Abstract
Most tissue-resident macrophage populations develop during embryogenesis, self-renew in the steady-state and expand during type 2 immunity. Whether shared mechanisms regulate the proliferation of macrophages in homeostasis and disease is unclear. Here we found that the transcription factor Bhlhe40 was required in a cell-intrinsic manner for the self-renewal and maintenance of large peritoneal macrophages (LPMs), but not that of other tissue-resident macrophages. Bhlhe40 was necessary for the proliferation, but not the polarization, of LPMs in response to the cytokine IL-4. During infection with the helminth Heligmosomoides polygyrus bakeri, Bhlhe40 was required for cell cycling of LPMs. Bhlhe40 repressed the expression of genes encoding the transcription factors c-Maf and Mafb and directly promoted expression of transcripts encoding cell cycle-related proteins to enable the proliferation of LPMs. In LPMs, Bhlhe40 bound to genomic sites co-bound by the macrophage lineage-determining factor PU.1 and to unique sites, including Maf and loci encoding cell cycle-related proteins. Our findings demonstrate a tissue-specific control mechanism that regulates the proliferation of resident macrophages in homeostasis and type 2 immunity.
Tissue-resident macrophages are established during embryogenesis1,2,3 and are largely maintained by local self-renewal within each organ4,5. While some transcription factors specifying distinct macrophage lineages have been described3, differences in the transcriptional basis for self-renewal in distinct macrophage populations are not well understood. Established regulators of self-renewal in multiple macrophage lineages include the anti-proliferative transcription factors c-Maf and MafB6,7, as well as the pro-proliferative deacetylase Sirtuin18. The transcription factor GATA6 may exercise tissue-specific control of macrophage self-renewal, as loss of GATA6 causes large peritoneal macrophages (LPMs) to become multinucleated and impairs their proliferation9. However, deletion of GATA6 also causes changes in the morphology, surface markers and gene expression profile of LPMs9,10,11, illustrating that the study of tissue-specific control of resident macrophage self-renewal can be confounded by significant effects on macrophage identity. It remains unclear to what extent macrophage self-renewal is regulated in a tissue-specific manner and whether any tissue-specific regulation that does exist cooperates with broadly shared regulators.
In addition to their homeostatic self-renewal capacity, resident macrophages can become alternatively activated in response to type 2 cytokines produced in response to stimuli like helminth infection, resulting in dramatic proliferation concomitant with acquisition of a pro-repair or anti-helminth protein expression profile12,13,14,15. Until recently, proliferation of all macrophages elicited by type 2 immunity was assumed to be controlled by cytokine signaling through JAK kinases and STAT transcription factors. While it remains unclear whether tissue-specific, cell-intrinsic transcriptional regulation influences this process, the collagens SP-A and C1q act through the receptor Myo18a to mediate extrinsic, tissue-specific regulation of proliferation and alternative activation15. Signaling through receptors for apoptotic cells also influences these processes in tissue-resident macrophages16. Furthermore, differences in alternative activation between monocyte-derived and resident macrophages indicate that ontogeny influences responses during type 2 immunity17,18. Whether there are common regulators of macrophage self-renewal at steady-state and proliferation during disease remains unknown.
The transcription factor Bhlhe40 is expressed in some hematopoietic and non-hematopoietic cell types19,20, including select resident macrophage populations21. Bhlhe40 binds to DNA at class B E-box motifs and functions primarily as a transcriptional repressor22,23, although examples of transcriptional activation have been described24,25. Bhlhe40 is dysregulated in some cancers and may regulate cell cycling in specific contexts20. A variety of hematopoietic cell types are regulated by Bhlhe40, including NKT cells and B cells26,27,28,29, and it controls cytokine production in T cells during infection and autoimmunity21,30,31,32,33. Bhlhe40 and c-Maf may be interconnected in the regulation of the cytokine IL-10, but how this would occur is unclear32,34. Despite an emerging view that Bhlhe40 is an important regulator of immunity, little is known regarding its role in myeloid cells. Bhlhe40 has been proposed as a tissue-specific binding partner of PU.1 in LPMs, but this has not been directly tested35.
Here we found that Bhlhe40 had a unique and cell-intrinsic role in LPMs to regulate self-renewal, proliferation and accumulation during type 2 immunity. In LPMs, Bhlhe40 bound a subset of genomic sites bound by the macrophage lineage-specifying transcription factor PU.1, but also many unique sites, including loci encoding cell cycle-related proteins such as c-Maf. Loss of Bhlhe40 in LPMs led to higher expression of Maf and Mafb mRNA and lower expression of cell cycle-related transcripts. Our findings establish Bhlhe40 as a tissue-specific transcriptional regulator of LPM proliferation active in both homeostatic self-renewal and upon rapid cell cycling during type 2 immunity.
Results
Loss of Bhlhe40 selectively reduces LPMs
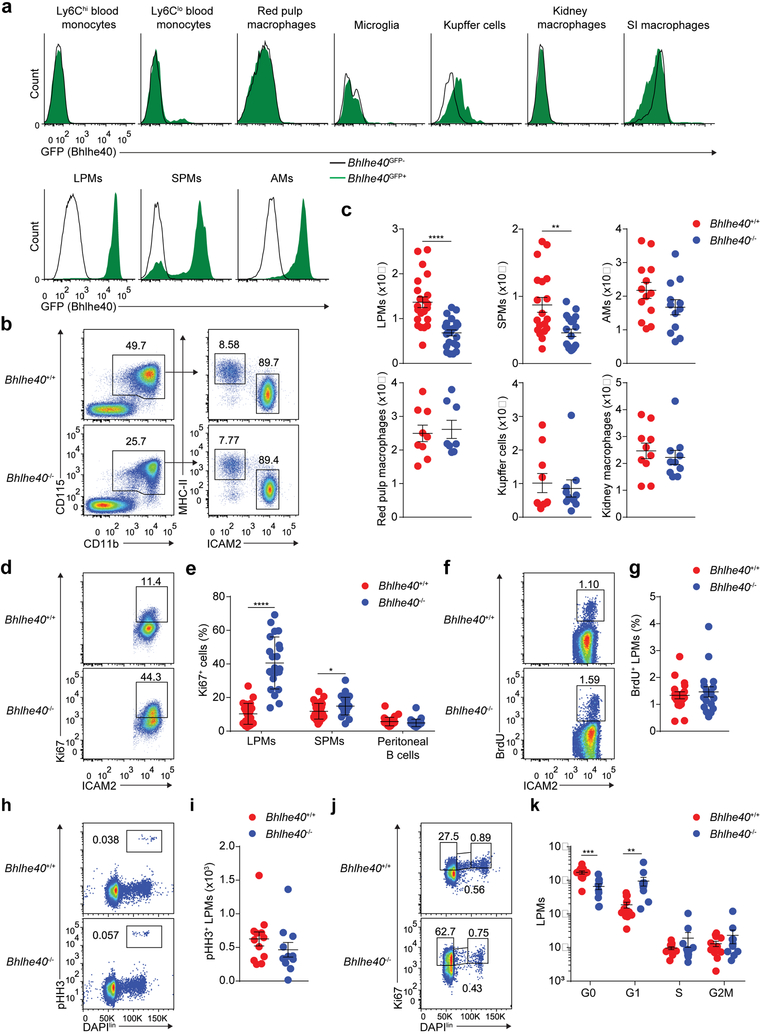
Because Bhlhe40 expression has been observed in select resident macrophage populations21,36,37, we examined macrophages from mice transgenic for a Bhlhe40GFP bacterial artificial chromosome (Bhlhe40GFP+ mice hereafter)21. We observed low or undetectable GFP expression in Ly6G−CD115+ Ly6Chi and Ly6Clo blood monocytes, F4/80hi splenic red pulp macrophages (hereafter red pulp macrophages), CD45intCD11b+ central nervous system microglia, CD45+CD11bloF4/80hi liver Kupffer cells (hereafter Kupffer cells), CD45+Ly6C−CD11b+F4/80hi kidney macrophages (hereafter kidney macrophages) and CD45+Ly6C−F4/80+CD64+MHC-II+ small intestinal lamina propria macrophages (hereafter SI macrophages), but found high expression of GFP in CD45+Siglec-F+CD11c+ lung alveolar macrophages (hereafter AMs), CD115+CD11b+ICAM2+MHC-IIint large peritoneal macrophages (hereafter LPMs), CD115+CD11b+MHC-II+ICAM2− small peritoneal macrophages (hereafter SPMs), CD115+CD11b+ICAM2+MHC-IIint large pleural macrophages (hereafter large pleural macrophages) and CD115+CD11b+MHC-II+ICAM2− small pleural macrophages (hereafter small pleural macrophages) (Fig. 1a and Supplementary Fig. 1). Of the populations examined, only LPMs and SPMs were decreased in Bhlhe40−/− compared to Bhlhe40+/+ mice (Fig. 1b,c and Supplementary Fig. 1). In some resident macrophage populations, including LPMs, Tim4 is a marker of embryonically-derived, long-lived resident macrophages38,39,40, while CD226 marks mature SPMs41. Decreases in Tim4+ LPMs and CD226+ SPMs largely accounted for the reduced number of peritoneal macrophages in Bhlhe40−/− mice (Supplementary Fig. 1). The number of peritoneal CD115−MHC-II+CD19+ B cells (hereafter B cells) was not reduced in Bhlhe40−/− compared to Bhlhe40+/+ mice (Supplementary Fig. 1). Therefore, loss of Bhlhe40 selectively reduced the number of LPMs and SPMs.
Figure 1. Loss of Bhlhe40 dysregulates the cell cycle in LPMs.
a, Flow cytometry of Bhlhe40GFP transgene reporter expression in blood monocytes (representative of 2 experiments, n=5 Bhlhe40GFP+, 2 Bhlhe40GFP-); red pulp macrophages, microglia, Kupffer cells, kidney macrophages, SI macrophages, and peritoneal macrophages (representative of 2 experiments, n=4 Bhlhe40GFP+, 2 Bhlhe40GFP-); and AMs (representative of 3 experiments, n=6 Bhlhe40GFP+, 3 Bhlhe40GFP-) from Bhlhe40GFP+ and Bhlhe40GFP- mice. b, Flow cytometry of peritoneal macrophage subsets in Bhlhe40+/+ and Bhlhe40−/− mice (representative of 6 experiments, n=22/group). c, Numbers of LPMs as in b, SPMs (pooled from 5 experiments, n=19/group), AMs (pooled from 4 experiments, n=13 Bhlhe40+/+, 12 Bhlhe40−/−), red pulp macrophages (pooled from 3 experiments, n=9 Bhlhe40+/+, 8 Bhlhe40−/−), Kupffer cells, and kidney macrophages (both pooled from 2 experiments, n=10/group) from Bhlhe40+/+ and Bhlhe40−/− mice. d, Flow cytometry of Ki67 expression by Bhlhe40+/+ and Bhlhe40−/− LPMs (representative of 7 experiments, n=24 Bhlhe40+/+, 22 Bhlhe40−/−). e, Frequency of Ki67+ LPMs as in d, SPMs, and B cells (both pooled from 8 experiments, n=30 Bhlhe40+/+, 29 Bhlhe40−/−) from Bhlhe40+/+ and Bhlhe40−/− mice. f, Flow cytometry of BrdU incorporation by Bhlhe40+/+ and Bhlhe40−/− LPMs (representative of 5 experiments, n=18/group). g, Frequency of BrdU+ LPMs as in f. h, Flow cytometry of pHH3 expression by Bhlhe40+/+ and Bhlhe40−/− LPMs (representative of 4 experiments, n=12 Bhlhe40+/+, 11 Bhlhe40−/−). i, Frequency of pHH3+ LPMs as in h. j, Flow cytometry for discrimination of cell cycle phases of Bhlhe40+/+ and Bhlhe40−/− LPMs (representative of 4 experiments, n=12 Bhlhe40+/+, 11 Bhlhe40−/−). k, Numbers of LPMs in each phase of the cell cycle as in j. Data in c,e,g,i,k are mean ± s.e.m; each symbol represents an individual mouse (c,e,g,i,k). *P ≤ 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001, unpaired two-sided Student’s t-test.
Bhlhe40 is required in LPMs for self-renewal
To address whether the loss of LPMs in Bhlhe40−/− mice was due to impaired proliferation, we stained peritoneal cells from Bhlhe40+/+ and Bhlhe40−/− mice for Ki67, a marker of cycling cells. We observed a 4-fold increase in the frequency of Ki67+ LPMs (Fig. 1d,e), but little change in the frequency of Ki67+ SPMs and peritoneal B cells in Bhlhe40−/− compared to Bhlhe40+/+ mice (Fig. 1e). Despite normal numbers, we found an increase in the frequency of Ki67+ large pleural macrophages in Bhlhe40−/− compared to Bhlhe40+/+ mice (Supplementary Fig. 1). There was no difference in the uptake of bromodeoxyuridine (BrdU), which is incorporated during the S phase, in LPMs from Bhlhe40+/+ and Bhlhe40−/− mice 3 hours after injection with BrdU (Fig. 1f,g). Staining for the mitosis marker phosphohistone H3 (pHH3) was similar in Bhlhe40+/+ and Bhlhe40−/− LPMs (Fig. 1h,i). When using Ki67 and the nuclear stain 4′,6-diamidino-2-phenylindole (DAPI) to separate the phases of the cell cycle, we observed an increased number of LPMs in the G1 phase (Fig. 1j,k), but similar numbers of LPMs in the S, G2 and M phases in Bhlhe40−/− compared to Bhlhe40+/+ mice (Fig. 1j,k), suggesting that Bhlhe40−/− LPMs were impaired in progressing from G1, but proliferated sufficiently to maintain a stable, although reduced, population of LPMs. The proportion of LPMs staining for the viability dye 7-aminoactinomycin D (7-AAD) was similar in Bhlhe40+/+ and Bhlhe40−/− mice (Supplementary Fig. 1). Taken together, these data indicate that Bhlhe40 was required for normal proliferation of LPMs.
Bhlhe40 is required intrinsically in LPMs for cell cycling
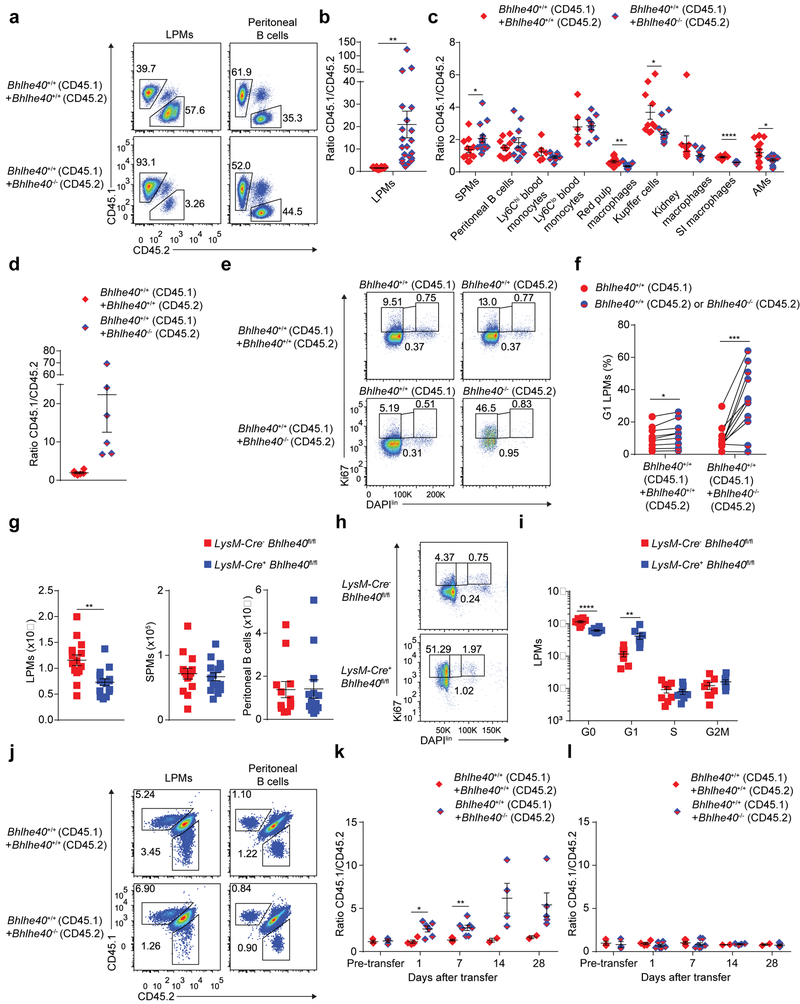
To address whether the role of Bhlhe40 in LPMs was cell-intrinsic, we generated mixed bone marrow chimeras by co-transfer of equal numbers of Bhlhe40+/+ (CD45.1) plus either Bhlhe40+/+ (CD45.2) or Bhlhe40−/− (CD45.2) total bone marrow cells into irradiated Bhlhe40+/+ CD45.1/CD45.2 mice, which were allowed to reconstitute for >8 weeks. Out of peritoneal, blood, splenic, liver, kidney, SI lamina propria, lung and pleural hematopoietic populations examined, only LPMs and large pleural macrophages exhibited Bhlhe40-dependent reconstitution (Bhlhe40+/+ outnumbering Bhlhe40−/− cells by more than 10:1) (Fig. 2a–d, Supplementary Fig. 2 and data not shown). The small number of Bhlhe40−/− LPMs in mixed chimeras accumulated in the G1 phase (Fig. 2e,f), indicating that the alterations in cell cycling in Bhlhe40−/− LPMs were cell-intrinsic. Next, we bred LysM-Cre+ Bhlhe40fl/fl mice to delete Bhlhe40 in myeloid cells. Compared to LysM-Cre− Bhlhe40fl/fl mice, LysM-Cre+ Bhlhe40fl/fl mice had a nearly 2-fold reduction in the number of LPMs, with no change in SPMs or peritoneal B cells (Fig. 2g) and an increased proportion of LPMs in the G1 phase (Fig. 2h,i). Finally, we co-transferred Bhlhe40+/+ (CD45.1) plus either Bhlhe40+/+ (CD45.2) or Bhlhe40−/− (CD45.2) bulk peritoneal cells into resting Bhlhe40+/+ (CD45.1/CD45.2) mice at ratios calculated to result in the transfer of equal numbers of LPMs (200,000–300,000) from each donor. Over four weeks, the relative proportion of transferred Bhlhe40+/+ (CD45.1) to Bhlhe40−/− (CD45.2) LPMs was increased (Fig. 2j,k and Supplementary Fig. 2), while the relative proportion of transferred Bhlhe40+/+ (CD45.1) to transferred Bhlhe40−/− (CD45.2) B cells was maintained in the peritoneum (Fig. 2j,l and Supplementary Fig. 2), supporting a cell-intrinsic role for Bhlhe40 in mature LPMs. Thus, Bhlhe40 was cell-intrinsically required in LPMs for normal proliferation and maintenance.
Figure 2. Bhlhe40 is cell-intrinsically required in LPMs to regulate the cell cycle.
a, Flow cytometry for the discrimination of donor and recipient LPMs (representative of 6 experiments, n=18 [Bhlhe40+/+ (CD45.1) +Bhlhe40+/+ (CD45.2)], 21 [Bhlhe40+/+ (CD45.1) +Bhlhe40−/− (CD45.2)]) and peritoneal B cells (representative of 4 experiments, n=12 [Bhlhe40+/+ (CD45.1) +Bhlhe40+/+ (CD45.2)], 13 [Bhlhe40+/+ (CD45.1) +Bhlhe40−/− (CD45.2)]) from Bhlhe40+/+ (CD45.1) plus either Bhlhe40+/+ (CD45.2) or Bhlhe40−/− (CD45.2) mixed bone marrow chimeras. b, Ratio of CD45.1 to CD45.2 LPMs as in a. c, Ratios of CD45.1 cells to CD45.2 cells for SPMs (pooled from 4 experiments, n=12 [Bhlhe40+/+ (CD45.1) +Bhlhe40+/+ (CD45.2)], 13 [Bhlhe40+/+ (CD45.1) +Bhlhe40−/− (CD45.2)]), peritoneal B cells as in a, blood monocytes (pooled from 2 experiments, n=6 [Bhlhe40+/+ (CD45.1) +Bhlhe40+/+ (CD45.2)], 8 [Bhlhe40+/+ (CD45.1) +Bhlhe40−/− (CD45.2)]), red pulp macrophages, AMs (both pooled from 6 experiments, n=13 [Bhlhe40+/+ (CD45.1) +Bhlhe40+/+ (CD45.2)], 15 [Bhlhe40+/+ (CD45.1) +Bhlhe40−/− (CD45.2)]), Kupffer cells, kidney macrophages (both pooled from 3 experiments, n=10 [Bhlhe40+/+ (CD45.1) +Bhlhe40+/+ (CD45.2)], 11 [Bhlhe40+/+ (CD45.1) +Bhlhe40−/− (CD45.2)]), and SI macrophages (pooled from 2 experiments, n=6 [Bhlhe40+/+ (CD45.1) +Bhlhe40+/+ (CD45.2)], 7 [Bhlhe40+/+ (CD45.1) +Bhlhe40−/− (CD45.2)]) from mixed bone marrow chimeras. d, Ratio of CD45.1 to CD45.2 large pleural macrophages (pooled from 2 experiments, n=6/group) from mixed bone marrow chimeras. e, Flow cytometry for discrimination of cell cycle phases of Bhlhe40+/+ (CD45.1), Bhlhe40+/+ (CD45.2), or Bhlhe40−/− (CD45.2) LPMs from mixed bone marrow chimeras (representative of 3 experiments, n=9 [Bhlhe40+/+ (CD45.1) +Bhlhe40+/+ (CD45.2)], 11 [Bhlhe40+/+ (CD45.1) +Bhlhe40−/− (CD45.2)]). f, Frequency of G1 LPMs as in e, with LPMs from each donor recovered from the same recipient connected by a line. g, Numbers of LPMs, SPMs (both pooled from 6 experiments, n=14 LysM-Cre− Bhlhe40fl/fl, 15 LysM-Cre+ Bhlhe40fl/fl) and peritoneal B cells (5 experiments, n=12 LysM-Cre− Bhlhe40fl/fl, 13 LysM-Cre+ Bhlhe40fl/fl) from LysM-Cre− Bhlhe40fl/fl and LysM-Cre+ Bhlhe40fl/fl mice. h, Flow cytometry for discrimination of cell cycle phases of LysM-Cre− Bhlhe40fl/fl and LysM-Cre+ Bhlhe40fl/fl LPMs (representative of 3 experiments, n=8/group). i, Numbers of LPMs in each phase of the cell cycle as in h. j, Flow cytometry for the discrimination of donor and recipient LPMs and peritoneal B cells from CD45.1/CD45.2 recipients of mixed peritoneal cells transferred from Bhlhe40+/+ (CD45.1) plus either Bhlhe40+/+ (CD45.2) or Bhlhe40−/− mice (CD45.2) mice (representative of 2 experiments, n=3 [Bhlhe40+/+ (CD45.1) +Bhlhe40+/+ (CD45.2)], 5 [Bhlhe40+/+ (CD45.1) +Bhlhe40−/− (CD45.2)]). k,l, Ratio of CD45.1 LPMs to CD45.2 LPMs (k) and CD45.1 peritoneal B cells to CD45.2 peritoneal B cells (l) as in j (pooled from 2–3 experiments, n≥3 for all time points, except day 14 [Bhlhe40+/+ (CD45.1) +Bhlhe40+/+ (CD45.2)], n=2). Data in b,c,d,f,g,i,k,l are mean ± s.e.m; (b,c,d,g,i,k,l) each symbol or (f) paired symbols represent an individual mouse. *P ≤ 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001, unpaired (b,c,d,g,i,k,l) or paired (f) two-sided Student’s t-test.
Loss of Bhlhe40 dysregulates a unique set of genes in LPMs
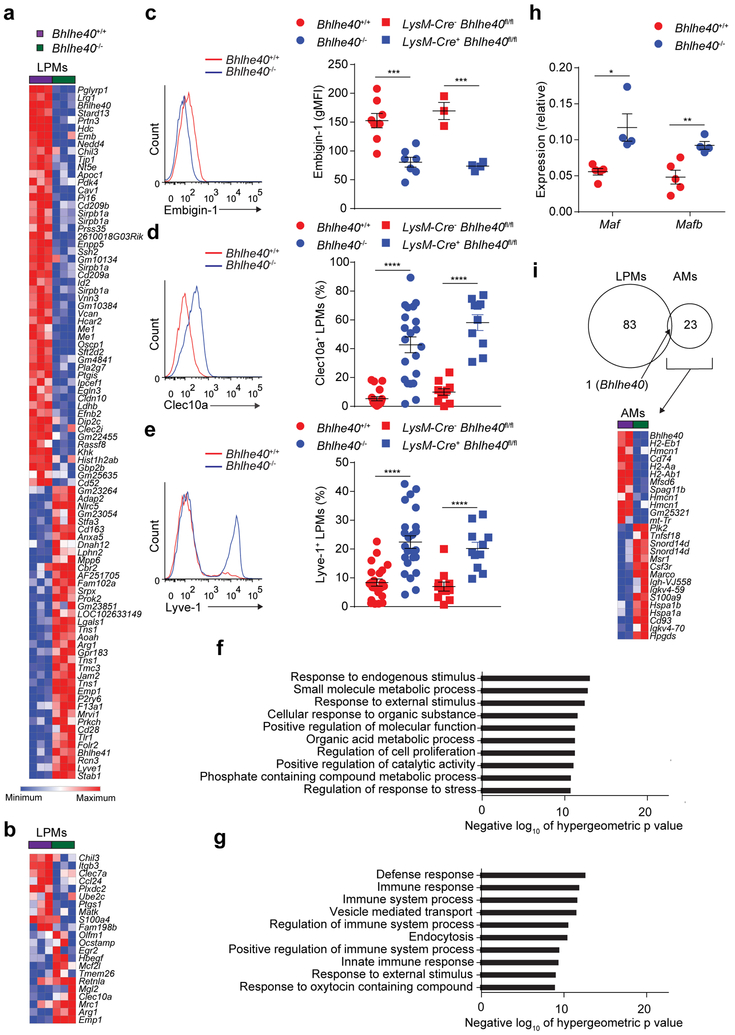
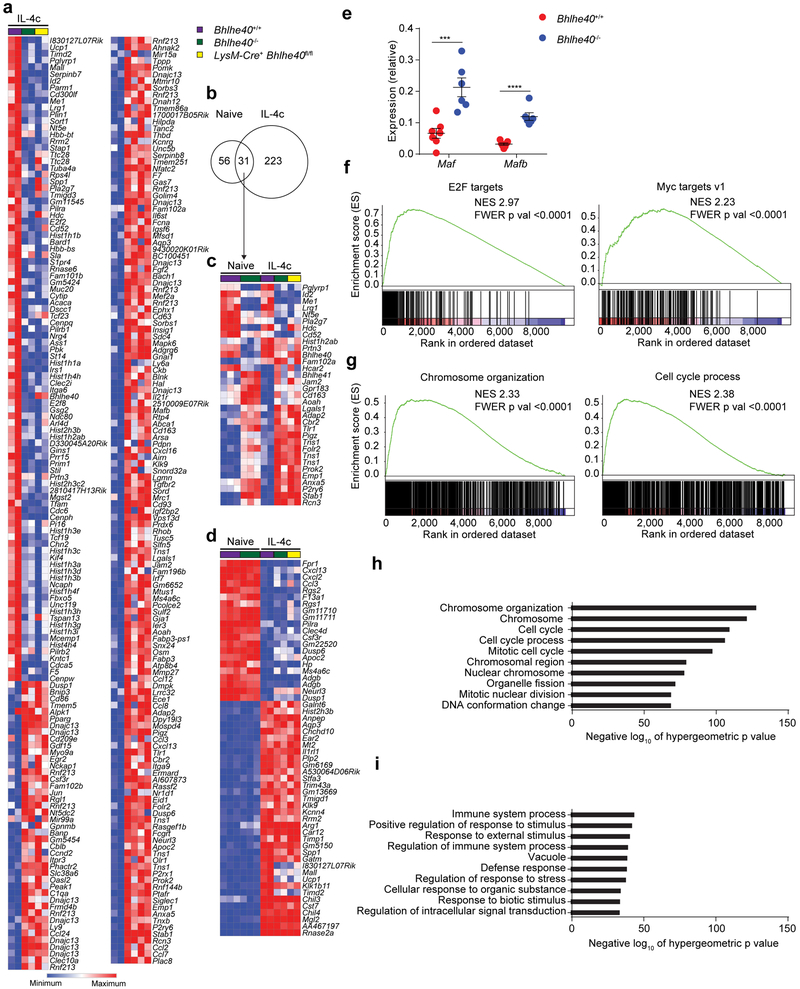
Next, we performed gene expression microarrays to determine the transcriptional differences between LPMs from Bhlhe40+/+ and Bhlhe40−/− mice. 84 genes were dysregulated by 2-fold or more in Bhlhe40−/− compared to Bhlhe40+/+ LPMs (Fig. 3a), including expression changes in several genes related to macrophage alternative activation, such as Chil3, Clec10a, Mrc1 and Arg1 (Fig. 3b). We validated these data by flow cytometry for several proteins encoded by differentially expressed genes (Emb, Clec10a, Lyve1) (Fig. 3c–e). When we assessed the expression of gene ontology sets42 in the absence of Bhlhe40 using the list of genes that were differentially expressed ≥1.5-fold between Bhlhe40+/+ and Bhlhe40−/− LPMs, we found that the Regulation of cell proliferation gene set was enriched in Bhlhe40+/+ LPMs, while some immune response-related gene sets were enriched in Bhlhe40−/− LPMs (Fig. 3f,g). We validated higher expression of Maf and Mafb mRNA in Bhlhe40−/− compared to Bhlhe40+/+ LPMs in our microarrays (data not shown) by qRT-PCR (Fig. 3h), consistent with impaired proliferation of Bhlhe40−/− LPMs.
Figure 3. Bhlhe40 regulates a distinct set of genes related to alternative activation in LPMs.
a,b, Gene expression microarray data were analyzed for genes differentially expressed by ≥2-fold (a) and expression of a macrophage alternative activation gene signature in Bhlhe40+/+ and Bhlhe40−/− LPMs (b). c, Flow cytometry of Embigin-1 expression and quantitation of geometric mean fluorescence intensity (gMFI) on LPMs (pooled from 4 experiments, n=8 Bhlhe40+/+, 7 Bhlhe40−/−; 1 experiment, n=3 LysM-Cre− Bhlhe40fl/fl, 4 LysM-Cre+ Bhlhe40fl/fl). d, Flow cytometry of Clec10a expression and frequency of Clec10a+ LPMs (pooled from 6 experiments, n=19 Bhlhe40+/+ and 21 Bhlhe40−/−; 4 experiments, n=10 LysM-Cre− Bhlhe40fl/fl and LysM-Cre+ Bhlhe40fl/fl). e Flow cytometry of Lyve-1 expression and frequency of Lyve-1+ LPMs (pooled from 7 experiments, n=22 Bhlhe40+/+, 24 Bhlhe40−/−; 5 experiments, n=11 LysM-Cre− Bhlhe40fl/fl and LysM-Cre+ Bhlhe40fl/fl) from Bhlhe40+/+, Bhlhe40−/−, LysM-Cre− Bhlhe40fl/fl, and LysM-Cre+ Bhlhe40fl/fl LPMs. f,g, MSigDB C5 gene set enrichment was analyzed using the lists of genes expressed at ≥1.5-fold in Bhlhe40+/+ vs. Bhlhe40−/− (f) or Bhlhe40−/− vs. Bhlhe40+/+ LPMs (g). h, qRT-PCR of Maf and Mafb expression relative to Hprt in Bhlhe40+/+ and Bhlhe40−/− LPMs (pooled from 2 experiments, n=5 Bhlhe40+/+, 4 Bhlhe40−/−). i, Gene expression microarray data were analyzed for shared and unique Bhlhe40-dependent genes in Bhlhe40+/+ and Bhlhe40−/− LPMs and AMs (≥2-fold differentially expressed, depicted as a Venn diagram). Heat map depicts all genes differentially expressed by ≥2-fold in Bhlhe40+/+ and Bhlhe40−/− AMs. Microarray data from LPMs (n=3/group) and AMs (n=2/group) are from a single experiment. Data in c-e,h are mean ± s.e.m; each symbol represents an individual mouse (c-e,h). *P ≤ 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001, (c-e,h) unpaired two-sided Student’s t-test and (f,g) one-sided hypergeometric test.
Because the transcription factor GATA6 is an important regulator of LPMs9,10,11, we reanalyzed our microarray data and published microarray data11 from LysM-Cre− Gata6fl/fl and LysM-Cre+ Gata6fl/fl LPMs to look for differentially expressed genes regulated by both transcription factors. Expression of Gata6 mRNA was not substantially changed in Bhlhe40−/− LPMs (log2 expression, Bhlhe40+/+ 10.24, Bhlhe40−/− 10.10; data not shown), nor did loss of GATA6 cause substantial changes in the expression of Bhlhe40 in LPMs (log2 expression, LysM-Cre− Gata6fl/fl 10.04, LysM-Cre+ Gata6fl/fl 9.81; data not shown). Furthermore, the majority of Bhlhe40-dependent genes were not dependent on GATA6 and the converse was likewise true (Supplementary Fig. 2).
Next, we performed transcriptome analysis of AMs, which have high expression of Bhlhe40, from Bhlhe40+/+ and Bhlhe40−/− mice. Compared to LPMs, Bhlhe40 controlled a largely distinct and smaller group of genes in AMs, mostly encoding proteins involved in antigen presentation by MHC class II (H2-Aa, H2-Ab1, H2-Eb1, Cd74; Fig. 3i). A set of genes selectively expressed in LPMs relative to AMs, splenic red pulp macrophages and microglia has been previously curated36. Bhlhe40 regulated the expression of only a small subset of these genes (Lrg1, Stard13, Nedd4) (Supplementary Fig. 2). Therefore, Bhlhe40 regulated a cell type-specific set of genes in LPMs, but was dispensable for identity.
Bhlhe40 is required for LPM responses during type 2 immunity
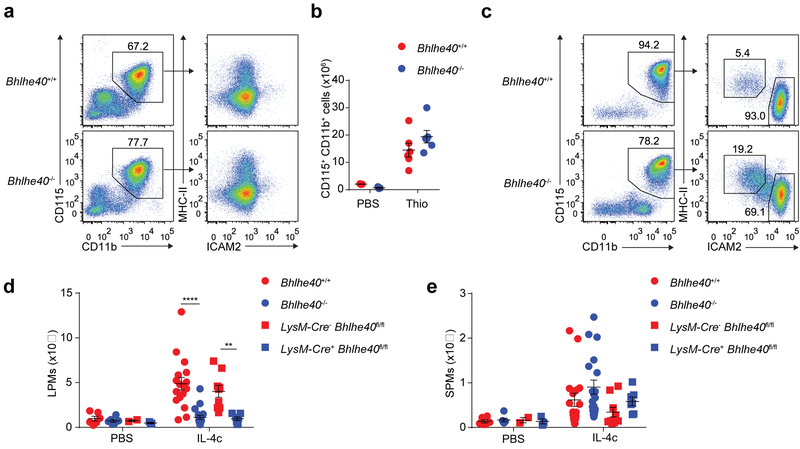
We then asked whether Bhlhe40 was required for macrophage accumulation during peritoneal immune responses characterized either by the differentiation of monocyte-derived macrophages or the local proliferation of LPMs. Intraperitoneal (i.p.) injection of thioglycollate, which elicits the recruitment and differentiation of blood-derived monocytes to the peritoneum independent of proliferation17,43, resulted in equivalent accumulation of CD115+CD11b+ICAM2lo thioglycollate-elicited macrophages (hereafter thioglycollate-elicited macrophages) in Bhlhe40+/+ and Bhlhe40−/− mice after 4 days (Fig. 4a,b), while the i.p injection of IL-4+anti-IL-4 antibody complexes (hereafter IL-4c), which elicit the robust proliferation of resident macrophages12, caused a 5-fold increase in the number of LPMs in Bhlhe40+/+ mice compared to Bhlhe40−/− mice after 4 days (Fig. 4c,d). Similar findings were obtained in LysM-Cre− Bhlhe40fl/fl and LysM-Cre+ Bhlhe40fl/fl mice (Fig. 4d), suggesting Bhlhe40 was required for the proliferation of resident LPMs in a cell-intrinsic manner. SPMs were not reduced in Bhlhe40−/− and LysM-Cre+ Bhlhe40fl/fl compared to Bhlhe40+/+ and LysM-Cre− Bhlhe40fl/fl mice in response to IL-4c (Fig. 4e). Because Bhlhe40 represses the production of IL-10 in T cells and myeloid cells21,31,32,33, we injected IL-4c i.p. into Bhlhe40−/− Il10−/− mice to test whether lack of IL-10 restored the IL-4c-driven accumulation of LPMs in the absence of Bhlhe40. Similar to Bhlhe40−/− mice, Bhlhe40−/− Il10−/− mice had poor accumulation of LPMs after injection of IL-4c (Supplementary Fig. 3), indicating IL-10 did not contribute to the impaired response of Bhlhe40−/− LPMs. Taken together, these data indicate that Bhlhe40 was required for normal accumulation of LPMs in response to IL-4c.
Figure 4. Bhlhe40 is required for normal accumulation of resident, but not recruited, macrophages in the peritoneum.
a, Flow cytometry of peritoneal macrophage subsets from Bhlhe40+/+ and Bhlhe40−/− mice treated with thioglycollate (Thio) (representative of 3 experiments, n=6/group). b, Numbers of CD115+CD11b+ peritoneal macrophages from Bhlhe40+/+ and Bhlhe40−/− mice treated with PBS or Thio as in a (pooled from 3 experiments, n=3/group for PBS, 6/group for Thio). c, Flow cytometry of peritoneal macrophage subsets from Bhlhe40+/+ and Bhlhe40−/− mice treated with IL-4c (representative of 6 experiments, 17 IL-4c-treated Bhlhe40+/+, 19 IL-4c-treated Bhlhe40−/−). d,e, Numbers of LPMs (d) and SPMs (e) as in c from Bhlhe40+/+, Bhlhe40−/−, LysM-Cre− Bhlhe40fl/fl, and LysM-Cre+ Bhlhe40fl/fl mice treated with PBS or IL-4c (pooled from 5 experiments, 6 PBS-treated Bhlhe40+/+ and Bhlhe40−/−; IL-4c-treated as in c for Bhlhe40+/+ and Bhlhe40−/−; 2 experiments, 2 PBS-treated LysM-Cre− Bhlhe40fl/fl; 3 PBS-treated LysM-Cre+ Bhlhe40fl/fl; 9 IL-4c-treated LysM-Cre− Bhlhe40fl/fl; 7 IL-4c-treated LysM-Cre+ Bhlhe40fl/fl). Data in b,d,e are mean ± s.e.m; each symbol represents an individual mouse (b,d,e). **P < 0.01; ****P < 0.0001, unpaired two-sided Student’s t-test.
Bhlhe40 regulates proliferation, but not polarization, of LPMs
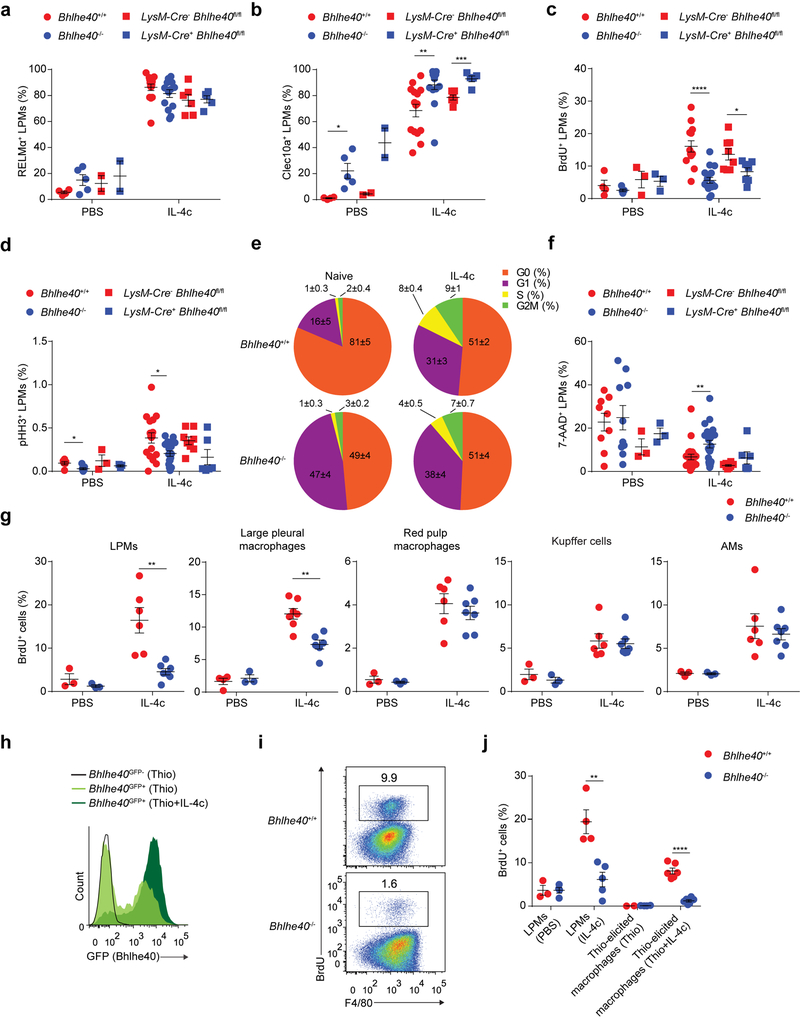
We next assessed whether Bhlhe40 regulated induction of the alternative activation markers RELMα and Clec10a in LPMs in response to IL-4c. LPMs from Bhlhe40+/+, Bhlhe40−/−, LysM-Cre− Bhlhe40fl/fl and LysM-Cre+ Bhlhe40fl/fl mice all induced these proteins following i.p. injection with IL-4c (Fig. 5a,b). In contrast, IL-4c increased the proportions of BrdU+ LPMs and pHH3+ LPMs by approximately 2-fold in Bhlhe40+/+ and LysM-Cre− Bhlhe40fl/fl mice compared to Bhlhe40−/− and LysM-Cre+ Bhlhe40fl/fl mice (Fig. 5c,d). IL-4c treatment also elicited a greater increase in the fraction of LPMs in the G1, S and G2M phases of the cell cycle in Bhlhe40+/+ compared to Bhlhe40−/− mice (Fig. 5e). Immunoblot analysis of cyclins D1–D3, cyclin dependent kinase (CDK) 2, CDK4, CDK6 and the transcription factor E2F2, which regulate the G1 and S phases of the cell cycle44, showed increases in cyclin D3, CDK2 and CDK4 in LPMs from IL-4c-treated compared to naïve mice (Supplementary Fig. 3); however, their abundance was generally similar in Bhlhe40+/+ and Bhlhe40−/− LPMs (Supplementary Fig. 3). In contrast to cyclins and CDKs, E2F2 was similar in LPMs from mice injected or not with IL-4c (Supplementary Fig. 3). 7-AAD+ necrotic LPMs were somewhat increased in IL-4c-injected Bhlhe40−/− and LysM-Cre+ Bhlhe40fl/fl mice compared to Bhlhe40+/+ and LysM-Cre− Bhlhe40fl/fl mice (Fig. 5f). In mixed bone marrow chimeras (generated and reconstituted as in Fig. 2a–f) injected i.p. with IL-4c, a lower proportion of Bhlhe40−/− LPMs incorporated BrdU compared to Bhlhe40+/+ LPMs within the same recipient (Supplementary Fig. 3).
Figure 5. Bhlhe40 is required for normal cycling, but not polarization, of peritoneal macrophages during type 2 immunity.
a-d, Frequency of RELMα+ LPMs (a) (pooled from 3 experiments, n=4 PBS-treated Bhlhe40+/+ and Bhlhe40−/−; 15 IL-4c-treated Bhlhe40+/+ and Bhlhe40−/−; 2 experiments, 2 PBS-treated LysM-Cre− Bhlhe40fl/fl and LysM-Cre+ Bhlhe40fl/fl; 6 IL-4c-treated LysM-Cre− Bhlhe40fl/fl; 5 IL-4c-treated LysM-Cre+ Bhlhe40fl/fl), Clec10a+ LPMs (b) (pooled as in a), BrdU+ LPMs (c) (pooled from 3 experiments, n=4 PBS-treated Bhlhe40+/+; 5 PBS-treated Bhlhe40−/−; 13 IL-4c-treated Bhlhe40+/+; 17 IL-4c-treated Bhlhe40−/−; 3 PBS-treated LysM-Cre− Bhlhe40fl/fl and LysM-Cre+ Bhlhe40fl/fl; 8 IL-4c-treated LysM-Cre− Bhlhe40fl/fl; 7 IL-4c-treated LysM-Cre+ Bhlhe40fl/fl), and pHH3+ LPMs (d) (pooled from 3 experiments, n=6 PBS-treated Bhlhe40+/+; 7 PBS-treated Bhlhe40−/−; 16 IL-4c-treated Bhlhe40+/+; 18 IL-4c-treated Bhlhe40−/−; 3 PBS-treated LysM-Cre− Bhlhe40fl/fl and LysM-Cre+ Bhlhe40fl/fl; 8 IL-4c-treated LysM-Cre− Bhlhe40fl/fl; 6 IL-4c-treated LysM-Cre+ Bhlhe40fl/fl) from Bhlhe40+/+, Bhlhe40−/−, LysM-Cre− Bhlhe40fl/fl, and LysM-Cre+ Bhlhe40fl/fl mice treated with PBS or IL-4c. e, Proportion of LPMs in each phase of the cell cycle from Bhlhe40+/+ and Bhlhe40−/− mice unstimulated or treated with IL-4c (pooled from 5 experiments, n=6/group for unstimulated; 16 IL-4c-treated Bhlhe40+/+; 15 IL-4c-treated Bhlhe40−/−). f, Frequency of 7-AAD+ LPMs from Bhlhe40+/+, Bhlhe40−/−, LysM-Cre− Bhlhe40fl/fl, and LysM-Cre+ Bhlhe40fl/fl mice treated with PBS or IL-4c (pooled from 3 experiments, n=5 PBS-treated Bhlhe40+/+ and Bhlhe40−/−; 13 IL-4c-treated Bhlhe40+/+; 17 IL-4c-treated Bhlhe40−/−; 3 PBS-treated LysM-Cre− Bhlhe40fl/fl and LysM-Cre+ Bhlhe40fl/fl; 8 IL-4c-treated LysM-Cre− Bhlhe40fl/fl; 7 IL-4c-treated LysM-Cre+ Bhlhe40fl/fl). g, Frequency of BrdU+ LPMs, large pleural macrophages, red pulp macrophages, Kupffer cells, and AMs from Bhlhe40+/+ and Bhlhe40−/− mice treated with PBS or IL-4c (pooled from 2 experiments, n=3/group for PBS; 6 IL-4c-treated Bhlhe40+/+; 7 IL-4c-treated Bhlhe40−/−; except for pleura, 3 experiments, n=4 PBS-treated Bhlhe40+/+; 3 PBS-treated Bhlhe40−/−; 7 IL-4c-treated Bhlhe40+/+; 6 IL-4c-treated Bhlhe40−/−). h, Flow cytometry of Bhlhe40GFP transgene reporter expression in thioglycollate (Thio)-elicited macrophages from Bhlhe40GFP+ and Bhlhe40GFP- mice treated with Thio or Thio and IL-4c (1 experiment, n=2 Thio-treated Bhlhe40GFP+; 3 Thio and IL-4c-treated Bhlhe40GFP+; 1 Thio-treated Bhlhe40GFP-). i, Flow cytometry of BrdU incorporation by Thio-elicited macrophages from Bhlhe40+/+ and Bhlhe40−/− mice treated with Thio and IL-4c (representative of 2 experiments, n=6/group). j, Frequency of BrdU+ LPMs and Thio-elicited macrophages from Bhlhe40+/+ and Bhlhe40−/− mice treated with PBS, IL-4c, Thio, or Thio and IL-4c (pooled from 2 experiments, n=3 PBS-treated Bhlhe40+/+, 4 PBS-treated Bhlhe40−/−, 4 IL-4c-treated Bhlhe40+/+, 5 IL-4c-treated Bhlhe40−/−, 2 Thio-treated Bhlhe40+/+, 4 Thio-treated Bhlhe40−/−, Thio and IL-4c-treated as in i). Data in a-f,g,j are mean ± s.e.m; each symbol represents an individual mouse (a-d,f,g,j). *P ≤ 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001, unpaired two-sided Student’s t-test.
We used transmission electron microscopy of bulk peritoneal cells from naïve and IL-4c-treated Bhlhe40+/+ and Bhlhe40−/− mice to assess cell morphology. Bhlhe40+/+ and Bhlhe40−/− LPMs from IL-4c-treated mice showed increases in cell size and endoplasmic reticulum (ER) extent compared to naïve LPMs (Supplementary Fig. 4). We observed no distinct morphology between naïve Bhlhe40−/− and Bhlhe40+/+ LPMs, while LPMs from IL-4c-treated Bhlhe40−/− mice were somewhat larger and more vacuolated than LPMs from IL-4c-treated Bhlhe40+/+ mice, without any severe morphologic defects (Supplementary Fig. 4). Therefore, Bhlhe40 was required for LPMs to rapidly cycle in response to IL-4c, but was dispensable for normal morphology and induction of alternative activation markers.
Monocytes can acquire a Bhlhe40-dependent proliferative program
Next we asked whether Bhlhe40 was required for the IL-4c-induced proliferation of other macrophages. IL-4c injection i.p. into Bhlhe40GFP+ mice did not change the expression of GFP in LPMs, SPMs, AMs, kidney macrophages, red pulp macrophages and Kupffer cells compared to these populations in PBS-treated Bhlhe40GFP+ mice (Supplementary Fig. 5). IL-4c injection i.p. into Bhlhe40+/+ and Bhlhe40−/− mice resulted in equivalent BrdU incorporation by, and numbers of, red pulp macrophages, Kupffer cells and AMs (Fig. 5g and Supplementary Fig. 5), in contrast to LPMs and large pleural macrophages, which required Bhlhe40 for a normal population of BrdU-incorporating cells in response to IL-4c (Fig. 5g and Supplementary Fig. 5).
Injection of thioglycollate and IL-4c i.p. causes monocyte-derived macrophages to proliferate and acquire alternative activation markers17,18. When we asked whether Bhlhe40 was expressed in these monocyte-derived macrophages, we found that the combination of thioglycollate and IL-4c induced marked expression of GFP in the thioglycollate-elicited macrophages in Bhlhe40GFP+ mice compared to a lower expression of GFP in macrophages elicited by thioglycollate alone (Fig. 5h). After treatment with thioglycollate and IL-4c, Bhlhe40−/− mice had severely reduced proportions of BrdU+ and pHH3+ thioglycollate-elicited macrophages compared to Bhlhe40+/+ mice (Fig. 5i,j and Supplementary Fig. 6), while RELMα was acquired normally (Supplementary Fig. 6). Thus, these data indicate that Bhlhe40 is a specific regulator of large serous cavity macrophage proliferation in response to IL-4c and that monocyte-derived macrophages can acquire a Bhlhe40-dependent proliferative program similar to that of serous cavity resident macrophages.
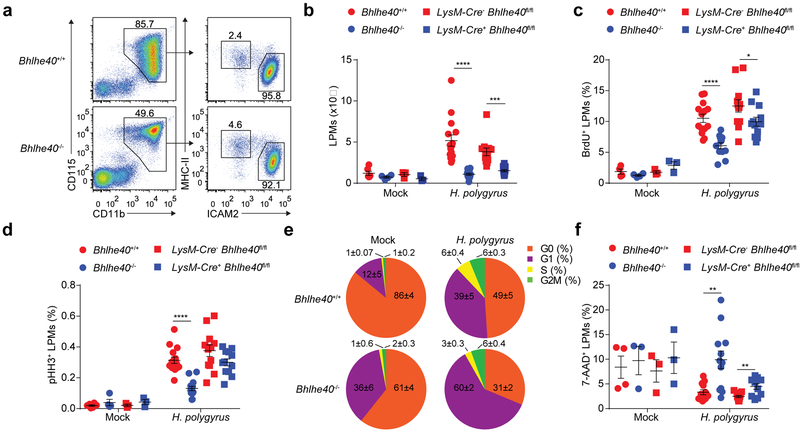
Bhlhe40 regulates LPM proliferation in response to H. polygyrus
The intestinal helminth Heligmosomoides polygyrus bakeri (H. polygyrus) is a natural mouse pathogen that elicits robust proliferation of LPMs following oral infection14. Infection with H. polygyrus caused a 4-fold increase in the number of LPMs in infected Bhlhe40+/+ mice compared to Bhlhe40−/− mice after 8 days (Fig. 6a,b). Similar findings were obtained in LysM-Cre− Bhlhe40fl/fl and LysM-Cre+ Bhlhe40fl/fl mice (Fig. 6b). Furthermore, after H. polygyrus infection, the proportions of BrdU+ LPMs and pHH3+ LPMs were reduced in Bhlhe40−/− and LysM-Cre+ Bhlhe40fl/fl compared to Bhlhe40+/+ and LysM-Cre− Bhlhe40fl/fl mice (Fig. 6c,d). H. polygyrus infection elicited a greater increase in the fraction of LPMs in the G1, S and G2M phases of the cell cycle in Bhlhe40+/+ compared to Bhlhe40−/− mice (Fig. 6e). The proportion of 7-AAD+ necrotic LPMs was also increased in Bhlhe40−/− and LysM-Cre+ Bhlhe40fl/fl compared to Bhlhe40+/+ and LysM-Cre− Bhlhe40fl/fl infected mice (Fig. 6f). These data suggested that Bhlhe40 is essential for the proliferation of LPMs during type 2 immunity.
Figure 6. Bhlhe40 is required for LPM proliferation in response to H. polygyrus.
a, Flow cytometry of peritoneal macrophage subsets from Bhlhe40+/+ and Bhlhe40−/− mice infected with H. polygyrus (representative of 4 experiments, n=15 Bhlhe40+/+, 14 Bhlhe40−/−). b, Numbers of LPMs from Bhlhe40+/+, Bhlhe40−/−, LysM-Cre− Bhlhe40fl/fl, and LysM-Cre+ Bhlhe40fl/fl mice mock- or H.polygyrus-infected as in a (pooled from 3 experiments, n=6 mock-infected Bhlhe40+/+; 4 mock-infected Bhlhe40−/−; H. polygyrus-infected as in a for Bhlhe40+/+ and Bhlhe40−/−; 3 mock-infected LysM-Cre− Bhlhe40fl/fl and LysM-Cre+ Bhlhe40fl/fl; 12 H. polygyrus-infected LysM-Cre− Bhlhe40fl/fl and LysM-Cre+ Bhlhe40fl/f). c,d, Frequency of BrdU+ LPMs (c) (pooled from 3 experiments, n=4 mock-infected Bhlhe40+/+ and Bhlhe40−/−; 14 H. polygyrus-infected Bhlhe40+/+; 12 H. polygyrus-infected Bhlhe40−/−; 3 mock-infected LysM-Cre− Bhlhe40fl/fl and LysM-Cre+ Bhlhe40fl/fl; 12 H. polygyrus-infected LysM-Cre− Bhlhe40fl/fl and LysM-Cre+ Bhlhe40fl/fl) and pHH3+ LPMs (d) (pooled from 3 experiments, n=6 mock-infected Bhlhe40+/+; 4 mock-infected Bhlhe40−/−; 15 H. polygyrus-infected Bhlhe40+/+; 14 H. polygyrus-infected Bhlhe40−/−; 3 mock-infected LysM-Cre− Bhlhe40fl/fl and LysM-Cre+ Bhlhe40fl/fl; 12 H. polygyrus-infected LysM-Cre− Bhlhe40fl/fl and LysM-Cre+ Bhlhe40fl/fl) from Bhlhe40+/+, Bhlhe40−/−, LysM-Cre− Bhlhe40fl/fl, and LysM-Cre+ Bhlhe40fl/fl mice mock- or H. polygyrus-infected. e, Proportion of LPMs in each phase of the cell cycle from Bhlhe40+/+ and Bhlhe40−/− mice mock- or H. polygyrus-infected (pooled from 2 experiments, n=3 mock-infected Bhlhe40+/+, 2 mock-infected Bhlhe40−/−, 7 H. polygyrus-infected Bhlhe40+/+, 6 H. polygyrus-infected Bhlhe40−/−). f, Frequency of 7-AAD+ LPMs from Bhlhe40+/+, Bhlhe40−/−, LysM-Cre− Bhlhe40fl/fl, and LysM-Cre+ Bhlhe40fl/fl mice mock- or H. polygyrus-infected (pooled from 3 experiments, n=4 mock-infected Bhlhe40+/+; 3 mock-infected Bhlhe40−/−; 12 H. polygyrus-infected Bhlhe40+/+ and Bhlhe40−/−; 3 mock-infected LysM-Cre− Bhlhe40fl/fl and LysM-Cre+ Bhlhe40fl/fl; 11 H. polygyrus-infected LysM-Cre− Bhlhe40fl/fl and LysM-Cre+ Bhlhe40fl/fl). Data in b-f are mean ± s.e.m; each symbol represents an individual mouse (b-d,f). *P ≤ 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001, unpaired two-sided Student’s t-test.
Bhlhe40 controls cell cycle-related transcription
To determine the effects of Bhlhe40 on the expression profile of LPMs during type 2 immunity, we performed gene expression microarrays on sorted LPMs from IL-4c-treated Bhlhe40+/+, Bhlhe40−/− and LysM-Cre+ Bhlhe40fl/fl mice after 4 days. More genes (254; Fig. 7a–c) were differentially expressed by 2-fold or more between LPMs from IL-4c-treated Bhlhe40+/+ and Bhlhe40−/− mice compared to LPMs from naive Bhlhe40+/+ and Bhlhe40−/− mice (87 genes; Fig. 7a–c). To ask whether the transcriptional changes that occurred in alternatively activated LPMs were dependent on Bhlhe40, we selected the 55 genes most differentially expressed (≥10-fold different) between naive and IL-4c-treated Bhlhe40+/+ LPMs, including Mgl2, Chil3, Arg1 and Il1rl1 (Fig. 7d). These genes were generally normally expressed in Bhlhe40+/+, Bhlhe40−/− and LysM-Cre+ Bhlhe40fl/fl LPMs from IL-4c-treated mice (Fig. 7d), and we also found no defect in the expression of Myo18a, C1qa, C1qb and C1qc, which encode known regulators of LPM proliferation during type 2 immune responses15 (Supplementary Fig. 7). Consistent with impaired proliferation, Maf (3.2-fold) and Mafb (3.8-fold) were more highly expressed in Bhlhe40−/− compared to Bhlhe40+/+ LPMs by qRT-PCR (Fig. 7e). Gene set enrichment analysis (GSEA) for Hallmark gene sets45 showed prominent enrichment of gene sets related to proliferation, including the E2F targets and Myc targets v1 gene sets in the gene expression data between LPMs from IL-4c-treated Bhlhe40+/+ and Bhlhe40−/− mice (Fig. 7f). Further comparison of differentially expressed genes to the C5 gene ontology sets indicated that LPMs from IL-4c-treated Bhlhe40+/+ mice were substantially enriched for cell cycle and chromosome-related gene sets (Cell cycle, Cell cycle process, Mitotic cell cycle), while LPMs from IL-4c-treated Bhlhe40−/− mice showed enrichment for the Vacuole gene set (Fig. 7g–i), consistent with increased vacuolar area by electron microscopy. Therefore, Bhlhe40 was required in LPMs for normal regulation of cell cycle-related gene expression.
Figure 7. Bhlhe40 regulates gene expression to modulate proliferation, but not alternative activation, in LPMs during type 2 immunity.
a, Gene expression microarray data were analyzed for genes differentially expressed by ≥2-fold in LPMs from Bhlhe40+/+, Bhlhe40−/−, and LysM-Cre+ Bhlhe40fl/fl mice treated with IL-4c. b-d, Gene expression microarray data were analyzed for shared and unique Bhlhe40-dependent genes (≥2-fold differentially expressed, depicted as a Venn diagram) (b), shared Bhlhe40-dependent genes (c), and expression of the LPM alternative activation signature (d) in LPMs from Bhlhe40+/+, Bhlhe40−/−, and LysM-Cre+ Bhlhe40fl/fl mice unstimulated or treated with IL-4c. e, qRT-PCR of Maf and Mafb expression relative to Hprt in Bhlhe40+/+ and Bhlhe40−/− LPMs from mice treated with IL-4c (pooled from 3 experiments, n=7 Bhlhe40+/+, 6 Bhlhe40−/−). f,g, GSEA of gene expression microarray data for representative Hallmark (f) and C5 gene sets (g) enriched in Bhlhe40+/+ vs. Bhlhe40−/− LPMs from IL-4c-treated mice. NES, normalized enrichment score. FWER, family-wise error rate. h,i, MSigDB C5 gene set enrichment was analyzed using the lists of genes expressed at ≥1.5-fold in Bhlhe40+/+ vs. Bhlhe40−/− (h) or Bhlhe40−/− vs. Bhlhe40+/+ LPMs from IL-4c-treated mice (i). Microarray data from naïve LPMs (n=3/group, reanalyzed from Fig. 3) and in vivo IL-4c-stimulated LPMs (n=2/group) are from single separate experiments. Data in e are mean ± s.e.m; each symbol represents an individual mouse (e). ***P < 0.001; ****P < 0.0001, (e) unpaired two-sided Student’s t-test, (f,g) NES and FWER, and (h,i) one-sided hypergeometric test.
Bhlhe40 targets cell cycle-related loci directly
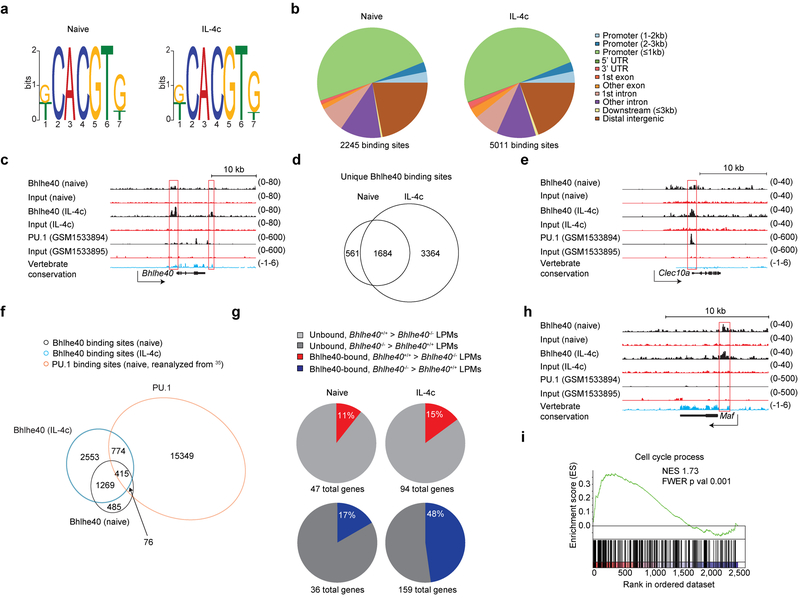
We next addressed whether Bhlhe40 regulated LPM gene expression via direct binding to gene loci by sorting LPMs from naïve and IL-4c-treated Bhlhe40+/+ mice after 4 days for Bhlhe40 chromatin immunoprecipitation sequencing (ChIP-seq). Motif analysis of the called peaks identified the expected CACGTG E-box sequence (Fig. 8a) and a majority of Bhlhe40 peaks (naïve 2,245 total peaks; IL-4c-treated 5,011 total peaks) were promoter-associated in both samples (Fig. 8b). In both conditions, Bhlhe40 bound sites in the Bhlhe40 (single peak, within promoter) and Il10 (single peak, 1 kilobase (kb) downstream of locus) loci previously described in T cells (Fig. 8c and Supplementary Fig. 7)33. We also identified a novel Bhlhe40 binding site 1.5kb downstream of the Bhlhe40 locus that was occupied only in LPMs from IL-4c-treated mice (Fig. 8c). Many Bhlhe40 peaks were shared between LPMs from naïve and IL-4c-treated mice (1,684 sites, including peaks proximal to the Klf4, Nr1d1, Plac8 and Yy1 loci) (Fig. 8d), but the majority were unique to LPMs from IL-4c-treated mice (3,364 sites, including peaks proximal to the Klf4 and Nr1d1 loci) (Fig. 8d), often in association with shared peaks (as for the Bhlhe40 locus).
Figure 8. Bhlhe40 directly regulates gene expression in LPMs in an activation state-dependent manner.
a, Bhlhe40 ChIP-seq data were analyzed for consensus binding motifs in LPMs from naive (left) and IL-4c-treated mice (right). b, Bhlhe40 ChIP-seq data were analyzed for locations of Bhlhe40 peaks in the genome in LPMs from naive (left) and IL-4c-treated mice (right). UTR, untranslated region. c, Tracings of Bhlhe40 binding, PU.1 binding, and vertebrate conservation at the Bhlhe40 locus. d, Bhlhe40 ChIP-seq data were analyzed for shared and unique Bhlhe40 binding sites in LPMs from naive and IL-4c-treated mice (depicted as a Venn diagram). e, Tracings of Bhlhe40 binding, PU.1 binding, and vertebrate conservation at the Clec10a locus. f, Naïve LPM Bhlhe40 ChIP-seq data, in vivo IL-4c-stimulated LPM Bhlhe40 ChIP-seq data, and naïve LPM PU.1 ChIP-seq data were analyzed for shared and unique Bhlhe40 and PU.1-bound genes between the three samples (depicted as a Venn diagram). g, The proportion of Bhlhe40-bound, Bhlhe40-dependent genes (≥2-fold differentially expressed in Bhlhe40+/+ and Bhlhe40−/− LPMs) in LPMs from naïve mice and Bhlhe40-bound, Bhlhe40-dependent genes (≥2-fold differentially expressed in Bhlhe40+/+ and Bhlhe40−/− LPMs) in LPMs from IL-4c-treated mice. h, Tracings of Bhlhe40 binding, PU.1 binding, and vertebrate conservation at the Maf locus. i, GSEA of gene expression microarray data for Bhlhe40-bound genes from LPMs from Bhlhe40+/+ and Bhlhe40−/− mice treated with IL-4c for the C5 Cell Cycle Process gene set. NES, normalized enrichment score. FWER, family-wise error rate. LPM Bhlhe40 ChIP-seq data (n=1/group) and microarray data from in vivo IL-4c-stimulated LPMs (n=2/group) are from single separate experiments. LPM PU.1 ChIP-seq data reanalyzed from35. (i) NES and FWER.
Because Bhlhe40 and PU.1 may cooperate in LPMs35, we compared our ChIP-seq data with previously published PU.1 ChIP-seq performed on LPMs from naïve C57BL/6 mice35. PU.1 peaks overlapped with 22% or 24% of Bhlhe40 peaks in LPMs from naïve or IL-4c-treated mice, respectively, including the Clec10a, Ccl2 and Plac8 loci (Fig. 8e,f and Supplementary Fig. 7). However, the majority of Bhlhe40 peaks (naïve 1,754 peaks; IL-4c 3,822 peaks) were not associated with PU.1 binding (Fig. 8f), including at the Bhlhe40, Maf and Il10 loci. When we assessed whether Bhlhe40 bound directly to genes with Bhlhe40-dependent expression, we found that Bhlhe40 bound a small fraction of genes differentially expressed by two-fold or more between LPMs from naïve Bhlhe40+/+ and Bhlhe40−/− mice (11% of genes downregulated in Bhlhe40−/− LPMs, 17% of genes upregulated in Bhlhe40−/− LPMs, Fig. 8g and Supplementary Fig. 7) and bound a greater fraction of genes differentially expressed between LPMs from IL-4c-treated Bhlhe40+/+ and Bhlhe40−/− mice (15% of genes downregulated in Bhlhe40−/− LPMs, 48% of genes upregulated in Bhlhe40−/− LPMs, Fig. 8g and Supplementary Fig. 7), suggesting a direct role for Bhlhe40 in regulating gene expression in LPMs.
Further analysis of our ChIP-Seq data identified a Bhlhe40 peak within the Maf promoter in LPMs from naïve or IL-4c-treated mice (Fig. 8h), as well as two additional peaks closest to the Maf locus (200kb downstream (naïve and IL-4c) and 300kb downstream (IL-4c) of the locus) (Supplementary Fig. 7), suggesting that Bhlhe40 repressed the Maf locus. There was no clear Bhlhe40 peak uniquely associated with the Mafb locus (Supplementary Fig. 7). To address whether Bhlhe40 directly regulated other cell cycle-related loci, we performed GSEA analysis for the subset of genes directly bound by Bhlhe40 using the gene expression data from LPMs from IL-4c-treated Bhlhe40+/+ and Bhlhe40−/− mice. We found that differential expression of these genes between Bhlhe40+/+ and Bhlhe40−/− LPMs from IL-4c-treated mice largely recapitulated the enrichment of cell cycle-related modules observed when all gene expression data were analyzed (Fig. 8i and Supplementary Fig. 8). Thus, Bhlhe40 functioned in LPMs as a direct transcriptional regulator of numerous genomic loci, including those encoding cell cycle-related proteins.
Discussion
Here we found that the transcription factor Bhlhe40 was an essential cell-intrinsic regulator of proliferation in LPMs. In the steady-state, Bhlhe40−/− LPMs were reduced in number and a higher proportion accumulated in the G1 phase compared to Bhlhe40+/+ LPMs. During type 2 immunity, Bhlhe40 was essential for normal proliferation and accumulation, with a reduced proportion of Bhlhe40−/− LPMs in the S and M phases compared to Bhlhe40+/+ LPMs, but Bhlhe40 was dispensable for acquisition of alternative activation markers. Bhlhe40 mediated repression of Maf and activation of multiple proliferation-related loci to allow LPM cell cycling. Bhlhe40 was a tissue-specific regulator of proliferation of LPMs, but could be acquired by peritoneal monocyte-derived macrophages to support a proliferative program.
How deletion of Bhlhe40 impairs cell cycle progression of LPMs remains unclear. We observed that a higher proportion of Bhlhe40−/− LPMs were Ki67+ compared to Bhlhe40+/+ LPMs, suggesting that Bhlhe40−/− LPMs might inappropriately enter the cell cycle. However, our data are not consistent with this notion, as we saw a selective increase in the proportion of Bhlhe40−/− LPMs in the G1 phase, without a commensurate increase in LPMs in the S, G2, or M phases. Both Bhlhe40+/+ and Bhlhe40−/− LPMs upregulated cyclins and CDKs when mice were treated with IL-4c. In contrast, Bhlhe40+/+ and Bhlhe40−/− LPMs had low expression of cyclins and CDKs at steady-state. These data support the notion that impaired progression from the G1 phase rather than enhanced proliferation was the primary cause of accumulation of G1 phase LPMs in naïve and likely IL-4c-treated Bhlhe40−/− mice. As expression of cyclin D and CDKs were comparable between Bhlhe40+/+ and Bhlhe40−/− LPMs, alterations solely in the expression of these regulators likely do not explain the effect of Bhlhe40 deficiency on LPM proliferation. Instead, the phenotype of Bhlhe40−/− LPMs was probably due to the impaired transcriptional regulation of a broad set of cell cycle-related genes caused by loss of Bhlhe40 and upregulation of c-Maf and MafB.
Bhlhe40 and c-Maf may functionally interact in T cells32,34. Our data suggest that Bhlhe40 is a transcriptional repressor of Maf in LPMs. In contrast to T cells and other tissue-resident macrophages, LPMs require Bhlhe40 to support normal proliferation, suggesting that Bhlhe40-mediated repression of Maf has distinct effects in different cell types. Expression of both Bhlhe40 and c-Maf in LPMs results in a unique regulatory interaction not detected in macrophage subsets lacking expression of one of these transcription factors. Downregulation of Maf and Mafb expression is critical for proliferation in macrophages6,7. Our data are most consistent with a role for Bhlhe40 in repressing Maf and Mafb to permit LPM cell cycling, along with Bhlhe40-mediated regulation of a wider set of target genes, some of them co-bound by PU.1, as previously proposed35. It is likely that specific networks of integrated transcriptional regulators control the development and function of resident macrophages in each tissue. In LPMs, this network would include Bhlhe40, PU.135, c-Maf7 and MafB7, as well as GATA69,10,11 and C/EBPβ46, two transcription factors whose loss results in impaired development of LPMs.
In addition to LPMs and large pleural macrophages, AMs and, in some contexts, monocyte-derived macrophages expressed Bhlhe40. Bhlhe40−/− AMs only showed minor transcriptional differences compared to Bhlhe40+/+ AMs and no evidence of a proliferative defect. c-Maf and MafB were poorly expressed in AMs7 and were not induced in Bhlhe40−/− AMs (data not shown), suggesting that Bhlhe40 was not required to repress these transcription factors in AMs. It is also possible that Bhlhe41, which is expressed highly in AMs but not LPMs (data not shown) and can partially substitute for Bhlhe4028, may compensate for the absence of Bhlhe40 in AMs. In contrast to AMs, monocyte-derived macrophages can acquire a Bhlhe40-dependent proliferative program in response to thioglycollate and IL-4c. The similarities of this Bhlhe40-regulated transcriptional program in monocyte-derived macrophages to that of LPMs remain to be explored.
Whether common regulators can control macrophage cell cycling in response to different stimuli during homeostasis (e.g. CSF-1) or type 2 immunity (e.g. IL-4, IL-5, or IL-13)13 is unclear. Our findings demonstrate the existence of shared regulation of macrophage proliferation in the steady-state and disease, as well as a crucial role for tissue-specific transcriptional regulation acting in concert with more broadly shared regulators like c-Maf. This suggests the possibility of therapeutically targeting the proliferation of select macrophage populations, including tumor-associated macrophages (TAMs), which are known to partly derive from tissue-resident macrophages and locally proliferate47,48,49,50.
Our results illustrate the complexity of tissue-specific control of macrophages, demonstrating that tissue-specific transcription factors are critical for the regulation of macrophage proliferation in health and disease. Our data provide direct evidence that resident macrophages are under constant control by a partnership of shared and tissue-specific transcription factors, with possible implications for therapies.
Methods
Mice
C57BL/6 (Taconic), B6.SJL (CD45.1, Taconic or Jackson), Il10−/− (B6.129P2-Il10tm1Cgn/J, Jackson) and LysM-Cre (B6N.129P2(B6)-Lyz2tm1(cre)Ifo/J, Jackson) mice were obtained from the vendors listed. Bhlhe40−/− (10 generations backcrossed to the C57BL/6 background)31,51, Bhlhe40GFP+(10 generations backcrossed to the C57BL/6 background21) and Bhlhe40fl/fl(33) mice have been previously reported. The Bhlhe40GFP+ mouse strain, originally defined as STOCK Tg(Bhlhe40-EGFP)PX84Gsat/Mmucd, identification number 034730-UCD, was obtained from the Mutant Mouse Regional Resource Center (MMRRC), a NCRR-NIH funded strain repository, and was donated to the MMRRC by the NINDS funded GENSAT BAC transgenic project (The GENSAT Project, NINDS Contract #N01NS02331 to the Rockefeller University). All mice were maintained in our specific pathogen free animal facility. Sex-matched littermates were used for experiments whenever possible, although in some cases mice from multiple litters were used in a single experiment. All animal experiments were approved by the Animal Studies Committee of Washington University in St. Louis.
Bone marrow chimeras
Bhlhe40+/+ CD45.1/CD45.2 mice were lethally irradiated with 1,000 rads from a gamma irradiator, followed by same-day i.v. transfer of 16 million total bone marrow cells (8 million Bhlhe40+/+ (CD45.1) cells plus either 8 million Bhlhe40+/+ (CD45.2) or Bhlhe40−/− (CD45.2) cells). Mice were given drinking water containing sulfamethoxazole (1.3 mg/ml) and trimethoprim (0.26 mg/ml) for 2 weeks after irradiation and were allowed to reconstitute for at least 8 weeks. In some experiments, chimeras were also made with CD45.1 recipients using CD45.1/CD45.2 and CD45.2 donor bone marrow cells.
Peritoneal cell transfers
Peritoneal cells were lavaged from the peritonea of Bhlhe40+/+ (CD45.1), Bhlhe40+/+ (CD45.2), and Bhlhe40−/− (CD45.2) donors, and aliquots of cells were analyzed by flow cytometry to determine the frequency of LPMs. Bulk peritoneal cells were then transferred i.p. into resting Bhlhe40+/+ recipients (CD45.1/CD45.2) at ratios resulting in the transfer of equal numbers of LPMs from each donor (200,000–300,000 LPMs).
Treatment of mice with thioglycollate and interleukin-4 complexes (IL-4c)
A 3% solution of thioglycollate was prepared in water, autoclaved and aged for three or more months43. Mice received 1 ml i.p. to induce peritonitis or a control injection of PBS43. IL-4c were prepared fresh as described12,52. IL-4 (Shenandoah Biotechnology #200–18, resuspended in 0.1% BSA in water) and anti-IL-4 antibody (clone 11B11; Leinco I-1071 or BioXCell BE0045) were combined in a 1:5 ratio by mass and a 1:1 ratio by volume, using ~1 mg/ml cytokine and ~5 mg/ml antibody. Complexes were incubated for ~2 minutes at room temperature (RT), diluted in 1x Dulbecco’s PBS (DPBS) and injected i.p. Control injections were 0.1% BSA diluted in 1x DPBS, while naïve mice were also used for assessment of cell cycling, due to acquisition of Ki67+ by LPMs 2 days after PBS injection as described13. Mice received injections on day 0 and day 2, followed by sacrifice on day 4 as described12. For treatment with thioglycollate and IL-4c complexes, mice were injected i.p. with thioglycollate on day 0 and IL-4c on days 0 and 2, as previously described17,18.
H. polygyrus infections
H. polygyrus bakeri third-stage larvae (L3) were prepared as described53. Mice were orally gavaged with 200 L3 or water (mock) with a 20-gauge ball-tipped gavage needle. Mice were sacrificed on day 8 of infection for assessment of peritoneal cells.
Leukocyte collection from tissues
Peritoneal and pleural cells were collected from body cavities by lavage. Bone marrow was collected by flushing hind limb femurs and tibias. Blood was collected by submandibular bleeding into EDTA or lithium heparin tubes. Lungs, liver, spleen and kidney were excised, placed in Iscove’s Modified Dulbecco’s Medium (IMDM) containing 5% fetal bovine serum (FBS), minced finely, and digested at 37 °C for an hour with mechanical disruption with a stir bar and enzymatic digestion (lung and kidneys, 4 mg/ml collagenase D (Roche); spleen, 0.25 g/ml collagenase B (Roche) and 30U/ml DNase I (EMD); liver, 4 mg/ml collagenase D and 30U/ml DNase I). Microglia21 and small intestinal lamina propria cells54 were isolated as described. After digestion, enzymes were inactivated with 5 mM EDTA and samples were incubated on ice for 5 minutes.
All cells were passed through a 70 μm cell strainer before analysis. If necessary, tissues were treated with ACK lysis buffer to lyse red blood cells. Cells were counted with a hemocytometer using 3% acetic acid (naïve peritoneum and pleura) or trypan blue (all others).
Flow cytometry
Cell surface staining was conducted in sterile 1x PBS with 0.5% BSA and 2 mM EDTA (hereafter FACS buffer). In brief, cells were washed in FACS buffer, blocked with α-CD16/32 (clone 2.4G2, BioXCell) for 10 minutes at 4 °C, stained for 20 min at 4 °C and washed with FACS buffer before flow cytometry. In some experiments to assess cell death, 7-AAD (1:20 of a 50 μg/mL solution, BioLegend or BD) was added to cells for 15 minutes prior to flow cytometry. Flow cytometry was performed on FACSCanto II, LSRFortessa, LSRFortessa X20 and LSR II instruments (all BD). FlowJo software (Treestar) was used for analysis. Antibodies and fluorescent dyes used in this study are listed in Supplementary Table 1.
Gating of cell populations was as follows (all analysis pre-gated on FSC/SSC and a FSC-W/FSC-A singlet gate). Blood monocytes were gated as Ly6G−CD115+ and then divided by Ly6C expression. Peritoneal and pleural macrophages were gated as CD115+CD11b+, then divided into ICAM2+MHC-IIint large macrophages and ICAM2−MHC-II+ small macrophages. Thioglycollate-elicited macrophages were gated as CD115+CD11b+ICAM2lo. Liver Kupffer cells were gated as CD45+CD11bloF4/80hi, and in some contexts as Ly6C−. Kidney macrophages were gated as CD45+Ly6C−CD11b+F4/80hi. AMs were gated as CD45+Siglec-F+CD11c+, and in some contexts as F4/80+CD11b−. Red pulp macrophages were gated as F4/80hi and negative or low for other markers (CD11blo, MHC-IIlo, or CD11clo). Microglia were gated as CD45intCD11b+. Small intestinal lamina propria macrophages were gated as CD45+Ly6C−F4/80+CD64+MHC-II+. Peritoneal B cells were gated as CD115−MHC-II+CD19+. Analysis of cells from the Bhlhe40GFP+ reporter mouse used viability dyes (Po pro 1 or 7-AAD) when necessary to exclude dead cells.
Intracellular staining for flow cytometry
For Ki67, DAPI, RELMα, BrdU and pHH3 staining, the eBioscience FoxP3/Transcription Factor Staining Buffer set (00-5523-00) or the BioLegend True-Nuclear Transcription Factor Buffer set (424401) was used. In brief, after surface staining, cells were fixed with 1x Fix Concentrate buffer in provided Fix Diluent for 30 minutes at 4 °C. Cells were then washed with FACS buffer and stored overnight. To permeabilize the cells, samples were washed with 1x Perm buffer diluted in water. Following blocking with 2% rat serum, samples were stained for 1 hour at RT, except for DAPI and secondary antibodies (20 minutes at RT), followed by washing in 1x Perm buffer and FACS buffer before flow cytometry.
For BrdU staining, mice were given 1 mg of BrdU i.p. (from BD kit, 552598, or Sigma, B5002) three hours before sacrifice as described12. After sacrifice of mice and peritoneal lavage, samples were processed, fixed and stored overnight as for other intracellular antigens. BrdU-labelled cells were washed in 1x Perm buffer, incubated in DNase I (from BD kit, 552598 or Sigma, D4513) in 1x DPBS for 30 min at 37 °C, washed in 1x Perm buffer, blocked with 2% rat serum and stained for 1 hour at RT with α-BrdU antibody (BD, 552598), followed by washing in 1x Perm buffer and FACS buffer. Mice that did not receive BrdU were used as negative controls.
Transmission electron microscopy
For ultrastructural analyses, peritoneal cells were fixed in 2% PFA/2.5% glutaraldehyde (Polysciences Inc.) in 100 mM sodium cacodylate buffer, pH 7.2 for 1 hour at RT. Samples were washed in sodium cacodylate buffer at RT and postfixed in 1% osmium tetroxide (Polysciences Inc.) for 1 hour. Samples were rinsed in distilled water prior to en bloc staining with 1% aqueous uranyl acetate (Ted Pella Inc.) for 1 hour. Following rinsing in distilled water, samples were dehydrated in a graded series of ethanol and embedded in Eponate 12 resin (Ted Pella Inc.). Sections of 95 nm were cut with a Leica Ultracut UCT ultramicrotome, stained with uranyl acetate and lead citrate and viewed on a JEOL 1200 EX transmission electron microscope (JEOL USA Inc.) equipped with an AMT 8 megapixel digital camera and AMT Image Capture Engine V602 software (Advanced Microscopy Techniques).
For morphological analysis, images were blinded and randomized. LPMs were identified as large cells with abundant cytoplasm and were distinguished from rare peritoneal mast cells by the absence of electron-dense granules. For measurement statistics, the ObjectJ plugin was used in ImageJ software (NIH). In brief, cell and vesicle cross-sectional area were calculated by tracing the outline of the cell or vesicles, respectively, and calculating the enclosed area. ER cross-sectional extent was calculated by tracing the ER with lines and adding these lengths together. For assessment of ER luminal width, a randomly placed grid was used to subdivide the cell into sections. Representative measurements were then taken across the lumen of the ER, and the measurements from each section were averaged.
Microarrays
The following cell populations were sorted on a FACSAria II (BD) into FBS: for naïve LPM microarrays, B220−F4/80+CD11b+ICAM2+ LPMs from untreated mice; for in vivo IL-4c-stimulated LPM microarrays, CD115+CD11b+ICAM2+MHC-IIint LPMs from mice treated with IL-4c at days 0 and 2, with peritoneal cells collected at day 4; for naïve AM microarrays, CD45+Ly6G−Siglec-F+CD11c+CD11blo AMs from untreated mice. Cells were lysed and RNA was purified using the E.Z.N.A. MicroElute Total RNA kit (Omega Bio-Tek). Total RNA was submitted to the Genome Technology Access core at Washington University for cDNA synthesis (NuGen Pico SL) followed by microarray analysis on the Affymetrix Mouse Gene 1.0 ST platform. Data were analyzed using the DNASTAR ArrayStar program. Genes with an expression value of <5 (in log 2 scale) in all replicates were considered not expressed. For analysis of naïve microarrays, which were conducted on three biologic replicates, the differentially expressed gene list was also filtered on genes with a p-value significance of ≤0.05 by the moderated t-test. For analysis of in vivo IL-4c-stimulated LPM microarrays, which were conducted on two biologic replicates, no p-value filtering was applied. For comparison of naïve to in vivo IL-4c-stimulated LPMs, CEL files were normalized together to generate expression data. Heatmaps were generated with Morpheus (software.broadinstitute.org/morpheus/). Venn diagrams were generated with the Venn Diagram Plotter tool (Pacific Northwest National Laboratories, omics.pnl.gov). Multiple differentially expressed probe sets representing a single gene were presented in heat maps without exclusion, but only unique genes were counted in Venn diagrams.
The macrophage alternative activation gene signature used to assess naïve macrophages was generated from GSE69607 comparing M0, M1 and M2 bone marrow-derived macrophages (BMDMs)55. The 29 genes 20-fold upregulated in M2 vs. M1 BMDMs were used to define a set of macrophage alternative activation-related genes. The LPM gene signature was previously published36. For the LPM alternative activation gene signature used to assess in vivo IL-4c-stimulated LPMs, we compared our microarray data from naïve and in vivo IL-4c-stimulated Bhlhe40+/+ LPMs and defined an alternative activation signature for LPMs, composed of the 55 unique genes up- or down-regulated by ten-fold or more. For comparison of the gene expression signature of Bhlhe40- or GATA6-deficient LPMs, our data was analyzed in parallel with GSE3744811 as above, as both data sets were generated on the Affymetrix Mouse Gene 1.0 ST platform.
Quantitative real-time polymerase chain reaction (qRT-PCR)
LPMs were sorted as for microarrays. RNA was isolated with the E.Z.N.A. MicroElute Total RNA kit (Omega Bio-Tek), and cDNA was synthesized with 50 ng RNA using the High Capacity RNA to cDNA kit (Invitrogen). RNA concentration was assessed with a Nanodrop 2000 spectrophotometer (ThermoFisher). qRT-PCR was performed with Power SYBR Green PCR Master Mix (Applied Biosystems) using a StepOnePlus Real-Time PCR machine (Applied Biosystems). Gene expression was determined relative to Hprt by the ΔCT method. The following primers were used: Hprt, forward 5′-TCAGTCAACGGGGGACAT AAA-3′, reverse 5′-GGGGCTGTACTGCTTAACCAG-3′; Maf forward 5’-GGAGACCGACCGCATCATC-3’ reverse 5’-TCATCCAGTAGTAGTCTTCCAGG-3’; Mafb forward 5’-TTCGACCTTCTCAAGTTCGACG-3’, reverse 5’-TCGAGATGGGTCTTCGGTTCA-3’.
Immunoblotting
LPMs were sorted as for microarrays, pooled from multiple mice and lysed at a concentration of 25 million cells/mL using the RIPA lysis buffer system (Santa Cruz Biotechnology). Laemmli buffer (Bio-Rad, with added 2-mercaptoethanol) was added to the samples, which were then boiled for 10 minutes, and run on a Bio-Rad Miniprotean TGX gel with Precision Plus Dual Color molecular weight standards (Bio-Rad). Proteins were transferred to a BioBlot polyvinylidine fluoride membrane (Costar). Blots were blocked for 1–2 hours with 5% milk, followed by overnight staining with primary antibodies in 5% milk at 4 °C with shaking. After washing, blots were stained with horseradish peroxidase-conjugated secondary antibodies for 45–60 minutes at RT with shaking. After washing, blots were developed with either Super Signal West Femto Maximum Sensitivity Substrate (Thermo Fisher) or Clarity Western ECL Substrate (Bio-Rad). Images were captured on a Chemidoc system (Bio-Rad) and inverted on Adobe Illustrator for presentation. Blots were stripped with Restore PLUS Western Blot Stripping Buffer (ThermoFisher). Blots were then washed, reblocked and restained. Antibodies used are listed in Supplementary Table 1.
Gene set enrichment analysis
Lists of differentially expressed genes (≥1.5-fold up- or downregulated) were cross-referenced to the C5 gene sets in the MSigDB database. To further examine the enrichment of gene sets, the GSEA software from the Broad Institute was used to analyze all expressed genes or all Bhlhe40-bound expressed genes for gene set enrichment using the Hallmark and C5 databases42,45.
ChIP-seq
Anti-Bhlhe40 ChIP-seq was performed as previously published33. LPMs were sorted as for microarrays and pooled from multiple mice. Cells were fixed for 10 min at RT in 1% PFA with shaking. Cross-linking was stopped with glycine added to 0.125 M, cells were pelleted and dry pellets were stored at −80 °C. Cross-linked chromatin was sonicated and immunoprecipitated using rabbit anti-Dec1 (Bhlhe40) antibody (NB100–1800, Lot C1; Novus Biologicals). Following immunoprecipitation, the GenElute PCR cleanup kit (Sigma) was used to purify DNA. Library construction was followed by single-read sequencing on a HiSeq3000 (Illumina) at the Genome Technology Access Center at Washington University in St. Louis. Read length was 50 base pairs (bp). Quality control of FASTQ files used FastQC (0.11.3). Bowtie (1.1.1) was used to map reads onto the mm10 mouse reference genome. Input DNA samples were used for peak calling on Bhlhe40-immunoprecipitation samples using MACS v1.4 with default settings56. Generated peaks were additionally required to have fold-enrichment ≥5 and reads from unmapped regions (chrUn_xxxxx) were excluded.
Normalized tracks were generated with Deeptools (2.5.3), and tracks were visualized with the UCSC Genome Browser. Discriminative Regular Expression Motif Elicitation (DREME) (5.0.1)57 was used for motif enrichment analysis using 250 bp flanked summits of all acquired peaks. To annotate peaks, R package ChIPseeker (1.14.1) was used. The intersect function from the BEDtools suite (v2.25.0) was used to find shared peaks. The shared peak count was defined as the number of overlapping peaks in the naïve LPM Bhlhe40 ChIP-seq sample compared to the in vivo IL-4c-stimulated LPM Bhlhe40 ChIP-seq sample. Two group Venn diagrams were generated with the Venn Diagram Plotter tool.
ChIP-seq data for PU.1 performed on LPMs (GSM1533894) and the corresponding input sample (GSM1533895)35 were downloaded in SRA format and converted to FASTQ format using the fastq-dump function (v2.8.1) from the SRA Toolkit. Subsequent processing and filtration was performed as described above. The shared peak count was defined as the number of overlapping peaks in the naïve LPM Bhlhe40 ChIP-seq sample compared to each of the other samples, except for the comparison of in vivo IL-4c-stimulated LPM Bhlhe40 ChIP-seq and LPM PU.1 ChIP-seq samples, which was defined as the number of overlapping peaks in the IL-4c Bhlhe40 sample. Three group Venn diagrams were generated with the eulerAP3 v3 tool (www.eulerdiagrams.org/eulerAPE/)58.
Statistical analysis
All data are from at least two independent experiments, unless otherwise indicated. Data were analyzed by paired or unpaired two-tailed Student’s t-tests (Prism 7; GraphPad Software, Inc.) as indicated in the figure legends, with p ≤ 0.05 considered significant. For relevant comparisons where no p-value is shown, the p-value was > 0.05. For analysis of gene lists against the MSigDB database, the hypergeometric test performed by the Investigate Gene Sets tool was used to determine significance. For GSEA analysis, the NES score calculated by the GSEA software was used to account for set size effects when determining enrichment. The GSEA-calculated FWER p-value was used to determine significance, as this statistic is more conservative than the False Discovery Rate (FDR). Horizontal bars represent the mean and error bars represent the standard error of the mean (s.e.m.).
Reporting summary
Further information on experimental design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The data that support the findings of this study are available from the corresponding author upon request. The microarray and ChIP-sequencing data have been deposited in the GEO repository under accession code GSE125730.
Supplementary Material
Acknowledgements
This work was supported by the National Institute of Allergy and Infectious Disease (NIAID) (AI113118 and AI132653) (B.T.E.) and a Burroughs Wellcome Fund Career Award for Medical Scientists (B.T.E.). N.N.J. was supported by grant 5T32AI007163 from the NIAID. C.-C.L. was supported by the McDonnell International Scholars Academy at Washington University. S.C.-C.H. was supported by the Case Comprehensive Cancer Center American Cancer Society IRG Award (IRG-16-186-21). M.E.C. was supported by the National Science Foundation Graduate Research Fellowship program (DGE-1745038). Research reported in this publication was supported by the Washington University Institute of Clinical and Translational Sciences grant UL1TR002345 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official view of the NIH. We thank E. Lantelme, A. Cullen and D. Brinja for help with fluorescence activated cell-sorting. We thank W. Beatty and L. Mwaghore for electron microscopy. We thank S. Van Dyken and C. Farnsworth for critical reading of the manuscript. We thank the members of the G. Randolph laboratory for helpful discussions about this project. We thank J. Bando, M. Robinette and T. Ai for help with flow cytometry of the gut.
Footnotes
Competing Interests
The authors declare no competing financial interests.
References
- 1.Ginhoux F et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330, 841–845 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schulz C et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science 336, 86–90 (2012). [DOI] [PubMed] [Google Scholar]
- 3.T’Jonck W, Guilliams M & Bonnardel J Niche signals and transcription factors involved in tissue-resident macrophage development. Cell. Immunol 330, 43–53 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hashimoto D et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity 38, 792–804 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yona S et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 38, 79–91 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aziz A, Soucie E, Sarrazin S & Sieweke MH MafB/c-Maf deficiency enables self-renewal of differentiated functional macrophages. Science 326, 867–871 (2009). [DOI] [PubMed] [Google Scholar]
- 7.Soucie EL et al. Lineage-specific enhancers activate self-renewal genes in macrophages and embryonic stem cells. Science 351, 680–693 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Imperatore F et al. SIRT1 regulates macrophage self-renewal. EMBO J. 36, 2353–2372 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosas M et al. The transcription factor Gata6 links tissue macrophage phenotype and proliferative renewal. Science 344, 645–648 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okabe Y & Medzhitov R Tissue-specific signals control reversible program of localization and functional polarization of macrophages. Cell 157, 832–844 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gautier EL et al. Gata6 regulates aspartoacylase expression in resident peritoneal macrophages and controls their survival. J. Exp. Med 211, 1525–1531 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jenkins SJ et al. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science 332, 1284–1288 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jenkins SJ et al. IL-4 directly signals tissue-resident macrophages to proliferate beyond homeostatic levels controlled by CSF-1. J. Exp. Med 210, 2477–2491 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruckerl D & Allen JE Macrophage proliferation, provenance, and plasticity in macroparasite infection. Immunol. Rev 262, 113–133 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Minutti CM et al. Local amplifiers of IL-4Ralpha-mediated macrophage activation promote repair in lung and liver. Science 356, 1076–1080 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bosurgi L et al. Macrophage function in tissue repair and remodeling requires IL-4 or IL-13 with apoptotic cells. Science 356, 1072–1076 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gundra UM et al. Alternatively activated macrophages derived from monocytes and tissue macrophages are phenotypically and functionally distinct. Blood 123, e110–122 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gundra UM et al. Vitamin A mediates conversion of monocyte-derived macrophages into tissue-resident macrophages during alternative activation. Nat. Immunol 18, 642–653 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kato Y, Kawamoto T, Fujimoto K & Noshiro M DEC1/STRA13/SHARP2 and DEC2/SHARP1 coordinate physiological processes, including circadian rhythms in response to environmental stimuli. Curr. Top. Dev. Biol 110, 339–372 (2014). [DOI] [PubMed] [Google Scholar]
- 20.Ow JR, Tan YH, Jin Y, Bahirvani AG & Taneja R Stra13 and sharp-1, the non-grouchy regulators of development and disease. Curr. Top. Dev. Biol 110, 317–338 (2014). [DOI] [PubMed] [Google Scholar]
- 21.Lin CC et al. IL-1-induced Bhlhe40 identifies pathogenic T helper cells in a model of autoimmune neuroinflammation. J. Exp. Med 213, 251–271 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun H & Taneja R Stra13 expression is associated with growth arrest and represses transcription through histone deacetylase (HDAC)-dependent and HDAC-independent mechanisms. Proc. Natl. Acad. Sci. USA 97, 4058–4063 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.St-Pierre B, Flock G, Zacksenhaus E & Egan SE Stra13 homodimers repress transcription through class B E-box elements. J. Biol. Chem 277, 46544–46551 (2002). [DOI] [PubMed] [Google Scholar]
- 24.Li Y et al. The expression of antiapoptotic protein survivin is transcriptionally upregulated by DEC1 primarily through multiple sp1 binding sites in the proximal promoter. Oncogene 25, 3296–3306 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qian Y, Zhang J, Jung YS & Chen X DEC1 coordinates with HDAC8 to differentially regulate TAp73 and DeltaNp73 expression. PLoS One 9, e84015 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seimiya M et al. Impaired lymphocyte development and function in Clast5/Stra13/DEC1-transgenic mice. Eur. J. Immunol 34, 1322–1332 (2004). [DOI] [PubMed] [Google Scholar]
- 27.Kanda M et al. Transcriptional regulator Bhlhe40 works as a cofactor of T-bet in the regulation of IFN-γ production in iNKT cells. Proc. Natl. Acad. Sci. USA 113, E3394–3402 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kreslavsky T et al. Essential role for the transcription factor Bhlhe41 in regulating the development, self-renewal and BCR repertoire of B-1a cells. Nat. Immunol 18, 442–455 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Camponeschi A et al. DEC1/STRA13 is a key negative regulator of activation-induced proliferation of human B cells highly expressed in anergic cells. Immunol. Lett 198, 7–11 (2018). [DOI] [PubMed] [Google Scholar]
- 30.Martinez-Llordella M et al. CD28-inducible transcription factor DEC1 is required for efficient autoreactive CD4+ T cell response. J. Exp. Med 210, 1603–1619 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin CC et al. Bhlhe40 controls cytokine production by T cells and is essential for pathogenicity in autoimmune neuroinflammation. Nat. Commun 5, 3551 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu F et al. The transcription factor Bhlhe40 is a switch of inflammatory versus antiinflammatory Th1 cell fate determination. J. Exp. Med 215, 1813–1821 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huynh JP et al. Bhlhe40 is an essential repressor of IL-10 during Mycobacterium tuberculosis infection. J. Exp. Med 215, 1823–1838 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gabryšová L & O’Garra A Regulating the regulator: Bhlhe40 directly keeps IL-10 in check. J. Exp. Med 215, 1767–1769 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gosselin D et al. Environment drives selection and function of enhancers controlling tissue-specific macrophage identities. Cell 159, 1327–1340 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gautier EL et al. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat. Immunol 13, 1118–1128 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lavin Y et al. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell 159, 1312–1326 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scott CL et al. Bone marrow-derived monocytes give rise to self-renewing and fully differentiated Kupffer cells. Nat. Commun 7, 10321 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bain CC et al. Long-lived self-renewing bone marrow-derived macrophages displace embryo-derived cells to inhabit adult serous cavities. Nat. Commun 7, 11852 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shaw TN et al. Tissue-resident macrophages in the intestine are long lived and defined by Tim-4 and CD4 expression. J. Exp. Med 215, 1507–1518 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim KW et al. MHC II+ resident peritoneal and pleural macrophages rely on IRF4 for development from circulating monocytes. J. Exp. Med 213, 1951–1959 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Subramanian A et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 102, 15545–15550 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gautier EL, Ivanov S, Lesnik P & Randolph GJ Local apoptosis mediates clearance of macrophages from resolving inflammation in mice. Blood 122, 2714–2722 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bertoli C, Skotheim JM, & de Bruin RA Control of cell cycle transcription during G1 and S phases. Nat. Rev. Mol. Cell. Biol 14, 518–528 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liberzon A et al. The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell. Syst 1, 417–425 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cain DW et al. Identification of a tissue-specific, C/EBPβ-dependent pathway of differentiation for murine peritoneal macrophages. J. Immunol 191, 4665–4675 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Franklin RA & Li MO Ontogeny of tumor-associated macrophages and its implication in cancer regulation. Trends Cancer 2, 20–34 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhu Y et al. Tissue-resident macrophages in pancreatic ductal adenocarcinoma originate from embryonic hematopoiesis and promote tumor progression. Immunity 47, 323–338 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Loyher PL et al. Macrophages of distinct origins contribute to tumor development in the lung. J. Exp. Med 215, 2536–2553 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mantovani A, Marchesi F, Malesci A, Laghi L & Allavena P Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol 14, 399–416 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Online Methods References
- 51.Sun H, Lu B, Li RQ, Flavell RA & Taneja R Defective T cell activation and autoimmune disorder in Stra13-deficient mice. Nat. Immunol 2, 1040–1047 (2001). [DOI] [PubMed] [Google Scholar]
- 52.Finkelman FD et al. Anti-cytokine antibodies as carrier proteins. Prolongation of in vivo effects of exogenous cytokines by injection of cytokine-anti-cytokine antibody complexes. J. Immunol 151, 1235–1244 (1993). [PubMed] [Google Scholar]
- 53.Camberis M, Le Gros G & Urban J Jr. Animal model of Nippostrongylus brasiliensis and Heligmosomoides polygyrus. Curr. Protoc. Immunol Chapter 19, Unit 19.12 (2003). [DOI] [PubMed] [Google Scholar]
- 54.Bando JK et al. The tumor necrosis factor superfamily member RANKL suppresses effector cytokine production in group 3 innate lymphoid cells. Immunity 48, 1208–1219 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jablonski KA et al. Novel markers to delineate murine M1 and M2 Macrophages. PLoS One 10, e0145342 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang Y et al. Model-based analysis of ChIP-Seq (MACS). Genome Biol. 9, R137 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bailey TL DREME: motif discovery in transcription factor ChIP-seq data. Bioinformatics 27, 1653–1659 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Micallef L & Rodgers P EulerAPE: drawing area-proportional 3-Venn diagrams using ellipses. PLoS One 9, e101717 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon request. The microarray and ChIP-sequencing data have been deposited in the GEO repository under accession code GSE125730.