Summary
Regulated activation of the cytokine TGFβ by integrins αvβ6 and αvβ8 expressed on keratinocytes is required for residence of epidermal-resident memory T cells, but whether skin-derived signals also affect recirculating memory cells in the skin remains unclear. Here, we show that after resolution of skin vaccinia virus (VV) infection, antigen-specific circulating memory CD8+ T cells migrated into skin. In mice lacking αvβ6 and αvβ8 integrins (Itgb6−/−Itgb8fl/fl-K14-cre), the absence of epidermal activated TGFβ resulted in a gradual loss of E- or P-selectin-binding central and peripheral memory populations, that was rescued when skin entry was inhibited. Skin recirculating memory cells were required for optimal host defense against skin VV infection. These data demonstrate that skin migration can persist after resolution of local skin infection and that the cytokine environment within this nonlymphoid tissue shapes the differentiation state and persistence of the central and peripheral memory T cell pool.
Keywords: skin, CD8+ T cell memory, Transforming growth factor beta, αvβ6, αvβ8, keratinocytes
eToc:
Whether circulating memory CD8+ T cells enter uninflamed skin remains unclear. Hirai et al. show that after resolution of skin Vaccinia Virus infection, circulating CD8+ memory T cells migrate through the skin and require the cytokine TGFβ for their persistence. These findings demonstrate that the peripheral tissue environment shapes central and peripheral memory T cell pools.
Graphical Abstract
INTRODUCTION
A central feature of adaptive immunity is the establishment of memory T cells specific for past pathogen encounters that provide an accelerated and augmented response upon subsequent encounters. In defense against intracellular pathogens such as viruses, CD8+ T cells are the principle cell type involved in host cell-mediated immunity. Following an initial pathogen encounter, naïve CD8+ T cells that encounter their cognate antigen proliferate and differentiate into short-lived terminal effectors (TE) as well as several types of long-lived memory cells (Jameson and Masopust, 2018). Tissue resident memory (Trm) cells remain positioned within non-lymphoid sites of initial pathogen encounter and provide a rapid response to local reinfection. Memory T cells that are found in blood can be divided into central memory (Tcm) cells that express high endothelial venule (HEV) homing molecules and constitutively recirculate through secondary lymphoid organs (SLO), and effector memory (Tem) cells that lack HEV homing molecules (Masopust and Schenkel, 2013). Recently, this nomenclature has been refined to segregate nonlymphoid tissue recirculating memory T cells (peripheral memory cells, Tpm cells, that constitutively enter and leave) from Tem cells that are confined to blood-contiguous compartments. In addition to surveying peripheral tissues Tpm cells can give rise to Tcm cells (Gerlach et al., 2016).
Understanding the mechanisms that underlie the differentiation and maintenance of CD8+ T cell memory has been a long-standing objective given the potential utility of augmenting memory development in the context of vaccines and cancer immunotherapy or inhibiting memory in the context of CD8+ T cell-mediated autoimmune disease. A key cytokine regulating CD8+ T cell memory is TGFβ (Sanjabi et al., 2017). Experiments using genetic methods to block TGFβ-signaling in CD8+ T cells have found that TGFβ limits numbers of TEs by inducing apoptosis (Sanjabi et al., 2009) and appears to be required for maintenance of non-resident memory cells (Ma and Zhang, 2015). In addition, Trm cells in the skin, gut, and lung require TGFβ for differentiation (Casey et al., 2012; Mackay et al., 2013; Wakim et al., 2015).
TGFβ is produced by many cell types always in an inactive form and is complexed with the latency-associated peptide that requires activation for bioactivity (Worthington et al., 2011). In the epidermis, TGFβ activation is mediated exclusively by the integrins αvβ6 and αvβ8 (Aluwihare et al., 2009; Yang et al., 2007). αvβ6 is expressed by barrier epithelia including intrafollicular keratinocytes (KC) and αvβ8 is expressed by several cell types including follicular KC (Mohammed et al., 2016; Worthington et al., 2011). In Itgb6−/−Itgb8ΔKC (Itgb6−/−Itgb8fl/fl-K14-cre mice that lack both TGFβ-activating integrins in the skin, CD8+ effectors are efficiently recruited into the skin but Trm cells fail to persist (Mohammed et al., 2016). Thus, transactivation of TGFβ represents a tissue-derived signal that is required for long-term survival of Trm cells within the skin. Whether skin-derived signals also affect recirculating memory cells that are only transiently in the skin remains unclear.
Using the well-defined vaccinia virus (VV) skin scarification model (Liu et al., 2010), we report that after resolution of skin infection, circulating antigen-specific skin-homing memory CD8+ T cells entered the skin where they encountered integrin-activated TGFβ. In the absence of activated TGFβ, normal expansion and differentiation of CD8+ T cells occured but skin-homing KLRG1−CD127+ memory precursors (MP), CXCR3+CX3CR1− Tcm cells, and CXCR3+CX3CR1int Tpm cells were lost from the secondary lymphoid tissues. This loss could be rescued if skin entry was inhibited. These data demonstrate that after resolution of skin infection, recirculating CD8+ T cells transit through the skin where local production of TGFβ is required for persistence of Tcm and Tpm memory subsets.
RESULTS
Vaccinia virus-specific tissue resident and recirculating memory CD8+ T cells require αvβ6 and αvβ8
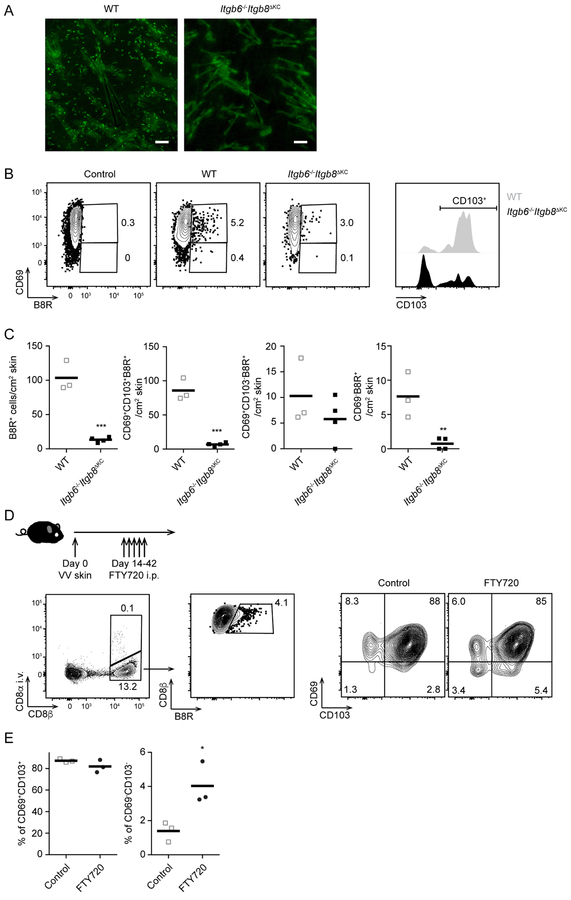
We previously showed that in the context of a systemic LCMV infection and epicutanteous application of the sensitizing hapten 1-Fluoro-2,4-dinitrobenzene (DNFB) to “pull” non-antigen specific cells into the skin, epidermal persistence of Trm cells required KC-mediated activation of TGFβ by the integrins αvβ6 and αvβ8 (Mohammed et al., 2016). To determine whether the presence of antigen in the target tissue could affect the integrin requirement for Trm cells persistence (Muschaweckh et al., 2016), we infected cohorts of Itgb6−/−Itgb8ΔKC mice that have a global absence of αvβ6 and a KC-specific deletion of αvβ8 with recombinant vaccinia virus expressing the immunodominant OVA257–264 peptide from ovalbumin (VV-OVA) using skin scarification (Liu et al., 2010). A time course measuring viral load in the skin demonstrated viral clearance by day 14 post infection with similar kinetics in Itgb6−/−Itgb8ΔKC and WT mice, thereby indicating that infectivity was not affected by the absence of αvβ6 and αvβ8 (Figure S1A). We next adoptively transferred 105 Thy1.1+ OVA257–264-specific OT-I transgenic CD8+ T cells into naïve WT or Itgb6−/−Itgb8ΔKC mice followed by skin infection with VV-OVA. Epidermal whole mounts obtained 42 days post infection revealed numerous Trm cells in WT epidermis but very few in Itgb6−/−Itgb8ΔKC epidermis consistent with our previous observations (Figure 1A). A similar result was obtained when WT and Itgb6−/−Itgb8ΔKC were infected with VV and antigen-specific cells in the skin were analyzed by flow cytometry using the VV-specific B8R tetramer. B8R+ CD8+ T cells (B8R+ cells) were substantially decreased in the skin of Itgb6−/−Itgb8ΔKC mice compared to WT mice at day 68 post infection (Figure 1B and 1C). As expected the majority of CD69+ B8R+ cells from WT mice expressed CD103 in WT mice indicative of Trm cells (Mackay et al., 2013). CD103+ B8R+ cells were largely lost in Itgb6−/−Itgb8ΔKC mice with a preferential sparing of CD103− cells (Figure 1B and 1C). Because TGFβ signal is required for the expression of CD103 and CD103−/− T cells cannot persist in the skin (Mackay et al., 2013), our observation is consistent with a model in which TGFβ activation by αvβ6 and αvβ8 integrins is required for both CD103 expression and maintenance of Trm cells in the skin.
Figure 1. Trm cells and recirculating CD8+ T cells are decreased in the skin of Itgb6−/−Itgb8ΔKC mice after skin vaccinia virus (VV)-infection.
(A) Representative epidermal whole mounts from OT-I adoptive transferred WT and Itgb6−/−Itgb8ΔKC mice at day 42 after VV-OVA skin infection stained for OT-I cells (Thy1.1, green). Scale bar=100 μm. (B) VV-infected skin at day 68 post infection were analyzed by flow cytometry. Plots (left panels) are gated on live CD45+CD3+TCRb+CD8+ T cells (WT and Itgb6−/−Itgb8ΔKC) or CD45+CD3+TCRb+CD8− T cells (control), and B8R+ CD69+ (right panel). (C) Total number of B8R+ cells from (B) are shown. (D) Experimental scheme for testing skin recirculating CD8+ T cells in VV-infected skin. Anti-CD8α antibody was intravenously injected to stain intravascular CD8+ T cells before harvesting VV-infected skin for flow cytometric analysis one day after the last treatment of FTY720. Flow plots gated on live CD90+ T cells (left panel), i.v. CD8α−CD8β+ (middle panel), and B8R+ cells (right panels). (E) The frequency of i.v. CD8α−B8R+ gated cells in the skin are shown. Data are representative of two independent experiments. Each dot represents an individual mouse. Flow cytometry plots are shown as concatenations for all mice in each group within the same experiment. *p < 0.05, **p < 0.01, and ***p < 0.001. See also Figure S1.
In addition to the loss of CD103+ Trm cells in the skin of Itgb6−/−Itgb8ΔKC mice, we noted the near-absence of CD69− B8R+ cells (Figure 1B and 1C). CD69− T cells in peripheral tissues are assumed to be recirculating cells that egress in a sphingosine 1-phosphate receptor-1 (S1P1) -dependent manner, though CD69 is an imperfect marker of Trm cells (Mackay et al., 2015; Steinert et al., 2015; Takamura et al., 2016). To determine whether CD69− CD8+ T cells are recirculating, we skin infected WT mice with VV and used FTY720-treatments to block skin egress starting on day 14 after the resolution of the acute infection coupled with i.v. anti-CD8α staining to identify intravascular cells (Figure 1D) (Anderson et al., 2014). Very few cells were labeled by i.v. anti-CD8α indicating that our skin preparations had little contamination from intravascular cells. FTY720 treatments increased the frequency of CD69− cells, but not CD69+CD103+ Trm cells, consistent with a similar report in the lung (Figure 1D and 1E) (Takamura et al., 2016). These data suggest that CD69− cells in the skin represent recirculating cells rather than intravascular or Trm cells and raised the possibility that the absence of cutaneous TGFβ activation in Itgb6−/−Itgb8ΔKC mice could affect circulating CD8+ T cell memory.
CD8+ memory T cells decay in Itgb6−/−Itgb8ΔKC mice
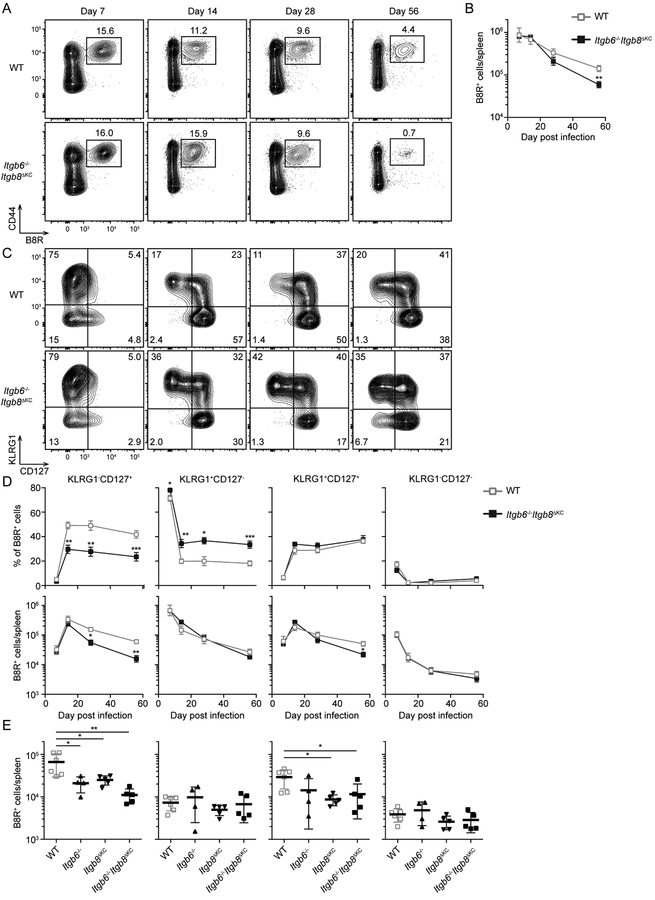
To test whether CD8+ T cell expansion or memory phenotype are altered in Itgb6−/−Itgb8ΔKC mice after vaccinia virus infection, we performed a time course comparing B8R+ CD8+ T cells in spleen of WT and Itgb6−/−Itgb8ΔKC mice (Figure 2A–B). Expansion of B8R+ cells was similar in both groups but beyond day 14 post skin VV infection we noted a gradual and persistent reduction in the number of splenic B8R+ cells in Itgb6−/−Itgb8ΔKC mice.
Figure 2. Vaccinia specific splenic CD8+ T cells are not maintained in Itgb6−/−Itgb8ΔKC mice.
Spleens from WT or Itgβ6−/−Itgβ8ΔKC mice were analyzed by flow cytometry at the indicated time point after skin VV infection. Representative plots gated on live CD3+CD8+ cells (A) the total number of B8R+ cells (B) at each time point are shown. (C) Representative plots gated on B8R+ cells from (A) are shown. (D) The frequency (upper panels) and number (lower panels) of splenic B8R+ cells are shown. (E) Splenic B8R+ cells from WT, Itgβ6−/−, Itgβ8ΔKC, or Itgβ6−/−Itgβ8ΔKC mice were analyzed by flow cytometry at 68 days after skin VV infection. Data are representative of two or three independent experiments with at least four mice per time point. Data are means ± s.e.m. *p < 0.05, **p < 0.01, and ***p < 0.001. See also Figure S2.
We used expression of the markers KLRG1 and CD127 to further phenotype B8R+ cell subsets (Figure 2C–D). The decrease of B8R+ cells was primarily due to loss of KLRG1−CD127+ MP both in relative percentage and in total number of B8R+ cells starting after day 14 post infection (Figure 2D). The percentage but not total numbers of KLRG1+CD127− terminal effectors (TE) were increased in Itgb6−/−Itgb8ΔKC mice from day 14 post infection reminiscent of the higher TE:MP ratio seen in CD8+ T cells rendered insensitive to TGFβ (Figure 2D) (Ma and Zhang, 2015). A similar, though less pronounced phenotype was also observed in draining lymph nodes (dLNs) (Figure S2). Therefore, we concluded that systemic B8R+ KLRG1−CD127+ MP in Itgb6−/−Itgb8ΔKC mice were decreased at later time points after skin VV.
To test the individual contribution of αvβ6 and αvβ8, we compared the phenotype of B8R+ cells at day 68 post VV skin infection in WT, Itg◻6−/−, Itgb8ΔKC, and Itgb6−/−Itgb8ΔKC mice. Both Itgb6−/− and Itg◻8ΔKC had significantly fewer B8R+ KLRG1−CD127+ MP compared to WT mice, though the reduction was not as great compared to double integrin-deficient Itgb6−/−Itgb8ΔKC mice (Figure 2E, left panel). This is consistent with our earlier data showing that keratinocytes express either αvβ6 or αvβ8 depending on their location in the epidermis (Mohammed et al., 2016). Because Itgβ6−/− mice have a global absence of Itgb6, we cannot exclude some potential role for αvβ6 in non-skin tissues. However, the fact that Itgβ8ΔKC mice with a skin-specific ablation of Itgb8 have reduced numbers of MP and that a partial phenotype in single integrin-deficient mice is predicted based on the expression of both Itgb6 and Itgb8 by KC argues that loss of integrin expression in the skin of Itgb6−/−Itgb8ΔKC mice accounts for the loss of KLRG1−CD127+ B8R+ cells.
VV-specific T cells are not decreased after intravenous VV infection in Itgb6−/−Itgb8ΔKC mice
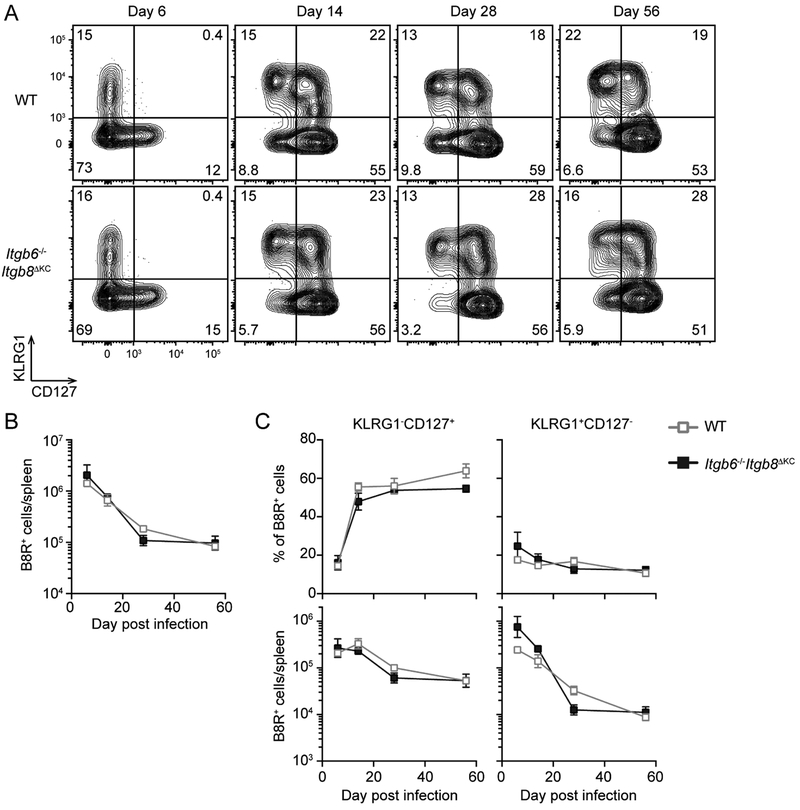
Circulating memory CD8+ T cells are unaffected in Itgb6−/−Itgb8ΔKC mice following systemic LCMV infection (Mohammed et al., 2016). Because expression of Itgb6 is limited to barrier epithelia (Worthington et al., 2011) and ablation of Itgb8 is confined to the skin through K14 driven expression of Cre, we hypothesized that systemic VV infection of Itgb6−/−Itgb8ΔKC mice would have no effect on KLRG1−CD127+ MP. Consistent with this hypothesis, kinetic analysis of subsets of B8R+ cells in the spleen was equivalent in WT and Itgb6−/−Itgb8ΔKC mice following i.v. VV infection (Figure 3A and B). Thus, the loss of KLRG1−CD127+ MP occurs following skin but not systemic infection and Itgb6−/−Itgb8ΔKC mice do not have an intrinsic immune defect that could explain their phenotype.
Figure 3. Intravenous vaccinia virus (VV)-induced CD8+ T cells are maintained normally in Itgb6−/−Itgb8ΔKC mice.
Splenic cells at the indicated time following intravenous VV infection in WT or Itgb6−/−Itgb8ΔKC mice were analyzed for KLRG1−CD127+ memory precursors and KLRG1+CD127− terminal effectors by flow cytometry. (A) Representative plots gated on B8R+ cells are shown. (B) Total number of B8R+ cells, and (C) frequency (top panels) and total number (bottom panels) of KLRG1−CD127+ or KLRG1+CD127− B8R+ cells are shown. Data are representative of two separate experiments with at least four mice per each time point. Data are means ± s.e.m.
Loss of CD8+ MP is not due to altered priming
Although we did not observe altered expansion of B8R+ cells in Itgb6−/−Itgb8ΔKC mice (Figure 2), we next looked more carefully at early time points for potential subtle changes in Itgb6−/−Itgb8ΔKC mice. B8R-specific CD8+ T cell expansion in blood peaked by day 9 post skin vaccinia virus infection followed by the expected contraction phase (Figure S3A). Numbers of all subsets were equivalent in WT and Itgb6−/−Itgb8ΔKC mice following skin or i.v. infection until day 14 when the decrease of KLRG1−CD127+ B8R+ cells in skin infected Itgb6−/−Itgb8ΔKC mice became evident. It has been reported that increased plasma TGFβ following Listeria monocytogenes infection can reduce the number of KLRG1+CD127− CD8+ T cells (Sanjabi et al., 2009). This does not appear to occur following VV infection as serum TGFβ levels are unchanged (Figure S3B).
To test for possible defects in priming, we next analyzed B8R+ cells in dLNs on day 7 post infection (Figure S4). As expected, the number of B8R+ subsets as well as phenotypic markers were largely unchanged between WT and Itgb6−/−Itgb8ΔKC mice. Induction of T-bet, which is a critical determinant of effector differentiation (Joshi et al., 2007), was identical between the groups.
As TGFβ is known to regulate Langerhans cell migration, we next enumerated dendritic cell (DC) subsets in dLNs on day 3 and 7 post infection. Numbers of skin migratory DCs and resident DCs in dLNs as well as skin-resident DCs were equivalent in WT and Itgb6−/−Itgb8ΔKC mice with the expected exception of Langerhans cells that are decreased in Itgb6−/−Itgb8ΔKC mice (Figure S5B–C). The absence of Langerhans cells in Itgb6−/−Itgb8ΔKC mice, however, did not explain the loss of MP as the number of splenic B8R+ cells at day 56 post skin VV infection were equivalent in WT and Langerhans cells-deficient mice (Figure S5D–E). Thus, we concluded that the decrease of VV-specific MP in Itgb6−/−Itgb8ΔKC mice was not due to altered differentiation in early infection.
Loss of MP is maintained after adoptive transfer
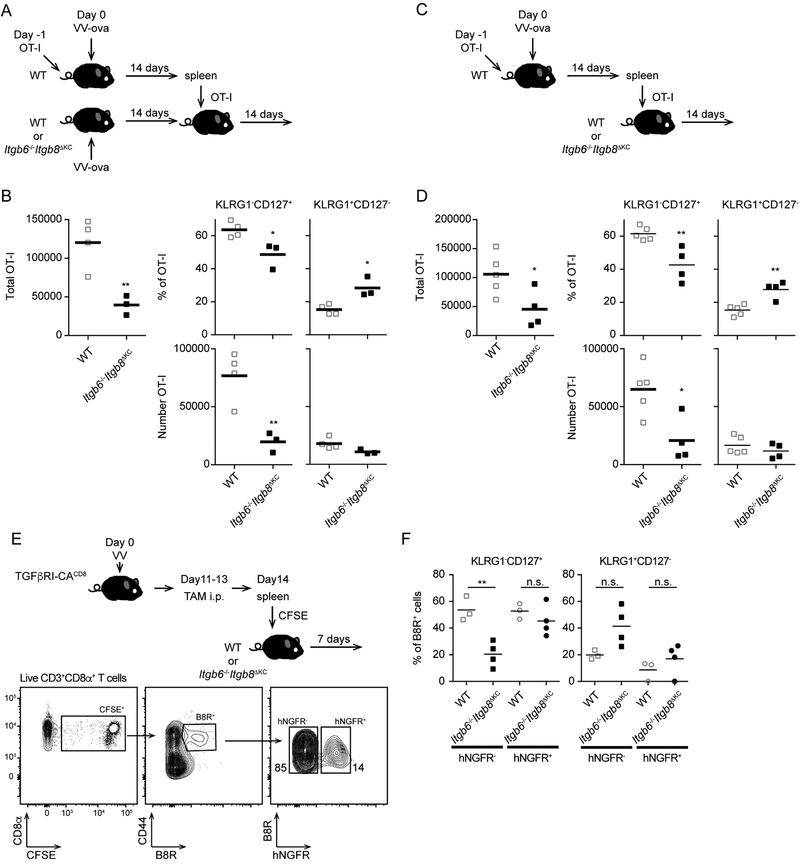
To determine whether cells primed in WT mice maintained their phenotype after transfer, we transferred 105 naïve Thy1.1+ OT-I cells to WT mice followed by skin VV-OVA infection. On day 14 post infection, a time point at which the virus has been cleared (Figure S1A), we purified splenic CD8+ T cells and adoptively transferred 2 × 106 OT-I cells into infection-matched WT or Itgb6−/−Itgb8ΔKC recipients (Figure 4A). On day 14 post transfer, total numbers of splenic OT-I cells were decreased in Itgb6−/−Itgb8ΔKC mice compared to WT mice due primarily to the loss of KLRG1−CD127+ MP, thus recapitulating our earlier findings (Figure 4B). We repeated a similar series of experiments in which OT-I cells from WT infected mice were adoptively transferred into naïve WT or Itgb6−/−Itgb8ΔKC mice (Figure 4C). Total OT-I cells and KLRG1−CD127+ MP but not KLRG1+CD127− TE were reduced in Itgb6−/−Itgb8ΔKC recipients (Figure 4D). These results demonstrate that the loss of KLRG1−CD127+ MP in Itgb6−/−Itgb8ΔKC mice after skin VV infection results from the absence of these integrins at time points beyond 14 days and that alterations in priming, possible effects on CD4+ helper T cells (Laidlaw et al., 2016), potential effects of persistent antigen (Zammit et al., 2006), or possible effects resulting from the VV infection itself cannot explain the observation. These data, together with the observed gradual decay over time of MP in Itgb6−/−Itgb8ΔKC mice suggest a scenario in which MP are recruited into the skin at times after the resolution of infection where they must receive αvβ6- and αvβ8-dependent signals for persistence.
Figure 4. KLRG1−CD127+ Memory precursors are not maintained following adoptive transfer into integrin-deficient mice.
(A) The experimental scheme for adoptive transfer into matched infected is shown. OT-I adoptive transfer WT mice were infected with VV-OVA. On day 14 post infection, splenic CD8+ T cells were isolated and CD8+ T cells containing 2 × 106 OT-I cells were adoptively transferred into matched infected WT or Itgb6−/−Itgb8ΔKC mice. (B) Total numbers of Thy1.1+OT-I cells (left panel) from the spleens of recipient WT or Itgb6−/−Itgb8ΔKC mice 28 days after infection (14 days post transfer) are shown (left panel). The percentage (top panels) and total number (bottom panels) of KLRG1+CD127− and KLRG1−CD127+ OT-I cells are shown. (C) The experimental scheme for adoptive transfer into naive mice is shown. As in (A) except cells were adoptively transferred into naïve WT or Itgb6−/−Itgb8ΔKC recipients. (D) Total numbers of Thy1.1+OT-I cells from the spleens of recipient WT or Itgb6−/−Itgb8ΔKC mice 28 days after infection (14 days post transfer) is shown (left panel). The percentage (top, right panels) and total number (bottom, right panels) of KLRG1+CD127− and KLRG1−CD127+ OT-I cells is shown. (E) Experimental scheme for testing a direct requirement for TGFβ on skin recirculating memory cells. Flow plots shows gating strategy for analysis of the transferred CFSE+B8R+ cells expressing TGFβRI-CA (hNGFR+) or control (hNGFR−) in spleen day 7 post transfer. (F) Frequency of the indicated subsets of CFSE+B8R+ cells are shown. Data are representative of two to four independent experiments. Each dot represents an individual mouse. *p < 0.05 and **p < 0.01. See also Figure S3, S4, and S5.
To determine whether loss of KLRG1−CD127+ MP in Itgb6−/−Itgb8ΔKC mice is the direct result of reduced active TGFβ, we employed mice expressing CreERT2 recombinase driven by a combination of the core E8I enhancer and the CD8a promoter (E8I-CreERT2). These mice were bred with mice that contain the gene for a constitutively active, ligand independent form of the TGFβ receptor I (TGFβR-CA) behind a lox-stop-lox cassette (Bartholin et al., 2008) and mice containing the reporter human nerve growth factor receptor (hNGFR) in the Rosa26 locus also behind a lox-STOP-lox cassette. The resulting mice (TGFβRI-CACD8) allow for tamoxifen-induced expression of TGFβR-CA in CD8+ T cells that can be monitored by expression of hNGFR. TGFβRI-CACD8 mice were infected with VV and treated with tamoxifen i.p. on days 11–13 after the infection. On day 14 post infection, CD8+ T cells were purified from the spleen, labeled with CFSE for tracking and then adoptively transferred into naïve WT or Itgb6−/−Itgb8ΔKC recipient mice. Seven days after transfer approximately 10–20% of the transferred splenic B8R+ cells expressed hNGFR indicative of Cre activity (Figure 4E). The percentage of KLRG1+CD127− and KLRG1−CD127+ B8R+ CD8 T cells was equivalent in hNGFR− and hNGFR+ populations in WT recipients. In contrast, in Itgb6−/−Itgb8ΔKC recipients, KLRG1−CD127+ MP were reduced in the hNGFR− population but remained at levels similar to that seen in WT mice in the hNGFR+ population (Figure 4F). Thus, expression of TGFβRCA was able to rescue the loss of MP cells in Itgb6−/−Itgb8ΔKC mice. This demonstrated that αvβ6- and αvβ8-mediated activation of TGFβ acts directly on CD8+ T cells to maintain MP numbers following VV infection.
Skin-homing memory cells are selectively decreased in Itgb6−/−Itgb8ΔKC mice
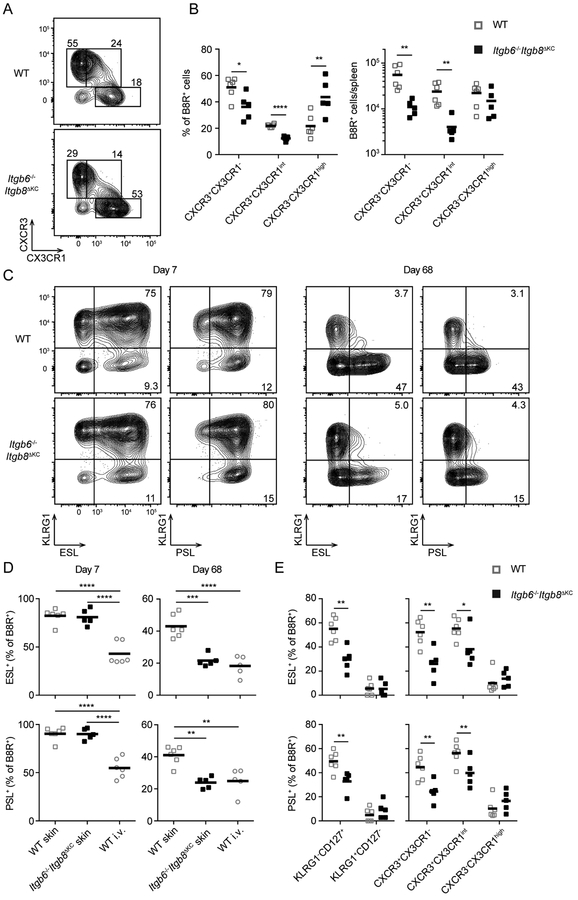
Recently, a revised strategy to subset CD8+ T cells has been proposed based on expression of CX3CR1 and CXCR3: CXCR3+CX3CR1− Tcm cells, CXCR3+CX3CR1int Tpm cells, and CXCR3−CX3CR1high Tem cells (Gerlach et al., 2016). Using this strategy, we observed that on day 68 following VV infection, numbers of B8R+ Tcm and Tpm cells were reduced in Itgb6−/−Itgb8ΔKC mice (Figure 5A and B). This is consistent with the observation that Tem cells inefficiently recirculate peripherally (Gerlach et al., 2016).
Figure 5. Skin-homing memory cells are decreased in Itgb6−/−Itgb8ΔKC mice.
(A and B) Splenic B8R+ cells from WT or Itgb6−/−Itgb8ΔKC mice were analyzed for memory subsets based on expression of CXCR3 and CX3XR1 by flow cytometry at day 68 post skin vaccinia virus (VV) infection: CXCR3+CX3CR1− Tcm cells, CXCR3+CX3CR1int Tpm cells and CXCR3−CX3CR1high Tem cells. (A) Representative flow cytometry plots gated on B8R+ cells and the (B, left panel) frequency and (B, right panel) number of each memory subset of B8R+ cells are shown. (C and D) E-selectin or P-selectin binding (ESL+ or PSL+ respectively) of splenic B8R+ cells were analyzed by flow cytometry following skin VV infection. Representative flow plots (C) and the frequency of ESL+ (top panels) or PSL+ (bottom panels) B8R+ cells day 7 and 68 post infection are shown. (D) Frequency of ESL+ (top panels) or PSL+ (bottom panels) among each subset of memory B8R+ cells in the spleen are shown. Data are representative of two separate experiments. Each dot represents an individual mouse. *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001. See also Figure S6.
To directly assess skin-homing cells, we examined skin-homing markers, E-selectin ligands (ESL) and P-selectin ligands (PSL) on B8R+ cells (Fuhlbrigge et al., 1997; Woodland and Kohlmeier, 2009). The frequency of E- or P-selectin binding cells (ESL+ and PSL+ respectively) were identical at day 7 post skin VV infection, consistent with data showing no phenotype at this time, but were significantly decreased in Itgb6−/−Itgb8ΔKC mice at day 68 post infection (Figure 5C and D). Notably, skin infection induced significantly more ESL+ and PSL+ B8R+ cells compared to i.v. infection. The loss of ESL+ and PSL+ cells on day 68 in Itgb6−/−Itgb8ΔKC mice reduced levels down to that observed following i.v. infection. ESL+ and PSL+ cells were enriched in KLRG1−CD127+, CXCR3+CX3CR1−, and CXCR3+CX3CR1int populations in WT mice, the same subsets that show a selectively decreased in Itgb6−/−Itgb8ΔKC mice (Figure 5E). As expected, in the skin the majority of memory CD69− cells were KLRG1-CD127+ and ESL+ which were greatly enriched compared to blood circulating cells (Figure S6). FTY720 treatments further enriched this same population in skin. From these data we conclude that skin, but not i.v. infection imprints skin-homing capacity of CD8+ T cells consistent with earlier observations (Gebhardt et al., 2011; Liu et al., 2010; Woodland and Kohlmeier, 2009) and that skin-homing Tpm and Tcm cells are reduced in Itgb6−/−Itgb8ΔKC.
Skin recirculating CD8+ T cells require integrin-mediated TGFβ activation for maintenance
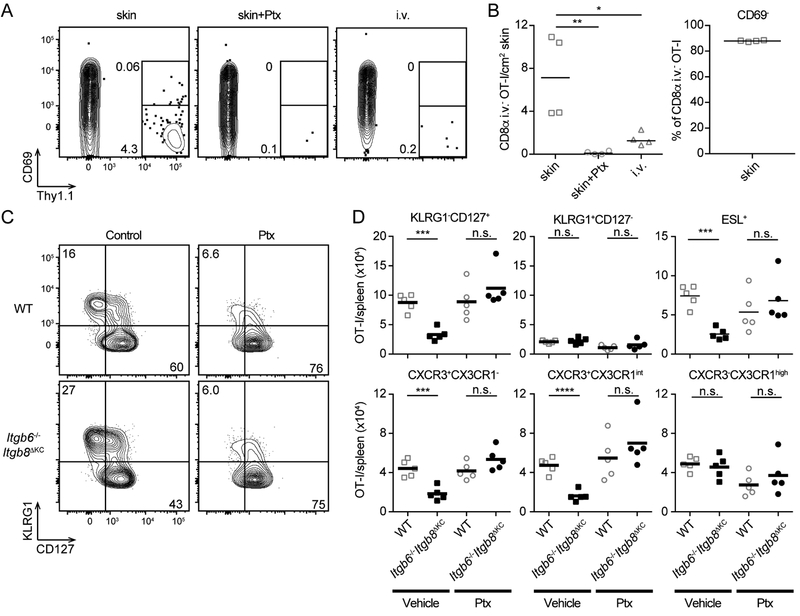
To directly demonstrate that systemic CD8+ T cells recirculate through skin, we used the same OT-I adaptive transfer model into naïve WT recipients as in Figure 4C. On day 7 after adoptive transfer of cells from infected WT hosts (21 days after priming) there was a clearly evident population of i.v. CD8α− OT-I CD8+ T cells in skin (Figure 6A–B). OT-I cells treated prior to transfer with pertussis toxin (Ptx) that prevents entry into peripheral tissue (Ward et al., 1998) or OT-I cells transferred from mice following i.v. VV infection were much less abundant in recipient skin. Furthermore, the vast majority of these cells were CD69−. From these data we conclude that circulating CD8+ T cells have the capacity to enter skin in the absence of infection or other stimuli and they can be identified based on their lack of CD69 expression.
Figure 6. Skin recirculating CD8+ T cells require TGF-β activation by keratinocytes for maintenance.
(A and B) Naïve Thy1.1+OT-I cells were transferred to WT mice followed by skin or intravenous vaccinia virus (VV)-OVA infection. Total CD8+ T cells were collected from spleens 14 days post VV-OVA infection and 106 OT-I cells treated with vehicle or pertussis toxin (Ptx) (100 ng/mL) were adoptively transferred to naïve WT recipient mice. Anti-CD8α antibody was intravenously injected to stain intravascular CD8+ T cells before harvesting skin for flow cytometric analysis. (A) Representative plot of OT-I cells from the skin of recipient mice 7 days post transfer gated on live i.v. CD8α−CD8β+ cells are shown. (B) The total number of OT-I cells and frequency of CD69− OT-I cells in the skin is shown. (C and D) Naïve Thy1.1+OT-I cells were transferred to WT mice followed by skin VV-OVA infection. Total CD8+ T cells were isolated from spleens 14 days post skin VVOVA infection and treated with vehicle or pertussis toxin (Ptx) (100 ng/mL) immediately before adoptive transfer (106 OT-I/mouse) into naïve WT or Itgb6−/−Itgb8ΔKC recipients. (C) Representative plots and (D) total numbers of OT-I cells in the spleen 7 days after transfer are shown. Data are representative of two or three separate experiments. Each dot represents an individual recipient. n.s.; not significant, *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001. See also Figure S7.
To confirm that memory CD8+ T cells recirculating through the skin require αvβ6- and αvβ8-activated TGFβ for maintenance, we repeated the adoptive transfer of cells from day 14 infected WT mice into WT and Itgb6−/−Itgb8ΔKC mice (as in Figure 4C) but pretreated the transferred cells with Ptx or vehicle. Since memory CD8+ T cells survive without TGFβ in a systemic Listeria monocytogenes infection model (Ma and Zhang, 2015), we hypothesized that those cells that could not circulate through the skin would no longer require TGFβ and thus persist in Itgb6−/−Itgb8ΔKC mice. On day 7 post transfer, numbers of KLRG1−CD127+ OT-I cells in WT recipients of Ptx-treated OT-I cells were not altered compared to WT recipients of vehicle treated control cells (Figure 6C and D, open squares). In contrast, numbers of KLRG1−CD127+ OT-I cells in Itgb6−/−Itgb8ΔKC recipients were significantly decreased but were rescued by treatment with Ptx resulting in numbers comparable to WT mice (Figure 6D, closed circles). A similar result was observed with ESL+, CXCR3+CX3CR1− Tcm cells and CXCR3+CX3CR1int, Tpm cells (Figure 6D and S7). Taken together, we concluded that αvβ6- and αvβ8-mediated activation of TGFβ is required for maintenance of circulating memory cells that home to the skin.
Skin-homing circulating memory CD8+ T cells contribute to host defense against skin VV infection
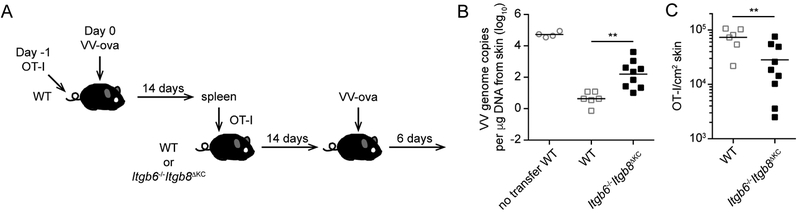
To determine whether the loss of skin-homing circulating CD8+ memory T cells in Itgb6−/−Itgb8ΔKC mice has a functional consequence, we transferred OT-I cells into WT mice followed by a skin VV-OVA infection. On day 14 post infection, splenic CD8+ T cells were isolated and adoptively transferred into naïve WT and Itgb6−/−Itgb8ΔKC mice. Fourteen days following transfer, recipient mice were skin infected with VV-OVA. Six days after infection, skin was harvested and analyzed for number of OT-I cells by flow cytometry and VV DNA by qPCR (Figure 7A). As expected, WT mice that received CD8+ T cells from previously infected WT mice show approximately 4 logs of protection compared with no transfer WT mice (Figure 7B). Notably, there was approximately a 2 log greater viral burden in Itgb6−/−Itgb8ΔKC recipient mice. Itgb6−/−Itgb8ΔKC recipients did show VV resistance compared with no transfer mice. Analysis of the number of OT-I cells in the skin of infected recipients confirmed that the number of OT-I cells in Itgb6−/−Itgb8ΔKC recipient mice was significantly lower than WT recipients consistent with the lower numbers of circulating memory CD8+ T cells in Itgb6−/−Itgb8ΔKC mice (Figure 7C). From these data we conclude that skin-homing memory CD8+ T cells have the capacity to provide host defense against VV and that the loss of skin recirculating cells in Itgb6−/−Itgb8ΔKC mice results in reduced protection to VV.
Figure 7. Skin-recirculating memory CD8+ T cells contribute to host defense against skin VV infection.
(A) The experimental scheme is shown. OT-I adoptive transfer WT mice were infected with skin vaccinia virus (VV)-OVA. On day 14 post infection, splenic CD8+ T cells were isolated and CD8+ T cells containing 106 OT-I cells were adoptively transferred into naive WT or Itgb6−/−Itgb8ΔKC recipient mice. Fourteen days after the transfer, the mice were challenged with skin VV-OVA and the infected skin were harvested 6 days after the challenge. (B) qPCR for VV DNA at the infected skin site of WT and Itgb6−/−Itgb8ΔKC mice is shown. (C) Total numbers of OT-I cells identified by flow cytometry in the skin are shown. Data are representative of two separate experiments. Each symbol represents an individual recipient. **p < 0.01.
DISCUSSION
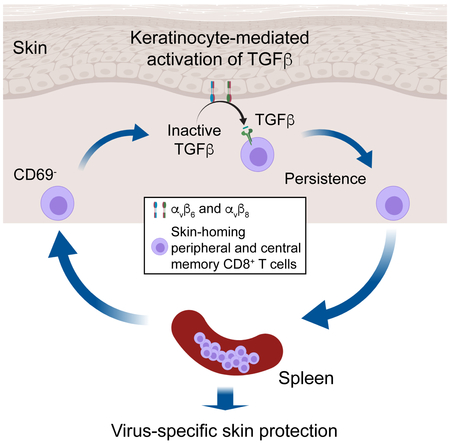
We have demonstrated that following resolution of vaccinia virus skin infection in Itgb6−/−Itgb8ΔKC mice lacking the TGFβ-activating integrins αvβ6 and αvβ8 in the skin, there was a gradual loss of antigen-specific, skin-homing KLRG1−CD127+ MP, CXCR3+CX3CR1− Tcm cells, and CXCR3+CX3CR1int Tpm cells from the secondary lymphoid tissues that was first evident during the contraction phase. In addition, CD69− CD8+ T cells were absent from the skin of previously infected Itgb6−/−Itgb8ΔKC mice. CD69− CD8+ T cells represent a population of skin recirculating cells as evidenced by their presence in the skin of naïve mice following adoptive transfer of splenic CD8+ T cells isolated from previously infected mice and by their augmented numbers in skin when egress is inhibited by FTY720. The lack of skin CD69− CD8+ T cells in VV-infected Itgb6−/−Itgb8ΔKC mice when combined with the selective loss of skin homing memory CD8+ T cell populations that can be rescued when skin recirculation is inhibited by pre-treatment with pertussis toxin allows us to conclude that at time points after the resolution of skin infection, circulating skin-homing memory CD8+ T cells transit through the skin. In the skin, recirculating memory cells require αvβ6- or αvβ8-activated TGFβ for their long-term persistence. These data reveal a contribution by nonlymphoid tissue to the shaping of recirculating memory CD8+ T cell populations.
It is notable that Tpm and Tcm cells are lost when adoptively transferred into naïve Itgb6−/−Itgb8ΔKC recipients. As recipient mice lack VV-derived antigens, the effects of TGFβ must be cell-intrinsic and occur independently of TCR engagement. This is further supported by the ability of constitutive TGFβ signaling to rescue memory CD8 T cells in Itgb6−/−Itgb8ΔKC recipients. Our efforts have focused on adoptive transfer shortly after resolution of infection. It remains possible that circulation of CD8+ T cells through skin and their TGFβ-dependence may occur only during a specific window of time following infection. Nevertheless, we demonstrate that 1) T-cell intrinsic homing to skin was encoded during priming in the context of a skin (but not i.v.) infection, 2) that this homing program was retained upon transfer to naïve mice and thus did not require ongoing antigen presentation or inflammation in the skin, and that 3) the cytokine milieu within the skin impacted the differentiation state and survival of the systemic T cell pool and this would obviously be long-lasting.
Tpm cells (CX3CR1int) comprise a recently defined memory T cell subset thought to be primarily responsible for recirculation between nonlymphoid tissues and blood. We observed a selective loss of skin-homing Tpm cells and also Tcm cells in Itgb6−/−Itgb8ΔKC mice. Given the known conversion of Tpm cells into Tcm cells, the loss of skin recirculating Tpm cells may give rise to a concomitant loss of Tcm cells. Alternatively, the relatively high binding of E- and P-selectin on Tcm cells, observations that Tcm cells can be recovered from human skin (Gehad et al., 2018; Watanabe et al., 2015), and the proposed use of CCR7 as a nonlymphoid tissue exit mechanism (Bromley et al., 2005; Debes et al., 2005) suggests that Tcm cells may circulate through the skin and also depend on KC-mediated TGFβ activation. Notably, Tem cells defined as CXCR3−CX3CR1hi expressed low levels of skin-homing markers and were not lost in Itgb6−/−Itgb8ΔKC mice supporting the concept that they circulate inefficiently through peripheral tissues (Gerlach et al., 2016).
TGFβ is well known to control CD8+ T cell biology at multiple stages including limiting TE numbers by inducing apoptosis and by promoting Trm cells differentiation in the skin, gut, and lung (Casey et al., 2012; Mackay et al., 2013; Sanjabi et al., 2009; Wakim et al., 2015). In the skin and gut Trm cells also depend on TGFβ for persistence (Mohammed et al., 2016). Our data indicates that in addition to Trm cells, skin-recirculating non-resident memory CD8+ T cells also have a TGFβ dependence for their long-term maintenance. The decline of non-resident skin-homing memory cells in Itgb6−/−Itgb8ΔKC mice could be averted by inhibition of skin homing, suggesting the necessity of KC-mediated TGFβ occurs during skin recirculation. This suggests that memory CD8+ T cells in the skin including both resident and non-resident populations are dependent on TGFβ for persistence. This could potentially be related to the more “harsh” environment encountered at barrier surfaces (e.g. commensal and pathogen exposure, mechanical injury, high O2, and UV exposure) (Woodland and Kohlmeier, 2009). TGFβRII ablation in memory CD8+ T cells following systemic Listeria monocytogenes infection increased the TE:MP ratio reminiscent of the effects of skin homing memory cells in Itgb6−/−Itgb8ΔKC mice but without the loss of total cell number (Ma and Zhang, 2015). Thus, non-resident memory CD8+ T cells that circulate though tissues other than skin may also depend on active TGFβ in their target tissue to maintain a memory phenotype.
Unlike other nonlymphoid tissues, skin supports significant memory CD4+ T cell recirculation (Bromley et al., 2013; Gebhardt et al., 2011). The function of recirculating memory CD8+ T cells in skin is overall poorly understood. They are very unlikely to serve as a source of Trm cells under steady-state conditions in the skin since parabiotic experiments have demonstrated little Trm cells contribution from the parabiont and Trm cells have been shown to be able to self-renew (Jiang et al., 2012; Park et al., 2018). Recirculating memory CD8+ T cells could potentially still be recruited into the Trm cells population in the context of stimuli that deplete Trm cells from skin (e.g. UV irradiation) (Mohammed et al., 2016). This may differ for other peripheral tissues such as the lung where recirculating memory CD8+ T cells are required for maintenance of Trm cells (Slütter et al., 2017) and could potentially explain how skin immunization produces Trm cells at the mucosal surfaces (Gaide et al., 2015; Zaric et al., 2017).
In the setting of amnestic host defense to epicutaneous vaccinia virus infection the majority of host defense is provided by Trm cells but circulating memory cells do provide some measure of host defense, particularly at later timepoints (Jiang et al., 2012). We find that in the absence of Trm cells, circulating skin-homing memory cells provide significant host defense against VV-skin challenge that is attenuated in Itgb6−/−Itgb8ΔKC mice with reduced number of skin homing memory cells. Recirculating memory cells may, thus, provide an important source of protection at skin locations distant from the initial infection site where Trm cells may be low.
In addition to providing host defense, memory CD8+ T cells in skin also participate in autoimmune disease pathogenesis (Clark, 2015). Blockade of TGFβ activation through inhibition of αvβ6 and αvβ8 can successfully deplete Trm cells in skin and gut (Mohammed et al., 2016) thereby providing a potential approach for therapeutic Trm cells depletion that would have minimal side effects, particularly if administered topically. The dependence of skin homing Tpm and Tcm cells on integrin-mediated TGFβ activation suggests that inhibition of αvβ6 and αvβ8 in the skin may also selectively deplete these populations. Thus, in addition to defining a role for TGFβ-mediated tissue imprinting of skin-homing circulating memory CD8+ T cells, this work also provides a potential therapeutic avenue to deplete populations of both resident and recirculating pathogenic memory CD8+ T cells in the context of autoimmune skin diseases.
STAR METHODS
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Daniel H. Kaplan (dankaplan@pitt.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Itgb6−/− and Itgb8loxP mice were provided by Dean Sheppard (University of California, San Francisco). E8ICreERT2 and ROSA26.LSL.hNGFR reporter mice were provided by Dario A. Vignali (University of Pittsburgh). Human Langerin-DTA and TGFβRI-CA mice have been previously described (Kaplan et al., 2005; Bartholin et al., 2008). C57BL/6 (WT), Tg(KRT14-cre)1Amc/J (K14-Cre), C57BL/6-Tg(TcraTcrb)1100Mjb/J (OT-I), B6.129S7-Rag1tm1Mom/J (Rag−/−), CBy.PL(B6)-Thy1a/ScrJ (Thy1.1) mice were purchased from Jackson Laboratories. We crossed K14-Cre mice with Itgb8loxP and Itgb6−/− mice to obtain Itgb6−/−Itgb8ΔKC mice (Mohammed et al., 2016). E8ICreERT2 mice were crossed with ROSA26.LSL.hNGFR mice and TGFβRI-CA mice to obtain TGFβRI-CACD8 mice. We generated Thy1.1+Rag−/−OT-I mice by crossing OT-I mice with Rag−/− and Thy1.1 mice. We used age- and sex-matched (males and female) mice that were between 6 and 12 weeks of age in all experiments. All mice were maintained under specific-pathogen-free conditions and all animal experiments were approved by University of Pittsburgh Institutional Animal Care and Use Committee.
METHOD DETAILS
Infections
Mice were infected by skin scarification (skin infection) or intravenous injection (i.v. infection) with 2 × 106 plaque-forming units recombinant vaccinia virus expressing the SIINFEKL peptide of ovalbumin (VV-OVA). For skin scarification, 30 mL of VV was applied to shaved left flank (4–5 cm2) and the skin were gently scratched 100 times with 27 G needle under anesthesia. For FTY720 treatments, mice were intraperitoneally injected FTY720 (Cayan chemical) (1 μg/g) in saline with 0.5% DMSO daily.
Quantitative PCR for determination of viral load
Vaccinia viral load was evaluated by quantitative real-time PCR as described by others (Freyschmidt et al., 2007). The skin was harvested and DNA was purified with the DNeasy Blood & Tissue Kit (Qiagen) according to the manufacturer’s instructions. PCR was performed with the StepOnePlus Real-Time PCR System (Applied Biosystems, Foster City, CA). The primers and TaqMan probe used in the quantitative PCR assay are specific for the vaccinia ribonucleotide reductase Vvl4L. The sequences of the primers are: forward, 5’-GAC ACT CTG GCA GCC GAA AT-3’; and reverse, 5’-CTG GCG GCT AGA ATG GCA TA-3’. The TaqMan probe was synthesized by Applied Biosystems with 5’-labeled with FAM and 3’-labeled with TAMRA. The sequence of the probe is: 5’-AGC AGC CAC TTG TAC TAC ACA ACA TCC GGA-3’. Amplification reactions were performed in the MicroAmp Fast Optical 96-Well Reaction Plate (Applied Biosystems) in a 20 μL volume containing 2x TaqMan Master Mix (Applied Biosystems), 500 nM forward primer, 500 nM reverse primer, 150 nM probe, and the template DNA. Thermal cycling conditions were 50°C for 2 min and 95°C for 10 min for one cycle. Subsequently, 40 cycles of amplification were performed at 95°C for 15 s and 60°C for 1 min. Viral load was determined by a stan dard curve from DNA of a VV stock.
Adoptive transfers
For Naïve OT-I cells transfer, OT-I cells were purified from spleen and lymph nodes of Thy1.1+Rag−/−OT-I mice by MojoSort Mouse CD8 T Cell Isolation Kit (Biolegend) according to the manufacturer’s instructions and 1 × 105 OT-I cells were intravenously transferred to WT or Itgb6−/−Itgb8ΔKC mice followed by VV-OVA infection one day later. For transfer of OT-I cells from the infected mice, spleen was harvested from WT mice day 14 post VV-OVA infection with OT-I cells and total CD8+ T cells were purified by MojoSort Mouse CD8 T Cell Isolation Kit (Biolegend). Frequency of OT-I cells in the CD8+ T cells were determined by flow cytometry and CD8+ T cells containing 106 OT-I cells were intravenously transferred into matched infected (day 14 post VV-OVA infected) or naïve WT or Itgb6−/−Itgb8ΔKC mice. For transfer of CD8+ T cells from infected TGFβRI-CACD8 mice, TGFβRI-CACD8 mice were infected with skin VV and treated intraperitoneal tamoxifen (50 mg/kg) (Sigma-Aldrich) in 1:9 200 proof ethanol:corn oil (Sigma-Aldrich) daily from day 11 to 13 post infection. The spleen was harvested from TGFβRI-CA mice day 14 post VV infection, total CD8+ T cells were purified by MojoSort Mouse CD8 T Cell Isolation Kit (Biolegend). The CD8+ T cells were resuspended in PBS at a concentration of 4 × 106 cells/mL and CFSE (CellTrace™ CFSE Cell Proliferation Kit; Invitrogen) was mixed at final concentration of 5 μM for 5 min at room temperature. The cells were washed with RPMI1640 supplemented with 10% FBS (Hyclone, Logan, UT) three times and adoptively transferred into recipient mice (4 × 106 CD8+ T cells/mouse). For experiments with pertussis toxin, the purified CD8+ T cells (3–4 × 106 cells/mL) were treated with 100 ng/ml pertussis toxin (Sigma-Aldrich) for 90 min at 37°C in complete RPMI1640 supplemented with 10% FBS (Hyclone, Logan, UT), 2-mercaptoethanol (50 μM; Sigma-Aldrich) and 1x MEM Non-Essential Amino Acids (Gibco), followed by three times wash and transferred to recipient mice (106 OT-I cells/mouse).
Immunofluorescence of epidermis
Epidermal sheets were prepared as previously described (Mohammed et al., 2016). Briefly, shaved defatted flank skin was affixed to slides with double-sided adhesive (3M, St. Paul, MN). Slides were incubated in 10 mM EDTA in PBS for 90 min at 37°C, followed by physical removal of the dermis. The epidermal sheets were fixed in 4% PFA at RT for 30 min. The eidermal sheets were blocked with PBS containing 0.1% tween-20, 2% BSA and 2% rat serum for 2 h at RT before staining overnight with antibodies in PBS containing 0.1% tween-20 and 0.5% BSA. Images were captured on a IX83 fluorescent microscope (Olympus Tokyo, Japan) using a x10 objective; image analysis was performed using cellSens Dimension software (Olympus).
Flow cytometry
Preparation of single cells suspension from tissues were performed as previously described (Mohammed et al., 2016). Briefly, for preparing single cells suspension from the skin, shaved skin was harvested and fat tissues was removed mechanically by forceps. The skin was minced finely with scissors and resuspended in RPMI1640 (Gibco, Grand Island, NY) with 2.5 mg/ml collagenase XI (Sigma-Aldrich), 0.25 mg/ml hyaluronidase (Sigma-Aldrich), 0.1 mg/ml DNase (Sigma-Aldrich), 0.01 M HEPES (Sigma-Aldrich), and 10% FBS followed by incubation in a shaking incubator for 1 h at 37°C a t 250 rpm. The resulting cells were filtered through a 40 mm cell strainer (BD Biosciences). Lymph nodes were incubated in 400 U/mL Collagenase D (Roche Applied Science) and 0.1 mg/ml DNase in RPMI1640 with 10% FBS for 40 min at 37°C a nd then mashed through a 40 mm cell strainer. Spleen were mechanically mashed through a 40 mm cell strainer followed by red blood cell lysing buffer (Sigma-Aldrich) treatment. Blood were collected with heparin (Sigma-Aldrich) and treated with red blood cell lysing buffer (Sigma-Aldrich). Single-cell suspensions were blocked with 2.4G2 culture supernatant (American Type Culture Collection) and were stained with antibodies to extracellular markers, B8R tetramer (2.5 μg/mL) (biotinylated monomer was obtained from NIH Tetramer Core Facility and tetramized with APC-streptavidin; Prozyme), E- or P- selectin Fc Chimera Protein (5 and 2 μg/mL respectively) and/or Fixable Viability Dye (eFluor 780) (eBioscience) in PBS with 2% calf serum (Hyclone) and 0.05% Azide for 30 min at 4°C o r 37°C (B8R staining) followed by incubation with Goat Anti-Human IgG, Fcγ Fragment Specific (Jackson ImmunoResearch, West Grove, PA) for detecting E-selectin or P-selectin binding. For T-bet staining, cells were fixed and permeabilized with true nuclear transcripion factor buffer set (Biolegend) according to the manufacturer’s instructions. For Granzyme B staining, cells were fixed and permeabilized with Cytofix/Cytoperm kit (BD Biosciences) according to the manufacturer’s instructions. For intracellular cytokine staining, cells were incubated with 1 μg/mL B8R20–27 TSYKFESV (AnaSpec, Fremont, CA) for 1 h at 37°C an d then incubated another 4 h with additional Protein Transport Inhibitor Cocktail (eBioscience). The intracellular cytokine staining was performed with Cytofix/Cytoperm kit (BD Biosciences) according to the manufacturer’s instructions. Anti-KLRG1 (2F1/KLRG1), CD127 (A7R34), CD8α (53–6.7), CD44 (IM7), TCRβ (H57–597), CXCR3 (CXCR3–173), CX3CR1 (SA011F11), CD3 (17A2), Thy1.2 (30-H12), Thy1.1 (OX-7), granzyme B (GB11), T-bet (4B10), CD69 (H1.2F3), CD103 (2E7), CD45.2 (104), CD27 (LG.3A10), CD122 (TM-β1), IL-2 (JES6–5H4), TNF-α (MP6-XT22), MHCII (M5/114.15.2), and CD11b (M1/70) were purchased from Biolegend. Anti-CD8α (53–6.7), CD8β (H35–17.2), CD44 (IM7), CXCR3 (CXCR3–173), and Thy1.1 (OX-7) were purchased from BD Biosciences. Anti-IFN-γ (XMG1.2) and CD11c (N418) were purchased from TONBO bioscience (San Diego, CA). Anti-Langerin (929F3.01) was purchased from Novus Biologicals (Littleton, CO). A BD LSRFORTESSA (BD Biosciences) and Flowjo software (TreeStar, Ashland, OR) were used for analysis.
Intravascular staining
Staining intravascular CD8+ T cells was performed as previously described (Anderson et al., 2014). Animals were injected intravenously with BUV737-conjugated anti-CD8α (53–6.7) (3 μg/mouse) through the tail vein. Three minutes’ post-injection, animals were sacrificed, and tissues were harvested. Isolation of cells from tissues was done as described.
ELISA
Blood were collected with heparin and centrifuged at 5,000g at 4 °C for 15 min to obtain the plasma. Plasma TGFβ was activated by 1 N HCI and the acidified samples was neutralized by 1.2 N NaOH/0.5 M HEPES. Total TGFβI in the samples were measured by ELISA kit (R&D systems) according to the manufacturer’s instructions.
QUANTIFICATION AND STATISTICAL ANALYSIS
Groups were compared with Prism software (GraphPad) using the two-tailed unpaired Student’s t test for comparison of two groups or Dunnett’s test for comparisons of more than two groups with the control. Data are presented as each data point and mean or mean ± standard error of the mean (s.e.m.). p < 0.05 was considered significant.
Supplementary Material
Key Points.
CD69− CD8+ T cells in skin are recirculating memory cells
TGFβ-activation in skin is required for recirculating memory CD8+ T cell persistence
The peripheral tissue environment shapes central and peripheral memory T cell pools
Loss of recirculating memory T cells impairs host defense to skin infection
Acknowledgments
We thank the members of the Kaplan laboratory, Vignali laboratory and members throughout the departments of Dermatology and Immunology for helpful discussions. We also thank the Division of Laboratory Animal Resources of the University of Pittsburgh for excellent animal care. This work benefitted from SPECIAL BD LSRFORTESSATM funded by NIH 1S10OD011925–01. TH was supported by JSPS Overseas Research Fellowships and DHK by NIH R01AR060744.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests
The authors declare no competing interests.
REFERENCES
- Aluwihare P, Mu Z, Zhao Z, Yu D, Weinreb PH, Horan GS, Violette SM, Munger JS, 2009. Mice that lack activity of alphavbeta6- and alphavbeta8-integrins reproduce the abnormalities of Tgfb1- and Tgfb3-null mice. J. Cell. Sci 122, 227–232. doi: 10.1242/jcs.035246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KG, Mayer-Barber K, Sung H, Beura L, James BR, Taylor JJ, Qunaj L, Griffith TS, Vezys V, Barber DL, Masopust D, 2014. Intravascular staining for discrimination of vascular and tissue leukocytes. Nature Protocols 9, 209–222. doi: 10.1038/nprot.2014.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholin L, Cyprian FS, Vincent D, Garcia CN, Martel S, Horvat B, Berthet C, Léon SG, Treilleux I, Rimokh R, Marie JC, 2008. Generation of mice with conditionally activated transforming growth factor beta signaling through the TβRI/ALK5 receptor. genesis 46, 724–731. doi: 10.1002/dvg.20425 [DOI] [PubMed] [Google Scholar]
- Bromley SK, Thomas SY, Luster AD, 2005. Chemokine receptor CCR7 guides T cell exit from peripheral tissues and entry into afferent lymphatics. Nature Immunology 6, 895–901. doi: 10.1038/ni1240 [DOI] [PubMed] [Google Scholar]
- Bromley SK, Yan S, Tomura M, Kanagawa O, Luster AD, 2013. Recirculating memory T cells are a unique subset of CD4+ T cells with a distinct phenotype and migratory pattern. J Immunol 190, 970–976. doi: 10.4049/jimmunol.1202805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey KA, Fraser KA, Schenkel JM, Moran A, Abt MC, Beura LK, Lucas PJ, Artis D, Wherry EJ, Hogquist K, Vezys V, Masopust D, 2012. Antigen-Independent Differentiation and Maintenance of Effector-like Resident Memory T Cells in Tissues. J Immunol 188, 4866–4875. doi: 10.4049/jimmunol.1200402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RA, 2015. Resident memory T cells in human health and disease. Science Translational Medicine 7, 269rv1–269rv1. doi: 10.1126/scitranslmed.3010641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins N, Jiang X, Zaid A, Macleod BL, Li J, Park CO, Haque A, Bedoui S, Heath WR, Mueller SN, Kupper TS, Gebhardt T, Carbone FR, 2016. Skin CD4(+) memory T cells exhibit combined cluster-mediated retention and equilibration with the circulation. Nat Commun 7, 11514. doi: 10.1038/ncomms11514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debes GF, Arnold CN, Young AJ, Krautwald S, Lipp M, Hay JB, Butcher EC, 2005. Chemokine receptor CCR7 required for T lymphocyte exit from peripheral tissues. Nature Immunology 6, 889–894. doi: 10.1038/ni1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freyschmidt E-J, Mathias CB, MacArthur DH, Laouar A, Narasimhaswamy M, Weih F, Oettgen HC, 2007. Skin inflammation in RelB−/− mice leads to defective immunity and impaired clearance of vaccinia virus. Journal of Allergy and Clinical Immunology 119, 671–679. doi: 10.1016/j.jaci.2006.12.645 [DOI] [PubMed] [Google Scholar]
- Fuhlbrigge RC, Kieffer JD, Armerding D, Kupper TS, 1997. Cutaneous lymphocyte antigen is a specialized form of PSGL-1 expressed on skin-homing T cells. Nature 389, 978–981. doi: 10.1038/40166 [DOI] [PubMed] [Google Scholar]
- Gaide O, Emerson RO, Jiang X, Gulati N, Nizza S, Desmarais C, Robins H, Krueger JG, Clark RA, Kupper TS, 2015. Common clonal origin of central and resident memory T cells following skin immunization. Nature Medicine 21, 647–653. doi: 10.1038/nm.3860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhardt T, Whitney PG, Zaid A, Mackay LK, Brooks AG, Heath WR, Carbone FR, Mueller SN, 2011. Different patterns of peripheral migration by memory CD4+ and CD8+ T cells. Nature 477, 216–219. doi: 10.1038/nature10339 [DOI] [PubMed] [Google Scholar]
- Gehad A, Teague JE, Matos TR, Huang V, Yang C, Watanabe R, O’Malley JT, Trimble CL, Kupper TS, Clark RA, 2018. A primary role for human central memory cells in tissue immunosurveillance. Blood Advances 2, 292–298. doi: 10.1182/bloodadvances.2017011346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach C, Moseman EA, Loughhead SM, Alvarez D, Zwijnenburg AJ, Waanders L, Garg R, la Torre, de JC, Andrian, von UH, 2016. The Chemokine Receptor CX3CR1 Defines Three Antigen-Experienced CD8 T Cell Subsets with Distinct Roles in Immune Surveillance and Homeostasis. Immunity 45, 1270–1284. doi: 10.1016/j.immuni.2016.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jameson SC, Masopust D, 2018. Understanding Subset Diversity in T Cell Memory. Immunity 48, 214–226. doi: 10.1016/j.immuni.2018.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Clark RA, Liu L, Wagers AJ, Fuhlbrigge RC, Kupper TS, 2012. Skin infection generates non-migratory memory CD8+ TRM cells providing global skin immunity. Nature 483, 227–231. doi: 10.1038/nature10851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM, 2007. Inflammation Directs Memory Precursor and Short-Lived Effector CD8+ T Cell Fates via the Graded Expression of T-bet Transcription Factor. Immunity 27, 281–295. doi: 10.1016/j.immuni.2007.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan DH, Jenison MC, Saeland S, Shlomchik WD, Shlomchik MJ, 2005. Epidermal Langerhans cell-deficient mice develop enhanced contact hypersensitivity. Immunity 23, 611–620. doi: 10.1016/j.immuni.2005.10.008 [DOI] [PubMed] [Google Scholar]
- Laidlaw BJ, Craft J, Kaech SM, 2016. The multifaceted role of CD4+ T cells in the regulation of CD8+ T cell memory maturation. Nat. Rev. Immunol 16, 102–111. doi: 10.1038/nri.2015.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Zhong Q, Tian T, Dubin K, Athale SK, Kupper TS, 2010. Epidermal injury and infection during poxvirus immunization is crucial for the generation of highly protective T cell-mediated immunity. Nature Medicine 16, 224–227. doi: 10.1038/nm.2078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C, Zhang N, 2015. Transforming growth factor-β signaling is constantly shaping memory T-cell population. Proc. Natl. Acad. Sci. U.S.A. 112, 11013–11017. doi: 10.1073/pnas.1510119112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay LK, Braun A, Macleod BL, Collins N, Tebartz C, Bedoui S, Carbone FR, Gebhardt T, 2015. Cutting edge: CD69 interference with sphingosine-1-phosphate receptor function regulates peripheral T cell retention. J Immunol 194, 2059–2063. doi: 10.4049/jimmunol.1402256 [DOI] [PubMed] [Google Scholar]
- Mackay LK, Rahimpour A, Ma JZ, Collins N, Stock AT, Hafon M-L, Vega-Ramos J, Lauzurica P, Mueller SN, Stefanovic T, Tscharke DC, Heath WR, Inouye M, Carbone FR, Gebhardt T, 2013. The developmental pathway for CD103(+)CD8+ tissue-resident memory T cells of skin. Nature Immunology 14, 1294–1301. doi: 10.1038/ni.2744 [DOI] [PubMed] [Google Scholar]
- Masopust D, Schenkel JM, 2013. The integration of T cell migration, differentiation and function. Nat. Rev. Immunol 13, 309–320. doi: 10.1038/nri3442 [DOI] [PubMed] [Google Scholar]
- Mohammed J, Beura LK, Bobr A, Astry B, Chicoine B, Kashem SW, Welty NE, Igyártó BZ, Wijeyesinghe S, Thompson EA, Matte C, Bartholin L, Kaplan A, Sheppard D, Bridges AG, Shlomchik WD, Masopust D, Kaplan DH, 2016. Stromal cells control the epithelial residence of DCs and memory T cells by regulated activation of TGF-[beta]. Nature Immunology 17, 414–421. doi: 10.1038/ni.3396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschaweckh A, Buchholz VR, Fellenzer A, Hessel C, König P-A, Tao S, Tao R, Heikenwälder M, Busch DH, Korn T, Kastenmuller W, Drexler I, Gasteiger G, 2016. Antigen-dependent competition shapes the local repertoire of tissue-resident memory CD8+ T cells. J. Exp. Med 213, 3075–3086. doi: 10.1084/jem.20160888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SL, Zaid A, Hor JL, Christo SN, Prier JE, Davies B, Alexandre YO, Gregory JL, Russell TA, Gebhardt T, Carbone FR, Tscharke DC, Heath WR, Mueller SN, Mackay LK, 2018. Local proliferation maintains a stable pool of tissue-resident memory T cells after antiviral recall responses. Nature Immunology 16, 79–191. doi: 10.1038/s41590-017-0027-5 [DOI] [PubMed] [Google Scholar]
- Sanjabi S, Mosaheb MM, Flavell RA, 2009. Opposing effects of TGF-beta and IL-15 cytokines control the number of short-lived effector CD8+ T cells. Immunity 31, 131–144. doi: 10.1016/j.immuni.2009.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanjabi S, Oh SA, Li MO, 2017. Regulation of the Immune Response by TGF-β: From Conception to Autoimmunity and Infection. Cold Spring Harb Perspect Biol 9, a022236. doi: 10.1101/cshperspect.a022236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slütter B, Van Braeckel-Budimir N, Abboud G, Varga SM, Salek-Ardakani S, Harty JT, 2017. Dynamics of influenza-induced lung-resident memory T cells underlie waning heterosubtypic immunity. Sci Immunol 2, eaag2031–12. doi: 10.1126/sciimmunol.aag2031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert EM, Schenkel JM, Fraser KA, Beura LK, Manlove LS, Igyártó BZ, Southern PJ, Masopust D, 2015. Quantifying Memory CD8 T Cells Reveals Regionalization of Immunosurveillance. Cell 161, 737–749. doi: 10.1016/j.cell.2015.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamura S, Yagi H, Hakata Y, Motozono C, McMaster SR, Masumoto T, Fujisawa M, Chikaishi T, Komeda J, Itoh J, Umemura M, Kyusai A, Tomura M, Nakayama T, Woodland DL, Kohlmeier JE, Miyazawa M, 2016. Specific niches for lung-resident memory CD8+ T cells at the site of tissue regeneration enable CD69-independent maintenance. J. Exp. Med 213, 3057–3073. doi: 10.1084/jem.20160938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakim LM, Smith J, Caminschi I, Lahoud MH, Villadangos JA, 2015. Antibody-targeted vaccination to lung dendritic cells generates tissue-resident memory CD8 T cells that are highly protective against influenza virus infection. Mucosal Immunol 8, 1060–1071. doi: 10.1038/mi.2014.133 [DOI] [PubMed] [Google Scholar]
- Ward SG, Bacon K, Westwick J, 1998. Chemokines and T Lymphocytes: More than an Attraction. Immunity 9, 1–11. doi: 10.1016/S1074-7613(00)80583-X [DOI] [PubMed] [Google Scholar]
- Watanabe R, Gehad A, Yang C, Scott LL, Teague JE, Schlapbach C, Elco CP, Huang V, Matos TR, Kupper TS, Clark RA, 2015. Human skin is protected by four functionally and phenotypically discrete populations of resident and recirculating memory T cells. Science Translational Medicine 7, 279ra39–279ra39. doi: 10.1126/scitranslmed.3010302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodland DL, Kohlmeier JE, 2009. Migration, maintenance and recall of memory T cells in peripheral tissues. Nat. Rev. Immunol 9, 153–161. doi: 10.1038/nri2496 [DOI] [PubMed] [Google Scholar]
- Worthington JJ, Klementowicz JE, Travis MA, 2011. TGFβ: a sleeping giant awoken by integrins. Trends Biochem. Sci 36, 47–54. doi: 10.1016/j.tibs.2010.08.002 [DOI] [PubMed] [Google Scholar]
- Yang Z, Mu Z, Dabovic B, Jurukovski V, Yu D, Sung J, Xiong X, Munger JS, 2007. Absence of integrin-mediated TGFbeta1 activation in vivo recapitulates the phenotype of TGFbeta1-null mice. J. Cell Biol. 176, 787–793. doi: 10.1083/jcb.200611044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit DJ, Turner DL, Klonowski KD, Lefrançois L, Cauley LS, 2006. Residual antigen presentation after influenza virus infection affects CD8 T cell activation and migration. Immunity 24, 439–449. doi: 10.1016/j.immuni.2006.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaric M, Becker PD, Hervouet C, Kalcheva P, Yus BI, Cocita C, O’Neill LA, Kwon S-Y, Klavinskis LS, 2017. Long-lived tissue resident HIV-1 specific memory CD8+ T cells are generated by skin immunization with live virus vectored microneedle arrays. Journal of Controlled Release 268, 166–175. doi: 10.1016/j.jconrel.2017.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.