Summary
Background
Prosthetic joint infection is a devastating complication of knee replacement. The risk of developing a prosthetic joint infection is affected by patient, surgical, and health-care system factors. Existing evidence is limited by heterogeneity in populations studied, short follow-up, inadequate power, and does not differentiate early prosthetic joint infection, most likely related to the intervention, from late infection, more likely to occur due to haematogenous bacterial spread. We aimed to assess the overall and time-specific associations of these factors with the risk of revision due to prosthetic joint infection following primary knee replacement.
Methods
In this cohort study, we analysed primary knee replacements done between 2003 and 2013 in England and Wales and the procedures subsequently revised for prosthetic joint infection between 2003 and 2014. Data were obtained from the National Joint Registry linked to the Hospital Episode Statistics data in England and the Patient Episode Database for Wales. Each primary replacement was followed for a minimum of 12 months until the end of the observation period (Dec 31, 2014) or until the date of revision for prosthetic joint infection, revision for another indication, or death (whichever occurred first). We analysed the data using Poisson and piecewise exponential multilevel models to assess the associations between patient, surgical, and health-care system factors and risk of revision for prosthetic joint infection.
Findings
Of 679 010 primary knee replacements done between 2003 and 2013 in England and Wales, 3659 were subsequently revised for an indication of prosthetic joint infection between 2003 and 2014, after a median follow-up of 4·6 years (IQR 2·6–6·9). Male sex (rate ratio [RR] for male vs female patients 1·8 [95% CI 1·7–2·0]), younger age (RR for age ≥80 years vs <60 years 0·5 [0·4–0·6]), higher American Society of Anaesthesiologists [ASA] grade (RR for ASA grade 3–5 vs 1, 1·8 [1·6–2·1]), elevated body-mass index (BMI; RR for BMI ≥30 kg/m2vs <25 kg/m2 1·5 [1·3–1·6]), chronic pulmonary disease (RR 1·2 [1·1–1·3]), diabetes (RR 1·4 [1·2–1·5]), liver disease (RR 2·2 [1·6–2·9]), connective tissue and rheumatic diseases (RR 1·5 [1·3–1·7]), peripheral vascular disease (RR 1·4 [1·1–1·7]), surgery for trauma (RR 1·9 [1·4–2·6]), previous septic arthritis (RR 4·9 [2·7–7·6]) or inflammatory arthropathy (RR 1·4 [1·2–1·7]), operation under general anaesthesia (RR 1·1 [1·0–1·2]), requirement for tibial bone graft (RR 2·0 [1·3–2·7]), use of posterior stabilised fixed bearing prostheses (RR for posterior stabilised fixed bearing prostheses vs unconstrained fixed bearing prostheses 1·4 [1·3–1·5]) or constrained condylar prostheses (3·5 [2·5–4·7]) were associated with a higher risk of revision for prosthetic joint infection. However, uncemented total, patellofemoral, or unicondylar knee replacement (RR for uncemented vs cemented total knee replacement 0·7 [95% CI 0·6–0·8], RR for patellofemoral vs cemented total knee replacement 0·3 [0·2–0·5], and RR for unicondylar vs cemented total knee replacement 0·5 [0·5–0·6]) were associated with lower risk of revision for prosthetic joint infection. Most of these factors had time-specific effects, depending on the time period post-surgery.
Interpretation
We have identified several risk factors for revision for prosthetic joint infection following knee replacement. Some of these factors are modifiable, and the use of targeted interventions or strategies could lead to a reduced risk of revision for prosthetic joint infection. Non-modifiable factors and the time-specific nature of the effects we have observed will allow clinicians to appropriately counsel patients preoperatively and tailor follow-up regimens.
Funding
National Institute for Health Research.
Introduction
Knee replacement is one of the most common elective surgical procedures worldwide. The National Joint Registry for England, Wales, Northern Ireland and the Isle of Man recorded 102 777 knee replacements performed in 2017, and the secular trend continues to increase.1 Deep infection is a rare but devastating complication affecting approximately 4% of primary and 15% of revision knee replacements.2 The most common causative organisms remain coagulase-negative staphylococci and Staphylococcus aureus, which are usually sensitive to a range of antibiotics.3 Treatment of prosthetic joint infection is expensive and protracted, and both the infection and the treatment have profoundly negative effects on patients and their families.4, 5, 6, 7 Treatment options include antibiotic suppression, debridement and retention of implants, excisional arthroplasty, and one-stage or two-stage revision.8, 9 However, all of these treatment options are associated with substantial morbidity and a high risk of adverse outcomes. As knee replacements have become more common, the number of revision operations for infection between 2005 and 2013 in England and Wales has risen by more than threefold, with more than 1000 revision procedures due to prosthetic joint infection of the knee done annually since 2011.10
Research in context.
Evidence before this study
Prosthetic joint infection, although a rare complication following total joint replacement, is associated with devastating consequences. Evidence suggests that the risk of developing prosthetic joint infection following total hip or knee replacement is likely to be affected by patient-related, surgery-related, and health-care system-related factors. However, since total hip and knee replacements are two different operations involving patients with differing risk profiles, whether these factors affect prosthetic joint infection rates differentially in these patient groups remains uncertain. In a meta-analysis of 66 studies comprising more than 500 000 total joint replacements and published by our group in 2016, patient-related factors such as male sex, high body-mass index (BMI), steroid use, diabetes, rheumatoid arthritis, congestive heart failure, depression, and smoking and alcohol intake were each found to be associated with an increased risk of prosthetic joint infection. In a single cohort prospective study published in September, 2018, and comprising more than 600 000 primary hip replacements, we confirmed previous findings and showed several additional patient factors (eg, younger age, chronic pulmonary disease, and liver disease) and surgical factors (eg, surgery type, lateral surgical approach, and non-ceramic bearing surfaces) to be associated with an increased risk of infection. We also demonstrated that these factors exhibit specific time effects following surgery. However, the evidence for total knee replacements is less robust. We searched MEDLINE, Embase, and Web of Science from the date of the last search of the 2016 review (Sept 1, 2015) up to August, 2018, for observational cohort studies and systematic reviews and meta-analyses reporting on associations of patient-related, surgery-related, or health-care system-related factors with risk of prosthetic joint infection following total knee replacement. We used search terms related to the exposures (eg, “risk factor”, “body mass index”, and “comorbidity”) with those related to prosthetic joint infection (eg, “peri-prosthetic joint infection” and “prosthetic joint infection”). Our search was not restricted by language. We identified several registry studies and a meta-analysis based on 16 studies, whose findings are consistent with previous work on the topic. Existing evidence for the role of patient-related, surgery-related, or health-care system-related factors on prosthetic joint infection risk following total knee replacement is limited by inadequate sample sizes, short follow-up durations, inadequate adjustment for confounders, substantial inter-study heterogeneity, and inability to account for time-specific effects during follow-up.
Added value of this study
Using a single observational cohort of 679 010 primary total knee replacements, this study evaluated the overall and time-specific associations of patient, surgical, and health-care system factors on the risk of revision for prosthetic joint infection. Over a median follow-up of 4·6 years, 3659 knees were revised for prosthetic joint infection. Patient factors such as male sex, younger age (<60 years), high BMI (≥25 kg/m2), chronic pulmonary disease, diabetes, liver disease, connective tissue or rheumatic disease, and peripheral vascular disease were each associated with an increased risk of revision for prosthetic joint infection. Surgical factors such as indications for the primary procedure, type of procedure, and implant fixation and constraint or bearing significantly affected the risk of revision for prosthetic joint infection. Patients who received general anaesthesia or a tibial bone graft had an increased risk of revision, whereas the risk was lower for those who received a spinal anaesthetic. On the role of health-care system characteristics, high-volume hospitals had an increased risk of revision for prosthetic joint infection and privately funded procedures carried a lower risk of revision than operations funded by the NHS. Factors such as male sex and younger age had a consistent effect during the entire postoperative period, whereas other factors (such as indications for the primary procedure, type of procedure, and implant fixation and constraint or bearing) exhibited time-specific effects on revision for prosthetic joint infection.
Implications of all the available evidence
With the ageing population and a projected increase in total knee replacements, the burden of prosthetic joint infection will rise proportionately. The development of a prosthetic joint infection following total knee replacement is influenced by several modifiable and non-modifiable factors, which also seem to exhibit time-specific effects. Awareness of these factors and their time-specific effects should assist clinicians in appropriate counselling of patients preoperatively, optimisation of patients before surgery, as well as enhanced monitoring of at-risk patients after surgery.
The risk of developing infection after any form of arthroplasty is affected by both modifiable and non-modifiable patient, surgical, and health-care system factors. A recent systematic review of patient risk factors for prosthetic joint infection in both hip and knee replacements identified male sex, smoking, increasing body-mass index (BMI), steroid use, previous joint surgery, and comorbidities such as diabetes, rheumatoid arthritis, and depression, as notable risk factors for infection.11 However, limitations of this study and other reviews include short follow-up, pooled estimates based on variably adjusted data, and evidence of substantial heterogeneity between studies.11, 12
In view of these limitations, there is a need for large-scale cohort studies with adequate power to provide evidence about the nature and magnitude of the associations of potential risk factors with prosthetic joint infection. We recently published one such study about infection following hip replacement, which highlighted the importance of disentangling the time-specific effects of factors associated with early onset of prosthetic joint infection that are likely to be the consequence of the primary intervention versus factors associated with later onset that are more likely to result from haematogenous spread.9, 13
Although they are often studied together, hip and knee osteoarthritis are in some regards very different diseases14 with varying responses to joint replacement.15 Orthopaedic surgeons often specialise in either hip or knee replacement, and surgical techniques and implants aim to address specific issues relating to joint structure and function. Furthermore, patient recovery,16 outcomes,17 and rates of complications including prosthetic joint infection10, 18 differ between hip and knee replacement.
In this study, we aimed to assess the overall and post-operative period-specific associations of patient, surgical, and health-care setting factors with the risk of revision due to prosthetic joint infection in prospectively collected observational data of primary knee replacements done in England and Wales. We also aimed to investigate whether these factors differed from those associated with revision for prosthetic joint infection after hip replacement.
Methods
Study design and data sources
In this observational cohort study, we report analyses of data for England and Wales from the National Joint Registry for England, Wales, Northern Ireland, and the Isle of Man between April 1, 2003, and December 31, 2014. Data collection for Northern Ireland and the Isle of Man could not be considered due to their low number of procedures and insufficient duration of follow-up.
National Joint Registry data were linked to Hospital Episode Statistics in England and the Patient Episode Database for Wales to obtain data about inpatient and day case admissions. Data from the Office for National Statistics were linked to obtain the date of death.
Patient consent was obtained for data collection and linkage by the National Joint Registry. According to the NHS Health Research Authority, separate consent and ethics approval were not required for this study.
Procedures
We analysed primary knee replacements done between April 1, 2003, and Dec 31, 2013, and revision procedures for prosthetic joint infection that occurred after the primary replacement between April 1, 2003, and Dec 31, 2014. The reason for revision is recorded by clinicians at the time of the revision procedure and reflects a clinical judgement sufficient to lead the surgeon to do an invasive procedure tailored to treat prosthetic joint infection. The diagnosis and treatment strategy for prosthetic joint infection is at the discretion of the surgeon and treating unit and is reflective of contemporary practice during the study period, with raised inflammatory markers, joint-specific symptoms, sinuses, and positive microbiological cultures19 being common diagnostic features during that period.
Each primary replacement was followed for a minimum of 12 months until the end of the observation period (Dec 31, 2014) or until the date of revision for prosthetic joint infection, revision for another indication, or death (whichever occurred first). Revisions for prosthetic joint infection included debridement and implant retention with modular exchange, or a single-stage or two-stage revision procedure.20
Patient characteristics21 considered were age, sex, ethnicity, BMI, American Society of Anesthesiologists (ASA) grade, and comorbidities. Ethnicity and comorbidities were obtained from the Hospital Episode Statistics records. We used International Classification of Diseases, 10th revision codes to classify comorbidities for which patients had been admitted to hospital in the 5 years preceding their primary operation (appendix p 2).22
Surgical factors21 considered were indication for surgery, anaesthesia type, thromboprophylaxis regime, surgical approach, knee replacement type, fixation, degree of constraint, use of bone graft, and occurrence of intraoperative complications (appendix p 2).
Health-care system factors21 considered were hospital type, funding source (National Health Service [NHS] or private), country, operating surgeon grade, consultant involvement, and the volume of knee surgeries (categorised into quartiles) done by the operating surgeon and surgeon in charge of the procedure in the preceding 12 months.
Statistical analysis
The associations between the risk factors and risk of revision for prosthetic joint infection were first investigated during the overall follow-up period. Poisson multilevel models accounting for clustering at the unit level (random intercept) were used. Clustering at the surgeon level was negligible and therefore not considered further.
Prosthetic joint infection management can vary according to the time elapsed since the primary procedure at the time of onset of infection. Early onset of infection (within 2 years of the primary procedure) is generally believed to result from the primary intervention. Later onset of infection (2 years or longer after the primary procedure) is more likely to be due to haematogenous spread.9 For patients with early postoperative or acute haematogenous infection and a short duration of symptoms, debridement, modular exchange, and implant retention rather than full revision is appropriate if the joint replacement is well fixed.9 The associations between risk factors and risk of revision were therefore re-investigated over several at-risk post-operative periods: 0–3 months, 3–6 months, 6–12 months, 12–24 months, and more than 24 months. Each participant's at-risk period (defined as the time elapsed between their primary procedure and the endpoint) was split according to the time spent in each of these periods and the revision for prosthetic joint infection status (revised for prosthetic joint infection vs not) was assigned to the relevant period. We used a piecewise exponential multilevel model with period-specific effects to assess these associations—ie, their rate ratios (RRs) and 95% CIs across these time periods.23, 24 Analyses were done by running MLwiN from Stata version 14.1 using Markov Chain Monte Carlo methods.25 To account for test multiplicity, adjusted p values were derived using Simes' false-discovery rate testing controlling procedure.26, 27 To be confident that 95% of the effects tested were not due to chance, evidence of association was only discussed for adjusted p values of up to 0·05.
The analyses were done on the overall sample for all exposures except for ethnicity and comorbidities, which were only investigated in the patients operated on in England with record of hospital admission in Hospital Episode Statistics, but not in the Patient Episode Database for Wales and no evidence of residency outside England. The regressions were adjusted for age, sex, ASA grade, and BMI. BMI is an important risk factor for prosthetic joint infection but has substantial missing data in the National Joint Registry (47%), partly because it was not included as a variable in the early data collection forms. A missing at random mechanism was assumed to account for observed factors associated with the propensity of BMI to be missing and avoid the use of a complete-case analysis, which would have produced biased estimates: mean time at risk in missing BMI group 5·9 years (SD 2·8) versus 4·1 years (2·3) in the complete BMI group; incidence of revision for prosthetic joint infection 0·58 (95% CI 0·55–0·62) versus 1·68 (1·62–1·75); uncemented total knee replacement 6·0% versus 4·1%; other type of total knee replacement 1·6% versus 0·8%, unicondylar procedure 7·9% versus 9·3%. This approach also allowed us to use the entire study sample and investigate the rare exposure, something precluded with a complete case approach. A multiple imputation strategy was used to impute BMI using a Gaussian normal regression imputation model with the above factors used as covariates as well as the log of the observed event or censoring time, knee replacement type, and revision for prosthetic joint infection status. Because of the computational time required by each multilevel piecewise model, five imputations were computed and no sensitivity analyses of our missing at random approach were done. Estimates were combined by Rubin's rules. Unadjusted and adjusted models without BMI are available on request. To avoid over-adjustment, models investigating the effect of comorbidities were not adjusted for ASA grade, a proxy indicator of comorbidity profile.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. EL had full access to all the data in the study. AWB is the guarantor and had final responsibility for the decision to submit for publication.
Results
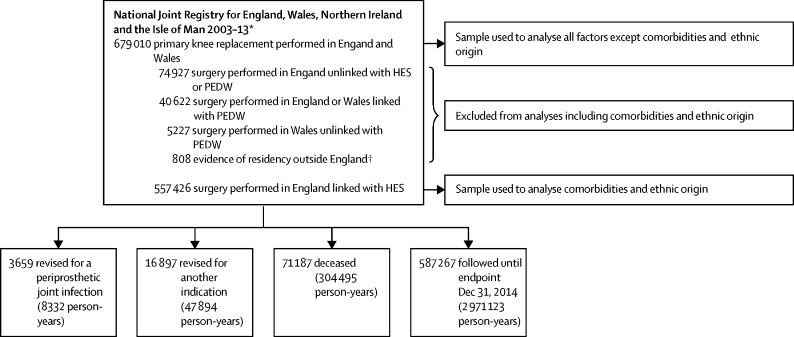
Baseline study sample characteristics are presented in figure 1 and the table. 679 010 primary knee procedures were done in 449 different surgical units with a median of 1142 procedures (IQR 564–2144) per unit. Baseline characteristics were assessed in all 679 010 primary knee procedures, except for ethnicity and comorbidities, which were only investigated in the 557 426 patients operated on in England with record of hospital admission in Hospital Episode Statistics, but not in the Patient Episode Database for Wales and no evidence of residency outside England (figure 1, appendix p 2). 3659 index procedures were subsequently revised for an indication of prosthetic joint infection after a median follow-up of 4·6 years (IQR 2·6–6·9): 245 (6·7%) of these within 3 months, 238 (6·5%) between 3 and 6 months, 628 (17·2%) between 6 and 12 months, 970 (26·5%) between 1 and 2 years, and 1578 (43·1%) beyond 2 years from the index procedure. The median patient age was 70 years (IQR 63–76). The sample is presented by time periods in appendix pp 4–9. In 792 (28%) of the 2833 two-stage revision procedures done for prosthetic joint infection, only a second stage procedure was recorded in the National Joint Registry. Patients with incompletely registered two-stage procedures did not differ from those with complete procedures and their time to first stage procedure was estimated (appendix p 1).
Figure 1.
Study sample
HES=Hospital Episode Statistics for England. PEDW=Patient Episode Database for Wales. *In this research, only data for England and Wales were considered; data collection for Northern Ireland commenced on Feb 1, 2013, and primary and revision procedures from this country could not be considered because of their low number and short follow-up. Data collection for the Isle of Man commenced on July 1, 2015, which was after the endpoint of the study and therefore these data were not considered. †As recorded in HES for the 5 years preceding the primary knee replacement.
Table.
Sample description and incidence rates
| n | Person-years of follow-up | Cases | Incidence rate per 1000 person-years (95% CI) | ||
|---|---|---|---|---|---|
| Patient characteristics | |||||
| Sex | |||||
| Female | 386 047 | 1 913 858 | 1564 | 0·82 (0·78–0·86) | |
| Male | 292 963 | 1 418 437 | 2095 | 1·48 (1·41–1·54) | |
| Age, years | |||||
| <60 | 109 000 | 537 080 | 793 | 1·48 (1·38–1·58) | |
| 60–69 | 229 107 | 1 143 670 | 1342 | 1·17 (1·11–1·24) | |
| 70–79 | 247 636 | 1 233 478 | 1223 | 0·99 (0·94–1·05) | |
| ≥80 | 93 267 | 418 066 | 301 | 0·72 (0·64–0·81) | |
| Ethnic origin | |||||
| White | 515 098 | 2 491 245 | 3066 | 1·23 (1·19–1·28) | |
| Black African origin | 6011 | 27 217 | 52 | 1·91 (1·43–2·51) | |
| South Asian | 15 510 | 69 500 | 94 | 1·35 (1·09–1·66) | |
| Other and mixed | 6513 | 28 414 | 40 | 1·41 (1·01–1·92) | |
| Unclear | 14 294 | 71 896 | 30 | 0·42 (0·28–0·60) | |
| Unavailable* | 121 584 | 644 023 | 377 | 0·59 (0·53–0·65) | |
| BMI, kg/m2 | |||||
| <25 | 40 333 | 167 416 | 212 | 1·27 (1·10–1·45) | |
| 25–29·9 | 131 560 | 548 505 | 849 | 1·55 (1·45–1·66) | |
| ≥30 | 205 134 | 840 041 | 1558 | 1·85 (1·76–1·95) | |
| Missing | 301 983 | 1 776 333 | 1040 | 0·59 (0·55–0·62) | |
| ASA grade | |||||
| 1 | 92 441 | 523 023 | 490 | 0·94 (0·86–1·02) | |
| 2 | 484 992 | 2 347 559 | 2460 | 1·05 (1·01–1·09) | |
| 3–5 | 101 577 | 461 713 | 709 | 1·54 (1·42–1·65) | |
| Chronic pulmonary disease | |||||
| No | 478 788 | 2 350 416 | 2822 | 1·20 (1·16–1·25) | |
| Yes | 78 638 | 337 856 | 460 | 1·36 (1·24–1·49) | |
| Unavailable* | 121 584 | 644 023 | 377 | 0·59 (0·53–0·65) | |
| Diabetes | |||||
| No | 490 521 | 2 398 439 | 2809 | 1·17 (1·13–1·22) | |
| Yes | 66 905 | 289 833 | 473 | 1·63 (1·49–1·79) | |
| Unavailable* | 121 584 | 644 023 | 377 | 0·59 (0·53–0·65) | |
| Dementia | |||||
| No | 555 783 | 2 682 651 | 3274 | 1·22 (1·18–1·26) | |
| Yes | 1643 | 5621 | 8 | 1·42 (0·61–2·80) | |
| Unavailable* | 121 584 | 644 023 | 377 | 0·59 (0·53–0·65) | |
| Liver disease | |||||
| No | 553 389 | 2 672 866 | 3237 | 1·21 (1·17–1·25) | |
| Yes | 4037 | 15 406 | 45 | 2·92 (2·13–3·91) | |
| Unavailable* | 121 584 | 644 023 | 377 | 0·59 (0·53–0·65) | |
| Congestive heart failure | |||||
| No | 546 613 | 2 643 872 | 3211 | 1·21 (1·17–1·26) | |
| Yes | 10 813 | 44 399 | 71 | 1·60 (1·25–2·02) | |
| Unavailable* | 121 584 | 644 023 | 377 | 0·59 (0·53–0·65) | |
| Connective tissue–rheumatic disease | |||||
| No | 526 493 | 2 547 415 | 3059 | 1·20 (1·16–1·24) | |
| Yes | 30 933 | 140 856 | 223 | 1·58 (1·38–1·81) | |
| Unavailable* | 121 584 | 644 023 | 377 | 0·59 (0·53–0·65) | |
| Cancer | |||||
| No | 534 534 | 2 590 793 | 3170 | 1·22 (1·18–1·27) | |
| Non-metastatic cancer | 20 364 | 87 849 | 95 | 1·08 (0·87–1·32) | |
| Metastatic cancer | 2528 | 9629 | 17 | 1·77 (1·03–2·83) | |
| Unavailable* | 121 584 | 644 023 | 377 | 0·59 (0·53–0·65) | |
| Cerebrovascular disease | |||||
| No | 546 047 | 2 640 626 | 3221 | 1·22 (1·18–1·26) | |
| Yes | 11 379 | 47 646 | 61 | 1·28 (0·98–1·64) | |
| Unavailable* | 121 584 | 644 023 | 377 | 0·59 (0·53–0·65) | |
| Myocardial infarction | |||||
| No | 541 849 | 2 619 164 | 3186 | 1·22 (1·17–1·26) | |
| Yes | 15 577 | 69 107 | 96 | 1·39 (1·13–1·70) | |
| Unavailable* | 121 584 | 644 023 | 377 | 0·59 (0·53–0·65) | |
| Paraplegia and hemiplegia | |||||
| No | 555 229 | 2 678 669 | 3263 | 1·22 (1·18–1·26) | |
| Yes | 2197 | 9603 | 19 | 1·98 (1·19–3·09) | |
| Unavailable* | 121 584 | 644 023 | 377 | 0·59 (0·53–0·65) | |
| Peptic ulcer disease | |||||
| No | 549 071 | 2 649 116 | 3229 | 1·22 (1·18–1·26) | |
| Yes | 8355 | 39 155 | 53 | 1·35 (1·01–1·77) | |
| Unavailable* | 121 584 | 644 023 | 377 | 0·59 (0·53–0·65) | |
| Peripheral vascular disease | |||||
| No | 547 096 | 2 645 530 | 3207 | 1·21 (1·17–1·25) | |
| Yes | 10 330 | 42 742 | 75 | 1·75 (1·38–2·20) | |
| Unavailable* | 121 584 | 644 023 | 377 | 0·59 (0·53–0·65) | |
| Renal disease | |||||
| No | 539 605 | 2 628 852 | 3197 | 1·22 (1·17–1·26) | |
| Yes | 17 821 | 59 419 | 85 | 1·43 (1·14–1·77) | |
| Unavailable* | 121 584 | 644 023 | 377 | 0·59 (0·53–0·65) | |
| Surgical characteristics | |||||
| Osteoarthritis | |||||
| No | 18 529 | 92 371 | 157 | 1·70 (1·44–1·99) | |
| Yes | 660 481 | 3 239 923 | 3502 | 1·08 (1·05–1·12) | |
| Trauma | |||||
| No | 675 193 | 3 313 911 | 3613 | 1·09 (1·05–1·13) | |
| Yes | 3817 | 18 383 | 46 | 2·50 (1·83–3·34) | |
| Previous knee infection | |||||
| No | 678 522 | 3 329 986 | 3644 | 1·09 (1·06–1·13) | |
| Yes | 488 | 2309 | 15 | 6·50 (3·64–10·72) | |
| Avascular necrosis | |||||
| No | 676 515 | 3 319 900 | 3638 | 1·10 (1·06–1·13) | |
| Yes | 2495 | 12 394 | 21 | 1·69 (1·05–2·59) | |
| Inflammatory arthropathy | |||||
| No | 663 410 | 3 251 205 | 3534 | 1·09 (1·05–1·12) | |
| Yes | 15 600 | 81 089 | 125 | 1·54 (1·28–1·84) | |
| Other indication | |||||
| No | 675 312 | 3 317 114 | 3636 | 1·10 (1·06–1·13) | |
| Yes | 3698 | 15 181 | 23 | 1·52 (0·96–2·27) | |
| Surgical approach | |||||
| Medial parapatellar | 629 891 | 3 093 847 | 3 420 | 1·11 (1·07–1·14) | |
| Midvastus | 19 384 | 89 860 | 76 | 0·85 (0·67–1·06) | |
| Lateral parapatellar | 7992 | 42 506 | 47 | 1·11 (0·81–1·47) | |
| Subvastus | 9403 | 48 926 | 49 | 1·00 (0·74–1·32) | |
| Other approach | 12 340 | 57 155 | 67 | 1·17 (0·91–1·49) | |
| Procedure | |||||
| Primary TKR cemented | 569 737 | 2 760 945 | 3227 | 1·17 (1·13–1·21) | |
| Primary TKR uncemented | 33 754 | 188 639 | 153 | 0·81 (0·69–0·95) | |
| Primary TKR other | 7699 | 48 490 | 52 | 1·07 (0·80–1·41) | |
| Unicondylar | 58 885 | 291 906 | 211 | 0·72 (0·63–0·83) | |
| Patellofemoral | 8935 | 42 314 | 16 | 0·38 (0·22–0·61) | |
| Constraint | |||||
| Unconstrained fixed | 397 175 | 1 918 707 | 1987 | 1·04 (0·99–1·08) | |
| Unconstrained mobile | 47 532 | 262 875 | 278 | 1·06 (0·94–1·19) | |
| Posterior stabilised fixed | 144 960 | 705 782 | 981 | 1·39 (1·30–1·48) | |
| Posterior stabilised mobile | 9714 | 51 054 | 54 | 1·06 (0·79–1·38) | |
| Constrained condylar | 2968 | 11 498 | 43 | 3·74 (2·71–5·04) | |
| Fixed | 16 703 | 74 967 | 67 | 0·89 (0·69–1·14) | |
| Mobile | 41 297 | 212 086 | 141 | 0·66 (0·56–0·78) | |
| Undetermined | 18 661 | 95 326 | 108 | 1·13 (0·93–1·37) | |
| General anaesthesia | |||||
| No | 381 156 | 1 788 490 | 1889 | 1·06 (1·01–1·10) | |
| Yes | 297 854 | 1 543 804 | 1770 | 1·15 (1·09–1·20) | |
| Nerve block anaesthesia | |||||
| No | 547 783 | 2 644 393 | 2914 | 1·10 (1·06–1·14) | |
| Yes | 131 227 | 687 902 | 745 | 1·08 (1·01–1·16) | |
| Epidural anaesthesia | |||||
| No | 621 572 | 2 987 043 | 3311 | 1·11 (1·07–1·15) | |
| Yes | 57 438 | 345 251 | 348 | 1·01 (0·90–1·12) | |
| Spinal anaesthesia | |||||
| No | 283 120 | 1 499 422 | 1724 | 1·15 (1·10–1·21) | |
| Yes | 395 890 | 1 832 873 | 1935 | 1·06 (1·01–1·10) | |
| Thromboprophylaxis regimen | |||||
| Chemical | 606 001 | 2 870 437 | 3204 | 1·12 (1·08–1·16) | |
| Non-chemical | 73 009 | 461 857 | 455 | 0·99 (0·90–1·08) | |
| Femoral bone graft | |||||
| No | 675 147 | 3 317 925 | 3635 | 1·10 (1·06–1·13) | |
| Yes | 3863 | 14 370 | 24 | 1·67 (1·07–2·49) | |
| Tibial bone graft | |||||
| No | 676 271 | 3 319 182 | 3629 | 1·09 (1·06–1·13) | |
| Yes | 2739 | 13 112 | 30 | 2·29 (1·54–3·27) | |
| Intraoperative event | |||||
| No | 675 089 | 3 314 501 | 3636 | .. | |
| Yes | 3921 | 17 794 | 23 | 1·29 (0·82–1·94) | |
| Health system characteristics | |||||
| Country of surgery | |||||
| England | 638 835 | 3 136 010 | 3461 | 1·10 (1·07–1·14) | |
| Wales | 40 175 | 196 285 | 198 | 1·01 (0·87–1·16) | |
| Funding | |||||
| NHS | 574 433 | 2 722 013 | 3091 | 1·14 (1·10–1·18) | |
| Private | 75 507 | 395 514 | 362 | 0·92 (0·82–1·01) | |
| Unspecified | 29 070 | 214 768 | 206 | 0·96 (0·83–1·10) | |
| Grade of operating surgeon | |||||
| Consultant | 572 464 | 2 767 937 | 3032 | 1·10 (1·06–1·14) | |
| Other | 106 546 | 564 357 | 627 | 1·11 (1·03–1·20) | |
| Consultant involvement | |||||
| Operating | 572 464 | 2 767 937 | 3032 | 1·10 (1·06–1·14) | |
| Assisting | 38 327 | 188 754 | 223 | 1·18 (1·03–1·35) | |
| Not involved | 68 219 | 375 604 | 404 | 1·08 (0·97–1·19) | |
| Total volume (operating surgeon) of knee replacements done in previous 12 months | |||||
| ≤25 | 173 288 | 988 694 | 1091 | 1·10 (1·04–1·17) | |
| >25–50 | 160 104 | 815 890 | 983 | 1·20 (1·13–1·28) | |
| >50–85 | 170 157 | 788 139 | 816 | 1·04 (0·97–1·11) | |
| >85 | 175 461 | 739 571 | 769 | 1·04 (0·97–1·12) | |
| Total volume (surgeon in charge) of knee replacements done in previous 12 months | |||||
| ≤38 | 173 204 | 1 010 739 | 1113 | 1·10 (1·04–1·17) | |
| >38–70 | 174 209 | 872 816 | 986 | 1·13 (1·06–1·20) | |
| >70–110 | 162 179 | 730 804 | 791 | 1·08 (1·01–1·16) | |
| >110 | 169 418 | 717 936 | 769 | 1·07 (1·00–1·15) | |
| Total volume (hospital) of knee replacements done in previous 12 months | |||||
| ≤150 | 167 930 | 1 008 852 | 984 | 0·98 (0·92–1·04) | |
| >150–285 | 174 288 | 863 114 | 973 | 1·13 (1·06–1·20) | |
| >285–440 | 169 780 | 743 102 | 893 | 1·20 (1·12–1·28) | |
| >440 | 167 012 | 717 227 | 809 | 1·13 (1·05–1·21) | |
ASA=American Society of Anesthesiologists. TKR=total knee replacement. NHS=National Health Service.
Information about ethnicity and comorbidities is only available for the 557 426 patients operated on in England with a Hospital Episodes Statistics record, no record in the Patient Episode Database for Wales, and no evidence of residency outside England. See figure 1 and appendix p 2 for more details.
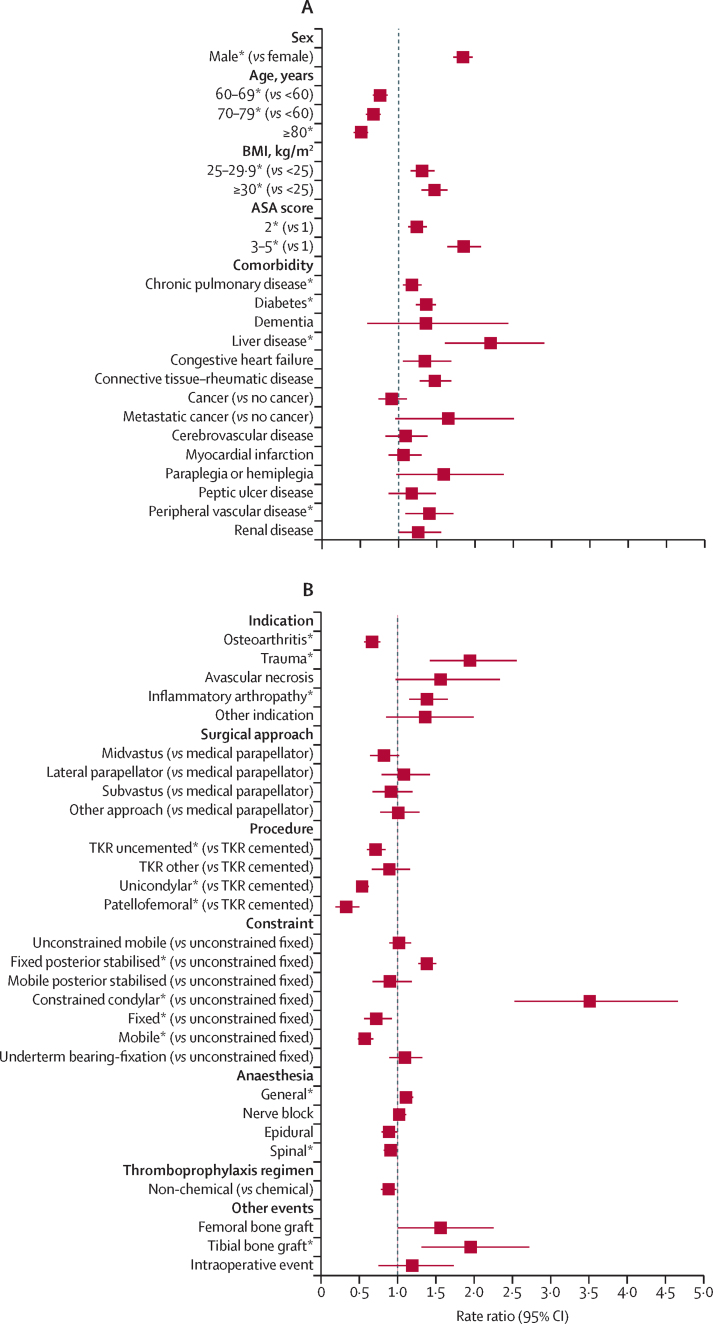
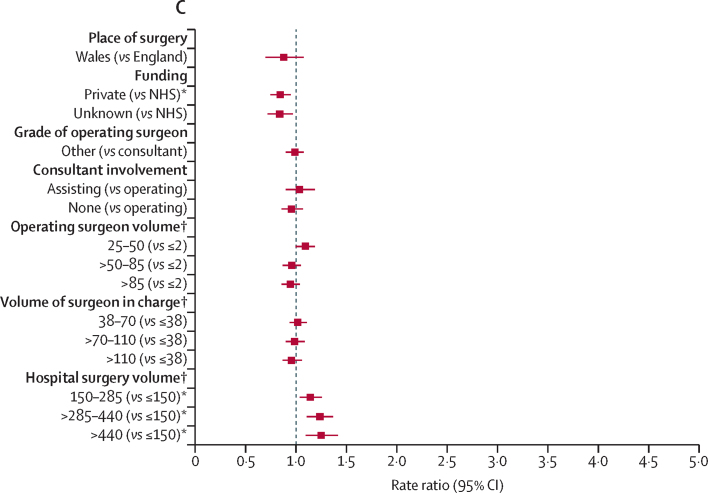
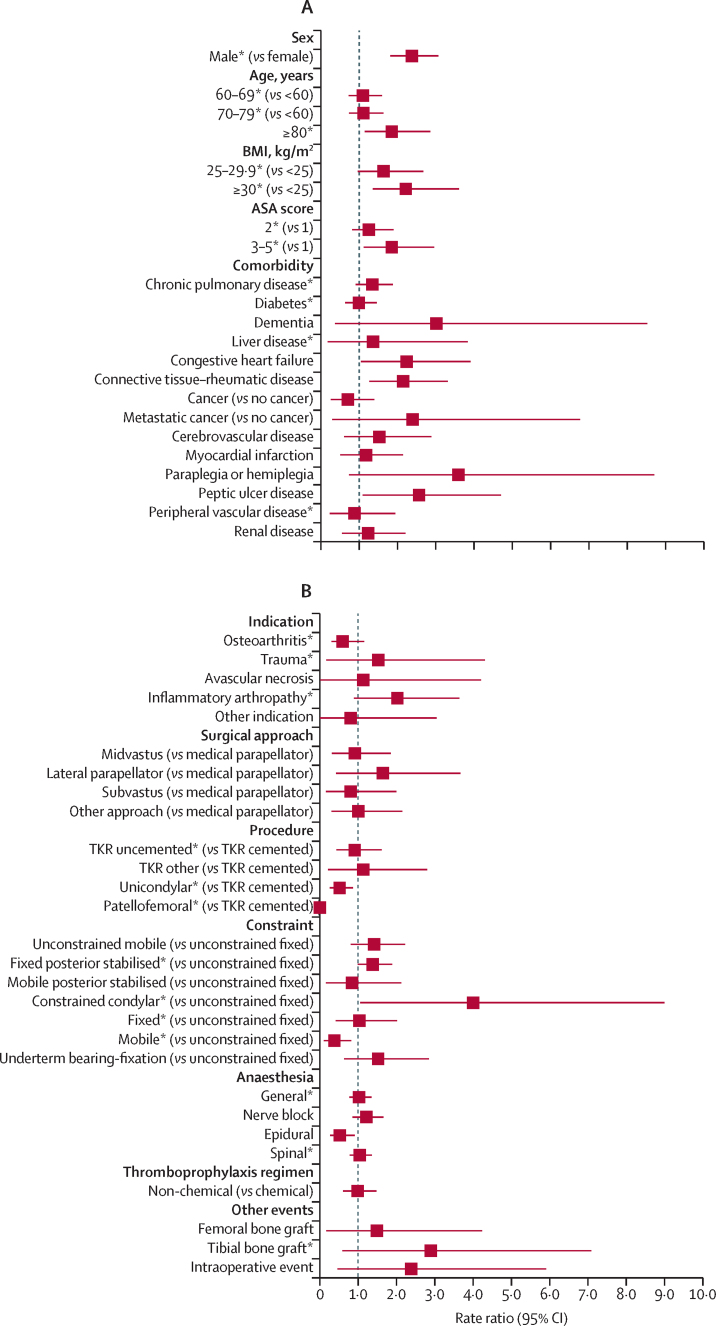
RRs of revision for prosthetic joint infection surgery are presented in appendix pp 10–12. Figure 2 provides RRs over the entire follow-up and figure 3 shows their effect within the first 3 postoperative months. Effects associated with other periods are presented in appendix pp 15–18.
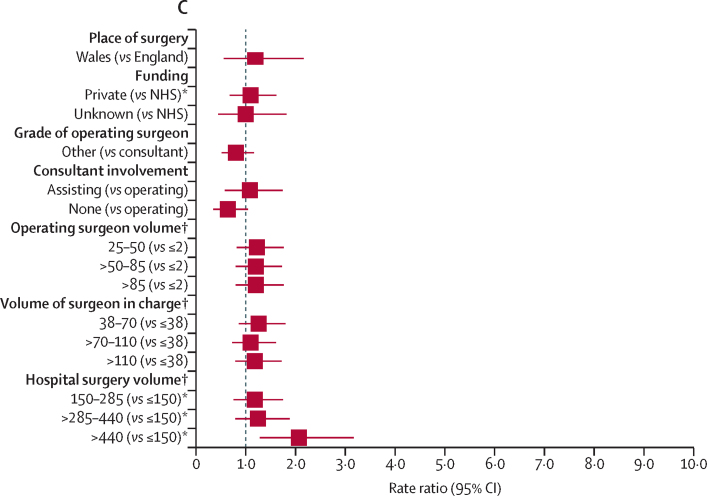
Figure 2.
Risk factors of revision for prosthetic joint infection during the overall postoperative period
(A) Patient factors. (B) Surgery factors. (C) Health-care system factors. Reference categories are in parentheses. BMI=body mass index. ASA=American Society of Anesthesiologists. TKR=total knee replacement. NHS=National Health Service. *Adjusted p value <0·05, detailed in appendix pp 10–12, alongside the rate ratios and 95% CIs. †Volume refers to the total volume of knee replacements done within the previous 12 months.
Figure 3.
Risk factors of revision for prosthetic joint infection in the first 3 postoperative months
(A) Patient factors. (B) Surgery factors. (C) Health-care system factors. Reference categories are in parentheses. BMI=body mass index. ASA=American Society of Anesthesiologists. TKR=total knee replacement. NHS=National Health Service. *Adjusted p value <0·05, detailed in appendix pp 10–12, alongside the rate ratios and 95% CIs. †Volume refers to the total volume of knee replacements performed within the previous 12 months.
In terms of the role of patient characteristics, men were at a higher risk of revision for prosthetic joint infection in all time periods (figure 2). During the entire follow-up, the risk was lower for patients aged 60 years and older than in patients younger than 60 years of age. Patients aged 80 years and older were at increased risk of early revision for prosthetic joint infection but were at lower risk of revision thereafter (appendix pp 10, 15–18). Patients aged 60–79 years were at reduced risk of long-term revision for prosthetic joint infection (appendix pp 10, 15–18).
BMI of 30 kg/m2 or higher was associated with an increased risk of revision for prosthetic joint infection compared with BMI lower than 25 kg/m2 (figure 2), especially after the first year (appendix pp 10, 15–18). Compared with healthy patients, those with an ASA of 2 or higher were at increased risk (figure 2), especially beyond 6 months for ASA 3–5 and after 2 years for ASA 2 (appendix pp 10, 16–18).
Patients with a pre-existing history of chronic pulmonary disease, diabetes, liver disease, connective tissue or rheumatic disease, or peripheral vascular disease had a higher risk than those without pre-existing histories of these diseases (figure 2). Patients with a history of rheumatic disease had a higher risk of revision at most postoperative periods (appendix p 10). Those with liver disease were at higher risk of long-term revision than those without liver disease (appendix pp 10, 15–18). No or inconsistent time-specific effects were observed for the other comorbidities.
In terms of surgical characteristics, risk of revision for prosthetic joint infection varied according to the indication for the primary procedure. Patients operated on for osteoarthritis were less likely to be revised for prosthetic joint infection (figure 2). Those operated on for trauma, history of previous infection in the operated joint (appendix p 11), or an inflammation arthropathy were at increased risk of revision for prosthetic joint infection (figure 2), especially from 2 years post-operation (figure 3; appendix pp 11, 15–17). The indication for surgery did not affect the risk of early revision for prosthetic joint infection (appendix p 11). Patients operated on for trauma or with a history of previous infection were at an increased risk of revision from 1 year onwards (appendix pp 11, 17–18).
The risk of revision varied according to the type of procedure, implant fixation and constraint or bearing, with more extensive and complex procedures associated with an increased risk. Compared with cemented total knee replacement, patients who had an uncemented total knee replacement, a patellofemoral replacement, or an unicondylar replacement were at lower risk of revision for prosthetic joint infection (figure 2). From 6 months onwards, those with a unicondylar procedure were at lower risk of revision for prosthetic joint infection; the reduced risk of revision was observed from 1 year and 2 years onwards respectively for patellofemoral and uncemented total knee replacement procedures (appendix pp 11, 16–18).
The risk of revision was increased for patients with a posterior stabilised fixed-bearing implant or a constrained condylar implant compare with those with an unconstrained (or cruciate-retaining) fixed-bearing implant (figure 2): from 6 postoperative months onwards with a posterior stabilised fixed implant and beyond 1 year post-surgery for a constrained condylar implant (appendix pp 11, 16–18). The risk of revision was lower for patients with fixed or mobile bearing implants, and this finding was particularly evident from 6 months onwards for mobile bearing implants (appendix pp 11, 16–18).
The risk of revision for prosthetic joint infection was higher for patients who had received a general anaesthetic or tibial bone graft, and lower for those who had received a spinal anaesthetic. Little or no difference in the risk of revision for prosthetic joint infection was found for other anaesthetic techniques, thromboprophylaxis regimen, use of femoral bone graft, occurrence of intraoperative complication, or surgical approach.
In terms of health-care system characteristics, the risk of revision for prosthetic joint infection did not differ between Wales and England (figure 2). Privately funded procedures had a lower risk of revision than procedures funded by the NHS (figure 2), especially beyond 2 years (appendix pp 11, 18).
Revision for prosthetic joint infection was not affected by the grade of the operating surgeon, the presence or absence of a consultant surgeon during surgery, or by the volume of all knee procedures done by the operating surgeon or the surgeon in charge (Figure 2, Figure 3, appendix pp 11, 15–18).
The overall risk of revision for prosthetic joint infection was higher in high-volume hospitals than in low-volume hospitals (figure 2). Compared with hospitals with a small volume of activity, the risk of revision was higher in the first 3 months after primary surgery in hospitals that had done more than 440 knee procedures in the year preceding the index surgery (figure 3). No specific difference in the RRs were found beyond this period or for units doing lower volumes of knee procedures (appendix pp 11, 15–18).
Discussion
The revision burden for prosthetic joint infection after knee replacement is higher than that after hip replacement in England and Wales.10, 18 In our cohort of 679 010 knee replacements, 3659 (0·53%) underwent revision for prosthetic joint infection compared with 2707 out of 623 253 (0·43%) hip replacements studied during the same period.13 However, revision within the first 3 months is proportionately less common (6·7% of knee replacements had revision surgery for infection vs 13·8% of hip replacements).
At the patient level, male patients, younger patients, and those with high BMI or more severe systemic disease, indicated by their ASA grade, had higher risk of revision for prosthetic join infection; however older patients (aged ≥80 years) were at high risk of early revision for prosthetic joint infection. This finding might reflect a tendency to treat older patients non-operatively with suppressive antibiotics in the longer term. Comorbidities that increased the risk of revision for prosthetic joint infection included chronic pulmonary disease, diabetes, liver disease, connective tissue or rheumatic diseases, and peripheral vascular disease. Treatment of these comorbidities and elevated BMI can potentially be optimised before surgery. A targeted preoperative intervention for male patients with high BMI and specific comorbidities could be a particularly relevant approach. Long-term vigilance seems to be important in those with liver disease. Our patient-level findings are concordant with the results of our study of infection after hip replacement,13 another large study of knee replacement,28 and systematic reviews.11, 12 Thus, these results might be generalisable to a wide population of patients undergoing implant surgery of various types.
At the surgical level, some of our results are consistent with those in hip surgery, but others are not. In particular, different surgical approaches in knee replacement are not associated with revision for prosthetic joint infection, but the use of general anaesthetic is. In general, factors that are a surrogate marker for operative duration and complexity, such as general anaesthetic, the need for additional constraint, total rather than partial knee replacement, and the use of tibial bone grafts, are associated with increased risk of revision for prosthetic joint infection. Concordant with hip surgery and previous studies,11, 12, 28 patients undergoing joint replacement for trauma or inflammatory arthritis have an increased risk of revision for infection. This finding is unsurprising because inflammatory arthropathies such as rheumatoid arthritis and drugs used to treat these conditions, such as disease-modifying antirheumatic drugs are known to be immunosuppressive.29 The substantially higher risk of prosthetic joint infection in those with historical infection of the knee is a new finding, but again unsurprising, and might be due to quiescent bacteria or other immune conditions that predispose individuals to infection.
Factors at the health-care system level seem to be less important than patient or surgical characteristics, with no notable sustained associations across the time periods studied. As previously reported,28 higher volume centres seemed to have a higher overall risk of revision for prosthetic joint infection and in the early postoperative period, but this association was not seen in the time-specific analysis or when test multiplicity was accounted for, indicating that this effect is not significant and might reflect more rapid diagnosis and early management of prosthetic joint infection in these centres. Privately funded patients were associated with lower long-term risks than those whose treatment was funded by the NHS—a finding not mirrored in our hip study. This difference is likely to reflect residual confounding with variables not available in our analysis because of case selection. The funding source of the primary procedure might therefore be a proxy for socioeconomic status, a patient factor not directly measured in the National Joint Registry.
Smoking has previously been identified as a risk factor for prosthetic joint infection,11, 30 and, although we did not have information about this, the surrogate comorbidity of chronic pulmonary disease was associated with increased risk. Evidence of an association between alcohol intake and increased risk has been inconsistent.31, 32 We noted a higher risk in patients with liver disease, but this outcome might represent a number of pathologies, including alcohol-related liver disease, and non-alcoholic-related disease such as fatty liver disease, hepatitis, haemochromatosis, or primary biliary cirrhosis. Our study corroborates the previous findings of increased risk in patients with diabetes and rheumatoid arthritis.11
The current study has several strengths. To our knowledge, this is the largest and most comprehensive evaluation of patient-related, surgical-related, and healthcare-related factors and their associations with the risk of revision for prosthetic joint infection of the knee. We used a large-scale cohort design comprising of a larger number of participants (>600 000) than those of the most up-to-date reviews on the topic (n=375 895 and n=512 508 hip and knee replacements)11, 12 or individual articles.33 Other strengths include the long-term follow-up of the cohort (median 4·6 years) and advanced statistical analyses, which include the evaluation of the effects of these potential risk factors in time-specific periods, which is appropriate because we have demonstrated that risk is not constant over time. There are also several limitations to our study. Although prospectively collected, our data are observational, and we can only draw inferences about the nature and magnitude of the associations, but not establish causation. In the UK, no agreed national gold standards are available to orthopaedic surgeons for the diagnosis of prosthetic joint infection. As such, the reported indication for revision of prosthetic joint infection in the National Joint Registry might vary between units. The approach used to diagnose prosthetic joint infection is, however, reflective of contemporary practice, with raised inflammatory markers, joint-specific symptoms, sinuses, and positive microbiological cultures.19 The diagnosis of prosthetic joint infection reflects a clinical judgement sufficient to lead the surgeon to conduct a very severe and invasive procedure tailored to tackle the infection. We also acknowledge issues relating to under-reporting of revision for prosthetic joint infection, and thus potentially lower incidence estimates.34 Linkage of the National Joint Registry data to microbiology data could reduce a posteriori any misdiagnoses of prosthetic joint infection, but has been shown to be of limited generalisability, with only 11·8% linkage achievable.35 The associations that we have identified might vary with different causative pathogens, but unfortunately we do not have the data to explore this concept. Our findings should be considered as conservative estimates of the risk factors with the strongest effects. The investigation into the effects of comorbidities was limited to a subset of National Joint Registry patients linked to Hospital Episode Statistics. This subset had higher ASA grade and therefore higher rate of revision for prosthetic joint infection than those excluded from these investigations, but they did not differ in terms of age, sex, BMI, or surgical characteristics, suggesting little evidence of differential selection bias. All other factors were investigated in the entire sample.
We have done appropriate modelling to adjust for known relevant confounders, but the possibility of residual confounding does exist. We had no specific data about confounders such as smoking and alcohol consumption, but have surrogate markers for these such as chronic pulmonary disease and liver disease. BMI data were not collected in the early years of the registry, necessitating imputation of the missing data, as with a previous study on this dataset.36 The duration of surgery is not collected in the National Joint Registry but the surgical characteristics influencing revision for prosthetic joint infection show that this factor is likely to have an important role: knee replacement type, fixation, and constraint are all associated with the duration, and complexity of surgery. This has previously been shown.28 Competing risk due to revision for another cause or death, which in combination affected 13% of the index primary knee replacements in the dataset during the period of observation (figure 1), could not be accounted for in the modelling strategy. This was a pragmatic decision because we chose a strategy focusing on time-specific effects while accounting for the clustering nature of the data, to disentangle the effects associated with surgical factors (likely to be more substantial in the short-term to mid-term follow-up) from those associated with health risk behaviours (likely to be more influential in the mid-term to long-term follow-up period). This strategy was optimal because there was evidence of non-proportional hazard rates (figure 3, appendix pp 15–18). Finally, it was not possible to investigate any ethnic disparities in terms of revision for prosthetic joint infection because of the small number of ethnic minority patients who underwent revision for prosthetic joint infection.
Knee replacement is an effective intervention to address the symptoms arising from degenerative knee conditions such as osteoarthritis. Although successful, complications can occur and prosthetic joint infection is a devastating example. Strategies should therefore be adopted to reduce the risk of infection. Modifiable risk factors could be ameliorated with targeted interventions that could lead to a reduction in the incidence of prosthetic joint infection. When risk factors are not modifiable, they should form part of the information used to counsel and prepare patients for surgery and can form the basis of targeted follow-up and monitoring strategies. The time period-specific effects of the identified risk factors should also form an integral part of the preparation for and management of knee replacement surgery. Overall, the results of this large cohort study could help to better inform the practice and delivery of knee replacement surgery.
Data sharing
Data are accessible via application to the National Joint Registry Research Sub-Committee.
Acknowledgments
Acknowledgments
We thank the patients and staff of all the hospitals who have contributed data to the National Joint Registry, and the Healthcare Quality Improvement Partnership, the National Joint Registry Steering Committee, and staff at the National Joint Registry for facilitating this work. This article presents independent research funded by the National Institute for Health Research (NIHR) under its Programme Grants for Applied Research programme (RP-PG-1210-12005). This study was supported by the NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol (Bristol, UK). The views expressed in this article are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health, or of the National Joint Registry Steering Committee or Healthcare Quality Improvement Partnership, who do not vouch for how the information is presented.
Contributors
EL, MRW, ADB, SKK, PF, MP, and AWB designed the study. The data were extracted by Northgate (Hemel Hempstead, UK). EL, MRW, ADB, SKK, and AWB did the literature search. EL did the data analysis. All authors interpreted data, drafted, and reviewed the final manuscript. All authors approved the submitted manuscript. EL had full access to all the data and AWB is the guarantor.
Declaration of interests
MP is Medical Director of the National Joint Registry and a member of the Programme Steering Committee for the National Institute for Health Research programme grant for applied research (RP-PG-1210-12005). We declare no competing interests.
Supplementary Material
References
- 1.NJR Steering Commitee . National Joint Registry Centre; Hemel Hempstead: 2018. National Joint Registry for England, Wales, Northern Ireland and the Isle of Man: 15th annual report, 2017. [Google Scholar]; NJR Steering Commite, ational Joint Registry for England, Wales, Northern Ireland and the Isle of Man: 15th annual report, 2017 201, 8 National Joint Registry Centr, emel Hempstead
- 2.Blom AW, Brown J, Taylor AH, Pattison G, Whitehouse S, Bannister GC. Infection after total knee arthroplasty. J Bone Joint Surg Br. 2004;86:688–691. doi: 10.1302/0301-620x.86b5.14887. [DOI] [PubMed] [Google Scholar]; AW Blom, J Brown, AH Taylor, G Pattison, S Whitehouse, GC Bannister. Infection after total knee arthroplasty. J Bone Joint Surg Br, 86, 2004, 688–691 [DOI] [PubMed]
- 3.Nickinson RS, Board TN, Gambhir AK, Porter ML, Kay PR. The microbiology of the infected knee arthroplasty. Int Orthop. 2010;34:505–510. doi: 10.1007/s00264-009-0797-y. [DOI] [PMC free article] [PubMed] [Google Scholar]; RS Nickinson, TN Board, AK Gambhir, ML Porter, PR Kay. The microbiology of the infected knee arthroplasty. Int Orthop, 34, 2010, 505–510 [DOI] [PMC free article] [PubMed]
- 4.Boddapati V, Fu MC, Mayman DJ, Su EP, Sculco PK, McLawhorn AS. Revision total knee arthroplasty for periprosthetic joint infection is associated with increased postoperative morbidity and mortality relative to noninfectious revisions. J Arthroplasty. 2018;33:521–526. doi: 10.1016/j.arth.2017.09.021. [DOI] [PubMed] [Google Scholar]; V Boddapati, MC Fu, DJ Mayman, EP Su, PK Sculco, AS McLawhorn. Revision total knee arthroplasty for periprosthetic joint infection is associated with increased postoperative morbidity and mortality relative to noninfectious revisions. J Arthroplasty, 33, 2018, 521–526 [DOI] [PubMed]
- 5.Kapadia BH, Berg RA, Daley JA, Fritz J, Bhave A, Mont MA. Periprosthetic joint infection. Lancet. 2016;387:386–394. doi: 10.1016/S0140-6736(14)61798-0. [DOI] [PubMed] [Google Scholar]; BH Kapadia, RA Berg, JA Daley, J Fritz, A Bhave, MA Mont. Periprosthetic joint infection. Lancet, 387, 2016, 386–394 [DOI] [PubMed]
- 6.Kunutsor SK, Beswick AD, Peters TJ. Health care needs and support for patients undergoing treatment for prosthetic joint infection following hip or knee arthroplasty: a systematic review. PLoS One. 2017;12:e0169068. doi: 10.1371/journal.pone.0169068. [DOI] [PMC free article] [PubMed] [Google Scholar]; SK Kunutsor, AD Beswick, TJ Peters. Health care needs and support for patients undergoing treatment for prosthetic joint infection following hip or knee arthroplasty: a systematic review. PLoS One, 12, 2017, e0169068 [DOI] [PMC free article] [PubMed]
- 7.Mallon CM, Gooberman-Hill R, Moore AJ. Infection after knee replacement: a qualitative study of impact of periprosthetic knee infection. BMC Musculoskelet Disord. 2018;19:352. doi: 10.1186/s12891-018-2264-7. [DOI] [PMC free article] [PubMed] [Google Scholar]; CM Mallon, R Gooberman-Hill, AJ Moore. Infection after knee replacement: a qualitative study of impact of periprosthetic knee infection. BMC Musculoskelet Disord, 19, 2018, 352 [DOI] [PMC free article] [PubMed]
- 8.Kunutsor SK, Whitehouse MR, Lenguerrand E, Blom AW, Beswick AD, Team I. Re-infection outcomes following one- and two-stage surgical revision of infected knee prosthesis: a systematic review and meta-analysis. PLoS One. 2016;11:e0151537. doi: 10.1371/journal.pone.0151537. [DOI] [PMC free article] [PubMed] [Google Scholar]; SK Kunutsor, MR Whitehouse, E Lenguerrand, AW Blom, AD Beswick, I Team. Re-infection outcomes following one- and two-stage surgical revision of infected knee prosthesis: a systematic review and meta-analysis. PLoS One, 11, 2016, e0151537 [DOI] [PMC free article] [PubMed]
- 9.Zimmerli W, Trampuz A, Ochsner PE. Prosthetic-joint infections. N Engl J Med. 2004;351:1645–1654. doi: 10.1056/NEJMra040181. [DOI] [PubMed] [Google Scholar]; W Zimmerli, A Trampuz, PE Ochsner. Prosthetic-joint infections. N Engl J Med, 351, 2004, 1645–1654 [DOI] [PubMed]
- 10.Lenguerrand E, Whitehouse MR, Beswick AD. Description of the rates, trends and surgical burden associated with revision for prosthetic joint infection following primary and revision knee replacements in England and Wales: an analysis of the National Joint Registry for England, Wales, Northern Ireland and the Isle of Man. BMJ Open. 2017;7:e014056. doi: 10.1136/bmjopen-2016-014056. [DOI] [PMC free article] [PubMed] [Google Scholar]; E Lenguerrand, MR Whitehouse, AD Beswick. Description of the rates, trends and surgical burden associated with revision for prosthetic joint infection following primary and revision knee replacements in England and Wales: an analysis of the National Joint Registry for England, Wales, Northern Ireland and the Isle of Man. BMJ Open, 7, 2017, e014056 [DOI] [PMC free article] [PubMed]
- 11.Kunutsor SK, Whitehouse MR, Blom AW, Beswick AD, Team I. Patient-related risk factors for periprosthetic joint infection after total joint arthroplasty: a systematic review and meta-analysis. PLoS One. 2016;11:e0150866. doi: 10.1371/journal.pone.0150866. [DOI] [PMC free article] [PubMed] [Google Scholar]; SK Kunutsor, MR Whitehouse, AW Blom, AD Beswick, I Team. Patient-related risk factors for periprosthetic joint infection after total joint arthroplasty: a systematic review and meta-analysis. PLoS One, 11, 2016, e0150866 [DOI] [PMC free article] [PubMed]
- 12.Kong L, Cao J, Zhang Y, Ding W, Shen Y. Risk factors for periprosthetic joint infection following primary total hip or knee arthroplasty: a meta-analysis. Int Wound J. 2017;14:529–536. doi: 10.1111/iwj.12640. [DOI] [PMC free article] [PubMed] [Google Scholar]; L Kong, J Cao, Y Zhang, W Ding, Y Shen. Risk factors for periprosthetic joint infection following primary total hip or knee arthroplasty: a meta-analysis. Int Wound J, 14, 2017, 529–536 [DOI] [PMC free article] [PubMed]
- 13.Lenguerrand E, Whitehouse MR, Beswick AD. Risk factors associated with revision for prosthetic joint infection after hip replacement: a prospective observational cohort study. Lancet Infect Dis. 2018;18:1004–1014. doi: 10.1016/S1473-3099(18)30345-1. [DOI] [PMC free article] [PubMed] [Google Scholar]; E Lenguerrand, MR Whitehouse, AD Beswick. Risk factors associated with revision for prosthetic joint infection after hip replacement: a prospective observational cohort study. Lancet Infect Dis, 18, 2018, 1004–1014 [DOI] [PMC free article] [PubMed]
- 14.Dieppe PA, Lohmander LS. Pathogenesis and management of pain in osteoarthritis. Lancet. 2005;365:965–973. doi: 10.1016/S0140-6736(05)71086-2. [DOI] [PubMed] [Google Scholar]; PA Dieppe, LS Lohmander. Pathogenesis and management of pain in osteoarthritis. Lancet, 365, 2005, 965–973 [DOI] [PubMed]
- 15.Sayers A, Wylde V, Lenguerrand E. Rest pain and movement-evoked pain as unique constructs in hip and knee replacements. Arthritis Care Res. 2016;68:237–245. doi: 10.1002/acr.22656. [DOI] [PMC free article] [PubMed] [Google Scholar]; A Sayers, V Wylde, E Lenguerrand. Rest pain and movement-evoked pain as unique constructs in hip and knee replacements. Arthritis Care Res, 68, 2016, 237–245 [DOI] [PMC free article] [PubMed]
- 16.Lenguerrand E, Wylde V, Gooberman-Hill R. Trajectories of pain and function after primary hip and knee arthroplasty: the ADAPT cohort study. PLoS One. 2016;11:e0149306. doi: 10.1371/journal.pone.0149306. [DOI] [PMC free article] [PubMed] [Google Scholar]; E Lenguerrand, V Wylde, R Gooberman-Hill. Trajectories of pain and function after primary hip and knee arthroplasty: the ADAPT cohort study. PLoS One, 11, 2016, e0149306 [DOI] [PMC free article] [PubMed]
- 17.Blom AW, Artz N, Beswick AD. NIHR Journals Library; Southampton, UK: 2016. Improving patients' experience and outcome of total joint replacement: the RESTORE programme. [PubMed] [Google Scholar]; AW Blom, N Artz, AD Beswick. Improving patients' experience and outcome of total joint replacement: the RESTORE programm, e 201, 6 NIHR Journals Librar, outhampton, UK [PubMed]
- 18.Lenguerrand E, Whitehouse MR, Beswick AD, Jones SA, Porter ML, Blom AW. Revision for prosthetic joint infection following hip arthroplasty: evidence from the National Joint Registry. Bone Joint Res. 2017;6:391–398. doi: 10.1302/2046-3758.66.BJR-2017-0003.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]; E Lenguerrand, MR Whitehouse, AD Beswick, SA Jones, ML Porter, AW Blom. Revision for prosthetic joint infection following hip arthroplasty: evidence from the National Joint Registry. Bone Joint Res, 6, 2017, 391–398 [DOI] [PMC free article] [PubMed]
- 19.Parvizi J, Fassihi SC, Enayatollahi MA. Diagnosis of periprosthetic joint infection following hip and knee arthroplasty. Orthop Clin North Am. 2016;47:505–515. doi: 10.1016/j.ocl.2016.03.001. [DOI] [PubMed] [Google Scholar]; J Parvizi, SC Fassihi, MA Enayatollahi. Diagnosis of periprosthetic joint infection following hip and knee arthroplasty. Orthop Clin North Am, 47, 2016, 505–515 [DOI] [PubMed]
- 20.Matthews PC, Berendt AR, McNally MA, Byren I. Diagnosis and management of prosthetic joint infection. BMJ. 2009;338:b1773. doi: 10.1136/bmj.b1773. [DOI] [PubMed] [Google Scholar]; PC Matthews, AR Berendt, MA McNally, I Byren. Diagnosis and management of prosthetic joint infection. BMJ, 338, 2009, b1773 [DOI] [PubMed]
- 21.National Joint Registry Data collection forms. June 4, 2018. http://www.njrcentre.org.uk/njrcentre/Healthcare-providers/Collecting-data/Data-collection-forms; National Joint Registr, ata collection forms, http://www.njrcentre.org.uk/njrcentre/Healthcare-providers/Collecting-data/Data-collection-form, une 4, 2018
- 22.Quan H, Sundararajan V, Halfon P. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]; H Quan, V Sundararajan, P Halfon. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care, 43, 2005, 1130–1139 [DOI] [PubMed]
- 23.Blossfeld H, Golsch K, Rohwer G. Piecewise constant exponential model. 5.4. Models with period-specific effects. In: Blossfeld H, Golsch K, Rohwer G, editors. Event history analysis with Stata. Psychology Press; Hove: 2009. pp. 123–127. [Google Scholar]; H Blossfeld, K Golsch, G Rohwer. Piecewise constant exponential model. 5.4. Models with period-specific effects, H Blossfeld K Golsch G Rohwer, Event history analysis with Stat, a 200, 9 Psychology Pres, ov, e 123–127
- 24.Sera F, Ferrari P. A multilevel model to estimate the within- and the between-center components of the exposure/disease association in the EPIC study. PLoS One. 2015;10:e0117815. doi: 10.1371/journal.pone.0117815. [DOI] [PMC free article] [PubMed] [Google Scholar]; F Sera, P Ferrari. A multilevel model to estimate the within- and the between-center components of the exposure/disease association in the EPIC study. PLoS One, 10, 2015, e0117815 [DOI] [PMC free article] [PubMed]
- 25.Leckie G, Charlton C. runmlwin: a program to run the MLwiN multilevel modeling software from within Stata. J Stat Softw. 2012;52(11):1–40. [Google Scholar]; G Leckie, C Charlton. runmlwin: a program to run the MLwiN multilevel modeling software from within Stata. J Stat Softw, 52 11 2012, 1–40
- 26.Benjamini Y, Hochber D. The control of the false discovery rate in multiple testing under dependency. Ann Stat. 2001;29:1165–1188. [Google Scholar]; Y Benjamini, D Hochber. The control of the false discovery rate in multiple testing under dependency. Ann Stat, 29, 2001, 1165–1188
- 27.Newson RB. Frequentist q-values for multiple-test procedures. Stata J. 2010;10:568–584. [Google Scholar]; RB Newson. Frequentist q-values for multiple-test procedures. Stata J, 10, 2010, 568–584
- 28.Namba RS, Inacio MC, Paxton EW. Risk factors associated with deep surgical site infections after primary total knee arthroplasty: an analysis of 56,216 knees. J Bone Joint Surg Am. 2013;95:775–782. doi: 10.2106/JBJS.L.00211. [DOI] [PubMed] [Google Scholar]; RS Namba, MC Inacio, EW Paxton. Risk factors associated with deep surgical site infections after primary total knee arthroplasty: an analysis of 56,216 knees. J Bone Joint Surg Am, 95, 2013, 775–782 [DOI] [PubMed]
- 29.Baker JF, George MD. Prevention of infection in the perioperative setting in patients with rheumatic disease treated with immunosuppression. Curr Rheum Rep. 2019;21:17. doi: 10.1007/s11926-019-0812-2. [DOI] [PMC free article] [PubMed] [Google Scholar]; JF Baker, MD George. Prevention of infection in the perioperative setting in patients with rheumatic disease treated with immunosuppression. Curr Rheum Rep, 21, 2019, 17 [DOI] [PMC free article] [PubMed]
- 30.Everhart JS, Altneu E, Calhoun JH. Medical comorbidities are independent preoperative risk factors for surgical infection after total joint arthroplasty. Clin Orthop Relat Res. 2013;471:3112–3119. doi: 10.1007/s11999-013-2923-9. [DOI] [PMC free article] [PubMed] [Google Scholar]; JS Everhart, E Altneu, JH Calhoun. Medical comorbidities are independent preoperative risk factors for surgical infection after total joint arthroplasty. Clin Orthop Relat Res, 471, 2013, 3112–3119 [DOI] [PMC free article] [PubMed]
- 31.Maradit Kremers H, Kremers WK, Berry DJ, Lewallen DG. Social and behavioral factors in total knee and hip arthroplasty. J Arthroplasty. 2015;30:1852–1854. doi: 10.1016/j.arth.2015.04.032. [DOI] [PubMed] [Google Scholar]; H Maradit Kremers, WK Kremers, DJ Berry, DG Lewallen. Social and behavioral factors in total knee and hip arthroplasty. J Arthroplasty, 30, 2015, 1852–1854 [DOI] [PubMed]
- 32.Poultsides LA, Ma Y, Della Valle AG, Chiu YL, Sculco TP, Memtsoudis SG. In-hospital surgical site infections after primary hip and knee arthroplasty—incidence and risk factors. J Arthroplasty. 2013;28:385–389. doi: 10.1016/j.arth.2012.06.027. [DOI] [PubMed] [Google Scholar]; LA Poultsides, Y Ma, AG Della Valle, YL Chiu, TP Sculco, SG Memtsoudis. In-hospital surgical site infections after primary hip and knee arthroplasty—incidence and risk factors. J Arthroplasty, 28, 2013, 385–389 [DOI] [PubMed]
- 33.George DA, Drago L, Scarponi S, Gallazzi E, Haddad FS, Romano CL. Predicting lower limb periprosthetic joint infections: a review of risk factors and their classification. World J Orthop. 2017;8:400–411. doi: 10.5312/wjo.v8.i5.400. [DOI] [PMC free article] [PubMed] [Google Scholar]; DA George, L Drago, S Scarponi, E Gallazzi, FS Haddad, CL Romano. Predicting lower limb periprosthetic joint infections: a review of risk factors and their classification. World J Orthop, 8, 2017, 400–411 [DOI] [PMC free article] [PubMed]
- 34.Gundtoft PH, Overgaard S, Schonheyder HC, Moller JK, Kjaersgaard-Andersen P, Pedersen AB. The “true” incidence of surgically treated deep prosthetic joint infection after 32,896 primary total hip arthroplasties: a prospective cohort study. Acta Orthop. 2015;86:326–334. doi: 10.3109/17453674.2015.1011983. [DOI] [PMC free article] [PubMed] [Google Scholar]; PH Gundtoft, S Overgaard, HC Schonheyder, JK Moller, P Kjaersgaard-Andersen, AB Pedersen. The “true” incidence of surgically treated deep prosthetic joint infection after 32,896 primary total hip arthroplasties: a prospective cohort study. Acta Orthop, 86, 2015, 326–334 [DOI] [PMC free article] [PubMed]
- 35.Holleyman RJ, Baker P, Charlett A, Gould K, Deehan DJ. Microorganisms responsible for periprosthetic knee infections in England and Wales. Knee Surg Sports Traumatol Arthrosc. 2016;24:3080–3087. doi: 10.1007/s00167-015-3539-2. [DOI] [PubMed] [Google Scholar]; RJ Holleyman, P Baker, A Charlett, K Gould, DJ Deehan. Microorganisms responsible for periprosthetic knee infections in England and Wales. Knee Surg Sports Traumatol Arthrosc, 24, 2016, 3080–3087 [DOI] [PubMed]
- 36.Hunt LP, Ben-Shlomo Y, Clark EM. 90-day mortality after 409,096 total hip replacements for osteoarthritis, from the National Joint Registry for England and Wales: a retrospective analysis. Lancet. 2013;382:1097–1104. doi: 10.1016/S0140-6736(13)61749-3. [DOI] [PubMed] [Google Scholar]; LP Hunt, Y Ben-Shlomo, EM Clark. 90-day mortality after 409,096 total hip replacements for osteoarthritis, from the National Joint Registry for England and Wales: a retrospective analysis. Lancet, 382, 2013, 1097–1104 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are accessible via application to the National Joint Registry Research Sub-Committee.