Abstract
Substance use disorders are global health problems with few effective treatment options. Unfortunately, most potential pharmacological treatments are hindered by abuse potential of their own, limited efficacy, or adverse side effects. As a consequence, there is a pressing need for the development of addiction treatments with limited abuse potential and fewer off target effects. Given the difficulties in developing new pharmacotherapies for substance use disorders, there has been growing interest in medications that act on non-traditional targets. Recent evidence suggests a role for dysregulated immune signaling in the pathophysiology of multiple psychiatric diseases. While there is evidence that immune responses in the periphery and the central nervous system are altered by exposure to drugs of abuse, the contributions of neuroimmune interactions to addictive behaviors are just beginning to be appreciated. In this review, we discuss the data on immunological changes seen in clinical populations with substance use disorders, as well as in translational animal models of addiction. Importantly, we highlight those mechanistic findings showing causal roles for central or peripheral immune mediators in substance use disorder and appropriate animal models. Based on the literature reviewed here, it is clear that brain-immune system interactions in substance use disorders are much more complex and important than previously understood. While much work remains to be done, there are tremendous potential therapeutic implications for immunomodulatory treatments in substance use disorders.
Keywords: addiction, immune effects of drugs of abuse, inflammation, microbiome
Introduction
Substance use disorders affect millions of individuals in the United States and around the world. The consequences of illicit drug use, including death from overdose (Rudd et al., 2016), the spread of infectious disease (Alcabes & Friedland, 1995), and shorter life expectancies (Smyth et al., 2006) all contribute to the tremendous cost of addiction and create significant social and economic burden for users, their families, and society at large (Patel et al., 2016; Jiang et al., 2017). While overall illicit drug use rates have remained relatively steady, use of certain drugs of abuse, including opioids, has been on the rise and rates of pathological drug use remain dangerously high (Substance Abuse and Mental Health Services Administration, 2008).
Despite the dangers associated with addiction, few pharmacological treatments are available for substance use disorders and these treatments often have limited efficacy. Varenicline and bupropion have been approved for nicotine use disorder (Gómez-Coronado et al., 2018), while naltrexone, acamprosate, and disulfiram (Soyka & Müller, 2017) are approved for alcohol use disorder. For opioid use disorder the primary means of treatment is with opioid replacement therapies such as methadone or buprenorphine. While opioid replacement therapy is often effective as a means of risk reduction, there are myriad associated adverse effects and potential complications (Mattick et al., 2014; Schuckit, 2016). For psychostimulant use disorders there are currently no FDA-approved pharmacotherapies. Agonist therapies targeting dopaminergic systems have been heavily investigated for treatment of psychostimulant use disorders, however, the potential for significant abuse potential and side effects have limited their use (Castells et al., 2016). Similarly, antipsychotic and/or antidepressant medications have been tested for multiple substance use disorders, but these trials have been hampered by limited efficacy and high rates of undesirable side-effects including sexual dysfunction and weight gain (Tollefson, 1991; Solmi et al., 2017). Given the negative health consequences and economic burden ascribed to addiction, there is clearly a pressing need for more effective treatment options with fewer side effects.
In recent years, there has been a growing understanding that interactions of the immune system and the central nervous system are likely playing causal roles in the pathophysiology of multiple psychiatric illnesses. As a result of observational clinical studies and translational animal studies, we are moving closer to understanding the mechanistic links between immune system dysregulation and psychiatric pathology. These findings have led to a recent uptick in clinical trials investigating immune or inflammatory treatment targets in psychiatric disease. While the role of inflammatory processes is better understood in other psychiatric conditions, such as depression and schizophrenia (Hodes et al., 2015; Prata et al., 2017), recent work has identified neuroimmune mechanisms as possibly important in addictive disorders as well. Here, we will present a brief overview of the literature delineating the role of neuroimmune processes in psychiatric pathology and will then discuss clinical and translational evidence for these processes in illicit substance use disorders. For the purposes of this review we will focus primarily on psychostimulants and opioids as these are the illicit substances that account for the greatest levels of morbidity and mortality. The role of the immune system in alcoholism has been reviewed thoroughly elsewhere (Mayfield et al., 2013; Montesinos et al., 2016; Crews et al., 2017). Thus, this review will provide a perspective on the clinical and basic science literature of inflammation in addictive disorders with the goal of highlighting promising translational strategies that have potential for rapid clinical translation.
Inflammation and the brain
The immune system is the major regulator of inflammatory processes and is composed of the innate and adaptive immune systems. The innate system contains cells of the myeloid lineage, including granulocytes, monocytes, macrophages and natural killer cells (Galli et al., 2011; Montaldo et al., 2014) while the adaptive immune system is largely composed of T and B lymphocytes (den Haan et al., 2014). The innate immune system is available to mount rapid inflammatory response to either sterile or microbial injuries. This occurs primarily via pattern recognition receptors (PRR) that detect the presence of foreign entities via pathogen-associated molecular patterns (PAMPs) and endogenous danger-associated molecular patterns (DAMPs) that come from damaged cells (Frank et al., 2015; Portou et al., 2015). The response to DAMPs and PAMPs in the central nervous system (CNS) is primarily mediated by tissue resident macrophages called microglia (Salter & Stevens, 2017), although astrocytes have also been shown to contribute to immune responses (Bylicky et al., 2018). Upon activation of PRRs, these cells produce and release cytokines, which have myriad effects. Pro-inflammatory cytokines (e.g., IL-1b & tumor necrosis factor-alpha, TNF-a) are necessary to launch appropriate immune responses to pathogens or injury and aid in the proliferation and recruitment of phagocytic cells at the site of insult (Shi & Pamer, 2011; Zhang & Wang, 2014). Conversely, anti-inflammatory cytokines (e.g., IL-10) are associated with tissue repair and help maintain normal tissue homeostasis (Khanna et al., 2010; Ferrante & Leibovich, 2012). To properly respond to pathogen exposure and tissue damage, organisms must have a balanced pro- to anti-inflammatory profile. The inability to mobilize and recruit macrophages to a site of injury or infection, present when pro-inflammatory cytokines are low, can impair an organism’s ability to fight invading pathogens. However, prolonged inflammation, present when pro-inflammatory cytokines are high, can cause tissue damage and this pro-inflammatory state significantly contributes to several chronic diseases, including psychiatric pathologies (Dinarello, 2011; Hodes et al., 2015).
Inflammation and addiction
The concept of immune system derangements in psychiatric conditions has been around for many years, but recently has begun to gain more attention both for its role in the pathophysiology of mental illness and also as a potential therapeutic strategy (Hodes et al., 2015; Miller & Goldsmith, 2017). Indeed, inflammatory changes have been seen in multiple neuropsychiatric conditions such as autism spectrum disorder (Vargas et al., 2005), bipolar disorder (Sayana et al., 2017), Alzheimer’s disease (Salter & Stevens, 2017), and schizophrenia (Ripke et al., 2014; Wray & Sullivan, 2017). Research into the role of neuroimmune interactions in substance use disorders has been more limited than in the psychiatric conditions described above. Despite this, it has been known for some time that neuroimmune interactions can lead to important changes in brain systems that are key to the development of substance use disorders. In particular, there is a wealth of literature demonstrating that inflammatory changes can affect dopamine signaling within the brain. Healthy controls given a typhoid vaccination showed increased activation of their substantia nigra by fMRI, a change that correlated with increased serum levels of IL-6 seen after the vaccine (Brydon et al., 2008). Similar effects have been reported in patients receiving infusions of the interferon-a (IFN-a) pro-inflammatory cytokine (Capuron et al., 2012). It was previously shown that inflammatory conditions are capable of decreasing dopamine synthesis by affecting important cofactors and may even affect dopamine transporter function. This literature linking inflammation with altered dopaminergic signaling is reviewed in much more detail elsewhere (Felger & Miller, 2012; Felger & Treadway, 2017). Given that drugs of abuse all work via increasing mesolimbic dopamine release (Chao & Nestler, 2004), the possibility of inflammation and drug interactions to heighten these effects is a real possibility.
Inflammation has also been found to affect glutamatergic signaling, which is another key neurotransmitter system in drug addiction and relapse (Dong et al., 2017). High levels of peripheral inflammation in patients with major depressive disorder are correlated with decreased functional connectivity of the glutamatergic projection neurons between the frontal cortex and ventral striatum (Felger et al., 2016). Also, pro-inflammatory cytokines downregulate glutamate transporters at the synapse thus disrupting the homeostasis of glutamate signaling in the circuit (Tilleux & Hermans, 2007; Felger & Treadway, 2017). Work in animal models of addiction has also shown that glutamate transporters are downregulated in cocaine treated animals, and that this increase in synaptic glutamate can contribute to relapse behaviors (Moussawi et al., 2009; Scofield & Kalivas, 2014). Electrophysiological studies have demonstrated that the TNF-α cytokine is an important regulator of synaptic strength in striatal medium spiny neurons (Lewitus et al., 2014). Given the importance of corticostriatal signaling in both drug reward and relapse to drug seeking, the effects of inflammation on this circuit could have major implications for the development and persistence of substance use disorders.
Inflammation and psychostimulants
Psychostimulants such as cocaine and amphetamines are highly addictive substances that increase levels of dopamine in the CNS and the periphery via inhibition or reversal of the dopamine transporter (Kalivas et al., 2005). Many populations of leukocytes including B cells, T cells, and monocytes are known to express different subtypes of dopamine receptor, and stimulation with dopamine can affect their production of cytokines and other inflammatory mediators (Arreola et al., 2016). In recent years, there is additional evidence that cocaine itself may engage PRRs and create an inflammatory response independent of its effects on dopamine (Northcutt et al., 2015). Cocaine is well established as a factor that increases HIV replication rates in infected microglia and macrophages (Webber et al., 1999; Tyagi et al., 2016). Stimulants also lead to activation of the hypothalamic-pituitary-adrenal axis leading to the release of cortisol which has acutely anti-inflammatory effects and chronically pro-inflammatory effects (Manetti et al., 2014). While stimulants can affect immune function via multiple mechanisms, the role of these inflammatory changes in the development and persistence of addictive behaviors is a matter of ongoing research.
Clinical findings
There are a number of studies that have examined the effects of cocaine on peripheral expression of inflammatory mediators (Summarized in Table 1), including studies showing that acute cocaine alters cytokine expression in serum or in isolated peripheral leukocytes. In abstinent users that there are decreases in serum levels of the chemokine monocyte chemoattractant protein 1 (MCP-1) and a number of pro-inflammatory cytokines including IL-6, IL-17, and TNF-a among others (Pedraz et al., 2015; Maza-Quiroga et al., 2017; Gupta et al., 2018). Of note, chemokines are frequently discussed using two different nomenclatures, which can lead to confusion. In an attempt to simplify, we have provided a side-by-side comparison of the naming structure of all chemokines in this review in Table 2. Intravenous infusion of cocaine suppressed expression of serum IL-6 for four hours in healthy controls (Halpern et al., 2003). This is corroborated by a finding that isolated monocytes from patients who are actively using cocaine have a decreased IL-6 and TNF-a pro-inflammatory response to a bacterial antigen challenge, an effect that was made more pronounced by acute infusion of cocaine (Irwin et al., 2007). However, not all studies show this same pattern of decreased inflammatory markers. More recently studies have shown that active cocaine users express higher levels of IL-6 and decreased levels of the anti-inflammatory cytokine IL-10 (Levandowski et al., 2016; Moreira et al., 2016). Of particular interest, recent studies have shown that patients with psychostimulant use disorders exhibit increased serum expression of pro-inflam-matory markers in response to drug cues (Fox et al., 2012) or in response to unpleasant images (Ersche et al., 2014) – an effect not seen in healthy controls. These studies suggest that while the immune system of drug users may be inhibited in certain situations it may also be primed to respond to certain environmental stimuli in a pro-inflammatory manner. While far from conclusive, it is possible that these changes in peripheral immune function may be playing a role in the development or persistence of psychostimulant use disorders.
Table 1.
Cytokines regulated by cocaine or opioids
| Drug | Species | Target | Direction | Location | Administration | Citation |
|---|---|---|---|---|---|---|
| Cocaine | Animal | G-CSF | ↑ | Peripheral/Central | Repeated | Calipari et al. (2018) |
| Cocaine | Human | IL-10 | ↓ | Peripheral | Active users | Moreira et al. (2016) |
| Cocaine | Human | IL-17 | ↓ | Peripheral | Abstinence | Maza-Quiroga et al., (2017) |
| Cocaine | Human | IL-6 | ↓ | Peripheral | Acute | Halpern et al., (2003) |
| Abstinence | Pedraz et al., (2015) | |||||
| Cocaine | Human | IL-6 | ↑ | Peripheral | Active users | Levandowski et al., (2016) |
| ↓ | Moreira et al., (2016) | |||||
| Cocaine | Human | MCP-1 | Peripheral | Acute | Gupta et al., (2018) | |
| Abstinence | Pedraz et al., (2015) | |||||
| Cocaine | Human | MIP-1α | ↓ | Peripheral | Abstinence | Maza-Quiroga et al., (2017) |
| Cocaine | Animal | SDF-1 | ↑ | Peripheral | Repeated | Araos et al., (2015) |
| Cocaine | Human | TGF-α | ↓ | Peripheral | Abstinence | Maza-Quiroga et al., 20170 |
| Cocaine | Animal | TNF-α | ↑ | Central | Repeated | Lewitus et al., (2016) |
| Cocaine | Human | TNF-α | ↓ | Peripheral | Abstinence | Pedraz et al., (2015) |
| Opioid | Animal | ECP | ↑ | Central | Acute | Schwarz et al., (2011) |
| Opioid | Animal | Fractalkine | ↑ | Central | Repeated | Hutchinson et al., (2008a) |
| Opioid | Animal | GRO/KC | ↑ | Central | Repeated | Hutchinson et al., (2008a) |
| Opioid | Animal | IFN-γ | ↑ | Peripheral/Central | Acute | Pacifici et al., (2000) |
| Schwarz et al., (2011) | ||||||
| Opioid | Animal | IL-10 | ↑ | Peripheral/Central | Repeated | Hutchinson et al., (2008a) |
| ↑ | Acute | Schwarz et al., (2011) | ||||
| Opioid | Animal | IL-17A | Peripheral | Chronic | Meng et al., (2015) | |
| Opioid | Animal | IL-1β | ↑ | Peripheral/Central | Acute | Eidson et al., (2016) |
| Repeated | Hutchinson et al., (2008a) | |||||
| Chronic | Johnston et al., (2004) | |||||
| Chronic | Kang et al., (2017) | |||||
| Repeated | Raghavendra et al., (2002) | |||||
| Opioid | Animal | IL-2 | ↓ | Peripheral | Acute | Lysle et al., (1993) |
| Opioid | Animal | IL-2 | ↑ | Peripheral | Acute | Pacifici et al., (2000) |
| Opioid | Animal | IL-6 | Peripheral/Central | Repeated | Hutchinson et al., (2008a) | |
| Chronic | Johnston et al., (2004) | |||||
| Chronic | Meng et al., (2015) | |||||
| Repeated | Raghavendra et al., (2002) | |||||
| Chronic | Zhang et al., (2011) | |||||
| Opioid | Animal | MCP-1 | ↑ | Central | Repeated | Hutchinson et al., (2008a) |
| Opioid | Animal | MCP-5 | ↑ | Central | Acute | Schwarz et al., (2011) |
| Opioid | Animal | MIG | ↑ | Central | Acute | Schwarz et al., (2011) |
| Opioid | Animal | MIP-1α | ↑ | Central | Repeated | Hutchinson et al., (2008a,b) |
| Opioid | Animal | MIP-1β | ↑ | Central | Acute | Schwarz et al., (2011) |
| Opioid | Animal | RANTES | ↑ | Central | Repeated | Campbell et al., (2013) |
| Opioid | Animal | TARC | ↑ | Central | Acute | Schwarz et al., (2011) |
| Opioid | Animal | TECK | ↑ | Central | Acute | Schwarz et al., (2011) |
| Opioid | Animal | TNF-α | ↑ | Peripheral/Central | Chronic | Johnston et al., (2004) |
| Repeated | Niwa et al., (2007) | |||||
| Acute | Pacifici et al., (2000) | |||||
| Repeated | Raghavendra et al., (2002) | |||||
| Chronic | Zhang et al., (2011) |
A number of cytokines and chemokines have been shown to be differentially regulated by stimulants or opioids in the periphery or in the CNS of human subjects and animal models. This table outlines the known changes in these factors by substance, location, direction and subjects.
Table 2.
Modern and traditional names of chemokines regulated by cocaine or opioids
| Traditional | Modern |
|---|---|
| MCP-1 (monocyte chemoattractant protein 1) | CCL2 |
| MIP-1α (macrophage inflammatory protein-1 alpha) | CCL3 |
| MIP-1β (macrophage inflammatory protein-1 beta) | CCL4 |
| RANTES (regulated on activation, normal T cell expressed and secreted) | CCL5 |
| MCP-2 (monocyte chemoattractant protein 2) | CCL8 |
| ECP (eosinophil chemotactic protein) | CCL11 |
| MCP-5 (monocyte chemoattractant protein 5) | CCL12 |
| TARC (thymus and activation-regulated chemokine) | CCL17 |
| TECK (thymus-expressed chemokine) | CCL25 |
| GRO/KC (growth-related oncogene/keratinocyte chemoattractant) | CXCL1 |
| MIG (monokine induced by gamma interferon) | CXCL9 |
| SDF-1 (stromal cell-derived factor 1) | CXCL12 |
| Fractalkine | CX3CL1 |
CCL2, chemokine (C-C motif) ligand 2; CCL3, chemokine (C-C motif) ligand 3; CCL4, chemokine (C-C motif) ligand 4; CCL5, chemokine (C-C motif) ligand 5; CCL8, chemokine (C-C motif) ligand 8; CCL11, chemokine (C-C motif) ligand 11; CCL12, chemokine (C-C motif) ligand 12; CCL17, chemokine (C-C motif) ligand 17; CCL25, chemokine (C-C motif) ligand 25; CXCL1, chemokine (C-X-C motif) ligand 1; CXCL9, chemokine (C-X-C motif) ligand 9; CXCL12, chemokine (C-X-C motif) ligand 12; CX3CL1, chemokine (C-X3-C motif) ligand 1.
Given that diverse populations of leukocytes express dopamine receptors, it is entirely possible that changes in peripheral inflammation seen after psychostimulants will not necessarily correlate with central inflammatory processes. However, it is important to note that populations of peripheral monocytes and T cells have been shown to have marked effects on brain and behavior and may even be able to extravasate into the CNS parenchyma (Hodes et al., 2015; Menard et al., 2017). Evidence for central inflammatory mechanisms in human populations with psychostimulant use disorder is some-what sparse. Postmortem examination of the midbrain of cocaine addicts demonstrated an increase in both activated microglia, CD68 + activated macrophages, and decreased number of dopamine cell bodies (Little et al., 2009). This is suggestive of a central pro-inflammatory mechanism engaged by cocaine use, but this finding is subject to confounds from both the lifestyle of the patients as well as the myriad issues with histological analysis of postmortem tissue. More recently there have been two positron emission tomography (PET) studies performed in human subjects with substance use disorder using tracers believed to bind to activated glial cells (Banati, 2002). Subjects with methamphetamine use disorder with 1–12 years of reported abstinence were found to have increased markers of microglial activation, with a negative correlation between time of abstinence and microglial activity (Sekine et al., 2008). A similar study looked at patients with long-term cocaine use disorder who had at least two weeks of abstinence by urine toxicology and found no differences in binding of microglial radiotracers between controls and patients with cocaine use disorder (Narendran et al., 2014). While important, these studies should be viewed with some caution given the very different lengths of abstinence, and that these PET tracers are known to not be fully specific for activated microglia (Cosenza-Nashat et al., 2009).
Animal models
In addition to the clinical studies outline above, there has been a growing body of research investigating the role of neuroinflammatory changes in animal models of psychostimulant use disorder. In particular there has been interest in the role of psychostimulants and activating microglia. There is also considerable evidence for psychostimulant activation of astrocytes and other glial cells reviewed more extensively by Lacagnina et al., (2017b). Methamphetamine has been repeatedly shown to induce microglial activation throughout the brain in a dose-dependent manner (Thanos et al., 2016). Studies have found that inhibition of microglial activation via the anti-inflammatory antibiotic minocycline, or via the phosphodiesterase inhibitor and antagonist of the toll-like receptor 4 (TLR-4) ibudilast reduce the rewarding properties of stimulants (Chen et al., 2009; Snider et al., 2013; Attarzadeh-Yazdi et al., 2014). While the exact mechanism of microglial activation by psychostimulants is not fully clear, work is ongoing to make this determination.
Recently, there has been growing evidence that cocaine in particular may bind to TLR4, a PRR expressed on microglia in the CNS (Zhang et al., 2014). Northcutt et al., (2015) did in silico and in vitro modeling to determine that cocaine binds the TLR4 receptor. Additionally, they found that signaling through the TLR4 receptor was important for cocaine-induced dopamine release as well as conditioned place preference (CPP) and self-administration of cocaine (Fig. 1). Subsequent studies have shown that TLR4 is necessary for basal synaptic transmission and NMDA-dependent synaptic plasticity in the nucleus accumbens (NAc; Kashima & Grueter, 2017). Another study found that TLR4 activity in the ventral tegmental area (VTA) affects reinstatement of cocaine seeking in a manner dependent on IL-1 signaling (Brown et al., 2018). However, another study from Tanda et al., (2016) disputes the effects of the TLR4 antagonists (+)-naloxone on dopamine release and reports the effects on behavior to be non-specific (Tanda et al., 2016). While the role of TLR4-mediated signaling in models of substance use remains a subject of ongoing research, it does represent a potentially important line of translational research in the neurobiology of addiction.
Fig. 1.
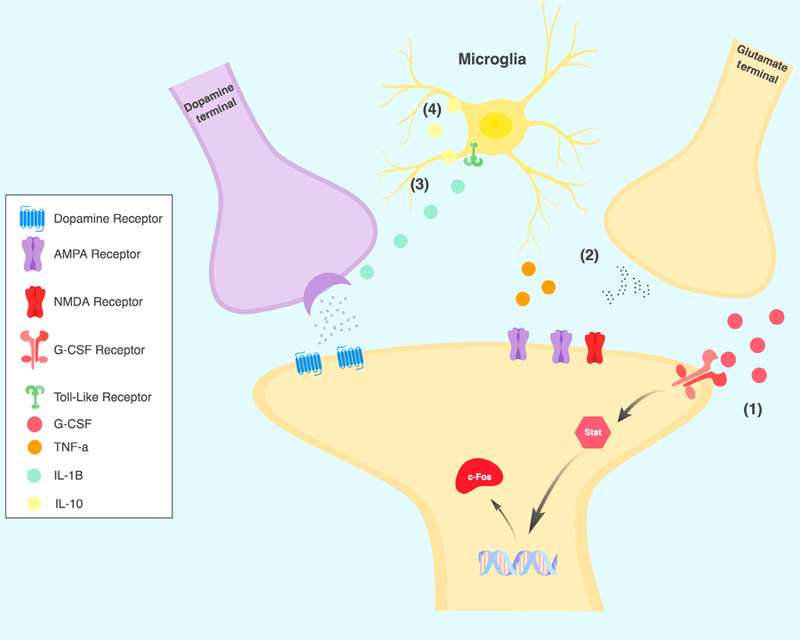
Emerging synaptic mechanisms of inflammation and addiction in the nucleus accumbens. Recent evidence has demonstrated multiple potential mecha-nisms of inflammatory processes affecting synaptic function in models of substance use disorder. (1) Levels of G-CSF are increased by cocaine, and G-CSF enhances neuronal activation and production of immediate early gene c-Fos in response to cocaine. (2) Cocaine and potentially opioids lead to increases in microglial production of TNF-a which inhibit AMPA receptor function in D1-expressing medium spiny neurons. (3) Via direct action on the toll-like receptors cocaine and opioids increase levels of IL-1b, enhancing synaptic dopamine release. (4) The anti-inflammatory cytokine IL-10 is upregulated by opioids in rats with a history of stress. IL-10 inhibits production of pro-inflammatory mediators by toll-like receptors.
In addition to the studies on TLR signaling, there has also been considerable interest in the role of cytokine and chemokine signaling in models of psychostimulant use disorders. These immune mediators are expressed by, and have receptors on, myriad cell types in the brain and can have diverse and complex effects on plasticity and behavior that are only beginning to be understood (Wohleb et al., 2016; Peineau et al., 2018). Recently, our group identified granulocyte colony-stimulating factor (G-CSF) as a cytokine upregulated in both serum and brain after prolonged cocaine administration (Calipari et al., 2018). Interestingly, G-CSF treatment lead to increased neuronal activation in the NAc and prefrontal cortex after an acute cocaine injection. Behaviorally, G-CSF enhanced cocaine CPP, locomotor sensitization and self-administration (Fig. 1) while inhibition of G-CSF signaling in the NAc abolished the formation of cocaine CPP. Others have found a role for canonical pro-inflammatory cytokines in the neuronal and behavioral response to cocaine. Lewitus et al. (2016) demonstrated that microglial production of TNF-a is increased in NAc after cocaine where it inhibits AMPA receptor function specifically in dopamine D1 receptor containing medium spiny neurons (Fig. 1). Additionally, TNF-a knockouts showed increased locomotor sensitization to cocaine and inhibition of TNF-a signaling reduced cocaine sensitization. While the paper from Lewitus and colleagues found that TNF-a seemed to be inhibitory to the behavioral and neurophysiologic sequelae of cocaine, the above mentioned paper found that signaling via TLR-4 via IL-1b enhanced behavioral response to cocaine and was necessary for cocaine-induced dopamine release into the NAc (Northcutt et al., 2015). Given that both TNF-a and IL-1b are known to be down-stream of the TLR4 receptor, these findings appear somewhat contradictory. While the Lewitus group looked at locomotor sensitization and glutamatergic plasticity in the NAc, the Northcutt group focused primarily on the rewarding effects of cocaine and dopamine release from VTA neurons. This raises the possibility that these two closely-related signaling pathways may have differing effects on various aspects of the response to drugs of abuse, and certainly suggests that a more nuanced understanding of the molecular pathways will be crucial.
In addition to these cytokines, chemokine signaling has also been implicated. Infusions of MCP-1 into the midbrain result in increased locomotor activity and increased striatal dopamine release (Guyon et al., 2009), whereas knockout of MCP-1 receptor C-C chemokine receptor 2 (CCR2) leads to decreased locomotor sensitization to cocaine and decreased activation of the ERK signaling cascade in the striatum (Trocello et al., 2011). Additionally, levels of stromal cell-derived factor 1 (SDF-1) are increased in both humans and mice after prolonged exposure to cocaine (Araos et al., 2015), and intraventricular or VTA infusion of SDF-1 protein potentiates the locomotor response to cocaine (Trecki & Unterwald, 2009).
The microbiome and psychostimulants
A topic of growing interest in the field is in the interactions between the gut microbiome, the immune system and the brain – the so-called gut-immune-brain axis (Fung et al., 2017). While the majority of work looking at the role of the microbiome in neuropsychiatric diseases has focused on affective (Foster et al., 2017), neurodevelopmental (Vuong & Hsiao, 2017), and neurodegenerative (Sampson et al., 2016) disorders, there is growing evidence that alterations in the gut microbiome might be playing a role in the development of addictive disorders via their interactions with the immune system. Recently, Volpe et al. (2014) examined patients with cocaine use disorder and healthy controls with and without HIV and found that cocaine use disorder was an independent predictor of an altered gut microbiome – with users having markedly increased representation of the Bacteroidetes phylum. Additionally, cocaine users had a strong trend toward higher levels of bacterial DNA in their serum, which is suggestive of increased bacterial translocation from the gut that may result in a pro-inflammatory state.
While evidence of interactions between the gut microbiome and psychostimulants has been relatively limited, there is growing evidence from translational studies that the gut microbiome can affect the behavioral response to drugs of abuse, and in turn the microbiome can be altered by psychostimulant treatment. Recently, our group found that knockdown of the gut microbiome with broad spectrum antibiotics altered CPP and locomotor sensitization to cocaine, finding that microbiome depleted animals were more sensitive to the rewarding and sensitizing effects of cocaine at low doses (Kiraly et al., 2016). We also found that depletion of the microbiome affected gene expression in the NAc, resulting in increased levels of brain derived neurotrophic factor (BDNF), D1 and D2 dopamine receptors, and GluR2 AMPA receptors. This corroborated a number of previous reports suggesting that alterations in the gut microbiome affect brain expression of BDNF and dopamine receptors (Bercik et al., 2011; Heijtz et al., 2011; Savignac et al., 2013; Desbonnet et al., 2015). Given the importance of these path-ways in the pathophysiology of addictive behaviors, further investigation is needed to establish causal links between the microbiome and BDNF and dopamine signaling in the brain. While a number of groups are currently investigating the effect of psychostimulant drugs on the composition of the gut microbiome, the only currently published study is one in which rats were injected every other day with methamphetamine. This study finds modest increases in bacterial diversity after methamphetamine with small shifts in families of bacteria (Ning et al., 2017). Additionally, they found a decrease in caecal content of the short chain fatty acid propionate. The production of short chain fatty acids by the gut microbiota has been implicated in the integrity of the blood–brain barrier, and was found to play a key role in the behavioral effects of the microbiota and cocaine in the (2016) Kiraly et al. paper. While there is clearly more work to be done, evidence for gut-immune-brain connection in addiction is mounting.
Inflammation and opioids
Clinical findings
Opioids are used as firstline treatment for many types of acute and chronic pain (Zuckerman & Harris, 1994; Roberts et al., 2012; Mesgarpour et al., 2014) and are often prescribed to individuals suffering from other chronic illnesses with weakened immune systems. Despite their abundant use in pain management, recent evidence suggests that opioids might be impairing recovery due to their interactions with inflammatory pathways. Several clinical studies have noted that opioid abusers have worse overall health and are more susceptible to infectious disease complications including HIV infection (Donahoe & Vlahov, 1998; Risdahl et al., 1998). The potentiated negative health outcomes, resulting from opioid exposure are likely due, in part, to general immunosuppression (Budd, 2006; Sacerdote, 2008). As it pertains to HIV, one study demonstrated that heroin users have lower levels of the retrovirus restriction factors tripartite motif-containing 22 (TRIM22), tripartite motif-containing 5a (TRIM5a), apolipoprotein B mRNA editing enzyme catalytic polypeptide-like 3G (APOBEC3G), and IFN-a, which leads to decreased the ability to combat retroviral infection (Zhu et al., 2017). Additionally, leukocytes and granulocytes exposed to morphine show decreased levels of activation and have impaired chemotactic ability (Perez-Castrillon et al., 1992; Makman et al., 1995; Grimm et al., 1998).
Studies measuring cytokine release in human cells have generated mixed results (Table 1). First, heroin users have higher levels of circulatory inflammatory cytokines such as TNF-a, transforming growth factor a (TGF-a), and IL-8 (Piepenbrink et al., 2016). Conversely, giving morphine to healthy volunteers results in natural killer cells that produce less cytotoxic response compared to pla-cebo-treated volunteers (Yeager et al., 1995). When administered alone, a mu opioid receptor selective agonist increased release of several cytokines including TGF-b1 and regulated on activation, normal T cell expressed and secreted (RANTES) from lymphocytes (Happel et al., 2008) and morphine increased MCP-1 expression in cultured brain cells (Rock et al., 2006). Yet, when opioids are administered concurrently or before toxin exposure, they act to dampen immune cell activation. Cells from heroin abusers have lower levels of pro-inflammatory cytokines such as IL-1b, IL-6, TNF-a, and IFN-c after in vitro stimulation with the bacterial endotoxin lipopolysaccharide (LPS; Meijerink et al., 2015). Similarly, serum from chronic heroin users produced less IFN-c and more IL-10 than healthy controls when stimulated with LPS (Azarang et al., 2007). Cultured human lymphocytes pretreated with morphine decreased production of IFN-c in response to the toxin Concanavilin A (Con-A; Peterson et al., 1987) and dendritic cells produced less IL-10 but more IL-12 in response to LPS after morphine compared to LPS alone (Messmer et al., 2006).
In addition to the possible immunosuppressive effects of opioids and increased risk of chronic illness, chronic use of opioids comes with other undesirable side effects, including tolerance, dependence, and addiction. Opioids produce their rewarding effects centrally by binding to opioid receptors and inhibiting GABA neurons in the VTA, resulting in disinhibition of dopamine neurons (Johnson & North, 1992). Opioids also directly bind to opioid receptors in spinal cord to reduce pain (Yaksh, 1981). While the data discussed above focused on peripheral cytokine release or cytokine production in vitro, changes in cytokine expression within the nervous system might more accurately reflect changes in complex behavior. In the CNS, microglia are known to play important roles in regulating both analgesia and addiction (Miguel-Hidalgo, 2009). These cells, along with astrocytes, are likely to be a source of cytokine upregulation in the brain after opioid exposure due to their general role in modulating inflammation (Sofroniew, 2013; Salter & Stevens, 2017). To this end, glial inhibitors are being investigated for their potential to decrease opioidrelated side effects, namely: addiction and physical dependence (Bachtell et al., 2017). Ibudilast, a glial inhibitor known to decrease TNF-a release (Suzumura et al., 1999), attenuated subjective morphine withdrawal (Cooper et al., 2016) and motivation for low-dose oxycodone in a progressive ratio task conducted on heroin-dependent individuals (Metz et al., 2017).
Animal models
In rodents, morphine produces an upregulation of peripheral IL-1b acute or chronic administration (Pacifici et al., 2000; Wang et al., 2012; Kang et al., 2017), IL-6 after chronic administration (Meng et al., 2015), IL-17A after chronic administration (Meng et al., 2015), IL-10 after acute administration (Schwarz et al., 2011), TNF-a, IL-2, and IFN-c after acute administration (Pacifici et al., 2000). However, two groups have observed decreased IL-2 release from peripheral immune cells after acute and chronic morphine (Lysle et al., 1993; Wang et al., 2007). As seen in humans, opioids impair immune response to infection or injury in rodent models. Animals injected with morphine and infected with pneumonia (Wang et al., 2005), Candida (Szabo et al., 1993), or peripherally injured by caecal ligation (Meng et al., 2015) all have decreased survival rates compared to controls. Rodent peripheral blood mononuclear cells or splenic leukocytes treated with opioids also produce less IFN-c in response to LPS or Con-A applied in vitro (Peterson et al., 1987; Lysle et al., 1993; Fecho et al., 2000; Carrigan et al., 2004). However, morphine in combination with HIV protein Tat-1 potentiates Tat-1-induced IL-6, IL-10, MCP-1, MCP-5, and RANTES (El-Hage et al., 2005).
While only a few studies have measured cytokine expression after opioid exposure using cells from the human nervous system (Rock et al., 2006), considerable work has been done to measure these in rodents. The majority of published work demonstrates upregulation and/or enhanced release of cytokines in CNS preparations, including whole brain sections from areas that regulate reward and analgesic functions such as: periaqueductal grey (Eidson et al., 2016), NAc (Niwa et al., 2007; Schwarz et al., 2011; Zhang et al., 2011), hip-pocampus (Liu et al., 2011), and spinal cord (Hutchinson et al., 2008a). The majority of upregulated cytokines are pro-inflammatory and occur after acute, repeated, and chronic opioid administration, including IL-1b (Raghavendra et al., 2002; Johnston et al., 2004; Hutchinson et al., 2008a; Liu et al., 2011; Eidson et al., 2016), IL-6 (Raghavendra et al., 2002; Johnston et al., 2004; Hutchinson et al., 2008a; Zhang et al., 2011), and TNF-a (Raghavendra et al., 2002; Johnston et al., 2004; Niwa et al., 2007; Hutchinson et al., 2008a; Zhang et al., 2011). However, the anti-inflammatory cyto-kine IL-10 is also upregulated after acute and repeated opioid exposure (Hutchinson et al., 2008a; Schwarz et al., 2011). In addition to the traditional cytokines, several chemokines also show differential expression after opioids. Campbell et al. (2013) measured enhanced RANTES release in cortex and striatum after repeated morphine, while Hutchinson et al. (2008a,b) measured several differentially regulated chemokines in spinal cord: monokine induced by gamma interferon (MIG), eosinophil chemotactic protein (ECP), MCP-1, MCP-5, thymus-expressed chemokine (TECK), thymus and activation regulated chemokine (TARC), macrophage inflammatory protein-1a and b (MIP-1a and b), growth-related oncogene/keratinocyte chemoattractant (GRO/KC), and fractalkine (Hutchinson et al., 2008a; Schwarz et al., 2011). Finally, some studies have observed conflicting results, suggesting that some pro-inflammatory cytokines are downregulated after morphine. One such study measured a reduction in prefrontal cortex IL-1b in BALB/c mice only (Liu et al., 2011). Some discrepancies can be explained by altered timing of cytokine measurements (Pacifici et al., 2000), whether animals went through a withdrawal period (Campbell et al., 2013), and the strain of mouse utilized (Liu et al., 2011), as all these factors affected experimental outcome when they were the subject of study.
As mentioned above, inhibition of microglia or astrocytes has been shown to decrease a variety of opioid side effects, including the development of tolerance (Song & Zhao, 2001; Raghavendra et al., 2003; Hutchinson et al., 2008a; Eidson & Murphy, 2013; Fukagawa et al., 2013), physical dependence (Bland et al., 2009; Hutchinson et al., 2009), morphine reward (Hutchinson et al., 2008b; Zhang et al., 2012), and reinstatement of morphine CPP (Schwarz et al., 2011). IL-1b seems to be a crucial mediator of morphine’s analgesic effects. Inhibition of IL-1 enhanced morphine’s analgesic effect and attenuated tolerance (Johnston et al., 2004; Shavit et al., 2005). Additionally, inhibition of fractalkine and IL-6 also produced a reduction in morphine tolerance (Hutchinson et al., 2008a). The importance of TNF-a is not as clear. Some studies demonstrate that inhibition of TNF-a reduced or reversed morphine tolerance (Hutchinson et al., 2008a; Eidson et al., 2016). However, another study suggested that knockout of TNF-a did not affect acute nociceptive responses or tolerance (Niwa et al., 2007).
Enhanced pro-inflammatory signaling seems to potentiate opioid reward mechanisms. When given IFN-a alone, rats showed potentiated reinstatement of morphine CPP (Lang et al., 2009). However, upregulation of anti-inflammatory processes may counteract inflammatory actions and reduce the rewarding effects of opioids. Overexpression of the anti-inflammatory cytokine IL-10 decreased activation of microglia, reinstatement of morphine CPP, and acquisition of self-administration of the short-acting opioid remifentanil (Schwarz et al., 2011; Lacagnina et al., 2017a).
Morphine and other opioids are known to bind opioid receptors to produce reward, dependence, and analgesia (Matthes et al., 1996); however; there is no consensus about which receptor morphine binds in order to disrupt inflammatory signaling. Recent evidence suggests that (+)- and ( )-isomers of opioids bind TLR4 and opioid receptors (Hutchinson et al., 2010), respectively, and that this stereo-specificity might contribute to conflicting reports on the role of inflammation in morphine-induced behaviors. (+)-Naloxone and (+)-naltrexone are putative antagonists at TLR4 that do not bind opioid receptors (Wang et al., 2016). In behavioral studies both compounds have been shown to reverse morphine tolerance (Eidson & Murphy, 2013) and enhance morphine’s analgesic effect (Wang et al., 2012). More selective pharmacological disruption of TLR4 signaling impaired the development of morphine CPP, self-administration of remifentanil (Hutchinson et al., 2012), and incubation of heroin craving (Theberge et al., 2013). However, TLR4 knockout mice still show enhanced microglial activation in response to morphine (Fukagawa et al., 2013), as well as normal morphine tolerance (Fukagawa et al., 2013; Mattioli et al., 2014) and dependence (Mattioli et al., 2014). Additionally, Tanda et al. (2016) have recently noted that (+)-naloxone and (+)-naltrexone do not affect opioid reward at clinically relevant doses.
The microbiome and opioids
Alterations in the gastrointestinal tract have been noted in human opioid users. In patients with hepatic encephalopathy (HE), opioid use, not degree of liver disease, predicted hospital readmission (Acharya et al., 2017). Opioid users in this cohort displayed significantly different patterns of bacterial populations compared to non-opioid users with HE (Acharya et al., 2017). Recent studies in rodents have confirmed these findings and have identified opioid-induced alterations in the microbiome that impact the behavioral effects of opioids. For example, experimenter-administered morphine increased systemic spread of bacteria through compromised intestinal epithelium (Meng et al., 2015; Banerjee et al., 2016; Kang et al., 2017) and altered the proportion of gram-negative to gram-positive bacteria present in the gastrointestinal tract (Meng et al., 2015; Wang et al., 2018). This disruption in bacterial balance directly contributes to the development of morphine analgesic tolerance. Administration of the gram-positive bacteria Enterococcus faecalus to mice accelerated the development of morphine tolerance (Wang et al., 2018) while oral antibiotics, which reduced systemic bacterial spread, attenuated morphine tolerance (Kang et al., 2017). The effect of morphine on bacterial spread and intestinal compromise are dependent on IL-17A. Treatment with IL-17A antibody increased survival after caecal ligation, decreased bacterial spread, decreased IL-6 production, and restored the intestinal epithelial barrier (Meng et al., 2015).
In conclusion, opioids influence inflammation and immune reactivity in a variety of ways that are purported to affect opioid-related behaviors. Some of these factors include upregulation of pro-inflammatory cytokines after short- or prolonged opioid exposure (Hutchinson et al., 2008a), enhanced activation of microglia (Zhang et al., 2011; Fukagawa et al., 2013), reduction in the immune response to pathogens (Azarang et al., 2007; Meijerink et al., 2015), and disruption of intestinal permeability leading to systemic bacterial spread (Meng et al., 2015). The increase in pro-inflammatory cytokines and enhanced activation of microglia resulting from opioid intake most likely contribute to the negative consequences of opioid use, such as tolerance (Johnston et al., 2004; Shavit et al., 2005), dependence (Bland et al., 2009; Hutchinson et al., 2009), and reward (Hutchinson et al., 2008b; Zhang et al., 2012), although the exact cellular mechanisms have yet to be elucidated.
Conclusions
Based on our review of the literature it is clear that psychostimulants and opioids have robust and complex effects on immune system function. As demonstrated in Table 1, the effects of drugs of abuse on immune system function is highly dependent on timing after administration, chronicity of use, and a number of other factors. In fact, a number of inflammatory markers differ depending on whether they are being assessed peripherally or centrally. Despite the complexity of these interactions, we are beginning to develop more of a mechanistic understanding of the role that inflammatory and immune-related mechanisms may be playing in the development and persistence of substance use disorders.
As diagrammed in Fig. 1, there are now multiple different mechanisms that have begun to be outlined for how drugs of abuse affect specific synaptic functions in animal models of substance use disorders. While there is evidence that all of these pathways can be activated by treatment with drugs of abuse, there is clearly a subtlety to how they affect the neuronal and behavioral response. At this time the evidence suggests that the distinction is not as simples as provs. anti-inflammatory; pro-inflammatory IL-1b drives increased behavioral response to cocaine and opioids (Hutchinson et al., 2012; Northcutt et al., 2015) while pro-inflammatory TNF-α inhibits response to cocaine (Lewitus et al., 2016). Additionally, anti-inflammatory IL-10 reduces behavioral response for opioids (Lacagnina et al., 2017a) while G-CSF, which is primarily anti-inflammatory, enhances behavioral responses to cocaine (Calipari et al., 2018).
The findings outlined in the figure stem primarily from studies during the development of addiction-like behaviors or shortly after drug administration. This raises the interesting discrepancy between the clinical and animal literature in this field, in that the majority of studies on patients are on patients with chronic substance use disorders, often with some period of abstinence, while the majority of animal studies are performed after relatively shorter periods of drug exposure (Table 2). Of the pathways outlined in animals it seems that only the TLR-4 signaling pathway has been examined after abstinence and during reinstatement of drug seeking (Theberge et al., 2013; Brown et al., 2018). Further animal studies that more closely mirror what is seen in the clinical literature will be crucial for identification of potential biomarkers and translational research strategies.
At this time there have been relatively few neuroimmune pathways that have been examined in animal models of both psychostimulant and opioid use (Table 1). However, there may be commonalities in inflammatory cascades that affect behavioral response to different drugs of abuse. Increases in TNF-α inhibit behavioral responding for both psychostimulants (Lewitus et al., 2016) and opioids (Niwa et al., 2007). Conversely, signaling through the IL-1b pathway enhances behavioral response to both cocaine (Northcutt et al., 2015) as well as opioids (Hutchinson et al., 2012). Given the shared changes in neurocircuitry seen in models of drug abuse this is not surprising but will be important for possible clinical translation.
While far from complete, our understanding of how neuroimmune interactions are affecting the pathophysiology of substance use disorders is expanding rapidly. Further study is required to clarify which specific signaling pathways are affected and affect neuronal and behavioral response to drugs at different phases of the addiction cycle – including during relapse/reinstatement. Unraveling the intricacies of this signaling will be crucial for our understanding of the neurobiology of addiction and has great potential to help us understand the possible translational potential of immunetargeted therapies for substance use disorders in the clinical population.
Acknowledgements
This work was supported by NIH grants to SJR (MH104559) and DDK (DA044308) as well as by funds from the Brain and Behavior Research Foundation, the Leon Levy Foundation, and the Seaver Family Foundation all to DDK. The figure was made using BioRender software with full license to publish.
Abbreviations
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- APO-BEC3G
Apolipoprotein B mRNA editing enzyme catalytic polypeptide-like 3G
- BDNF
Brain-derived neurotrophic factor
- CCR2 C-C
chemokine receptor type 2
- CNS
Central nervous system
- Con-A
Concanavilin A
- CPP
Conditioned place preference
- DAMP
Danger-associated molecular pattern
- ECP
Eosinophil chemotactic protein
- ERK
Extracellular-regulated kinase
- fMRI
Functional magnetic resonance imaging
- G-CSF
Granulocyte colony-stimulating factor
- GRO/KC
Growth-related oncogene/keratinocyte chemoattractant
- HE
Hepatic encephalopathy
- IFN-α
Interferon alpha
- IFN-γ
Interferon gamma
- IL-10
Interleukin-10
- IL-12
Interleukin-12
- IL-17
Interleukin-17
- IL-1β
Interleukin-1 beta
- IL-2
Interleukin-2
- IL-6
Inter-leukin-6
- IL-8
Interleukin-8
- LPS
Lipopolysaccharide
- MCP-1
Monocyte chemoattractant protein 1
- MCP-5
Monocyte chemoattractant protein 5
- MDD
Major depressive disorder
- MIG-1
Monokine induced by gamma interferon
- MIP-1α
Macrophage inflammatory protein 1 alpha
- MIP-1β
Macrophage inflammatory protein 1 beta
- NAc
Nucleus accumbens
- NMDA
N-methyl-D-aspartate
- PAMP
Pathogen-associated molecular pattern
- PET
Positron emission tomography
- PRR
Pattern recognition receptor
- RANTES
Regulated on activation, normal T cell expressed and secreted
- SDF-1
Stro-mal cell-derived factor 1
- TARC
Thymus and activation-regulated chemokine
- TECK
Thymus-expressed chemokine
- TGF-α
Transforming growth factor alpha
- TGF-β1
Transforming growth factor beta
- TLR4
Toll-like receptor 4
- TNF-α
Tumor necrosis factor alpha
- TRIM22
Tripartite motif-containing 22
- TRIM5α
Tripartite motif-containing 5α
- VTA
Ventral tegmental area
Footnotes
Conflict of interests
The authors declare no competing interests.
Data accessibility
There are no original data contained in this manuscript.
All peer review communications can be found with the online version of the article.
References
- Acharya C, Betrapally NS, Gillevet PM, Sterling RK, Akbarali H, White MB, Ganapathy D, Fagan A et al. (2017) Chronic opioid use is associated with altered gut microbiota and predicts readmissions in patients with cirrhosis. Aliment. Pharmacol. Ther, 45, 319–331. [DOI] [PubMed] [Google Scholar]
- Alcabes P & Friedland G (1995) Injection drug use and human immunodeficiency virus infection. Clin. Infect. Dis, 20, 1467–1479. [DOI] [PubMed] [Google Scholar]
- Araos P, Pedraz M, Serrano A, Lucena M, Barrios V, Garcıa-Marchena N, Campos-Cloute R, Ruiz JJ et al. (2015) Plasma profile of pro-inflammatory cytokines and chemokines in cocaine users under outpatient treatment: influence of cocaine symptom severity and psychiatric co-morbidity. Addict. Biol, 20, 756–772. [DOI] [PubMed] [Google Scholar]
- Arreola R, Alvarez-Herrera S, Perez-Sanchez G, Becerril-Villanueva E, Cruz-Fuentes C, Flores-Gutierrez EO, Garces-Alvarez ME, de la Cruz-Aguilera DL et al. (2016) Immunomodulatory effects mediated by dopamine. J. Immunol. Res, 2016, 3160486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attarzadeh-Yazdi G, Arezoomandan R & Haghparast A (2014) Minocy-cline, an antibiotic with inhibitory effect on microglial activation, attenu-ates the maintenance and reinstatement of methamphetamine-seeking behavior in rat. Prog. Neuro-Psychopha, 53, 142–148. [DOI] [PubMed] [Google Scholar]
- Azarang A, Mahmoodi M, Rajabalian S, Shekari MA, Nosratabadi J & Rezaei N (2007) T-helper 1 and 2 serum cytokine assay in chronic opioid addicts. Eur. Cytokine Netw, 18, 210–214. [DOI] [PubMed] [Google Scholar]
- Bachtell RK, Jones JD, Heinzerling KG, Beardsley PM & Comer SD (2017) Glial and neuroinflammatory targets for treating substance use disorders. Drug Alcohol Depen, 180, 156–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banati RB (2002) Visualising microglial activation in vivo. Glia, 40, 206–217. [DOI] [PubMed] [Google Scholar]
- Banerjee S, Sindberg G, Wang F, Meng J, Sharma U, Zhang L, Dauer P, Chen C et al. (2016) Opioid-induced gut microbial disruption and bile dysregulation leads to gut barrier compromise and sustained systemic inflammation. Mucosal Immunol, 9, 1418–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bercik P, Denou E, Collins J, Jackson W, Lu J, Jury J, Deng Y, Blennerhassett P et al. (2011) The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology, 141, 599–609. [DOI] [PubMed] [Google Scholar]
- Bland ST, Hutchinson MR, Maier SF, Watkins LR & Johnson KW (2009) The glial activation inhibitor AV411 reduces morphine-induced nucleus accumbens dopamine release. Brain Behav. Immun, 23, 492–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KT, Levis SC, O’Neill CE, Northcutt AL, Fabisiak TJ, Watkins LR & Bachtell RK (2018) Innate immune signaling in the ventral tegmental area contributes to drug-primed reinstatement of cocaine seeking. Brain Behav. Immun, 67, 130–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brydon L, Harrison NA, Walker C, Steptoe A, & Critchley HD (2008). Peripheral inflammation is associated with altered substantia nigra activity and psychomotor slowing in humans. Biol. Psychiatry, 63, 1022–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budd K (2006) Pain management: is opioid immunosuppression a clinical problem? Biomed. Pharmacother, 60, 310–317. [DOI] [PubMed] [Google Scholar]
- Bylicky MA, Mueller GP & Day RM (2018) Mechanisms of endogenous neuroprotective effects of astrocytes in brain injury. Oxid. Med. Cell. Longev, 2018, 6501031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari ES, Godino A, Peck EG, Salery M, Mervosh NL, Landry JA, Russo SJ, Hurd YL et al. (2018) Granulocyte-colony stimulating factor controls neural and behavioral plasticity in response to cocaine. Nat. Commun, 9, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell LA, Avdoshina V, Rozzi S & Mocchetti I (2013) CCL5 and cytokine expression in the rat brain: Differential modulation by chronic morphine and morphine withdrawal. Brain Behav. Immun, 34, 130–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L, Pagnoni G, Drake DF, Woolwine BJ, Spivey JR, Crowe RJ, Votaw JR, Goodman MM, & Miller AH (2012). Dopaminergic mechanisms of reduced basal ganglia responses to hedonic reward during interferon alfa administration. Arch. Gen. Psychiatry, 69, 1044–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrigan KA, Saurer TB, Ijames SG & Lysle DT (2004) Buprenorphine produces naltrexone reversible alterations of immune status. Int. Immunopharmacol, 4, 419–428. [DOI] [PubMed] [Google Scholar]
- Castells X, Cunill R, PerezMaña C, Vidal X & Capella D (2016) Psychostimulant drugs for cocaine dependence. Cochrane Database Syst. Rev, 9, CD007380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao J, & Nestler EJ (2004). Molecular neurobiology of drug addiction. Annu. Rev. Med, 55, 113–132. [DOI] [PubMed] [Google Scholar]
- Chen H, Uz T & Manev H (2009) Minocycline affects cocaine sensitization in mice. Neurosci. Lett, 452, 258–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ZD, Johnson KW, Pavlicova M, Glass A, Vosburg SK, Sullivan MA, Manubay J, Martinez D et al. (2016) The effects of ibudilast, a glial activation inhibitor, on opioid withdrawal symptoms in opioid-dependent volunteers. Addict. Biol, 21, 895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosenza-Nashat M, Zhao M-L, Suh H-S, Morgan J, Natividad R, Morgello S & Lee SC (2009) Expression of the translocator protein of 18 kDa by microglia, macrophages and astrocytes based on immunohisto-chemical localization in abnormal human brain. Neuropathol. Appl. Neurobiol, 35, 306–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Lawrimore CJ, Walter TJ & Coleman LG (2017) The role of neuroimmune signaling in alcoholism. Neuropharmacology, 122, 56–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desbonnet L, Clarke G, Traplin A, O’Sullivan O, Crispie F, Moloney RD, Cotter PD, Dinan TG et al. (2015) Gut microbiota depletion from early adolescence in mice: Implications for brain and behaviour. Brain Behav. Immun, 48, 165–173. [DOI] [PubMed] [Google Scholar]
- Dinarello CA (2011) A clinical perspective of IL-1b as the gatekeeper of inflammation. Eur. J. Immunol, 41, 1203–1217. [DOI] [PubMed] [Google Scholar]
- Donahoe RM & Vlahov D (1998) Opiates as potential cofactors in progression of HIV-1 infections to AIDS. J. Neuroimmunol, 83, 77–87. [DOI] [PubMed] [Google Scholar]
- Dong Y, Taylor JR, Wolf ME, & Shaham Y (2017). Circuit and synaptic plasticity mechanisms of drug relapse. J. Neurosci, 37, 10867–10876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eidson LN & Murphy AZ (2013) Blockade of toll-like receptor 4 attenuates morphine tolerance and facilitates the pain relieving properties of morphine. J. Neurosci, 33, 15952–15963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eidson LN, Inoue K, Young LJ, Tansey MG & Murphy AZ (2016) Toll-like receptor 4 mediates morphine-induced neuroinflammation and tolerance via soluble tumor necrosis factor signaling. Neuropsychopharmacology, 42, 661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hage N, Gurwell JA, Singh IN, Knapp PE, Nath A & Hauser KF (2005) Synergistic increases in intracellular Ca(2+), and the release of MCP-1, RANTES, and IL-6 by astrocytes treated with opiates and HIV-1 Tat. Glia, 50, 91–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Hagan CC, Smith DG, Abbott S, Jones PS, ApergisSchoute AM & Döffinger R (2014) Aberrant disgust responses and immune reactivity in cocainedependent men. Biol. Psychiatry, 75, 140–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fecho K, Nelson CJ & Lysle DT (2000) Phenotypic and functional assessments of immune status in the rat spleen following acute heroin treatment. Immunopharmacology, 46, 193–207. [DOI] [PubMed] [Google Scholar]
- Felger JC, Li Z, Haroon E, Woolwine BJ, Jung MY, Hu X, & Miller AH (2016). Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Molecular Psychiatry, 21, 1358–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, & Miller AH (2012). Cytokine effects on the basal ganglia and dopamine function: the subcortical source of inflammatory malaise. Front Neuroendocrinol, 33, 315–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, & Treadway MT (2017). Inflammation effects on motivation and motor activity: role of dopamine. Neuropsychopharmacology, 42, 216–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrante CJ & Leibovich SJ (2012) Regulation of macrophage polarization and wound healing. Adv. Wound Care, 1, 10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster JA, Rinaman L & Cryan JF (2017) Stress & the gut-brain axis: regulation by the microbiome. Neurobiol. Stress, 7, 124–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, D’Sa C, Kimmerling A, Siedlarz KM, Tuit KL, Stowe R & Sinha R (2012) Immune system inflammation in cocaine dependent individuals: implications for medications development. Hum. Psychopharmacol. Clin. Exp, 27, 156–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Weber MD, Watkins LR & Maier SF (2015) Stress sounds the alarmin: the role of the danger-associated molecular pattern HMGB1 in stress-induced neuroinflammatory priming. Brain Behav. Immun, 48, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukagawa H, Koyama T, Kakuyama M & Fukuda K (2013) Microglial activation involved in morphine tolerance is not mediated by toll-like receptor 4. J. Anesth, 27, 93–97. [DOI] [PubMed] [Google Scholar]
- Fung TC, Olson CA & Hsiao EY (2017) Interactions between the microbiota, immune and nervous systems in health and disease. Nat. Neurosci, 20, 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli SJ, Borregaard N & Wynn TA (2011) Phenotypic and functional plasticity of cells of innate immunity: macrophages, mast cells and neutrophils. Nat. Immunol, 12, 1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Coronado N, Walker AJ, Berk M & Dodd S (2018) Current and emerging pharmacotherapies for cessation of tobacco smoking. Pharmacother. J. Hum. Pharmacol. Drug Ther, 38, 235–258. [DOI] [PubMed] [Google Scholar]
- Grimm MC, Ben-Baruch A, Taub DD, Howard OMZ, Wang JM & Oppenheim JJ (1998) Opiate inhibition of chemokine-induced chemotaxis. Ann. N.Y. Acad. Sci, 840, 9–20. [DOI] [PubMed] [Google Scholar]
- Gupta K, Sharma R, Singh V, Masoomi R, Dileepan KN, He J, Smith DD, Dawn B et al. (2018) Intravenous cocaine results in an acute decrease in levels of biomarkers of vascular inflammation in humans. Cardiovasc. Toxicol, 18, 295–303. [DOI] [PubMed] [Google Scholar]
- Guyon A, Skrzydelski D, De Giry I, Rovere C, Conductier G, Tro-cello JM, Dauge V, Kitabgi P et al. (2009) Long term exposure to the chemokine CCL2 activates the nigrostriatal dopamine system: a novel mechanism for the control of dopamine release. Neuroscience, 162, 1072–1080. [DOI] [PubMed] [Google Scholar]
- den Haan JMM, Arens R & van Zelm MC (2014) The activation of the adaptive immune system: cross-talk between antigen-presenting cells, T cells and B cells. Immunol. Lett, 162, 103–112. [DOI] [PubMed] [Google Scholar]
- Halpern JH, Sholar MB, Glowacki J, Mello NK, Mendelson JH & Siegel AJ (2003) Diminished interleukin-6 response to proinflammatory challenge in men and women after intravenous cocaine administration. J. Clin. Endocrinol. Metab, 88, 1188–1193. [DOI] [PubMed] [Google Scholar]
- Happel C, Steele AD, Finley MJ, Kutzler MA & Rogers TJ (2008) DAMGO-induced expression of chemokines and chemokine receptors: the role of TGF-b1. J. Leukoc. Biol, 83, 956–963. [DOI] [PubMed] [Google Scholar]
- Heijtz RD, Wang S, Anuar F, Qian Y, Björkholm B, Samuelsson A, Hibberd ML, Forssberg H et al. (2011) Normal gut microbiota modulates brain development and behavior. Proc. Natl Acad. Sci. USA, 108, 3047–3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodes GE, Kana V, Menard C, Merad M & Russo SJ (2015) Neuroimmune mechanisms of depression. Nat. Neurosci, 18, 1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Coats BD, Lewis SS, Zhang Y, Sprunger DB, Rezvani N, Baker EM, Jekich BM et al. (2008a) Proinflammatory cytokines oppose opioid-induced acute and chronic analgesia. Brain Behav. Immun, 22, 1178–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Northcutt AL, Chao LW, Kearney JJ, Zhang Y, Berkelhammer DL, Loram LC, Rozeske RR et al. (2008b) Minocycline suppresses morphine-induced respiratory depression, suppresses mor-phine-induced reward, and enhances systemic morphine-induced analgesia. Brain Behav. Immun, 22, 1248–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Lewis SS, Coats BD, Skyba DA, Crysdale NY, Berkelhammer DL, Brzeski A, Northcutt A et al. (2009) Reduction of opioid withdrawal and potentiation of acute opioid analgesia by systemic AV411 (ibudilast). Brain Behav. Immun, 23, 240–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Zhang Y, Shridhar M, Evans JH, Buchanan MM, Zhao TX, Slivka PF, Coats BD et al. (2010) Evidence that opioids may have toll-like receptor 4 and MD-2 effects. Brain Behav. Immun, 24, 83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Northcutt AL, Hiranita T, Wang X, Lewis SS, Thomas J, van Steeg K, Kopajtic TA et al. (2012) Opioid activation of toll-like receptor 4 contributes to drug reinforcement. J. Neurosci, 32, 11187–11200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Olmos L, Wang M, Valladares EM, Motivala SJ, Fong T, Newton T, Butch A et al. (2007) Cocaine dependence and acute cocaine induce decreases of monocyte proinflammatory cytokine expression across the diurnal period: autonomic mechanisms. J. Pharmacol. Exp. Ther, 320, 507–515. [DOI] [PubMed] [Google Scholar]
- Jiang R, Lee I, Lee TA & Pickard AS (2017) The societal cost of heroin use disorder in the United States. PLoS ONE, 12, e0177323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SW & North RA (1992) Opioids excite dopamine neurons by hyperpolarization of local interneurons. J. Neurosci, 12, 483–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston IN, Milligan ED, Wieseler-Frank J, Frank MG, Zapata V, Campisi J, Langer S, Martin D et al. (2004) A role for proinflammatory cytokines and fractalkine in analgesia, tolerance, and subsequent pain facilitation induced by chronic intrathecal morphine. J. Neurosci, 24, 7353–7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Volkow N & Seamans J (2005) Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron, 45, 647–650. [DOI] [PubMed] [Google Scholar]
- Kang M, Mischel RA, Bhave S, Komla E, Cho A, Huang C, Dewey WL & Akbarali HI (2017) The effect of gut microbiome on tolerance to morphine mediated antinociception in mice. Sci. Rep, 7, 42658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashima DT & Grueter BA (2017) Toll-like receptor 4 deficiency alters nucleus accumbens synaptic physiology and drug reward behavior. Proc. Natl Acad. Sci. USA, 114, 8865–8870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna S, Biswas S, Shang Y, Collard E, Azad A, Kauh C, Bhasker V, Gordillo GM et al. (2010) Macrophage dysfunction impairs resolution of inflammation in the wounds of diabetic mice. PLoS ONE, 5, e9539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiraly DD, Walker DM, Calipari ES, Labonte B, Issler O, Pena CJ, Ribeiro EA, Russo SJ et al. (2016) Alterations of the host microbiome affect behavioral responses to cocaine. Sci. Rep, 6, 35455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacagnina MJ, Kopec AM, Cox SS, Hanamsagar R, Wells C, Slade S, Grace PM, Watkins LR et al. (2017a) opioid self-administration is attenuated by early-life experience and Gene therapy for anti-inflammatory IL-10 in the nucleus accumbens of male rats. Neuropsychopharmacology, 42, 2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacagnina MJ, Rivera PD & Bilbo SD (2017b) Glial and neuroimmune mechanisms as critical modulators of drug use and abuse. Neuropsy-chopharmacology, 42, 156–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang J-Y, Wang J-S, Zhai H-F, Fang Q, Wu P & Lu L (2009) Interferon-alpha reinstates morphine-conditioned place preference through opioid receptors in rats. Behav. Pharmacol, 20, 166–173. [DOI] [PubMed] [Google Scholar]
- Levandowski ML, Hess ARB, Grassi-Oliveira R & de Almeida RMM (2016) Plasma interleukin-6 and executive function in crack cocaine-dependent women. Neurosci. Lett, 628, 85–90. [DOI] [PubMed] [Google Scholar]
- Lewitus GM, Pribiag H, Duseja R, St-Hilaire M & Stellwagen D (2014) An adaptive role of TNF in the regulation of striatal synapses. J. Neurosci, 34, 6146–6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewitus GM, Konefal SC, Greenhalgh AD, Pribiag H, Augereau K & Stellwagen D (2016) Microglial TNFa suppresses cocaine-induced plasticity and behavioral sensitization. Neuron, 90, 483–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little KY, Ramssen E, Welchko R, Volberg V, Roland CJ & Cassin B (2009) Decreased brain dopamine cell numbers in human cocaine users. Psychiatry Res, 168, 173–180. [DOI] [PubMed] [Google Scholar]
- Liu L, Coller JK, Watkins LR, Somogyi AA & Hutchinson MR (2011) Naloxone-precipitated morphine withdrawal behavior and brain IL-1b expression: comparison of different mouse strains. Brain Behav. Immun, 25, 1223–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lysle DT, Coussons ME, Watts VJ, Bennett EH & Dykstra LA (1993) Morphine-induced alterations of immune status: dose dependency, compartment specificity and antagonism by naltrexone. J. Pharmacol. Exp. Ther, 265, 1071–1078. [PubMed] [Google Scholar]
- Makman MH, Bilfinger TV & Stefano GB (1995) Human granulocytes contain an opiate alkaloid-selective receptor mediating inhibition of cyto-kine-induced activation and chemotaxis. J. Immunol, 154, 1323–1330. [PubMed] [Google Scholar]
- Manetti L, Cavagnini F, Martino E & Ambrogio A (2014) Effects of cocaine on the hypothalamic–pituitary–adrenal axis. J. Endocrinol. Invest, 37, 701–708. [DOI] [PubMed] [Google Scholar]
- Matthes HWD, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, Befort K, Dierich A et al. (1996) Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the l-opioid-receptor gene. Nature, 383, 819. [DOI] [PubMed] [Google Scholar]
- Mattick RP, Breen C, Kimber J & Davoli M (2014) Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst. Rev, 6, CD002207. [DOI] [PubMed] [Google Scholar]
- Mattioli TA, Leduc-Pessah H, Skelhorne-Gross G, Nicol CJB, Milne B, Trang T & Cahill CM (2014) Toll-like receptor 4 mutant and null mice retain morphine-induced tolerance, hyperalgesia, and physical dependence. PLoS ONE, 9, e97361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayfield J, Ferguson L & Harris RA (2013) Neuroimmune signaling: a key component of alcohol abuse. Curr. Opin. Neurobiol, 23, 513–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maza-Quiroga R, Garcıa-Marchena N, Romero-Sanchiz P, Barrios V, Pedraz M, Serrano A, Nogueira-Arjona R, Ruiz JJ et al. (2017) Evaluation of plasma cytokines in patients with cocaine use disorders in abstinence identifies transforming growth factor alpha (TGFa) as a potential biomarker of consumption and dual diagnosis. PeerJ, 5, e3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijerink H, Indrati A, Utami F, Soedarmo S, Alisjahbana B, Netea MG, van Crevel R, Wisaksana R et al. (2015) Heroin use is associated with suppressed pro-inflammatory cytokine response after LPS exposure in HIV-infected individuals. PLoS ONE, 10, e0122822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menard C, Pfau ML, Hodes GE, Kana V, Wang VX, Bouchard S, Takahashi A, Flanigan ME et al. (2017) Social stress induces neurovascular pathology promoting depression. Nat. Neurosci, 20, 1752–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng J, Banerjee S, Li D, Sindberg GM, Wang F, Ma J & Roy S (2015) Opioid exacerbation of gram-positive sepsis, induced by gut microbial modulation, is rescued by IL-17A neutralization. Sci. Rep, 5, 10918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesgarpour B, Griebler U, Glechner A, Kien C, Strobelberger M, Van Noord MG & Michalek-Sauberer A (2014) Extended-release opioids in the management of cancer pain: a systematic review of efficacy and safety. Eur. J. Pain (United Kingdom), 18, 605–616. [DOI] [PubMed] [Google Scholar]
- Messmer D, Hatsukari I, Hitosugi N, Schmidt-Wolf IGH & Singhal PC (2006) Morphine reciprocally regulates IL-10 and IL-12 production by monocyte-derived human dendritic cells and enhances T cell activation. Mol. Med, 12, 284–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz VE, Jones JD, Manubay J, Sullivan MA, Mogali S, Segoshi A, Madera G, Johnson KW et al. (2017) Effects of ibudilast on the subjective, reinforcing, and analgesic effects of oxycodone in recently detoxified adults with opioid dependence. Neuropsychopharmacology, 42, 1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ (2009) The role of glial cells in drug abuse. Curr. Drug Abuse Rev, 2, 72–82. [PubMed] [Google Scholar]
- Miller BJ, & Goldsmith DR (2017). Towards an immunophenotype of schizophrenia: progress, potential mechanisms, and future directions. Neu-ropsychopharmacology, 42, 299–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montaldo E, Vacca P, Moretta L & Mingari MC (2014) Development of human natural killer cells and other innate lymphoid cells. Semin. Immunol, 26, 107–113. [DOI] [PubMed] [Google Scholar]
- Montesinos J, Alfonso-Loeches S & Guerri C (2016) Impact of the innate immune response in the actions of ethanol on the central nervous system. Alcohol. Clin. Exp. Res, 40, 2260–2270. [DOI] [PubMed] [Google Scholar]
- Moreira FP, Medeiros JRC, Lhullier AC, Souza L.D.de.M., Jansen K, Portela LV, Lara DR, Silva R.A.da. et al. (2016) Cocaine abuse and effects in the serum levels of cytokines IL-6 and IL-10. Drug Alcohol Depen, 158, 181–185. [DOI] [PubMed] [Google Scholar]
- Moussawi K, Pacchioni A, Moran M, Olive MF, Gass JT, Lavin A, & Kalivas PW (2009). N-Acetylcysteine reverses cocaine-induced metaplasticity. Nat. Neurosci, 12, 182–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendran R, Lopresti BJ, Mason NS, Deuitch L, Paris J, Himes ML, Kodavali CV & Nimgaonkar VL (2014) Cocaine abuse in humans is not associated with increased microglial activation: an 18-kDa translocator protein positron emission tomography imaging study with [(11)C]PBR28. J. Neurosci, 34, 9945–9950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ning T, Gong X, Xie L & Ma B (2017) Gut microbiota analysis in rats with methamphetamine-induced conditioned place preference. Front. Microbiol, 8, 1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa M, Nitta A, Yamada Y, Nakajima A, Saito K, Seishima M, Noda Y & Nabeshima T (2007) Tumor necrosis factora and its inducer inhibit morphine-induced rewarding effects and sensitization. Biol. Psychiatry, 62, 658–668. [DOI] [PubMed] [Google Scholar]
- Northcutt AL, Hutchinson MR, Wang X, Baratta MV, Hiranita T, Cochran TA, Pomrenze MB, Galer EL et al. (2015) DAT isn’t all that: cocaine reward and reinforcement require Toll-like receptor 4 signaling. Mol. Psychiatry, 20, 1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacifici R, di Carlo S, Bacosi A, Pichini S & Zuccaro P (2000) Phar-macokinetics and cytokine production in heroin and morphine-treated mice. Int. J. Immunopharmacol, 22, 603–614. [DOI] [PubMed] [Google Scholar]
- Patel V, Chisholm D, Parikh R, Charlson FJ, Degenhardt L, Dua T, Ferrari AJ, Hyman S et al. (2016) Addressing the burden of mental, neurological, and substance use disorders: key messages from Disease Control Priorities, 3rd edition. Lancet, 387, 1672–1685. [DOI] [PubMed] [Google Scholar]
- Pedraz M, Araos P, Garcıa-Marchena N, Serrano A, Romero-Sanchiz P, Suarez J, Castilla-Ortega E, Mayoral-Cleries F et al. (2015) Sex differences in psychiatric comorbidity and plasma biomarkers for cocaine addiction in abstinent cocaine-addicted subjects in outpatient settings. Front. Psychiatry, 6, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peineau S, Rabiant K, Pierrefiche O & Potier B (2018) Synaptic plasticity modulation by circulating peptides and metaplasticity: Involvement in Alzheimer’s disease. Pharmacol. Res, 130, 385–401. [DOI] [PubMed] [Google Scholar]
- Perez-Castrillon JL, Perez-Arellano JL, Garcıa-Palomo JD, Jimenez-Lopez A & Castro S.De. (1992) Opioids depress in vitro human monocyte chemotaxis. Immunopharmacology, 23, 57–61. [DOI] [PubMed] [Google Scholar]
- Peterson PK, Sharp B, Gekker G, Brummitt C & Keane WF (1987) Opioid-mediated suppression of interferon-gamma production by cultured peripheral blood mononuclear cells. J. Clin. Invest, 80, 824–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piepenbrink MS, Samuel M, Zheng B, Carter B, Fucile C, Bunce C, Kiebala M, Khan AA et al. (2016) Humoral dysregulation associated with increased systemic inflammation among injection heroin users. PLoS ONE, 11, e0158641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portou MJ, Baker D, Abraham D & Tsui J (2015) The innate immune system, toll-like receptors and dermal wound healing: a review. Vascular Pharmacol, 71, 31–36. [DOI] [PubMed] [Google Scholar]
- Prata J, Santos SG, Almeida MI, Coelho R & Barbosa MA (2017) Bridging Autism Spectrum Disorders and Schizophrenia through inflammation and biomarkers – pre-clinical and clinical investigations. J. Neuroinflammation, 14, 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavendra V, Rutkowski MD & DeLeo JA (2002) The role of spinal neuroimmune activation in morphine tolerance/hyperalgesia in neuropathic and sham-operated rats. J. Neurosci, 22, 9980–9989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavendra V, Tanga FY & DeLeo JA (2003) Attenuation of mor-phine tolerance, withdrawal-induced hyperalgesia, and associated spinal inflammatory immune responses by propentofylline in rats. Neuropsychopharmacology, 29, 327. [DOI] [PubMed] [Google Scholar]
- Ripke S, Neale BM, Corvin A, Walters JTR, Farh KH, Holmans PA, Lee P, Bulik-Sullivan B et al. (2014) Biological insights from 108 schizophrenia-associated genetic loci. Nature, 511, 421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risdahl JM, Khanna KV, Peterson PK & Molitor TW (1998) Opiates and infection. J. Neuroimmunol, 83, 4–18. [DOI] [PubMed] [Google Scholar]
- Roberts M, Brodribb W & Mitchell G (2012) Reducing the pain: a systematic review of postdischarge analgesia following elective orthopedic surgery. Pain Med, 13, 711–727. [DOI] [PubMed] [Google Scholar]
- Rock RB, Hu S, Sheng WS & Peterson PK (2006) Morphine stimulates CCL2 production by human neurons. J. Neuroinflammation, 3, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd RA, Aleshire N, Zibbell JE & Matthew Gladden R. (2016) Increases in drug and opioid overdose deaths—United States, 2000–2014. Am. J. Transplant, 16, 1323–1327. [DOI] [PubMed] [Google Scholar]
- Sacerdote P (2008) Opioid-induced immunosuppression. Curr. Opin. Support Pa, 2, 14–18. [DOI] [PubMed] [Google Scholar]
- Salter MW & Stevens B (2017) Microglia emerge as central players in brain disease. Nat. Med, 23, 1018. [DOI] [PubMed] [Google Scholar]
- Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE, Challis C, Schretter CE et al. (2016) Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell, 167, 1469–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savignac HM, Corona G, Mills H, Chen L, Spencer JPE, Tzortzis G & Burnet PWJ (2013) Prebiotic feeding elevates central brain derived neurotrophic factor, N-methyl-d-aspartate receptor subunits and d-serine. Neurochem. Int, 63, 756–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayana P, Colpo GD, Simões LR, Giridharan VV, Teixeira AL, Quevedo J, & Barichello T (2017). A systematic review of evidence for the role of inflammatory biomarkers in bipolar patients. J. Psychiatr. Res, 92, 160–182. [DOI] [PubMed] [Google Scholar]
- Schuckit MA (2016) Treatment of opioiduse disorders. N. Engl. J. Med, 375, 357–368. [DOI] [PubMed] [Google Scholar]
- Schwarz JM, Hutchinson MR & Bilbo SD (2011) Early-life experience decreases drug-induced reinstatement of morphine CPP in adulthood via microglial-specific epigenetic programming of anti-Inflammatory IL-10 expression. J. Neurosci, 31, 17835–17847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scofield MD, & Kalivas PW (2014). Astrocytic dysfunction and addiction: consequences of impaired glutamate homeostasis. Neuroscientist, 20, 610–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekine Y, Ouchi Y, Sugihara G, Takei N, Yoshikawa E, Nakamura K, Iwata Y, Tsuchiya KJ et al. (2008) Methamphetamine causes microglial activation in the brains of human abusers. J. Neurosci, 28, 5756–5761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shavit Y, Wolf G, Goshen I, Livshits D & Yirmiya R (2005) Inter-leukin-1 antagonizes morphine analgesia and underlies morphine tolerance. Pain, 115, 50–59. [DOI] [PubMed] [Google Scholar]
- Shi C & Pamer EG (2011) Monocyte recruitment during infection and inflammation. Nat. Rev. Immunol, 11, 762–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth B, Fan J & Hser Y (2006) Life expectancy and productivity loss among narcotics addicts thirty-three years after index treatment. J. Addict. Dis, 25, 37–47. [DOI] [PubMed] [Google Scholar]
- Snider SE, Hendrick ES & Beardsley PM (2013) Glial cell modulators attenuate methamphetamine self-administration in therat. Eur. J. Pharmacol, 701, 124–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew MV (2013) Multiple roles for astrocytes as effectors of cytokines and inflammatory mediators. Neuroscience, 20, 160–172. [DOI] [PubMed] [Google Scholar]
- Solmi M, Murru A, Pacchiarotti I, Undurraga J, Veronese N, Fornaro M, Stubbs B, Monaco F et al. (2017) Safety, tolerability, and risks associated with first- and second-generation antipsychotics: a state-of-the-art clinical review. Ther. Clin. Risk Manag, 13, 757–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song P & Zhao Z-Q (2001) The involvement of glial cells in the development of morphine tolerance. Neurosci. Res, 39, 281–286. [DOI] [PubMed] [Google Scholar]
- Soyka M & Müller CA (2017) Pharmacotherapy of alcoholism – an update on approved and off-label medications. Expert Opin. Pharma-cother, 18, 1187–1199. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (2008) The NSDUH Report, Treatment for Depression Among Adults. Office of Applied Studies, Substance Abuse and Mental Health Services Administration, Dept. of Health & Human Services, Rockville, MD. [Google Scholar]
- Suzumura A, Ito A, Yoshikawa M & Sawada M (1999) Ibudilast suppresses TNFa production by glial cells functioning mainly as type III phosphodiesterase inhibitor in the CNS. Brain Res, 837, 203–212. [DOI] [PubMed] [Google Scholar]
- Szabo I, Rojavin M, Bussiere JL, Eisenstein TK, Adler MW & Rogers TJ (1993) Suppression of peritoneal macrophage phagocytosis of Candida albicans by opioids. J. Pharmacol. Exp. Ther, 267, 703–706. [PubMed] [Google Scholar]
- Tanda G, Mereu M, Hiranita T, Quarterman JC, Coggiano M & Katz JL (2016) Lack of specific involvement of (+)-Naloxone and (+)-Naltrex-one on the reinforcing and neurochemical effects of cocaine and opioids. Neuropsychopharmacology, 41, 2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanos PK, Kim R, Delis F, Ananth M, Chachati G, Rocco MJ, Masad I, Muniz JA et al. (2016) Chronic methamphetamine effects on brain structure and function in rats. PLoS ONE, 11, e0155457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theberge FR, Li X, Kambhampati S, Pickens CL, Laurent R, Bossert JM, Baumann MH, Hutchinson MR et al. (2013) Effect of chronic delivery of the toll-like receptor 4 antagonist (+)-naltrexone on incubation of heroin craving. Biol. Psychiatry, 73, 729–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilleux S, & Hermans E (2007). Neuroinflammation and regulation of glial glutamate uptake in neurological disorders. J. Neurosci. Res, 85, 2059–2070. [DOI] [PubMed] [Google Scholar]
- Tollefson G (1991) Antidepressant treatment and side effect considerations. J. Clin. Psychiatry, 52, 4–13. [PubMed] [Google Scholar]
- Trecki J & Unterwald EM (2009) Modulation of cocaine-induced activity by intracerebral administration of CXCL12. Neuroscience, 161, 13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trocello JM, Rostene W, Melik-Parsadaniantz S, Godefroy D, Roze E, Kitabgi P, Kuziel WA, Chalon S et al. (2011) Implication of CCR2 chemokine receptor in cocaine-induced sensitization. J. Mol. Neu-rosci, 44, 147–151. [DOI] [PubMed] [Google Scholar]
- Tyagi M, Weber J, Bukrinsky M & Simon GL (2016) The effects of cocaine on HIV transcription. J. Neurovirol, 22, 261–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, & Pardo CA (2005). Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann. Neurol, 57, 67–81. [DOI] [PubMed] [Google Scholar]
- Volpe GE, Ward H, Mwamburi M, Dinh D, Bhalchandra S, Wanke C & Kane AV (2014) Associations of cocaine use and HIV infection with the intestinal microbiota, microbial translocation, and inflammation. J. Stud. Alcohol Drugs, 75, 347–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuong HE & Hsiao EY (2017) Emerging roles for the gut microbiome in autism spectrum disorder. Biol. Psychiatry, 81, 411–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Barke RA, Charboneau R & Roy S (2005) Morphine impairs host innate immune response and increases susceptibility to Streptococcus pneumoniae lung infection. J. Immunol, 174, 426–434. [DOI] [PubMed] [Google Scholar]
- Wang J, Barke RA & Roy S (2007) Transcriptional and epigenetic regulation of interleukin-2 gene in activated T cells by morphine. J. Biol. Chem, 282, 7164–7171. [DOI] [PubMed] [Google Scholar]
- Wang X, Loram LC, Ramos K, de Jesus AJ, Thomas J, Cheng K, Reddy A, Somogyi AA et al. (2012) Morphine activates neuroinflammation in a manner parallel to endotoxin. Proc. Natl Acad. Sci. USA, 109, 6325–6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhang Y, Peng Y, Hutchinson MR, Rice KC, Yin H & Watkins LR (2016) Pharmacological characterization of the opioid inactive isomers (+)-naltrexone and (+)-naloxone as antagonists of toll-like receptor 4. Br. J. Pharmacol, 173, 856–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Meng J, Zhang L, Johnson T, Chen C & Roy S (2018) Morphine induces changes in the gut microbiome and metabolome in a morphine dependence model. Sci. Rep, 8, 3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webber MP, Schoenbaum EE, Gourevitch MN, Buono D & Klein RS (1999) A prospective study of HIV disease progression in female and male drug users. Aids, 13, 257–262. [DOI] [PubMed] [Google Scholar]
- Wohleb ES, Franklin T, Iwata M & Duman RS (2016) Integrating neuroimmune systems in the neurobiology of depression. Nat. Rev. Neurosci, 17, 497. [DOI] [PubMed] [Google Scholar]
- Wray NR, Ripke S, Mattheisen M, Trzaskowski M, Byrne EM, Abdellaoui A, Adams MJ, Agerbo E et al. (2018). Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat. Genet, 50, 668–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaksh TL (1981) Spinal opiate analgesia: characterstics and principles of action. Pain, 11, 293. [DOI] [PubMed] [Google Scholar]
- Yeager MP, Colacchio TA, Yu CT, Hildebrandt L, Howell AL, Weiss J & Guyre PM (1995) Morphine inhibits spontaneous and cytokine-enhanced natural killer cell cytotoxicity in volunteers. Anesthesiology, 83, 500–508. [DOI] [PubMed] [Google Scholar]
- Zhang L & Wang C-C (2014) Inflammatory response of macrophages in infection. Hepatob. Pancreat. Dis. Int, 13, 138–152. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Li H, Li Y, Sun X, Zhu M, Hanley G, LeSage G & Yin D (2011) Essential role of toll-like receptor 2 in morphine-induced microglia activation in mice. Neurosci. Lett, 489, 43–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X-Q, Cui Y, Cui Y, Chen Y, Na X-D, Chen F-Y, Wei X-H, Li Y-Y et al. (2012) Activation of p38 signaling in the microglia in the nucleus accumbens contributes to the acquisition and maintenance of morphine-induced conditioned place preference. Brain Behav. Immun, 26, 318–325. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O’Keeffe S, Phatnani HP, Guarnieri P et al. (2014) An RNA-sequencing tran-scriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci, 34, 11929–11947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J-W, Liu F-L, Mu D, Deng D-Y & Zheng Y-T (2017) Heroin use is associated with lower levels of restriction factors and type I inter-feron expression and facilitates HIV-1 replication. Microbes Infect, 19, 288–294. [DOI] [PubMed] [Google Scholar]
- Zuckerman RL, & Harris AP, (1994) Anesthesia in obstetrics. Curr. Opin. Obstet. Gynecol, 6, 408–413. [PubMed] [Google Scholar]


