Abstract
Background:
Transition to enteral feeding is difficult for very low-birth-weight (VLBW; ≤1500 g) infants, and optimal nutrition is important for clinical outcomes.
Method:
Data on feeding practices and short-term clinical outcomes (growth, necrotizing enterocolitis [NEC], mortality) in VLBW infants were collected from 13 neonatal intensive care units (NICUs) in 5 continents (n = 2947). Specifically, 5 NICUs in Guangdong province in China (GD), mainly using formula feeding and slow feeding advancement (n = 1366), were compared with the remaining NICUs (non-GD, n = 1581, Oceania, Europe, United States, Taiwan, Africa) using mainly human milk with faster advancement rates.
Results:
Across NICUs, large differences were observed for time to reach full enteral feeding (TFF; 8–33 days), weight gain (5.0–14.6 g/kg/day), Δz-scores (−0.54 to −1.64), incidence of NEC (1%–13%), and mortality (1%–18%). Adjusted for gestational age, GD units had longer TFF (26 vs 11 days), lower weight gain (8.7 vs 10.9 g/kg/day), and more days on antibiotics (17 vs 11 days; all P < .001) than non-GD units, but NEC incidence and mortality were similar.
Conclusion:
Feeding practices for VLBW infants vary markedly around the world. Use of formula and long TFF in South China was associated with more use of antibiotics and slower weight gain, but apparently not with more NEC or higher mortality. Both infant- and hospital-related factors influence feeding practices for preterm infants. Multicenter, randomized controlled trials are required to identify the optimal feeding strategy during the first weeks of life.
Keywords: antibiotics, formula, growth, milk, NEC, parenteral, preterm infants
Introduction
Preterm birth is a major cause of morbidity and mortality, especially for very low-birth-weight (VLBW; ≤1500 g) infants.1 Survival of VLBW infants has increased in recent years because of novel perinatal interventions, but clear evidence for the optimal postnatal nutrition strategy is lacking. Optimal nutrition status may be critical to prevent adverse in-hospital outcome and support long-term development (eg, neurodevelopment).2–5 Early and fast increase in enteral feeding is preferred, relative to prolonged use of parenteral nutrition (PN), to reduce sepsis, liver problems, and persistent gut immaturity.6,7 Cochrane reviews show that early introduction and progressive advancement of enteral feeding are not associated with more necrotizing enterocolitis (NEC) than slow feeding,8–11 but the evidence remains inconsistent.12–14 Many feeding guidelines recommend early and progressive enteral feeding,15,16 but it is challenging to adhere to such guidelines due to fear of NEC and nonspecific signs of feeding intolerance, especially for very immature infants17 in hospitals with limited access to human milk.
There is a consensus that own mother’s milk (MM) is the best diet during the first weeks after birth for highrisk preterm infants.18,19 The evidence in favor of human donor milk (DM) is more variable,20 whereas infant formula (IF) is inferior to MM with regard to clinical outcomes (eg, NEC, sepsis, mortality).21 Therefore, in neonatal intensive care units (NICUs) with low availability of human milk, the clinical concern for NEC may be greater and the time to reach full enteral feedings (TFF) longer. In addition, limited access to nutrition, medical, and surgical support in NICUs in low- and middle-income countries may influence the chosen nutrition practices.22
In this observational cohort study, we explored the nutrition practices and short-term clinical outcomes in 13 NICUs in 9 countries from 5 continents. Before this study, we informally observed that NICUs in the Guangdong province in South China (GD) had limited access to human milk and a slow feeding advancement, relative to other NICUs (non-GD) in our research network focusing on preterm nutrition. Thus, we specifically aimed to investigate whether the short-term clinical outcomes (growth, NEC incidence, mortality) differed between GD and non-GD NICUs. In addition, the GD region has a rapidly growing population and economy, with new hospital infrastructure, making it important to study how hospital-related factors may influence feeding practices and clinical outcomes. Data included time to full enteral feeding, diet type, duration of PN, and prebiotics and antibiotics use, together with growth, NEC, and mortality rates.
Methods
Study Design and Subjects
The NEOMUNE-NeoNutriNet was a combined retrospective and prospective cohort study to describe nutrition practices and short-term clinical outcomes in VLBW infants. Inclusion criteria were infants with a body weight (BW) ≤1500 g and admitted to the participating NICUs within 24 hours after birth. Exclusion criteria included major congenital abnormalities, metabolic disease, and transfer to another hospital within 24 hours of birth. Thirteen hospitals participated in the study across Europe (3), Oceania (2), North America (1), Africa (1), and Asia (6). Selection of the participating units was based on personal contact with the lead investigators and participation in the NEOMUNE research network (http://www.neomune.ku.dk).
Ethics
The study was approved by the ethical committee of VU University Medical Center, Amsterdam, the Netherlands, and at individual hospitals when required. Parental informed consents were obtained in 1 hospital but were not necessary in others because collected data were anonymous and were routinely collected as part of clinical care. A contract research organization was responsible for regulatory and safety aspects of the database (Academic Medical Center, Amsterdam, the Netherlands).
Data Collection and Outcomes
Entry into the web-based database started on September 15, 2013. For each unit the aim was to include at least 100 infants, born consecutively between January 1, 2011, and September 15, 2014. Case report forms were used to collect information from medical and nursing records including infant demographics (gestational age [GA] and anthropometrics at birth, gender, delivery mode, antenatal corticosteroids), nutrition regimens (timing and type of enteral nutrition [EN] and PN, time to reach enteral feeding volumes of 120 [TFF120] or 150 mL/kg/day [TFF150]), use of probiotics, and clinical outcomes (use of antibiotics, respiratory support, NEC incidence, defined as Bell stage ≥II23), all-cause mortality, and weekly anthropometric data (eg, weight, length, head circumference). Small for gestational age (SGA) was defined as < 10th percentile for weight at birth. The Newcastle unit did not record TFF120, and the Taiwan site did not collect data on infants who died. All data were collected until postconceptional age (PCA) 37 weeks, or less if infants were transferred to a step-down unit, discharged to home, discharged on parental request before reaching discharge criteria, or died. Complying with the recommendations on reporting growth-related outcomes in preterm infants,24 the growth was reported from birth with the Fenton 2013 growth chart25 as the growth reference. Weight was reported in z-scores, and Δz-scores from birth to 28 days were used to assess changes in weight over time. The exponential method by Patel et al26 was used to calculate weight gain velocity.
Statistical Analysis
All statistical analyses were performed using the R software package (version 3.2.2). Data were summarized using means and SDs, medians and interquartile ranges (IQRs), numbers and percentages, as appropriate. Median and IQR were used in cases of right-censored time-to-event data (eg, TFF). Comparisons of demographic characteristics listed in Table 1 between GD and non-GD NICUs were based on analysis of variance for continuous outcomes (eg, BW) and logistic regression models for binary outcomes (eg, SGA). In Table 2, all outcome comparisons between GD and non-GD units were performed with or without adjustments for GA. Time-to-event data were compared using Cox proportional hazard models, and other continuous outcomes (eg, weight) were compared based on analysis of variance. Binary outcomes (eg, mortality) were compared using logistic regression models. Adjustment for GA was done using the groups <28, 28, 29, 30, 31, 32, and >32 weeks because of the nonlinear relation between GA and clinical outcomes. Nonparametric Kruskal-Wallis test was used when normal distribution could not be achieved. A P-value <.001 was considered significant, whereas .01 < P < .001 was considered as a tendency to an effect. We used this very restrictive approach to statistical differences, considering the large number of comparisons performed, and the possible inflation of significancy by cluster effects.
Table 1.
Demographic Characteristics at Birth and Use of Antenatal Steroids, Milk Fortification, and Probiotics in Guangdong (GD) NICUs and in Other Units Around the World (Non-GD).
| Hospital | FOS | SWC | SNP | BWC | PWC | AUC | CHI | COP | AMS | NEW | PER | TAI | IBA | GD | Non-GD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total included | 407 | 458 | 93 | 167 | 241 | 160 | 177 | 284 | 270 | 139 | 152 | 249 | 150 | 1366 | 1581 |
| Gestational age, weeks | 30.4 (2.1) | 30.3 (2.2) | 30.4 (2.0) | 29.7 (2.0) | 30.5 (2.1) | 29.0 (2.7) | 28.3 (2.5) | 28.0 (2.5) | 28.6 (2.2) | 28.4 (2.8) | 29.4 (2.5) | 29.9 (2.6) | 30.5 (2.4) | 30.3 (2.1) | 29.0 (2.6)* |
| Birth weight g | 1237 (184) | 1254 (177) | 1245 (219) | 1250 (183) | 1262 (172) | 1101 (262) | 1025 (252) | 990 (267) | 1070 (258) | 1043 (280) | 1127 (257) | 1165 (236) | 1223 (191) | 1250 (182) | 1086 (262)* |
| z-Score | −0.56 (0.99) | −0.44 (1.06) | −0.66 (0.90) | −0.20 (0.84) | −0.55 (1.05) | −0.32 (1.03) | −0.25 (0.94) | −0.31 (0.93) | −0.24 (0.95) | −0.34 (0.92) | −0.45 (0.9) | −0.56 (1.05) | −0.62 (1.24) | −0.48 (1.01) | −0.38 (1.00) |
| Birth length cm | 38 (4) | 39 (4) | 38 (3) | 39 (3) | 38 (3) | 37 (4) | 36 (3) | 36 (4) | 36 (3) | UNK | 37 (4) | 38 (3) | 38 (3) | 39 (3) | 37 (4)* |
| z-Score | −0.43 (1.48) | −0.02 (1.59) | −0.64 (1.18) | 0.06 (1.04) | −0.57 (1.45) | −0.12 (1.24) | −0.31 (1.08) | −0.2 (1.12) | −0.43 (1.18) | UNK | −0.23 (1.14) | −0.32 (1.30) | −0.51 (1.30) | −0.27 (1.47) | −0.30 (1.20) |
| Birth head circumference cm | 26.7 (1.9) | 27.1 (2.2) | 27.3 (1.8) | 27.0 (1.7) | 27.3 (1.8) | 26.3 (2.1) | 25.1 (2.1) | 25.3 (2.7) | 25.9 (2.4) | 25.6 (1.9) | 26.2 (2.4) | 26.3 (2.1) | 27.3 (1.8) | 27.0 (2.0) | 26.0 (2.3)* |
| z-Score | −0.63 (1.41) | −0.27 (1.62) | −0.33 (1.16) | −0.02 (1.08) | −0.27 (1.36) | 0.05 (1.06) | −0.33 (1.11) | −0.11 (0.98) | −0.02 (1.08) | −0.14 (1.06) | −0.29 (1.12) | −0.50 (1.23) | −0.21 (1.34) | −0.36 (1.44) | −0.20 (1.14) |
| Small for gestational age, n (%) | 84 (21) | 86 (19) | 24 (26) | 19 (11) | 52 (22) | 27 (17) | 28 (16) | 49 (17) | 40 (15) | 24 (17) | 28 (18) | 61 (24) | 40 (27) | 265 (19) | 297 (19) |
| Male, n (%) | 230 (57) | 231 (51) | 43 (46) | 108 (65) | 153 (63) | 80 (50) | 95 (54) | 130 (46) | 135 (50) | 71 (51) | 72 (47) | 116 (47) | 66 (44) | 765 (56) | 765 (48)* |
| Singleton, n (%) | 278 (69) | 270 (59) | 72 (78) | 124 (74) | 164 (68) | 115 (72) | 151 (85) | 165 (58) | 204 (76) | 29 (73) | 116 (76) | 170 (68) | 82 (55) | 908 (67) | 1032 (70) |
| Caesarean section, n (%) | 251 (62) | 312 (68) | 62 (67) | 65 (39) | 142 (59) | 104 (65) | 117 (66) | 193 (68) | 154 (57) | 71 (52) | 99 (65) | 178 (71) | 66 (44) | 832 (61) | 982 (62) |
| Any steroids, n (%) | 155 (38) | 106 (70) | 74 (80) | 85 (52) | 92 (38) | 157 (98) | 164 (93) | 203 (88) | 235 (87) | 131 (94) | 141 (93) | 193 (78) | 47 (31) | 512 (49) | 1271 (83)* |
| Complete steroids, n (%) | 82 (20) | 75 (50) | 52 (57) | 37 (22) | 65 (27) | 119 (74) | 124 (70) | 170 (73) | 154 (57) | 97 (70) | 118 (78) | 155 (62) | 28 (19) | 311 (30) | 965 (63)* |
| Human milk fortifier n (%) | 0 (0) | 167 (37) | 22 (24) | 0 (0) | 8 (3) | 138 (86) | 135 (76) | 163 (57) | 187 (69) | UNKa | 140 (92) | 197 (79) | 0 (0) | 197 (14) | 960 (67)* |
| Day of life | — | 24 (8) | 23 (8) | — | 22 (8) | 10 (7) | 20(9) | 21(9) | 12(5) | UNKa | 11(5) | 15(9) | — | 24 (8) | 15 (8)* |
| Probiotics, n (%) | 67 (17) | 38 (11) | 0 (0) | 79 (47) | 78 (33) | 106 (67) | 0 (0) | 227 (81) | 0 (0) | 34 (81) | 109 (72) | 43 (17) | 0 (0) | 262 (21) | 515 (35)* |
| Observation period,b days | 35 (18) | 40 (17) | 32 (16) | 42 (18) | 37 (18) | 38 (26) | 58 (20) | 46 (27) | 30 (23) | 45 (28) | 46 (22) | 49 (18) | 23 (12) | 38 (18) | 42 (25)* |
Data are presented as mean (SD) unless otherwise stated.
AMS, Amsterdam UMC, Vrije Universiteit, Emma Children’s Hospital; AUC, Newborn Service, National Women’s Health, Auckland; BWC, Shenzhen Bao’an Maternal and Child Health Hospital; CHI, Rush University Children’s, Hospital, Chicago; COP, Rigshospitalet, Copenhagen; FOS, Foshan Woman and Children’s Hospital; GD, Guangdong province in China; IBA, University College Hospital, Ibadan; NEW, Newcastle Hospitals NHS Foundation Trust; PER, Kang Edward Memorial Hospital, Perth; PWC, Guangdong Women and Children Hospital; SNP, Shenzhen Nanshan People’s Hospital; SWC, Shenzhen Maternity & Child Health Care Hospital; TAI, Children’s Hospital of China Medical University, Taichung; UNK, unknown.
Significant difference between GD and non-GD (P < .001).
Human milk fortifier was used, but exact data are missing.
Data were collected from <24 hours after birth to postconceptional age 37 weeks, or less if infants were transferred to a different (step-down) unit, discharged to home (including discharge on parental request), or died earlier.
Table 2.
Anthropometric and Clinical Outcomes in Guangdong (GD) NICUs and Other NICUs Around the World (Non-GD).
| Hospital | FOS | SWC | SNP | BWC | PWC | AUC | CHI | COP | AMS | NEW | PER | TAI | IBA | GD | Non-GD | P1 | P2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mortality (%) | 42 (10) | 7 (2) | 3 (3) | 14 (8) | 7 (3) | 2 (1) | 1 (1) | 38 (13) | 29 (11) | 7 (5) | 6 (4) | UNK | 27 (18) | 73 (5) | 110 (7) | NS | NS |
| Intubation ventilation n (%) | 182 (46) | 131 (30) | 26 (28) | 98 (59) | 145 (60) | 84 (53) | 141 (80) | 144 (51) | 170 (63) | 90 (65) | 85 (56) | 78 (32) | 6 (4) | 582 (43) | 798 (51) | * | NS |
| Daysa | 3 (7) | 1 (3) | 2 (3) | 4 (6) | 3 (5) | 5 (10) | 11 (16) | 5 (11) | 8 (14) | 8 (13) | 3 (9) | 4 (11) | 0 (0) | 2 (5) | 6 (12) | * | NS |
| Start enteral nutrition n (%) | 371 (92) | 441 (97) | 83 (89) | 165 (99) | 227 (94) | 156 (99) | 177 (100) | 282 (99) | 268 (100) | 137 (99) | 149 (99) | 249 (100) | 144 (96) | 1287 (95) | 1562 (99) | * | * |
| DOL, median (IQR) | 4 (2–6) | 2 (2–3) | 3 (3–6) | 2 (2–3) | 3 (1–6) | 2 (1–2) | 3 (3–5) | 1 (1–2) | 1 (1–1) | 4 (2–5) | 2 (2–3) | 2 (2–4) | 3 (3–5) | 3 (2–5) | 2 (1–3) | * | * |
| TFF (120 mL/kg/day) n (%) | 233 (58) | 392 (86) | 58 (62) | 126 (75) | 160 (66) | 141 (88) | 176 (99) | 235 (83) | 204 (76) | UNK | 146 (96) | 245 (98) | 119 (79) | 969 (71) | 1266 (88) | * | * |
| DOL, median (IQR) | 33 (23–45) | 24 (20–29) | 26 (19–37) | 26 (17–38) | 27 (20–41) | 11 (9–14) | 15 (12–22) | 12 (8–16) | 11 (9–15) | UNK | 8 (7–11) | 12 (9–17) | 11 (8–14) | 26 (20–37) | 11 (9–16) | * | * |
| TFF (150 mL/kg/day) n (%) | 155 (38) | 373 (82) | 41 (44) | 90 (54) | 124 (51) | 138 (86) | 171 (97) | 222 (78) | 173 (64) | 117 (85) | 144 (95) | 166 (67) | 110 (73) | 783 (58) | 1241 (79) | * | * |
| DOL, median (IQR) | 47 (30–60) | 30 (24–36) | 38 (25–49) | 37 (26–61) | 37 (28–50) | 12 (10–16) | 19 (14–28) | 14 (10–20) | 15 (12–22) | 13 (10–17) | 11 (8–16) | 35 (20–68) | 13 (11–18) | 34 (26–49) | 15 (11–25) | * | * |
| Parenteral amino acids n (%) | 389 (96) | 455 (100) | 92 (99) | 167 (100) | 233 (97) | 154 (96) | 176 (99) | 231 (81) | 269 (100) | 131 (94) | 148 (97) | 236 (95) | 76 (51) | 1336 (98) | 1421 (90) | * | * |
| Daysa | 27 (15) | 24 (10) | 25 (12) | 28 (13) | 24 (15) | 15 (11) | 22 (15) | 11 (9) | 15 (11) | 14 (14) | 11 (7) | 11 (7) | 3 (5) | 25 (13) | 13 (11) | * | * |
| Parenteral lipids n (%) | 293 (72) | 453 (99) | 90 (97) | 159 (95) | 185 (77) | 151 (94) | 176 (99) | 221 (78) | 269 (100) | 131 (94) | 145 (95) | 80 (32) | 0 (0) | 1180 (86) | 1173 (74) | * | * |
| Daysa | 18 (16) | 20 (10) | 24 (12) | 21 (12) | 16 (14) | 13 (11) | 22 (15) | 9 (S) | 14 (11) | 14 (14) | 9 (7) | 4 (7) | 0 | 19 (13) | 11 (12) | * | * |
| Weight GV g/kg/day | 8.7 (4.1) | 9.3 (2.3) | 12.7 (3.1) | 10.5 (3.4) | 5.0 (3.5) | 14.6 (4.1) | 12.1 (3.8) | 11.2 (3.8) | 11.0 (4.6) | 10.0 (4.4) | 12.6 (4.0) | 8.0 (4.3) | 8.8 (5.7) | 8.7 (4.2) | 10.9 (4.6) | * | * |
| Δz-Scoreb | −1.24 (0.48) | −1.18 (0.31) | −0.62 (0.38) | −1.04 (0.41) | −1.64 (0.42) | −0.54 (0.49) | −0.83 (0.51) | −0.86 (0.48) | −0.83 (0.50) | −0.88 (0.45) | −0.73 (0.41) | −1.25 (0.42) | −1.30 (0.67) | −1.23 (0.50) | −0.91 (0.53) | * | * |
| Length GV cm/week | 0.8 (0.6) | 1.0 (0.6) | 0.9 (0.6) | 0.9 (0.4) | UNK | 1.0 (0.5) | 0.8 (0.5) | 0.9 (0.5) | 0.7 (0.5) | UNK | 1.2 (0.5) | 0.7 (0.5) | 0.0 (0.0) | 0.9 (0.5) | 0.8 (0.5) | NS | NS |
| Δz-Scoreb | −0.96 (1.12) | −0.52 (0.95) | −0.52 (0.89) | −0.80 (0.59) | UNK | −0.54 (0.83) | −0.86 (0.76) | −0.74 (0.84) | −1.04(0.86) | UNK | −0.22 (0.74) | −1.08 (0.85) | −2.46 (0.2) | −0.77 (0.90) | −0.92 (0.85) | NS | NS |
| Head circumference | 0.5 (0.4) | 0.7 (0.3) | 0.6 (0.3) | 0.5 (0.3) | UNK | 0.5 (0.3) | 0.5 (0.3) | 0.7 (0.3) | 0.5 (0.3) | UNK | 0.6 (0.3) | 0.3 (0.3) | 0.6 (0.4) | 0.5 (0.3) | 0.5 (0.3) | NS | NS |
| GV cm/week | |||||||||||||||||
| Δz-Scoreb | −1.19 (1.32) | −0.43 (0.92) | −0.72 (0.79) | −1.18 (0.81) | UNK | −1.32 (0.90) | −1.18 (0.85) | −0.73 (0.93) | −1.09 (0.77) | UNK | −0.75 (1.07) | −1.81 (1.10) | −0.96 (1.26) | −0.97 (1.03) | −1.29 (1.05) | * | NS |
| NECn(%) | 8 (2) | 5 (1) | 5 (5) | 6 (4) | 25 (10) | 5 (3) | 10 (6) | 20 (7) | 32 (12) | 18 (13) | 1 (1) | 2 (1) | 2 (1) | 49 (4) | 90 (6) | NS | NS |
| NEC scores, Bell II/III | 7/0 | 4/0 | 5/0 | 2/4 | 20/5 | 3/2 | UNK | 8/12 | 26/6 | 6/12 | 0/1 | 2/0 | 2/0 | 39/9 | 47/33 | NS | NS |
| Day of NEC onset | 12 (9) | 11 (8) | 11 (12) | 15 (9) | 14 (11) | 27 (18) | 25 (9) | 18 (16) | 23 (16) | 17 (15) | 13 (-) | 20 (10) | 8 (1) | 13 (10) | 21 (15) | * | NS |
| Postconceptional age at | 225 (20) | 214 (12) | 215 (15) | 228 (15) | 221 (13) | 203 (28) | 215 (14) | 202 (17) | 217 (15) | 199 (15) | 221 (-) | 226 (6) | 235 (7) | 221 (16) | 210 (18) | * | NS |
| NEC onset | |||||||||||||||||
| Surgery for NEC, n(%) | 0 (0) | 0 (0) | 0 (0) | 3 (43) | 5 (20) | 3 (60) | 5(50) | 10 (50) | 8 (25) | 11 (61) | 1 (100) | 0 (0) | 0 (0) | 8 (16) | 38 (42) | NS | NS |
| First stool, DOL | 2 (1) | 2 (1) | 1 (0) | 2 (1) | 2 (1) | 3 (2) | UNK | 4 (3) | 3 (1) | UNK | 3 (2) | 1 (1) | 2 (1) | 2 (1) | 3 (2) | * | * |
| Antibiotics n (%) | 399 (99) | 456 (100) | 93 (100) | 165 (99) | 235 (98) | 149 (93) | 174 (98) | 233 (82) | 256 (95) | 125 (90) | 143 (94) | 233 (94) | 147 (98) | 1348 (99) | 1460 (92) | * | * |
| Daysa | 19 (12) | 12 (10) | 25 (13) | 23 (13) | 16 (10) | 11 (12) | 13 (12) | 12 (11) | 13 (12) | 8 (9) | 7(7) | 8 (8) | 16 (9) | 17 (12) | 11 (11) | * | * |
| Antifungal medicine n (%) | 59 (15) | 109 (24) | 12 (13) | 65 (39) | 29 (14) | 148 (93) | UNKc | 9 (3) | 28 (10) | 34 (81) | 112 (74) | 18 (7) | 35 (24) | 274 (21) | 384 (29) | * | NS |
| Daysa | 1 (3) | 2 (4) | 1 (4) | 2 (4) | 1 (5) | 15 (14) | UNK | 0 (4) | 1 (4) | 12 (19) | 22 (26) | 3 (10) | 3 (9) | 1 (4) | 8 (16) | * | ND |
Data are presented as mean (SD) unless otherwise stated.
AMS, Amsterdam UMC, Vrije Universiteit, Emma Children’s Hospital; AUC, Newborn Service, National Women’s Health, Auckland; BWC, Shenzhen Bao’an Maternal and Child Health Hospital; CHI, Rush University Children’s Hospital, Chicago; COP, Rigshospitalet, Copenhagen; DOL, day of life; FOS, Foshan Woman and Children’s Hospital; GD, Guangdong province in China; GV, growth velocity; IBA, University College Hospital, IQR, interquartile range; Ibadan; ND, not done; NEC, necrotizing enterocolitis; NEW, Newcastle Hospitals NHS Foundation Trust; NICU, neonatal intensive care unit; NS, nonsignificant P1, unadjusted P-value comparing GD and non-GD units; P2, P-value comparing GD and non-GD units, adjusted for gestational age; PER, King Edward Memorial Hospital, Perth; PWC, Guangdong Women and Children Hospital; SNP, Shenzhen Nanshan People’s Hospital; SWC, Shenzhen Maternity & Child Health Care Hospital; TAI, Children’s Hospital of China Medical University, Taichung; TFF, time to full feeding; UNK, unknown.
Significant differences between GD and non-GD, P < .001
Deceased infants were excluded from analysis.
Days 0–28.
All infants with body weight < 1000 g received prophylactic fluconazole from birth until the central line was removed, but exact data are missing.
Results
A total of 2947 infants (GD, n = 1366; non-GD, n = 1581) were collected from the 13 NICUs that each included >100 consecutively born VLBW infants (except 1 unit with n = 93; Table 1). Mean GA and BW ranged widely among NICUs and specifically, infants in the 5 GD units had higher GA and BWs (30.3 ± 2.1 weeks, 1250 ± 182 g) than the infants from non-GD units (29.0 ± 2.6 weeks, 1086 ± 262 g, both P < .001; Table 1). Likewise, birth length and head circumference were higher in GD infants (both P < .001; Table 1). z-Scores of BW, birth length, and head circumference did not differ (Table 1). The overall mean values for proportion of SGA infants (≈20%), singletons (≈70%), and caesarean deliveries (≈60%) were similar between GD and non-GD units (Table 1), whereas the proportion of males was higher in the GD units (56% vs 48% in non-GD units; P< .001). Use of antenatal steroids was less prevalent in GD vs non-GD units (49% vs 83%), and likewise there was less use of probiotics (21% vs 35%; all P < .001; Table 1).
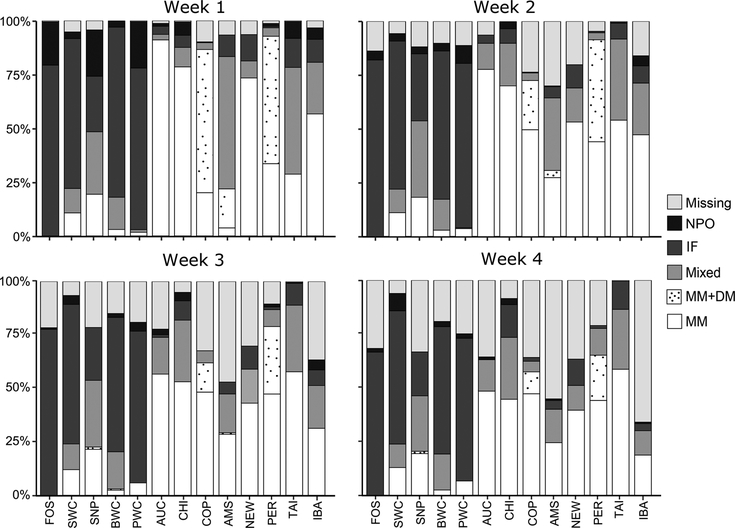
As predicted before the study, most infants in the 5 GD units were formula-fed during the first 4 weeks (Figure 1). The proportion of infants receiving exclusively IF during the first 4 weeks was 74% in GD vs 5% in non-GD units (P< .001). Provision of MM varied widely among NICUs, and DM was available in only 3 units (Amsterdam, Copenhagen, and Perth). Nine units used human milk fortifier for some infants (14% in GD and 67% in non-GD units), being initiated from 10 to 24 days after birth (Table 1). The GD units reported more early discharge on parental request, relative to non-GD units (22% vs 1%; P < .001).
Figure 1.
Type of enteral nutrition 0–28 days after birth in 13 neonatal intensive care units (NICUs) around the world. AMS, Amsterdam UMC, Vrije Universiteit, Emma Children’s Hospital; AUC, Newborn Service, National Women’s Health, Auckland; BWC, Shenzhen Bao’an Maternal and Child Health Hospital, Auckland; CHI, Rush University Children’s Hospital, Chicago; COP, Rigshospitalet, Copenhagen; FOS, Foshan Woman and Children’s Hospital; IBA, University College Hospital, Ibadan; IF, exclusive infant formula; MM, exclusively own mother’s milk; MM+DM, own mother’s milk and/or donor milk; mixed, MM+DM and IF; missing, missing values due to lack of data, infant death, or discharge; NEW, Newcastle Hospitals NHS Foundation Trust; NPO, nil per os; PER, King Edward Memorial Hospital, Perth; PWC, Guangdong Women and Children Hospital; SNP, Shenzhen Nanshan People’s Hospital; SWC, Shenzhen Maternity & Child Health Care Hospital; TAI, Children’s Hospital of China Medical University, Taichung.
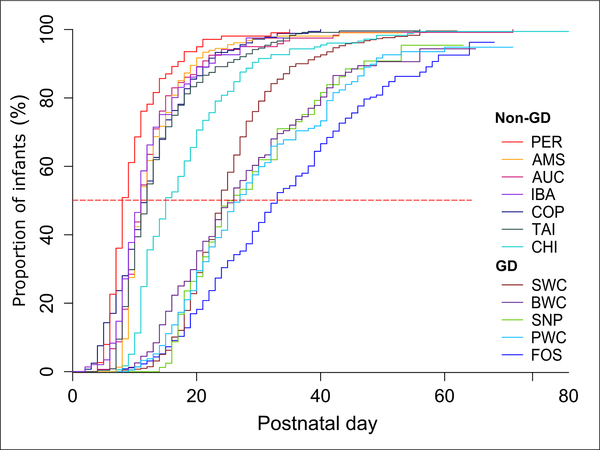
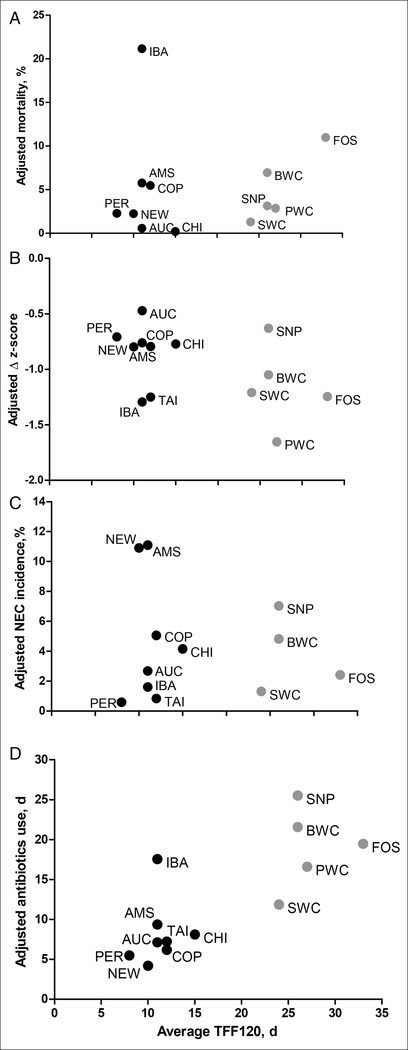
Among the clinical outcomes (Table 2), mortality (1%–18% across all NICUs) was similar between GD and non-GD units (5% vs 7%), with or without adjustment for GA. The frequency and duration of intubated ventilation were less in GD units (43% vs 51% and 2 vs 6 days, respectively; both P < .001), but the significances disappeared after GA adjustment. The median age for introduction of enteral feeding was older in GD vs non-GD units (3 vs 2 days; P < .001), both with and without GA adjustment. TFF120 and TFF150 were 26 and 34 days, respectively, compared with 11 and 15 days for the non-GD units (both P < .001; Table 2). Figure 2 shows TFF120 in relation to postnatal days for all units, and the median TFF120 and TFF150 were 8–33 and 11–47 days, respectively (Table 2). In Figure 3, TFF120 for the individual units are depicted together with 4 key clinical outcomes adjusted for the differences in GA among infants (mortality, growth rate, NEC incidence, antibiotics use).
Figure 2.
Proportion of infants reaching enteral feeding volumes of 120 mL/kg/day relative to infant age in South China units (GD) and other units (non-GD) around the world. AMS, Amsterdam UMC, Vrije Universiteit, Emma Children’s Hospital; AUC, Newborn Service, National Women’s Health, Auckland; BWC, Shenzhen Bao’an Maternal and Child Health Hospital; CHI, Rush University Children’s Hospital, Chicago; COP, Rigshospitalet, Copenhagen; FOS, Foshan Woman and Children’s Hospital; GD, Guangdong province in China; IBA, University College Hospital, Ibadan; PER, King Edward Memorial Hospital, Perth; PWC, Guangdong Women and Children Hospital; SNP, Shenzhen Nanshan People’s Hospital; SWC, Shenzhen Maternity & Child Health Care Hospital; TAI, Children’s Hospital of China Medical University, Taichung.
Figure 3.
Association between time to full feeding (median days to 120 mL/kg/day) and gestation age-corrected mortality (A), weight Δz-scores at 0–28 days (B), necrotizing enterocolitis incidence (C), and days receiving antibiotics (D) in South China (Guangdong) hospitals (gray symbols) and other hospitals around the world (black symbols). AMS, Amsterdam UMC, Vrije Universiteit, Emma Children’s Hospital; AUC, Newborn Service, National Women’s Health, Auckland; BWC, Shenzhen Bao’an Maternal and Child Health Hospital; CHI, Rush University Children’s Hospital, Chicago; COP, Rigshospitalet, Copenhagen; FOS, Foshan Woman and Children’s Hospital; IBA, University College Hospital, Ibadan; NEW, Newcastle Hospitals NHS Foundation Trust; PER, King Edward Memorial Hospital, Perth; PWC, Guangdong Women and Children Hospital; SNP, Shenzhen Nanshan People’s Hospital; SWC, Shenzhen Maternity & Child Health Care Hospital; TAI, Children’s Hospital of China Medical University, Taichung; TFF120, time to reach enteral feeding volumes of 120 mL/kg/day.
PN was administered for a longer time in GD vs non- GD units, with or without GA adjustment (25 vs 13 days; P < .001; Table 2). Growth velocity for weight was 5.0–14.6 g/kg/day (0–28 days) and Δz-scores −0.54 to −1.64, and both variables were lower in GD vs non-GD hospitals (8.7 vs 10.9 g/kg/day and −1.23 vs −0.91, respectively), with or without GA adjustment (Table 2). Growth velocity and z-score change for length did not differ between GD and non-GD units. The z-score for head circumference decreased less in GD vs non-GD units, but the difference disappeared after GA adjustment (Table 2).
Antibiotics were used more in GD units compared with non-GD units (99% vs 92% and 17 vs 11 days; both P < .001; Table 2). The incidence of NEC varied widely among hospitals (1%–13%; Table 2), with the mean day and PCA at onset being 8–27 days and 28.4–33.6 weeks, respectively. NEC rates tended to be lower in GD vs non-GD hospitals (4% vs 6%; P = .009), with fewer severe NEC cases (Bell score II/III, 39/9 vs 47/33; P = .007), earlier age at onset (13 vs 21 days; P < .001), and fewer NEC surgeries (16% vs 42%; P = .001). However, these differences became insignificant when adjusted for GA (P2-values in Table 2). The first stool was passed earlier in GD units with or without adjustment for GA (2 vs 3 days in non-GD units; P < .001).
There was considerable heterogeneity in demographic and outcome data among the 8 non-GD units that practiced early and fast progression of human milk feeding (Figure 1, Tables 1 and 2). Particularly, Ibadan (n = 150, Africa) tended to differ from the others (n = 1431 from mainly Western countries) with a higher mortality rate (18% vs 6%), GA (30.5 vs 28.8 weeks), and BW (1223 vs 1072 g); more SGA infants (27% vs 18%); and fewer singletons (55% vs 71%), caesarean deliveries (44% vs 64%), antenatal steroid treatments (31% vs 89%), infants provided intubated respiratory support (4% vs 56%), and parenteral amino acids (51% vs 94%; all P < .01; Tables 1 and 2).
Discussion
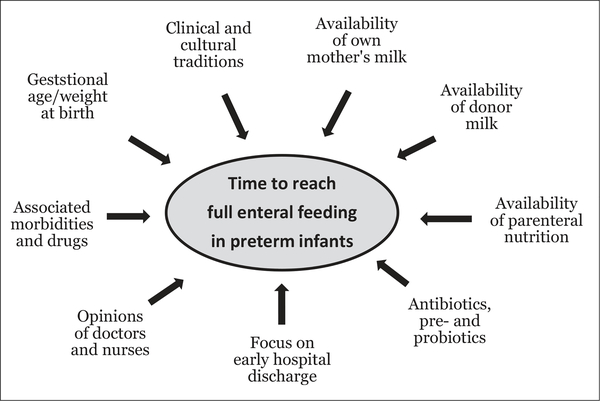
Our study demonstrates marked differences in nutrition practices and short-term clinical outcomes for hospitalized VLBW infants around the world. Specifically, the predominant use of formula, and a long TFF for the 5 NICUs in South China, was associated with more use of antibiotics and less weight gain during the first 4 weeks of life, but this apparently did not affect the in-hospital NEC or mortality rates, compared with the remaining units. Although the TFF depends on the infant’s clinical conditions, the significant variability among NICUs demonstrates that many other factors contribute to the chosen nutrition practice. These include availability of human milk and PN, physical infrastructure of NICUs, clinical and cultural traditions, and the perception of doctors and nurses toward signs of feeding intolerance and NEC. Thus, a combination of infant- and hospital-related factors determines the time to full enteral feeding in NICUs (Figure 4). It remains important to investigate whether these differences for in-hospital feeding regimens and short-term clinical outcomes have long-term effects on infant health and development.
Figure 4.
Many different factors influence the time taken to reach full enteral feeding in preterm infants. These factors are related to the infants, but also to the parents, clinical staff, and hospital conditions.
The more conservative approach to volume advancement in South China resulted in a TFF that was more than twice of that in other hospitals. Although longer PN was used to compensate for slow enteral feeding advancement, a reduced weight gain was observed in GD units, reflecting the difficulties in achieving adequate growth during the first 4 weeks using mainly PN. Importantly, this was associated with 50% more days receiving antibiotics, probably because of an increased risk for infections from indwelling catheters for PN or differences in clinical guidelines. Differences in use of antenatal steroids and respiratory support might also have influenced both TFF and NEC rates. Unknown confounders, such as hospital-specific gut colonization, genetics, differences in diagnostic criteria (Bell scoring), and access to surgery for NEC may also contribute to differences among units. Further, we did not know the NEC incidence for infants discharged early to step-down units or to home after parental request. Consequently, the differences in NEC incidences must be interpreted with caution.
Formula feeding has been associated with a higher risk for feeding interruption because of emesis, abdominal distension, bloody stools, or suspicion of NEC,27 and increased NEC rates.28 Consequently, predominant use of formula feeding may have contributed to the more conservative enteral feeding in the GD units. Moreover, clinical features of feeding intolerance, used as early signs of NEC, are nonspecific and vary widely among units and clinicians. This may lead to unjustified delay in enteral feeding, regardless of diet.29 More research is required to evaluate and predict the clinical consequences of the variable symptoms of feeding intolerance. Nevertheless, it may be important to alter feeding advancement rate and criteria for withholding feeding according to the availability of human milk. However, our data cannot be used to provide direct evidence to support specific diet and feeding regimens, because this would require large randomized clinical trials (RCTs).
The advantages of MM for preterm infants are well-known, leading health authorities around the world to strongly recommend the use of MM for VLBW infants.16,30 Unfortunately, not all mothers can provide their infants with sufficient amounts of MM, especially when initiation of lactation is delayed after preterm birth.31,32 It is difficult to increase the availability of human milk if the hospital infrastructure does not allow cohospitalization of mothers, lactation support, or DM banking. The African NICU (Ibadan) had a relatively high mortality rate, despite early feeding with MM, which may be related to sepsis22 and more limited use of some clinical treatments (eg, respiratory and PN support). The presence of human milk banks may positively influence breast feeding rates for preterm infants, and several feeding guidelines propose to use DM when MM is not available or insufficient.33,34 The number of countries with DM banks has increased,35,36 and in the 3 non-GD units with access to DM, TFF120 was reached already after 8–11 days. At 2 of these units, enteral feeding started on the first day after birth, compared with days 2–4 in units without access to DM. Delaying feeding introduction until MM becomes available is common (rather than starting on IF), especially for the smallest infants, but limited scientific evidence is available.14,17 Delayed start of enteral feeding often reflects a concern for NEC, but there is also a risk that enteral fasting after birth will delay intestinal maturation, induce mucosal atrophy, and reduce the digestive capacity.4 In a recent randomized trial, there was no difference in NEC and sepsis rates between preterm infants who received DM or IF as supplement to MM for the first 10 days after birth.20 Intestinal maturation may not be optimal unless the mother’s own colostrum is given as the first feed.37 Interestingly, bovine colostrum, given as a supplement to MM during the first 14 days, reduced TFF in 2 GD units in a recent pilot trial.38
Our study has several strengths and limitations. A major strength is the wide geographic variation in the participating NICUs with a large number of infants, which enabled a comparison of nutrition practices across several continents. Limitations include that the design was primarily retrospective, with some missing data and potentially important unmeasured confounders. Clinical outcomes (eg, NEC, mortality) were unclear for infants subjected to early discharge on parental request in GD units and discharged to step-down units in European units. In some GD units, parents asked for early discharge if their infants were stable before reaching the required BW for discharge (ie, 2000 g). Other reasons for discharge on parental request may include economic or social limitations, leading to giving up further treatments or transferring to another hospital near home. Finally, it is important to note that participating NICUs in this study may not be representative of those in their respective countries or continents, and clinical routines may be changing rapidly in newly urbanized regions, like South China. Different hospital settings and infant demographics and genetics may have a different balance of risks and benefits for selected treatments. Thus, trials performed in 1 hospital or region cannot always be translated to other settings.
In summary, nutrition practices and clinical outcomes varied markedly among NICUs worldwide. Much longer time to full enteral feeding, more use of formula feeding and antibiotics, prolonged use of PN, and lower weight gain were observed in NICUs in the GD region in South China, but this did not appear to be associated with increased risk for in-hospital NEC or death. It may be important to vary nutrition strategies (eg, advancement rate, criteria for withholding feeding) according to different NICU settings (eg, availability of human milk and PN, access to surgery for NEC). Large RCTs are required to identify the optimal nutrition strategies for VLBW preterm infants, and our data provide valuable information for planning such trials. The results have contributed to the design of an ongoing large randomized trial comparing supplemental bovine colostrum with IF during the first 14 days on TFF in some GD hospitals (ClinicalTrials.gov identifier: NCT03085277). It is also relevant, based on the present observational results, to investigate in RCTs how slow vs fast feeding advancement rate, different feeding intolerance assessments, and time and duration of antibiotics use may affect clinical outcomes, especially in hospitals with limited access to human milk. However, clinical routines for VLBW infants are changing rapidly (eg, use of human milk and antibiotics), both in China and in many other areas of the world. Thus, it may soon become relevant in follow-up observational studies to compare the present data from the NEOMUNE-NeoNutriNet database with updated data on clinical routines and outcomes for VLBW infants.
Clinical Relevancy Statement.
Early transition to enteral nutrition, especially using mother’s own milk, is believed to be important for growth, health, and development of preterm infants. Many factors may interact to determine the feeding practices at neonatal intensive care units (NICUs) around the world, including clinical traditions, available milk diets, and structural limitations at individual hospitals. Our cohort study shows that a relatively long time to reach full enteral feeding in 5 NICUs in South China with predominantly infant formula feeding was associated with more frequent use of antibiotics and less weight gain during the first 4 weeks of age, but apparently not with increased risk for necrotizing enterocolitis or death, relative to 8 other NICUs around the world, using faster feeding advancement rates with predominantly human milk. It may be important to vary nutrition strategies (eg, feeding advancement rate, criteria for withhold of feeding) according to different NICU settings (eg, availability of human milk).
Acknowledgments
We thank Britt Andersen, Elena Cester, Lars Christensen, Marisa Duong, Alexander Erichsen, Patricia Halladin, Judy Janes, Maj-Britt Jorgensen, Bridget Little, Yanwei Liu, Elise Mank, Xiaoyu Pan, Hui Hui Phua, Simone Repstock, and Julie Schrøder for their help with the data collection.
Financial disclosure: The study was part of the NEOMUNE project sponsored by the Innovation Fund Denmark (grant 12-132401). Data from 1 neonatal intensive care unit were provided by support from National Health Institute grant NR010009.
Footnotes
Conflicts of interest: J. B. van Goudoever is the director of the Dutch Human Milk Bank and is a member of the National Health Council. B. E. Cormack serves on scientific advisory boards for Nestlé Nutrition Institute and Danone/Nutricia. K. Simmer is the Director of the Human Milk Bank in Perth and has received support from Medela and Nestlé Nutrition Institute. P. T. Sangild has received grant support from ARLA Foods, Medela, Danone/Nutricia, Biofiber-Damino, Mead Johnson Nutrition, and Nestlé Nutrition Institute. F. H. Bloomfield has received travel support for invited lectures from Abbot Nutrition and Nestlé Nutrition Institute. N. D. Embleton has received speakers’ honoraria from Nestlé Nutrition Institute and Danone/Nutricia, and his department has received research support from Prolacta Bioscience and Danone/Nutricia. The other authors declare no conflicts of interest.
References
- 1.Blencowe H, Cousens S, Chou D, et al. ; Born Too Soon Preterm Birth Action Group. Born too soon: the global epidemiology of 15 million preterm births. Reprod Health. 2013;10(suppl 1):S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neu J Gastrointestinal development and meeting the nutritional needs of premature infants. Am J Clin Nutr. 2007;85(2):629S–634S. [DOI] [PubMed] [Google Scholar]
- 3.Embleton NE, Pang N, Cooke RJ. Postnatal malnutrition and growth retardation: an inevitable consequence of current recommendations in preterm infants? Pediatrics. 2001;107(2):270–273. [DOI] [PubMed] [Google Scholar]
- 4.Hay WW Jr. Strategies for feeding the preterm infant. Neonatology. 2008;94(4):245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ong KK, Kennedy K, Castaneda-Gutierrez E, et al. Postnatal growth in preterm infants and later health outcomes: a systematic review. Acta Paediatr. 2015;104(10):974–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aynsley-Green A. Hormones and postnatal adaptation to enteral nutrition. J Pediatr Gastroenterol Nutr. 1983;2(3):418–427. [DOI] [PubMed] [Google Scholar]
- 7.Berseth CL. Neonatal small intestinal motility: motor responses to feeding in term and preterm infants. J Pediatr. 1990;117(5): 777–782. [DOI] [PubMed] [Google Scholar]
- 8.Morgan J, Bombell S, McGuire W. Early trophic feeding versus enteral fasting for very preterm or very low birth weight infants. Cochrane Database Syst Rev. 2013;(3): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morgan J, Young L, McGuire W. Delayed introduction of progressive enteral feeds to prevent necrotising enterocolitis in very low birth weight infants. Cochrane Database Syst Rev. 2014;(12): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morgan J, Young L, McGuire W. Slow advancement of enteral feed volumes to prevent necrotising enterocolitis in very low birth weight infants. Cochrane Database Syst Rev. 2015;(10): [DOI] [PubMed] [Google Scholar]
- 11.Oddie SJ, Young L, McGuire W. Slow advancement of enteral feed volumes to prevent necrotising enterocolitis in very low birth weight infants. Cochrane Database Syst Rev. 2017;(8): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berseth CL, Bisquera JA, Paje VU. Prolonging small feeding volumes early in life decreases the incidence of necrotizing enterocolitis in very low birth weight infants. Pediatrics. 2003;111(3):529–534. [DOI] [PubMed] [Google Scholar]
- 13.Viswanathan S, McNelis K, Super D, Einstadter D, Groh-Wargo S, Collin M. Standardized slow enteral feeding protocol and the incidence of necrotizing enterocolitis in extremely low birth weight infants. JPEN J Parenter Enteral Nutr. 2015;39(6):644–654. [DOI] [PubMed] [Google Scholar]
- 14.Klingenberg C, Embleton ND, Jacobs SE, O’Connell LA, Kuschel CA. Enteral feeding practices in very preterm infants: an international survey. Arch Dis Child Fetal Neonatal Ed. 2012;97(1):F56–F61. [DOI] [PubMed] [Google Scholar]
- 15.Agostoni C, Buonocore G, Carnielli VP, et al. Enteral nutrient supply for preterm infants: commentary from the European Society of Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J Pediatr Gastroenterol Nutr. 2010;50(1):85–91. [DOI] [PubMed] [Google Scholar]
- 16.Working Group of Pediatrics Chinese Society of Parenteral and Enteral Nutrition, Working Group of Neonatology Chinese Society of Pediarics, Working Group of Neonatal Surgery Chinese Society of Pediatric Surgery. CSPEN guidelines for nutrition support in neonates. Asia Pac J Clin Nutr. 2013;22(4):655–663. [DOI] [PubMed] [Google Scholar]
- 17.Salas AA, Li P, Parks K, Lal CV, Martin CR, Carlo WA. Early progressive feeding in extremely preterm infants: a randomized trial. Am J Clin Nutr. 2018;107(3):365–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hylander MA, Strobino DM, Dhanireddy R. Human milk feedings and infection among very low birth weight infants. Pediatrics. 1998;102(3):E38. [DOI] [PubMed] [Google Scholar]
- 19.Lucas A, Cole TJ. Breast milk and neonatal necrotising enterocolitis. Lancet. 1990;336(8730):1519–1523. [DOI] [PubMed] [Google Scholar]
- 20.Corpeleijn WE, de Waard M, Christmann V, et al. Effect of donor milk on severe infections and mortality in very low-birth-weight infants: the Early Nutrition Study randomized clinical trial. JAMA Pediatr. 2016;170(7):654–661. [DOI] [PubMed] [Google Scholar]
- 21.Cortez J, Makker K, Kraemer DF, Neu J, Sharma R, Hudak ML. Maternal milk feedings reduce sepsis, necrotizing enterocolitis and improve outcomes of premature infants. J Perinatol. 2018;38(1): 71–74. [DOI] [PubMed] [Google Scholar]
- 22.Onwuanaku CA, Okolo SN, Ige KO, Okpe SE, Toma BO. The effects of birth weight and gender on neonatal mortality in north central Nigeria. BMC Res Notes. 2011;4:562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bell MJ, Ternberg JL, Feigin RD, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg. 1978;187(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cormack BE, Embleton ND, van Goudoever JB, Hay WW Jr., Bloomfield FH. Comparing apples with apples: it is time for standardized reporting of neonatal nutrition and growth studies. Pediatric Res. 2016;79(6):810–820. [DOI] [PubMed] [Google Scholar]
- 25.Fenton TR, Kim JH. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013;13:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patel AL, Engstrom JL, Meier PP, Jegier BJ, Kimura RE. Calculating postnatal growth velocity in very low birth weight (VLBW) premature infants. J Perinatol. 2009;29(9):618–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Assad M, Elliott MJ, Abraham JH. Decreased cost and improved feeding tolerance in VLBW infants fed an exclusive human milk diet. J Perinatol. 2016;36(3):216–220. [DOI] [PubMed] [Google Scholar]
- 28.Cristofalo EA, Schanler RJ, Blanco CL, et al. Randomized trial of exclusive human milk versus preterm formula diets in extremely premature infants. J Pediatrics. 2013;163(6):1592–1595. [DOI] [PubMed] [Google Scholar]
- 29.Li YF, Lin HC, Torrazza RM, Parker L, Talaga E, Neu J. Gastric residual evaluation in preterm neonates: a useful monitoring technique or a hindrance? Pediatr Neonatol. 2014;55(5):335–340. [DOI] [PubMed] [Google Scholar]
- 30.ESPGHAN Committee on Nutrition; Agostoni C, Braegger C, et al. Breast-feeding: a commentary by the ESPGHAN Committee on Nutrition. J Pediatr Gastroenterol Nutr. 2009;49(1):112–125. [DOI] [PubMed] [Google Scholar]
- 31.Corpeleijn WE, Kouwenhoven SM, Paap MC, et al. Intake of own mother’s milk during the first days of life is associated with decreased morbidity and mortality in very low birth weight infants during the first 60 days of life. Neonatology. 2012;102(4):276–281. [DOI] [PubMed] [Google Scholar]
- 32.Scott JA, Binns CW, Oddy WH. Predictors of delayed onset of lactation. Matern Child Nutr. 2007;3(3):186–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arslanoglu S, Ziegler EE, Moro GE. Donor human milk in preterm infant feeding: evidence and recommendations. J Perinat Med. 2013;38(4):347–351. [DOI] [PubMed] [Google Scholar]
- 34.Johnston M, Landers S, Noble L, Szucs K, Viehmann L. Breastfeeding and the use of human milk. Pediatrics. 2012;129(3):e827–e841. [DOI] [PubMed] [Google Scholar]
- 35.Ostergaard MV, Cilieborg MS, Skovgaard K, Schmidt M, Sangild PT, Bering SB. Preterm birth reduces nutrient absorption with limited effect on immune gene expression and gut colonization in pigs. J Pediatr Gastroenterol Nutr. 2015;61(4):481–490. [DOI] [PubMed] [Google Scholar]
- 36.Nguyen DN, Jiang P, Jacobsen S, Sangild PT, Bendixen E, Chatterton DE. Protective effects of transforming growth factor beta2 in intestinal epithelial cells by regulation of proteins associated with stress and endotoxin responses. PLoS One. 2015;10(2):e0117608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meier PP, Engstrom JL, Patel AL, Jegier BJ, Bruns NE. Improving the use of human milk during and after the NICU stay. Clin Perinatol. 2010;37(1):217–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Juhl SM, Ye X, Zhou P, et al. Bovine colostrum for preterm infants in the first days of life: a randomized controlled pilot trial. J Pediatr Gastroenterol Nutr. 2018;66(3):471–478. [DOI] [PubMed] [Google Scholar]