 2013;504(7480):432–436. [DOI] [PubMed] [Google Scholar]
- 70.Wilkinson IB, Qasem A, McEniery CM, Webb DJ, Avolio AP, Cockcroft JR. Nitric oxide regulates local arterial distensibility in vivo. Circulation. 2002;105(2):213–217. [DOI] [PubMed] [Google Scholar]
- 71.Stewart AD, Millasseau SC, Kearney MT, Ritter JM, Chowienczyk PJ. Effects of inhibition of basal nitric oxide synthesis on carotid-femoral pulse wave velocity and augmentation index in humans. Hypertension. 2003;42(5):915–918. [DOI] [PubMed] [Google Scholar]
- 72.McEniery CM, Qasem A, Schmitt M, Avolio AP, Cockcroft JR, Wilkinson IB. Endothelin-1 regulates arterial pulse wave velocity in vivo. J Am Coll Cardiol. 2003;42(11):1975–1981. [DOI] [PubMed] [Google Scholar]
- 73.Li JS, Lariviere R, Schiffrin EL. Effect of a nonselective endothelin antagonist on vascular remodeling in deoxycorticosterone acetate-salt hypertensive rats. Evidence for a role of endothelin in vascular hypertrophy. Hypertension. 1994;24(2):183–188. [DOI] [PubMed] [Google Scholar]
- 74.Amiri F, Virdis A, Neves MF, et al. Endothelium-restricted overexpression of human endothelin-1 causes vascular remodeling and endothelial dysfunction. Circulation. 2004;110(15):2233–2240. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Graphical Abstract: Epidemiologic and genetic associations of arterial stiffness with blood pressure and coronary artery disease.
Association between phenotypic ASI, and separately, genotypic ASI (from the ASI GRS), with systolic and diastolic blood pressures, as well as with incident and prevalent CAD. Blood pressure results are adjusted by age, sex, smoking status, prevalent hypercholesterolemia, prevalent diabetes, heart rate, vegetable intake, alcohol intake, and exercise frequency. Blood pressure effect estimates represent mmHg increase in blood pressure resulting from 1 SD increase in ASI phenotype, and separately, 1 SD increase in genetically-mediated ASI from the ASI GRS. Incident CAD associations in the UK Biobank, as well as prevalent CAD associations using the CARDIOGRAMplusC4D consortium, with phenotypic ASI and the ASI GRS are provided. Incident CAD results were derived using individual-level data from the UK Biobank and adjusting by cardiometabolic risk factors (age, sex, smoking status, prevalent hypertension, prevalent hypercholesterolemia, prevalent diabetes, heart rate, vegetable intake, alcohol intake, and exercise frequency). Prevalent CAD results were derived from summary-level genome-wide association data from the CARDIOGRAMplusC4D consortium using robust, penalized inverse-variance weighted 2-sample Mendelian randomization.
ASI = Arterial stiffness index, CAD = coronary artery disease, DBP = diastolic blood pressure, GRS = genetic risk score, SBP = systolic blood pressure, SD = standard deviation