Abstract
Purpose
Hepatocellular adenoma (HCA) is a rare benign monoclonal neoplasm, recently categorized on genetic and histopathological basis into four subtypes with different biological behaviors. Since contrast-enhanced ultrasonography (CEUS) is nowadays a well-established technique for liver nodule characterization, the aim of our study was to assess CEUS features of HCAs to identify criteria that correlate with different HCA subtypes as compared to histopathologic examination and other imaging modalities.
Methods
We retrospectively analyzed data of patients with histology-proven HCA who underwent CEUS, computed tomography or magnetic resonance imaging (MRI) in seven different Italian ultrasound units.
Results
The study enrolled 19 patients (16 females; 69% with concomitant/prior use of oral contraceptives): the mean size of all HCAs was 4.2 cm (range 1.6–7.1 cm); 14/19 had inflammatory HCAs (I-HCA), 1/19 β-catenin-activated HCA, and the others unclassified HCAs. On CEUS, during the arterial phase, all but one HCA displayed a rapid enhancement, with 89% of these showing centripetal and 11% centrifugal filling pattern, whereas during the portal and late venous phase 58% of HCA showed washout and the remaining 42% displayed persistent enhancement. In particular, among I-HCAs 7/14 showed no washout, 3/14 and 4/14 showed washout in the portal or late phase, respectively.
Conclusions
This dataset represents one of the few published experiences on HCAs and CEUS in Italy and shows that HCAs are hypervascularized in the arterial phase usually with a centripetal flow pattern and have a heterogeneous behavior in portal and late phase. In particular, occurrence of delayed washout on CEUS but not on MRI is frequently observed in the subtype of I-HCA.
Keywords: Contrast-enhanced ultrasound, Hepatocellular adenoma, Phenotype classification, Benign liver lesion, Magnetic resonance imaging
Riassunto
Introduzione
L′adenoma epatico (HCA) rappresenta una rara neoplasia primitiva del fegato, recentemente classificata in quattro diversi sottotipi sulla base delle caratteristiche istopatologiche e del comportamento biologico. In considerazione dell’ampio e diffuso utilizzo dell’ecografia con mezzo di contrasto ecografico (CEUS) nella valutazione non-invasiva delle lesioni focali epatiche l’obiettivo di questo studio è stato quello di documentare in una casistica multicentrica le caratteristiche CEUS di lesioni focali epatiche già caratterizzate come HCA e di valutare le eventuali correlazioni con i diversi sottotipi istologici e con altre metodiche di imaging (CT/MRI).
Metodi
Sono stati raccolti retrospettivamente le informazioni su pazienti con diagnosi istologica di HCA sottoposti a CEUS e CT ± MR in sette diversi centri italiani di ecografia.
Risultati
Sono stati inclusi nello studio 19 pazienti con diagnosi istologica di HCA (16 donne; 69% con storia attuale e/o pregressa di utilizzo di farmaci estroprogestinici): 14/19 adenomi sottotipo “infiammatori” (IHCA), 1/19 β-catenin-activated HCA e i restanti erano HCA non classificabili. L’esame CEUS ha mostrato nella quasi totalità dei casi (18/19) un rapido enhancement arterioso di tipo centripeto (89%) o centrifugo (11%). Durante la fase portale e tardiva si è dimostrato un wash-out contrastografico rispettivamente nel 58% degli HCA; invece nel 42% dei rimanenti casi non è stato osservato wash-out in nessuna delle fasi contrastografiche. In particolare è stato evidenziato che nel sottotipo I-HCA 7/14 non presentavano washout in nessuna delle fasi contrastografiche, mentre 3/14 e 4/14 mostravano rispettivamente un washout nelle fasi portali o tardive.
Conclusioni
La nostra casistica rappresenta una delle poche esperienze italiane presenti in letteratura riguardo all’utilizzo della CEUS negli adenomi epatici, confermando l’aspetto di ipervascolarizzazione nella fase arteriosa (soprattutto con un flusso centripeto) ed il comportamento eterogeneo nelle fasi portali e tardive. In particolare, nel caso di I-HCA un comportamento contrastografico caratterizzato da washout in fase tardiva è frequente con l’utilizzo della CEUS ma non con l’utilizzo della MRI.
Introduction
Hepatocellular adenoma (HCA) is a rare benign monoclonal neoplasm of the liver with an estimated incidence of 1–1.3 million cases per year in North America and Europe and a male-to-female ratio of approximately 1:9/10 [1]. It occurs mainly in females of childbearing age taking oral contraceptives for a long period (> 2 years), in the setting of androgenic steroid therapy and type I, III and IV glycogen storage disease (GSD) [2–4]. In recent years, a growing incidence of HCAs has been reported, especially in males, linked to the rising prevalence of metabolic syndrome and anabolic substance misuse related to sport [5, 6].
Unlike other benign neoplasms, HCA requires long-term follow-up and eventually surgical resection due to the potential for hemorrhage and malignant transformation into hepatocellular carcinoma (HCC), which occur in about 27.2% and 4.2% of cases, respectively [7, 8]. As a consequence, HCA differentiation from other hepatic tumors and identification of major risk lesions are of great significance, because of different outcomes and management strategies for the patient. However, it still represents a diagnostic challenge because HCAs may show a wide range of imaging appearances related to fat content, hemorrhage or malignant degeneration.
A better understanding of the natural history of this neoplasm came from the results of comprehensive genotype–phenotype analyses which have indicated that HCA is not a single entity but a heterogeneous group of tumors encompassing four distinct subtypes on the basis of genetic and histopathological features: hepatocyte nuclear factor-1 alpha inactivated HCA (H-HCA), inflammatory HCA (I-HCA), β-catenin-activated HCA (B-HCA) and unclassified HCA (UNC-HCA). They account for 40–55%, 30–40%, 10–20% and 5–10% of all HCAs, respectively, and show different biological behaviors [9–11]. In particular, I-HCAs carry the highest risk of hemorrhage, while malignant transformation has been observed mainly in B-HCAs and rarely in H-HCAs [8]. As a consequence, identification of HCA subtype is mandatory, since each molecular subgroup is likely to be differently managed [10].
Magnetic resonance imaging (MRI) is considered the technique of choice for HCA diagnosis. Previous MRI studies reported specific features of two main subtypes, owing to fat repartition in H-HCAs and telangiectasia component in I-HCAs [12–14]. Contrast-enhanced sonography (CEUS) has been established as a potential alternative to MRI for the study of focal liver lesions, allowing continuous imaging over the whole enhancement period with the advantages related to cost-effectiveness, ready availability and safety in multiple clinical settings. Small studies have recently reported a good correlation between CEUS and MRI findings in H-HCAs and some discordance when I-HCAs were analyzed [15, 16]. However, since HCAs are rare lesions, information about CEUS behavior of distinct HCA subgroups are limited and mainly related to small monocentric series [17].
The aim of this multicenter study was to document the dynamic behavior of HCAs on CEUS, to identify non-invasive criteria that correlate with different HCA subtypes and to create a database for future analysis. For this purpose, we retrospectively analyzed the data of 19 histology-proven HCAs comparing CEUS features with histopathologic examination.
Materials and methods
Patient population
This retrospective study was approved by the institutional review board of the Catholic University of the Sacred Heart of Rome and involved seven different Italian ultrasound units. Requirement for informed consent was waived. The study group included 19 patients (16 females), who underwent CEUS for diagnosis of focal liver lesions between 2008 and 2017. Histopathologic examination of specimens obtained by percutaneous needle biopsy or hepatic resection was mandatory to confirm diagnosis and to classify the case into the four known subtypes based on morphological and/or immunohistochemical criteria. Demographic and clinical information were also collected.
Image technique
Contrast-enhanced ultrasonography was performed by physicians expert in CEUS and in case of patients with more than one lesion, the largest one was chosen for the examination. All patients underwent a baseline grayscale US to identify each hepatic lesion, and the following data were recorded: US pattern and steatosis degree of the liver parenchyma; number of tumors; location, size, US pattern and color Doppler appearances of the lesion evaluated with CEUS. The target lesion was then observed after bolus injection of 2.4 mL of a microbubble agent (SonoVue, Bracco, Italy) followed by a 10-mL saline flush through a peripheral vein. The arterial phase was defined as the interval between 10 and 30–45 s after the completion of the flush. The portal venous phase was defined as the interval between 30–45 and 120 s, and the subsequent late phase was observed until 5 min after injection in accordance with the last EFSUMB guidelines for the use of ultrasound contrast agents [18]. The following parameters were analyzed: arterial phase enhancement pattern and filling direction, presence of washout during portal and late venous phase. Washout was defined as a reduction in enhancement as compared to the adjacent parenchyma, while lesions with a complete or incomplete degree washout (i.e., with the presence of non-enhancing regions) in the portal and/or late venous phase were both considered as having washout for the sake of the analysis.
Results were compared with the following MRI parameters: signal intensity of the lesion on unenhanced T1- and T2-weighted (w) images compared to the adjacent parenchyma, enhancement pattern in arterial, portal and late phase and homogeneity of the enhancement. CEUS findings were also compared with dynamic TC pattern, if available or if MRI was not performed.
Statistical analysis
Due to low prevalence of HCA in the general population, we designed a multicenter study to obtain an adequate power of detecting clinically relevant differences between groups. Twenty-eight subjects per group of HCA histotypes were needed to detect at least 40% difference in US or baseline characteristics with alpha 0.05 and power 0.8. Despite this, the sample size was not sufficient to carry out an inferential analysis. Therefore, only descriptive statistics was run on the population under study.
Results
Nineteen patients evaluated in seven participating centers were included in the analysis. Clinical characteristics of the patients and the mode of HCA discovery are reported in Table 1. Among the patients, 16 were female (male-to-female ratio 1:5/6) and 69% of them have been taking oral contraceptives for a long period (from 1.5 to more than 20 years). One of the males reported prior use of anabolic steroids. Age at diagnosis ranged from 28 to 51 years (mean age 36.7 ± 7 years). Seven patients (36%) had a BMI ≥ 25 kg/m2. No one reported a history of alcohol abuse. A bright liver echo pattern was detected in eight patients (42%) in whom a diagnosis of non-alcoholic fatty liver disease (NAFLD) was established. Three patients (16%) had GSD. Twelve patients had single HCA, whereas the remaining seven patients had more than one lesion. Diagnosis was incidental in 63% of cases, and less frequently it was made in the setting of abdominal pain (16%) or abnormal liver function test (21%). No one presented with acutely bleeding lesion and only one asymptomatic patient with liver function test abnormalities showed previous hemorrhagic necrosis within the lesion. The histological diagnosis was achieved with percutaneous biopsy in 15/19 cases and with hepatic resection in the other cases; 14 lesions were I-HCA, 1 was B-HCA and the other 4 were UNC-HCA.
Table 1.
Patients’ characteristics
| Case no. | Age (years) | Gender | BMI (kg/m2) | Diabetes | Alcohol intake | Oral contraceptive use (duration) | Chronic hepatic disease | Mode of discovery | Single or multiple nodules | Other |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 51 | F | 23.9 | No | No | Yes (6 years) | No | Abdominal pain | Multiple | NA |
| 2 | 31 | F | 28.3 | No | No | Yes (12 years) | NAFLD | Abdominal pain | Multiple | NA |
| 3 | 33 | F | 24.7 | No | No | Yes (15 years) | No | Incidental | Multiple | NA |
| 4 | 34 | M | 25.9 | No | No | NA | No | Abnormal LFT | Single | Prior use of anabolic steroids |
| 5 | 38 | F | 22.7 | No | No | No | No | Incidental | Single | NA |
| 6 | 29 | F | 20.3 | No | No | No | No | Incidental | Single | NA |
| 7 | 28 | M | 28.1 | No | No | NA | NAFLD | Incidental | Single | NA |
| 8 | 41 | M | 25 | No | No | NA | NAFLD | Incidental | Single | GSD |
| 9 | 36 | F | > 30 | No | No | Yes (10 years) | NAFLD | Incidental | Multiple | NA |
| 10 | 51 | F | 31.2 | Yes | No | Yes (20 years) | NAFLD | Incidental | Single | NA |
| 11 | 39 | F | 24.1 | No | No | Yes (20 years) | No | Abnormal LFT | Multiple | NA |
| 12 | 40 | F | 20.3 | No | No | Yes (10 years) | No | Incidental | Multiple | NA |
| 13 | 43 | F | 22 | No | No | No | No | Incidental | Single | NA |
| 14 | 44 | F | 25 | No | No | Yes (20 years) | No | Incidental | Single | NA |
| 15 | 31 | F | 26.8 | No | No | Yes (5 years) | NAFLD | Abdominal pain | Multiple | NA |
| 16 | 37 | F | 23.8 | No | No | Yes (1.5 years) | No | Incidental | Single | NA |
| 17 | 29 | F | 20.3 | No | No | No | NAFLD | Abnormal LFT | Single | GSD |
| 18 | 31 | F | 22.3 | No | No | No | No | Abnormal LFT | Single | GSD |
| 19 | 31 | F | 20.8 | No | No | Yes (2.5 years) | NAFLD | Incidental | Single | NA |
BMI body mass index, NA not applicable, NAFLD non-alcoholic fatty liver disease, LFT liver function tests, GSD glycogen storage disease
The imaging features of the HCAs are reported in Table 2. The mean size of all HCAs was 4.2 cm, ranging from 1.6 to 7.1 cm. On standard B-mode imaging, 47.4% of HCAs were hypoechoic (6/14 I-HCA, 3/4 UNC-HCAs), while 31.6% were hyperechoic (4/14 I-HCAs, 1/1 B-HCA, 1/1 UNC-HCA). Color Doppler flow imaging detected arterial and/or venous flow signals in the majority of HCAs and no signaling in 5/19 (26.3%) cases. Most lesions showed only perilesional Doppler signals, whereas exclusively intralesional or both intralesional and perilesional Doppler flow signals were observed only in I-HCAs (5/14 and 2/14, respectively). On CEUS, during arterial phase all HCAs but one (94.7%) displayed a rapid enhancement, with 89% of these showing centripetal and 11% centrifugal filling pattern. The only one lesion without arterial enhancement was an UNC-HCA. During portal and/or late venous phase, 58% of HCAs showed complete or partial and mainly central washout and the remaining 42% displayed persistent enhancement. In particular, among I-HCAs 7/14, showed no washout, 3/14 and 4/14 showed washout in the portal/late phases or only in the late phase, respectively. The B-HCA and all the UNC-HCAs except one showed portal or late washout.
Table 2.
Radiologic features of the hepatocellular adenomas
| Case no. | Size (cm) | US pattern (echogenicity) | Color Doppler flow pattern | CEUS arterial phase filling | CEUS portal phase washout | CEUS late phase washout | MRI non-contrast pattern (T1W intensity–T2W intensity) | MRI contrast pattern (arterial–portal–venous T1 W intensity) | Histological diagnosis |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 6.0 | Hyper | No FS | Centrifugal | No | Yes | NA | NA | I-HCA |
| 2 | 2.5 | Hypo | Intralesional AS and VS | Centripetal | No | No | NA | NA | I-HCA |
| 3 | 1.6 | Hypo | Intralesional AS and VS | Centripetal | No | No | Iso–Hyper | Hyper–Hypo–Hypo | I-HCA |
| 4 | 5.1 | Mixed | Perilesional AS and VS, intralesional VS | Centripetal | No | No | Hypo–Hyper | Hyper–Iso–Iso with inhomogeneous internal area suspected for hemorrhagic necrosis | I-HCA |
| 5 | 2.8 | Iso | Perilesional AS/VS | Centripetal | No | No | Hypo–Hyper | Hyper–Hypo–Iso | I-HCA |
| 6 | 3.2 | Hypo | Perilesional AS/VS | Centrifugal | No | Yes | Hypo–Hyper | Hyper–Hypo–Iso | UNC-HCA |
| 7 | 5.6 | Hyper | Perilesional VS | Centripetal | Yes | Yes | Iso–Hyper | Hyper–Hypo–Hypo | B-HCA |
| 8 | 4.8 | Hypo | Perilesional VS | Centripetal | No | Yes | NA | NA | UNC-HCA |
| 9 | 2.1 | Hypo | No FS | Centripetal | No | No | NA | Hyper–Hyper–Hyper | I-HCA |
| 10 | 4.4 | Hypo | No FS | Centripetal | Yes | Yes | Mixed–Mixed | Hyper–Hyper–Hyper with internal hypointense areas | I-HCA |
| 11 | 4.0 | Hyper | Perilesional AS, intralesional AS | Centripetal | No | No | Hypo–Hyper | Hyper–Iso–Iso | I-HCA |
| 12 | 4.0 | Hyper | No FS | No filling | Yes | Yes | NA | NA | UNC-HCA |
| 13 | 3.0 | Hypo | No FS | Centripetal | No | Yes | Hypo–Hyper | Hyper–Hyper–Hyper | I-HCA |
| 14 | 3.9 | Hypo | Perilesional AS | Centripetal | No | No | NA | NA | UNC-HCA |
| 15 | 7.1 | Hyper | Perilesional AS | Centripetal | Yes | Yes | Hypo–Hyper | Hyper–Iso–Hyper | I-HCA |
| 16 | 5.3 | Hyper | Intralesional AS | Centripetal | Yes | Yes | Hypo–Hyper | Hyper–Hyper–Hyper | I-HCA |
| 17 | 6.0 | Mixed | Perilesional AS | Centripetal | No | No | Hyper–Hyper | Hyper–Hyper–Iso | I-HCA |
| 18 | 6.2 | Iso | Intralesional VS | Centripetal | No | Yes | Hyper–Hyper | Hyper–Hyper–Iso | I-HCA |
| 19 | 2.5 | Hypo | Intralesional AS | Centripetal | No | Yes | Iso–Hyper | Hyper–Iso–Iso | I-HCA |
US ultrasound, CEUS contrast-enhanced ultrasound, MRI magnetic resonance imaging, FS flow signals, NA not available, AS arterial signals, VS venous signals, T1W T1-weighted, T2W T2-weighted, I-HCA inflammatory hepatocellular adenoma, UNC-HCA unclassified hepatocellular adenoma, B-HCA β-catenin-activated hepatocellular adenoma
Nine HCAs were examined with computed tomography (CT); in all cases arterial enhancement was detected; hypoattenuation in portal/late phases was detected in three cases whereas all the other nodules resulted to be hyper- or iso-dense. MRI was available in 14 HCAs (12 I-HCAs). Lesions were mainly hypointense in T1w sequences (7/12), whereas they always appeared hyperintense in T2w sequences and during arterial phase. Concerning the 12 I-HCAs, five nodules were hyperintense, six isointense and only one hypointense in portal/venous phases. The B-HCA and the single UNC-HCA in which MRI was available were both hypointense in portal/venous phases.
Discussion
The current study retrospectively investigated CEUS features of HCAs, in relation to clinico-pathologic characteristics, including histological subtype. Our data confirm that HCAs are prevalent in women (84.2% of the patients) most of whom (11/16, 69%) had a long clinical history of oral contraceptives intake. Recent data suggest a possible association of HCAs with obesity and metabolic syndrome and our results are in agreement with these findings, as 36% of the patients had increased BMI (≥ 25 kg/m2), 42% were affected by NAFLD and majority of the patients (10/19, 52.6%) had increased BMI or NAFLD or both [2, 3, 5, 6, 11]. Three patients had GSD which is a well-recognized risk factor for HCA [4]. However, none of the cases that reported a history of high alcohol consumption (> 1 g/kg of body weight) was previously linked to a major risk of I-HCA development [11]. Despite the high risk of bleeding, which occurs in up to 30% of cases owing to the presence of marked sinusoidal dilatation, abnormal thick-walled arteries, and peliotic areas on histopathology, none of our patients presented with acute hemorrhage and only one presented with areas of intralesional hemorrhagic necrosis in the context of asymptomatic abnormal liver tests [19]. Malignant transformation into HCC was reported in none of our patients. However, since specific beta-catenin mutations have been described in almost 12% of all I-HCAs, and both B-HCA and UNC-HCA have a high risk of neoplastic transformation, high medical attention should be paid in the follow-up of these lesions and liver biopsy should be performed to exclude new mutation onset in case of increasing size and/or change in the imaging appearance [20].
The US pattern of HCAs is non-specific and our series confirm this finding as half of the nodules were hypoechoic, whereas the remaining resulted in becoming hyperechoic, isoechoic or showed a mixed pattern. In the same way, the color Doppler flow pattern was non-specific even if intralesional with or without perilesional flow signals were only detected in I-HCA and not in B-HCA and UNC-HCA. However, the most interesting findings of our HCA series are concerned with the HCA’s dynamic behavior on CEUS. Indeed, it has been shown that on CEUS, HCAs usually display a homogeneous arterial enhancement with rapid and usually complete centripetal filling [11]. Our results confirm these findings, as 18/19 HCAs had arterial enhancement that was centripetal in 16 cases and centrifugal in 2 cases. This also applies to I-HCAs that showed arterial centripetal pattern in 92.8% of cases. It is worth mentioning that centrifugal filling in HCAs, although very rare, can be present, as a consequence it does not allow to rule out confidently HCA diagnosis [21]. It should be pointed out that CEUS is the only contrast-enhanced imaging technique that allows continuous monitoring of the filling pattern of each focal lesion and that rapid centripetal filling during arterial phase is rarely reported in other liver lesions (with the exception of high-flow hemangiomas, which are usually easily characterized). Thus, we think that the detection of such pattern in a liver mass should always raise the suspicion of HCA especially in patients at increased clinical risk for this lesion. On the other hand, during portal and late phases, the CEUS pattern of HCAs was more heterogeneous as 11 lesions showed washout (including the only one HCA without arterial enhancement) and 8 resulted in becoming persistently iso- or hyper-enhanced. Even within the subgroup of I-HCA, the CEUS pattern was heterogeneous with seven lesions showing complete or partial washout, and seven having persistent enhancement (Figs. 1, 2). The only interesting finding could be the high prevalence of washout in the subgroup of five B-HCA/UNC-HCA (4/5 lesions showing washout), but the number of the lesions evaluated was too small to draw any conclusion. On the whole, the heterogenous pattern of HCAs and particularly of I-HCA in portal/venous phases is probably related to the variable presence of dystrophic vessels, dilated sinusoids and inflammatory content within the lesion and we can confirm that CEUS does not seem to be sufficiently accurate to differentiate between HCA subtypes but may be useful in some cases to distinguish between HCA and other benign liver neoplasms such as focal nodular hyperplasia [11].
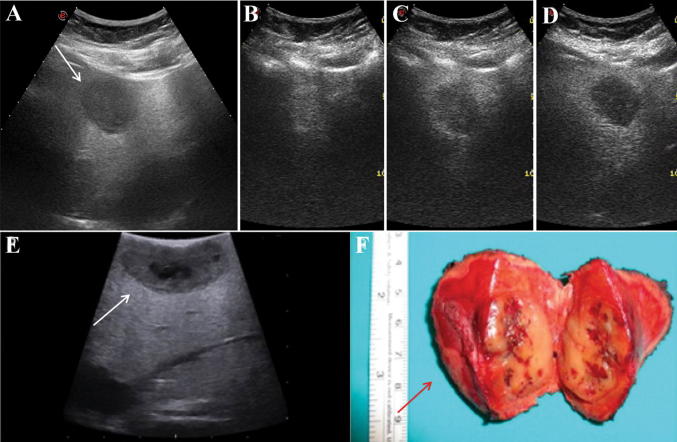
Fig. 1.
A 51-year-old woman with a new incidental lesion discovered during follow-up for atypical haemangioma and NAFLD with severe liver steatosis at B-mode ultrasound (> 20 years of oral contraceptives intake). a Grayscale ultrasound showing a hypoechoic nodule of 4.4 cm in the left lobe of the liver (white arrow). b Contrast-enhanced ultrasound showed a rapid enhancement and centripetal filling pattern in the arterial phase (24 s after SonoVue injection). c Almost complete washout with peripheral enhancement ring was observed in the portal phase (51 s after SonoVue injection). d Complete washout was observed in the late venous phase (≈ 3 min after SonoVue injection). e, f A histological diagnosis of inflammatory hepatocellular adenoma was established after intraoperative ultrasound (white arrow) and surgical resection (red arrow)
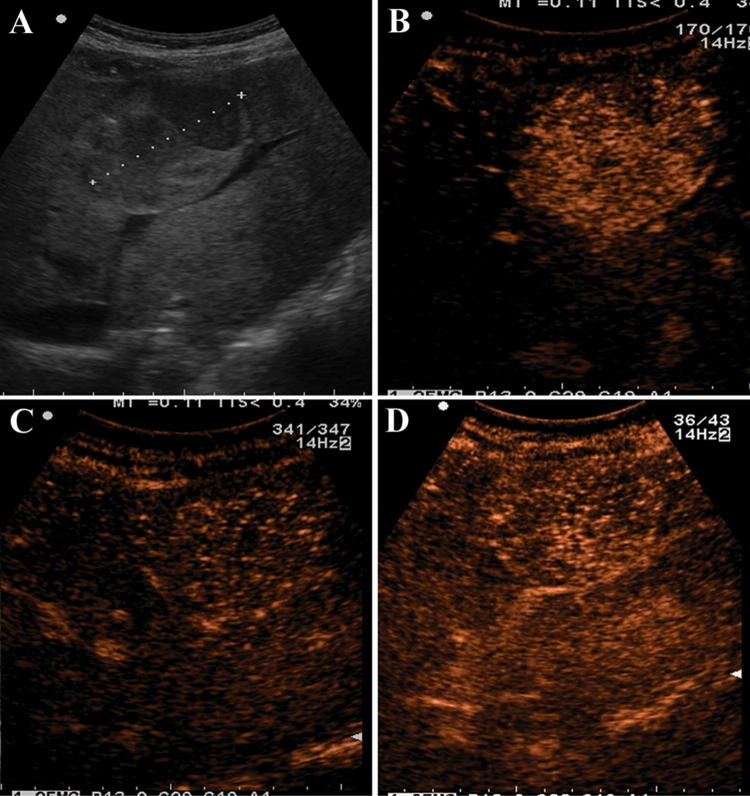
Fig. 2.
A 29-year-old woman who had glycogen storage disease underwent ultrasound for raised liver function tests (no previous oral contraceptives intake). a Grayscale ultrasound showing a single nodule of 6 cm with a mixed echogenicity in the right lobe of the liver (white arrow). b Contrast-enhanced ultrasound showed a rapid enhancement and centripetal filling pattern in the arterial phase (21 s after SonoVue injection). c Contrast-enhanced ultrasound in the portal phase showed no washout (50 s after SonoVue injection). d In the late venous phase the nodule is still iso/hyper-enhanced as compared to the surrounding liver parenchyma (≈ 2 min after SonoVue injection)
MRI is at present considered superior to all other imaging modalities in the diagnosis of HCA. Specific features of two main HCA subtypes (I-HCA and H-HCA) have been reported on previous MRI studies. In particular, I-HCAs resulted hyperintense in T2w images, with no or patchy intratumoural steatosis, and hyperintense during arterial phase with enhancement persisting into the portal and delayed phase either diffusely or as a rim-like band, due to teleangectasic components [12–14]. Furthermore, recent studies have proposed gadoxetic acid, a new liver-specific hepatobiliary contrast agent, for HCA diagnosis and subtyping, reporting low signal intensity in the hepatobiliary phase, in 100% of H-HCAs, 92% of UNC-HCAs, 75% of I-HCAs and 59% of B-HCAs [22]. Our results on I-HCAs confirmed previous findings, showing that lesions had a variable aspect on T1w images, while they were all but one hyperintense on T2w images. During dynamic sequences, they showed a strong hyper-enhancement during arterial phase, and persistent enhancement in the portal or late phase in all but two cases, which displayed washout in the portal phase. A peripheral rim of late sustained enhancement with internal washout was observed only in one case. In all three patients who underwent gadoxetic acid-enhanced liver MRI, I-HCAs appeared hypointense compared with surrounding liver parenchyma on the hepatobiliary phase.
Furthermore, our data confirm a discordance between CEUS and MRI pattern in portal and late enhanced phases previously described in some cases of I-HCAs [15, 16, 23]. Indeed, in our study among 12 patients with I-HCAs who underwent both CEUS and MRI, washout in portal and/or late phase was reported in seven and two cases, respectively. It has been postulated that blood flow is impaired in congested sinusoids of the central area of I-HCAs, with MRI contrast material diffusing through the vascular endothelium into the tumor interstitium concealing washout and sonographic microbubbles remaining intravascular showing washout [23]. However, explanations remain still unclear and larger studies are required to confirm these results.
This study has some limitations, first of all the limited number of cases included in the analysis. The retrospective design brings inherent limitations of bias, such as the non-consecutive series of patients and the ‘a posteriori’ collection of data. Moreover, the use of different sets of ultrasound devices and software in the various centers may potentially lead to non-homogenous CEUS imaging. Nevertheless, this dataset represent one of the few published experiences on HCAs and CEUS and inclusion of different ultrasound units may reflect real-life clinical scenarios.
Conclusion
Our study confirms that oral contraceptive use, overweight and NAFLD are often encountered in association with HCA. On CEUS, such lesions show arterial centripetal hyper-enhancement in the vast majority of cases, whereas the behavior in the portal and late phases appears to be heterogeneous even in the subgroup of I-HCAs in which washout is more frequently detected on CEUS than on MRI.
Funding
All authors received no specific funding for this work.
Compliance with ethical standards
Conflict of interest
All authors declare no conflict of interest.
Ethical approval
The study was approved by the Ethics Committee of the Catholic University of Sacred Heart. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required.
References
- 1.Lin H, van den Esschert J, Liu C, et al. Systematic review of hepatocellular adenoma in China and other regions. J Gastroenterol Hepatol. 2011;26(1):28–35. doi: 10.1111/j.1440-1746.2010.06502.x. [DOI] [PubMed] [Google Scholar]
- 2.Rooks JB, Ory HW, Ishak KG, et al. Epidemiology of hepatocellular adenoma. The role of oral contraceptive use. JAMA. 1979;242:644–648. doi: 10.1001/jama.1979.03300070040020. [DOI] [PubMed] [Google Scholar]
- 3.Nakao A, Sakagami K, Nakata Y, et al. Multiple hepatic adenomas caused by long-term administration of androgenic steroids for aplastic anemia in association with familial adenomatous polyposis. J Gastroenterol. 2000;35:557–562. doi: 10.1007/s005350070081. [DOI] [PubMed] [Google Scholar]
- 4.Labrune P, Trioche P, Duvaltier I, et al. Hepatocellular adenomas in glycogen storage disease type I and III: a series of 43 patients and review of the literature. J Pediatr Gastroenterol Nutr. 1997;24:276–279. doi: 10.1097/00005176-199703000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Socas L, Zumbado M, Perez-Luzardo O, et al. Hepatocellular adenomas associated with anabolic androgenic steroid abuse in bodybuilders: a report of two cases and a review of the literature. Br J Sports Med. 2005;39(5):e27. doi: 10.1136/bjsm.2004.013599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang CY, Hernandez-Prera JC, Roayaie S, et al. Changing epidemiology of hepatocellular adenoma in the United States: review of the literature. Int J Hepatol. 2013;2013:604860. doi: 10.1155/2013/604860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Aalten SM, De Man RA, IJzermans JN, et al. Systematic review of haemorrhage and rupture of hepatocellular adenomas. Br J Surg. 2012;99(7):911–916. doi: 10.1002/bjs.8762. [DOI] [PubMed] [Google Scholar]
- 8.Stoot JH, Coelen RJ, De Jong MC, et al. Malignant transformation of hepatocellular adenomas into hepatocellular carcinomas: a systematic review including more than 1600 adenoma cases. HPB. 2010;12(8):509–522. doi: 10.1111/j.1477-2574.2010.00222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zucman-Rossi J, Jeannot E, Nhieu JT, et al. Genotype–phenotype correlation in hepatocellular adenoma: new classification and relationship with HCC. Hepatology. 2006;43(3):515–524. doi: 10.1002/hep.21068. [DOI] [PubMed] [Google Scholar]
- 10.Bioulac-Sage P, Laumonier H, Couchy G, et al. Hepatocellular adenoma management and phenotypic classification: the Bordeaux experience. Hepatology. 2009;50(2):481–489. doi: 10.1002/hep.22995. [DOI] [PubMed] [Google Scholar]
- 11.European Association for the Study of the Liver (EASL) EASL Clinical Practice Guidelines on the management of benign liver tumours. J Hepatol. 2016;65(2):386–398. doi: 10.1016/j.jhep.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 12.Hussain SM, van den Bos IC, Dwarkasing RS, et al. Hepatocellular adenoma: findings at state-of-the-art magnetic resonance imaging, ultrasound, computed tomography and pathologic analysis. Eur Radiol. 2006;16(9):1873–1886. doi: 10.1007/s00330-006-0292-4. [DOI] [PubMed] [Google Scholar]
- 13.Laumonier H, Bioulac-Sage P, Laurent C, et al. Hepatocellular adenomas: magnetic resonance imaging features as a function of molecular pathological classification. Hepatology. 2008;48:808–818. doi: 10.1002/hep.22417. [DOI] [PubMed] [Google Scholar]
- 14.Dharmana H, Saravana-Bawan S, Girgis S, et al. Hepatocellular adenoma: imaging review of the various molecular subtypes. Clin Radiol. 2017;72(4):276–285. doi: 10.1016/j.crad.2016.12.020. [DOI] [PubMed] [Google Scholar]
- 15.Laumonier H, Cailliez H, Balabaud C, et al. Role of contrast-enhanced sonography in differentiation of subtypes of hepatocellular adenoma: correlation with MRI findings. AJR Am J Roentgenol. 2012;199(2):341–348. doi: 10.2214/AJR.11.7046. [DOI] [PubMed] [Google Scholar]
- 16.Manichon AF, Bancel B, Durieux-Millon M, et al. Hepatocellular adenoma: evaluation with contrast-enhanced ultrasound and MRI and correlation with pathologic and phenotypic classification in 26 lesions. HPB Surg. 2012;2012:418745. doi: 10.1155/2012/418745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dong Y, Zhu Z, Wang WP, et al. Ultrasound features of hepatocellular adenoma and the additional value of contrast-enhanced ultrasound. Hepatobiliary Pancreat Dis Int. 2016;15(1):48–54. doi: 10.1016/S1499-3872(15)60039-X. [DOI] [PubMed] [Google Scholar]
- 18.Claudon M, Dietrich CF, Choi BI, et al. Guidelines and good clinical practice recommendations for Contrast Enhanced Ultrasound (CEUS) in the liver—update 2012: a WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. World Federation for Ultrasound in Medicine; European Federation of Societies for Ultrasound. Ultrasound Med Biol. 2013;39(2):187–210. doi: 10.1016/j.ultrasmedbio.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 19.Katabathina VS, Menias CO, Shanbhogue AK, et al. Genetics and imaging of hepatocellular adenomas: 2011 update. RadioGraphics. 2011;31(6):1529–1543. doi: 10.1148/rg.316115527. [DOI] [PubMed] [Google Scholar]
- 20.Rebouissou S, Amessou M, Couchy G, et al. Frequent in-frame somatic deletions activate gp130 in inflammatory hepatocellular tumours. Nature. 2009;457:200–204. doi: 10.1038/nature07475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taimr P, Bröker MEE, Dwarkasing RS, et al. A model-based prediction of the probability of hepatocellular adenoma and focal nodular hyperplasia based on characteristics on contrast-enhanced ultrasound. Ultrasound Med Biol. 2017;10:2144–2150. doi: 10.1016/j.ultrasmedbio.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 22.Guo Y, Li W, Cai W, et al. Diagnostic value of gadoxetic acid-enhanced MR imaging to distinguish HCA and its subtype from FNH: a systematic review. Int J Med Sci. 2017;14(7):668–674. doi: 10.7150/ijms.17865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson SR, Kim TK, Jang HJ, et al. Enhancement patterns of focal liver masses: discordance between contrast-enhanced sonography and contrast-enhanced CT and MRI. AJR Am J Roentgenol. 2007;189(1):W7–W12. doi: 10.2214/AJR.06.1060. [DOI] [PubMed] [Google Scholar]