Abstract
Objective.
FMO3 converts bacterial-derived trimethylamine to trimethylamine N-oxide (TMAO), an independent risk factor for cardiovascular disease. We generated FMO3 knockout (KO) mouse to study its effects on plasma TMAO, lipids, glucose/insulin metabolism, thrombosis, and atherosclerosis.
Approach and Results.
Previous studies with an antisense oligonucleotide (ASO) knockdown strategy targeting FMO3 in LDLRKO mice resulted in major reductions in TMAO levels and atherosclerosis, but also showed effects on plasma lipids, insulin, and glucose. While FMO3KO mice generated via CRISPR/Cas9 technology bred onto the LDLRKO background did exhibit similar effects on TMAO levels, the effects on lipid metabolism were not as pronounced as with the ASO knockdown model. These differences could result from either off-target effects of the ASO or from a developmental adaptation to the FMO3 deficiency. To distinguish these possibilities, we treated wild-type and FMO3KO mice with control or FMO3 ASOs. FMO3-ASO treatment led to the same extent of lipid lowering effects in the FMO3KO mice as the wild-type mice, indicating off-target effects. The levels of TMAO in LDLRKO mice fed an atherogenic diet are very low in both WT and FMO3KO mice, and no significant effect was observed on atherosclerosis. When FMO3KO and WT mice were maintained on a 0.5% choline diet, FMO3KO showed a marked reduction in both TMAO and in vivo thrombosis potential.
Conclusions.
FMO3KO markedly reduces systemic TMAO levels and thrombosis potential. However, the previously observed large effects of an FMO3 ASO on plasma lipid levels appear to be due partly to off-target effects.
Keywords: trimethylamine-N-oxide, atherosclerosis, antisense oligonucleotides, FMO3, plasma lipids, glucose, thrombosis
Subject codes: Animal models of human disease, lipids and cholesterol, cardiovascular disease, genetics, thrombosis, diabetes
Introduction
Flavin containing monooxygenase 3 (FMO3) is expressed primarily in the liver and oxidizes a variety of xenobiotics containing amines or sulfides, including the bacterial-derived metabolite trimethylamine (TMA)1–4. The oxidation product of TMA, trimethylamine N-oxide (TMAO), has been identified as an independent risk factor for cardiovascular disease, as well as other disorders2, 4–9. The possibility that elevated TMAO levels are causal for disease, rather than simply a marker, has been supported by experimental studies in mice2, 4, 5. In particular, a small molecule non-lethal inhibitor of TMA and TMAO generation resulted in atherosclerosis reduction in the apoEKO mouse model10. The proposed mechanisms by which TMAO promotes cardiovascular disease include promotion of foam cell formation by upregulation of macrophage scavenger receptors2, inhibition of reverse cholesterol transport5, induction of inflammation in the vascular cells by activation of the NF-κB pathway11, and inflammasome activation12, 13. Recently, TMAO was also shown to be a powerful potentiator of platelet activation and thrombosis potential at relatively low levels4. Moreover, a family of mechanism-based suicide substrate inhibitors that target gut microbial TMA production, which in turn reduces TMAO generation inhibited platelet hyper-responsiveness and enhanced thrombosis potential in vivo14.
Several groups, including ours, have examined the effects of FMO3 deficiency on atherosclerosis and related traits using an antisense oligonucleotide (ASO), that efficiently knocks down both transcript and protein levels of FMO3 in liver. Such FMO3 knockdown resulted in substantial reductions in not only plasma TMAO levels and atherosclerosis but also plasma lipids, glucose, and insulin levels15–17.
We have generated FMO3 knockout (FMO3KO) mice using the CRISPR-Cas9 technology. The FMO3KO mice exhibited decreased TMAO levels and reduced platelet reactivity and in vivo thrombosis potential, but they only partially recapitulated the effects on plasma lipid metabolism that were previously reported with an FMO3 ASO. However, treatment of FMO3KO mice with FMO3 ASO resulted in similar effects on lipid metabolism previously reported with the FMO3 ASO, strongly supporting an off-target effects hypothesis, Our results provide new clarification of the role of FMO3 in cardiometabolic disease.
Materials and Methods
Animal studies.
All animal experiments were approved by the UCLA, Cleveland Clinic, and University of Massachusetts Medical School Animal Care and Use Committees, in accordance with PHS guidelines. The FMO3KO mice, on the C57BL/6J background, were generated in our laboratory and we reported their preliminary characterization18. Briefly, they lacked FMO3 protein as judged by Western blot analysis18, exhibited significantly reduced TMAO levels and elevated TMA levels, and liver homogenates showed an overall 75% reduction in the ability to oxidize TMA18. Our previous study demonstrated that expression of FMO3 is sexually dimorphic, with females expressing more than 100-fold and 4-fold, respectively, higher FMO3 mRNA and activity levels in liver as compared to the male mice3. Therefore, in order to observe a larger phenotypic difference in TMAO levels, thrombosis, and atherosclerosis between the WT and FMO3KO mice, we chose to use only female mice in these experiments. We feel that this is a valid exception to the guidelines as described in the ATVB Council Statement19. For thrombosis related studies, age matched female FMO3KO and WT mice were fed a chow diet containing 0.5% choline (TD.140294, Envigo Teklad Diets, Madison, WI) for 2 weeks before traits were measured. In thrombosis study using a separate knockout, FMO3KO-2 (see below), the mice were fed a 1% choline containing chow diet (TD 09041, Envigo Teklad Diets, Madison, WI) for 2 weeks before traits were measured. For atherosclerosis studies, the FMO3 null mutation was crossed onto the LDLRKO background to generate FMO3KO/LDLRKO and LDLRKO littermates. Two-month-old female FMO3KO/LDLRKO and LDLRKO mice were fed a Western diet (RD-D12079B, Research Diets, New Brunswick, NJ) for 12 weeks before tissue collection. Sex- and age- matched Serpina1a-e knockout mice20 and WT mice maintained on a chow diet were fasted for 4 hrs before blood collection.
DNA sequence analysis of potential off-target genes in the genome of FMO3KO mice.
Genomic DNA of five FMO3KO mice were PCR amplified using primers specific for potential off-target genes, Olfr325, Myo15, Fmo6, and Carmn (Supplemental Table I). The PCR products were then purified and sequenced by GENEWIZ (South Plainfield, NJ). BLAST (https://blast.ncbi.nlm.nih.gov) was then used to compare the DNA sequence data obtained from the amplified DNA fragments of the FMO3KO mice with the C57BL/6J reference genome.
Generation of FMO3KO-2 mice.
To generate global Fmo3 knockout mice with conditional potential using homologous recombination, we used the “knockout first” gene targeting strategy21. This strategy relies on the identification of a critical exon common to all transcript variants that, when deleted, creates a frame-shift mutation. The KO-first allele is flexible and can produce reporter knockouts, conditional knockouts, and null alleles following exposure to site-specific recombinases Cre and Flp21. This targeting strategy incorporates a LacZ reporter cassette between exons 4 and 5, while allowing for the floxing of exon 5 for subsequent conditional knockout studies. To create a Fmo3 knockout first mouse model we purchased a promoter-driven knockout first targeting vector [Fmo3tm1a(KOMP)mbp] from the Wellcome Trust Institute (Project ID CSD80017). This vector was linearlized and electroporated into mouse-derived embryonic stem cells derived from the 129 background22, and G418-resistant colonies were selected by the Case Western Reserve University Transgenic & Targeting Core Facility. Following selection, embryonic stem cell colonies were expanded and screened for homologous recombination by standard and quantitative PCR strategies spanning genomic and LacZ cassette regions. Two positive ES cell clones were injected into C57BL/6 blastocysts for generation of chimeric mice. One chimeric male and two chimeric female mice with >70% agouti coat color were generated, and the male was successful in germline transmission of the targeted allele. Thereafter, breeders pairs were maintained as Fmo3WT/KO-first x Fmo3WT/KO-first to generate either Fmo3WT/WT, Fmo3WT/KO-first, or Fmo3KO-first/KO-first experimental mice. Genotype was confirmed by PCR of genomic DNA using the following primers: 5’-CATCACAGCATGGTAAAGAAAGG-3’, 5′-CCAACTGACCTTGGGCAAGAACAT- 3′, and 5’-GTGGTACTGAGGTGTGGATATAAG-3”. All studies here used global Fmo3 knockouts (designated as FMO3KO-2 in this study) that had not been crossed to Flp recombinase in a mixed C57BL/6J-129 background, and will be referred to as FMO3KO-2.
ASO injection study.
Four-month-old female WT or FMO3KO mice were treated with a control ASO (Ionis #141923, 5’-CCTTCCCTGAAGGTTCCTCC-3’, 50 mg/kg body weight per week) or a FMO3 ASO (Ionis #555847, 5’-TGGAAGCATTTGCCTTTAAA-3’, 50 mg/kg body weight per week) through weekly i.p. injection for a total of 8 injections before tissue collection.
Biochemical assays.
After 4 hours of fasting, blood samples were collected retro-orbitally from mice. Plasma total-, and HDL- cholesterol, triglyceride, glucose, insulin, bile acids, and AST levels of WT and FMO3KO mice were then determined as previously described16. HOMA-IR was calculated as [glucose (μM) x insulin (μU/L)]/22.5. Plasma TMA and TMAO levels were determined by mass spectrometry as previously described23.
Mouse ex vivo platelet aggregometry studies.
Mice were anesthetized with ketamine (90 mg/kg) and xylazine (15 mg/kg). Platelet rich plasma was generated from whole blood (600 μl) collected into 0.109 M sodium citrate (100 μl), and then diluted with an additional 500 μl Ca2±/Mg2± free modified Tyrode’s buffer and centrifugation at 100 x g for 10 min at 22°C as previously described4. Diluted platelet poor plasma was prepared by further centrifugation at 800 xg for 2 min. Platelets were counted using a hemocytometer and concentrations adjusted to 2 × 108/ml with platelet poor plasma. CaCl2 and MgCl2 (both 1 μM final concentration) were added immediately before platelet aggregation studies. Platelet aggregation in response to 1 μM ADP was assessed at 37°C in a dual channel Type 500 VS aggregometer (Chrono-log,) with stirring at 1200 rpm.
In vivo carotid artery thrombosis model.
After surgical isolation of the common carotid artery and fluorescent labeling of platelets with rhodamine 6G (100 μl; 0.5 mg/ml) via direct injection into the right jugular vein4, mice were subjected to common carotid artery injury by application of 10% FeCl3 for 1 min and thrombus formation monitored in real time using intravital fluorescence microscopy and continuous video image capture, as previously described4. Time to cessation of blood flow was determined through inspection of computer images as described4 by two data analysts blinded to animal group assignments.
RNA isolation and quantitative RT-PCR analyses.
Total RNA samples from tissues were isolated using Trizol reagent (Life Technologies, Carlsbad, CA) according to the manufacturer’s protocol. The cDNA was synthesized using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). Quantitative PCR was performed using gene-specific primers (Supplemental Table I) and the Roche SYBR green master mix in a Roche Lightcycler 480 system (Roche, Pleasanton, CA). The mRNA levels of specific genes were normalized to the mRNA levels of the housekeeping gene, β-actin, of the same sample.
Atherosclerosis.
The study adhered to the guidelines for experimental atherosclerosis studies described in the AHA Statement24. Power calculation estimated that the minimal sample size needed for each group was 13 to detect a 50% difference in atherosclerotic lesion size with a common standard deviation of 45%, power = 0.8, and significance level at 0.05. Quantification of the lesion area was performed in a blinded fashion. Atherosclerotic lesions in the proximal aorta were quantitated as described25. Briefly, the aorta was flushed with PBS and embedded in OCT. Frozen sections (10μM) were stained with Oil Red O and lesion area quantified every third section beginning at the aortic valves. The average of the first 10 stained sections was then used as a measure of lesion size.
Statistical analysis.
All analyses were performed using the GraphPad Prism 8 software (San Diego, CA). When data were not normally distributed, a logarithm or a square root transformation of the data was performed so that it followed normal distribution. If data from two groups followed a normal distribution and had equal variances, a Student’s t-test was used to compare the means of the two groups. If data from two groups followed a normal distribution but the groups had unequal variances, an unpaired t-test with Welch’s correction was used. When comparing two groups, for traits that could not be transformed to normality, the Mann Whitney test was used. For tests comparing multiple groups all data followed a normal distribution and were analyzed by a one-way or two-way ANOVA. Following a significant one-way ANOVA a post-hoc Tukey’s multiple comparisons test was used. A two-way ANOVA was used to estimate the contributions of ASO, FMO3 genotype, and their interaction to the variances of the traits obtained in the FMO3KO/WT ± ASOs study (Figure 1D and Table 2).
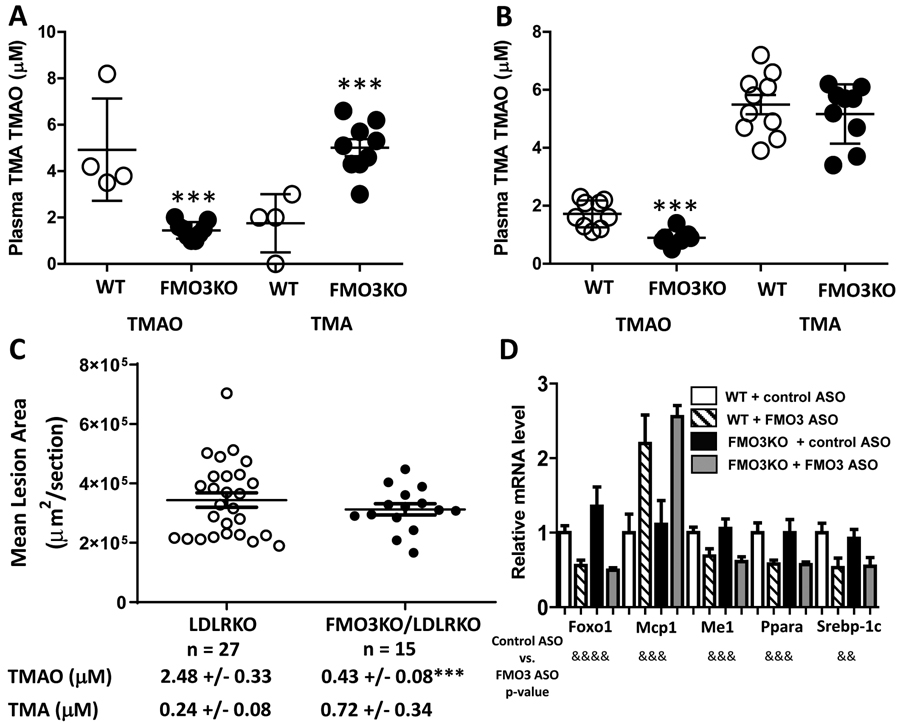
Figure 1.
Phenotypic comparisons of FMO3KO and WT mice, and WT and FMO3KO mice receiving a control ASO or a FMO3 ASO. Plasma TMAO and TMA levels of chow diet fed female (A, n = 4 to 9) and male (B, n = 9 to 10) WT and FMO3KO mice are shown. (C) Female LDLRKO and FMO3KO/LDLRKO mice were fed a western diet for 12 weeks before mean atherosclerotic lesion size at the aortic root region (n = 15 to 27) and plasma TMAO and TMA levels (mean ± SEM, n= 9 to 16) were measured. Symbol for (A) to (C): ***: p < 0.001 (Student’s t-test) between the two genotype groups. For (A) to (C), individual values (in circles) with group means (middle bars) ± SEM (upper and lower bars) are shown. (D) Hepatic gene expression analysis of WT and FMO3KO mice that received either a control or a FMO3 ASO (Ionis #555847). Sample sizes of each group range from 5 to 7 mice. Two-way ANOVA was used for analysis. Group means and SEMs are shown. Symbols for (D): &&: p < 0.01, &&&: p≤ 0.001, &&&&: p ≤ 0.0001.
Table 2.
Plasma lipid, glucose, insulin levels and HOMA-IR of WT and FMO3KO mice at baseline and after receiving 8 weekly injections of a control ASO or a FMO3 ASO
| Group (sample size) | Triglyceride mg/dL |
Total Chol. mg/dL |
HDL Chol. mg/dL |
VLDL/IDL/LDL Chol. mg/dL |
Glucose mg/dL |
Insulin pg/ml |
HOMA-IR |
|---|---|---|---|---|---|---|---|
| WT(14) | 29 ± 2 | 74 ± 2 | 53 ± 2 | 21 ± 1 | 190 ± 8 | 222 ± 20 | 3.1 ± 0.3 |
| FM03KO (10) | 27 ± 2 | 77 ± 2 | 56 ± 2 | 22 ± 2 | 215 ± 10 | 251 ± 39 | 3.9 ± 0.7 |
| p value | 0.54 | 0.47 | 0.36 | 0.61 | 0.07 | 0.47 | 0.25 |
| Trait | ASO | WT | FMO3KO |
|---|---|---|---|
| Triglyceride | Control ASO | 29 ± 6 | 26 ± 4 |
| FM03 ASO** | 16 ± 2 | 17 ± 2 | |
| Total Chol. | Control ASO | 74 ± 5 | 76 ± 6 |
| FMO3 ASO** | 61 ± 3 | 57 ± 6 | |
| HDL Chol. | Control ASO | 50 ± 3 | 50 ± 3 |
| FM03 ASO** | 41 ± 3 | 38 ± 4 | |
| VLDL/IDL/LDL Chol. | Control ASO | 24 ± 2 | 26 ± 3 |
| FM03 ASO** | 19 ± 1 | 19 ± 2 | |
| Glucose | Control ASO | 221 ± 22 | 222 ± 27 |
| FMO3 ASO** | 169 ± 11 | 162 ± 9 | |
| Insulin | Control ASO | 400 ± 72 | 611± 149 |
| FMO3ASO*** | 196 ± 32 | 211 ± 37 | |
| HOMA-IR | Control ASO | 5.8 ± 0.9 | 10 ± 3.1 |
| FMO3ASO*** | 2.2 ± 0.2 | 2.4 ± 0.4 |
Mice were fasted for 4 hours before blood collection. Values shown are mean ± standard error of each group. Student’s t-test was used for baseline dataset. Two-way ANOVA was used for ASO treatment dataset. Sample sizes were 5 to 7 per group in the ASO treatment study. Abbreviation: Chol.: cholesterol. Symbols:
: p<0.01,
: p≤0.001, two-way ANOVA, control ASO vs. FMO3 ASO.
Results
FMO3KO/LDLRKO mice exhibit minor changes in plasma lipid levels, decreased plasma TMAO levels, and similar atherosclerotic lesion size as LDLRKO mice
FMO3KO mice were generated by CRISPR/Cas9 technique using a guide RNA targeted to the exon2 of Fmo3 gene18. A number of different Fmo3 mutations were observed as shown in Supplemental Table II. The studies reported here utilized mice with a 7 bp deletion at exon 2 resulting in a frameshift mutation with premature termination at amino acid 98.
When maintained on a chow diet, female FMO3KO mice exhibited a 186% (p < 0.001) increase in circulating TMA levels and a 71% (p < 0.001) decrease in circulating TMAO levels as compared to the wild-type females (Figure 1A). Male FMO3KO mice, on the other hand, exhibited similar TMA levels and a significant 48% (p < 0.001) decrease in circulating TMAO levels (Figure 1B). The difference between females and males is due in part to the fact that adult male mice exhibit greatly reduced FMO3 expression due to testosterone-mediated repression of gene expression3. These data confirm that FMO3 is the major enzyme in mice that converts TMA to TMAO.
In order to study atherosclerosis and related traits, the FMO3KO mice were crossed with LDLRKO mice to generate LDLRKO and FMO3KO/LDLRKO mice. After 12 weeks of feeding of a Western diet to induce hypercholesterolemia, the female FMO3KO/LDLRKO mice exhibited similar plasma triglyceride and HDL-cholesterol levels as compared to the female LDLRKO littermates (Table 1). Plasma total bile acids and AST levels were also similar between the two groups of mice (Table 1). Moderate but significant decreases in total cholesterol (14%) and VLDL/IDL/LDL cholesterol (15%) were observed in the FMO3KO/LDLRKO mice (Table 1). Plasma glucose levels and HOMA-IR were significantly lower in the FMO3KO/LDLRKO mice whereas plasma insulin levels were similar between the two groups (Table 1). Hepatic gene expression analysis revealed that the FMO3KO/LDLRKO mice exhibited similar mRNA levels of genes involved in glucose and lipid metabolisms including Fasn, Fgf21, Foxo1, G6pc, Me1, Scd1, and Srebp-1c, as compared to the LDLRKO mice (Supplemental Figure I). In contrast, expression of the same set of genes were shown to be decreased in the livers of FMO3 ASO treated mice as compared to the control ASO group16. Circulating TMA levels were increased in the FMO3KO/LDLRKO mice and circulating TMAO levels were significantly decreased (p < 0.0001) as compared to the LDLRKO mice (Figure 1C). In male mice, we did not observe any significant differences in plasma triglyceride, total-, HDL-, and VLDL/IDL/LDL-cholesterol, glucose, insulin levels, or HOMA-IR between the FMO3KO/LDLRKO and LDLRKO mice (Supplemental Table III).
Table 1.
Plasma lipid, glucose, insulin, AST, and bile acid levels of female LDLRKO and FMO3KO/LDLRKO mice maintained on a Western diet for 3 months
| Genotype (n) | LDLRKO (27) | FMO3KO/LDLRKO (16) | p-value |
|---|---|---|---|
| Triglyceride (mg/dL) | 233 ± 21 | 197 ± 20 | 0.26 |
| Total Cholesterol (mg/dL) | 1504 ± 39 | 1292 ± 45 | 0.002 |
| HDL Cholesterol (mg/dL) | 85 ± 3 | 85 ± 3 | 0.97 |
| VLDL/IDL/LDL Cholesterol (mg/dL) | 1419 ± 39 | 1206 ± 44 | 0.0013 |
| Glucose (mg/dL) | 229 ± 9 | 201 ± 9 | 0.045 |
| Insulin (pg/ml) | 605 ± 65 | 506 ± 37 | 0.19* |
| HOMA-IR | 10.7 ± 1.2 | 7.8 ± 0.6 | 0.03* |
| AST (U/L) | 179 ± 14 | 170 ± 44 | 0.83 |
| Bile acids (μM) | 11.4 ± 0.7 | 16.1 ± 5 | 0.25 |
Plasma lipids, glucose, and insulin data were collected from 2 independent cohorts of mice. Mice were fasted for 4 hours before blood collection. Values shown are mean ± standard error of each group. Sample sizes for AST and bile acids were 17 and 9 for LDLRKO and FMO3KO/LDLRKO, respectively. Student’s t test was used for data analysis unless noted otherwise.
: Unpaired t test with Welch’s correction was used for data analysis due to unequal variances of the two groups.
Atherosclerotic lesion size at the aortic root region was similar between the female FMO3KO/LDLRKO and LDLRKO mice (Figure 1C). This result contrasts with our previous study with the FMO3 ASO (Ionis #555847) in the LDLRKO mice that resulted in a significant decrease in atherosclerotic lesion size, likely due to a combination of decreased circulating VLDL/IDL/LDL-cholesterol levels (75% decrease) as well as TMAO levels16. While FMO3KO/LDLRKO mice had significantly decreased plasma TMAO levels as compared to the LDLRKO mice (0.43 μM vs. 2.48 μM, respectively, p < 0.001), both of these values are very low and therefore not likely to have a major impact on atherogenesis. For example, studies showing an impact of TMAO on atherosclerosis involved ~10-fold higher levels of TMAO2, 5, 10.
Off-target effects of an FMO3 ASO on metabolic traits
We performed the following experiments to address the possibility that some of these differences between our present findings and previous studies with the FMO3 ASO were due to off-target effect of the guide RNA used for generation of the FMO3KO mice. Based on sequence similarity, there are 4 potential genes that may be targeted by the guide RNA used to mutate Fmo3: Olfr325, Myo15, Fmo6, or Carmn (Supplemental Table IV). However, we did not observe any mutations in these genes in the FMO3KO mice (Supplemental Table IV), suggesting specificity of this guide RNA toward Fmo3.
There are two likely mechanisms that can explain the differences observed between the genetic mutation of FMO3 and acute knockdown of FMO3 using an ASO: (1) the ASO utilized in our previous study may have off-target effects, or (2) there is genetic compensation induced in FMO3KO mice but not in FMO3 knockdown using an ASO. To address this issue, we performed 8 weekly control ASO or FMO3 ASO (Ionis #555847) injections into wild type (WT) or FMO3KO mice before tissue collection. Before ASO injection, WT and FMO3KO mice exhibited similar levels of plasma triglyceride, total cholesterol, HDL-cholesterol, VLDL/IDL/LDL-cholesterol, glucose, and insulin (Table 2). After 8 weekly injections of ASOs, we found that the FMO3 ASO substantially lowered plasma lipid, glucose, and insulin levels, and HOMA-IR, to the same extent in both WT and FMO3KO mice, suggesting that these effects are due to an off-target effect of the ASO (Table 2). Two-way ANOVA was used to determine the source of variance contributing to these metabolic traits. We found that FMO3 genotype did not significantly contribute to the variances observed in these plasma lipid, glucose, insulin or HOMA-IR traits (Supplemental Table V). The type of ASO (control ASO vs. FMO3 ASO) was responsible for between 35% to 48% of the variances observed in the metabolic traits, whereas the interaction between FMO3 genotype and ASO did not contribute significantly to the variance (Supplemental Table V).
Hepatic gene expression analyses showed that WT mice that received the FMO3 ASO exhibited substantial decreases in Foxo1, Me1, Ppara, and Srebp-1c mRNA levels and more than 2-fold increase in Mcp1 mRNA levels as compared to the control ASO groups (Figure 1D), confirming our previous observations16. Notably, the FMO3KO mice that received the FMO3 ASO exhibited similar changes in the expression of these genes as compared to the FMO3KO mice that received the control ASO (Figure 1D). Two-way ANOVA was performed and again we found that type of ASO contributed significantly to the variances of these mRNA levels (34% to 53%, Supplemental Table VI), whereas FMO3 genotype and interaction did not contribute significantly to the variances (Supplemental Table VI). These data indicate that the decreased expression of these glucose and lipid metabolism genes was due to the off-target effect of the FMO3 ASO, since it elicited a similar response in both WT and FMO3KO mice.
We identified potential off-target genes by searching for regions of similarity between the FMO3 ASO (TGGAAGCATTTGCCTTTAAA) and other gene transcript sequences using the publicly available Basic Local Alignment Search Tool (BLAST; https://blast.ncbi.nlm.nih.gov). The results demonstrated sequence similarity between the FMO3 ASO and the mRNA sequences of several genes including Rab5b [16/16 consecutive nucleotides (nt) matched], Serpina1a-e (14/14 consecutive nt matched to all five Serpina1 paralogs: Serpina1a, Serpina1b, Serpina1c, Serpina1d, and Serpina1e, abbreviated as Serpina1a-e), Slc38a9 (17/18 nt matched), Sh3bp4 (14/14 consecutive nt matched), and Zyg11b (14/14 consecutive nt matched). In follow-up studies, we observed marked decreases in the mRNA levels of these potential off-target genes in the livers of FMO3 ASO-treated mice as compared to those of the control ASO-treated mice (Supplemental Figure II). These data suggest that the FMO3 ASO significantly reduced the expression of the Rab5b, Serpina1a-e, Slc38a9, Sh3bp4, and/or Zyg11b genes, which could have mediated some of the metabolic effects observed using the ASO. We next obtained a second, independent ASO for FMO3 (Ionis #555926) from Ionis, Inc. This ASO was less efficient at knocking down hepatic FMO3, had some effect in reducing plasma triglyceride and HDL cholesterol level, but showed no effects on plasma total cholesterol, VLDL/IDL/LDL cholesterol, glucose, or insulin levels (Supplemental Figure III).
To further investigate the possibility of ASO-mediated off-target effects, we leveraged publicly available results of human genome-wide association studies for plasma lipid levels from the Global Lipids Genetics Consortium26. Of the 400kb regions encompassing each gene that we examined, SNPs located in between SERPINA6 and SERPINA2/SERPINA1 (the ortholog of the 5 mouse paralogs: Serpina1a-e) were associated with plasma LDL levels (Supplemental Figure IVA). The lead variant at this locus was rs4905179 (A>G) and yielded a p-value of 5.6×10−7, which exceeded the Bonferroni corrected significance threshold for the number of SNPs in this interval (0.05/621=8.1×10−5) by two orders of magnitude. Specifically, the A allele of rs4905179, which has a frequency of ~0.80 in subjects of European ancestry, was associated with decreased LDL levels and exhibited a comparably significant association (p=1.1×10−6) with decreased plasma total cholesterol (Supplemental Figure IVB). From the Cardiovascular Disease Knowledge Portal (http://broadcvdi.org/home/portalHome), we also found significant and suggestive associations of SERPINA1 SNPs with plasma lipids (LDL- and total-cholesterol, triglyceride) and Type 2 diabetes in human data sets (Supplemental Table VII).
To verify that Serpina1a-e explain the potential off-targets of the FMO3 ASO, we examined plasma lipids traits of the KO and WT mice. We observed that female Serpina1a-e KO mice had significantly decreased plasma triglyceride and VLDL/IDL/LDL cholesterol levels but no changes in plasma total- and HDL- cholesterol, glucose, or insulin levels, as compared to the WT mice (Supplemental Table VIII). Male Serpina1a-e KO mice had no changes in plasma total-, HDL-, and VLDL/IDL/LDL-cholesterol and glucose levels but significantly decreased plasma triglyceride, and increased plasma insulin and HOMA-IR as compared to the WT mice (Supplemental Table VIII). It is unclear why there is a paradoxical increase in plasma insulin levels in the male Serpina1a-e KO mice. These data suggest that part of the off-target effect of the FMO3 ASO, including the plasma triglyceride and VLDL/IDL/LDL cholesterol lowering effects, is mediated through the decreased expression of Serpina1a-e.
FMO3KO mice exhibit decreased platelet responsiveness and thrombosis potential
Recent studies have shown that TMAO promotes platelet hyper-responsiveness to multiple agonists and enhances thrombosis potential, suggesting that this is a likely mechanism by which TMAO may contribute to the development of atherosclerosis and cardiovascular disease risks4. We evaluated the impact of the FMO3 deficiency on platelet function and thrombosis potential by examining ex vivo platelet aggregation and in vivo carotid artery injury (FeCl3-induced) thrombosis in mice maintained on 0.5% choline to elevate plasma TMAO levels. We observed no difference in plasma triglyceride, total-, HDL-, and VLDL/IDL/LDL-cholesterol, and glucose levels between the WT and FMO3KO mice under non-fasting condition (cohort #1, housed at Cleveland Clinic, Supplemental Table IX). However, plasma insulin levels were significantly decreased in the FMO3KO mice as compared to the WT mice (cohort #1, Supplemental Table IX). In a separate cohort (cohort #2, housed at UCLA), we did not observe any significant differences in plasma lipids, glucose, or insulin levels between the 2 groups of mice (Supplemental Table IX), suggesting that differences in housing conditions, microbiome, and/or animal handling may have influenced these metabolic traits. As shown in Figure 2A, the lower TMAO levels observed in FMO3KO mice were accompanied by decreased platelet responsiveness (p<0.0001). In a different group of animals (FMO3KO versus WT controls), decreased TMAO levels in the FMO3KO mice were accompanied by slower clot formation and significantly longer times to vessel occlusion in the carotid artery FeCl3 injury experiments (p<0.001; Figure. 2B, 2C). We observed that aortas collected from the FMO3KO mice exhibited decreased expression levels of inflammatory genes including Ptgs2 (Cox2) and Cxcl2 (Supplemental Figure V). Among the genes that promote platelet adhesion/thrombosis, we observed that E-selectin (Sele) levels were decreased in the aortas of FMO3KO, but no change in Icam1, P-selectin (Selp), tissue factor (F3), or von Willebrand factor (Vwf) levels were observed (Supplemental Figure V). Our findings suggest that the reduced thrombosis potential observed in the FMO3KO mice is mainly due to TMAO’s effect on platelet responsiveness, with decreased vasculature inflammation playing a supporting role.
Figure 2.
FMO3 deficiency leads to decreased platelet responsiveness and decreased thrombosis potential in vivo. (A-C) FMO3KO and WT mice were fed with 0.5% of choline supplemented chow diet for 2 weeks. (A) Platelet aggregometry was monitored in platelet rich plasma following stimulation of sub-maximal (1μM final) ADP. Data represent the % of max aggregometry amplitude in response to 1μM ADP, along with mean (±SEM) plasma TMA and TMAO levels, for each of the mouse groups. (B, C) in vivo thrombosis potential was quantified using the FeCl3-induced carotid artery injury model. (B) Time to cessation of flow in the FeCl3 induced carotid artery injury model, along with plasma TMA and TMAO levels reported as mean (± SEM). (C) Shown are representative vital microscopy images of carotid artery thrombus formation at the indicated time points following arterial injury. Symbols: *: p < 0.05, **: p < 0.01, and ****: p < 0.0001 (Student’s t-test), ####: p < 0.0001, unpaired t-test with Welch’s correction, $ $ $ $: p<0.0001, Mann Whitney test, between the two genotype groups. For (A) and (B), individual values (in circles) with group means (middle bars) ± SEM (upper and lower bars) are shown. (D) Another strain of FMO3 knockout mice, FMO3KO-2, independently created by Mark Brown’s laboratory, exhibited decreased thrombosis potential as compared to the WT and heterozygous (HET) mice in the FeCl3-induced carotid artery injury model. One-way ANOVA was used to compare 3 groups. Following a significant one-way ANOVA, Tukey’s post hoc test was then used to compare between any two groups of mice. For TMAO and TMA, any 2 groups that share the same letters are significantly different (p<0.05) in means.
To further confirm that the anti-thrombotic effects that we observed in the FMO3KO mice are due to FMO3 deficiency but not an off-target gene effect or an artifact of the CRISPR-Cas9 mutagenesis, we studied an independently generated strain of FMO3KO mice, FMO3KO-2 created using homologous recombination (see Materials and Methods). After feeding a 1% choline diet for 2 weeks, the FMO3KO-2 mice exhibited significantly lower plasma TMAO levels and longer times to vessel occlusion in the carotid artery FeCl3 injury experiments as compared to the WT and FMO3KO-2 heterozygous (HET) mice (Figure 2D), confirming our results using the CRISPR-Cas9 generated FMO3KO mice.
Discussion
Numerous human studies have revealed a significant association between TMAO levels and cardiovascular disease, including myocardial infarction and stroke6, 7, and experimental studies in mice have provided evidence that TMAO is causal for the disease2,10. Several recent meta analyses have systematically examined the many clinical studies involving TMAO and confirmed that elevated TMAO levels are associated with both enhanced cardiovascular disease and mortality risk in subjects7–9. Several potential mechanisms by which TMAO can contribute to cardiovascular disease have been identified, including alterations in cholesterol and lipid metabolism2, 5, 16, 17, glucose metabolism and diabetes-related traits15–17, vascular inflammation11, and thrombosis4, 27. The initial studies implicating FMO3 in modulation of lipid and glucose metabolism were based on studies with an ASO (Ionis #555847), showing that a reduction in FMO3 coincided with substantially decreased levels of plasma cholesterol, insulin, and glucose15–17. Recently, we generated FMO3KO mice and were surprised to see that plasma lipids, glucose, and insulin levels were not affected as compared to the WT mice under chow diet and 4 hr-fasting conditions (Table 2). On the hyperlipidemic LDLRKO mouse background, we observed that female FMO3KO/LDLRKO mice had moderate but significant decreases in plasma total cholesterol (14%), VLDL/IDL/LDL cholesterol (15%), and glucose (12%) levels but no change in plasma triglyceride, HDL-cholesterol, or insulin levels as compared to the LDLRKO mice (Table 1). In addition, male FMO3KO/LDLRKO mice did not exhibit any differences in plasma lipids, glucose, or insulin levels as compared to the LDLRKO mice (Supplemental Table III).
We then examined the basis of the differences observed between ASO treatment and genetic deficiency of FMO3. To determine whether the ASO exhibited off-target effects, we treated the FMO3KO mice with the ASO. The ASO still exhibited effects on plasma cholesterol, lipid, glucose and insulin levels in the absence of FMO3 (Table 2). Other instances of discordance between phenotypes resulting from ASO treatment as compared to genetic mutations have been reported28, 29. To determine the nature of the off-target effects, we identified several genes exhibiting strong homology to the ASO sequence that showed reduced expression following ASO administration. The human ortholog of Serpina1a-e, SERPINA1, exhibits natural genetic variation in human populations that was associated with plasma lipids and type 2 diabetes traits. Consistent with this, decreased plasma triglyceride (both female and male) and VLDL/IDL/LDL-cholesterol (female only) levels were observed in the Serpina1a-e knockout mice as compared to the WT mice (Supplemental Table VIII), suggesting that the off-target triglyceride and VLDL/IDL/LDL-cholesterol lowering effect of the FMO3 ASO (Ionis #555847) is partly mediated through Serpina1a-e knockdown.
Our previous study16 demonstrated that treatment of the second FMO3 ASO (Ionis #555926) at 100 mg/kg body weight/week significantly decreased plasma triglyceride, total- and HDL- cholesterol, glucose, and insulin levels in male C57BL/6J mice maintained on a chow diet. However, in our current study we found that using the same FMO3 ASO at 75 mg/kg/week to treat female LDLRKO mice maintained on a Western diet significantly decreased only plasma triglyceride and HDL-cholesterol, not total- or VLDL/IDL/LDL cholesterol, glucose, or insulin levels as compared to the control ASO group (Supplemental Figure III). The differences in gender (male vs. female), ASO dose (100 vs. 75 mg/kg/week), genetic background (wild-type vs. LDLRKO), and diet (chow vs. Western diet) may have contributed to the different outcomes of these 2 studies.
We wish to emphasize that our studies do not negate the previous conclusions15–18 that FMO3 has metabolic effects in addition to its effect on TMA/TMAO metabolism. While it appears that the large decrease in plasma lipids levels observed with the FMO3 ASO (Ionis #555847) is due at least partly to an off-target effect, the other reported metabolic phenotypes were observed in the FMO3KO mice under certain conditions, including plasma glucose and VLDL/IDL/LDL cholesterol levels (on the LDRKO background, female only), plasma insulin (Supplemental Table IX, C57BL/6J background, choline diet, non-fasted, only in 1 cohort) and adiposity (both on the C57BL6/J and LDLRKO background)18. There is experimental evidence for these phenotypes that is independent of the FMO3 ASO, including FMO3 transgenic mouse studies16, tissue culture overexpression studies16, and studies with a second ASO16. Most of the findings, but not all, were consistent with the present results. For example, FMO3 transgenic mice showed effects on plasma triglyceride levels16 that were not observed here in the FMO3KO mice. Such differences could well result from factors such as the diet, differences in the chosen hyperlipidemic background (LDLRKO vs. apo-E Leiden transgenic mice), or gut microbiomes, which likely exhibit small differences between studies. Since FMO3 is expressed in many tissues, there may also be tissue specific effects that differ between studies. For example, the FMO3 ASO is likely much more effective in some tissues than others, whereas the FMO3KO will abolish expression in all tissues. Lastly, we can not rule out the possibility of developmental adaptation that occurs in the FMO3KO mice since certain traits such as plasma triglyceride and HDL cholesterol levels were decreased by treatment of both of the FMO3 ASOs but not in the FMO3KO mice. Therefore, it is possible that there are certain genes involved in lipid and glucose metabolism whose expression are altered to compensate for the loss of Fmo3 during development.
We did not observe any changes in atherosclerotic lesion development in the FMO3KO/LDLRKO mice as compared to the LDLRKO littermates, in contrast to previous studies16. This difference is probably due to the fact that there was a 75% decrease in plasma VLDL/IDL/LDL-cholesterol levels associated with the FMO3 ASO knockdown, but only a minor decrease (15%) in the FMO3KO/LDLRKO mice. It is important to note, however, that the TMAO levels observed in the present studies were quite low (mean = 2.48 μM for LDLRKO mice and 0.43 μM for FMO3KO/LDLRKO mice), well below the median TMAO level (3.7 μM) reported in human subjects6, and also well below levels of TMAO observed in numerous mouse studies where effects on atherosclerotic plaque development have been observed2, 5, 10. We note that recent studies examining inhibition of TMA and TMAO production via use of an inhibitor of gut microbial conversion of choline into TMA significantly reduced atherosclerosis in the apoEKO mouse model10. It will be important in future studies to test the effect of the FMO3KO on atherosclerosis under conditions of elevated TMAO, such as feeding choline or carnitine, or in the presence of impaired renal function.
Next we performed a series of thrombosis experiments in mice fed a 0.5% choline diet to elevate TMAO. Here we observed that the FMO3KO mice fed a 0.5% choline diet had a significant decrease in plasma TMAO level and robustly decreased both platelet hyper-responsiveness and thrombosis potential. Specifically, we observed that the FMO3KO mice had reduced platelet aggregation in response to ADP and that in a FeCl3 model of thrombosis, the rate of clot formation was decreased and time to vessel occlusion was significantly increased. Furthermore, similar findings of decreased thrombosis potential in vivo were observed using a second, independently generated, FMO3KO mouse strain, confirming that these findings are directly due to FMO3 deficiency. The mechanism by which FMO3 deficiency leads to attenuated thrombosis potential is likely due to decreased circulating TMAO levels. Previously, we showed that direct exposure of platelets to TMAO enhanced sub-maximal stimulus-dependent platelet activation from multiple agonists through augmented Ca2± release from intracellular stores4. This is likely the main reason that FMO3KO mice exhibit attenuated platelet function.
Collectively, there is now strong evidence that the TMAO pathway is associated with cardiovascular disease risk in humans7–9. In parallel, the fact that feeding TMAO promotes atherosclerosis and thrombosis in mouse models2, 4, 5, 10, provides support that TMAO serves as both a biomarker and causative factor in atherothrombotic diseases. Our findings clarify the specific role of the TMAO-producing enzyme FMO3 in lipid and glucose metabolism and atherothrombotic disease. It is important to note that our previous observation of a reduction in atherosclerosis following ASO treatment16 may have been due to the off-target effect on plasma VLDL/IDL/LDL cholesterol levels rather than TMAO levels. This is an important lesson that warns against the use of a single drug or knockdown approach and reinforces the power of mouse genetics in defining mechanism of action. Given the findings of this work, it will be important to continue investigation in FMO3KO mice to fully understand how this multi-functional enzyme impacts cardiometabolic disease.
Supplementary Material
Highlights.
FMO3 knockout mice exhibited elevated TMA levels and reduced TMAO levels.
The previously observed effect of an FMO3 antisense oligonucleotide on plasma lipid levels is due primarily to an off-target effect.
The FMO3 knockout mice exhibited reduced platelet activation and reduced thrombosis in a FeCl3 model.
Acknowledgments
We thank Sarada Charugundla and Zhiqiang Zhou for excellent technical support.
Sources of Funding: This work was supported by NIH grants HL30568, HL28481, HL103866, HL126827, HL122283, AA024333, HL071776, R01-DK098252, R01-HL131471, and R01-NS088689, and the Leducq Foundation.
Disclosures: Drs. Hazen and Wang report being named as co-inventors on pending and issued patents held by the Cleveland Clinic relating to cardiovascular diagnostics or therapeutics, and having the right to receive royalty payment for inventions or discoveries related to cardiovascular diagnostics or therapeutics. Dr. Hazen also reports having been paid as a consultant for P&G, and receiving research funds from Astra Zeneca, P&G, Pfizer Inc., Roche Diagnostics and Takeda
Abbreviations:
- ASO
antisense oligonucleotide
- AST
aspartate aminotransferase
- Cas9
CRISPR associated protein 9
- Chol.
Cholesterol
- CRISPR
Clustered Regularly Interspaced Short Palindromic Repeats
- FMO3
flavin containing monooxygenase 3
- HOMA-IR
Homeostatic Model Assessment of Insulin Resistance
- KO
knockout
- NF-κB
Nuclear Factor kappa-light-chain enhancer of B cells
- TMA
Trimethylamine
- TMAO
Trimethylamine N-oxide
- WT
wild type
References
- 1.Hamman MA, Haehner-Daniels BD, Wrighton SA, Rettie AE, Hall SD. Stereoselective sulfoxidation of sulindac sulfide by flavin-containing monooxygenases. Comparison of human liver and kidney microsomes and mammalian enzymes. Biochemical pharmacology. 2000;60:7–17. [DOI] [PubMed] [Google Scholar]
- 2.Wang Z, Klipfell E, Bennett BJ, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bennett BJ, de Aguiar Vallim TQ, Wang Z, Shih DM, Meng Y, Gregory J, Allayee H, Lee R, Graham M, Crooke R, Edwards PA, Hazen SL, Lusis AJ. Trimethylamine-n-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab. 2013;17:49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu W, Gregory JC, Org E, et al. Gut microbial metabolite tmao enhances platelet hyperreactivity and thrombosis risk. Cell. 2016;165:111–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koeth RA, Wang Z, Levison BS, et al. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19:576–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368:1575–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qi J, You T, Li J, Pan T, Xiang L, Han Y, Zhu L. Circulating trimethylamine n-oxide and the risk of cardiovascular diseases: A systematic review and meta-analysis of 11 prospective cohort studies. Journal of cellular and molecular medicine. 2017:185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heianza Y, Ma W, Manson JE, Rexrode KM, Qi L. Gut microbiota metabolites and risk of major adverse cardiovascular disease events and death: A systematic review and meta-analysis of prospective studies. Journal of the American Heart Association. 2017;6:e004947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schiattarella GG, Sannino A, Toscano E, Giugliano G, Gargiulo G, Franzone A, Trimarco B, Esposito G, Perrino C. Gut microbe-generated metabolite trimethylamine-n-oxide as cardiovascular risk biomarker: A systematic review and dose-response meta-analysis. European heart journal. 2017;38:2948–2956. [DOI] [PubMed] [Google Scholar]
- 10.Wang Z, Roberts AB, Buffa JA, et al. Non-lethal inhibition of gut microbial trimethylamine production for the treatment of atherosclerosis. Cell. 2015;163:1585–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seldin MM, Meng Y, Qi H, Zhu W, Wang Z, Hazen SL, Lusis AJ, Shih DM. Trimethylamine n-oxide promotes vascular inflammation through signaling of mitogen-activated protein kinase and nuclear factor-kappab. Journal of the American Heart Association. 2016;5:e002767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boini KM, Hussain T, Li PL, Koka S. Trimethylamine-n-oxide instigates nlrp3 inflammasome activation and endothelial dysfunction. Cell Physiol Biochem. 2017;44:152–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen ML, Zhu XH, Ran L, Lang HD, Yi L, Mi MT. Trimethylamine-n-oxide induces vascular inflammation by activating the nlrp3 inflammasome through the sirt3-sod2-mtros signaling pathway. Journal of the American Heart Association. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roberts AB, Gu X, Buffa JA, et al. Development of a gut microbe-targeted nonlethal therapeutic to inhibit thrombosis potential. Nat Med. 2018;24:1407–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miao J, Ling AV, Manthena PV, Gearing ME, Graham MJ, Crooke RM, Croce KJ, Esquejo RM, Clish CB, Morbid Obesity Study G, Vicent D, Biddinger SB. Flavin-containing monooxygenase 3 as a potential player in diabetes-associated atherosclerosis. Nature communications. 2015;6:6498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shih DM, Wang Z, Lee R, et al. Flavin containing monooxygenase 3 exerts broad effects on glucose and lipid metabolism and atherosclerosis. Journal of lipid research. 2015;56:22–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Warrier M, Shih DM, Burrows AC, et al. The tmao-generating enzyme flavin monooxygenase 3 is a central regulator of cholesterol balance. Cell reports. 2015:326–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schugar RC, Shih DM, Warrier M, et al. The tmao-producing enzyme flavin-containing monooxygenase 3 regulates obesity and the beiging of white adipose tissue. Cell reports. 2017;19:2451–2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robinet P, Milewicz DM, Cassis LA, Leeper NJ, Lu HS, Smith JD. Consideration of sex differences in design and reporting of experimental arterial pathology studies-statement from atvb council. Arterioscler Thromb Vasc Biol. 2018;38:292–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borel F, Sun H, Zieger M, Cox A, Cardozo B, Li W, Oliveira G, Davis A, Gruntman A, Flotte TR, Brodsky MH, Hoffman AM, Elmallah MK, Mueller C. Editing out five serpina1 paralogs to create a mouse model of genetic emphysema. Proceedings of the National Academy of Sciences of the United States of America. 2018;115:2788–2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skarnes WC, Rosen B, West AP, et al. A conditional knockout resource for the genome-wide study of mouse gene function. Nature. 2011;474:337–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagy A, Rossant J, Nagy R, Abramow-Newerly W, Roder JC. Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:8424–8428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Z, Levison BS, Hazen JE, Donahue L, Li XM, Hazen SL. Measurement of trimethylamine-n-oxide by stable isotope dilution liquid chromatography tandem mass spectrometry. Analytical biochemistry. 2014;455:35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daugherty A, Tall AR, Daemen M, Falk E, Fisher EA, Garcia-Cardena G, Lusis AJ, Owens AP 3rd, Rosenfeld ME, Virmani R, American Heart Association Council on Arteriosclerosis T, Vascular B, Council on Basic Cardiovascular S. Recommendation on design, execution, and reporting of animal atherosclerosis studies: A scientific statement from the american heart association. Arterioscler Thromb Vasc Biol. 2017;37:e131–e157. [DOI] [PubMed] [Google Scholar]
- 25.Shih DM, Xia YR, Wang XP, Miller E, Castellani LW, Subbanagounder G, Cheroutre H, Faull KF, Berliner JA, Witztum JL, Lusis AJ. Combined serum paraoxonase knockout/apolipoprotein e knockout mice exhibit increased lipoprotein oxidation and atherosclerosis. The Journal of biological chemistry. 2000;275:17527–17535. [DOI] [PubMed] [Google Scholar]
- 26.Willer CJ, Schmidt EM, Sengupta S, et al. Discovery and refinement of loci associated with lipid levels. Nature genetics. 2013;45:1274–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu W, Wang Z, Tang WHW, Hazen SL. Gut microbe-generated trimethylamine n-oxide from dietary choline is prothrombotic in subjects. Circulation. 2017;135:1671–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kok FO, Shin M, Ni CW, et al. Reverse genetic screening reveals poor correlation between morpholino-induced and mutant phenotypes in zebrafish. Developmental cell. 2015;32:97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rossi A, Kontarakis Z, Gerri C, Nolte H, Holper S, Kruger M, Stainier DY. Genetic compensation induced by deleterious mutations but not gene knockdowns. Nature. 2015;524:230–233. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.