Abstract
Avian influenza H9N2 (AIV-H9N2) and Infectious bronchitis (IB) viruses are the most commonly isolated viruses from poultry flocks suffering from respiratory signs with mortalities. The outcome of co-infection with both viruses hasn’t been yet well understood. In this study, eighty 1-day-old specific pathogen free chicks were divided into four distinct groups. Group 1 remained uninfected as negative control group; groups 2, 3 and 4 were inoculated with either AIV-H9N2 or IBV or co infected with AIV-H9N2 followed by IBV three days post inoculation respectively. Chicks were monitored for clinical and pathological changes, virus shedding and both Interleukin-6 (IL6) and Interferon gamma (IFNγ) cytokines immune responses. Clinical signs varied from mild to moderate respiratory signs in all challenged groups but were more severe in group 4 with mortalities in groups 3 and 4. Tracheal shedding of both viruses washigher in group 4 than group 2 and 3. Mean AIV-H9 virus titer in lung and kidney was higher in group 4 than group 2 in all time points. IFNγ mRNA gene expression in lung was significantly lower in groups3 and 4. In conclusion, this study reports that co-infection of chicks with both viruses enhances the pathogenicity, increases both viruses shedding and extend AIV-H9 replication with impairment of IFNγ stimulation in lung.
Keywords: Avian influenza, H9N2, IBV, Co-infection, Cytokines
Introduction
Influenza viruses are segmented, negative-sense, single-stranded RNA viruses that belong to the family Orthomyxoviridae. Type A influenza viruses are further divided into subtypes based on the surface glycoproteins hemagglutinin (H) and neuraminidase (N) [27]. Avian influenza viruses can be divided into two distinct groups on the basis of their ability to cause disease. The very virulent viruses cause Highly Pathogenic Avian Influenza disease and low pathogenic viruses that cause a much milder disease [7]. Low pathogenic AIV-H9N2 was detected in Egyptian farms from early 2011 and based on genetic analysis of HA gene, all of AIVs-H9N2 belong to G1-like lineage.The virus was detected all over Egypt causing great economic losses especially when accompanied with other viruses or bacteria [10]. Infectious bronchitis virus (IBV) is an enveloped virus and has a linear positive sense non-segmented single-stranded RNA genome that belong to the family Coronaviridae. Four main structural proteins construct the IBV particles; namely the nucleoprotein (N), the membrane protein (M), the spike glycoprotein (S) and the small membrane protein (E). The S glycoprotein cleaves into 2 separate subunits; the S1 and S2 polypeptides [6]. In Egypt, two Egyptian strains, named Egy/Var-I and Egy/Var-II were reported from 2011 as a new IBV variants resembling IS/885 and IS/1494/06 strains according to the sequence of the hypervariable region-3 of the S1 subunit [1]. Recently in 2016, depending on the full S1 sequence, a research study clustered the Egyptian variant strains in the GI-23 lineage which represents the unique wild-type cluster geographically confined to the Middle East [24].
AIV-H9N2 and IBV are considered of major significance and have a great economic impact because they are able to induce disease state independently or in association with each other and have been frequently reported from different broiler chicken flocks in Egypt [10]. AIV-H9N2 enhanced the morbidity and mortality when accompanied with infectious bronchitis (IB) infection [10]. The mechanism of this enhancement still not well understood. However, two different hypotheses could explain this phenomenon. Firstly, through mechanical damage of the ciliated cells and the epithelial lining of the respiratory tract which in turn diminish the microbial clearance and enhance pathogens attachments and colonization [3], secondly, functionally, via alteration of the innate immune response including impairment of phagocytic activity [8].
Interleukin-6 (IL6) is a Proinflammatory cytokine that is released at the site of inflammation under the effect of interleukin-1β production [15], then promotes the inflammation response implicated in pulmonary tissue pathology [14], and therefore enhances tissue damage associated with H9N2 infection severity [26], while interferon gamma (IFNγ) is a typical cytokine which is critical during intracellular infection and produced by type 1 helper T cells and cytotoxic T cells. Cytotoxic T cell activity is required for destroying virus infected cells and inhibits the replication of AIV-H9N2 in chicken embryo fibroblasts [28], also IFNγ stimulates direct upregulation of antigen processing and presentation then subsequently facilitate the trafficking of T cells and natural killer cells to the site of inflammation [22].
In this study, pathogenesis of both single and mixed infections with AIV-H9N2 and IBV under experimental conditions was investigated in SPF chickens. Experimentally infected SPF chicks with AIV-H9N2 and/or recent Egyptian variant of IBV were monitored for different aspects of pathogenicity and immunogenicity including clinical signs, postmortem lesions, viruses shedding and replication in lung and kidney, histopathological lesions, serological response and cytokine response (IL6 and IFNγ) in internal organs.
Materials and methods
Virus
AIV-H9N2 Egyptian virus A/chicken/Egypt/700 V/2015 (Genbankaccession no. MH236438) which belongs to G1-like lineage viruses and a variant Egyptian IBV, IBV-Eg/141242F-SP1 (Genbankaccession no. KY119258) were used for experimental infection. Both viruses were propagated and titrated in 10 days old specific pathogen free embryonated chicken eggs (SPF-ECE). The viruses’ titer were determined by calculating the 50% egg infectious dose (EID50) per ml of virus stock, using the method of Reed and Munch [21].
Experimental design
The experimental protocol used in this work was evaluated and approved by the Research committee of the Animal Health Research Institute. Eighty SPF chicks (one day old) from SPF-ECE delivered from SPF poultry project, Komoshim, EL-Fayoum Governorate were divided randomly into four groups; each group included twenty chicks that were kept in separate isolators and received food and water ad libitum.
Group1 was kept as non-infected negative control group. Group 2 was infected intranasally with 0.1 ml of107 EID50/ml of AIV-H9N2 at 14 days old. Group 3 was infected intranasally with 0.1 ml of 106 EID50/ml of IBV at 17 days old and group 4 was infected intranasally with 0.1 ml of 107 EID50/ml of AIV-H9N2 at 14 days old then infected intranasally with 0.1 ml of 106 EID50/ml of IBV at 17 days old.
Samples collection and preparation
Tracheal swabs were collectedfrom ten experimentally infected chicks for further viruses detection and quatification at 17, 19, 21, 24 and 27 days old for group 2 and at 19, 21, 24 and 27 days old for both groups 3 and 4. Swabs samples were suspended in 0.5 ml sterile phosphate buffer saline (pH 7.2) andvortexed.
Three chicks from each group were humanely euthanized for collection of organs (spleen, trachea, lung and kidney) at 17, 20, 24 and 27 days old for groups 1 and 2 and at 20, 24 and 27 days old for groups 3 and 4. Spleen samples were tested for cytokines gene expression and histopathological examination; tracheal samples were tested for histopathology; while lung and kidney samples were used for viruses’ detection and quantification, cytokines gene expression and histopathological examination. Targeted organs for viral quantification and cytokines mRNA relative quantification were collected in RNA later solution(Qiagen, Gmbh, Germany) according to manufacturer instructions while organs targeted for histopathological examination were collected on formalin 10%, then embedded in paraffin and sections were cut for hematoxylin and eosin (H&E) staining [4]. Serum samples were collected from ten experimentally infected chicks at 24, 31 and 38 days old from groups 2, 3 and 4 for AIV-H9N2 and IBV antibodies detection.
Tracheal virus shedding, viral load and cytokines mRNA relative quantification in organs
Viral RNA was extracted from swabs samples using QIAamp viral RNA mini kit and from tissue samples using RNeasy minikit (Qiagen, Germany)in accordance with manufacturer’s instructions. Verso 1-Step qRT-PCR Kit (Thermo scientific, USA)and specific oligonucleotide primers and taqman probes assays were used for detection and quantification of AIV-H9N2 [5] and nucleoprotein gene of IBV [17]. To determine AIV-H9N2 and IBV viruses shedding and load titers, a standard curve for each virus was generated using titrated viruses in SPF-ECE. Also, the same kits were used for relative quantification of IL6 and IFNγ that were normalized to chicken 28SrRNA house-keeping gene [13].
The qRT-PCR reaction volume was 25 μl containing 2 μl of extracted RNA, 12.5 μl 2 × One-step RT-PCR ready mix, 1.25 μl RT enhancer, 0.25 μl Verso enzyme mix, 0.5 μl of 50 pmol of both forward and reverse primers, 0.125 μl of specific probe of 30 pmolconc. And 7.875 nuclease free water. The thermal profile included a reverse transcription step at 50 °C for 30 min followed by 15 min at 95 °C. The PCR cycling was performed in 40 cycles of denaturation at 95 °C for 15 s, annealing and extension at 60 °C for 1 min. QRT-PCR was performed using Mx3005P real time PCR machine (Agilent Technologies, Santa Clara, CA, USA).
AIV-H9N2 and IBV post challenge serology
Sera were collected for determinationof the antibody titer against AIV-H9N2 using HI test [20] using locally produced antigen by Reference Laboratory for Veterinary Quality Control on Poultry Production (RLQP), and against IBV using ELISA (ProFLOK, Synbiotics, USA) according to manufacturer instruction.
Statistical analysis
The differences in HI antibody titers of AIV-H9N2 and IBV ELISA titers between single and mixed infected groups were statisticallyanalyzedusing one-way ANOVA to determine significant difference at each time points.
The fold changes in relative cytokines genes expression were calculated using 2−ΔΔct method [16]. Samples were tested in triplicate to calculate the standard error for infected and non-infected chicks at each time point. One-way ANOVA using SPSS20 software was used to determine significant differences between fold change values of the infected chicks with single and mixed infections at each time point in relation to the control group. P value less than 0.05 was considered to be statistically significant.
Results
Clinical signs and gross lesions
Experimentally infected chicks were monitored for clinical signs. Until 10 days post infection (dpi). No clinical signs were observed in group 2, while group 3 chicks showed depression, ruffled feathers that started from 2 dpi to 8 dpi with death of one chick at 4 dpi. Chicks of group 4 showed depression, ruffled feather; 2 chicks suffered from mouth breathing and conjunctivitis with death of one chick at 6 dpi.
Three chicks from each group were killed humanely at 3, 7 and 10 dpi and examined for gross lesions and histopathological examination; chicks of group 2 had mild congestion in lungs at 3 dpi, while chicks of group 3 had mild congestion in lungs in addition to swollen kidneys and distended ureters with ureates at 3 and 7 dpi. Chicks of group 4 had moderate lungs congestion, with swollen kidneys and distended ureters with ureates from 3 till 10 dpi.
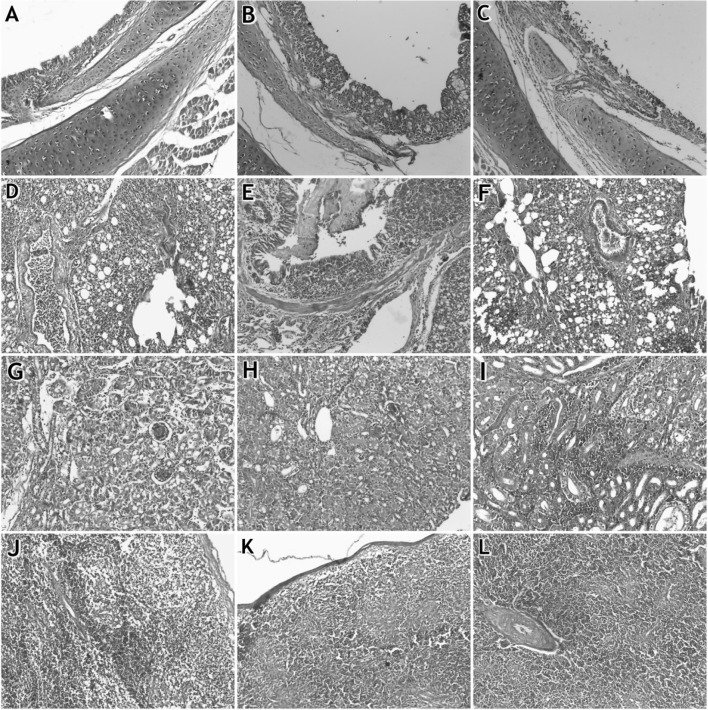
Histopathological examination of trachea, lung, kidney and spleen of 4 groups showed that the organs of group 1 were normal without any pathological changes. For group 2, trachea had mild deciliation of mucosa with infiltration of inflammatory cells in submucosa, congestion and necrosis of epithelial cells. Lung showed congestion of blood vessels with infiltration of cellular exudate. Kidney showed renal tubular necrosis with infiltration of inflammatory cells; also, spleen showed lymphoid depletion (Fig. 1a, d, g, j).
Fig. 1.
Histopathological examination in organs of infected chicks: a–c refer to tracheas of groups 2, 3 and 4, respectively. d–f refer to lungs of groups 2, 3 and 4, respectively. g–i refer to kidneys of groups 2, 3 and 4, respectively. j–l refer to Spleens of groups 2, 3 and 4, respectively. Magnifications 20×
For group 3, Trachea showed deciliation and hyperplasia of glandular epithelium with infiltration of inflammatory cells in submucosa; lung showed congestion of alveolar capillary; bronchi were filled with fibrinous exudate; kidney showed degeneration of renal tubules and filled lumen with proteinous cast; spleen showed lymphoid depletion (Fig. 1b, e, h, k). While in group 4, trachea showed sloughing of epithelial cells with infiltration of inflammatory cells in submucosa. Lung showed congestion of blood vessels and the bronchioles were filled with cellular exudates; kidneys showed degenerative changes in renal tubules with severe infiltration of inflammatory cellsandspleen showed lymphoid depletion with thickening of the wall of blood vessels (Fig. 1c, f, i, l).
Results of tracheal virus shedding by qRT-PCR
Quantification of virus shedding from trachea by real time PCR was done in relation to standard curve generated by the challenge strains (A/Ck/Eg/V700/15 for H9 and IBV-Eg-F1242-15 for IBV).
Mean of AIV-H9 virus shedding at 22 days old in group 4 (2.9 × 104) from 100% of chicks was higher than that of group 2 (1 × 103) from 80% of chicks (Table 1).
Table 1.
Tracheal virus shedding mean of AIV-H9 and IBV of infected groups using qRT-PCR
| Age of chick* | Group 2 (H9) | Group 3 (IBV) | Group 4 (mixed) | |||||
|---|---|---|---|---|---|---|---|---|
| H9N2 virus | IBV | |||||||
| % of shedders | Virus titer mean** | % of shedders | Virus titer mean | % of shedders | Virus titer mean | % of shedders | Virus titer mean | |
| 17 days old | 100 | 1.5 × 103 | Nd3 | Nd*** | 100 | 1.3 × 103 | Nd | Nd |
| 19 days old | 80 | 1 × 103 | 100 | 6.4 × 104 | 100 | 2.9 × 104 | 100 | 1.9 × 104 |
| 21 days old | 10 | 7 × 102 | 100 | 5.9 × 103 | 20 | 2.7 × 102 | 100 | 9 × 103 |
| 24 days old | 0 | 0 | 100 | 7.2 × 102 | 0 | 0 | 100 | 2.9 × 103 |
| 27 days old | Nd | Nd | 70 | 2.1 × 102 | Nd | Nd | 90 | 4 × 102 |
*H9 infection was done at 14 days old, while IBV infection was done at 17 days old
**Virus titer mean with EID50/ml
***Nd not done
The IBV shedding mean at 19 days old was higher in group 3 than group 4, but in group 4, the virus shedding mean were higher than group 3 at 21, 24 and 27 days old(Table 1).
Results of qRT-PCR forvirus replication in lung and kidney
In group 2, AIV-H9 was detected in lung up to 20 days old and then disappeared. While it was detected in kidney for a longer duration until 27 days old. In group 4, AIV-H9 was detected in lung and kidney until 27 days old, and it showed higher titer in kidney than lungs (Table 2). The Mean H9 titer in lung and kidneys was higher in group 4 than group 2 at each time points (Table 2).
Table 2.
Means of AIV-H9 and IBV titer in lung and kidney of infected groups using qRT-PCR
| Age of chicks | H9N2 mean titers* | IBV mean titers* | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Group 2 | Group 4 | Group 3 | Group 4 | ||||||
| Lung | Kidney | Lung | Kidney | Lung | Kidney | Lung | Kidney | ||
| 20 days old | No of positive/total | 2/3 | 2/3 | 2/3 | 2/3 | 3/3 | 3/3 | 3/3 | 3/3 |
| Mean titers | 3.5 × 103 | 4.8 × 103 | 6.8 × 103 | 7.5 × 106 | 1.4 × 105 | 1.7 × 104 | 6 × 104 | 2.2 × 104 | |
| 24 days old | No of positive/total | 0/3 | 2/3 | 2/3 | 2/3 | 2/3 | 3/3 | 3/3 | 3/3 |
| Mean titers | – | 4.8 × 103 | 1.2 × 103 | 9.6 × 103 | 6.3 × 103 | 5 × 106 | 2.5 × 104 | 1.4 × 108 | |
| 27 days old | No of positive/total | 0/3 | 3/3 | 1/3 | 2/3 | 3/3 | 3/3 | 2/3 | 3/3 |
| Mean titers | – | 3 × 103 | 9 × 103 | 9.6 × 103 | 3 × 104 | 3.4 × 105 | 1.7 × 105 | 1.3 × 105 | |
*Virus mean titer with EID50/gram
For group 3, IBV mean titer was higher in lung than in kidney at 20 days old while it was higher in kidney than lung at 24 and 27 days old. In group 4, IBV mean titers in lung were higher than group 3 at 24 and 27 days old, also IBV mean titers in kidney in group 4 were higher than group 3 at 20 and 24 days old. The highest titer was recorded at 24 days old in kidney of group 4 (Table 2).
Serological response
Serum samples from negative control group were free from AIV-H9 and IBV antibodies. HI mean titers of AIV-H9 in group 4 (7.3 and 7.6) were non significantly higher than that of group 2 (6.5 and 7.2) at 24 and 31 days old, while it was less than H9 mean titer in H9 group in 38 days old (Table 3) without significant variation.
Table 3.
AIV-H9N2 and IBV antibody titer mean of infected groups
| Age of chicks | Group 2* | Group 3 | Group 4 | |||||
|---|---|---|---|---|---|---|---|---|
| % of seropositive chicks | Mean antibody titer | % of seropositive | Mean antibody titer | AIV-H9N2* | IB | |||
| % of seropositive | Mean antibody titer | % of seropositive | Mean antibody titer | |||||
| 24 days old | 100 | 6.5 | 0 | 0 | 100 | 7.3 | 0 | 0 |
| 31 days old | 100 | 7.2 | 90 | 2874 | 10 | 7.6 | 90 | 3419 |
| 38 days old | 100 | 7.77 | 80 | 1897 | 100 | 7.25 | 100 | 3093 |
*H9 titer log2
No IB antibody response detected in 24 days old in groups 3 and 4 by ELISA, but geometric mean of IB antibody titers was higher but non-significant in group 4 (3419, 3093) than in group 3 (2874, 1897) in 31 and 38 days old respectively.
Results of cytokine mRNA gene expression in spleen, lung and kidney
The mRNA expressions of cytokines (IL-6 and IFN-γ) genes were evaluated in spleen, lung and kidney tissues from chicks infected with AIV-H9N2 or IBV or both viruses and compared toexpression in negative control chicks.
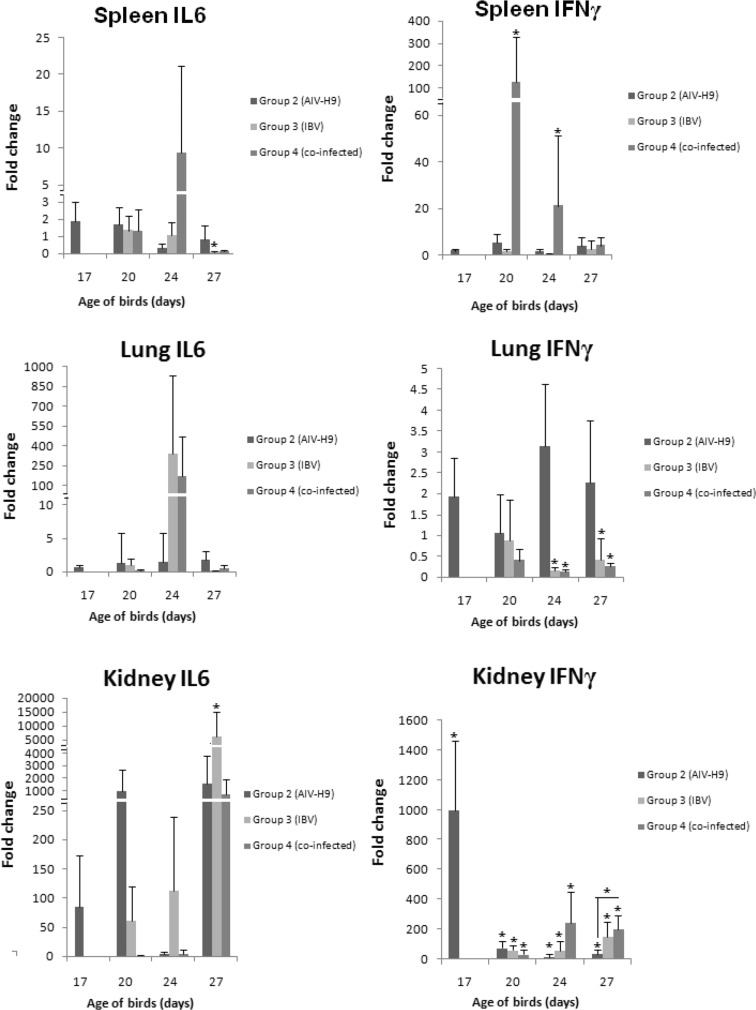
Regarding IL6 gene expression in spleen, infection of group 2 and 4 induced no significant change, while group 3 infection induced significant downregulation at 10th dpi. For spleen IFNγ mRNA gene expression; it was upregulated significantly in group 4 at 20 and 24 days old.
In lung, there was no significant difference in IL6 expression in any group at all time points. Also, there was no significant difference in IFNγ expression in group 2. While significant down regulation was recorded at 24 and 27 days old in groups 3 and 4.
In kidney, IL6 expression was upregulated in group 3 at 27 days old with no significant differences in group 2 and 4. IFNγ expression of group 2, 3 and 4 increased significantly at all time points. There was significant increase in IFNγ expression in group 4 than group 2 at 27 days old.
Discussion
Co-infection of AIV-H9N2 and IBV was one of the most common causes of respiratory problems and mortalities in the Egyptian poultry flocks during last few years. The lack of vaccination against AIV-H9N2 with its potential immunosuppressive effect may be the main reason for the high prevalence of respiratory infections [10]. The present study was designed to investigate the pathogenicity and immunogenicity of AIV-H9N2 and IBV in SPF chicks in single and mixed infections. In this study, experimentally infected chicks of group 2 showed no clinical signs, however mild respiratory signs were shown in group 3. Death of one bird was observed in groups 3 and 4 in addition to severe clinical signs in group 4. AIV-H9N2 or IBV-variant single infection showed mild respiratory signs unless complicated with other bacterial or viral infections [9].
Post mortem examination of infected chicks of group 2 showed mild congestion in lung at 3 dpi, as AIV-H9N2 which circulates in the Middle East is low pathogenic causing slight hyperemia and congestion in trachea and lungs [23]. In group 3, lesions were found in lungs and kidneys till 7 dpi. Mild hyperaemia and oedema in tracheal mucosa with pale and swollen kidneys were observed after IBV infection in old ages unlike infection of one day old chicks that produced severe lesions with high mortality [29].While in group 4, lesions were more severe and extended for longer period than group 3 as previously reported [11]. This may be due to higher replication of both viruses in lung and kidney of co-infected group (Table 2).
Histopathologically, trachea and lung in group 4 showed severe changes with sloughing of tracheal epithelium and accumulation of exudates in bronchiole. In comparison to other groups 2 and 3, they had milder changes which was also observed by previous work [11]. Pathological changes in kidney revealed that co-infection of AIV-H9N2 and IBV variant II viruses caused severe nephritis than other infected groups then subsequently acute renal failure was observed which may be the main cause of death as reported by previous studies [23, 29]. Lymphoid depletion was also observed in all infected groups which may indicate that AIV-H9N2 and IBV had immunosuppressive effect on immune system that facilitated secondary infections.
In our study, AIV-H9 tracheal shedding was detected by real time PCR from 17 till 24 days old in both groups 2 and 4. AIV-H9 virus mean titer shedding was higher in group 4 at 19 days old than that of group 2 by one log (Table 1)which indicates that IBV infection may facilitate H9 virus replication and shedding. In other studies, classic or variant IBV increased the mean titer shedding of AIV-H9 virus than AIV-H9 single infection, In addition, IBV vaccine extended H9 shedding 4 days more than H9 single infection [9, 11]. While IB virus shedding was detected until 27 days old with higher virus mean titer shedding in group 4 at 21, 24 and 27 days old than that of group 3 at the same time points (Table 1). Also, number of shedders was higher in group 4 (8/10) than group 3 (6/10) at 27 days old which increased the time of chicks’ exposure to virus and delayed its recovery.
In the current study, AIV-H9 was detected in lung of group 2 until 20 days old only, while in group 4 it was detected in lung until 27 days old. AIV-H9 was also detected in kidney of group 2 and 4 in all time points; however virus mean titer in kidney of group 4 was higher than that of group 2 in all time points (Table 2) which indicates the effect of IBV to enhance replication and pathogenesis of AIV-H9 in lung and kidney of infected chicks. On the other hand, the AIV-H9 replicated in kidney with higher titer than lung. Lung and kidney are the two main target organs for AIV-H9 replication which persists in kidney for longer time [23]. It has been reported that a trypsin-like serin protease domain is encoded by IBV [19] which may be the cause of higher AIV-H9 virus load in organs of group 4 than group 2 in addition to downregulation of IFNγ expression in lung of group 4 (Fig. 2).
Fig. 2.
Relative expression of cytokines genes in spleen, lung and kidney of infected chicks (group 2 at 17, 20, 24 and 27 days old, group 3 and 4 at 20, 24 and 27 days old), fold change values of the infected chicks at each time point in relation to the negative control group.*P < 0.05, asterisk over error bars represents significant difference between infected and non-infected chicks organs. Showed data are the mean with calculated error bar from three replicates at each time point
In the current study, IBV was detected in lung of group 4 with higher mean titer than group 3 at 24 and 27 days old. Also IBV was detected in kidney of groups 3 and 4 at all time points with different virus loads. Variant IBV genome was detected in trachea, lung and kidney while classical strains were isolated from respiratory organs only following experimental infection of chicken [29].
IL6 mRNA gene expression in group 2 wasn’t significantly changed in any organ at each time point. In previous studies, IL6 expression was positively correlated with AIV-H9N2 replication and pathogenicity [26]. For group 3, significant down regulation of IL6 in spleen at 27 days old, while IL6 was upregulated significantly in kidney at 27 days old. The IL6 upregulation with high viral load and obvious renal lesions that was previously observed and differed according to IBV genotype and genetic lines of chickens [2, 12]. While in group 4, no significant changes in IL6 gene expression was reported in any organ at each time point. These results suggested that IL6 mRNA gene expression was expressed clearly in kidney with high and extended virus replication. The non-significant observed changes may be due to transient expression of IL6 and individual variation [30].
IFNγ mRNA gene expression in group 2 was increased significantly in kidney only at each time point. Interestingly, it is the first study on the effect of AIV-H9N2 on IFNγ gene expression in kidney; and these results may be due to extended virus replication in kidney until 27 days old. In previous studies, AIV-H9N2 induced IFNγ mRNA expression in trachea, lung and intestine of infected chicken [18]. In this work, IFNγ decreased significantly in lung at 24 and 27 days old of group 3 and 4. IFNγ downregulation in lung may be due to immuno suppersive effect of IBV that was associated with extended AIV-H9 replication in group 4. These results disagree with an increased production of IFNγ in lungs of IBV-M41-infected birds [25]. While it was increased in kidney at all time points in both group 3 and 4 with higher virus replication and renal lesions. These results indicated the significant increase of IFNγ more efficiently in kidney which may be due to high and prolonged virus replication and latency at that site.
In conclusion, AIV-H9N2 increased the shedding of IBV in experimentally infected chicks; also IBV potentiated prolonged AIV-H9N2 replication in lung with impairing IFNγ expression. Further researches are needed to elucidate more pathological and immunological factors associated with mixed infection.
Acknowledgements
The authors would like to acknowledge Mohamed Ahmed and Mai Morsi for their kind support.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures involving animals were reviewed and approved by the Research committee of the Animal Health Research Institute.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Abdel-Moneim AS, Afifi MA, El-KadyMF. Emergence of a novel genotype of avian infectious bronchitis virus in Egypt. ArchVirol. 2012;157: 2453–7. 10.1007/s00705-012-1445. (PMID: 22903394). [DOI] [PubMed]
- 2.Asif M, Lowenthal JW, Ford ME, Schat KA, Kimpton WG, Bean AG. Interleukin-6 expression after infectious bronchitis virus infection in chickens. Viral Immunol. 2007;20:479–486. doi: 10.1089/vim.2006.0109. [DOI] [PubMed] [Google Scholar]
- 3.Bakaletz LO. Viral potentiation of bacterial superinfection of the respiratory tract. Trendsin Microbiol. 1995;3:110–114. doi: 10.1016/S0966-842X(00)88892-7. [DOI] [PubMed] [Google Scholar]
- 4.Bancroft JD, Gamble M. Theory and practices of histologic techniques. Philadelphia, PA: Elsevier; 2008. [Google Scholar]
- 5.Ben Shabat M, Meir R, Haddas R, Lapin E, Shkoda I, Raibstein I, Perk S, Davidson I. Development of a real-time TaqMan RT-PCR assay for the detection of H9N2 avian influenza viruses. J Virolog Methods. 2010;168(1–2):72–77. doi: 10.1016/j.jviromet.2010.04.019. [DOI] [PubMed] [Google Scholar]
- 6.Cavanagh D, Davis PJ, Cook JK, Li D, Kant A, Koch G. Location of the amino acid differences in the S1 spike glycoprotein subunit of closely related serotypes of infectious bronchitis virus. Avian Pathol. 1992;21:33–43. 10.1080/03079459208418816. (PMID: 18670913). [DOI] [PubMed]
- 7.Capua I, Marangon S. The avian influenza epidemic in Italy, 1999–2000: a review. Avian Pathol. 2000;29:289–294. doi: 10.1080/03079450050118403. [DOI] [PubMed] [Google Scholar]
- 8.Engelich G, White M, Hartshorn KL. Role of the respiratory burst in co-operativereduction in neutrophil survival by influenza A virus and Escherichia coli. J Med Microbiol. 2002;51:484–490. doi: 10.1099/0022-1317-51-6-484. [DOI] [PubMed] [Google Scholar]
- 9.Haghighat-Jahromi M, Asasi K, Nili H, Dadras H. Role of infectious bronchitis live vaccine on pathogenicity of H9N2 avian influenza virus. Int J Poult Sci. 2007;6(11):838–841. ISSN 1682-8356.
- 10.Hassan KE, Shany SA, Ali A, Dahshan AHM, Azza A, El-Kady MF. Prevalence of avian respiratory viruses in broiler flocks in Egypt. Poult Sci. 2016;95:1271–1280. doi: 10.3382/ps/pew068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hassan KE, Ali A, Shany Salama AS, El-Kady MF. Experimental co-infection of infectious bronchitis and low pathogenic avian influenza H9N2 viruses in commercial broiler chickens. Res Vet Sci. 2017;115:356–362. doi: 10.1016/j.rvsc.2017.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jang H, Koo B-S, Jeon E-O, Lee H-R, Lee S-M, Mo I-P. Altered pro-inflammatory cytokine mRNA levels in chickens infected with infectious bronchitis virus. Poult Sci. 2013;92:2290–2298. doi: 10.3382/ps.2013-03116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaiser P, Rothwell L, Galyov EE, Barrow PA, Burnside J, Wigley P. Differential cytokine expression in avian cells in response to invasion by Salmonella typhimurium, Salmonella enteritidis and Salmonella gallinarum. Microbiology. 2000;146:3217–3226. doi: 10.1099/00221287-146-12-3217. [DOI] [PubMed] [Google Scholar]
- 14.Kim KS, Jung H, Shin IK, Choi BR, Kim DH. Induction of interleukin1 beta (IL1β) is a critical component of lung inflammation during influenza A (H1N1) virus infection. J Med Virol. 2015;87:1104–1112. doi: 10.1002/jmv.24138. [DOI] [PubMed] [Google Scholar]
- 15.Liu J, Li N, Meng D, Hao M, Wei L, Chai T. The mRNA and proteins expression levels analysis of TC-1 cells immune response to H9N2 avian influenza virus. Front Microbiol. 2016;7:1039. doi: 10.3389/fmicb.2016.01039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Livak JK, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 17.Meir R, Maharat O, Farnushi Y, Simanov L. Development of a real-time TaqMan® RT-PCR assay for the detection of infectious bronchitis virus in chickens, and comparison of RT-PCR and virus isolation. J Virol Methods. 2010;163:190–194. doi: 10.1016/j.jviromet.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nang NT, Lee JS, Song BM, Kang YM, Kim HS, Seo SH. Induction of inflammatory cytokines and Toll-like receptors in chickens infected with avian H9N2 influenza virus. Vet Res. 2011;42:64. doi: 10.1186/1297-9716-42-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ng LFP, Liu DX. Further characterization of the coronavirus infectious bronchitis virus C-like proteinase and determination of a new cleavage site. Virology. 2000;272:27–39. doi: 10.1006/viro.2000.0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.OIE. Chapter 2.3.4. Avian influenza. http://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/2.03.04_AI.pdf (2014). Accessed 22 Nov 2014.
- 21.Reed LJ, Muench H. A simple method of estimating fifty percent end points. Am J Hyg. 1938;27:493–497. [Google Scholar]
- 22.Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75:163–189. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- 23.Subtain SM, Iqbal CZ, Ahmad AA, Azhar M, Umer S. Study on pathogenesis of low pathogenic avian influenza virus H9 in broiler chickens. Pak J Zool. 2011;43(5):999–1008. [Google Scholar]
- 24.Valastro V, Holmes EC, Britton P, Fusaro A, Jackwood MW, Cattoli G, Monne I. S1 gene-based phylogeny of infectious bronchitis virus: an attempt to harmonize virus classification. Infect Genet Evol. 2016;39:349–364. doi: 10.1016/j.meegid.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vervelde L, Matthijs MG, Van Haarlem DA, et al. Rapid NK-cell activation in chicken after infection with infectious bronchitis virus M41. Vet Immunol Immunopathol. 2013;151:337–341. doi: 10.1016/j.vetimm.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang J, Cao Z, Guo X, Zhang Y, Wang D, Xu S, Yin Y. Cytokine expression in 3 chicken host systems infected with H9N2 influenza viruses with different pathogenicities. Avian Pathol. 2016;45(6):630–639. doi: 10.1080/03079457.2016.1193665. [DOI] [PubMed] [Google Scholar]
- 27.Wood GW, McCauley JW, Bashiruddin JB, Alexander DJ. Deduced amino acid sequences at the hemagglutinin cleavage site of avian influenza A viruses of H5 and H7 subtypes. Arch Virol. 1993;130:209–217. doi: 10.1007/BF01319010. [DOI] [PubMed] [Google Scholar]
- 28.Yuk SS, Lee DH, Park JK, Tseren-Ochir EO, Kwon JH, Noh JY, Lee JB, Park SY, Choi IS, Song CS. Pre-immune state induced by chickeninterferon gamma inhibits the replicationof H1N1 human and H9N2 avian influenzaviruses in chicken embryo fibroblasts. Virol J. 2016;13:71. doi: 10.1186/s12985-016-0527-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zanaty A, Arafa A-S, Hagag N, El-Kady M. Genotyping and pathotyping of diversified strains of infectious bronchitis viruses circulating in Egypt. World J Virol. 2016;5(3): 125–134. ISSN 2220-3249. [DOI] [PMC free article] [PubMed]
- 30.Zhou H, Chen S, Yan B, Chen H, Wang M, Jia R, Zhu D, Liu M, Liu F, Yang Q, Wu Y, Sun K, Chen X, Jing B, Cheng A. LPAIV H9N2 drives the differential expression of goose interferons and proinflammatory cytokines in both in vitro and in vivo studies. Front Microbiol. 2016;7:166. doi: 10.3389/fmicb.2016.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]