Abstract
Background & Aims:
There are few serum biomarkers to identify patients with Crohn’s disease (CD) who are at risk for stricture development. The extracellular matrix components, collagen type III alpha 1 chain (COL3A1) and cartilage oligomeric matrix protein (COMP), could contribute to intestinal fibrosis. We investigated whether children with inflammatory CD (B1) who later develop strictures (B2) have increased plasma levels of COL3A1 or COMP at diagnosis, compared to children who remain B1. We compared results to previously studied biomarkers, including autoantibodies against colony stimulating factor 2 (CSF2).
Methods:
We selected 161 subjects (mean age, 12.2 years; 62% male) from the Risk Stratification and Identification of Immunogenic and Microbial Markers of Rapid Disease Progression in Children with Crohn’s cohort, completed at 28 sites in the United States and Canada from 2008 through 2012. The children underwent colonoscopy and upper endoscopy at diagnosis and were followed every 6 months for 36 months; plasma samples were collected at baseline. Based on CD phenotype, children were separated to group 1 (B1 phenotype at diagnosis and follow up), group 2 (B2 phenotype at diagnosis), or group 3 (B1 phenotype at diagnosis who developed strictures during follow up). Plasma samples were collected from patients and 40 children without inflammatory bowel disease (controls) at baseline and analyzed by ELISA to measure COL3A1 and COMP. These results were compared with those from a previous biomarker study. Kruskal-Wallis test and pairwise Dunn’s tests with Bonferroni correction were used to compare differences among groups.
Results:
The median baseline concentration of COL3A1 was significantly higher in plasma from group 3 vs group 1 (P<.01) and controls (P=.01). Median baseline plasma concentrations of COMP did not differ significantly among groups. A model comprising baseline concentrations of COL3A1 and anti-CSF2 identified patients with B2 vs B1 CD with an area under the curve of 0.80 (95% CI, 0.71–0.89); the combined concentration identified patients with strictures with a sensitivity value of 0.70 (95% CI, 0.55–0.83) and a specificity value of 0.83 (95% CI 0.67–0.93).
Conclusion:
We found median plasma concentrations of COL3A1, measured by ELISA at diagnosis, to be significantly higher in patients with CD who later developed strictures than in patients without strictures. The combination of concentrations of COL3A1 and anti-CSF2 might be used to identify pediatric patients at CD diagnosis who are at risk for future strictures.
Keywords: procollagen III, IBD, fibrosis, complication, biomarker
Introduction:
Crohn’s disease (CD) has a relapsing and remitting course, during which the bowel is cyclically injured and potentially repaired and healed. This constant remodeling of the bowel wall may leave no scar tissue in some patients, but in others may lead to an accumulation of fibrotic tissue that eventually causes bowel stricturing.1, 2 In the RISK cohort, 9% of patients developed stricturing complications within 3 years of CD diagnosis.3 Other studies have reported rates of stricturing disease as high as 30-50% within 5 years of CD diagnosis.2, 4,5 Disease progression from inflammatory to stricturing phenotype is more severe and rapid in children compared to adults.6 The presence of stricturing disease is associated with a 4-5 times higher risk for surgical resection in adults and 3 times higher risk in children.7, 8 The fibrotic tissue consists primarily of collagen fibrils, and increased collagen deposition is associated with fibrosis in other diseases.9
Extracellular matrix (ECM) components play a vital role in the development of intestinal fibrosis and strictures in Crohn’s disease.10 Collagens I and III are the major ECM proteins implicated in intestinal fibrosis.11 Genes involved in extracellular matrix accumulation, including collagens, are upregulated in pediatric patients with inflammatory (B1) disease who later develop stricturing (B2) disease.3
Terminal propeptides are cleaved from procollagen once secreted into the ECM and have been used to estimate the rate of collagen formation.12 Collagen type III alpha 1 chain (COL3A1), also called procollagen III N-terminal propeptide, concentrations were significantly decreased in CD patients after surgical resection of intestinal strictures.13 In IBD patients undergoing surgical bowel stricture resection, splanchnic COL3A1 was elevated, showing a positive gradient between peripheral circulation and mesenteric veins draining the affected intestinal segments.14 COL3A1 was used as part of a biomarker panel to predict hepatic fibrosis with 92% specificity15 Cartilage oligomeric matrix protein (COMP) binds with high affinity to collagen I and II, which plays an important role in collagen fibril formation.16 Clinically, COMP has been associated with skin, liver and lung fibrosis.17-20.
Other potential circulating biomarkers for predicting CD phenotypes include anti- Saccharomyces cerevisiae antibodies (ASCA) IgG and IgA, perinuclear anti-neutrophil cytoplasmic antibodies (pANCA), anti-CBir1 (anti-flagellin), anti-outer membrane protein C precursor (OmpC) and autoantibodies against colony stimulating factor 2 (anti-CSF2) . ASCA IgG and IgA, anti-CBir1, and anti-CSF2 were associated with stricturing disease in the recently published Risk Stratification and Identification of Immunogenic and Microbial Markers of Rapid Disease Progression in Children with Crohn’s (RISK) study.3
Currently only ASCA and CBir1 serologies are used in a clinical setting to predict the risk for development of strictures in patients with inflammatory (B1) Crohn’s disease. Our study aims to evaluate the predictive utility of circulating biomarkers including COL3A1 and COMP along with other previously studied serology markers in the development of CD strictures. For any prediction studies, a prospective inception cohort is necessary with the availability of the biomarker at baseline before the event occurs with accurate phenotyping, hence the RISK cohort was used. We examined the association of stricturing complications and COL3A1 and COMP plasma concentrations at diagnosis in this same Crohn’s disease cohort.
Methods:
Patient Population and Classification.
Subjects were selected from the RISK cohort. The RISK cohort is a multicenter pediatric Crohn’s disease inception cohort where treatment naive patients less than 18 years of age were enrolled at 28 sites in the U.S. and Canada from 2008-2012 (ClinicalTrials.gov identifier NCT00790543). Subjects were accurately phenotyped with colonoscopy and upper endoscopy at diagnosis and over 80% had cross-sectional imaging. They were followed prospectively every 6 months for 36 months. Follow up endoscopy and imaging to detect suspected intestinal stricture or fibrosis was at the discretion of the individual investigator. Two large audits were conducted to clean the data and resolve or exclude questionable or indeterminate phenotypes. The Montreal classification system was used for phenotyping, where a stricturing disease (B2) was defined as luminal narrowing with pre-stenotic dilation by imaging and inflammatory phenotype was defined as no evidence of B2 or internal penetrating behavior (B3).21
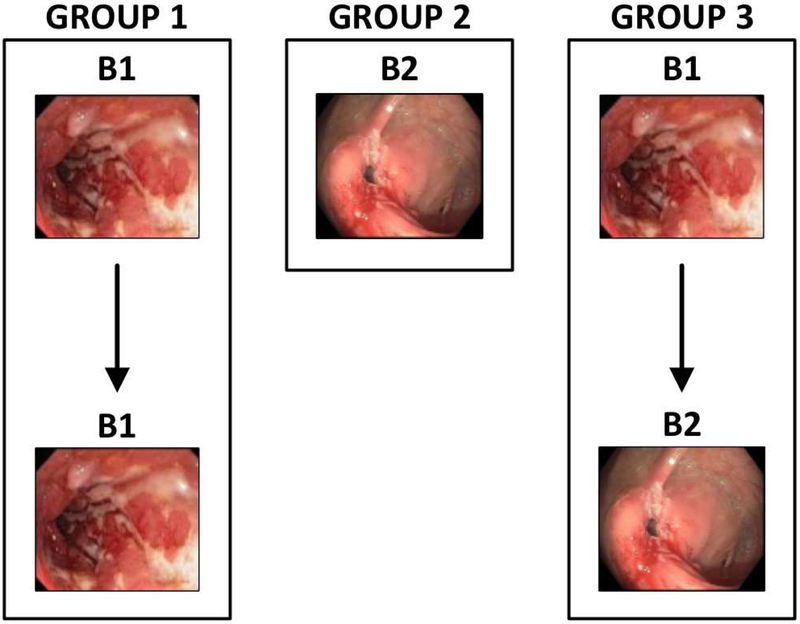
Within this RISK cohort, we separated subjects into 3 main groups (Figure 1). Group 1 included children with inflammatory or B1 phenotype at diagnosis who never developed B2 disease, group 2 included children with stricturing or B2 phenotype at diagnosis, and group 3 included children with B1 phenotype at diagnosis who developed strictures (B2) at any time 90 days or more after diagnosis during the 36-month follow up. Controls were children who presented to outpatient clinics at RISK sites and had normal gross and histological findings on upper and lower endoscopy excluding IBD as a diagnosis. A separate exploratory group (Group 4) was identified for further investigation of COL3A1 variability. This group included children who developed strictures during follow-up and had plasma collected at a follow-up visit that occurred within 90 days of stricture diagnosis. Group 4 was not directly comparable with the other groups as there was no baseline data or serum collected.
Figure 1: Description of CD phenotype group.
Demographic data including age, sex, race, and Tanner stage was collected from each subject at diagnosis. C-reactive protein (CRP), erythrocyte sedimentation rate (ESR) and physician global assessment (PGA) were collected from each subject at diagnosis and used to estimate disease activity. Anti-Saccharomyces cerevisiae antibodies (ASCA) IgG and IgA, perinuclear anti-neutrophil cytoplasmic antibodies (pANCA), anti-CBir1 (anti-flagellin), antiouter membrane protein C precursor (OmpC) and autoantibodies against colony stimulating factor 2 (anti-CSF2)were performed at Cedars-Sinai Hospital (Los Angeles, CA, USA) and Cincinnati Children’s Hospital Medical Center (OH, USA). ELISA for COL3A1 (Biomatik EKU06786, Wilmington, DE, USA) and COMP (BioVendor, Candler, NC, USA) was performed on plasma of the 3 main groups and 40 children without IBD (controls) at baseline. ELISA was performed on Group 4 plasma from follow up visits. ELISAs for COL3A1 were performed at Emory University (Atlanta, GA) and for COMP at the University of Michigan (Ann Arbor, MI).
Statistical Analysis.
Descriptive statistics of the baseline characteristics and predictors using medians with 25th and 75th percentiles for continuous variables and N with percentage for categorical variables were summarized by CD groups. Due to the skewed distribution usually seen in lab measurements, non-parametric Kruskal-Wallis test was used for testing overall among-group difference in the continuous (or ordinal) variables. If a significant p-value from the Kruskal-Wallis test is identified, Dunn’s test for pairwise comparisons with Bonferroni multiple adjustment is then performed. Chi-square tests were used for categorical variables. The discriminant power of the biomarkers for stricture development were evaluated directly using Area under the Receiver Operating Characteristic curve (AUC of ROC), sensitivity and specificity. DeLong’s test was used for comparing two correlated ROC curves. We also used logistic regression and Classification and Regression Trees (CART) methods to build a multivariable discriminant model. The continuously measured discriminants were also tested for their dichotomized version using the cutoffs suggested by the CART models. Graphical presentations such as ROC curves were also provided to help visualize the data and the models.
Results:
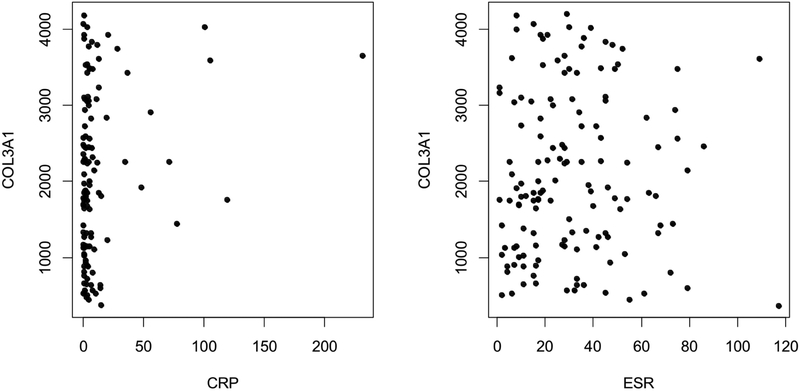
Crohn’s disease groups and controls were not statistically different for age, sex, and race (Table 1). Controls were healthy with normal ESR, CRP and higher tanner stage. Disease burden among the CD groups was similar, with no significant difference in PGA (p=0.66) and CRP (p=0.22). The median ESR and CRP in all three CD groups were significantly different from the control group (all p<0.01). Plasma COL3A1 concentration (Table 2) was significantly higher in subjects who developed strictures during follow-up (group 3) when compared to subjects who never developed strictures (2294 vs 1763 pg/mL; p<0.01) and controls (2294 vs 1739 pg/mL; p=0.01). COL3A1 concentration in plasma collected within 90 days of stricture diagnosis (exploratory group 4) was not statistically different than baseline COL3A1 concentration in group 3, where subjects developed stricture at any time during 36 month follow up (2444 vs 2293 pg/mL). Plasma COL3A1 did not correlate with CRP or ESR as displayed in the Figure 2 scatter plots.
Table 1:
Baseline characteristics of the study cohort by groups*
| Age | Sex, M | Race, Black/Whitet† | Tanner Stage | PGA, Mod/Sev | ESR | CRP | |
|---|---|---|---|---|---|---|---|
| Group1 (n=40) | 11.29 (9.63,13.77) | 26 (65%) | 6(15%)/ 26(65%) | 1 (1,2) | 27 (68%) | 40 (26,55) | 3.1 (0.9,6.71) |
| Group2 (n=33) | 13.25 (11.08,15) | 21 (64%) | 9(27%)/ 22(67%) | 1 (1,2.25) | 24 (73%) | 23.5 (15,32.5) | 3.1 (1.92,8.48) |
| Group3 (n=48) | 13.54 (11.54,15.1) | 31 (65%) | 6(12%)/ 39(81%) | 2 (1,3) | 33 (69%) | 35 (21,46) | 6 (1.17,19.25) |
| Control (n=40) | 12.71 (8.69,15.75) | 22 (55%) | 5(12%)/ 30(75%) | 3 (1,4.75) | NA | 9 (6,11) | 0.5 (0.1,0.7) |
| p-value** | 0.28 | 0.76 | 0.18 | 0.04 †† | 0.66 | <.001 †† | <.001†† |
Medians and quartiles were reported for continuous variable; Ns (%) were reported for the categorical variables
The p-values for categorical variables such as sex and race are from Chi-square test for overall association with the patient groups except for PGA, which only applies to the diseased groups. The p-values for continuous and ordinal variables are from Kruskal-Wallis test for difference in median among the patient groups.
Race has three categories: Black, White and other. Only black and white are reported in the table to save space.
If the overall p-value is significant, then Dunn’s test is done for pairwise comparisons with Bonferroni multiple adjustment. For Tanner stage, group 1 is significantly different from the control group (p=0.04). For ESR and CRP, all three disease groups are significantly different from the control group (all p<0.01).
PGA = physician global assessment, ESR = erythrocyte sedimentation rate (mm/hr), CRP = C-reactive protein (mg/L)
Table 2:
Plasma concentrations of the markers by CD phenotype
| COL3A1 | COMP | anti-CSF2 | ASCA-IgA | ASCA-IgG | OmpC | CBir | ANCA | ||
|---|---|---|---|---|---|---|---|---|---|
| Group1 (n=40) | 1762.53 (634.05,2722.56) | 88.67 (58.76,136.38) | 0.88 (0.38,2.85) | 8.31 (3.08,14.99) | 20.79 (8.22,41.03) | 5.98 (5.07,10.16) | 18.2 (7.53,43.27) | 15.16 (9.7,22.13) | |
| Group2 (n=33) | 1852.75 (1325.46,3043.74) | 54.5 (36.57,82.9) | 3.06 (1.62,6.86) | 22.12 (9.03,44.77) | 34.04 (21.2,62.59) | 8.63 (5.79,14.7) | 43.34 (16.74,110.94) | 13.02 (8.75,17.1) | |
| Group3 (n=48) | 2293.81 (1436.31,3675.97) | 77.88 (59.49,96.05) | 2.84 (1.36,7.18) | 14.26 (4.78,43.52) | 30.59 (7.82,66.64) | 10.28 (6.1,14.93) | 32.58 (14.29,87.78) | 12.87 (8.4,16.54) | |
| Control (n=40) | 1739.21 (1032.85,2286.66) | 57.93 (44.31,93.3) | 0.77 (0.41,2.12) | 1.44 (0.3,2.96) | 6.02 (2.81,9.35) | 5.11 (3.87,6.68) | 12.17 (7.99,19.43) | 12.64 (8,18.29) | |
| Overall p-value* | 0.003 | 0.003 | <.001 | <.001 | <.001 | <.001 | <.001 | 0.34 | |
| Pairwise comparisons using Dunn’s test with Bonferroni adjustment | |||||||||
| Group.1 vs. Group.2 | 0.879 | 0.007 | 0.012 | 0.062 | 0.189 | 0.660 | 0.073 | - | |
| Group.1 vs. Group.3 | 0.009 | 1.000 | 0.014 | 0.800 | 1.000 | 0.107 | 0.094 | - | |
| Group.1 vs. Control | 1.000 | 0.070 | 1.000 | <.001 | <.001 | 0.409 | 0.557 | - | |
| Group.2 vs. Group.3 | 0.807 | 0.065 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | - | |
| Group.2 vs. Control | 1.000 | 1.000 | 0.052 | <.001 | <.001 | 0.004 | <.001 | - | |
| Group.3 vs. Control | 0.012 | 0.461 | 0.076 | <.001 | <.001 | <.001 | <.001 | - | |
The p-values are all from non-parametric tests. Kruskal-Wallis test for difference in median among the patient groups. If the overall p-value is significant, then Dunn’s test is done for pairwise comparisons with Bonferroni multiple adjustment. These are reported in the sub-table below the main results.
OL3A1 = collagen type III alpha 1 chain (pg/mL), COMP = cartilage oligomeric matrix protein (ng/mL), Anti-CSF2 = autoantibodies against colony stimulating factor 2 (ng/mL), ASCA = anti- Socchoromyces cerevisiae antibodies (EU/mL), OmpC = anti-outer membrane protein C precursor (EU/mL), CBir = anti-flagellin (EU/mL), ANCA = perinuclear anti-neutrophil cytoplasmic antibodies
Figure 2: Scatter plot of COL3A1 with CRP (mg/L) and ESR (mm/hr).
Plasma COMP concentration in group 3 (Table 2), on the contrary, was not significantly different from group 1 (78 vs 89 ng/mL, p>0.99), group 2 (78 vs 55 ng/mL, p=0.07) or the controls (78 vs 58 ng/mL, p=0.46) after Bonferroni adjustment (sub-table 2).
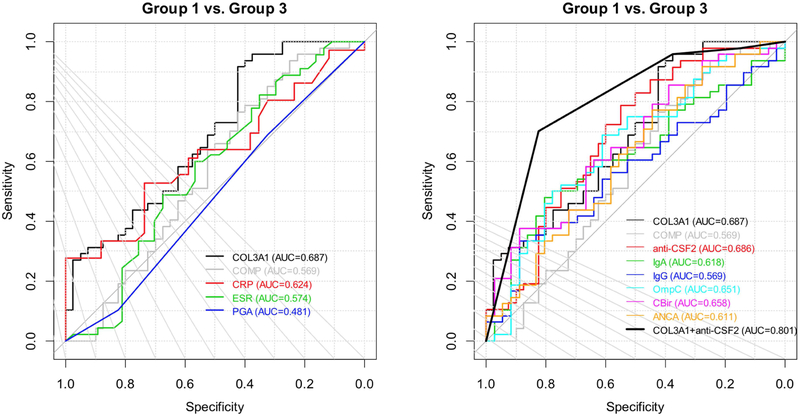
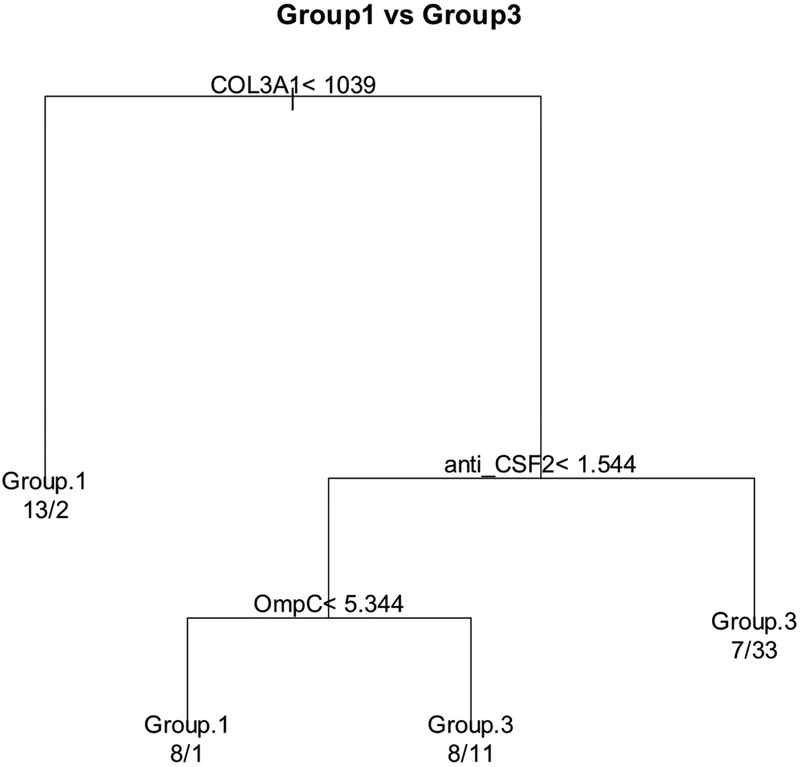
Figure 3 shows the ROC curves with their AUCs when using COL3A1, COMP, clinical burden variables (left panel) and serology markers (right panel) individually to differentiate group 3 from group 1 patients. Although COL3A1 alone gives numerically the highest AUC value (0.687 with 95% CI of [0.571, 0.79]), the performance from all the serologic predictors are fairly close. COL3A1 and anti-CSF2 individually had similar discriminant power for stricture development both with AUC 0.69 (95%CI 0.57, 0.79). Table 3 shows detailed performance of these individual predictors in predicting group 3 from group 1. The sensitivity and specificity are all unbalanced. COL3A1 and anti-CSF2 independently being the most promising predictors is verified by classification and regression tree (CART) method (Figure 4). To build a better classification model considering the limited sample sizes, a parsimonious model is desired. We further use logistic regressions with these two predictors as binary variables (thickened black line in Figure 3 and Table 5), where the cutoff values for COL3A1 (1039) and anti-CSF2 (1.6) were suggested by CART. This simple classification model gave better performance with AUC of 0.80 (95%CI [0.71-0.89]) in predicting the subjects who developed stricture during follow-up versus patients remaining B1 phenotype. Comparing the ROC curve of this model and that of COL3A1 alone, the difference is not statistical significant (p=0.12), but it is significantly better than that of anti- CSF2 alone (p=0.035). This model also improves the balance between sensitivity (0.70, 95% CI [0.55, 0.83]) and specificity (0.83, 95% CI [0.67, 0.93]).
Figure 3: ROC curves of various prediction models. Left panel: comparison of COL3A1 and clinical disease burden variables in prediction of group 1 vs. group 3. Right panel: comparison of prediction models using each serological marker vs. the model with COL3A1 + anti-CSF2.
COL3A1 = collagen type III alpha 1 chain, COMP = cartilage oligomeric matrix protein, PGA = Physician Global Assessment, ESR = erythrocyte sedimentation rate, CRP = C-reactive protein, anti-CSF2 = colony stimulating factor 2 antibodies, ASCA = anti-Saccharomyces cerevisiae antibodies, OmpC = anti-outer membrane protein C precursor, CBir = anti-flagellin, ANCA = perinuclear anti-neutrophil cytoplasmic antibodies
Table 3:
Discriminant power of individual and combined biomarkers as measured by AUC
| Group 1 vs Group 3 | |||
|---|---|---|---|
| Biomarker | N | AUC (95% CI) | Sensitivity, Specificity |
| COL3A1 | 88 | 0.687 (0.571, 0.79) | (0.917, 0.425) |
| COMP | 87 | 0.569 (0.443-0.694) | (0.766, 0.425) |
| PGA | 88 | 0.481 (0.365, 0.587) | (0.688, 0.325) |
| ESR | 82 | 0.574 (0.44, 0.693) | (0.822, 0.351) |
| CRP | 70 | 0.624 (0.485, 0.757) | (0.278, 1) |
| anti-CSF2 | 87 | 0.686 (0.569, 0.792) | (0.787, 0.55) |
| IgA ASCA | 84 | 0.618 (0.495, 0.74) | (0.479, 0.806) |
| IgG ASCA | 84 | 0.569 (0.437, 0.692) | (0.333, 0.889) |
| OmpC | 84 | 0.651 (0.531, 0.766) | (0.688, 0.611) |
| CBir | 84 | 0.658 (0.534, 0.771) | (0.375, 0.889) |
| ANCA | 84 | 0.611 (0.482, 0.728) | (0.771, 0.444) |
| COL3A1 (≥1039)* + anti-CSF2 (≥1.6)* | 87 | 0.801 (0.713, 0.889) | (0.702, 0.825) |
The cutoff of COL3A1 is based on the CART model. The cutoffs for other serology markers are the ones to define positivity in RISK study. This model is compared with the univariate COL3A1 (p=0.12) and anti-CSF2 (p=0.035) using DeLong's test for comparing two correlated ROC curves (Elisabeth R. DeLong, David M. DeLong and Daniel L. Clarke-Pearson“Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach”. Biometrics 1988, 44, 837–845).
COL3A1 = collagen type III alpha 1 chain, COMP = cartilage oligomeric matrix protein, PGA = Physician Global Assessment, ESR = erythrocyte sedimentation rate, CRP = C-reactive protein, Anti-CSF2 = autoantibodies against colony stimulating factor 2, ASCA = anti- Socchoromyces cerevisiae antibodies, OmpC = antiouter membrane protein C precursor, CBir = anti-flagellin, ANCA = perinuclear anti-neutrophil cytoplasmic antibodies
Figure 4. Prediction model using classification and regression tree (CART) method.
If a patient is evaluated as True for the condition at each level, s/he will be passed to the left side of the split, otherwise to the right side of the split. At the end of each branch, the group membership listed is the prediction label (predicted class using the criteria specified by that route). The numbers below the prediction label are the number of patients being predicted as the prediction label but actually belong to Group 1 and Group 3 separated by a slash. For example, the far-right route means with COL3A1≥1039 and anti-CSF2 ≥ 1.544, 40 patients were classified as Group 3 but only 33 of them are truly belong to Group 3.
Table 5:
Model information of the prediction model using dichotomized COL3A1and anti-CSF2 (the last row in Table 3).
| Data | Predictor | Odds Ratio (95% CI) | p-value |
|---|---|---|---|
| Group 3 vs. Group 1 (N=87) | COL3A1 (≥1039) vs. COL3A1 (<1039) | 19.17 (4.47, 137.04) | 0.0004 |
| anti-CSF2 (≥1.6) vs. anti-CSF2 (<1.6) | 4.63 (1.73, 13.45) | 0.0032 |
COL3A1 = collagen type III alpha 1 chain, Anti-CSF2 = autoantibodies against colony stimulating factor 2
Discussion:
The RISK cohort recruited a large sample of 1100 CD subjects at diagnosis with baseline plasma samples and other biomaterials and followed these patients prospectively for 36 months. Utilizing this unique resource, we have shown that baseline plasma COL3A1 concentrations are significantly elevated in subjects diagnosed as inflammatory (B1) Crohn’s disease who go on to develop strictures, as compared to subjects who remain B1 and healthy controls. These results are supported by the known pathogenesis of excess collagen deposition leading to tissue fibrosis.
Plasma COL3A1 was not elevated in subjects who presented with stricture at diagnosis compared to B1 patients and controls. This may be due to a decrease in active ECM degradation and production once a certain amount of bowel scarring has occurred. It is difficult to know when the process of bowel wall injury and remodeling began in patients who present with stricture. Stricture formation could occur over weeks to months or even years in certain patients. If active ECM degradation and production must be occurring for circulating COL3A1 to be detected, and so called “cold” or “mature” strictures are no longer undergoing this cyclical activity, than COL3A1 would not be elevated in patients with these types of mature strictures. This would suggest that COL3A1 may be a marker of active fibrogenesis and/or contribute to remodeling. When studied in combination, the ratio of COL3A1 to matrix metalloproteinase-9 (a marker of type III collagen degradation) has been shown to differentiate stricturing disease from penetrating disease.22 This is an important finding as mature strictures are identifiable with available imaging modalities, while early, active stricture formation may be missed by imaging, even with very experienced radiologists. Therefore, COL3A1 and other collagen markers may be useful to detect early, active fibrosis before a more clinically evident stricture forms.
Granulocyte macrophage colony-stimulating factor (GM-CSF) is a cytokine that promotes myeloid cell development and maturation, as well as intestinal epithelial wound healing. Colony stimulating factor 2 (CSF2) auto antibodies have been shown to impair neutrophil killing, increase rates of intestinal resection and accelerate surgical recurrence of Crohn’s disease.23-25 There is an association of serum anti-CSF2 in pediatric patients with IBD and their siblings, suggesting a possible genetic basis for variation in these antibodies.26 In our subjects, anti-CSF2 was the most predictive of stricture development of all the biomarkers studied. When anti-CSF2 antibodies were evaluated in a combined classification model with COL3A1, the AUC approached 0.8.
Surprisingly, COMP was highest in the B1 group that never developed stricture (Group 1), suggesting it may correlate with degree of mucosal inflammation rather than specify fibrosis. However, COMP did not correlate with markers of systemic inflammation, CRP or ESR. COMP plays a role in ECM structure, myofibroblast proliferation and collagen secretion, and has been shown to be overexpressed in fibrotic diseases of the skin, joints, lungs and liver.17-20, 27 Circulating serum COMP were elevated in adult patients with B2 phenotype as compared to B1; however, these results have not been replicated in children.28 COMP expression is increased in normal growth phases, especially long bone growth and development and may explain elevated levels in healthy or less-ill children. Bone growth may be a confounder for using COMP in the pediatric population, but even when controlling for age and Tanner stage, we were not able to demonstrate a difference in our phenotype groups. More research is needed to better understand the functions of this matrix protein and how it may reflect bowel fibrosis, systemic illness and bone growth.
It is unlikely that a single circulating biomarker will emerge as a clinical tool for prediction of strictures in CD. Focusing efforts on a combination panel to quickly identify individuals ‘at risk’ for the maturation of fibrosis leading to stricture and bowel resection may be a more readily attainable, incremental step towards biomarkers of fibrosis. COL3A1 is more sensitive and specific for stricture development than any clinical assessment tools being used currently, including inflammatory markers (ESR, CRP) and clinical disease assessments (PGA). When combined with anti-CSF2, COL3A1 had improved sensitivity and specificity with AUC approaching 0.8. We believe at present COL3A1 may be used to heighten clinician awareness that certain patients may be higher risk for stricture development and require closer monitoring with imaging and endoscopy rather than definitively diagnosing the absence or presence of bowel stricture. We know that over the past two decades the rates of surgery in pediatric and adult CD patients have not changed at the population levels, despite the introduction of multiple new therapies.5, 29 While anti-tumor necrosis factor drugs are very effective in treating inflammatory CD phenotypes and preventing the development of B3 (internal penetrating) disease, they may not be as effective in stricturing or fibrostenotic disease.3 Although intestinal fibrosis may not currently be preventable, COL3A1 could be used as another tool to risk-stratify patients at diagnosis and set expectations for disease course. Identifying patients with B2 phenotypes before a clinical stricture develops, and then tailoring anti-fibrotic therapy to prevent disease is the future of CD treatment.
Our study was limited by the relatively small sample size within each CD phenotype. While the RISK cohort was enrolled prospectively, COL3A1 and COMP concentrations were performed retrospectively, after the completion of the original study. Stricturing disease was not centrally defined in the RISK study but diagnosed using a combination of imaging and endoscopic modalities by clinicians at each individual site. This likely led to more heterogeneity within the B2 phenotype subjects.
In conclusion, we showed that plasma COL3A1 concentration was significantly elevated in children with B1 Crohn’s disease phenotype at diagnosis who developed strictures (B2) during 36 month follow up as compared to patients who remain B1 and controls. In a combined classification model, COL3A1 and anti-CSF2 were sensitive and specific for stricture development. We speculate that circulating serum COL3A1, in conjunction with clinical data and other biomarkers, may be useful to predict the development of strictures in patients with Crohn’s disease.
“What You Need To Know”.
Background: Genes involved in extracellular matrix (ECM) accumulation are upregulated in children with Crohn’s disease (CD) who develop strictures. Collagen type III alpha 1 chain (COL3A1) is an ECM component previously studied in structuring diseases.
Findings: COL3A1 was elevated at CD diagnosis in subjects who later developed strictures as compared to those who did not. Together, COL3A1 and granulocyte macrophage colony- stimulating factor auto-antibodies had an AUC of 0.79 when differentiating inflammatory and stricturing CD.
Implications for patient care: Along with clinical data, a panel of serologies including COL3A1, may be useful to stratify patient risk for stricturing complications at CD diagnosis and guide disease monitoring protocol.
Acknowledgments
Funding Source: Crohn’s and Colitis Foundation
Abbreviations:
- (COL3A1)
collagen type III alpha 1 chain
- (CD)
Crohn’s disease
- (COMP)
cartilage oligomeric matrix protein
- (RISK)
Risk Stratification and Identification of Immunogenic and Microbial Markers of Rapid Disease Progression in Children with Crohn’s
- (AUC)
area under curve
- (CSF2)
colony stimulating factor 2
- (ECM)
extra cellular matrix
- (ASCA)
anti-Saccharomyces cerevisiae antibodies
- (pANCA)
IgG and IgA, perinuclear anti-neutrophil cytoplasmic antibodies
- (anti-flagellin)
anti-CBir1
- (OmpC)
anti-outer membrane protein C precursor
- (CRP)
C- reactive protein
- (ESR)
erythrocyte sedimentation rate
- (PGA)
physician global assessment
- (ROC)
Receiver Operating Characteristic
- (CART)
Classification and Regression Trees
- (GM-CSF)
Granulocyte macrophage colony-stimulating factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Primary Author: Cortney R. Ballengee, MD.
Clinical Trial Registration: ClinicalTrials.gov identifier NCT00790543
Disclosures: Ms. Liu, Mr. Prince and Drs. Ballengee, Kim, Mondal, Baldassano, Leleiko and Kugathasan have nothing to disclose. Dr. Stidham serves as a consultant for Abbvie, Merck and Janssen. Dr. Dubinsky serves as a consultant for Abbvie, Janssen, Takeda and Pfizer. Dr. Hyams serves as a consultant for Janssen, Abbvie, Pfizer, Receptos, Boehringer Ingelheim, Allergan, Lilly and Roche. Dr. Markowitz serves as a consultant for Janssen, Celgene, and Lilly.
References
- 1.Cosnes J, Cattan S, Blain A, et al. Long-term evolution of disease behavior of Crohn's disease. Inflamm Bowel Dis 2002;8:244–50. [DOI] [PubMed] [Google Scholar]
- 2.Rieder F, Zimmermann EM, Remzi FH, et al. Crohn’s disease complicated by strictures: a systematic review. Gut 2013;62:1072–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kugathasan S, Denson LA, Walters TD, et al. Prediction of complicated disease course for children newly diagnosed with Crohn’s disease: a multicentre inception cohort study. Lancet 2017;389:1710–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rieder F, de Bruyn JR, Pham BT, et al. Results of the 4th scientific workshop of the ECCO (Group II): markers of intestinal fibrosis in inflammatory bowel disease. J Crohns Colitis 2014;8:1166–78. [DOI] [PubMed] [Google Scholar]
- 5.Burisch J, Kiudelis G, Kupcinskas L, et al. Natural disease course of Crohn’s disease during the first 5 years after diagnosis in a European population-based inception cohort: an Epi-IBD study. Gut 2018. [DOI] [PubMed] [Google Scholar]
- 6.Gower-Rousseau C, Vasseur F, Fumery M, et al. Epidemiology of inflammatory bowel diseases: new insights from a French population-based registry (EPIMAD). Dig Liver Dis 2013;45:89–94. [DOI] [PubMed] [Google Scholar]
- 7.Pittet V, Rogler G, Michetti P, et al. Penetrating or stricturing diseases are the major determinants of time to first and repeat resection surgery in Crohn's disease. Digestion 2013;87:212–21. [DOI] [PubMed] [Google Scholar]
- 8.Kerur B, Machan JT, Shapiro JM, et al. Biologics Delay Progression of Crohn's Disease, but Not Early Surgery, in Children. Clin Gastroenterol Hepatol 2018. [DOI] [PubMed] [Google Scholar]
- 9.Prockop DJ, Kivirikko KI, Tuderman L, et al. The Biosynthesis of Collagen and Its Disorders. New England Journal of Medicine 1979;301:77–85. [DOI] [PubMed] [Google Scholar]
- 10.Giuffrida P, Pinzani M, Corazza GR, et al. Biomarkers of intestinal fibrosis - one step towards clinical trials for stricturing inflammatory bowel disease. United European Gastroenterology Journal 2016;4:523–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alvarez-Lobos M, Arostegui JI, Sans M, et al. Crohn's disease patients carrying Nod2/CARD15 gene variants have an increased and early need for first surgery due to stricturing disease and higher rate of surgical recurrence. Ann Surg 2005;242:693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kjeldsen J, Schaffalitzky de Muckadell OB, Junker P. Seromarkers of collagen I and III metabolism in active Crohn's disease. Relation to disease activity and response to therapy. Gut 1995;37:805–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Simone M, Ciulla MM, Cioffi U, et al. Effects of surgery on peripheral N-terminal propeptide of type III procollagen in patients with Crohn's disease. J Gastrointest Surg 2007;11:1361–4. [DOI] [PubMed] [Google Scholar]
- 14.De Simone M, Cioffi U, Contessini-Avesani E, et al. Elevated serum procollagen type III peptide in splanchnic and peripheral circulation of patients with inflammatory bowel disease submitted to surgery. BMC Gastroenterol 2004;4:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenberg WM, Voelker M, Thiel R, et al. Serum markers detect the presence of liver fibrosis: a cohort study. Gastroenterology 2004;127:1704–13. [DOI] [PubMed] [Google Scholar]
- 16.Rosenberg K, Olsson H, Morgelin M, et al. Cartilage oligomeric matrix protein shows high affinity zinc-dependent interaction with triple helical collagen. J Biol Chem 1998;273:20397–403. [DOI] [PubMed] [Google Scholar]
- 17.Agarwal P, Schulz JN, Blumbach K, et al. Enhanced deposition of cartilage oligomeric matrix protein is a common feature in fibrotic skin pathologies. Matrix Biol 2013;32:325–31. [DOI] [PubMed] [Google Scholar]
- 18.Vuga LJ, Milosevic J, Pandit K, et al. Cartilage oligomeric matrix protein in idiopathic pulmonary fibrosis. PLoS One 2013;8:e83120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Magdaleno F, Arriazu E, Ruiz de Galarreta M, et al. Cartilage oligomeric matrix protein participates in the pathogenesis of liver fibrosis. J Hepatol 2016;65:963–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kobayashi M, Kawabata K, Kusaka-Kikushima A, et al. Cartilage Oligomeric Matrix Protein Increases in Photodamaged Skin. J Invest Dermatol 2016;136:1143–9. [DOI] [PubMed] [Google Scholar]
- 21.Satsangi J, Silverberg MS, Vermeire S, et al. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut 2006;55:749–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Haaften WT, Mortensen JH, Karsdal MA, et al. Misbalance in type III collagen formation/degradation as a novel serological biomarker for penetrating (Montreal B3) Crohn's disease. Aliment Pharmacol Ther 2017;46:26–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gathungu G, Kim MO, Ferguson JP, et al. Granulocyte-macrophage colony-stimulating factor autoantibodies: a marker of aggressive Crohn's disease. Inflamm Bowel Dis 2013;19:1671–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gathungu G, Zhang Y, Tian X, et al. Impaired granulocyte-macrophage colony-stimulating factor bioactivity accelerates surgical recurrence in ileal Crohn's disease. World J Gastroenterol 2018;24:623–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han X, Uchida K, Jurickova I, et al. Granulocyte-macrophage colony-stimulating factor autoantibodies in murine ileitis and progressive ileal Crohn's disease. Gastroenterology 2009;136:1261–71, e1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wright SS, Trauernicht A, Bonkowski E, et al. Familial Association of Granulocyte-Macrophage Colony Stimulating Factor Autoantibodies in Inflammatory Bowel Disease. J Pediatr Gastroenterol Nutr 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schulz JN, Nuchel J, Niehoff A, et al. COMP-assisted collagen secretion--a novel intracellular function required for fibrosis. J Cell Sci 2016;129:706–16. [DOI] [PubMed] [Google Scholar]
- 28.Stidham RW, Wu J, Shi J, et al. Serum Glycoproteome Profiles for Distinguishing Intestinal Fibrosis from Inflammation in Crohn’s Disease. PLoS One 2017;12:e0170506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rinawi F, Assa A, Hartman C, et al. Incidence of Bowel Surgery and Associated Risk Factors in Pediatric-Onset Crohn's Disease. Inflamm Bowel Dis 2016;22:2917–2923. [DOI] [PubMed] [Google Scholar]