Abstract
Rationale: More information on risk factors for death from tuberculosis in the United States could help reduce the tuberculosis mortality rate, which has remained steady for more than a decade.
Objective: To identify risk factors for tuberculosis-related death in adults.
Methods: We performed a retrospective study of 1,304 adults with tuberculosis who died before treatment completion and 1,039 frequency-matched control subjects who completed tuberculosis treatment in 2005 to 2006 in 13 states reporting 65% of U.S. tuberculosis cases. We used in-depth record abstractions and a standard algorithm to classify deaths in persons with tuberculosis as tuberculosis-related or not. We then compared these classifications to causes of death as coded in death certificates. We used multivariable logistic regression to calculate adjusted odds ratios for predictors of tuberculosis-related death among adults compared with those who completed tuberculosis treatment.
Results: Of 1,304 adult deaths, 942 (72%) were tuberculosis related, 272 (21%) were not, and 90 (7%) could not be classified. Of 847 tuberculosis-related deaths with death certificates available, 378 (45%) did not list tuberculosis as a cause of death. Adjusting for known risks, we identified new risks for tuberculosis-related death during treatment: absence of pyrazinamide in the initial regimen (adjusted odds ratio, 3.4; 95% confidence interval, 1.9–6.0); immunosuppressive medications (adjusted odds ratio, 2.5; 95% confidence interval, 1.1–5.6); incomplete tuberculosis diagnostic evaluation (adjusted odds ratio, 2.2; 95% confidence interval, 1.5–3.3), and an alternative nontuberculosis diagnosis before tuberculosis diagnosis (adjusted odds ratio, 1.6; 95% confidence interval, 1.2–2.2).
Conclusions: Most persons who died with tuberculosis had a tuberculosis-related death. Intensive record review revealed tuberculosis as a cause of death more often than did death certificate diagnoses. New tools, such as a tuberculosis mortality risk score based on our study findings, may identify patients with tuberculosis for in-hospital interventions to prevent death.
Keywords: cause of death, risk factors, death certificates
Americans still die of tuberculosis (TB), a preventable disease (1). On the basis of death certificate data, the TB mortality rate in the United States was 0.2/100,000 population, or 555 deaths, in 2013 and has not changed since 2003 (2). Despite the importance of TB mortality, limited information has been published about patients who die of TB and the frequency of potentially preventable deaths.
Many studies have documented the need for a systematic approach to measure TB-related deaths and determine the role that TB played in death. However, such studies often use death certificates as their main data source. Death certificates can misclassify the cause of death and underestimate the number who died with TB (3–7). One study of death certificates found TB listed as an underlying or contributing cause of death in only 34% of persons who died with TB (4). In a survey of resident physicians, only 33% who completed death certificates believed the death certificates were accurate; 20% who reported entering an incorrect cause of death stated they had done so because they were not familiar with the patient (5). Although some studies have examined TB-related mortality beyond death certificates, many were limited by narrowly defined populations or small size (6, 8–12).
Identifying risk factors for TB mortality could assist in the design of preventive interventions. Our primary objectives were to quantify and assess risk factors for TB-related deaths in the United States.
Methods
There were 1,304 adults with a verified diagnosis of TB (as reported to the Centers for Disease Control and Prevention [CDC]) who died in the 15 study catchment areas in 2005 and 2006 (see Table E1 in the online supplement). All patients were included who were reported as dead at diagnosis or if the reason for treatment cessation was “died” on the Report of Verified Case of Tuberculosis form.
For all of the aforementioned study subjects, trained data abstractors from each study site reviewed inpatient and outpatient medical and laboratory records and immediate, underlying, and contributing causes of death from the death certificates and entered into the abstraction form for the patient.
All persons evaluating the TB relatedness of death took a 2-hour training where the TB-relatedness algorithm was reviewed and sample cases were discussed to ensure consistency of review. In addition, all persons performing TB-relatedness determinations referred to a TB-relatedness flowchart and procedural manual to ensure consistency of classification. Two study team members, at least one of whom was a clinician with TB expertise, reviewed each data abstraction form and independently classified each death as possibly or definitely TB related, unlikely or definitely not related, or unclassifiable, using an a priori algorithm (Figure E1). Disagreements were resolved by a third independent reviewer. For the final analysis, definitely and possibly TB-related deaths were classified as TB-related deaths, and unlikely or definitely not TB-related deaths were classified as TB-unrelated deaths. This classification is used for all analyses.
A Kaplan-Meier curve was created to compare the time to death for patients who died with a TB-related death and patients who died with a TB-unrelated death.
To study risk factors for TB-related death during therapy, persons who died before diagnosis or treatment initiation were excluded from the cases. Only adults were included because of the small number (n = 14) of pediatric (<18 yr) deaths. Control subjects were selected from the group of patients with verified TB reported in the catchment areas from 2005 to 2006, who had completed TB treatment (which may last up to 2 years after treatment initiation; approximately 18,000 patients). Control patients were frequency matched 1:1 to cases by catchment area and age category. As with cases, control medical records were abstracted in each study site by reviewing inpatient and outpatient medical and laboratory records. All available data for each control were entered into the data abstraction form.
All subjects were designated as having severe or extensive TB disease if they had acid-fast bacilli on smear microscopy (smear-positive disease), respiratory failure, bilateral pulmonary disease, extensive pulmonary destruction, pulmonary and extrapulmonary disease, or TB meningitis.
We analyzed categorical variables with the chi-square or Fisher exact test and continuous variables with the Wilcoxon rank sum test. We used the kappa coefficient to compare agreement on TB-related deaths between study algorithm and death certificate.
We assessed risk factors known to contribute to TB-related death from previous studies as well as novel risk factors we hypothesized to be associated with TB-related death. Covariates associated with TB-related death in bivariable models (P < 0.2) were included in multivariable logistic regression models. Respiratory failure was excluded from all models because it is on the causal pathway for TB-related death (13). If covariates were correlated (phi value ≥ 0.3), only the variable associated with the highest proportion of patients was included in the model. We excluded covariates that represented limited exposure opportunities for patients who died within the first month of treatment (health department monitoring, adverse reactions to TB therapy, treatment interruptions). Age, sex, meningeal TB status, and human immunodeficiency virus (HIV) status were included a priori in all models.
Covariates were added in descending order of bivariable odds ratios and were retained if their addition yielded a Wald chi-square P value ≤ 0.05 and 1) changed the adjusted odds ratio associated with meningeal TB or HIV by at least 10% (14), or 2) improved the model fit as indicated by the likelihood ratio test. Interactions that were tested included HIV by meningeal TB, HIV by cognitive impairment, and HIV by sputum smear status, and absence of pyrazinamide in the initial treatment regimen by “liver condition” (defined as patient with cirrhosis or viral hepatitis or patient reported excess alcohol usage). Data were analyzed using SAS 9.3.
Institutional Review Boards at CDC and each study site approved the study. This study was funded by the Division of Tuberculosis Elimination, National Center for HIV, STD, and TB Prevention, Centers for Disease Control and Prevention, through a task order announced and managed by the Tuberculosis Epidemiologic Studies Consortium.
Results
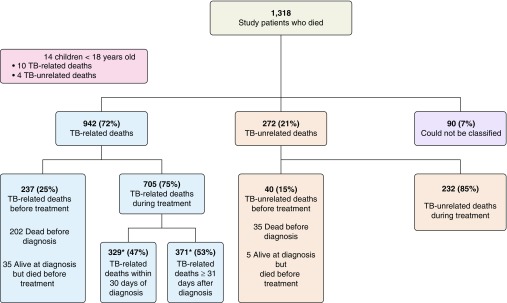
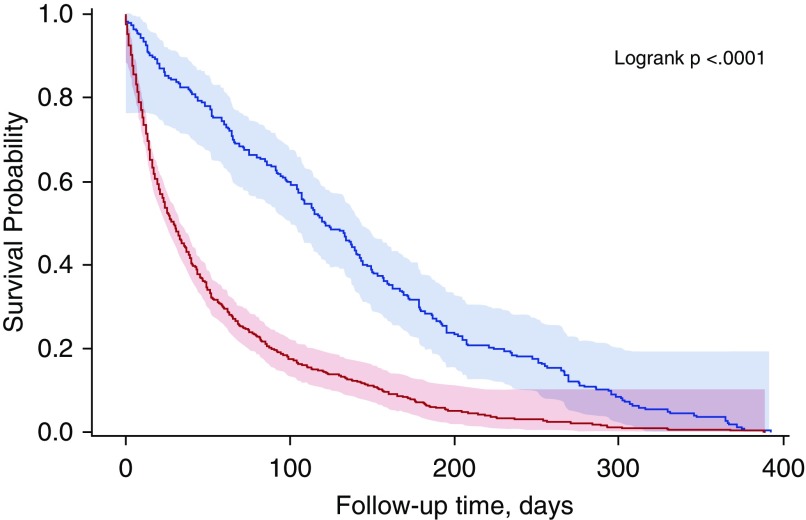
The 1,318 deaths included 14 children and accounted for 52% of all deaths among individuals with TB in the United States during 2005 to 2006. Of the 1,304 adult deaths, 942 (72%) were TB related, 272 (21%) were not, and 90 (7%) were unclassifiable (Figure 1). Review of 331 records indicated 87% agreement between the first and second reviewers on TB relatedness of death. TB-related death occurred during treatment for 705 decedents (75%) and before treatment began for 237 (25%), of whom 202 (85%) died before diagnosis. Of the 705 who died during treatment, 329 (47%) died within 30 days of diagnosis and 371 (53%) died later; 5 (0.7%) had missing dates (Figure 1). Figure 2 shows that those who had a TB-related death died sooner after their TB diagnosis than those who died of other causes.
Figure 1.
Tuberculosis (TB) relatedness and timing of deaths. *Five patients’ time-to-death could not be assessed due to missing dates. TB = tuberculosis.
Figure 2.
Kaplan-Meier curve comparing time to death for patients who died with tuberculosis (TB)-related death to patients who died with TB-unrelated death. The lower line represents the probability of survival for patients with TB-related death. The upper line represents the probability of survival for patients with TB-unrelated death.
Almost all (98%) of the 942 adult patients with TB-related deaths had culture-confirmed TB: 228 of 237 who died before TB treatment, and 692 of 705 who died during treatment. Of the 755 Mycobacterium tuberculosis isolates with drug susceptibility results, 58 (8%) were monoresistant (41 to isoniazid, 16 to pyrazinamide, and 1 to rifampin), 4 (0.5%) were resistant to more than one drug, and 14 (2%) displayed multidrug-resistant TB, defined as resistance to at least isoniazid and rifampin.
Among the 942 patients with TB-related deaths, 789 (84%) had at least one of the following: smear-positive disease (54%), bilateral pulmonary lesions (44%), extensive pulmonary destruction visible on chest image (31%), pulmonary and extrapulmonary disease (23%), meningeal TB (10%), or miliary TB (7%). Concurrent medical conditions included chronic obstructive pulmonary disease (COPD) (25%), diabetes mellitus (25%), coronary artery disease (20%), viral hepatitis (12%), cancer (11%), and cirrhosis (10%) (Table 1).
Table 1.
Characteristics associated with death before or during tuberculosis treatment among 942 adults whose deaths were tuberculosis related
| Characteristic | Patients Who Died before Treatment (n = 237) | Patients Who Died during Treatment (n = 705) | P Value |
|---|---|---|---|
| Demographic | |||
| Age, yr, mean, median (IQR) | 65.8, 69.4 (50.7–81.5) | 64.9, 66.1 (51.9–79.9) | 0.3 |
| Age group, yr | 0.014 | ||
| 18–44 | 45 (19) | 100 (14) | |
| 45–64 | 57 (24) | 237 (34) | |
| 65–85 | 96 (41) | 284 (40) | |
| >85 | 39 (16) | 84 (12) | |
| Male | 157 (66) | 481 (68) | 0.6 |
| White non-Hispanic | 95 (40) | 290 (41) | 0.8 |
| Black non-Hispanic | 85 (36) | 250 (35) | 0.9 |
| Asian | 54 (23) | 153 (22) | 0.7 |
| Hispanic | 45 (19) | 160 (23) | 0.2 |
| U.S. born | 124 (52) | 378 (54) | 0.7 |
| Socioeconomic/behavioral* | |||
| Unemployed | 162 (68) | 542 (77) | 0.01 |
| Homeless | 10 (4) | 64 (9) | 0.02 |
| Incarcerated | 5 (2) | 17 (2) | 0.8 |
| Injection drug use | 5 (2) | 34 (5) | 0.07 |
| Noninjection drug use | 13 (5) | 75 (11) | 0.02 |
| Excess alcohol | 35 (15) | 126 (18) | 0.3 |
| Long-term care facility | 29 (12) | 110 (16) | 0.2 |
| Clinical | |||
| Pulmonary TB only | 165 (70) | 440 (62) | 0.5 |
| Extrapulmonary TB only | 43 (18) | 74 (11) | 0.002 |
| Both pulmonary and extrapulmonary TB | 29 (12) | 191 (27) | <0.001 |
| Bilateral disease | 91 (38) | 328 (47) | 0.03 |
| Cavities on chest image | 24 (10) | 180 (26) | <0.001 |
| Effusion | 84 (35) | 272 (39) | 0.4 |
| Hemorrhage | 7 (3) | 7 (1) | 0.03 |
| Meningeal TB | 23 (10) | 75 (11) | 0.7 |
| Gastrointestinal TB | 1 (0.4) | 28 (4) | 0.01 |
| Peritoneal TB | 7 (3) | 26 (4) | 0.6 |
| Miliary TB | 20 (8) | 48 (7) | 0.4 |
| Pneumothorax | 3 (1) | 25 (4) | 0.07 |
| Extensive pulmonary destruction | 44 (19) | 252 (36) | <0.001 |
| Respiratory failure | 97 (41) | 352 (50) | 0.02 |
| Smear positive† | 88 (37) | 423 (60) | <0.001 |
| Multidrug-resistant TB‡ | 1 (0.4) | 13 (2) | 0.1 |
| History of TB | 9 (4) | 56 (8) | 0.03 |
| HIV status | <0.001 | ||
| HIV negative | 47 (20) | 283 (40) | |
| HIV positive | 29 (12) | 141 (20) | |
| HIV test not offered | 42 (18) | 98 (14) | |
| HIV test refused | 2 (1) | 24 (3) | |
| Unknown HIV test result | 117 (49) | 159 (23) | |
| Coronary artery disease | 46 (19) | 143 (20) | 0.8 |
| Cancer | 35 (15) | 66 (9) | 0.02 |
| COPD | 55 (23) | 184 (26) | 0.4 |
| Condition affecting the liver§ | 59 (25) | 215 (31) | 0.1 |
| Cirrhosis | 24 (10) | 70 (10) | 0.9 |
| Hepatitis (viral) | 23 (10) | 90 (13) | 0.2 |
| Diabetes mellitus | 52 (22) | 183 (26) | 0.2 |
| Cognitive impairment | 61 (26) | 198 (28) | 0.5 |
| Physical inability to self-medicate | 16 (7) | 108 (15) | <0.001 |
| Immunosuppressive medications|| | 5 (2) | 40 (6) | 0.03 |
| No reported TB symptom | 50 (21) | 48 (7) | <0.001 |
| Reported cough¶ | 111 (59) | 421 (64) | 0.2 |
| Reported fever¶ | 83 (44) | 358 (55) | 0.01 |
| Reported hemoptysis¶ | 18 (10) | 73 (11) | 0.6 |
| Reported night sweats¶ | 26 (14) | 178 (27) | <0.001 |
| Reported weight loss¶ | 81 (43) | 424 (65) | <0.001 |
| Health care | |||
| Early report** | 227 (96) | 695 (99) | 0.01 |
| No insurance | 25 (11) | 75 (11) | 0.9 |
| Competing diagnosis†† | 94 (40) | 304 (43) | 0.3 |
| NAAT not done | 132 (56) | 337 (48) | 0.04 |
| TST not done | 185 (78) | 341 (48) | <0.001 |
| Incomplete evaluation‡‡ | 120 (51) | 126 (18) | <0.001 |
Definition of abbreviations: AFB = acid-fast bacilli; COPD = chronic obstructive pulmonary disease; HIV = human immunodeficiency virus; IQR = interquartile range; NAAT = nucleic acid amplification test; TB = tuberculosis; TST = tuberculin skin test.
Data presented as n (%) unless otherwise noted.
Unemployed included all persons not seeking employment at the time of TB diagnosis; homeless included those without a stable home within 1 year up to TB diagnosis; incarcerated included those who were incarcerated within 1 year up to TB diagnosis; injection drug use, noninjection drug use, excess alcohol use: each involved substances used within 1 year up to TB diagnosis; long-term care facility included those who lived in a long-term care facility within 1 year up to TB diagnosis.
Smear-positive indicates positive on AFB smear microscopy. Comparison group was negative or unknown results on AFB smear microscopy.
Multidrug-resistant group was a subgroup of culture-confirmed cases (n = 920) with known drug susceptibility test results (n = 755).
Patient had cirrhosis or viral hepatitis or reported using excess alcohol.
Includes prednisone and tumor necrosis factor-α inhibitors.
Denominators are for patients who reported any TB symptom—patients who died: before treatment = 187; during treatment = 657.
Report case to state TB control program within 10 days of diagnosis.
A diagnosis other than TB was made before TB diagnosis.
Patient pulmonary evaluation did not include collection of three consecutive sputum samples for AFB smear microscopy within 4 days of hospitalization, or within 30 days if the patient with pulmonary disease was diagnosed as an outpatient. For patients with extrapulmonary TB only, no imaging or laboratory tests were documented.
Of the 237 cases with TB-related deaths who died before starting treatment, 82% had pulmonary/pleural involvement; 10% had cavitary lesions on chest radiographs. Notably, 40% had an initial diagnosis other than TB, 56% had no TB nucleic acid amplification test performed, and 51% did not have a full TB diagnostic evaluation. Compared with those who died during treatment, cases who died before starting treatment were more likely to have extrapulmonary TB, unknown HIV status, a diagnosis or history of cancer, or no reported TB symptoms (Table 1).
Adults with TB-related death within 30 days after starting TB treatment were older than those who died more than 30 days after starting TB treatment (Table E2).
Death certificates were available for 1,093 of the 1,214 patients whose deaths could be classified as TB related or not using the study algorithm. We classified 847 deaths (77% of 1,093) as TB related and 246 (23%) as TB unrelated. Of the 847 algorithm-defined TB-related deaths, 469 (55%) had TB listed on the death certificate as the immediate, underlying, or contributing cause of death. Among adult patients with TB-related deaths, early death was more common if the death certificate did not mention TB. Almost two-thirds (66%) of those with no mention of TB on the death certificate died before treatment or within 30 days of treatment start, compared with 54% of those whose death certificates mentioned TB (P = 0.0001).
Compared with death determined to be related to TB by the study algorithm, the sensitivity of death certificate data (i.e., TB listed as underlying, immediate, or contributing cause of death) was 55% and specificity was 75%. Agreement between study algorithm and death certificate was modest (κ = 0.21; 95% confidence interval [CI], 0.19–0.26) (Table E3).
In the case–control study, bivariable analysis showed that cases (adults with TB-related deaths who died during treatment) were more likely than control subjects (adults alive at least 2 years after treatment completion) to have severe or extensive disease: smear-positive disease (60% vs. 41%, P < 0.001), respiratory failure (50% vs. 3%, P < 0.001), bilateral disease (47% vs. 26%, P < 0.001), extensive pulmonary destruction (36% vs. 21%, P < 0.001), combined pulmonary and extrapulmonary disease (27% vs. 8%, P < 0.001), and meningeal disease (11% vs. 1%, P < 0.001).
Cases were also more likely to have been born in the United States (54% vs. 38%, P < 0.001), to have had an initial diagnosis other than TB (43% vs. 27% P < 0.001), to have refused treatment at any time during the treatment course (6% vs. 3%, P = 0.001), to have cognitive impairment (28% vs. 5% P < 0.001), to have had drug interactions with other medications (3% vs. 1%, P = 0.02), to report any TB symptom (93% vs. 85%, P < 0.001), and to report weight loss (65% vs. 56%, P = 0.001), fever (55% vs. 44%, P < 0.001), shortness of breath (49% vs. 29%, P < 0.001), or loss of appetite (40% vs. 25%, P < 0.001) (Table 2). Cases were more likely to be HIV-positive (20% vs. 5%, P < 0.001) or to have unknown HIV status (40% vs. 23%, P < 0.001). Cases were less likely to report cough (64% vs. 75%, P < 0.001), night sweats (27% vs. 33%, P = 0.01), or hemoptysis (11% vs. 16%, P = 0.003). There was no difference between the two groups in the proportion of patients with diabetes mellitus (DM) (26% vs. 23%, P = 0.11).
Table 2.
Comparison of adult patients with tuberculosis-related deaths who died during treatment and control patients who completed treatment
| Characteristic | Patients Who Died during Treatment (N = 705) | Control Subjects (N = 1,039) | P Value |
|---|---|---|---|
| Demographic | |||
| Age, yr, mean, median (IQR) | 64.9, 66.1 (51.9–79.9) | 62.5, 66.9 (50.6–76.4) | 0.01 |
| Male | 481 (68) | 666 (64) | 0.08 |
| White non-Hispanic | 290 (41) | 413 (40) | 0.6 |
| Black non-Hispanic | 250 (35) | 249 (24) | <0.001 |
| Asian | 153 (22) | 370 (36) | <0.001 |
| Hispanic | 160 (23) | 206 (20) | 0.2 |
| U.S. born | 378 (54) | 399 (38) | <0.001 |
| Socioeconomic/behavioral | |||
| Unemployed | 542 (77) | 633 (61) | <0.001 |
| Homeless | 64 (9) | 56 (5) | 0.003 |
| Incarcerated | 17 (2) | 33 (3) | 0.3 |
| Injection drug use | 34 (5) | 20 (2) | <0.001 |
| Noninjection drug use | 75 (11) | 60 (6) | <0.001 |
| Excess alcohol | 126 (18) | 110 (11) | <0.001 |
| Long-term care facility | 110 (16) | 43 (4) | <0.001 |
| Clinical | |||
| Pulmonary TB only | 440 (62) | 801 (77) | <0.001 |
| Extrapulmonary TB only | 74 (11) | 156 (15) | 0.006 |
| Both extrapulmonary and pulmonary TB | 191 (27) | 82 (8) | <0.001 |
| Bilateral disease | 328 (47) | 269 (26) | <0.001 |
| Cavities on chest image | 180 (26) | 254 (24) | 0.6 |
| Effusion | 272 (39) | 183 (18) | <0.001 |
| Hemorrhage | 7 (1) | 4 (0.4) | 0.1 |
| Meningeal TB | 75 (11) | 10 (1) | <0.001 |
| Gastrointestinal TB | 28 (4) | 14 (1) | <0.001 |
| Peritoneal TB | 26 (4) | 16 (1.5) | 0.004 |
| Miliary TB | 48 (7) | 4 (0.4) | <0.001 |
| Pneumothorax | 25 (4) | 2 (0.2) | <0.001 |
| Extensive pulmonary destruction | 252 (36) | 216 (21) | <0.001 |
| Respiratory failure | 352 (50) | 27 (3) | <0.001 |
| Smear positive* | 423 (60) | 423 (41) | <0.001 |
| Multidrug-resistant TB | 13 (2) | 0 | <0.001 |
| History of TB | 56 (8) | 71 (7) | 0.4 |
| HIV status | <0.001 | ||
| HIV negative | 283 (40) | 758 (73) | |
| HIV positive | 141 (20) | 47 (5) | |
| HIV test not offered | 98 (14) | 57 (5) | |
| HIV test refused | 24 (3) | 57 (5) | |
| Unknown HIV test result | 159 (23) | 120 (12) | |
| Coronary artery disease | 143 (20) | 109 (10) | <0.001 |
| Cancer | 66 (9) | 44 (4) | <0.001 |
| COPD | 184 (26) | 130 (13) | <0.001 |
| Condition affecting the liver† | 215 (31) | 166 (16) | <0.001 |
| Cirrhosis | 70 (10) | 20 (2) | <0.001 |
| Hepatitis (viral) | 90 (13) | 65 (6) | <0.001 |
| Diabetes mellitus | 183 (26) | 235 (23) | 0.1 |
| Renal failure | 24 (3) | 6 (0.6) | <0.001 |
| Immunosuppressive medications‡ | 40 (6) | 18 (2) | <0.001 |
| Cognitive impairment | 198 (28) | 53 (5) | <0.001 |
| Physical inability to self-medicate | 108 (15) | 27 (3) | <0.001 |
| No reported TB symptom | 48 (7) | 161 (15) | <0.001 |
| Reported cough§ | 421 (64) | 653 (75) | <0.001 |
| Reported fever§ | 358 (55) | 383 (44) | <0.001 |
| Reported weight loss§ | 424 (65) | 491 (56) | <0.001 |
| Reported night sweats§ | 178 (27) | 289 (33) | 0.01 |
| Reported shortness of breath§ | 348 (49) | 304 (29) | <0.001 |
| Reported loss of appetite§ | 285 (40) | 256 (25) | <0.001 |
| Reported chest pain§ | 128 (18) | 173 (17) | 0.4 |
| Reported hemoptysis§ | 73 (11) | 144 (16) | 0.003 |
| Health care | |||
| Competing diagnosis|| | 304 (43) | 280 (27) | <0.001 |
| TST not done | 341 (48) | 332 (32) | <0.001 |
| Incomplete evaluation¶ | 126 (18) | 110 (11) | <0.001 |
| Treatment characteristics | |||
| Initiated on RIPE | 610 (87) | 933 (90) | 0.04 |
| PZA was excluded from initial treatment regimen | 59 (8) | 37 (4) | <0.001 |
| DOT was initiated | 232 (33) | 874 (84) | <0.001** |
| DOT missing data | 414 (59) | 46 (4) | |
| Treatment interruptions | 92 (13) | 210 (20) | <0.001 |
| SAE from TB meds** | 76 (11) | 102 (10) | 0.5 |
| TB treatment discontinued due to SAE of TB meds | 64 (9) | 73 (7) | 0.1 |
| Drug interactions†† | 19 (3) | 12 (1) | 0.02 |
| Patient refused treatment at any time during treatment course | 42 (6) | 29 (3) | 0.001 |
| Health department activities‡‡ | |||
| Lost to follow-up ≥30 d | 10 (1.4) | 14 (1.3) | 0.9 |
Definition of abbreviations: AFB = acid-fast bacilli; COPD = chronic obstructive pulmonary disease; DOT = directly observed therapy; HIV = human immunodeficiency virus; IQR = interquartile range; PZA = pyrazinamide; RIPE = rifampin, isoniazid, pyrazinamide and ethambutol; SAE = severe adverse events; TB = tuberculosis; TST = tuberculin skin test.
Data presented as n (%) unless otherwise noted.
Smear-positive indicates positive on AFB smear microscopy. Comparison group was negative or unknown results on AFB smear microscopy.
Patient had cirrhosis or viral hepatitis or reported using excess alcohol.
Includes prednisone, tumor necrosis factor-α inhibitors.
Denominators are for patients who reported any TB symptom—patients who died during treatment = 657; control patients who completed treatment = 878.
A diagnosis other than TB was made before TB diagnosis.
Patient pulmonary evaluation did not include collection of three consecutive sputum samples for AFB smear microscopy within 4 days of hospitalization, or within 30 days if the patient with pulmonary disease was diagnosed as an outpatient. For patients with extrapulmonary TB only, no imaging or laboratory tests were documented.
SAEs documented to be possible due to TB treatment include hepatitis, hepatic failure, seizures, anaphylaxis, thrombocytopenia, severe dermatitis, cardiac event.
Drug interactions between TB medications and other medications that patient was taking were documented.
Of patients who died during treatment, 339 (48%) died within 30 days. Patients who died within 30 days had limited opportunity to have a tracking interview or home visit within 30 days.
Bivariate analyses also showed differences in receipt of health services. Cases were more likely to have been diagnosed as inpatients (89% vs. 54%, P < 0.001) and to have received a portion of the intensive phase of TB treatment as inpatients (79% vs. 25%, P < 0.001). Cases were less likely to have had a health department home visit, to have been interviewed within a week of the health department’s notification of their case, and to have had their home address recorded (11% vs. 1%, P < 0.001).
In multivariable analysis, factors associated with TB-related death while receiving TB treatment included receiving a portion of the intensive phase of therapy in the hospital (adjusted odds ratio [aOR], 6.5; 95% CI, 4.9–8.6), absence of pyrazinamide in the initial treatment regimen (aOR, 3.4; 95% CI, 1.9–6.0), cognitive impairment (aOR, 2.8; 95% CI, 1.9–4.2), receiving immunosuppressive medications such as prednisone or tumor necrosis factor-α inhibitors (aOR, 2.5; 95% CI, 1.1–5.6), COPD (aOR, 2.4; 95% CI, 1.7–3.3), bilateral pulmonary disease (aOR, 2.2; 95% CI, 1.6–2.9), no sputa collection for smear microscopy during pulmonary TB evaluation (aOR, 2.2; 95% CI, 1.5–3.3), liver conditions (aOR, 1.9; 95% CI, 1.4–2.7), cancer (aOR, 1.9; 95% CI, 1.9–5.4), and receiving a diagnosis other than TB before the TB diagnosis (aOR, 1.6; 95% CI, 1.2–2.2) (Table 3). None of the interaction terms achieved statistical significance; therefore, they were not maintained in the final model.
Table 3.
Factors associated with tuberculosis-related death on treatment for tuberculosis: multivariate logistic regression comparing patients who died during TB treatment to control patients who completed treatment
| Factor | Unadjusted OR (95% CI) | Adjusted OR (95% CI) |
|---|---|---|
| Age* | 1.01 (1.002–1.01) | 1.01 (1.003–1.02) |
| Sex (male vs. female)* | 1.2 (1.0–1.5) | 1.1 (0.8–1.5) |
| Meningeal TB* | 12.3 (6.3–23.9) | 13.2 (6.0–29.0) |
| HIV status*,† | ||
| HIV positive† | 8.0 (5.6–11.5) | 6.7 (4.1–10.8) |
| HIV test not offered† | 4.6 (3.2–6.6) | 4.3 (2.6–6.9) |
| HIV test refused† | 1.1 (0.7–1.9) | 1.4 (0.7–2.7) |
| HIV status unknown† | 3.6 (2.7–4.7) | 4.2 (2.9–6.1) |
| Miliary pattern on chest image | 18.9 (6.8–52.7) | 19.4 (5.4–69.8) |
| Initiated intensive phase of treatment as an inpatient | 11.2 (8.9–14.0) | 6.5 (4.9–8.6) |
| Cognitive impairment | 7.3 (5.3–10.0) | 2.8 (1.9–4.2) |
| Diagnosed in long-term care facility | 4.3 (3.0–6.2) | 2.1 (1.3–3.4) |
| Immunosuppressive medications‡ | 3.4 (1.9–6.0) | 2.5 (1.1–5.6) |
| COPD | 2.5 (1.9–3.2) | 2.4 (1.7–3.3) |
| Pyrazinamide was excluded from initial treatment regimen | 2.5 (1.6–3.8) | 3.4 (1.9–6.0) |
| Peritoneal TB | 2.4 (1.3–4.6) | 3.7 (1.6–8.4) |
| Cancer | 2.3 (1.6–3.5) | 3.2 (1.9–5.4) |
| Condition affecting the liver§ | 2.3 (1.8–2.9) | 1.9 (1.4–2.7) |
| Smear-positive|| | 2.2 (1.8–2.7) | 2.5 (1.8–3.3) |
| Bilateral disease | 2.2 (1.7–2.8) | 2.2 (1.6–2.9) |
| Competing diagnosis¶ | 2.1 (1.7–2.5) | 1.6 (1.2–2.2) |
| Incomplete evaluation** | 1.8 (1.4–2.4) | 2.2 (1.5–3.3) |
Definition of abbreviations: CI = confidence interval; COPD = chronic obstructive pulmonary disease; HIV = human immunodeficiency virus; OR = odds ratio; TB = tuberculosis.
Covariates were included in the model a priori.
Reference category: patients with known negative HIV test results.
Includes prednisone and tumor necrosis factor-α inhibitors.
Patient had cirrhosis or viral hepatitis or reported using excess alcohol.
Sputum sample demonstrated acid-fast bacilli with smear microscopy.
A diagnosis other than TB was made before TB diagnosis.
Patient pulmonary evaluation did not include collection of three consecutive sputum samples for acid-fast bacilli smear microscopy within 4 days of hospitalization, or within 30 days if the patient with pulmonary disease was diagnosed as an outpatient. For patients with extrapulmonary TB only, no imaging or laboratory tests were documented.
Discussion
Tuberculosis, a treatable and preventable disease, contributed to death in almost three-fourths of persons in our study who died with the disease in the United States. Persons who died from tuberculosis were less likely to have typical symptoms and signs of tuberculosis, yet, paradoxically, they had extensive disease. Clinicians must be aware of atypical presentations of tuberculosis, particularly in persons with extensive disease and in human immunodeficiency virus–infected persons, and have a low threshold for including tuberculosis in the differential diagnosis. For individuals without obvious risk factors for tuberculosis, additional tools are needed to help clinicians focus sooner on the possibility of tuberculosis.
Almost three-fourths of persons with tuberculosis-related death died before diagnosis or within a month after treatment initiation. Many who died did not report tuberculosis symptoms, and few who died before treatment had cavities on chest radiograph, which may have obscured the diagnosis of tuberculosis, an infrequent disease in the United States. Our finding was consistent with other studies that found an association between atypical chest radiographic findings (i.e., noncavitary infiltrate) and death at diagnosis and before treatment (15, 16). In our study, persons with tuberculosis-related deaths were more likely to have had an initial nontuberculosis diagnosis, such as pneumonia. This finding suggests patients may die or experience increased risk of death while providers pursue other diagnoses.
In a systematic review evaluating risk factors for tuberculosis death during treatment, Waitt and Squire hypothesized that studies demonstrating an association between increased comorbidities, social isolation, and death from tuberculosis may be a caused by delays in presenting to a provider with symptoms (17). The role of cognitive impairment in death during tuberculosis treatment may be explained by patient delays in presenting to a provider (8, 17), but could also be independently associated with mortality.
When compared with those dying before diagnosis, cases who died during treatment were more likely to be human immunodeficiency virus–positive, to have pulmonary and extrapulmonary disease, and to have extensive pulmonary destruction and bilateral disease on chest radiograph. Human immunodeficiency virus infection was an independent risk factor for tuberculosis-related death in our study and others (15, 16, 18, 19). However, many decedents had unknown human immunodeficiency virus status, either because the test was not performed or the results were unknown. This finding underscores the importance of human immunodeficiency virus testing of all patients being evaluated for tuberculosis and extensive pulmonary and extrapulmonary conditions without a definitive diagnosis, because management of both tuberculosis and human immunodeficiency virus concurrently increases the likelihood of a successful outcome (20, 21).
Findings of significant abnormalities on chest radiograph or combined pulmonary/extrapulmonary disease might increase the likelihood that the physician makes a tuberculosis diagnosis sooner. However, consistent with the extensive burden of disease, half the patients who died during treatment did so within a month of starting treatment.
Although previous studies identified diabetes mellitus as a risk factor for death in patients with tuberculosis (13, 22, 23), our study found that diabetes did not contribute to tuberculosis-related death. The literature has been inconsistent about the role of diabetes in tuberculosis treatment outcomes (24, 25).
Of note, 42 persons (6%) starting tuberculosis therapy had severe adverse reactions to antituberculosis medications, which may have exacerbated an underlying condition and led to death. In our study, the most common preexisting condition was liver disease. In a study evaluating mortality of patients with cirrhosis with and without tuberculosis, patients with cirrhosis were found to have higher mortality 30 days to 1 year after starting treatment, suggesting that hepatotoxicity from tuberculosis treatment may be a source of higher mortality (26). Careful monitoring of patients with cirrhosis early in their treatment could prevent mortality.
Even after adjusting for age and factors that increased the risk of liver injury, we found that absence of pyrazinamide in the initial treatment regimen was associated with tuberculosis-related death. Clinicians might not prescribe pyrazinamide to patients with risks for liver injury because of its potential hepatotoxicity (27). In fact, 24 of 59 (41%) patients who died during treatment without pyrazinamide had reported excess alcohol use or had viral hepatitis or cirrhosis, and only 3% had a pyrazinamide-resistant M. tuberculosis strain. Thus, the absence of pyrazinamide in the regimen may be partially explained by concerns about toxicity in patients with liver conditions, as drug resistance does not explain it. Further work is needed to understand the mechanism of pyrazinamide efficacy to develop drugs with a similar mechanism but without the toxicity.
As seen in previous studies, our study confirmed that death certificates might incompletely capture tuberculosis disease when it is related to death. As slightly more than half of patients with a death certificate and tuberculosis-related death had tuberculosis listed as an immediate, underlying, or contributing cause of death (55%), the sensitivity of the death certificate to find a tuberculosis-related death was modest (55%). One explanation for the lower detection of tuberculosis-related deaths by the death certificate is that tuberculosis might be diagnosed many weeks after death, when the culture becomes positive and the death certificate has already been completed.
Several features of tuberculosis-related death stand out in this study. Decedents often did not have the classic symptoms (cough, hemoptysis, cavities on chest radiograph), which made an illness that is uncommon in the United States even easier to miss. Human immunodeficiency virus infection and liver conditions complicate tuberculosis and are also potential markers for increased mortality risk. The risk factors identified in this study may form the basis for the development of a tuberculosis mortality risk score, analogous to those that have been used to predict mortality in patients with tuberculosis in the hospital (28) and intensive care unit (29, 30), which may help with earlier diagnosis, rapid treatment initiation, and enhanced care. A risk score for pulmonary tuberculosis using risk factors (similar to the ones we describe) that was developed using a derivation cohort and tested using a validation cohort was able to differentiate between low-, moderate-, and high-risk patients (31). Bilateral disease on chest radiograph; extensive pulmonary destruction; immunosuppression; meningeal, miliary, or peritoneal tuberculosis; cognitive impairment; and coexisting conditions of obstructive pulmonary disease, cancer, and underlying liver disease should be formally evaluated in scoring and predicting mortality. If scoring becomes routine, a high score could alert clinicians to patients who may benefit from more intensified management and monitoring. Even without a scoring system, when tuberculosis is diagnosed in a hospitalized patient, clinicians should recognize the increased risk of death.
Our study had limitations. We did not assess the impact of smoking on tuberculosis-related death and could not fully assess the impact of health department monitoring, because a large proportion of patients died before there was an opportunity for such intervention. Because few hospital records noted the provision of directly observed therapy, its potential protective role could not be evaluated. Similarly, we could not evaluate the association between provider type and patient outcome, because patients who died were overwhelmingly cared for in the hospital, with multiple providers during at least some portion of their diagnosis and treatment. Our analysis also assumes that our algorithm for tuberculosis-related death was more accurate than the death certificate. Given that our classification was based on data from an extensive chart review of medical and public health records and multiple independent reviewers, we feel confident that any residual bias is smaller than what might have occurred had the study been conducted with death certificates only in the classification. Because the study design required extensive medical record review, we were unable to assess risk factors for tuberculosis-related death before presentation to a healthcare provider. Finally, these data were collected on cases reported in 2005 to 2006; because of the extensive nature of the study organization and chart review required, more recent data are not available.
Our study underscores the importance of preventing tuberculosis deaths by preventing tuberculosis disease in the first place. In low-incidence nations with current diagnostic tools, tuberculosis diagnosis is often missed or delayed, and tuberculosis deaths most often occur within weeks of diagnosis. Current tuberculosis treatment regimens are not always sufficient to reverse extensive disease. Finally, many patients who successfully complete antituberculosis treatment often live less robust and shorter lives (32–34).
Fortunately, the public health and medical community have tools (more specific tests in the form of interferon-γ release assays and shorter treatment regimens that are more likely to be completed) to identify and treat latent tuberculosis infection (35–37). Increased testing and treatment for latent tuberculosis infection can reduce the number of persons who develop and die of this preventable disease.
Acknowledgments
Acknowledgment
The authors thank the following individuals, whose dedication made this manuscript possible: Veronica Abernathy; Sevim Ahmedov, M.P.A.; Winnie Alston; Holly Anger, M.P.H.; Elizabeth Barash, M.P.H.; Rob Berger (posthumously); Paul W. Colson, Ph.D.; David Conwill, M.D.; Mario Corro, M.S.; Wendy Cronin, Ph.D.; Kaitron Gordon; Neela D. Goswami, M.D., M.P.H.; Connie Hobbs, M.P.H.; Michael Holcombe, M.P.P.A.; Eileen Hufnagel, R.N., B.S.N.; Carol Jonda, R.N., B.S.N.; Gina Maltas, R.N., B.S.N.; Trini Matthew, M.D., M.P.H.; Wilson Miranda, M.B.B.S., M.P.H.; Lexa Moongrace; Guadalupe Munguia, M.D., M.P.H.; Ellen Murray, R.N., B.S.N.; Ellen O’Leary, R.N., B.S.N.; Margaret Oxtoby, M.D.; Jenny Pang, M.D.; Laura Romo, M.P.H.; Heather Rutz, M.C.R.P., M.H.S.; Katya Salcedo, M.P.H.; Shonita Savage; Rashmi Singh, M.D., Gisela Schechter, M.D., M.P.H.; Anna Sevilla, M.P.H.; Trudy Stein-Hart, M.P.H.; Tim Sterling, M.D.; Jane Tapia, R.N., B.S.N.; Larry Teeter, Ph.D.; Jennifer Vergeon; Kirsten Wall, M.H.S., M.P.H.; Stephen Weis, D.O.
Footnotes
Supported by the Division of Tuberculosis Elimination, National Center for HIV, STD, and TB Prevention, Centers for Disease Control and Prevention, through a task order announced and managed by the Tuberculosis Epidemiologic Studies Consortium. This information is distributed solely for the purpose of predissemination peer review under applicable information quality guidelines. It has not been formally disseminated by the Centers for Disease Control and Prevention/the Agency for Toxic Substances and Disease Registry. It does not represent and should not be construed to represent any agency determination or policy.
Author Contributions: S.F.B., L.P., A.L.D., J.M.M., Y.R.H.-M., J.E.G., H.M.B., R.M.W., R.A.R., S.E.B., M.K.L., P.C.W., R.W.B., S.E.H., J.V.W., S.F.W., M.L., P.M.B., D.J.K., D.O.G., E.A.G., and J.M.F. made substantial contributions to the conception and design of the work and the acquisition, analysis, and interpretation of data; authored and revised the work critically for important intellectual content; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. T.L.M. and S.R.K. made substantial contributions to the acquisition, analysis, and interpretation of data for the work; authored and revised the work critically for important intellectual content; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
Contributor Information
Collaborators: on behalf of the Tuberculosis Epidemiologic Studies Consortium, Veronica Abernathy, Sevim Ahmedov, Winnie Alston, Holly Anger, Elizabeth Barash, Rob Berger (posthumously), Paul W. Colson, David Conwill, Mario Corro, Wendy Cronin, Kaitron Gordon, Neela D. Goswami, Connie Hobbs, Michael Holcombe, Eileen Hufnagel, Carol Jonda, Gina Maltas, Trini Matthew, Wilson Miranda, Lexa Moongrace, Guadalupe Munguia, Ellen Murray, Ellen O’Leary, Margaret Oxtoby, Jenny Pang, Laura Romo, Heather Rutz, Katya Salcedo, Shonita Savage, Rashmi Singh, Gisela Schechter, Anna Sevilla, Trudy Stein-Hart, Tim Sterling, Jane Tapia, Larry Teeter, Jennifer Vergeon, Kirsten Wall, and Stephen Weis
References
- 1.Maher D, Watt CJ, Williams BG, Raviglione M, Dye C. Tuberculosis deaths in countries with high HIV prevalence: what is their use as an indicator in tuberculosis programme monitoring and epidemiological surveillance? Int J Tuberc Lung Dis. 2005;9:123–127. [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC) Atlanta, GA: U.S. Department of Health and Human Services, CDC; 2015. Reported tuberculosis in the United States, 2014. [Google Scholar]
- 3.Halanych JH, Shuaib F, Parmar G, Tanikella R, Howard VJ, Roth DL, et al. Agreement on cause of death between proxies, death certificates, and clinician adjudicators in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study. Am J Epidemiol. 2011;173:1319–1326. doi: 10.1093/aje/kwr033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Washko RM, Frieden TR. Tuberculosis surveillance using death certificate data, New York City, 1992. Public Health Rep. 1996;111:251–255. [PMC free article] [PubMed] [Google Scholar]
- 5.Wexelman BA, Eden E, Rose KM. Survey of New York City resident physicians on cause-of-death reporting, 2010. Prev Chronic Dis. 2013;10:1–12. doi: 10.5888/pcd10.120288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kattan JA, Sosa LE, Lobato MN. Tuberculosis mortality: death from a curable disease, Connecticut, 2007-2009. Int J Tuberc Lung Dis. 2012;16:1657–1662. doi: 10.5588/ijtld.12.0169. [DOI] [PubMed] [Google Scholar]
- 7.Gallivan MD, Lofy KH, Goldbaum GM. Use of death certificates to identify tuberculosis-related deaths in Washington State. J Public Health Manag Pract. 2017;23:e12–e15. doi: 10.1097/PHH.0b013e3182aaa1d8. [DOI] [PubMed] [Google Scholar]
- 8.Hansel NN, Merriman B, Haponik EF, Diette GB. Hospitalizations for tuberculosis in the United States in 2000: predictors of in-hospital mortality. Chest. 2004;126:1079–1086. doi: 10.1378/chest.126.4.1079. [DOI] [PubMed] [Google Scholar]
- 9.White MC, Portillo CJ. Tuberculosis mortality associated with AIDS and drug or alcohol abuse: analysis of multiple cause-of-death data. Public Health. 1996;110:185–189. doi: 10.1016/s0033-3506(96)80074-6. [DOI] [PubMed] [Google Scholar]
- 10.Rao VK, Iademarco EP, Fraser VJ, Kollef MH. The impact of comorbidity on mortality following in-hospital diagnosis of tuberculosis. Chest. 1998;114:1244–1252. doi: 10.1378/chest.114.5.1244. [DOI] [PubMed] [Google Scholar]
- 11.Franke MF, Appleton SC, Bayona J, Arteaga F, Palacios E, Llaro K, et al. Risk factors and mortality associated with default from multidrug-resistant tuberculosis treatment. Clin Infect Dis. 2008;46:1844–1851. doi: 10.1086/588292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oursler KK, Moore RD, Bishai WR, Harrington SM, Pope DS, Chaisson RE. Survival of patients with pulmonary tuberculosis: clinical and molecular epidemiologic factors. Clin Infect Dis. 2002;34:752–759. doi: 10.1086/338784. [DOI] [PubMed] [Google Scholar]
- 13.Erbes R, Oettel K, Raffenberg M, Mauch H, Schmidt-Ioanas M, Lode H. Characteristics and outcome of patients with active pulmonary tuberculosis requiring intensive care. Eur Respir J. 2006;27:1223–1228. doi: 10.1183/09031936.06.00088105. [DOI] [PubMed] [Google Scholar]
- 14.Greenland S. Modeling and variable selection in epidemiologic analysis. Am J Public Health. 1989;79:340–349. doi: 10.2105/ajph.79.3.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nguyen LT, Hamilton CD, Xia Q, Stout JE. Mortality before or during treatment among tuberculosis patients in North Carolina, 1993-2003. Int J Tuberc Lung Dis. 2011;15:257–262, i. [PMC free article] [PubMed] [Google Scholar]
- 16.Pascopella L, Barry PM, Flood J, DeRiemer K. Death with tuberculosis in California, 1994-2008. Open Forum Infect Dis. 2014;1:ofu090. doi: 10.1093/ofid/ofu090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waitt CJ, Squire SB. A systematic review of risk factors for death in adults during and after tuberculosis treatment. Int J Tuberc Lung Dis. 2011;15:871–885. doi: 10.5588/ijtld.10.0352. [DOI] [PubMed] [Google Scholar]
- 18.Marks SM, Magee E, Robison V. Patients diagnosed with tuberculosis at death or who died during therapy: association with the human immunodeficiency virus. Int J Tuberc Lung Dis. 2011;15:465–470. doi: 10.5588/ijtld.10.0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hannah HA, Miramontes R, Gandhi NR. Sociodemographic and clinical risk factors associated with tuberculosis mortality in the United States, 2009–2013. Public Health Rep. 2017;132:366–375. doi: 10.1177/0033354917698117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Branson BM, Handsfield HH, Lampe MA, Janssen RS, Taylor AW, Lyss SB, et al. Centers for Disease Control and Prevention. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55:1–17. [PubMed] [Google Scholar]
- 21.Taylor Z, Nolan CM, Blumberg HM American Thoracic Society; Centers for Disease Control and Prevention; Infectious Diseases Society of America. Controlling tuberculosis in the United States: recommendations from the American Thoracic Society, CDC, and the Infectious Diseases Society of America. MMWR Recomm Rep. 2005;54:1–81. [PubMed] [Google Scholar]
- 22.Choi H, Lee M, Chen RY, Kim Y, Yoon S, Joh JS, et al. Predictors of pulmonary tuberculosis treatment outcomes in South Korea: a prospective cohort study, 2005-2012. BMC Infect Dis. 2014;14:360. doi: 10.1186/1471-2334-14-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dooley KE, Tang T, Golub JE, Dorman SE, Cronin W. Impact of diabetes mellitus on treatment outcomes of patients with active tuberculosis. Am J Trop Med Hyg. 2009;80:634–639. [PMC free article] [PubMed] [Google Scholar]
- 24.Chiang CY, Bai KJ, Lin HH, Chien ST, Lee JJ, Enarson DA, et al. The influence of diabetes, glycemic control, and diabetes-related comorbidities on pulmonary tuberculosis. PLoS One. 2015;10:e0121698. doi: 10.1371/journal.pone.0121698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakamura A, Hagiwara E, Hamai J, Taguri M, Terauchi Y. Impact of underlying diabetes and presence of lung cavities on treatment outcomes in patients with pulmonary tuberculosis. Diabet Med. 2014;31:707–713. doi: 10.1111/dme.12414. [DOI] [PubMed] [Google Scholar]
- 26.Hung TH, Lay CJ, Tseng CW, Tsai CC, Tsai CC. The effect of tuberculosis on the mortality of cirrhotic patients: a population-based 3-year follow-up study. Medicine (Baltimore) 2014;93:e295. doi: 10.1097/MD.0000000000000295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saukkonen JJ, Cohn DL, Jasmer RM, Schenker S, Jereb JA, Nolan CM, et al. ATS (American Thoracic Society) Hepatotoxicity of Antituberculosis Therapy Subcommittee. An official ATS statement: hepatotoxicity of antituberculosis therapy. Am J Respir Crit Care Med. 2006;174:935–952. doi: 10.1164/rccm.200510-1666ST. [DOI] [PubMed] [Google Scholar]
- 28.Nagai K, Horita N, Sato T, Yamamoto M, Nagakura H, Kaneko T. Age, dehydration, respiratory failure, orientation disturbance, and blood pressure score predicts in-hospital mortality in HIV-negative non-multidrug-resistant smear-positive pulmonary tuberculosis in Japan. Sci Rep. 2016;6:21610. doi: 10.1038/srep21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Valade S, Raskine L, Aout M, Malissin I, Brun P, Deye N, et al. Tuberculosis in the intensive care unit: a retrospective descriptive cohort study with determination of a predictive fatality score. Can J Infect Dis Med Microbiol. 2012;23:173–178. doi: 10.1155/2012/361292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lanoix JP, Gaudry S, Flicoteaux R, Ruimy R, Wolff M. Tuberculosis in the intensive care unit: a descriptive analysis in a low-burden country. Int J Tuberc Lung Dis. 2014;18:581–587. doi: 10.5588/ijtld.13.0901. [DOI] [PubMed] [Google Scholar]
- 31.Bastos HN, Osório NS, Castro AG, Ramos A, Carvalho T, Meira L, et al. A prediction rule to stratify mortality risk of patients with pulmonary tuberculosis. PLoS One. 2016;11:e0162797. doi: 10.1371/journal.pone.0162797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller TL, Wilson FA, Pang JW, Beavers S, Hoger S, Sharnprapai S, et al. Mortality hazard and survival after tuberculosis treatment. Am J Public Health. 2015;105:930–937. doi: 10.2105/AJPH.2014.302431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoger S, Lykens K, Beavers SF, Katz D, Miller TL. Longevity loss among cured tuberculosis patients and the potential value of prevention. Int J Tuberc Lung Dis. 2014;18:1347–1352. doi: 10.5588/ijtld.14.0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shuldiner J, Leventhal A, Chemtob D, Mor Z. Mortality after anti-tuberculosis treatment completion: results of long-term follow-up. Int J Tuberc Lung Dis. 2016;20:43–48. doi: 10.5588/ijtld.14.0427. [DOI] [PubMed] [Google Scholar]
- 35.Shepardson D, Marks SM, Chesson H, Kerrigan A, Holland DP, Scott N, et al. Cost-effectiveness of a 12-dose regimen for treating latent tuberculous infection in the United States. Int J Tuberc Lung Dis. 2013;17:1531–1537. doi: 10.5588/ijtld.13.0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holland DP, Sanders GD, Hamilton CD, Stout JE. Costs and cost-effectiveness of four treatment regimens for latent tuberculosis infection. Am J Respir Crit Care Med. 2009;179:1055–1060. doi: 10.1164/rccm.200901-0153OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.World Health Organization. Framework towards tuberculosis elimination in low-incidence countries. Geneva: Switzerland: World Health Organization; 2014. [PubMed] [Google Scholar]