Abstract
Aim
Despite current treatments, high-grade meningiomas continue to have a poor prognosis. Immunotherapy targeting immune checkpoints, such as PD-L1, has demonstrated significant success in controlling numerous malignancies. In this study, we investigate the extent of systemic and local immunosuppression in meningiomas to assess the potential benefit of immune checkpoint inhibitors for the treatment of high-grade meningiomas.
Methods
Peripheral blood was collected from patients undergoing resection of meningiomas (WHO grade I, n = 18; grade II, n = 25; grade III, n = 10). Immunosuppressive myeloid cells (CD45+CD11b+PD-L1+), myeloid-derived suppressor cells (MDSCs) (CD11b+CD33+HLA-DRlow), and regulatory T cells (Tregs) (CD3+CD4+CD25+FoxP3+) were quantified through flow cytometry. Tissue sections from the same patients were assessed for PD-L1 expression and T cell infiltration via immunohistochemistry.
Results
Patients with grade III meningiomas demonstrated increased peripheral monocyte PD-L1 compared to patients with grade I/II meningiomas and healthy controls. Peripheral MDSC abundance was increased in grades II and III meningioma patients. PD-L1 staining of meningioma tissue demonstrated increased positivity in grade III meningiomas. Intratumoral PD-L1 was not associated with progression-free survival. High-grade meningiomas had increased T-cell infiltration. However, a significant proportion of these T cells were exhausted PD1+ T cells and immunosuppressive Tregs.
Conclusions
Patients with meningiomas exhibit signs of peripheral immunosuppression, including increased PD-L1 on myeloid cells and elevated MDSC abundance proportional to tumor grade. Additionally, the tumors express substantial PD-L1 proportional to tumor grade. These results suggest a role for immune checkpoint inhibitors targeting the PD-L1/PD-1 pathway in combination with standard therapies for the treatment of high-grade meningiomas.
Electronic supplementary material
The online version of this article (10.1007/s00262-019-02342-8) contains supplementary material, which is available to authorized users.
Keywords: Meningioma, Immune checkpoint inhibition, Immunotherapy, PD-L1, Programmed death-ligand 1
Introduction
Meningiomas are the most common primary intracranial tumor, the vast majority of which have World Health Organization (WHO) grade I benign pathology. Atypical (WHO grade II) meningiomas account for 5–15% of all tumors, and anaplastic (WHO grade III) meningiomas represent 1–3% [2]. Grade III meningiomas are more aggressive, resulting in higher frequencies of local invasion, recurrence, and metastasis. Recurrence rates of grade III meningiomas are as high as 80% with a median survival time of less than 2 years [3]. In addition, treatment options following recurrence are extremely limited. Currently, the primary treatments for meningiomas are surgery and radiotherapy. Targeted therapy, such as selective inhibitors of SMO and AKT, is only effective in cases where the tumor harbors a driver mutation in those genes. In addition, these driver mutations are most commonly seen in grade I meningiomas of the skull base [4, 5]. Thus, novel therapies for higher-grade meningiomas are necessary to improve patient prognoses.
One potential treatment strategy for high-grade meningiomas is immunotherapy, which has been applied to a variety of cancers with promising results. Investigators have only recently begun to explore the potential application of immune checkpoint inhibitors to intracranial meningiomas. Immune checkpoint proteins such as cytotoxic T lymphocyte antigen 4 (CTLA-4), programmed death 1 (PD-1), and programmed death-ligand 1 (PD-L1) are crucial for maintaining immune homeostasis and preventing autoimmunity. However, these checkpoint pathways are also used by cancer cells to evade the immune system [6]. To date, multiple clinical trials testing antibody-mediated inhibition of the immune checkpoints CTLA-4 and PD-1/PD-L1 have demonstrated great promise in a variety of cancers including lung and melanoma [7–9]. Recent studies have reported the presence of PD-L1 in meningiomas, as well as various subpopulations of infiltrating lymphocytes [10, 11]. However, it is unclear how these immune populations shift depending on tumor grade and how immune checkpoint expression correlates with clinical outcomes. Conflicting reports exist on whether CD3+ T-cell infiltration increases or decreases in higher-grade tumors [10, 12]. Therefore, we aimed to further characterize the tumor immune microenvironment of meningiomas as well as its relationship with the systemic immune phenotype.
Systemic immunosuppression occurs in patients with malignancy and has largely been attributed to the expansion of suppressive immune cells [13]. Among the myeloid lineage, expansion of suppressive myeloid cells, such as PD-L1+ monocytes and myeloid-derived suppressor cells (MDSCs), has been reported and associated with poor prognosis [13–15]. We previously demonstrated that circulating myeloid cells in patients with glioblastoma have increased PD-L1 surface expression, which was associated with worse survival in patients receiving a vaccine immunotherapy [15]. Whether similar immunosuppressive effects are seen peripherally in patients with meningiomas is unknown. Consequently, our study is the first to characterize the systemic immune phenotype of patients with meningiomas. This study of the peripheral markers of immune suppression has two purposes: first, to provide insight on the use of immune therapies for meningiomas; and second, to explore peripheral immune phenotype as a biomarker for meningioma grade.
Materials and methods
Patient cohorts, clinical tissue samples, and peripheral blood samples
Meningioma specimens from patients treated at the Northwestern Memorial Hospital between 2014 and 2017 were selected based on the availability of sufficient tissue for immunohistochemical analysis and matched peripheral blood mononuclear cells (PBMCs) for immunophenotyping. Formalin-fixed, paraffin-embedded (FFPE) tissue specimens and frozen PBMCs were obtained from the Northwestern University Nervous System Tumor Bank. Clinical information was obtained from review of the medical records. Recurrence was defined radiographically as a 20% increase in the size of existing residual tumor or the emergence of a new lesion. Of note, the cohort of patients in this study is relatively recent, incorporating patients treated between 2014 and 2017. Some patients have limited follow-up, and therefore, the number of evaluable patients at later time points decreases significantly. These patients are censored as part of the standard practice in Kaplan–Meier analysis for survival.
Flow cytometry
Frozen PBMCs were once thawed in a water bath at 37 °C prior to use. Cells were washed, re-suspended in a 2% bovine serum albumin solution, and flow cytometry staining was performed with antibodies following manufacturer instructions. The following anti-human antibodies were used: CD45 FITC (HI30, eBioscience), CD163 APC (eBioGHI/61, eBioscience), CD33 FITC (HIM3-4, eBioscience), HLA-DR APC (LN3, eBioscience), CD11b APC-eFluor 780 (ICRF44, eBioscience), PD-L1 PE (MIH1, eBioscience), CD3 PerCP-eFluor 710 (OKT3, eBioscience), CD4 PE (OKT4, eBioscience), CD25 APC (BC96, eBioscience), FoxP3 FITC (PCH101, eBioscience). For assays requiring intracellular stains, cells were permeabilized with a saponin-based Perm/Wash buffer (BD Biosciences). Sample data were collected by flow cytometry on an Attune NxT cytometer with the Attune NxT Software (Thermo Scientific). OneComp eBeads were used for compensation controls (Invitrogen). Fluorescence minus one (FMO) controls were used for appropriate gating of subsets. Post hoc analyses were performed with the FlowJo Software v10.3 (TreeStar).
Immunohistochemistry and immunofluorescence
The following primary antibodies were used in this study: PDL1 (0.8 μg/ml, Clone: SP142, Roche), IFN-γ (1:100, ab218426, Abcam), CD68 (1:100, ab199000, Abcam), Iba1/AIF1 (1:300, MABN92, Millipore), CD11b (1:500, 14-0196-82, eBioscience), CD3 (1:100, ab16669, Abcam), PD-1 (1:100, ab52587, Abcam), FoxP3 (1:300, ab20034, Abcam).
Tissue sections from FFPE blocks (5 μm thickness) were deparaffinized in xylene and rehydrated in serial baths of graded ethanol (100%, 95%, 70%). Heat-induced epitope retrieval was performed with an ethylenediaminetetraacetic acid buffer pH 8.0 in a decloaking chamber (Biocare Medical). Slides were blocked using a solution of 10% normal goat serum (Sigma-Aldrich) and incubated at room temperature for 10 min for PD-L1, and 4 °C overnight for all other primary antibodies. Visualization was performed with EXPOSE Rabbit specific HRP/DAB detection IHC kit (ab80437, Abcam) and EXPOSE Mouse specific AP (red) detection IHC kit (ab94740, Abcam).
For immunofluorescence staining, slides were incubated for 1 h at room temperature with secondary goat anti-rabbit (A-11034, ThermoFisher Scientific) and goat anti-mouse (A-11032, ThermoFisher Scientific) antibodies, conjugated to Alexa Fluor 488 and Alexa Fluor 594, respectively.
Image acquisition and analysis
Immunohistochemically stained samples were imaged using the PerkinElmer Nuance microscope. Immunofluorescent-stained slides were imaged using the Nikon A1R confocal microscope. Quantification of cell number for CD3 and PD-1 positivity was done manually. Quantification of CD68 cell number was performed using the automated cell counting function in ImageJ using a set of algorithms generated by a trained user (Size > 300 pixels2 and circularity 0.00–1.00).
Statistical analysis
Statistical analyses were performed using Prism7 (GraphPad Software). Expression of various markers between groups was compared using the two-sided Student’s t test or ANOVA. The Kaplan–Meier method was used to estimate survival distributions. Censored patients are indicated by vertical ticks on the survival plots. Differences in length of follow-up and censorship are accounted for in the Kaplan–Meier analysis, and the log-rank test remains significant where indicated by p < 0.05. p values < 0.05 were considered statistically significant.
Results
Patients
Fifty-three patients meeting the inclusion criteria were identified in our cohort. The median age at surgery was 61 years (range 25–85), and 34 patients (64%) were female. Eighteen patients had grade I, 25 patients had grade II, and 10 patients had grade III meningiomas. Of the 53 patients, 13 (25%) had recurrent tumors and 10 patients (19%) had radiation therapy in the past. Thirty-six patients (68%) had gross total resections, and the other 17 (32%) had subtotal resections. Table 1 summarizes the patients’ characteristics and prior therapies.
Table 1.
Clinical characteristics of patients included in this study
| Patient characteristics | n (53) | % |
|---|---|---|
| Age at surgery, median (range) | 61 (25–85) | |
| Sex | ||
| Female | 34 | 64.2 |
| Male | 19 | 35.8 |
| WHO grade | ||
| I | 18 | 34.0 |
| II | 25 | 47.2 |
| III | 10 | 18.8 |
| Karnofsky performance status | ||
| 90 + | 6 | 11.3 |
| 80 | 0 | 0 |
| 70 | 2 | 3.8 |
| < 70 | 0 | 0 |
| Unknown | 45 | 84.9 |
| Ki67/MIB1 status | ||
| 1–4.9% | 19 | 35.8 |
| 5–10% | 15 | 28.3 |
| > 10% | 18 | 34.0 |
| Unknown | 1 | 1.9 |
| Prior radiotherapy | ||
| Yes | 10 | 18.9 |
| No | 43 | 81.1 |
| Recurrence status | ||
| Recurrent tumor | 13 | 24.5 |
| Newly diagnosed tumor | 40 | 75.5 |
| Extent of resection | ||
| Gross total resection | 36 | 67.9 |
| Subtotal resection | 17 | 32.1 |
Peripheral immunosuppression in high-grade meningioma patients
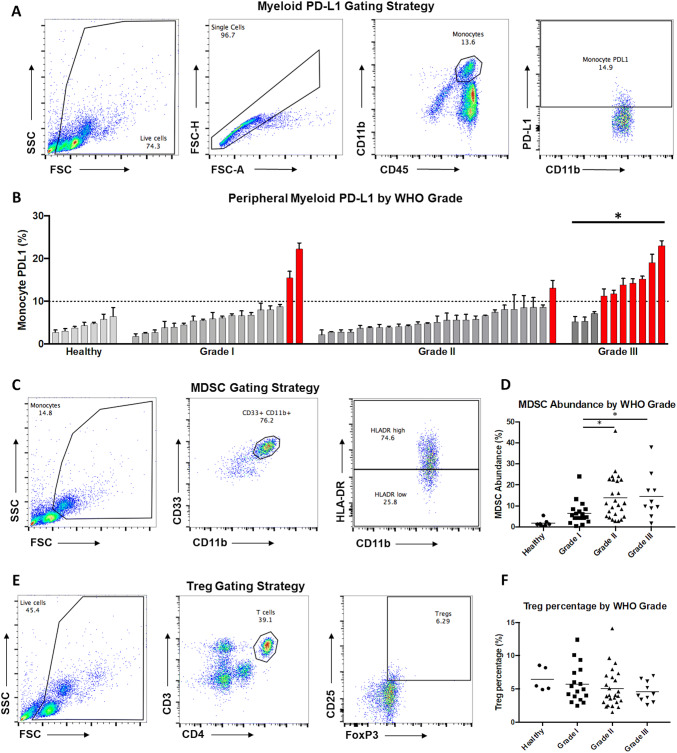
Monocyte PD-L1 (CD45+CD11b+PD-L1+), MDSC abundance (CD33+CD11b+HLA-DRlow), and regulatory T cell (Treg) abundance (CD3+CD4+CD25+FoxP3+) were examined for each patient via flow cytometry. Higher monocyte PD-L1 was seen in patients with grade III meningiomas; mean 6.9% for grade I, 5.6% for grade II, and 12.6% for grade III meningioma patients (ANOVA, p = 0.0002, Fig. 1a, b). The MDSC population was greater in patients with grades II and III meningiomas; mean 6.4% for grade I, 13.8% for grade II, and 14.4% for grade III meningioma patients (ANOVA, p = 0.023, Fig. 1c, d). Across all grades, the mean percentage of Tregs among CD4+ cells was 5.2%. There was no difference in Treg abundance based on the grade of tumor (Fig. 1e, f).
Fig. 1.
Peripheral immune profiling of patients with meningiomas. a Representative gating scheme for identification of myeloid cells from peripheral blood leukocytes by flow cytometry. Live cells were gated using forward and side scatter from the total population (left), followed by identification of single cells (left-center), gating for total myeloid population of CD45+/CD11b+ cells (right-center), and gating for PD-L1+ cells (right). b Summary of PD-L1 expression in monocytes from each of the 53 evaluated patients as well as healthy controls ordered by percent positive expression within each grade. Patients with grade III meningiomas had significantly elevated monocyte PD-L1 compared to patients with lower grade tumors (*p < 0.05). Patients with monocyte PD-L1 expression greater than 10% are colored in red. c Representative gating scheme for identification of MDSCs from peripheral blood leukocytes by flow cytometry. Monocytes were gated from the total population (left), followed by identification of single cells (not shown), gating for total myeloid population of CD33+/CD11b+ cells (center), and gating for MDSCs (HLA-Drlo; right). d Summary of MDSC abundance from each of the 53 evaluated patients as well as healthy controls grouped by grade. Patients with grades II and III meningiomas had increased MDSCs (*p < 0.05). e Representative gating scheme for identification of Tregs from peripheral blood leukocytes by flow cytometry. Live cells were gated from the total population (left), followed by identification of single cells (not shown), gating for CD4+ T-cell population (center), and gating for Tregs (CD25+, FoxP3+; right). f Summary of percentage of Tregs/CD4+ T cells from each of the 53 evaluated patients as well as healthy controls grouped by grade. Treg percentage did not vary significantly between grades
Peripheral markers of immune suppression are not independently associated with outcome
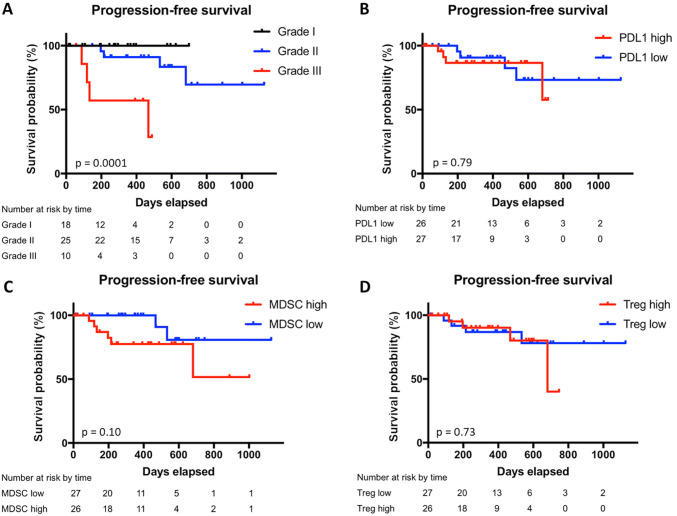
On univariate analysis, tumor grade was highly associated with time to progression (p = 0.0001), with grade III tumors having the shortest median progression-free survival (PFS) of 15.4 months (Fig. 2a). In addition, MIB-1 greater than 10% was associated with worse PFS (p = 0.001, data not shown). Neither peripheral monocyte PD-L1, MDSC abundance, nor Treg abundance was significantly associated with PFS (Fig. 2b–d).
Fig. 2.
Progression-free survival by WHO grade, PD-L1 expression, MDSC abundance, and Treg percentage. Kaplan–Meier estimates of progression-free survival in the subgroup of patients with peripheral blood analysis divided by WHO grade (a), expression of PD-L1 on peripheral myeloid cells (b), peripheral MDSC abundance (c), and peripheral Treg percentage (d). Vertical ticks indicate time points at which patients were censored
Intratumoral expression of PD-L1 in meningiomas
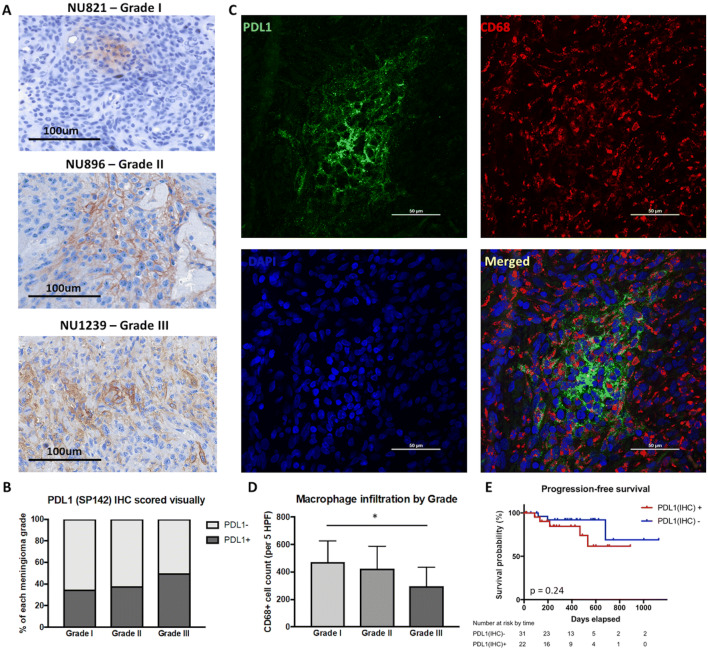
PD-L1 expression on meningioma tumors cells was evaluated using formalin-fixed paraffin-embedded tissue samples via immunohistochemistry. Overall, patients with higher-grade tumors had increased PD-L1 expression. PD-L1 expression was positive in 35% of grade I meningiomas, 38.5% of grade II meningiomas, and 50% of grade III meningiomas (Fig. 3a, b). Immunofluorescent co-staining with CD68 was performed to determine whether PD-L1 was primarily expressed on tumor cells or infiltrating macrophages. We did not find significant colocalization between PD-L1 and CD68 staining, indicating that tumor cells were the main source of PD-L1 expression within meningiomas (Fig. 3c). There was a significant decrease in the number of CD68+ cells in grade III meningiomas (473 cells/5 high powered fields (HPFs) in grade I, 422 cells/5 HPFs in grade II, and 295 cells/5 HPFs in grade III; ANOVA, p = 0.020, Fig. 3d). However, grade III tumors were far more necrotic compared to lower grades. CD68+ macrophage infiltration and intratumoral PD-L1 expression were not independently associated with outcomes (Fig. 3e).
Fig. 3.
Intratumoral expression of PD-L1 and its impact on outcomes. a Representative IHC staining of PD-L1 in FFPE tumor sections of grades I, II, and III meningiomas. b Quantification of intratumoral PD-L1 by grade. Grade III tumors (50%) had greater PD-L1 positivity compared to grade I (35%) and II (38%) tumors. c Representative immunofluorescent staining of PD-L1 and CD68 in FFPE tumor sections. d Quantification of macrophage infiltration by tumor grade. Compared to grade I meningiomas, grade III tumors had significantly decreased CD68+ cell infiltration (*p < 0.05). e Intratumoral PD-L1 was not correlated with PFS
T-cell infiltration in meningiomas
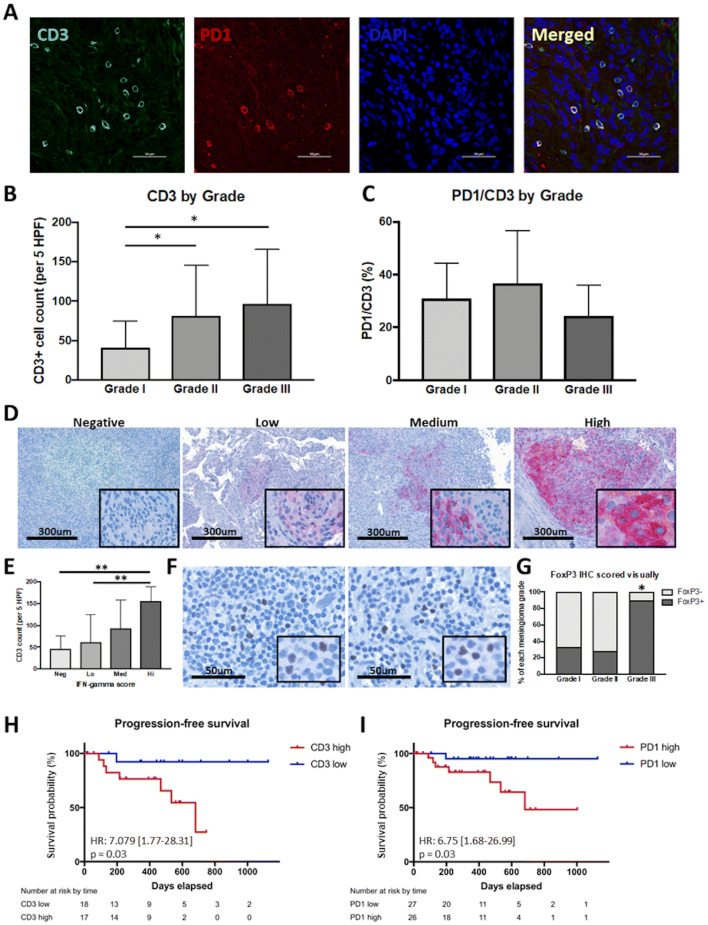
CD3 and PD-1 immunofluorescent co-staining was performed to characterize the T-cell infiltrate in meningiomas. CD3+ and CD3/PD-1 double positive cells were assessed over 5 HPFs. Grade II and grade III tumors had higher overall CD3 infiltration compared to grade I meningiomas with 81 cells/5 HPFs and 97 cells/5 HPFs vs. 41 cells/5 HPFs, respectively (ANOVA, p = 0.017, Fig. 4a, b). The number of PD-1+ cells as well as the proportion of PD-1+/CD3+ cells was not associated with the grade of the tumor (Fig. 4c). We also evaluated interferon-γ (IFN-γ) expression in the tumors. IFN-γ expression was semiquantitatively scored into categories of negative, low, medium, and high expression (Fig. 4d). While IFN-γ positivity did not correlate with tumor grade (data not shown), we found that the number of infiltrating CD3+ cells positively correlated with IFN-γ expression (Fig. 4e). Infiltration of tumors by Tregs was assessed by staining for FoxP3. We found 90% of grade III meningiomas had Treg infiltration compared to 33% of grade I and 28% of grade II meningiomas (p = 0.002, Fig. 4f, g).
Fig. 4.
T-cell infiltration of meningiomas. a Representative immunofluorescent staining of CD3 and PD1 in FFPE tumor sections. b Quantification of T-cell infiltration by grade. Grade II (81.4 cells/5 HPFs) and III (96.8 cells/5 HPFs) tumors had greater CD3+ T-cell infiltration compared to grade I (40.9 cells/5 HPFs) tumors (*p < 0.01). c Quantification of PD1 expression on T cells by grade. Expression of PD1 on CD3+ T cells did not vary significantly between tumor grades. d Representative IHC staining of IFN-γ in FFPE tumor sections with negative, low, medium, and high IFN-γ expression. e Summary of CD3+ T-cell infiltration grouped by degree of IFN-γ expression. Tumors with increased T-cell infiltration also had increased IFN-γ expression (**p < 0.01). f Representative IHC staining of FoxP3 in FFPE tumor sections. g Quantification of FoxP3 positivity by grade. Grade III tumors (90%) had significantly increased FoxP3 positivity compared to grade I (33%) and II (28%) tumors (*p < 0.001). h Kaplan–Meier estimates of progression-free survival by T-cell infiltration in grades II and III meningiomas. i Kaplan–Meier estimates of progression-free survival by PD1 positivity in all meningiomas
T-cells infiltration and PD-1 expression are independently associated with outcome
In univariate analysis, we found CD3 infiltration into the tumor to be a significant predictor of PFS in grades II and III meningioma patients (p = 0.031, Fig. 4h). In addition, PD-1 infiltrate was a significant predictor of PFS in all patients (p = 0.038, Fig. 4i). Treg infiltration was not independently associated with outcome when controlling for tumor grade.
Discussion
The expression of immune checkpoints within the microenvironment of solid tumors is now recognized as a major contributor to tumor-induced immunosuppression and evasion of the innate immune response to malignancy. Similar to many other neoplasms, meningiomas possess the ability to upregulate expression of PD-L1. In agreement with prior studies by Du et al. and Han et al. we found intratumoral PD-L1 expression to be highest in grade III meningiomas [10, 11]. In addition, the primary source of PD-L1 expression in the tumor microenvironment was on the tumor cell population, consistent with the results of tissue microarray analysis reported by Han et al. [11]. Despite increased expression of PD-L1 in high-grade tumors, PD-L1 expression across all tumor grades was not independently predictive of PFS. Du et al. also reported a lack of association between intratumoral PD-L1 expression and outcome [10]. However, by using a cohort enriched for high-grade meningiomas, Han et al. were able to demonstrate a worse prognosis associated with increased PD-L1 expression in the tumor [11]. They postulated that degree of PD-L1 expression is predictive of poor survival only in grades II and III tumors, requiring a large number of samples to identify the difference. Given the limited number of high-grade tumors in our cohort, we were unable to identify such an effect.
Our analysis of tumor samples also confirmed the presence of a significant population of infiltrating T cells in meningiomas, as reported in prior studies [10–12]. However, there is no consensus on how CD3 infiltration is associated with tumor grade. Holmes et al. reported a significant increase in total CD3+ T cells in grade III meningiomas [12]. In contrast, Du et al. reported grade III tumors having the lowest amount of CD3+ T-cell infiltration [10]. Han et al. reported no difference in the number of infiltrating CD3+ cells based on the grade of tumor [11]. In our analysis, we found increased CD3+ cell infiltration in higher-grade meningiomas, most consistent with the results of Holmes et al. In addition, we found that the CD3high cohort had a significantly shorter time to progression. This finding may be explained in part by the subtype of T cells that are present. In grade III meningiomas, 90% of tumor samples had positive staining for Tregs. These inhibitory T cells have been shown in various cancers to have a negative impact on survival [16–18]. In addition, higher degrees of total T-cell infiltration may be an indicator of a more aggressive tumor. In patients with breast cancer, increased T-cell infiltration has been associated with higher proliferation, higher histological grade, as well as larger tumor size [19]. Lastly, we provide evidence of an association between CD3+ T-cell infiltrate and the presence of IFN-γ in the tumor environment. IFN- γ is not only crucial for T-cell regulation, but has also been shown to be a key inducer of indoleamine 2,3-dioxygenase, the rate-limiting enzyme in a pathway that can enable tumor cells to evade host immune response [20]. Thus, it is possible that the higher amounts of CD3+ T-cell infiltrate predominantly seen in grades II and III meningiomas coupled with the expression of IFN-γ elicit a paradoxical increase in local immunosuppression. However, the presence of T cells in the tumor also suggests that if these immunosuppressive mechanisms can be overcome, innate immune responses may be active against the tumor.
We have demonstrated significant expression of PD-1 on infiltrating CD3+ cells, indicative of exhaustion. Upon binding of PD-L1, PD-1 inhibits T-cell effector functions, resulting in T-cell anergy and apoptosis [21]. However, despite the inhibitory effects of PD-1 activation, the prognostic impact of PD-1 expression has been controversial to date. In a study of head and neck cancer, the frequency of PD-1+ T cells has been shown to predict which patients will respond to immunotherapy [22]. Similarly, in our study, meningioma patients with higher numbers of PD-1+ CD3+ cells had significantly shorter PFS. These data may implicate a role for immune checkpoint inhibition targeting the PD-1/PD-L1 pathway in the treatment of high-grade meningiomas.
In addition to infiltration of T cells, another contributor to the efficacy of immunotherapy is the concept of a “hot” tumor. These tumors have high rates of somatic mutational load, express surface neoantigens, and provoke a strong immune response. For example, melanoma and lung cancer have the highest rates of somatic mutational burden and consequently both have had profound responses to immunotherapy [23]. Currently, the somatic mutational burden in meningiomas is not well studied. Dewan et al. recently demonstrated a low somatic burden in both grades I and II meningiomas through a whole-exome sequencing study of NF2-associated meningiomas [24]. Through whole-genome and whole-exome sequencing of 17 meningiomas, Brastianos et al. also found that most meningiomas had simple genomes with fewer mutations than other adult tumors. However, there was a subset of meningiomas that exhibited more genetic instability [5]. These findings suggest that while meningiomas may not be very immunogenic on average, at least in the group of patients with “hotter” tumors, there is a potential benefit from the use of immunotherapy.
We also investigated the myeloid cell infiltrate in the tumor microenvironment. We noted an abundance of CD68+ cells in meningioma tissue sections, similar to results reported by Han et al. [11]. The degree of positive staining was far in excess of the expected macrophage infiltrate based on H&E staining. To confirm the specificity of CD68+ staining for infiltrating myeloid cells, we also stained for alternative myeloid markers including CD11b and Iba1 (Supplemental Fig. 1). We reviewed slides with a neuropathologist (C. Horbinski) and confirmed the presence of tumor cells staining positive for all three myeloid markers. To our knowledge, this is the first account of meningioma tumor cells staining positive for three separate myeloid cell markers (CD68, CD11b, and Iba1). A study by Kanno et al. demonstrated aberrant expression of CD163 on tumor cells in meningioma but did not proceed to test other myeloid markers [25]. Although the etiology of our finding is unclear, it is important to recognize the cross-reactivity when attempting to characterize the myeloid population within meningiomas.
While we did not characterize the B-cell infiltrate in the tumor microenvironment, it is important to mention their significance. The T-cell infiltrate in meningiomas has been studied more than the B-cell infiltrate. However, it is recognized that B cells can enhance T-cell responses by producing antibodies, stimulatory cytokines and serving as antigen presenting cells [26]. Fang et al. demonstrated that the B-cell population in meningiomas was predominantly antigen-experienced and thus promoted immune responses [27]. They did not observe any significant associations between the tumor infiltrating lymphocyte characteristics and meningioma grade. They also note that the presence of B cells can sometimes have a paradoxical effect as resting B cells can inhibit T-cell activity. Interestingly, Ding et al. observed that meningiomas with a higher density of CD20+ B cells had an increased rate of recurrence [28]. Further studies will be needed to better elucidate the clinical significance of infiltrating B cells in meningiomas.
Although previous studies have evaluated the expression of immune checkpoints and suppressive cell populations within meningioma tissue, our study is the first to also evaluate the impact of these factors in the peripheral circulation of meningioma patients. We found an increased number of PD-L1+ myeloid cells and classic MDSCs in the circulation of patients with high-grade tumors. However, despite differences in expression of these targetable immunosuppressive factors between tumors of different grades, no differences in PFS were correlated with these factors.
While peripheral myeloid PD-L1 expression was not independently associated with outcome, it has the potential to be a critical biomarker guiding patient therapy. Using a threshold of 10% positivity, peripheral myeloid PD-L1 predicts the likelihood of a grade III meningioma with a positive predictive value of 93%. Sensitivity and specificity were 93% and 70%, respectively. Thus, in patients with a dural-based mass suggestive of meningioma, measuring myeloid PD-L1 expression via a blood test could provide insight into tumor grade preoperatively and assist with surgical planning. In addition, in patients who have undergone treatment for an atypical meningioma, following peripheral myeloid PD-L1 expression over time could serve as an adjunct to imaging, suggesting tumor recurrence and conversion to a grade III lesion.
The limitations of this study include those inherent to research utilizing banked tissue sections, including the possibility that the sections may not capture the heterogeneous nature of the tumor microenvironment. The peripheral immune phenotype of these patients was characterized primarily with flow cytometry; future studies utilizing functional assays of various immune subsets could provide further insight into the overall immune function of meningioma patients. Selection bias may have also been introduced as we only included patients with sufficient tumor tissue and matched PBMCs in our cohort. Our limited cohort with a relatively short follow-up time also limits the power of the analysis, possibly accounting for a lack of statistical correlation between immunosuppressive factors and PFS. Nonetheless, there is sufficient evidence of effector T-cell infiltration into meningiomas and elevated expression of immune checkpoints in the tumor and in circulation to warrant investigation of immune checkpoint inhibitors for the treatment of high-grade meningiomas.
Conclusion
Patients with meningiomas harbor multiple mediators of peripheral immunosuppression, including increased PD-L1 on myeloid cells and elevated MDSC abundance proportional to tumor grade. Additionally, the tumors express substantial PD-L1 proportional to tumor grade. Neither peripheral nor intratumoral PD-L1 expression correlates with outcomes when treated with standard interventions. However, high-grade meningiomas have significant CD3+ T-cell infiltration, suggesting a potential role of immune checkpoint modulators to enhance anti-tumor immunity. In addition, using a threshold of 10%, peripheral myeloid PD-L1 was a strong predictor of high-grade tumors and may be useful as a diagnostic biomarker.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank the Nervous System Tumor Bank at Northwestern University, without which the current study would not be possible. Imaging work was performed at the Northwestern University Center for Advanced Microscopy generously supported by National Cancer Institute (NCI) CCSG P30 CA060553 awarded to the Robert H. Lurie Comprehensive Cancer Center.
Abbreviations
- CTLA-4
Cytotoxic T lymphocyte antigen 4
- FFPE
Formalin-fixed, paraffin-embedded
- HPFs
High powered fields
- IFN-γ
Interferon-γ
- MDSC
Myeloid-derived suppressor cells
- NCI
National Cancer Institute
- NINDS
National Institute of Neurological Disorders and Stroke
- PBMC
Peripheral blood mononuclear cells
- PD-1
Programmed death 1
- PD-L1
Programmed death-ligand 1
- PFS
Progression-free survival
- Treg
Regulatory T cell
- WHO
World Health Organization
Author contributions
YDL took part in conceptualization, experimental design, data curation, formal analysis, funding acquisition, methodology, validation, and writing the manuscript; DV took part in experimental design and data curation; JBL took part in data curation, formal analysis, methodology, and validation. JBL took part in data curation and formal analysis; GK, and DB took part in data curation; CMH took part in formal analysis and validation; TJK took part in conceptualization and methodology; OB took part in conceptualization, funding acquisition, investigation, methodology, and supervision. All authors read and approved the final manuscript.
Funding
This work was supported by the Howard Hughes Medical Institute Medical Student Research Fellows program (Yuping Li) as well as by the NIH/NCI (National Cancer Institute) R01 (CA164714; Orin Bloch) and NIH/National Institute of Neurological Disorders and Stroke (NINDS) R00 (NS078055; Orin Bloch). The funding was received by National Cancer Institute (CA206413; Jonathan B. Lamano) and National Institute of Neurological Disorders and Stroke (NS101884; Gurvinder Kaur).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval and ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the Institutional Review Board of Northwestern University, Feinberg School of Medicine (STU00203854).
Informed consent
Written informed consent was obtained from all individual participants included in the study for the use of their blood and tumor specimen for research. Consent was not required for collection of patient characteristics as information was de-identified.
Footnotes
This work was previously published as an abstract at the 2018 American Association of Neurological Surgeons (AANS) Annual Scientific Meeting in New Orleans, LA, USA on April 29–May 2, 2018 [1].
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Li Y, Veliceasa D, Lamano J, Lamano JB, Kaur G, Smith B, DiDomenico J, Oyon D, Bloch O. Systemic and local immunosuppression in patients with high-grade meningiomas. J Neurosurg. 2018;128(4):961–1272. doi: 10.3171/2017.1.JNS162785. [DOI] [PubMed] [Google Scholar]
- 2.Saraf S, McCarthy BJ, Villano JL. Update on meningiomas. Oncologist. 2011;16(11):1604–1613. doi: 10.1634/theoncologist.2011-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perry A, Scheithauer BW, Stafford SL, Lohse CM, Wollan PC. “Malignancy” in meningiomas: a clinicopathologic study of 116 patients, with grading implications. Cancer. 1999;85(9):2046–2056. doi: 10.1002/(sici)1097-0142(19990501)85:9<2046::aid-cncr23>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 4.Clark VE, Erson-Omay EZ, Serin A, Yin J, Cotney J, Ozduman K, Avsar T, Li J, Murray PB, Henegariu O, Yilmaz S, Gunel JM, Carrion-Grant G, Yilmaz B, Grady C, Tanrikulu B, Bakircioglu M, Kaymakcalan H, Caglayan AO, Sencar L, Ceyhun E, Atik AF, Bayri Y, Bai H, Kolb LE, Hebert RM, Omay SB, Mishra-Gorur K, Choi M, Overton JD, Holland EC, Mane S, State MW, Bilguvar K, Baehring JM, Gutin PH, Piepmeier JM, Vortmeyer A, Brennan CW, Pamir MN, Kilic T, Lifton RP, Noonan JP, Yasuno K, Gunel M. Genomic analysis of non-NF2 meningiomas reveals mutations in TRAF7, KLF4, AKT1, and SMO. Science. 2013;339(6123):1077–1080. doi: 10.1126/science.1233009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brastianos PK, Horowitz PM, Santagata S, Jones RT, McKenna A, Getz G, Ligon KL, Palescandolo E, Van Hummelen P, Ducar MD, Raza A, Sunkavalli A, Macconaill LE, Stemmer-Rachamimov AO, Louis DN, Hahn WC, Dunn IF, Beroukhim R. Genomic sequencing of meningiomas identifies oncogenic SMO and AKT1 mutations. Nat Genet. 2013;45(3):285–289. doi: 10.1038/ng.2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perng P, Lim M. Immunosuppressive mechanisms of malignant gliomas: parallels at non-CNS sites. Front Oncol. 2015 doi: 10.3389/fonc.2015.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, Barlesi F, Kohlhaufl M, Arrieta O, Burgio MA, Fayette J, Lena H, Poddubskaya E, Gerber DE, Gettinger SN, Rudin CM, Rizvi N, Crino L, Blumenschein GR, Jr, Antonia SJ, Dorange C, Harbison CT, Graf Finckenstein F, Brahmer JR. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ott PA, Hodi FS, Robert C. CTLA-4 and PD-1/PD-L1 blockade: new immunotherapeutic modalities with durable clinical benefit in melanoma patients. Clin Cancer Res. 2013;19(19):5300–5309. doi: 10.1158/1078-0432.CCR-13-0143. [DOI] [PubMed] [Google Scholar]
- 9.Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob J-J, Cowey CL, Lao CD, Wagstaff J, Schadendorf D, Ferrucci PF, Smylie M, Dummer R, Hill A, Hogg D, Haanen J, Carlino MS, Bechter O, Maio M, Marquez-Rodas I, Guidoboni M, McArthur G, Lebbé C, Ascierto PA, Long GV, Cebon J, Sosman J, Postow MA, Callahan MK, Walker D, Rollin L, Bhore R, Hodi FS, Larkin J. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2017;377(14):1345–1356. doi: 10.1056/NEJMoa1709684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Du Z, Abedalthagafi M, Aizer AA, McHenry AR, Sun HH, Bray MA, Viramontes O, Machaidze R, Brastianos PK, Reardon DA, Dunn IF, Freeman GJ, Ligon KL, Carpenter AE, Alexander BM, Agar NY, Rodig SJ, Bradshaw EM, Santagata S. Increased expression of the immune modulatory molecule PD-L1 (CD274) in anaplastic meningioma. Oncotarget. 2015;6(7):4704–4716. doi: 10.18632/oncotarget.3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han SJ, Reis G, Kohanbash G, Shrivastav S, Magill ST, Molinaro AM, McDermott MW, Theodosopoulos PV, Aghi MK, Berger MS, Butowski NA, Barani I, Phillips JJ, Perry A, Okada H. Expression and prognostic impact of immune modulatory molecule PD-L1 in meningioma. J Neurooncol. 2016;130(3):543–552. doi: 10.1007/s11060-016-2256-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holmes M, Vides L, Chahine J, Harris B. The role of microglia and T cells in different grades of meningioma. Am J Clin Pathol. 2014;142(suppl_1):A001. doi: 10.1093/ajcp/142.suppl1.001. [DOI] [Google Scholar]
- 13.Vetsika E-K, Koinis F, Gioulbasani M, Aggouraki D, Koutoulaki A, Skalidaki E, Mavroudis D, Georgoulias V, Kotsakis A. A circulating subpopulation of monocytic myeloid-derived suppressor cells as an independent prognostic/predictive factor in untreated non-small lung cancer patients. J Immunol Res. 2014;2014:12. doi: 10.1155/2014/659294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang A, Zhang B, Wang B, Zhang F, Fan KX, Guo YJ. Increased CD14(+)HLA-DR−/low myeloid-derived suppressor cells correlate with extrathoracic metastasis and poor response to chemotherapy in non-small cell lung cancer patients. Cancer Immunol Immunother. 2013;62(9):1439–1451. doi: 10.1007/s00262-013-1450-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bloch O, Crane CA, Kaur R, Safaee M, Rutkowski MJ, Parsa AT. Gliomas promote immunosuppression through induction of B7-H1 expression in tumor-associated macrophages. Clin Cancer Res. 2013;19(12):3165–3175. doi: 10.1158/1078-0432.CCR-12-3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang Y, Liao H, Zhang Y, Yuan R, Wang F, Gao Y, Wang P, Du Z. Prognostic value of tumor-infiltrating FoxP3 + T cells in gastrointestinal cancers: a meta analysis. PLoS One. 2014;9(5):e94376. doi: 10.1371/journal.pone.0094376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santoiemma PP, Powell DJ., Jr Tumor infiltrating lymphocytes in ovarian cancer. Cancer Biol Ther. 2015;16(6):807–820. doi: 10.1080/15384047.2015.1040960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao HQ, Li WM, Lu ZQ, Yao YM. Roles of Tregs in development of hepatocellular carcinoma: a meta-analysis. World J Gastroenterol. 2014;20(24):7971–7978. doi: 10.3748/wjg.v20.i24.7971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miyan M, Schmidt-Mende J, Kiessling R, Poschke I, de Boniface J. Differential tumor infiltration by T-cells characterizes intrinsic molecular subtypes in breast cancer. J Transl Med. 2016 doi: 10.1186/s12967-016-0983-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zitron IM, Kamson DO, Kiousis S, Juhász C, Mittal S. In vivo metabolism of tryptophan in meningiomas is mediated by indoleamine 2,3-dioxygenase 1. Cancer Biol Ther. 2013;14(4):333–339. doi: 10.4161/cbt.23624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chikuma S, Terawaki S, Hayashi T, Nabeshima R, Yoshida T, Shibayama S, Okazaki T, Honjo T. PD-1-mediated suppression of IL-2 production induces CD8 + T cell anergy in vivo. J Immunol. 2009;182(11):6682–6689. doi: 10.4049/jimmunol.0900080. [DOI] [PubMed] [Google Scholar]
- 22.Kansy BA, Concha-Benavente F, Srivastava RM, Jie H-B, Shayan G, Lei Y, Moskovitz J, Moy J, Li J, Brandau S, Lang S, Schmitt NC, Freeman GJ, Gooding WE, Clump DA, Ferris RL. PD-1 status in CD8 + T cells associates with survival and anti-PD-1 therapeutic outcomes in head and neck cancer. Cancer Res. 2017;77(22):6353–6364. doi: 10.1158/0008-5472.CAN-16-3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Branca MA. Rekindling cancer vaccines. Nat Biotechnol. 2016;34:1019. doi: 10.1038/nbt.3690. [DOI] [PubMed] [Google Scholar]
- 24.Dewan R, Pemov A, Dutra AS, Pak ED, Edwards NA, Ray-Chaudhury A, Hansen NF, Chandrasekharappa SC, Mullikin JC, Asthagiri AR, Heiss JD, Stewart DR, Germanwala AV. First insight into the somatic mutation burden of neurofibromatosis type 2-associated grade I and grade II meningiomas: a case report comprehensive genomic study of two cranial meningiomas with vastly different clinical presentation. BMC Cancer. 2017;17(1):127. doi: 10.1186/s12885-017-3127-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanno H, Nishihara H, Wang L, Yuzawa S, Kobayashi H, Tsuda M, Kimura T, Tanino M, Terasaka S, Tanaka S. Expression of CD163 prevents apoptosis through the production of granulocyte colony-stimulating factor in meningioma. Neuro-oncology. 2013;15(7):853–864. doi: 10.1093/neuonc/not028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nelson BH. CD20 + B cells: the other tumor-infiltrating lymphocytes. J Immunol. 2010;185(9):4977–4982. doi: 10.4049/jimmunol.1001323. [DOI] [PubMed] [Google Scholar]
- 27.Fang L, Lowther DE, Meizlish ML, Anderson RCE, Bruce JN, Devine L, Huttner AJ, Kleinstein SH, Lee JY, Stern JNH, Yaari G, Lovato L, Cronk KM, O’Connor KC. The immune cell infiltrate populating meningiomas is composed of mature, antigen-experienced T and B cells. Neuro-oncology. 2013;15(11):1479–1490. doi: 10.1093/neuonc/not110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ding Y, Qiu L, Xu Q, Song L, Yang S, Yang T. Relationships between tumor microenvironment and clinicopathological parameters in meningioma. Int J Clin Exp Pathol. 2014;7(10):6973–6979. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.