Abstract
Objective:
Adverse cardiovascular (CV) events occur more frequently in the morning than at other times of the day. Vascular endothelial function (VEF), a robust CV risk marker is impaired during this morning period. We recently discovered that this morning impairment in VEF is not caused by either overnight sleep or the inactivity that accompanies sleep. We determined whether the endogenous circadian system is responsible for this morning impairment in VEF. We also assessed whether the circadian system affects mechanistic biomarkers, i.e. oxidative stress (malondialdehyde, MDA) and endothelin-1 (ET-1), blood pressure (BP) and heart rate (HR).
Approach:
21 (11 women) middle aged healthy participants completed a 5-day laboratory protocol in dim-light where all behaviors, including sleep and activity, and all physiological measurements were evenly distributed across the 24-h period. After baseline testing, participants underwent ten recurring 5-h 20-min ‘behavioral cycles’ of 2-h 40-min sleep opportunities and 2-h 40-min of standardized waking episodes. VEF, BP and HR were measured and venous blood was sampled immediately after awakening during each wake episode.
Results:
Independent of behaviors, VEF was significantly attenuated during the subjective night and across the morning (p=0.04). MDA and ET-1 exhibited circadian rhythms with increases across the morning vulnerable period and peaks around noon (p≤0.01). Both systolic (p=0.005) and diastolic BP (p=0.04), were rhythmic with peaks in the late afternoon.
Conclusions:
The endogenous circadian system impairs VEF and increases MDA and ET-1 in the morning vulnerable hours and may increase the risk of morning adverse CV events in susceptible individuals.
Keywords: Vascular endothelial function, flow mediated dilation, endogenous circadian rhythms, oxidative stress, adverse cardiovascular events, forced desynchrony
Subject codes: Endothelium, Oxidant Stress, Physiology, Vascular Biology, Ultrasound
Introduction:
Many epidemiological studies report that adverse cardiovascular (CV) events including sudden cardiac death and myocardial infarction occur most commonly in the morning soon after awakening1–3. The mechanisms underlying this vulnerable time for adverse CV events are unclear. It has been previously shown that the endogenous circadian system increases the pro-coagulation marker plasminogen activator-inhibitor-1 and the sympathetic reactivity to exercise across the morning vulnerable period, thereby potentially contributing to increased risk of adverse CV events4, 5. Vascular endothelial function (VEF)6 is a comprehensive marker of CV disease that is strongly associated with increased CV risk7. VEF has been found to be impaired during the morning vulnerable period8–11 as compared to other times of the day. The underlying mechanisms for this commonly observed morning impairment (e.g., prior sleep, inactivity during sleep, and the endogenous circadian system) are not well studied. In a recent study we aimed to determine if the decline in VEF across the night was the result of sleep or the inactivity12 that accompanies sleep13. We found that both nocturnal sleep and nocturnal inactivity did not result in decreased VEF13. We therefore speculated that the endogenous circadian system may be responsible for the previously observed decline in VEF across the night. Thus, in the current study, we determined whether or not the endogenous circadian system contributes to the morning decline in VEF while controlling for the effects of behaviors such as prior sleep, physical activity and food consumption. To better understand associated biological mechanisms, we also measured across the circadian cycle blood pressure (BP) heart rate (HR) and markers of oxidative stress (plasma levels of malondialdehyde adducts [MDA] and endothelin-1 [ET-1], both closely associated with impaired endothelial function)14–16. We hypothesized that the endogenous circadian system would impair VEF (measured as flow mediated dilation [FMD] and complementary low-flow mediated constriction [L-FMC]) in the vulnerable morning hours (between 06:00 and noon)3, 10, 17. Given the potential mechanistic link between increased oxidative stress, ET-1, and impaired VEF18, 19, we expected MDA and ET-1 to be rhythmic and peak during this potentially vulnerable period. We expected that BP and HR would be rhythmic with a peak in the evening as has been previously published20. Previous work on diurnal variation in VEF and was conducted largely in young lean individuals who are likely not to be at risk for CV diseases. Thus, we studied a potentially more relevant group of middle aged adults as these represent the age bracket in which adverse CV events predominate. Understanding the contributions of the endogenous circadian system to morning impairment in VEF in healthy middle-aged men and women is a necessary first step to studying individuals with existing CV disease, and may point to appropriate behavioral or pharmacological therapies against increased morning CV risk.
Material and Methods:
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study approval:
The experimental protocol was approved by the Institutional Review Board for human subject protection at Oregon Health & Science University. Written informed consent was obtained from all participants prior to participation in the studies.
We studied VEF, BP, HR, and related biomarkers across a circadian protocol (forced desynchrony, FD)21 that separates the effects of the endogenous circadian system from the effects caused by behaviors such as sleep, physical activity and food consumption.
Participants:
Volunteers were recruited using flyers on campus, internet research recruitment websites and word of mouth. We studied 21 participants (11 females; 4 post-menopausal/7 peri-menopausal) (Supplementary Table I). These data were collected as part of a larger study of the effects of the circadian system, sleep and activity on cardiovascular function in humans (ClinicalTrials.gov Identifier: NCT02202811). The endogenous circadian rhythm in another CV marker relevant to hypertension, i.e. plasma aldosterone has been previously published from this study21.
Screening and at-home routine:
Screening:
Participants were healthy (except for mild untreated hypertension in one female) based on self-report, psychological and physical examinations by a physician, 12 lead electrocardiogram, BP measured three times in the laboratory and across 48-h in the home environment (Spacelabs Healthcare, WA, USA), sleep apnea screening (WatchPAT, Itamar Medical Ltd., Israel), and laboratory testing of hematological and metabolic measures (i.e., hemoglobin, hematocrit levels, basic blood chemistry, and blood glucose). Exclusion criteria included report of any chronic disease, pregnancy, history of smoking or use of any prescription or non-prescription medications as verified by urinalysis of drugs (Drugsmart 12 panel cup, Speares Medical Inc, SC, USA) and cotinine (NicAlert®, Nymox Corporation, NJ, USA), and history of travel across more than three time zones in the last 3 months or shift work in the past 6 months (helping ensure stability of circadian rhythmicity before coming into the laboratory). To further stabilize sleep-wake patterns and circadian rhythmicity, participants maintained a stable self-selected 8-h sleep schedule (verified by actigraphy; ActiGraph wGT3X-BT, Actigraph Corporation, FL, USA; phone calls to a time stamped mailbox at bedtime and upon awakening; and a written sleep diary) for at least one week immediately prior to entering the laboratory. For this at-home period, participants refrained from any medication, food supplements, caffeinated food and beverages, alcohol, and light intensity physical activity >45 min per day. Participants were asked to not participate in structured exercise at least 24 hours before entering the laboratory and adherence was verified using self-report.
FD Protocol:
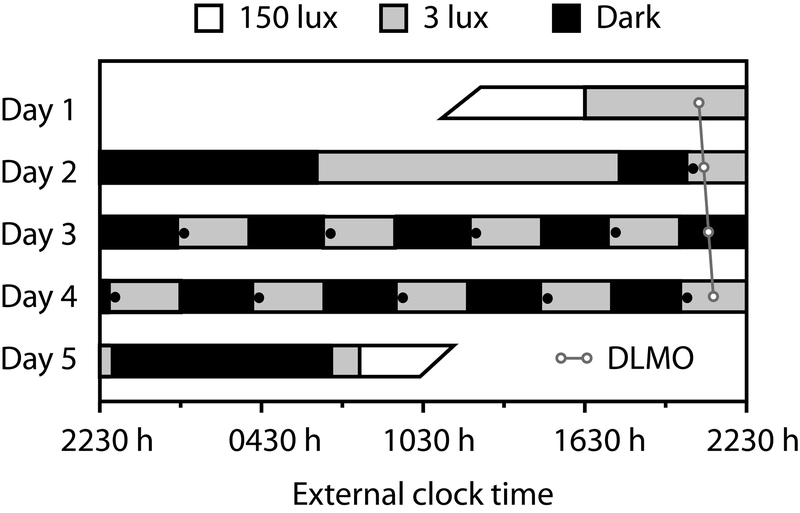
Upon admission to the Oregon Clinical and Translational Research Institute (OCTRI) laboratory, drug screen and pregnancy tests (in pre-menopausal females) were performed to ensure adherence to pre-admission instructions. Participants were familiarized with all testing procedures, and then completed a 5-day FD21 (Figure 1). The FD22 desynchronizes the sleep-wake schedule from the circadian pacemaker by delaying or advancing the daily sleep-wake schedule beyond the range of entrainment of the human circadian pacemaker23–26. This, in combination with dim light levels during wake periods (<3 lux), allows the human circadian pacemaker to ‘free-run’ at its intrinsic rate of ~24.1-h27. Participants are isolated from all external time cues including sunlight, but maintain contact with staff members. Repeated measurements of CV variables throughout scheduled wake/sleep cycles allow assessment of independent effects of endogenous circadian rhythms and of scheduled behaviors (reviewed in Thosar et al28).
Figure 1. Forced desynchrony (FD) Protocol.
Participants completed a 5-day protocol in time isolation. After a baseline 8-h control night, participants completed 10 alternating 2-h 40-min sleep and wake intervals, such that sleep opportunities were evenly spaced across the 24-h day. White bars represent wakefulness in typical room lighting (150 lux), black bars sleep opportunities (dark), and gray bars dim-light waking periods (<3 lux). Black circles represent measurements of VEF, MDA, ET-1, BP and HR. White circles represents a sample profile of dim-light melatonin onset (DLMO). The diagonal line represents an individual’s circadian time.
This FD consisted of an initial baseline 8-h sleep opportunity at the participant’s habitual sleep time and a baseline day followed by 10 identical recurring 5-h 20-min sleep/wake cycles (2-h 40-min sleep opportunity and 2-h 40-min scheduled wakefulness) to desynchronize the scheduled behaviors from the endogenous circadian system while permitting adequate sleep opportunity across all circadian phases29. Light levels were <3 lux at the horizontal angle of gaze during scheduled wakefulness to prevent light-induced phase shifts of the circadian clock30, and were <0.1 lux during scheduled sleep episodes. At the end of each scheduled sleep episode, participants were gently awoken in a standardized fashion by use of a mild auditory stimulus. Thereafter, across each 2-h 40-min period of wakefulness, all activities were scheduled with the same sequence and included: venous blood sample for oxidative stress, ET-1, and other biomarkers (e.g., aldosterone21), BP and HR assessment, VEF assessment, a short cognitive test battery, a 15 minute period of mild intensity cycle-ergometer exercise (<50% max predicted heart rate using the Karvonen’s formula31) and consumption of a small balanced meal representing 22.2% of the 24-h total isocaloric requirement (thereby totaling 100% isocaloric intake across 4.5 FD recurring 5-h 20-min cycles each 24 hour period). This meal was identical and fully consumed during each 5-h 20-min cycle. Measurements were identical during each recurring 5-h 20-min cycle.
Measurements
Vascular Endothelial Function:
VEF was assessed immediately upon awakening during each wake episode as brachial artery diameter FMD in accordance with current guidelines32 and as previously published by our group13. Low-flow mediated constriction (L-FMC) provides information on resting arterial tone33, 34 and was measured in a subset of the population (n = 14) as the percent reduction in brachial artery diameter from baseline to 15-seconds before cuff deflation when any constriction is maximal. All vascular images were coded before analysis and the analyses were performed in a blinded manner with respect to subject number, date and time of the image.
Blood pressure and heart rate:
BP and HR were measured in a supine position in the dominant arm after at least 20 minutes of quiet rest using an electronic transducer and the oscillometric method (Phillips SureSigns VS2+ vital signs monitor)35.
Polysomnography:
Standard full polysomnography (except leg electromyogram) was acquired and scored according to guidelines American Academy of Sleep Medicine36 as previously published by our group13.
Circadian phase and period:
Saliva was collected using Salivette® (Starstedt Inc, NC, USA) cotton swab, spun and frozen until analyses. Salivary melatonin was analyzed using a radioimmunoassay from Bühlmann Laboratories (Schönenbuch, Switzerland) employing the Kennaway G280 anti-melatonin antibody. The in-house interassay CV was 11.5%. The dim-light melatonin onset (DLMO) was used as the circadian phase marker for alignment of all other data. From the time series of salivary melatonin concentrations, for each day we determined the DLMO as the linear interpolated time-point when melatonin exceeded 3 pg/ml37. In one participant whose salivary melatonin never dipped below 3 pg/ml, 4 pg/ml37 was used as the threshold. Circadian period was calculated from the average differences between consecutive DLMOs.
Malondialdehyde adducts and ET-1.
Venous blood samples were taken via a 12-ft catheter tube, which permitted samples to be taken from an adjacent room during sleep periods without disturbing the participants. Venous blood was sampled just prior to measurement of BP, HR and VEF. Each sample was placed directly on ice and centrifuged for 15 minutes at 2500 x g at 5 C°. Subsequently, plasma was frozen at −80 C° until analysis. MDA was measured in duplicate (full profile available on n = 16) from EDTA plasma using Cell Biolabs, Inc. ELISA kit (San Diego, CA, USA) and ET-1 was measured in duplicate (full profile only available in n = 16 due to drop in hemoglobin/failure of intravenous line) from EDTA plasma using R&D Systems Quantikine ELISA kit (Minneapolis, MN, USA) as previously published13. To help control for possible degradation and variation over time, samples from the same participant were stored for the same duration of time before analysis and assayed with the same batch of reagents.
Statistics
Significance for all statistical analyses was set at p<0.05. Unless otherwise noted, data are reported as mean±SE. Confidence intervals (CI), where appropriate, are shown at the 95% level.
Our primary variable of interest was vascular endothelial function measured as FMD and the complementary L-FMC. Our mechanistic variables which can affect vascular endothelial function, were plasma levels of oxidative stress and endothelin-1, BP and HR. For these secondary variables, which were used for hypothesis generation, we did not adjust the threshold p values in order to avoid a Type II statistical error. Average habitual at-home sleep time for our participants was 22:27 ± 0:14, and average habitual wake time was 06:39 ± 0:15; the duration between these time constituting our participants’ subjective night. The average DLMO for all participants occurred at 20:51 ± 0:23. The average circadian period was 23:58 ± 00:05 h. For each individual, their DLMO was assigned circadian phase zero (0°), and all their data were assigned circadian phases (from 0–359°) based on their circadian period and time relative to the DLMO. For interpretation, 360° corresponds to 24-h with each hour corresponding to 15°.
Mixed model cosinor analyses were performed on all data to test for any systematic rhythmicity having an underlying sinusoidal shape across the 24-h period. These cosinor analyses included the fixed factors of circadian phase (2-harmonic parametrization)38 and time-into-experiment for each measurement, and the random factor of subject. In addition, to test for any systematic differences across the circadian cycle without pre-supposing a sinusoidal waveform, data were binned within each participant and across 6 circadian phase intervals (each interval is 60°, equivalent of ~4-h) in order to have data from each participant at each phase interval39, 40. For assessment of variation across the circadian cycle, mixed model analyses of variance41 were performed on the each of the outcomes of interest (with phase interval as the independent variable and subject as random factor). In the cosinor analysis as well as mixed model analysis of variance, the statistical analysis uncovers the effects of the circadian system because of the experimental design of distributing all behaviors evenly across the circadian cycle.
Results:
When data are available for only a subset of participants (e.g., 14 participants for L-FMC and 16 participants each for MDA and ET-1), there were no differences in age or body mass index (both p>0.2) in these subsets compared to the remaining participants.
Vascular endothelial function:
Flow mediated dilation:
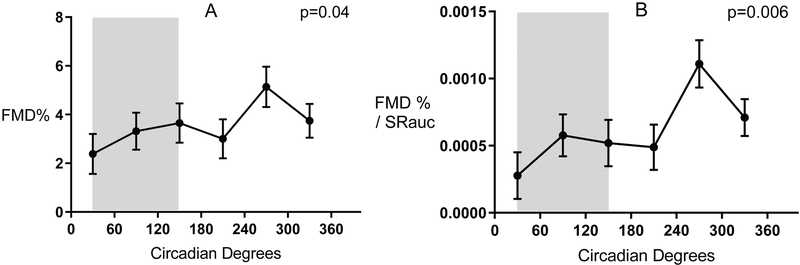
The circadian system significantly affected baseline brachial artery diameter, and all indices of VEF across a 24-h cycle, although the changes across the circadian cycle were not all sinusoidal in shape. FMD expressed as a percentage varied among the six circadian bin intervals [F (5, 145) = 2.4, p = 0.04, Figure 2A], with the lowest FMD in the subjective evening (2.4%; CI: 0.7 to 4% at phase interval 30°; corresponding to ~23:00) and a peak (5.1%; CI: 3.1 to 6.8%) at phase corresponding to ~15:00). In the four bins across the subjective night, FMD remained low (i.e., before, during and immediately after the times of the usual sleep period), yet almost doubled by early afternoon, then substantially declined again in the evening. FMD normalized to hyperemic shear also varied among the six circadian bin intervals [F (5, 145) = 3.5, p = 0.006, Figure 2B]. Again, normalized FMD remained low across the subjective night, doubled by early afternoon, and substantially declined in the evening. Neither FMD expressed as a percentage nor FMD normalized to hyperemic shear had significant sinusoidal variation (p = 0.08 and 0.07, respectively). There was a sinusoidal rhythm in baseline diameter [F (5, 153) = 1.6, p = 0.03] with least constriction (4.53 mm; CI: 4.43 to 4.62) at the circadian phase corresponding to ~01:00; and the most constriction (4.37 mm; CI: 4.28 to 4.47 cm) at the circadian phase corresponding to ~17:00.
Figure 2. Circadian variations in flow mediated dilation.
Circadian variations in FMD% (panel A) and FMD normalized to hyperemic shear rate (panel B). FMD and Normalized FMD are low during the subjective night and across the vulnerable morning period and recover during the afternoon. Grey shaded area represents habitual sleep time (~311 PM - ~7 AM) in our participants. Data are reported as mean ± SEM.
Low-Flow mediated constriction:
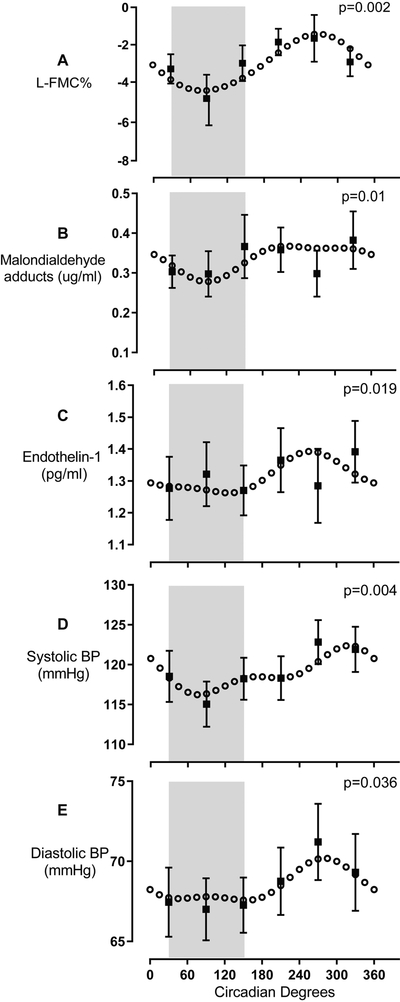
There was a significant sinusoidal rhythm in L-FMC [F (5, 88) = 2.04, p = 0.002], a measure of resting arterial tone, with greatest constriction (−4.45%; CI: −6.5 to −2.4) at circadian phase corresponding to ~03:00; and least constriction (−1.43%; CI: −3.5 to +0.6) at circadian phase corresponding to ~15:00; Figure 3A.
Figure 3. Sinusoidal variation in L-FMC, MDA, ET-1, SBP and DBP.
L-FMC (n = 14, panel A) displays a sinusoidal profile with a trough during the subjective night and a peak in the afternoon. Plasma levels of MDA (n = 16, panel B) and ET-1 (n = 16, panel C) displays sinusoidal variation with a trough during the subjective night and a peak around noon. Systolic BP (n = 21, panel D) and diastolic BP (n = 21, panel E) are rhythmic and sinusoidal with a trough during the biological night and a peak in the evening. Grey shaded area represents habitual sleep time (~11 PM- ~7 AM) in our participants. Circadian model is plotted from the cosinor analyses. Raw observed values are binned by circadian phase within subject and then averaged within group, and plotted as mean ± SEM.
Oxidative stress and Endothelin-1:
There was a significant sinusoidal rhythm in oxidative stress. For MDA, least oxidative stress (0.28 μg/ml; CI: 0.22 to 0.34 μg/ml) occurred during the subjective night, at circadian phase corresponding to ~03:00 [F (5, 118) = 4, p = 0.01] (Figure 3B), and remained consistently high across the subjective day. MDA also declined across the 5-day protocol (time into protocol p<0.001), supplementary figure IA. ET-1 also had a significant circadian rhythm (p = 0.019, Figure 3C), and was highest at circadian phase corresponding to ~14:00 (1.39 pg/ml; CI: 1.31 to 1.48 pg/ml) and low across the rest of the circadian cycle, which is a similar circadian profile as FMD (Figure 2). ET-1 also declined across the 5-day protocol (time into protocol p = 0.001), supplementary figure IB.
Blood pressure and heart rate:
There were significant sinusoidal rhythms in both systolic and diastolic BP with highest systolic BP (122 mmHg; CI: 118 to 126 mmHg) at circadian phase corresponding to ~18:00; and lowest systolic BP (116 mmHg; CI: 112 to 120 mmHg) at circadian phase corresponding to ~02:00 BP [F (5, 168) = 3.47, p = 0.004] Figure 3D. Highest diastolic BP (70 mmHg; CI: 68 to 72 mmHg) occurred at a circadian phase corresponding to ~16:00 (p = 0.036] Figure 3E) and was relatively low across the rest of the circadian cycle i.e., a similar circadian profile as normalized FMD (Figure 2) and ET-1. There was no significant sinusoidal rhythm in heart rate or any variation across circadian bins (p > 0.4)
Discussion:
This is the first study to systematically examine the association of the endogenous circadian system to the early morning impairment in VEF and related mechanistic biomarkers in humans. There were three main findings: 1) We discovered an impairment in VEF across the entire subjective night and into the vulnerable morning period, and this nocturnal/morning attenuation in VEF was due to the endogenous circadian system; 2) Plasma oxidative stress and endothelin-1 are rhythmic in humans with lowest values across the subjective night and increases across the morning vulnerable hours; and 3) BP has a peak in the subjective evening, but the rhythm is advanced by ~3 hours in the middle-aged population studied in the present experiment compared to a younger group of healthy volunteers20.
Circadian rhythms and CV risk:
We found that the circadian system attenuates FMD across the night and into the morning hours, and almost doubles by the late afternoon. The absolute difference of ~3% points is approximately equal to the difference in VEF between healthy controls and those with hypertension and therefore clinically significant42. We also see a robust circadian variation in VEF when FMD is normalized to hyperemic shear. Slight impairment of VEF leads to a host of adverse downstream effects including impairment in vascular tone, increase in platelet aggregation and fibrinolysis43–45. We have previously shown that the endogenous circadian system contributes to this pro-thrombotic milieu in the morning hours of CV vulnerability5, 46. Although low VEF may not be of concern across the subjective night because this is usually a period of sleep and low CV demand, the fact that it remains low across the vulnerable morning period may be more important. Indeed, it is possible that combination of a pro-thrombotic state and low VEF driven by the endogenous circadian system and the typical morning activities such as sudden change in posture, or sudden increases in physical activity can increase the risk of adverse CV events in vulnerable individuals47. Otto et al have previously discovered that VEF is impaired in the morning hours of CV vulnerability10 and we have recently shown that this impairment is not due to prior sleep or inactivity that usually accompanies sleep13. It is also interesting to note that even though of small amplitude (mean peak to trough difference 0.15 mm), we find an endogenous circadian rhythm in baseline brachial diameter with the lowest diameter at ~18:00 when the endogenous rhythm in BP peaks. Indeed, using two different circadian protocols, forced desynchrony and constant routine, it has been previously shown that there is an endogenous circadian rhythm in BP with a peak in the subjective evening and a trough in the early morning hours20. In this study of middle-aged people, we find a similarly significant circadian rhythm, however the rhythm appears to be phase advanced by ~ 3 hours compared to a younger group of healthy volunteers20.
Theoretically, increased BP during periods of constricted vasculature can increased after-load on the myocardium and increase CV risk. Therefore, it is possible that constricted conduit artery in the evening along with the circadian increase in BP could play be associated with the epidemiologically observed secondary peak in adverse CV events during this time of the day3.
Biomarkers affecting VEF and morning CV risk:
In this circadian protocol we discovered that plasma MDA and ET-1 both rise across the morning vulnerable hours and peak around noon. To put into perspective, the change in MDA across vulnerable morning hours (~7 AM- 12 noon) was approximately 6 times greater than the change in MDA across the subjective night (~11 PM- ~7 AM). Similarly, for ET-1, the change in the morning was 5.5 times greater than the change across the night. Thus, the morning period represents a period of low VEF and rapidly rising oxidative stress and ET-1 representing concomitant and perhaps additive risks of adverse CV events in people with CV vulnerabilities48. Prior studies measuring MDA and ET-1 across the 24-h period have reported peaks in plasma ET-1 at 8 AM and 8 PM and a peak in urinary MDA at ~7 PM (including diabetic and non-diabetic men), which is different than our data. This is likely at least in part due to the differences in degree of experimental control during assessments of circadian rhythmicity separate from behaviors, such as the sleep/wake or fasting/feeding cycles sleep and wakefulness. We used the gold standard FD protocol with standardization and equal distribution of activity and food across the circadian cycle, with all studies done in dim light to avoid shifting or resetting the circadian clock. We also found that MDA and ET-1 had a significant time into protocol decline (Supplementary Figure I). We have recently shown that oxidative stress decreases across sleep13 and the current protocol allowed 50% sleep opportunity, equivalent to 12 h every 24 h, which is more sleep opportunity than usual, which may be in part responsible for the decline in oxidative stress and ET-149, 50. Additionally, all meals in the protocol were isocaloric and comprised of ~30% fat, ~15% protein and ~55% carbohydrates. It is possible that our protocol induced a calorie restriction paradigm51 which may reduce oxidative stress and ET-152,53. ET-1 is a potent vasoconstrictor54 and a morning increase in ET-1 along with the behaviorally induced morning BP surge can increase CV risk in vulnerable people55, 56. Our findings, along with previously published reports5, 46 suggest that the endogenous circadian system may contribute to the increased risk of adverse CV events in the morning in vulnerable individuals.
Demographics of our participants:
Our participant pool was middle-aged and predominantly overweight (Supplementary Table I), and therefore at increased CV risk. This population is typical of a high-risk group compared to lean young healthy participants who are more often the participants in other physiological studies. As noted above, we find that the endogenous BP rhythm is advanced compared to our previously published report20 in which the participants were younger (mean age 51 y vs 26 y). Aging is known to alter the circadian profiles of core body temperature, melatonin, cortisol and testosterone57–60 and it is possible that that aging alters (blunts or advances) endogenous circadian rhythms in BP and HR which would potentially explain the lack of a significant rhythm in HR and the advance in the rhythm of BP in the current study, although this needs to be explicitly tested in a separate study.
Impaired VEF during the subjective night has implications for people with existing CV risk:
We find that VEF (FMD and L-FMC) is low during the subjective night (Figures 2A, 2B, and 3A) when most people would be sleeping. This may seem intuitive because the night is usually a state of low metabolic and CV demand. However, our findings may be relevant for shift workers who perform activity during the subjective night when VEF is impaired or in people with obstructive sleep apnea who have an earlier peak time of death according to epidemiological data61.
Relevance for measurement of FMD:
Most studies which use FMD as an outcome variable are conducted in the early morning hours due to theoretical and logistical considerations such as overnight fasting, lack of prior exercise and convenience. We recommend the following additions to the current guidelines32: 1) participants must be asked to maintain a strict 8-h in bed schedule before any measurement; 2) participants avoid bright-light exposure during the subjective evening/night to prevent resetting of the central circadian clock30; 3) measurements made at the same time for within-subject designs and based on the time of nocturnal sleep in between-subject designs. We also note that given these large circadian effects which may also occur in other animals62,63 , it is important for all CV researchers to consider and/or control for the internal circadian phase at which they make their measurements.
Strengths and Limitations:
This study involved intensive repeated measurements with each of 21 participants living in a “time-isolated” individual circadian laboratory suite in a constant environment with all behaviors (e.g., sleep, physical activity, food intake) scheduled evenly over 5 consecutive days. Thus, to our knowledge, our circadian study represents perhaps the most carefully controlled and intensive monitoring of human vascular function and associated mechanistic variables. We conclude that the circadian system (independent of the influences of sleep, physical activity and food intake) is responsible for the morning impairment in vascular function. We also found concomitant increases in oxidative stress and ET-1 that may be mechanistically linked to the daily changes in VEF. Additional important features of our study were the robust control of baseline and experimental conditions prior to the FD study, including history of sleep and activity, light exposure, meal size and timing, activity and sleep in the laboratory. Our study population is also of importance as it is perhaps the first circadian study investigating CV risk markers in middle-aged individuals, with predominantly overweight phenotype i.e., an age group in which individuals are at risk for CV disease. We also had a near equal distribution of men and women participants and although we are not powered to detect sex differences, because of the increased risk for CV disease in post-menopausal women64, this area requires further exploration. In addition to the traditional vascular function measurement FMD, we also assessed the complementary measure L-FMC which is likely mediated by cyclooxygenase and ET-1, and measures the response of the endothelium to resting shear stress33. We concurrently measured ET-1 and MDA levels in the plasma and discovered that these biomarkers are also endogenously rhythmic. Despite these clear innovations, there are some limitations. We measured blood biomarkers but our data are associative. It is impossible to use blockers of endothelin, cyclooxygenase and nitric oxide in a short circadian ‘day’, but these could be potentially tested in a circadian protocol with longer “day” length. Although this is the most comprehensive circadian study of VEF, we did not have a finer resolution (<4 h) of VEF data across the protocol. We used a well-established protocol in circadian biology4, 40, 41, 65, 66 in which each participant is their own control. However, we did not have an alternative control of having all participants in the lab for a 5-day period with a 16:8 wake/sleep ratio. Such a protocol could be used as an alternate control for FD in future studies. We only have one marker of oxidative stress (lipid peroxidation) due to the limited sampling of blood one can make per participant across a 5-day period. To help control for possible degradation and variation over time, samples from the same participant were stored for the same length of time before analysis and assayed with the same lot of reagents. Although the exceedingly careful experimental controls that we used are necessary to assess the contribution of the circadian system, this does not reflect normal daily life where other behaviors and the environmental factors may be more extreme and have greater effects than the circadian system. Furthermore, how the circadian system interacts with the behavioral and environment effects on VEF is largely unknown. Finally, due to the intensive nature of circadian protocols, it is necessary to establish the physiological endogenous circadian rhythms in healthy people before studying people with existing diseases. Thus, our study sets the stage for similar investigations of vascular function in people with existing chronic disease.
In conclusion, the endogenous circadian system affects VEF, oxidative stress, and ET-1 and may contribute to increased CV risk in the vulnerable morning period. More research is needed in vulnerable clinical populations such as those with hypertension or obstructive sleep apnea before selectively targeting the circadian system to reduce CV risk via chronotherapy.
Supplementary Material
Highlights:
Endogenous circadian system impairs vascular function across the night and into the morning, independent of behaviors such as sleep, wake, activity and food intake
Plasma markers of oxidative stress and endothelin 1 also show an endogenous circadian rhythm independent of behaviors, with a rise in the morning and a peak around noon
The endogenous circadian rhythm of blood pressure in middle aged individuals is slightly advanced than previously published rhythm of blood pressure in young adults; there is no endogenous circadian rhythm in heart rate.
Acknowledgements:
We thank Sally Roberts and Georgeann Booth for assistance with data collection and analysis. Author contributions: SST had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. SST, AMB, MXH, AWM, NPB, CMS, NAC, MPB, AAC, JSE and SAS contributed substantially in the study design and analyses, drafting the manuscript for intellectual content and approving the final version. All authors take accountability for the integrity of this work.
Sources of funding: This work was supported by the National Institutes of Health grant numbers R01-HL125893, R01-HL142064, HL125893-03S1, R01 HL140577 & K24-HL76446 F32-HL131308, F32DK107146, KL2TR002370 and UL1TR000128; National Space Biomedical Research Institute through NCC 9–58; Medical Research Foundation of Oregon; American Sleep Medicine Foundation; Ford Foundation Fellowship; and the Oregon Institute of Occupational Health Sciences via funds from the Division of Consumer and Business Services of the State of Oregon (ORS 656.630).
Abbreviation table:
- CV
Cardiovascular
- VEF
Vascular endothelial function
- FMD
Flow mediated dilation
- L-FMC
Low-flow mediated dilation
- FD
Forced Desynchrony
- DLMO
Dim-light onset of melatonin
- BP
Blood Pressure
- HR
Heart Rate
- MDA
Malondialdehyde adducts
- ET-1
Endothelin-1
Footnotes
Disclosure:
None.
TOC category: Clinical
TOC sub category Vascular Biology
References
- 1.Willich SN, Goldberg RJ, Maclure M, Perriello L, Muller JE. Increased onset of sudden cardiac death in the first three hours after awakening. The American Journal of Cardiology. 1992;70:65–68 [DOI] [PubMed] [Google Scholar]
- 2.Muller JE. Circadian variation in cardiovascular events. American Journal of Hypertension. 1999;12:35S–42S [DOI] [PubMed] [Google Scholar]
- 3.Muller JE, Ludmer PL, Willich SN, Tofler GH, Aylmer G, Klangos I, Stone PH. Circadian variation in the frequency of sudden cardiac death. Circulation. 1987;75:131–138 [DOI] [PubMed] [Google Scholar]
- 4.Scheer FA, Hu K, Evoniuk H, Kelly EE, Malhotra A, Hilton MF, Shea SA. Impact of the human circadian system, exercise, and their interaction on cardiovascular function. Proceedings of the National Academy of Sciences. 2010;107:20541–20546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scheer FA, Shea SA. Human circadian system causes morning peak in pro-thrombotic plasminogen activator inhibitor-1 (pai-1) independent of sleep/wake cycle. Blood. 2014;123:590–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson TJ, Uehata A, Gerhard MD, Meredith IT, Knab S, Delagrange D, Lieberman EH, Ganz P, Creager MA, Yeung AC. Close relation of endothelial function in the human coronary and peripheral circulations. Journal of the American College of Cardiology. 1995;26:1235–1241 [DOI] [PubMed] [Google Scholar]
- 7.Ras RT, Streppel MT, Draijer R, Zock PL. Flow-mediated dilation and cardiovascular risk prediction: A systematic review with meta-analysis. International Journal of Cardiology. 2013;168:344–351 [DOI] [PubMed] [Google Scholar]
- 8.Jones H, Lewis NC, Thompson A, Marrin K, Green DJ, Atkinson G. Diurnal variation in vascular function: Role of sleep. Chronobiology International. 2012;29:271–277 [DOI] [PubMed] [Google Scholar]
- 9.Bau PFD, Bau CHD, Naujorks AA, Rosito G, Fuchs FD. Diurnal variation of vascular diameter and reactivity in healthy young men. Brazilian Journal of Medical and Biological Research. 2008;41:500–503 [DOI] [PubMed] [Google Scholar]
- 10.Otto ME, Svatikova A, de Mattos Barretto RB, Santos S, Hoffmann M, Khandheria B, Somers V. Early morning attenuation of endothelial function in healthy humans. Circulation. 2004;109:2507–2510 [DOI] [PubMed] [Google Scholar]
- 11.Gaenzer H, Sturm W, Kirchmair R, Neumayr G, Ritsch A, Patsch J. Circadian variation of endothelium-dependent vasodilatation of the brachial artery as a confounding factor in the evaluation of endothelial function. Atherosclerosis. 2000;149:227. [DOI] [PubMed] [Google Scholar]
- 12.Nosova EV, Yen P, Chong KC, Alley HF, Stock EO, Quinn A, Hellmann J, Conte MS, Owens CD, Spite M. Short-term physical inactivity impairs vascular function. Journal of Surgical Research. 2014;190:672–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thosar SS, Berman AM, Herzig MX, Roberts SA, Lasarev MR, Shea SA. Morning impairment in vascular function is unrelated to overnight sleep or the inactivity that accompanies sleep. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2018;315:R986–R993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373. [DOI] [PubMed] [Google Scholar]
- 15.Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411. [DOI] [PubMed] [Google Scholar]
- 16.Donato AJ, Gano LB, Eskurza I, Silver AE, Gates PE, Jablonski K, Seals DR. Vascular endothelial dysfunction with aging: Endothelin-1 and endothelial nitric oxide synthase. American Journal of Physiology-Heart and Circulatory Physiology. 2009;297:H425–H432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones H, Green DJ, George K, Atkinson G. Intermittent exercise abolishes the diurnal variation in endothelial-dependent flow-mediated dilation in humans. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2010;298:R427–R432 [DOI] [PubMed] [Google Scholar]
- 18.Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE, Seals DR. Direct evidence of endothelial oxidative stress with aging in humans relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-κb. Circulation Research. 2007;100:1659–1666 [DOI] [PubMed] [Google Scholar]
- 19.Böhm F, Pernow J. The importance of endothelin-1 for vascular dysfunction in cardiovascular disease. Cardiovascular Research. 2007;76:8–18 [DOI] [PubMed] [Google Scholar]
- 20.Shea SA, Hilton MF, Hu K, Scheer FA. Existence of an endogenous circadian blood pressure rhythm in humans that peaks in the evening. Circulation Research. 2011;108:980–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thosar SS, Rueda JF, Berman AM, Lasarev MR, Herzig MX, Clemons NA, Roberts SA, Bowles NP, Emens JS, Ellison DH. Separate and interacting effects of the endogenous circadian system and behaviors on plasma aldosterone in humans. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2018;316:R157–R164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kleitman N. Sleep and wakefulness as alternating phases in the cycle of existence. 1939 [Google Scholar]
- 23.Czeisler CA, Duffy JF, Shanahan TL, Brown EN, Mitchell JF, Rimmer DW, Ronda JM, Silva EJ, Allan JS, Emens JS. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science. 1999;284:2177–2181 [DOI] [PubMed] [Google Scholar]
- 24.Dijk D-J, Czeisler CA. Paradoxical timing of the circadian rhythm of sleep propensity serves to consolidate sleep and wakefulness in humans. Neuroscience Letters. 1994;166:63–68 [DOI] [PubMed] [Google Scholar]
- 25.Dijk DJ, Duffy JF, Czeisler CA. Circadian and sleep/wake dependent aspects of subjective alertness and cognitive performance. Journal of Sleep Research. 1992;1:112–117 [DOI] [PubMed] [Google Scholar]
- 26.Wyatt JK, Ritz-De Cecco A, Czeisler CA, Dijk D-J. Circadian temperature and melatonin rhythms, sleep, and neurobehavioral function in humans living on a 20-h day. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 1999;277:R1152–R1163 [DOI] [PubMed] [Google Scholar]
- 27.Duffy JF, Cain SW, Chang AM, Phillips AJ, Munch MY, Gronfier C, Wyatt JK, Dijk DJ, Wright KP, Jr., Czeisler CA. Sex difference in the near-24-hour intrinsic period of the human circadian timing system. Proc Natl Acad Sci U S A. 2011;108 Suppl 3:15602–15608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thosar SS, Butler MP, Shea SA. Role of the circadian system in cardiovascular disease. The Journal of Clinical Investigation. 2018;128:2157–2167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson MP, Duffy JF, Dijk DJ, Ronda JM, Dyal CM, Czeisler CA. Short-term memory, alertness and performance: A reappraisal of their relationship to body temperature. J Sleep Res. 1992;1:24–29 [DOI] [PubMed] [Google Scholar]
- 30.St Hilaire MA, Gooley JJ, Khalsa SB, Kronauer RE, Czeisler CA, Lockley SW. Human phase response curve to a 1 h pulse of bright white light. J Physiol. 2012;590:3035–3045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karvonen MJ, Kentala E, Mustala O. The effects of training on heart rate; a longitudinal study. Annales Medicinae Experimentalis et Biologiae Fenniae. 1957;35:307. [PubMed] [Google Scholar]
- 32.Thijssen DH, Black MA, Pyke KE, Padilla J, Atkinson G, Harris RA, Parker B, Widlansky ME, Tschakovsky ME, Green DJ. Assessment of flow-mediated dilation in humans: A methodological and physiological guideline. American Journal of Physiology-Heart and Circulatory Physiology. 2011;300:H2–H12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gori T, Dragoni S, Lisi M, Di Stolfo G, Sonnati S, Fineschi M, Parker JD. Conduit artery constriction mediated by low flow: A novel noninvasive method for the assessment of vascular function. Journal of the American College of Cardiology. 2008;51:1953–1958 [DOI] [PubMed] [Google Scholar]
- 34.Spiro JR, Digby JE, Ghimire G, Mason M, Mitchell AG, Ilsley C, Donald A, Dalby MC, Kharbanda RK. Brachial artery low-flow-mediated constriction is increased early after coronary intervention and reduces during recovery after acute coronary syndrome: Characterization of a recently described index of vascular function. European Heart Journal. 2011;32:856–866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Borow KM, Newburger JW. Noninvasive estimation of central aortic pressure using the oscillometric method for analyzing systemic artery pulsatile blood flow: Comparative study of indirect systolic, diastolic, and mean brachial artery pressure with simultaneous direct ascending aortic pressure measurements. American Heart Journal. 1982;103:879–886 [DOI] [PubMed] [Google Scholar]
- 36.Iber C, Ancoli-Israel S, Chesson A, SftAAoSM Quan. The aasm manual for the scoring of sleep and associated events: Rules, terminology and technical specifications. 1st ed Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 37.Benloucif S, Burgess HJ, Klerman EB, Lewy AJ, Middleton B, Murphy PJ, Parry BL, Revell VL. Measuring melatonin in humans. Journal of Clinical Sleep Medicine: JCSM: official publication of the American Academy of Sleep Medicine. 2008;4:66. [PMC free article] [PubMed] [Google Scholar]
- 38.Hu K, Scheer FA, Laker M, Smales C, Shea SA. Endogenous circadian rhythm in vasovagal response to head-up tilt. Circulation. 2011;123:961–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scheer FA, Shea TJ, Hilton MF, Shea SA. An endogenous circadian rhythm in sleep inertia results in greatest cognitive impairment upon awakening during the biological night. J Biol Rhythms. 2008;23:353–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pavlova MK, Shea SA, Scheer FA, Bromfield EB. Is there a circadian variation of epileptiform abnormalities in idiopathic generalized epilepsy? Epilepsy Behav. 2009;16:461–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Butler MP, Smales C, Wu H, Hussain MV, Mohamed YA, Morimoto M, Shea SA. The circadian system contributes to apnea lengthening across the night in obstructive sleep apnea. Sleep. 2015;38:1793–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ghiadoni L, Huang Y, Magagna A, Buralli S, Taddei S, Salvetti A. Effect of acute blood pressure reduction on endothelial function in the brachial artery of patients with essential hypertension. Journal of Hypertension. 2001;19:547–551 [DOI] [PubMed] [Google Scholar]
- 43.Tofler GH, Brezinski D, Schafer AI, Czeisler CA, Rutherford JD, Willich SN, Gleason RE, Williams GH, Muller JE. Concurrent morning increase in platelet aggregability and the risk of myocardial infarction and sudden cardiac death. New England Journal of Medicine. 1987;316:1514–1518 [DOI] [PubMed] [Google Scholar]
- 44.Andreotti F, Davies GJ, Hackett DR, Khan MI, De Bart AC, Aber VR, Maseri A, Kluft C. Major circadian fluctuations in fibrinolytic factors and possible relevance to time of onset of myocardial infarction, sudden cardiac death and stroke. The American Journal of Cardiology. 1988;62:635–637 [DOI] [PubMed] [Google Scholar]
- 45.Panza JA, Epstein SE, Quyyumi AA. Circadian variation in vascular tone and its relation to α-sympathetic vasoconstrictor activity. New England Journal of Medicine. 1991;325:986–990 [DOI] [PubMed] [Google Scholar]
- 46.Scheer FA, Michelson AD, Frelinger AL 3rd, Evoniuk H, Kelly EE, McCarthy M, Doamekpor LA, Barnard MR, Shea SA. The human endogenous circadian system causes greatest platelet activation during the biological morning independent of behaviors. PLoS One. 2011;6:e24549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fenga D-L, Toiler GH. Diurnal physiologic processes and circadian variation of acute myocardial infarction. Journal of Cardiovascular Risk. 1995;2:494–498 [DOI] [PubMed] [Google Scholar]
- 48.Heitzer T, Schlinzig T, Krohn K, Meinertz T, Münzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation. 2001;104:2673–2678 [DOI] [PubMed] [Google Scholar]
- 49.Kanabrocki EL, Murray D, Hermida RC, Scott GS, Bremner WF, Ryan MD, Ayala DE, Third JL, Shirazi P, Nemchausky BA. Circadian variation in oxidative stress markers in healthy and type ii diabetic men. Chronobiology International. 2002;19:423–439 [DOI] [PubMed] [Google Scholar]
- 50.Weil BR, Mestek ML, Westby CM, Van Guilder GP, Greiner JJ, Stauffer BL, DeSouza CA. Short sleep duration is associated with enhanced endothelin-1 vasoconstrictor tone. Canadian Journal of Physiology and Pharmacology. 2010;88:777–781 [DOI] [PubMed] [Google Scholar]
- 51.Swinburn B, Sacks G, Ravussin E. Increased food energy supply is more than sufficient to explain the us epidemic of obesity. The American Journal of Clinical Nutrition. 2009;90:1453–1456 [DOI] [PubMed] [Google Scholar]
- 52.Maeda S, Jesmin S, Iemitsu M, Otsuki T, Matsuo T, Ohkawara K, Nakata Y, Tanaka K, Goto K, Miyauchi T. Weight loss reduces plasma endothelin-1 concentration in obese men. Experimental Biology and Medicine. 2006;231:1044–1047 [PubMed] [Google Scholar]
- 53.Qiu X, Brown K, Hirschey MD, Verdin E, Chen D. Calorie restriction reduces oxidative stress by sirt3-mediated sod2 activation. Cell Metabolism. 2010;12:662–667 [DOI] [PubMed] [Google Scholar]
- 54.Kiowski W, Lüscher TF, Linder L, Bühler F. Endothelin-1-induced vasoconstriction in humans. Reversal by calcium channel blockade but not by nitrovasodilators or endothelium-derived relaxing factor. Circulation. 1991;83:469–475 [DOI] [PubMed] [Google Scholar]
- 55.Metoki H, Ohkubo T, Kikuya M, Asayama K, Obara T, Hashimoto J, Totsune K, Hoshi H, Satoh H, Imai Y. Prognostic significance for stroke of a morning pressor surge and a nocturnal blood pressure decline: The ohasama study. Hypertension. 2006;47:149–154 [DOI] [PubMed] [Google Scholar]
- 56.Ziv I, Fleminger G, Djaldetti R, Achiron A, Melamed E, Sokolovsky M. Increased plasma endothelin-1 in acute ischemic stroke. Stroke. 1992;23:1014–1016 [DOI] [PubMed] [Google Scholar]
- 57.Bremner WJ, Vitiello MV, Prinz PN. Loss of circadian rhythmicity in blood testosterone levels with aging in normal men. The Journal of Clinical Endocrinology & Metabolism. 1983;56:1278–1281 [DOI] [PubMed] [Google Scholar]
- 58.Sharma M, Palacios-Bois J, Schwartz G, Iskandar H, Thakur M, Quirion R, Nair N. Circadian rhythms of melatonin and cortisol in aging. Biological Psychiatry. 1989;25:305–319 [DOI] [PubMed] [Google Scholar]
- 59.Vitiello MV, Smallwood RG, Avery DH, Pascualy RA, Martin DC, Prinz PN. Circadian temperature rhythms in young adult and aged men. Neurobiology of Aging. 1986;7:97–100 [DOI] [PubMed] [Google Scholar]
- 60.Van Cauter E, Leproult R, Kupfer DJ. Effects of gender and age on the levels and circadian rhythmicity of plasma cortisol. The Journal of Clinical Endocrinology & Metabolism. 1996;81:2468–2473 [DOI] [PubMed] [Google Scholar]
- 61.Kuniyoshi FHS, Garcia-Touchard A, Gami AS, Romero-Corral A, van der Walt C, Pusalavidyasagar S, Kara T, Caples SM, Pressman GS, Vasquez EC. Day–night variation of acute myocardial infarction in obstructive sleep apnea. Journal of the American College of Cardiology. 2008;52:343–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vukolic A, Antic V, Van Vliet BN, Yang Z, Albrecht U, Montani J-P. Role of mutation of the circadian clock gene per2 in cardiovascular circadian rhythms. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2010;R627–R634. [DOI] [PubMed] [Google Scholar]
- 63.Viswambharan H, Carvas JM, Antic V, Marecic A, Jud C, Zaugg CE, Ming X-F, Montani J-P, Albrecht U, Yang Z. Mutation of the circadian clock gene per2 alters vascular endothelial function. Circulation. 2007;115:2188–2195 [DOI] [PubMed] [Google Scholar]
- 64.Mikkola TS, Gissler M, Merikukka M, Tuomikoski P, Ylikorkala O. Sex differences in age-related cardiovascular mortality. PloS one. 2013;8:e63347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Scheer FA, Shea SA. Human circadian system causes a morning peak in prothrombotic plasminogen activator inhibitor-1 (pai-1) independent of the sleep/wake cycle. Blood. 2014;123:590–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proceedings of the National Academy of Sciences. 2009;106:4453–4458 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.