Abstract
Hepatocellular carcinoma (HCC) is a common malignant cancer and the second cause of cancer-related death worldwide. Glypican-3 (GPC3) is established as an important prognostic factor for HCC but the results are still controversial. Moreover, its utility as an immunohistochemical marker for HCC is not conclusive. Herein we aimed to find the prognostic significance of GPC3 in HCC patients. The PubMed, Web of Science, EMBASE, SCOPUS and Cochrane library databases were searched and eligible studies based on the GPC3 expression and survival outcome of HCC (odds ratios or hazard ratios) included in the current meta-analysis. The STATA 12.0 and RevMan 5.3 software were used for statistical evaluations. 17 articles contained 2618 patients, were included in the recent meta-analysis. Our findings revealed a significant association between tumor stage, higher tumor grade, presence of vascular invasion, shorter overall survival, shorter disease-free survival and high expression of GPC3. The subgroup analyses based on sample size, cutoffs and follow-up period were also conducted to examine the association between GPC3 and OS and also to increase the homogeneity of study. Current study found a significant association between GPC3 expression and poor prognosis of HCC and specially related to the HCC invasion and progression. It was recommended to design more prospective studies based on the relationship between GPC3 and HCC to confirm our results.
Electronic supplementary material
The online version of this article (10.1007/s13337-019-00517-6) contains supplementary material, which is available to authorized users.
Keywords: GPC3, HCC, Meta-analysis
Introduction
Hepatocellular carcinoma (HCC) as a common malignant cancer, is the second cause of cancer-related death, ranking fifth in the global incidence of malignant tumors. HCC is related to the 70–90% of primary liver cancer [30, 31]. Hepatitis B (HBV) and C (HCV) viruses are the main causes of HCC and is a prevalent malignancy in Asia [1]. The best treatments for HCC are surgical resection, transplantation, radiotherapy, chemotherapy, and radiofrequency but many patients lose the chance of treatment because their cancer is detected at advanced stages due to the lack of proper diagnostic methods and recurrence is frequent. On the other hand, the clinical manifestation of HCC is not clear and the probability of metastasis is very high. Therefore, the survival rate of patients reaches 5 years at 7% [37]. Early diagnosis of HCC is likely to improve the patient’s survival and prevent cancer. Nowadays, measurement of serum biomarkers and imaging techniques are the most common cancer screening techniques [32, 37]. However, the sensitivity and specificity of serological biomarkers are very low. For example, alpha fetoprotein (AFP), as one of the most common HCC markers, remains in normal range in 40% of patients with early stage and 15–30% with advanced HCC [51]. Also, in patients with chronic hepatitis B and/or C, AFP levels increase [33]. Ultrasonography is another cost-effective way to detect HCC in early stage, but, this method cannot detect nodules smaller than 3 cm [38]. CT and MRI are also improved sensitivity and specificity in the early stages to an acceptable level (91% and 96% respectively). However, these methods are expensive and because of exposure to radiation, routine use, especially in large-scale screening is not common [16]. Therefore, identification of a non-invasive and cost-effective diagnostic method is essential and introduction of high-sensitivity diagnostic markers also improves detection and screening techniques for HCC.
Glypican family comprises six members, GPC1 to GPC6, are proteins with heparan sulfate proteoglycan subunit and glycosyl-phosphatidylinositol anchor which can bind to the outer surface of the cell membrane [19, 33]. The GPC3 gene is located on the human X chromosome (Xq26) encoding a 70-kDa core protein. The mutation in the GPC3 gene is related to the human Simpson-Golabi-Behmel syndrome [36]. GPC3 is produced in the placenta and fetal liver, but it is not expressed in other adult tissues. The role of GPC3 in cancer is widespread and depends on cellular content as well as cellular signaling pathways. GPC3 is involved in signaling pathways such as tumor growth factor, Hedgehog, bone morphogenetic protein, Wnt/β-catenin and fibroblast growth factor through a lipase called Notum. GPC3 has different roles in various cancers. It can inhibit cell proliferation and induce pro-apoptotic functions in mesothelioma, breast and ovarian cancers [8]. In HCC, the expression of GPC3 is increased as an oncogene [25, 33]. GPC3 is a precise diagnostic marker for HCC and can differentiate between the early stage of HCC and precancerous state [9]. It can distinguish HCC from a number of pathological conditions such as cholangiocellular carcinoma, hepatocellular adenoma, focal nodular hyperplasia and cirrhosis [10, 24, 48]. In patients with hepatectomy, GPC3 is a strong diagnostic marker for HCC [11]. The cDNA microarray analysis has been revealed an overexpression of GPC3 in HCC, whereas its expression has been reduced in preneoplastic and non-neoplastic lesions [28, 42]. Some studies have reported that high GPC3 expression was related to the poor prognosis of HCC [11, 14, 43]. However, some other studies have revealed different results, distinctly [3, 18, 35, 44, 50]. The prognostic significance of GPC3 in patients with hepatocellular carcinoma is still unclear and it remains to be elucidated. Six retrospective studies and tow meta-analyses have reported that high GPC3 expression was related to the poor prognosis in patients with HCC [11, 14, 26, 34, 39, 43, 46, 49]. Moreover, there were also some studies reported different conclusions distinctly [3, 18, 27, 35, 44, 50]. In the current meta-analysis, we explored the correlation between GPC3 and prognostic significance in HCC by adding the latest data from current studies.
Methods and materials
Search strategy and inclusion criteria
The studies were included from PubMed, Web of Science, EMBASE, Cochrane Library and SCOPUS databases. These databases were systematically searched until November 1th, 2018 without time restriction. Resources search was done using the following keywords: (GPC3 [MESH] or GPC3 [TEXT WORD] or GPC3 protein or glypican-3 [All Fields]) AND (carcinoma, hepatocellular, liver cancer or hepatoma [MESH] or HCC [TEXT WORD]). If the study meet the following inclusion criteria, it would enter the meta-analysis: (1) the studies had to be published as cohort study in English with the full text available, (2) the technique for measuring GPC3 should be immunohistochemistry (IHC), (3) the sample size should preferably be greater than 20, (4) the studies included HCC patients with surgical resection (SR) or liver transplantation (LT), (5) the relationship between GPC3 and disease-free survival (DFS) and/or overall survival (OS) of patients with HCC and 95% confidence interval (CI) was evaluated or studies must provide sufficient information to estimate HR and 95% CI, (6) if several studies reused the same patient, the study that has the most data was included in the meta-analysis. Non-english-language papers, reviews, conference abstracts, or articles with insufficient information for calculating the HR and 95% CI of OS or DFS were considered as ineligible. The method for selecting eligible studies is shown in Supplementary Fig. 1.
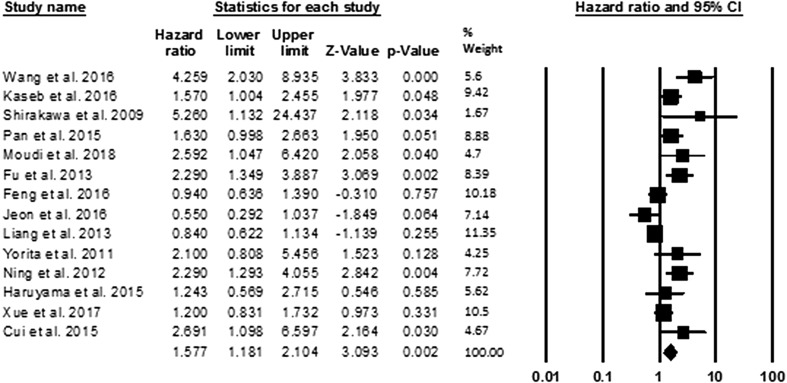
Fig. 1.
The association between high expression of GPC3 and OS in patients with HCC. OS was reported in 14 studies with a total of 2432 HCC patients. Because of the heterogeneity in the study, pooled HR was calculated by the random effect model. The summary HR and 95% CIs were shown. CI confidence interval, GPC3 glypican-3, HCC hepatocellular carcinoma, HR hazard ratio
Data extraction and quality assessment
Two reviewers, independently, extracted all the data based on the inclusion criteria. In cases where the two reviewers disagreed, the third reviewer announced the final decision. The information was extracted from the study included: first author, year of publication, country, the number of patients, the numbers of different clinicopathological parameters, cutoff, follow-up period, HR and OR with 95% CI. In articles where the survival data had not been directly examined, data were extracted using Kaplan–Meier curves and GetData Graph Digitizer 2.24 software (http://getdata-graphdigitizer.com). All analyzes were based on previously published studies and there was no need for informed consent and ethical approval. Macro- or microscopic vascular invasion was referred to the tumor vascular invasion. The interval between the medical treatment and last observation of patients/death was considered as OS. The interval between the treatment and detection of tumor recurrence was measured as DFS. Tumors were grouped according to the Edmondson Steiner grading system as follows: well/moderately (I/II) and poorly (III/IV) differentiated [6]. To find out whether GPC3 expression was low or high, we referred to the articles. Quality assessment was performed by the standard Newcastle–Ottawa quality assessment scale. Numbers from 0 to 9 were used to evaluate the quality of articles. Patient selection and ascertainment of outcome were awarded 1 point and comparability awarded 2 points. When it was rated 0 to 4 and 5 to 9, quality was considered low and high, respectively.
Statistical analysis
Meta-analysis was done using STATA 12.0 (StataCorp LP, College Station, TX, USA) and RevMan 5.3 software (Cochrane Collaboration, Oxford, UK). The heterogeneity between studies was shown using I2 statistic. We used random effect model if I2 ≥ 50% and fixed effect model in other situations. HR with 95% CI evaluated the association between GPC3 and HCC survival outcome. OR with 95% CI revealed the possible relationship between GPC3 and clinicopathological parameters for HCC. The stability of the results was assessed by analyzing the sensitivity. In this regard, we deleted 1 study each time and therefor examined the influence of each data on the pooled HR [23]. The bias was tested by the Egger linear regression using Begg funnel plots with significant publication bias defined as P < 0.05 [2].
Results
Selection and characteristics of literature
During the first process of retrieving the articles using the considered keywords, 367 articles on the relationship between GPC3 and HCC were obtained. In the next step, by evaluating the title and abstract of articles, 315 articles were deleted because they were not reported original articles and English language. The full texts were reviewed and evaluated and 5 papers were deleted due to a lack of information on patient survival data. In two studies, the same patients were used [43, 44]. To avoid duplicate counting, only the study was selected that contained more information. Finally, 17 articles contained 2618 patients, who had the inclusion criteria, were selected for the recent meta-analysis [3, 5, 7, 11, 14, 18, 21, 27, 33–35, 40, 44, 45, 47, 49, 50] (Fig. 1). Geographical distribution of articles was as follows: 9 literature from China [5, 7, 11, 27, 34, 35, 44, 45, 47], 4 from Japan [3, 14, 40, 49], 1 from USA [21], 1 from Iran [33], 1 from Taiwan [50] and 1 from Korea [18]. In all articles, IHC was used to evaluate GPC3 expression in the liver tissue. To evaluate low or high GPC3 expression, information from each article was used and the amount of cutoff was calculated. In this study, a unified amount of high GPC3 cutoff was not selected. In the selected articles, GPC3 was often expressed in the cytoplasm and in some articles, it was also expressed in the cell membrane. OS, DFS and their 95% CIs with HRs were extracted by the methods as mentioned above. The main treatment for HCC patients was SR that was performed in 13 articles and LT in 2 articles. The sample size varied from 31 to 362 in all studies. The number of patients with the highest levels of GPC3 expression were 20 to 270. The mean age was 43 to 69 years and the number of males was 29 to 324. The average follow-up time was 3 years. Quality assessment was performed by the standard Newcastle–Ottawa quality assessment scale and was 5 to 8. Primary parameters and clinicopathological properties of the 17 articles are summarized in Supplementary Table 1.
Relevance between high expression of GPC3 and clinicopathological features
In order to determine the effect of GPC3 on diagnosis of HCC, we assessed clinicopathological parameters. In many of the studies that were selected for the recent meta-analysis, the relationship between clinicopathological properties (HBV/HCV infection, tumor number, tumor size, histological grade, vascular invasion, Child–Pugh grade) and high expression of GPC3 was evaluated. Our findings revealed significant association between tumor stage and high expression of GPC3 (OR = 1.02, 95% CI 1.09–2.74, P = 0.04). Also, there was a significant relationship between high expression of GPC3 and higher tumor grade (OR = 1.86, 95% CI 1.42–3.45, P < 0.001). On the other hand, the presence of vascular invasion was significantly associated with the high expression of GPC3 (OR = 1.36, 95% CI 1.09–3.02, P = 0.04) (Table 1).
Table 1.
Association between high expression of GPC3 and clinicopathological features
| Effect model | OR (95% CI) | P value | Heterogeneity test | ||
|---|---|---|---|---|---|
| I2 (%) | P value | ||||
| HBV (±) | Fixed | 1.41 (0.98–1.84) | 0.09 | 38 | 0.12 |
| HCV (±) | Random | 1.05 (0.65–2.72) | 0.81 | 72 | 0.03 |
| Child–Pugh grade (B or C/A) | Fixed | 1.317 (0.87–2.01) | 0.19 | 0 | 0.57 |
| Tumor size (≥ 5 cm/< 5 cm) | Fixed | 1.08 (0.86–1.54) | 0.79 | 7 | 0.46 |
| Tumor number (multiple/single) | Random | 1.08 (0.76–2.52) | 0.23 | 59 | 0.01 |
| Hepatic cirrhosis (positive/negative) | Fixed | 1.42 (0.73–1.84) | 0.16 | 43 | 0.17 |
| Stage (III–IV/I–II) | Random | 1.02 (1.09–2.74) | 0.04 | 58 | 0.02 |
| Histological grade (G2–3/G1) | Fixed | 1.86 (1.42–3.45) | < 0.001 | 48 | 0.24 |
| Vascular invasion (positive/negative) | Random | 1.36 (1.09–3.02) | 0.04 | 55 | 0.006 |
This table evaluated the associations between GPC3 high expression and infection of HBV or HCV, Child–Pugh grade, tumor size, tumor number, stage, histological grade, and vascular invasion
CI confidence interval, GPC3 glypican-3, HBV hepatitis B virus, HCV hepatitis C virus, N number, OR odds ratio
Relevance between high expression of GPC3 and OS in patients with HCC
In this meta-analysis, 14 studies, including 2432 HCC patients, reported the relationship between GPC3 expression and OS. In evaluations the results, it was found that there is heterogeneity in the current study (I2 = 72%, P < 0.001), therefore, the random effect model was used for pooled HR calculation. Our findings revealed significant association between overexpression of GPC3 and decreased OS (pooled HR: 1.57, 95% CI 1.18–2.10, P = 0.002) (Fig. 1).
Relevance between high expression of GPC3 and DFS in patients with HCC
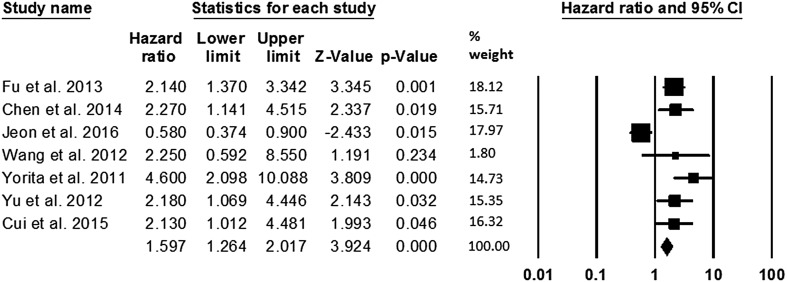
In this meta-analysis, 7 studies, including 829 HCC patients, reported the relationship between GPC3 expression and DFS. In evaluations the results, it was found that there is significant heterogeneity in the current study (I2 = 81.0%, P < 0.001), therefore, the random effect model was used for pooled DFS calculation. Our findings revealed a significant association between overexpression of GPC3 and poor DFS (pooled HR: 1.93, 95% CI 1.09–3.43, P = 0.02) (Fig. 2).
Fig. 2.
The association between high expression of GPC3 and DFS in patients with HCC. DFS was reported in 7 studies with a total of 829 HCC patients. Because of the heterogeneity in the study, pooled HR was calculated by the random effect model. The summary HR and 95% CIs were shown. CI confidence interval, GPC3 glypican-3, HCC hepatocellular carcinoma, HR hazard ratio
Subgroup analysis
The results of the subgroup analyses based on sample size, cutoffs and follow-up period are shown in Table 2 to examine the association between GPC3 and OS and also to increase the homogeneity of the study. In regard to the sample size, the combined HR of the studies with ≤ 200 cases was 1.49 (95% CI 1.32–2.21, P = 0.019) and the combined HR based on studies with more than 200 cases was 1.33 (95% CI 0.76–1.68, P = 0.814). In subgroup analyses based on the follow-up period, only studies with a shorter follow-up period (≤ 60 months) showed a significant association between GPC3 and poor OS with HR = 1.73 (95% CI 1.42–2.67, P = 0.001). Moreover, subgroup analysis related to the GPC3 cutoff values revealed that the pooled OS was varied in the included studies.
Table 2.
Subgroup analyses for GPC3 on HCC overall survival
| No. of studies | Effect model | HR (95% CI) | P value | Heterogeneity test | ||
|---|---|---|---|---|---|---|
| I2 (%) | P value | |||||
| Overall Sample size | 14 | Random | 1.62 (1.26–2.30) | 0.03 | 69.1 | 0.001 |
| ≤ 200 | 10 | Random | 1.49 (1.32–2.21) | 0.019 | 63.4 | 0.021 |
| > 200 | 4 | Random | 1.33 (0.76–1.68) | 0.814 | 53.7 | 0.125 |
| Duration of follow-up | ||||||
| ≤ 60 months | 6 | Fixed | 1.73 (1.42–2.67) | 0.001 | 41.5 | 0.12 |
| > 60 months | 6 | Random | 1.54 (0.57–1.69) | 0.196 | 70.2 | 0.001 |
| Cut-off value | ||||||
| 5% | 1 | – | 0.60 (0.33–1.29) | 0.071 | – | – |
| 10% | 8 | Random | 1.29 (0.86–2.10) | 0.81 | 71.4 | 0.008 |
| 20% | 4 | Fixed | 1.48 (0.71–2.67) | 0.176 | 0 | 0.601 |
| 25% | 1 | – | 2.14 (1.21–3.43) | 0.002 | – | – |
Subgroup analyses for the association between GPC3 and OS, based on sample size, follow-up period, and cut-offs, were conducted in this table
Publication bias and sensitivity analyses
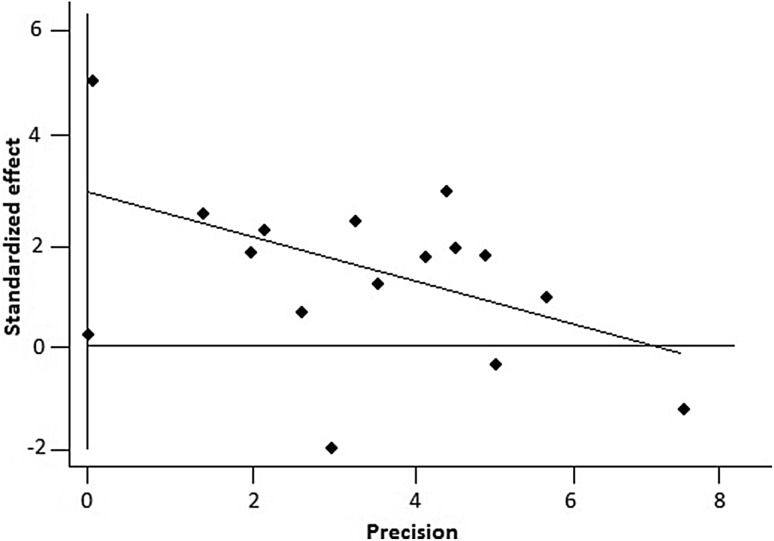
In our meta-analysis, the bias was tested by the Egger linear regression using Begg funnel plots with significant publication bias defined as P < 0.05. In all studies, an obvious symmetry was seen in the funnel plot (P = 0.05 for the Egger test) (Fig. 3) which means that the current meta-analysis did not have a significant publication bias.
Fig. 3.
Funnel plots of Egger to detect publication bias on overall estimate. Test of publication bias in our meta-analysis was performed using Begg funnel plot and Egger regression method. There was no asymmetry observed in funnel plot, indicating no evidence of significant publication bias
Discussion
A large number of literatures reported that the expression of GPC3 was lower or even absent in the normal tissue compared with malignant specimen and it distinctly expressed in HCC [15, 17, 20, 22]. In some tissues, GPC3 acts as a tumor suppressor gene, whereas in others, it acts as an oncofetal protein. GPC3 immunohistochemistry can aid in the differentiation of testicular germ cell tumors, being expressed in all yolk sac tumors but not in seminomas. GPC3 expression has also been identified in some squamous cell carcinomas of the lung and clear cell carcinomas of the ovary. The role of GPC3 in melanomas is still controversial [20]. Studies have shown that GPC3 expression contribute to the pathogenesis of HCC through proliferation, invasion, and progression cancer. However, there are contradictions in the results of various studies and the prognostic significance of GPC3 in patients with hepatocellular carcinoma is still unclear. Thus, in the current meta-analysis, we explored the correlation between GPC3 and prognostic significance in HCC using available researches. Current study, comprehensively revealed the relationship between GPC3 expression in liver tissue and HCC.
In this study, 17 papers containing 2618 patients were analyzed, with more cases than the previous studies [29, 46, 52]. Analysis the relevance between expression of GPC3 and clinicopathological properties indicated a significant association between higher expression of GPC3 and the presence of vascular invasion, later tumor stage and higher tumor grade. On the other hand, there was no significant relationship between GPC3 and ORs of some pathological properties (HBV and or HCV infection, tumor size, tumor number, Child–Pugh grade). It means that higher GPC3 levels can be a reliable marker for assessing the invasiveness of HCC. Our results are inconsistent with the results of Cheng et al. [4], Gauglhofer et al. [13], Galli et al. [12] and Sun et al. [41] studies. They showed that higher expression of GPC3 could promote the growth of cancer cells through FGF activity, insulin growth factor signaling and Wnt signaling pathway, in vivo and in vitro. Meanwhile, down regulation of GPC3 expression, decreases cell proliferation and cell cycle progression at the G1 phase through phosphorylation of SMAD2/3 in HCC cell lines [41]. Another valuable result found in the recent study was the promising relationship between GPC3 expression and the OS/DFS of HCC. Our results showed that higher GPC3 expression could increase the risk of poor OS and poor DFS 1.57 and 1.93 times, respectively compared to the patients with lower GPC3 expression. Our pooled HR for OS was different from previous meta-analysis. This discrepancy may be due to some reasons as follows: in our recent study, we analyzed more articles than previous meta-analysis, which can increase the statistical strength of the research and provide reliable conclusions. Also, in this study, more and varied geographical populations have been assessed than previous meta-analysis that can provide more valuable results. Although, a significant relationship was seen between higher GPC3 expression and poorer HCC survival in the current study, however, the diagnostic value of GPC3 should be discussed more than ever clinical situations.
In our meta-analysis, also subgroup analyses were carried out to examine the association between GPC3 and OS and also to increase the homogeneity of the study because our results had a significant heterogeneity. In subgroup analyses based on the follow-up period, only studies with follow-up period ≤ 60 months showed significant association between GPC3 and poor OS and studies with follow-up period > 60 months were not significant. It means that GPC3 expression may be able to predict short-termoutcome of HCC. Nevertheless, in regard to the sample size, the combined HR of the studies with ≤ 200 cases was significant and indicated a significant relationship between higher GPC3 and poor prognosis, which means that significant prognostic value of GPC3 was related to the studies with ≤ 200 patients. Therefore, in future studies, we needed to use a larger sample size to calculate the exact value of GPC3 in predicting the OS for HCC patients.
There are limitations in this study that should be taken into consideration. However, the publication bias was not significant in this study, but the most obvious limitation of the recent meta-analysis was publication bias because only the articles were used whit complete text and in English language. On the other hand, the method of HR extraction can be another factor of bias. In this regard, survival results related to the survival curves can reveal such imprecision. Also, in the eligible studies that were analyzed, the standard amount was not raised with cutoff values. Therefore, GPC3 cutoff values of each article were used to perform subgroup analysis. Moreover, given the fact that subgroup information was incomplete in some articles, we could not accurately report the amount of cutoff for predicting OS. Since the number of analyzed articles was limited, we were unable to include factors such as the primary antibody, treatments, laboratory infrastructure in subgroup analyses. All of the above factors can cause bias. To remove the bias sources, we need to increase the sample size and meta-analysis done in a multi-center manner.
In conclusion, despite all the limitations mentioned, the current study found a significant association between GPC3 expression and poor prognosis of HCC and specially related to the HCC invasion and progression. It was recommended to design more prospective studies based on the relationship between GPC3 and HCC to confirm our results.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This study was done according to a Dissertation Grant (Ph.D. Thesis of BM #7262, IR.ZAUMS.REC.1394.211) from the deputy for Research, Zahedan University of Medical Sciences.
Abbreviations
- GPC3
Glypican-3
- HCC
Hepatocellular carcinoma
- HR
Hazard ratio
- DFS
Disease-free survival
- OS
Overall survival
- OR
Odds ratio
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Bita Moudi, Email: bita.moudi@yahoo.com.
Zahra Heidari, Phone: 98-5433295794, Email: histology_iri@yahoo.com.
Hamidreza Mahmoudzadeh-Sagheb, Email: histology@ymail.com.
References
- 1.Alavian SM, Haghbin H. Relative importance of hepatitis b and c viruses in hepatocellular carcinoma in EMRO countries and the middle east: a systematic review. Hepat Mon. 2016;16(3):e35106. doi: 10.5812/hepatmon.35106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Begg CB. A comparison of methods to detect publication bias in meta-analysis by P. Macaskill, S. D. Walter and L. Irwig, Stat. Med. 2001;20:641–654. Stat Med. 2002;21(12):1803; author reply 4. [DOI] [PubMed]
- 3.Chen IP, Ariizumi S, Nakano M, Yamamoto M. Positive glypican-3 expression in early hepatocellular carcinoma predicts recurrence after hepatectomy. J Gastroenterol. 2014;49(1):117–125. doi: 10.1007/s00535-013-0793-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng W, Tseng CJ, Lin TT, Cheng I, Pan HW, Hsu HC, et al. Glypican-3-mediated oncogenesis involves the Insulin-like growth factor-signaling pathway. Carcinogenesis. 2008;29(7):1319–1326. doi: 10.1093/carcin/bgn091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cui X, Li Z, Gao PJ, Gao J, Zhu JY. Prognostic value of glypican-3 in patients with HBV-associated hepatocellular carcinoma after liver transplantation. Hepatobiliary Pancreat Dis Int. 2015;14(2):157–163. doi: 10.1016/S1499-3872(15)60349-6. [DOI] [PubMed] [Google Scholar]
- 6.Edmondson HA, Steiner PE. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer. 1954;7(3):462–503. doi: 10.1002/1097-0142(195405)7:3<462::AID-CNCR2820070308>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 7.Feng J, Zhu R, Chang C, Yu L, Cao F, Zhu G, et al. CK19 and Glypican 3 expression profiling in the prognostic indication for patients with HCC after surgical resection. PLoS One. 2016;11(3):e0151501. doi: 10.1371/journal.pone.0151501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Filmus J. Glypicans in growth control and cancer. Glycobiology. 2001;11(3):19r–23r. doi: 10.1093/glycob/11.3.19R. [DOI] [PubMed] [Google Scholar]
- 9.Filmus J. The contribution of in vivo manipulation of gene expression to the understanding of the function of glypicans. Glycoconj J. 2002;19(4–5):319–323. doi: 10.1023/A:1025312819804. [DOI] [PubMed] [Google Scholar]
- 10.Filmus J, Capurro M. Glypican-3: a marker and a therapeutic target in hepatocellular carcinoma. FEBS J. 2013;280(10):2471–2476. doi: 10.1111/febs.12126. [DOI] [PubMed] [Google Scholar]
- 11.Fu SJ, Qi CY, Xiao WK, Li SQ, Peng BG, Liang LJ. Glypican-3 is a potential prognostic biomarker for hepatocellular carcinoma after curative resection. Surgery. 2013;154(3):536–544. doi: 10.1016/j.surg.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 12.Galli A, Roure A, Zeller R, Dono R. Glypican 4 modulates FGF signalling and regulates dorsoventral forebrain patterning in Xenopus embryos. Development. 2003;130(20):4919–4929. doi: 10.1242/dev.00706. [DOI] [PubMed] [Google Scholar]
- 13.Gauglhofer C, Sagmeister S, Schrottmaier W, Fischer C, Rodgarkia-Dara C, Mohr T, et al. Up-regulation of the fibroblast growth factor 8 subfamily in human hepatocellular carcinoma for cell survival and neoangiogenesis. Hepatology. 2011;53(3):854–864. doi: 10.1002/hep.24099. [DOI] [PubMed] [Google Scholar]
- 14.Haruyama Y, Yorita K, Yamaguchi T, Kitajima S, Amano J, Ohtomo T, et al. High preoperative levels of serum glypican-3 containing N-terminal subunit are associated with poor prognosis in patients with hepatocellular carcinoma after partial hepatectomy. Int J Cancer. 2015;137(7):1643–1651. doi: 10.1002/ijc.29518. [DOI] [PubMed] [Google Scholar]
- 15.Hsu HC, Cheng W, Lai PL. Cloning and expression of a developmentally regulated transcript MXR7 in hepatocellular carcinoma: biological significance and temporospatial distribution. Can Res. 1997;57(22):5179–5184. [PubMed] [Google Scholar]
- 16.Jain D. Tissue diagnosis of hepatocellular carcinoma. J Clin Exp Hepatol. 2014;4(Suppl 3):S67–S73. doi: 10.1016/j.jceh.2014.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jakubovic BD, Jothy S. Glypican-3: from the mutations of Simpson-Golabi-Behmel genetic syndrome to a tumor marker for hepatocellular carcinoma. Exp Mol Pathol. 2007;82(2):184–189. doi: 10.1016/j.yexmp.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 18.Jeon Y, Kim H, Jang ES, Hong S, Kim JW, Yoon YS, et al. Expression profile and prognostic value of glypican-3 in post-operative South Korean hepatocellular carcinoma patients. APMIS: Acta Pathologica, Microbiologica, et Immunologica Scandinavica. 2016;124(3):208–215. doi: 10.1111/apm.12491. [DOI] [PubMed] [Google Scholar]
- 19.Jia HL, Ye QH, Qin LX, Budhu A, Forgues M, Chen Y, et al. Gene expression profiling reveals potential biomarkers of human hepatocellular carcinoma. Clin Cancer Res Off J Am Assoc Cancer Res. 2007;13(4):1133–1139. doi: 10.1158/1078-0432.CCR-06-1025. [DOI] [PubMed] [Google Scholar]
- 20.Kandil DH, Cooper K. Glypican-3: a novel diagnostic marker for hepatocellular carcinoma and more. Adv Anat Pathol. 2009;16(2):125–129. doi: 10.1097/PAP.0b013e3181992455. [DOI] [PubMed] [Google Scholar]
- 21.Kaseb AO, Hassan M, Lacin S, Abdel-Wahab R, Amin HM, Shalaby A, et al. Evaluating clinical and prognostic implications of Glypican-3 in hepatocellular carcinoma. Oncotarget. 2016;7(43):69916–69926. doi: 10.18632/oncotarget.12066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lage H, Dietel M. Cloning and characterization of human cDNAs encoding a protein with high homology to rat intestinal development protein OCI-5. Gene. 1997;188(2):151–156. doi: 10.1016/S0378-1119(96)00689-0. [DOI] [PubMed] [Google Scholar]
- 23.Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127(9):820–826. doi: 10.7326/0003-4819-127-9-199711010-00008. [DOI] [PubMed] [Google Scholar]
- 24.Lee HJ, Yeon JE, Suh SJ, Lee SJ, Yoon EL, Kang K, et al. Clinical utility of plasma glypican-3 and osteopontin as biomarkers of hepatocellular carcinoma. Gut Liver. 2014;8(2):177–185. doi: 10.5009/gnl.2014.8.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li L, Jin R, Zhang X, Lv F, Liu L, Liu D, et al. Oncogenic activation of glypican-3 by c-Myc in human hepatocellular carcinoma. Hepatology. 2012;56(4):1380–1390. doi: 10.1002/hep.25891. [DOI] [PubMed] [Google Scholar]
- 26.Li J, Gao JZ, Du JL, Wei LX. Prognostic and clinicopathological significance of glypican-3 overexpression in hepatocellular carcinoma: a meta-analysis. World J Gastroenterol. 2014;20(20):6336–6344. doi: 10.3748/wjg.v20.i20.6336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liang J, Ding T, Guo ZW, Yu XJ, Hu YZ, Zheng L, et al. Expression pattern of tumour-associated antigens in hepatocellular carcinoma: association with immune infiltration and disease progression. Br J Cancer. 2013;109(4):1031–1039. doi: 10.1038/bjc.2013.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Libbrecht L, Severi T, Cassiman D, Vander Borght S, Pirenne J, Nevens F, et al. Glypican-3 expression distinguishes small hepatocellular carcinomas from cirrhosis, dysplastic nodules, and focal nodular hyperplasia-like nodules. Am J Surg Pathol. 2006;30(11):1405–1411. doi: 10.1097/01.pas.0000213323.97294.9a. [DOI] [PubMed] [Google Scholar]
- 29.Liu H, Yang C, Lu W, Zeng Y. Prognostic significance of glypican-3 expression in hepatocellular carcinoma: a meta-analysis. Medicine. 2018;97(4):e9702. doi: 10.1097/MD.0000000000009702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maluccio M, Covey A. Recent progress in understanding, diagnosing, and treating hepatocellular carcinoma. CA Cancer J Clin. 2012;62(6):394–399. doi: 10.3322/caac.21161. [DOI] [PubMed] [Google Scholar]
- 31.Moudi B, Heidari Z, Mahmoudzadeh-Sagheb H. Impact of host gene polymorphisms on susceptibility to chronic hepatitis B virus infection. Infect Genet Evol. 2016;44:94–105. doi: 10.1016/j.meegid.2016.06.043. [DOI] [PubMed] [Google Scholar]
- 32.Moudi B, Heidari Z, Mahmoudzadeh-Sagheb H. Study of liver in HBV-related hepatocellular carcinoma: stereology shows quantitative differences in liver structure. Eur J Histochem EJH. 2018;62(3):238–246. doi: 10.4081/ejh.2018.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moudi B, Heidari Z, Mahmoudzadeh-Sagheb H, Alavian SM, Lankarani KB, Farrokh P, et al. Concomitant use of heat-shock protein 70, glutamine synthetase and glypican-3 is useful in diagnosis of HBV-related hepatocellular carcinoma with higher specificity and sensitivity. Eur J Histochem. 2018;62(1):2859. doi: 10.4081/ejh.2018.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ning S, Bin C, Na H, Peng S, Yi D, Xiang-hua Y, et al. Glypican-3, a novel prognostic marker of hepatocellular cancer, is related with postoperative metastasis and recurrence in hepatocellular cancer patients. Mol Biol Rep. 2012;39(1):351–357. doi: 10.1007/s11033-011-0745-y. [DOI] [PubMed] [Google Scholar]
- 35.Pan C, Wang X, Chen W, Tao C, Xu X, Jin L, et al. Reevaluation of glypican-3 as a prognostic marker in HCC using X-tile software. Med Oncol. 2015;32(1):359. doi: 10.1007/s12032-014-0359-z. [DOI] [PubMed] [Google Scholar]
- 36.Pilia G, Hughes-Benzie RM, MacKenzie A, Baybayan P, Chen EY, Huber R, et al. Mutations in GPC3, a glypican gene, cause the Simpson-Golabi-Behmel overgrowth syndrome. Nat Genet. 1996;12(3):241–247. doi: 10.1038/ng0396-241. [DOI] [PubMed] [Google Scholar]
- 37.Schwartz M, Roayaie S, Konstadoulakis M. Strategies for the management of hepatocellular carcinoma. Nat Clin Pract Oncol. 2007;4(7):424–432. doi: 10.1038/ncponc0844. [DOI] [PubMed] [Google Scholar]
- 38.Sheu JC, Sung JL, Chen DS, Lai MY, Wang TH, Yu JY, et al. Early detection of hepatocellular carcinoma by real-time ultrasonography: a prospective study. Cancer. 1985;56(3):660–666. doi: 10.1002/1097-0142(19850801)56:3<660::AID-CNCR2820560338>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 39.Shirakawa H, Suzuki H, Shimomura M, Kojima M, Gotohda N, Takahashi S, et al. Glypican-3 expression is correlated with poor prognosis in hepatocellular carcinoma. Cancer Sci. 2009;100(8):1403–1407. doi: 10.1111/j.1349-7006.2009.01206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shirakawa H, Kuronuma T, Nishimura Y, Hasebe T, Nakano M, Gotohda N, et al. Glypican-3 is a useful diagnostic marker for a component of hepatocellular carcinoma in human liver cancer. Int J Oncol. 2009;34(3):649–656. doi: 10.3892/ijo_00000190. [DOI] [PubMed] [Google Scholar]
- 41.Sun CK, Chua MS, He J, So SK. Suppression of glypican 3 inhibits growth of hepatocellular carcinoma cells through up-regulation of TGF-beta2. Neoplasia. 2011;13(8):735–747. doi: 10.1593/neo.11664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang XY, Degos F, Dubois S, Tessiore S, Allegretta M, Guttmann RD, et al. Glypican-3 expression in hepatocellular tumors: diagnostic value for preneoplastic lesions and hepatocellular carcinomas. Hum Pathol. 2006;37(11):1435–1441. doi: 10.1016/j.humpath.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 43.Wang Y, Shen Z, Zhu Z, Han R, Huai M. Clinical values of AFP, GPC3 mRNA in peripheral blood for prediction of hepatocellular carcinoma recurrence following OLT: AFP, GPC3 mRNA for prediction of HCC. Hepat Mon. 2011;11(3):195–199. [PMC free article] [PubMed] [Google Scholar]
- 44.Wang YL, Zhu ZJ, Teng DH, Yao Z, Gao W, Shen ZY. Glypican-3 expression and its relationship with recurrence of HCC after liver transplantation. World J Gastroenterol. 2012;18(19):2408–2414. doi: 10.3748/wjg.v18.i19.2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang L, Pan L, Yao M, Cai Y, Dong Z, Yao D. Expression of oncofetal antigen glypican-3 associates significantly with poor prognosis in HBV-related hepatocellular carcinoma. Oncotarget. 2016;7(27):42150–42158. doi: 10.18632/oncotarget.9892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xiao WK, Qi CY, Chen D, Li SQ, Fu SJ, Peng BG, et al. Prognostic significance of glypican-3 in hepatocellular carcinoma: a meta-analysis. BMC Cancer. 2014;14:104. doi: 10.1186/1471-2407-14-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xue R, Feng J, Meng Q, Lv F, Zhu Y, Yu H, et al. The significance of glypican-3 expression profiling in the tumor cellular origin theoretical system for hepatocellular carcinoma progression. J Gastroenterol Hepatol. 2017;32(8):1503–1511. doi: 10.1111/jgh.13736. [DOI] [PubMed] [Google Scholar]
- 48.Yao M, Yao DF, Bian YZ, Zhang CG, Qiu LW, Wu W, et al. Oncofetal antigen glypican-3 as a promising early diagnostic marker for hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2011;10(3):289–294. doi: 10.1016/S1499-3872(11)60048-9. [DOI] [PubMed] [Google Scholar]
- 49.Yorita K, Takahashi N, Takai H, Kato A, Suzuki M, Ishiguro T, et al. Prognostic significance of circumferential cell surface immunoreactivity of glypican-3 in hepatocellular carcinoma. Liver Int. 2011;31(1):120–131. doi: 10.1111/j.1478-3231.2010.02359.x. [DOI] [PubMed] [Google Scholar]
- 50.Yu MC, Lee YS, Lin SE, Wu HY, Chen TC, Lee WC, et al. Recurrence and poor prognosis following resection of small hepatitis B-related hepatocellular carcinoma lesions are associated with aberrant tumor expression profiles of glypican 3 and osteopontin. Ann Surg Oncol. 2012;19(Suppl 3):S455–S463. doi: 10.1245/s10434-011-1946-2. [DOI] [PubMed] [Google Scholar]
- 51.Zhang G, Ha SA, Kim HK, Yoo J, Kim S, Lee YS, et al. Combined analysis of AFP and HCCR-1 as an useful serological marker for small hepatocellular carcinoma: a prospective cohort study. Dis Mark. 2012;32(4):265–271. doi: 10.1155/2012/964036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang J, Zhang M, Ma H, Song X, He L, Ye X, et al. Overexpression of glypican-3 is a predictor of poor prognosis in hepatocellular carcinoma: an updated meta-analysis. Medicine. 2018;97(24):e11130. doi: 10.1097/MD.0000000000011130. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.