Abstract
Over 99% of cervical cancer cases are associated with genital infection by certain types of human papillomaviruses (HPVs). To outline optimal vaccination strategies and HPV based cervical cancer screening, synthesized data on the genotype distribution of HPV is fundamental that is otherwise missed in Ethiopia. The aim of this study is to compile the findings on HPV genotyping in Ethiopia. Published articles were systematically searched using comprehensive search strings from PubMed/Medline and SCOPUS. Further, Google Scholar and the Google databases were also searched manually for grey literature. The included studies in the review employed 859 women (age range 15–85 years) with different kinds of cervical abnormalities. A total of 534 HPV sequences were reported; the proportion of high risk HPVs was varied 80.4–100%. The top five identified genotypes were HPV 16 (45.3%; 95% CI 41.1–49.6%), HPV 52 (9.4%; 95% CI 7.2–12.1%), HPV 18 (8.2%; 95% CI 6.2–10.9%), HPV 58 (6.9%; 95% CI 5.1–9.4%) and HPV 45 (5.2%; 95% CI 3.7–7.5%). The combined prevalence of HPV 16/18 was at 53.6% (95% CI 49.3–57.8%). In this review, HPV 16 in particular, but also HPV 52 and 18, warrant exceptional consideration in vaccination and HPV based screening programs in Ethiopia. To the best of our knowledge, this study represents the first of its kind to establish the genotype distribution of HPV from different kinds of cervical lesions in Ethiopia although it was synthesized out of few studies. Hence, additional nationwide data are needed to strengthen our finding.
Electronic supplementary material
The online version of this article (10.1007/s13337-019-00527-4) contains supplementary material, which is available to authorized users.
Keywords: Human papillomaviruse, HPV, Genotype distribution, Ethiopia
Introduction
Cervical cancer is the leading cause of cancer-related mortality among women, causing an estimated 273,000 deaths worldwide each year. Eighty-three percent of these deaths occur in developing countries, where there is quite limited research in the field (http://screening.iarc.fr/doc/Human%20Papillomavirus%20and%20Related%20Cancers.pdf). More than 99% of cervical cancer cases are epidemiologically associated with high risk human papillomaviruses (HR-HPV) infection (https://www.ncbi.nlm.nih.gov/books/NBK195239/) which is one of the common sexually transmitted infections that most women are experiencing soon after they become sexually active [21]. To date, over 150 different HPVs (based on sequence differences within their L1 gene) have been characterized and completely sequenced. Moreover, these viruses are categorized according to their oncogenic potential (high risk and low risk). Of all types of HPVs, about 30–40 are sexually transmitted and infect the genital areas of both men and women [13, 25].
The HR-HPV types include 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 68 and 59. Some other groups are classified as potential or probable high risk (pHR) types including HPV types 53, 66, 70, 73, and 82. HPV 16 and 18 are the well-known oncogenic virus types, causing over 70% of all cervical cancer burdens around the world. About seven types of HR-HPVs are associated with approximately 87% of all cervical cancer cases worldwide (http://screening.iarc.fr/doc/Human%20Papillomavirus%20and%20Related%20Cancers.pdf) (https://www.ncbi.nlm.nih.gov/books/NBK195239/).
HPV has been associated with a range of malignancies including anogenital cancers, such as cervical cancer and possibly a subset of cancers of the aerodigestive tract. These small, non-enveloped, double-stranded DNA viruses primarily infect the epithelium and induce benign as well as malignant lesions of the mucosa and skin. Some HPV types (like HPV 16 and HPV 18) are considered to be high-risk due to their strong implication in carcinogenesis, particularly the malignant progression of cervical tumors. The recognition of papillomaviruses as a major etiologic agent for human cancers has increased their medical importance and stimulated research into developing strategies for screening, diagnosis, prevention and treatment of HPV-associated diseases [3, 6].
Ethiopia has a population of over 29 million women aged 15 years and older who are at risk of developing cervical cancer (http://afcrn.org/membership/membership-list/100-addisababa) (https://www.iccp-portal.org/system/files/plans/Guideline%20Eth%20Final.pdf). It is the leading cause of cancer mortality among Ethiopian women over the age of 30. Records show that 7600 Ethiopian women are diagnosed annually with cervical cancer and of these, 6000 die of the disease each year (https://www.pathfinder.org/publications/combating-cervical-cancer-ethiopia-addis-tesfa/).
In Sub-Saharan Africa, cervical screening coverage ranges from 2.0 to 20.2% in urban areas and 0.4 to 14.0% in rural areas [20]. In Ethiopia where there is no well-established cervical cancer screening and vaccination program the visual inspection with acetic acid (VIA) based screening coverage for targeted women is at 0.6% (among all women aged 18–69 years, screened every 3 years) (http://screening.iarc.fr/doc/Human%20Papillomavirus%20and%20Related%20Cancers.pdf) which is actually less than the African average. The main reasons for absence of implementation of the HPV vaccination might be the high cost of the vaccine.
Despite the high burden of cervical cancer related morbidity and mortality, robust compiled data on HPV genotype distribution among Ethiopian women is missing. Taking this knowledge gap under consideration, this systematic review was conducted aimed at determining the molecular epidemiology of HPVs in Ethiopia. In order to draw up optimal vaccination plans and HPV based cervical cancer screening test recommendations, it is crucial to identify the HPV genotypes circulating in the country. Hence, the main aim of this systematic review-study was to determine the genotype distribution of Human papillomavirus among women with different kinds of cervical pathologies in Ethiopia. The finding of this study will serve as a baseline data for policy makers and other stakeholders as a gate for vaccination programs and to plan HPV based cervical cancer screening.
Materials amd methods
Protocol registration
In accordance with the PRISMA guidelines, our systematic review protocol was registered by the International Prospective Register of Systematic Reviews (PROSPERO) on 28 August 2018 and was last updated on 30 August, 2018 (registration number CRD42018104943).
Eligibility criteria
Studies were selected according to the following criteria; study design: quantitative studies like cross-sectional studies reporting the genotype distribution of HPVs from cervical samples were considered. Participants: we included studies that examined women irrespective of the age group. Interventions: our interest was genotypic profile of HPV. The molecular epidemiology or genotype distribution of HPV was defined as the type of HPV genotype identified using a molecular method from women with different kinds of cervical pathologies. Setting: we included studies with the outcome of interest reported in Ethiopia. Language and publication: We considered peer-reviewed articles, governmental documents and unpublished articles (thesis) that reported in English language irrespective of the year of publication.
Information sources and search strategy
This review was designed following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis Protocols (PRISMA) Guideline [30]. Published research papers were systematically and compressively searched in PubMed/Medline and SCOPUS, the last search was conducted on 27th of July, 2018. Manual search from Google scholar and Google databases was also done for grey literature screening. The search terms were developed in line with the Medical Subject Headings (MeSH) thesaurus using a combination of big ideas (key terms) driven from the research question. The reference lists of retrieved articles were probed (forward and back ward searching) to identify articles that were not retrieved from databases and our manual search. The first two authors (AD and DM) independently searched the articles.
The domains of the search terms were Human papillomavirus/HPV, cervical cancer, cervical precancerous lesion, molecular epidemiology, genotype distribution and Ethiopia. We combined Human papillomavirus and cervical cancer/lesions with the Boolean operator “OR”, and the result was combined with the other terms with “AND”. Full search strategy for the two databases is given in Supplement 1.
Study selection
The research papers that have reported the molecular epidemiology (genotype distribution) of identified HPVs among women with normal cervical cytology, cervical precancerous lesions and cervical cancer in Ethiopian context were included.
Searched articles were directly imported and handled using EndNote X5 citation manager (Thomson Reuters, New York, USA). Based on the PRISMA approach, duplicated articles were excluded and the titles and abstracts of the remaining papers were screened independently for inclusion in full text evaluation by the first two authors (AD, DM). Differences between the reviewers were resolved through discussion. In case of disagreements the decision was determined by the last author.
Data collection process and data items
The Joanna Briggs Institute (JBI) data extraction tool was adopted for data extraction in this review. The first two authors extracted the data independently. In case where there are differences between the two authors with regard to the extracted data, the difference was solved through discussion. Data such as the name of the first author, year of publication, age group of women participants, study area/region, study design, the total number of women involved in the study and the type of HPVs identified were extracted from the included articles.
Quality appraisal
To assess the risk of bias, the two authors independently used the nine items (each score one point) based on the Joanna Briggs Institute (JBI) Critical Appraisal tools (http://joannabriggs.org/research/critical-appraisal-tools.html) for prevalence studies. We assumed that papers that scored > 50% (i.e. ≥ 5 of 9 scores) of the weighted value of the tool considered as good quality.
Data synthesis
The data extracted from eligible studies were logged into a Microsoft Excel spread sheet and were presented in terms of: (1) the prevalence of HPV from each study, (2) the proportion of HR-HPVs from identified genotypes and (3) the proportion of all identified HPV genotypes. A systematic narrative synthesis was provided in which summary results were presented using text, table and figures. Counts, ranges and percentages were used to describe the synthesized data.
Results
Search results
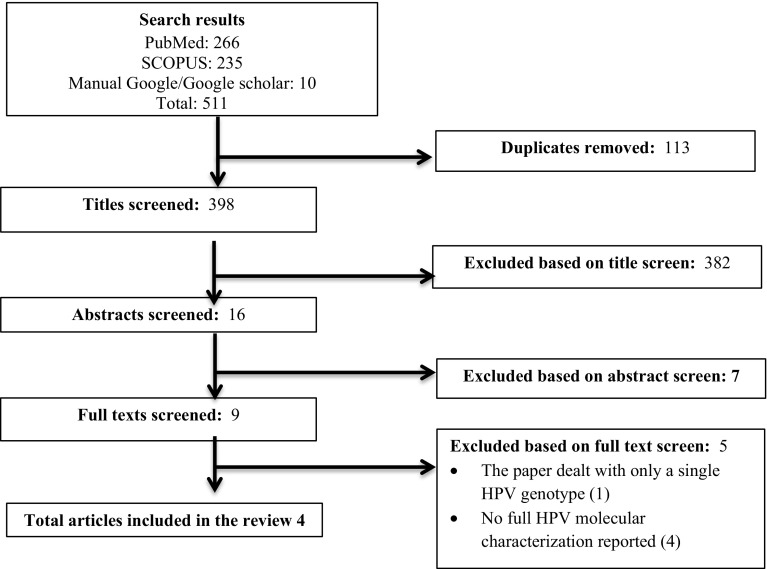
From the searched data bases and other sources, a total of 511 articles were retrieved and screened. After removing the duplicate, 398 were screened by title then 382 were removed. Consequently, 7 were removed by abstract and 5 by full text with reasons. Finally, a total of 4 studies [1, 4, 19, 24] met our inclusion criterion which in fact implies that, data is limited on HPV genotype distribution in Ethiopia.
Screening was based on the PRISMA flow chart which was adapted from the PRISMA guideline [30] (Fig. 1).
Fig. 1.
The PRISMA flow diagram of literature selection. A total of 511 articles were retrieved from the searched data bases and other sources. After removing the duplicates, 398 were screened by title then 382 were removed. Accordingly, 7 were removed by abstract and 5 by full text with justification. Finally, 4 studies met our inclusion criterion for analysis in this review. Un*: Undetermined. The x-axis of the figure indicates the HPV types
Study characteristics
The description of each study characteristic, together with the reported prevalence of HPV and the proportion of HR-HPV is presented in Table 1.
Table 1.
Characteristics of the included studies, 2010–2014
| References | Study area | Recruitment setting | Number of study participants | Age range of the participants | Prevalence of HPV | Proportion of HR-HPVs |
|---|---|---|---|---|---|---|
| Bekele et al. [4] | Jimma | Women with cervical dysplasia | 132 | 32–65 | 82 (67.8%) | 82 (100%) |
| Abate et al. [1] | Addis Ababa | Paraffin embedded cervical biopsy | 170 | 21–85 | 149 (93%) | 139 (93.5%) |
| Mihret et al. [24] | Addis Ababa | Women having different cervical intraepithelial neoplasia | 20 | 23–60 | 17 (85%) | 17 (100%) |
| Leyh-Bannurah et al. [19] | Atta | Women who visited the gynecological outpatient department | 537 | 15–64 | 107 (19.9%) | 86 (80.4%) |
The studies were undertaken in three different study areas (Addis Ababa, Jimma and Atta (which is located in southern part of the country) in the last few years (2010–2014) which might suggest an increasing interest in cervical cancer research in Ethiopia. These studies were conducted in the nation’s biggest hospital and community-based district hospitals which permitted to appreciate a better representative of women in the country.
All the included articles were published in peer-reviewed journals. The primary interest of the included papers was to determine the molecular profile of HPV from cervical samples. In total, data of 859 women (age range 15–85 years) were included.
One of the four studies [1] used paraffin embedded cervical biopsies while the remaining [4, 19, 24] employed cervical swabs for HPV DNA detection and molecular characterization. In three of the four studies [1, 4, 24], samples were taken from woman with different kinds of cervical dysplasia. In these studies the HPV detection level was between 67.8 and 93%. In the other study [19], the study subjects were women from the general population who visited the gynecology outpatient department of Atta hospital and as a result the prevalence of HPV was lower (19.9%). Of the total identified HPVs in the four studies, the proportion of high risk types (HR-HPVs) was between 80.4 and 100% (Table 1).
The included studies employed the following molecular techniques to characterize the genotype distribution of HPVs; reverse line blot hybridization assay [1], gel electrophoresis [4], Digene hybrid capture 2 HPV test with genotyping Kit HPV GP [19] and line probe assay (Inno-LiPA) [24].
Risk of bias within studies
The nine domain-based JBI Critical appraisal tool (http://joannabriggs.org/research/critical-appraisal-tools.html) for prevalence studies was used to test outcome level risk of bias of each studies. Each domain had a score of 1 point. The risk of bias for each individual domain was measured as ‘yes’, ‘no’, ‘unclear’ and ‘not applicable’. In this study, ‘yes’ scored 1 and ‘no’ ‘unclear’ and ‘not applicable’ scores zero. The total score is summarized below in Table 2. The total score therefore ranges from zero to nine, with higher scores indicating higher quality of outcome. Based on our assumption, all the included articles scored above 50% positive score (mean score 5.8). Hence, we considered all as good quality articles.
Table 2.
Quality of the included studies based on the JBI critical for prevalence studies, 2010–2014
Molecular type distribution of human papillomaviruses (HPVs)
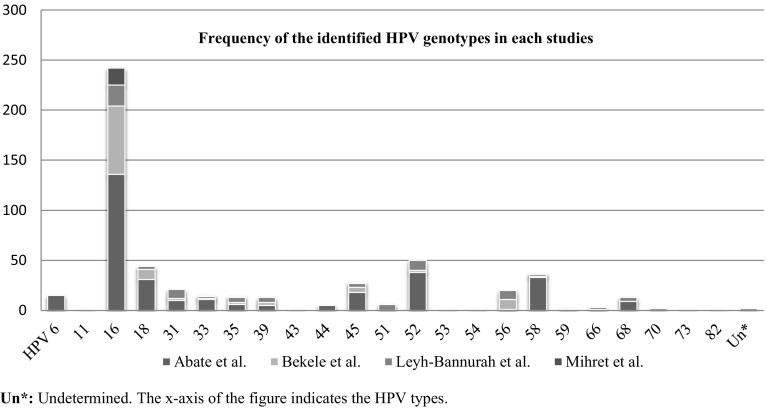
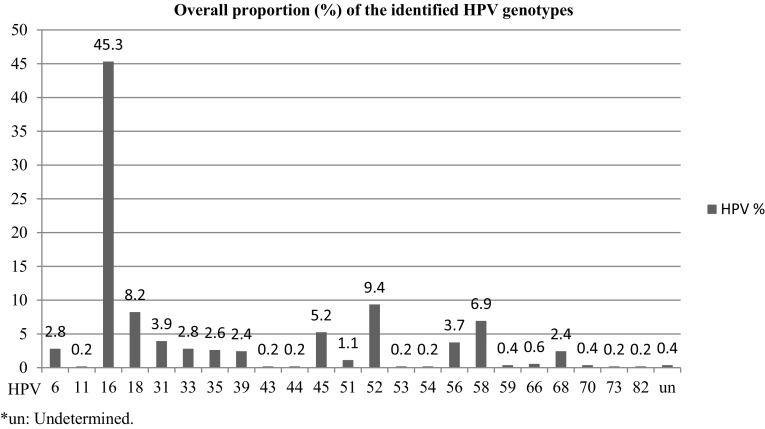
The molecular types of identified HPVs from each studies and their overall respective proportion are presented in Figs. 2 and 3, respectively. From the included studies, 23 kinds of a total of 534 HPVs were reported. The top five HPV genotypes were HPV 16 (45.3%; 95% CI 41.1–49.6%), HPV 52 (9.4%; 95% CI 7.2–12.1%), HPV 18 (8.2%; 95% CI 6.2–10.9%), HPV 58 (6.9%; 95% CI 5.1–9.4%) and HPV 45 (5.2%: 95% CI 3.7–7.5%). The combined prevalence of HPV 16/18 was at 53.6% (95% CI 49.3–57.8%). Some of other reported high risk HPV groups include HPV 31 (3.9%), HPV 33 (2.8%), HPV 39 (2.4%), HPV 51 (1.1%), HPV 56 (3.7%) and HPV 68 (2.4%). In contrast, from the low risk HPV types, HPV 6 at 2.8% (95% CI 1.7–4.9%) was predominant.
Fig. 2.
Frequency of HPV genotypes identified from included studies, in Ethiopia, 2010–2014. The figure basically also tells us about the relative abundances of the different genotypes in the Ethiopian areas. For example, HPV 58 is much more prevalent in Addis Ababa because it was picked up relatively more by the study of Abate et al. *un: Undetermined
Fig. 3.
HPV genotype distribution in Ethiopia, 2010–2014. The figure shows that HPV 16 at 45.3% followed by HPV 52 at 9.4% were the most commonly reported kind of high risk genotypes
Discussion
HPV infections by certain genotypes are known to cause cervical cancer globally. Although it is essential for pre-vaccine research, currently there is limited data on the prevalence and genotype distribution of HPVs in Ethiopia, where the government is planning to introduce HPV vaccination at the end of 2018 for girls. This review will be used to assess the impact of vaccine in the future and for planning which HPV based screening test to employ. With the advent of preventive HPV vaccines, several countries are using Cervarix™ (HPV 16 and 18), Gardasil ™ (HPV 6, 11, 16, 18) and the recent nonavalent vaccine Gardasil®9 (6, 11, 16, 18, 31, 33, 45, 52, and 58) that targets close to 90% of all HR-HPVs [35]. However, there is no HPV vaccination program in Ethiopia. However, it is currently under consideration and data from this review will be an important input on this regard.
Because of considerable geographical differences in the HPV genotype distribution globally, data are required on HPV genotyping for a specific nation not only for vaccination programs but also to support HPV based cervical cancer screening [18] as HPV genotyping may have the potential to improve the effectiveness of screening programs and to reduce overtreatment.
Despite the difference in methods employed and specimen used, it would have been nice to drive age specific prevalence and distribution of HPV. However, it was not possible in the present review because of lack of data. But, based the review of Smith and colleague, genital HPV infection in women is predominantly acquired in adolescence, and peak prevalence in middle-aged women appears to differ across geographical regions due to differences in sexual behavior [33].
In our review, one of the four studies [19] included study subjects from the general population who visited the gynecological outpatient department, from whom the prevalence of HPV was at 19.9%. Comparable findings were reported from different studies worldwide. For example, based on global based reviews the prevalence of HPV was between 11 and 12% (with higher rates in sub-Saharan Africa at 24%) in women without cervical abnormalities [7, 15]. Similarly, in other global based reviews analogous findings were reported on this regard at 22.1% in Africa, 20.4% in Central America and Mexico, 11.3% in northern America, 8.1% in Europe and 8.0–12.8% in Asia [12, 27], specifically 9.4% in Iran [22] and 14.71–44.3% in China [9, 32] and 42.3% in Burkina Faso [23]. The difference regarding the report from Burkina Faso and China could be explained by a population difference in which these studies might be included woman with cervical abnormalities.
In the present review the prevalence of HPV from woman with different kinds of cervical dysplasia was between 67.8 and 93%. Forman et al. [15] on his global based review reported close to 90% prevalence of HPV in women with different cervical abnormalities including neoplasia. Besides, a review by Pend et al. [27] conducted to demonstrate the HPV genotype among Asian women reported HPV prevalence at 84.8% from different kinds of advanced cervical lesions. Furthermore, other review studies from different corner of the globe reported the following ranges of HPV prevalence from different grades of cervical dysplasia; 87.2% (Burkina Faso) [23], between 70 and 80% (North America) [11], 78.1% (Central and Eastern Europe) [28] and between 64.8 and 90.1% (Thai) [18].
Taking the natural history of HPV infection under consideration, its prevalence increases in women with cervical pathology in proportion to the severity of the lesions. Although the different grades of cervical lesions were not specifically explored in our review, three of the four studies employed participants who had different kinds of cervical lesions which could make sense reporting (67.8–93%) HPV prevalence and is almost comparable with the above review findings on this regard.
In this review the reported genotypes in Ethiopia based on their descending proportion were HPV 16 (45.3%), HPV 52 (9.4%), HPV 18 (8.2%), HPV 58 (6.9%) and HPV 45 (5.2%), HPV 31 (3.9%), HPV 33 (2.8%), HPV 39 (2.4%), HPV 51 (1.1%), HPV 56 (3.7%) and HPV 68 (2.4%). Such findings are basically desirable to predict how HPV vaccination and HPV based screening will effect cervical cancer prevention in Ethiopia. This implies that further HPV vaccine studies should target the first predominant genotypes to prevent infections associated with them. This is the case with the nonavalent Cervarix vaccine which targets the seven first HPV types. Nevertheless, HPV types 39, 51, 56 and 68 counts up to 9.6%, meaning that, in accordance to other studies, vaccination with the broadest vaccine currently available would protect against about 90% of HR-HPV types.
Based on review articles and series of original studies somewhere in the globe, the genotype distribution of HPVs in different countries from different kinds of cervical lesions is presented in Table 3 for comparison purpose.
Table 3.
Comparison of our review result with other reported top five HPV genotypes with (%) distribution in different regions worldwide
| Our review result, HPV type (%) | Africa [26] | Asia [27] | N. America [11] | Israel [31] | Latin America and Caribbean [10] |
|---|---|---|---|---|---|
| HPV 16 (45.3) | HPV 16 (31.2) | HPV16 (23.9) | HPV16 (26.3) | HPV16 (46.5) | HPV 16 (46.5) |
| HPV 52 (9.4) | HPV 18 (13.9) | HPV18 (11) | HPV31 (11.5) | HPV18 (11.3) | HPV 18 (8.9) |
| HPV 18 (8.2) | HPV 31 (8.2) | HPV58 (9.4) | HPV51 (10.6) | HPV31 (7.0) | HPV 31 (8.0) |
| HPV 58 (6.9) | HPV 33 (10.3) | HPV 56 (6.3) | HPV53 (10.2) | HPV51 (5.6) | HPV 58 (8.7) |
| HPV 45 (5.2) | HPV 35 (13.4) | HPV 52 (5.3) | HPV45 (4.2) | HPV 33 (6.5) |
On top of this, series of studies in China had reported the following common HPV genotypes, HPV 16 (16.1–33.3%), HPV 52 (8.9–22.3%), HPV 58 (7.9–23.8%), HPV 18, 31 (8.8% each), HPV 33 (6.8%) [5, 8, 9, 32]. Similarly, in Qatar HPV 16 (22.2–25.0%), HPV 18 (22.2%), HPV 56 (22.2% each), HPV 59 (25.0%) [2, 14] and In Thailand the most common identified genotypes were HPV 16 (38.5%), HPV 58 (20.0%), and HPV 18 (5.5%) [18].
Variation in the HPV genotype distribution across the above studies is likely attributable to differences in population, severity of cervical lesions, age at screening initiation, frequency, coverage, and rates of follow-up of women with cervical abnormalities [34] but so far it is not clearly known how each HPV genotype is influenced by these factors.
In addition to the above findings, our review had also presented a high proportion of HR-HPV genotypes at 80.4–100%, which is significantly higher than the recent meta-analysis by Ogembo et al. [26] on pooled HPV prevalence in the Eastern Africa (42.2%). However, a review aimed at HPV profiling from women in India, Bangladesh, Sri Lanka and Nepal reported almost same proportion of high-risk HPV types with our finding at 97% [29].
Among HR-HPV groups HPV 16 and 18 are commonly implicated in cervical malignancies. In the present review, the combined prevalence of HPV 16/18 was at 53.6%. Similarly, a review by Ogembo et al. [26] showed HPV16/18 at 45.1% from high-grade cervical lesions and 67.7% of invasive cervical cancer among African women. Among HPV positive cases, the co-prevalence of HPV 16/18 was reported differently indifferent countries such as; 60% (in Israel) on both pre-neoplastic lesions and cervical cancer [31], 87.5% (in Central and Eastern Europe), [28], 80% (India) among high grade cervical lesions [29]. In Italy, HPV 16 (64%) and HPV 18 (7%) were frequently reported from women with abnormal cervical cytology [16].
In general, from the above paragraphs we can clearly learn that despite differences on the reported HPV genotype distribution, almost all studies suggest that HPV 16 is an important genotype that is most often involved in abnormal cervical cytology and cancer. An interesting review by Guan et al. [17] showed that the HPV 16 positivity increased steeply from normal to high grade cervical intra-neoplasia. Thus, a vaccine cocktails and HPV DNA based screening tool should always incorporate this genotype although some low-grade cervical lesions associated with certain other HPVs (like HPV 18, 52 and 58) may preferentially progress to cervical cancer as well. Our review would predict the future impact of predominantly identified genotypes (HPV 16, 52 and 18) in vaccination including the new nonavalent vaccine and HPV based screening test in Ethiopia.
To the best of our knowledge, this systematic review represents the first to present the genotype distribution of HPV in Ethiopia from different kinds of cervical lesions. The other strong suit of this review is that it includes studies from different settings, including the nation’s biggest hospital and community-based district hospitals that allowed incorporating a better representative of women in the country. In addition to the diversity of settings and the included age range of the study participants (15–85 years) in the included studies, the catchments of the health settings may have differed in terms of the severity of the cervical lesions and socioeconomic status, which as a consequence, may have had an impact on the type distribution of HPVs.
Our review should be interpreted in light of a couple of drawbacks including but not limited to the small number of included studies. As a result, the absence of data from some regions could compromise the overall picture of the HPV genotype distribution in the country. The other possible pitfall of this review is the variation in sensitivity/specificity of the HPV genotyping methods. Finally, the other curb relates to the point in time analysis of studies with cross-sectional study designs which inherently could affect the overall picture of the reported genotypes.
HPV 16 in particular, but also HPV 52 and 18, warrant exceptional consideration in vaccination and HPV based cervical cancer screening programs in Ethiopia. Additional data are needed to know the distribution of HPVs in other regions of Ethiopia and to bold HR-HPV related genital infections, especially cervical cancer, as a public health importance to introduce, implement and sustain effective prevention and control programs in the country.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Authors are delighted to thank Bahir Dar and Addis Ababa Universities and CDT-Africa for the opportunity we have provided to conduct this review article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Abate E, Aseffa A, El-Tayeb M, El-Hassan I, Yamuah L, Mihret W, et al. Genotyping of human papillomavirus in paraffin embedded cervical tissue samples from women in Ethiopia and the Sudan. J Med Virol. 2013;85(2):282–287. doi: 10.1002/jmv.23437. [DOI] [PubMed] [Google Scholar]
- 2.Bansal D, Elmi AA, Skariah S, Haddad P, Abu-Raddad LJ, Al Hamadi AH, et al. Molecular epidemiology and genotype distribution of human papillomavirus (HPV) among Arab women in the State of Qatar. J Transl Med. 2014;12(300):014–0300. doi: 10.1186/s12967-014-0300-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bekele A, Baay M, Mekonnen Z, Suleman S, Chatterjee S. Human papillomavirus type distribution among women with cervical pathology—a study over 4 years at Jimma Hospital, southwest Ethiopia. Trop Med Int Health. 2010;15(8):890–893. doi: 10.1111/j.1365-3156.2010.02552.x. [DOI] [PubMed] [Google Scholar]
- 4.Bekele A, Baay M, Mekonnen Z, Suleman S, Chatterjee S. Human papillomavirus type distribution among women with cervical pathology—a study over 4 years at Jimma Hospital, southwest Ethiopia. Trop Med Int Health. 2010;15(8):890–893. doi: 10.1111/j.1365-3156.2010.02552.x. [DOI] [PubMed] [Google Scholar]
- 5.Bi Q, Zhang L, Zhao Z, Mu X, Zhang M, Wang P. Human papillomavirus prevalence and genotypes distribution among female outpatients in Qingdao, East China. J Med Virol. 2015;87(12):2114–2121. doi: 10.1002/jmv.24281. [DOI] [PubMed] [Google Scholar]
- 6.Bradford L, Goodman A. Cervical cancer screening and prevention in low-resource settings. Clin Obstet Gynecol. 2013;56(1):76–87. doi: 10.1097/GRF.0b013e31828237ac. [DOI] [PubMed] [Google Scholar]
- 7.Bruni L, Diaz M, Castellsague X, Ferrer E, Bosch FX, de Sanjose S. Cervical human papillomavirus prevalence in 5 continents: meta-analysis of 1 million women with normal cytological findings. J Infect Dis. 2010;202(12):1789–1799. doi: 10.1086/657321. [DOI] [PubMed] [Google Scholar]
- 8.Chan PK, Li WH, Chan MY, Ma WL, Cheung JL, Cheng AF. High prevalence of human papillomavirus type 58 in Chinese women with cervical cancer and precancerous lesions. J Med Virol. 1999;59(2):232–238. doi: 10.1002/(SICI)1096-9071(199910)59:2<232::AID-JMV18>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 9.Chen X, Wallin KL, Duan M, Gharizadeh B, Zheng B, Qu P. Prevalence and genotype distribution of cervical human papillomavirus (HPV) among women in urban Tianjin, China. J Med Virol. 2015;87(11):1966–1972. doi: 10.1002/jmv.24248. [DOI] [PubMed] [Google Scholar]
- 10.Ciapponi A, Bardach A, Glujovsky D, Gibbons L, Picconi MA. Type-specific HPV prevalence in cervical cancer and high-grade lesions in Latin America and the Caribbean: systematic review and meta-analysis. PLoS ONE. 2011;6(10):4. doi: 10.1371/journal.pone.0025493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clifford GM, Rana RK, Franceschi S, Smith JS, Gough G, Pimenta JM. Human papillomavirus genotype distribution in low-grade cervical lesions: comparison by geographic region and with cervical cancer. Cancer Epidemiol Biomark Prev. 2005;14(5):1157–1164. doi: 10.1158/1055-9965.EPI-04-0812. [DOI] [PubMed] [Google Scholar]
- 12.de Sanjose S, Diaz M, Castellsague X, Clifford G, Bruni L, Munoz N, et al. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta-analysis. Lancet Infect Dis. 2007;7(7):453–459. doi: 10.1016/S1473-3099(07)70158-5. [DOI] [PubMed] [Google Scholar]
- 13.Doorbar J, Quint W, Banks L, Bravo IG, Stoler M, Broker TR, et al. The biology and life-cycle of human papillomaviruses. Vaccine. 2012;20(30):083. doi: 10.1016/j.vaccine.2012.06.083. [DOI] [PubMed] [Google Scholar]
- 14.Elmi AA, Bansal D, Acharya A, Skariah S, Dargham SR, Abu-Raddad LJ, et al. Human papillomavirus (HPV) Infection: molecular epidemiology, genotyping, seroprevalence and associated risk factors among Arab women in Qatar. PLoS ONE. 2017;12(1):e0169197. doi: 10.1371/journal.pone.0169197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forman D, de Martel C, Lacey CJ, Soerjomataram I, Lortet-Tieulent J, Bruni L, et al. Global burden of human papillomavirus and related diseases. Vaccine. 2012;20(30):055. doi: 10.1016/j.vaccine.2012.07.055. [DOI] [PubMed] [Google Scholar]
- 16.Giorgi Rossi P, Chini F, Borgia P, Guasticchi G, Carozzi FM, Confortini M, et al. Human papilloma virus (HPV), cervical cancer incidence and screening uptake: differences among Northern, Central and Southern Italy. Epidemiol Prev. 2012;36(2):108–119. [PubMed] [Google Scholar]
- 17.Guan P, Howell-Jones R, Li N, Bruni L, de Sanjose S, Franceschi S, et al. Human papillomavirus types in 115,789 HPV-positive women: a meta-analysis from cervical infection to cancer. Int J Cancer. 2012;131(10):2349–2359. doi: 10.1002/ijc.27485. [DOI] [PubMed] [Google Scholar]
- 18.Kietpeerakool C, Kleebkaow P, Srisomboon J. Human papillomavirus genotype distribution among Thai women with high-grade cervical intraepithelial lesions and invasive cervical cancer: a literature review. Asian Pac J Cancer Prev. 2015;16(13):5153–5158. doi: 10.7314/APJCP.2015.16.13.5153. [DOI] [PubMed] [Google Scholar]
- 19.Leyh-Bannurah S-R, Prugger C, de Koning MNC, Goette H, Lellé RJ. Cervical human papillomavirus prevalence and genotype distribution among hybrid capture 2 positive women 15 to 64 years of age in the Gurage zone, rural Ethiopia. Infect Agents Cancer. 2014;9:33. doi: 10.1186/1750-9378-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Louie KS, de Sanjose S, Mayaud P. Epidemiology and prevention of human papillomavirus and cervical cancer in sub-Saharan Africa: a comprehensive review. Trop Med Int Health. 2009;14(10):1287–1302. doi: 10.1111/j.1365-3156.2009.02372.x. [DOI] [PubMed] [Google Scholar]
- 21.Maine D, Hurlburt S, Greeson D. Cervical cancer prevention in the 21st century: cost is not the only issue. Am J Public Health. 2011;101(9):1549–1555. doi: 10.2105/AJPH.2011.300204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malary M, Moosazadeh M, Hamzehgardeshi Z, Afshari M, Moghaddasifar I, Afsharimoghaddam A. The prevalence of cervical human papillomavirus infection and the most at-risk genotypes among Iranian healthy women: a systematic review and meta-analysis. Int J Prev Med. 2016;7(70):2008–7802. doi: 10.4103/2008-7802.181756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maria H, Dana H, Francoise M, Michael P, Jurgen W. Human papillomaviruses in Western Africa: prevalences and risk factors in Burkina Faso. Arch Gynecol Obstet. 2018;17(10):018–4860. doi: 10.1007/s00404-018-4860-z. [DOI] [PubMed] [Google Scholar]
- 24.Mihret W, Yusuf L, Abebe M, Yamuah LK, Bekele L, Abate E, et al. A pilot study on detection and genotyping of human papilloma virus isolated from clinically diagnosed Ethiopian women having cervical intraepithelial neoplasia. Ethiop Med J. 2014;1:49–52. [PubMed] [Google Scholar]
- 25.Münger K, Baldwin A, Edwards KM, Hayakawa H, Nguyen CL, Owens M, et al. Mechanisms of human papillomavirus-induced oncogenesis. J Virol. 2004;78(21):11451–11460. doi: 10.1128/JVI.78.21.11451-11460.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ogembo RK, Gona PN, Seymour AJ, Park HS, Bain PA, Maranda L, et al. Prevalence of human papillomavirus genotypes among African women with normal cervical cytology and neoplasia: a systematic review and meta-analysis. PLoS ONE. 2015;10(4):e0122488. doi: 10.1371/journal.pone.0122488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peng RR, Li HM, Chang H, Li JH, Wang AL, Chen XS. Prevalence and genotype distribution of cervical human papillomavirus infection among female sex workers in Asia: a systematic literature review and meta-analysis. Sex Health. 2012;9(2):113–119. doi: 10.1071/SH11066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poljak M, Seme K, Maver PJ, Kocjan BJ, Cuschieri KS, Rogovskaya SI, et al. Human papillomavirus prevalence and type-distribution, cervical cancer screening practices and current status of vaccination implementation in Central and Eastern Europe. Vaccine. 2013;31(31):029. doi: 10.1016/j.vaccine.2013.03.029. [DOI] [PubMed] [Google Scholar]
- 29.Sankaranarayanan R, Bhatla N, Gravitt PE, Basu P, Esmy PO, Ashrafunnessa KS, et al. Human papillomavirus infection and cervical cancer prevention in India, Bangladesh, Sri Lanka and Nepal. Vaccine. 2008;19(26):005. doi: 10.1016/j.vaccine.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 30.Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647. doi: 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- 31.Shavit O, Roura E, Barchana M, Diaz M, Bornstein J. Burden of human papillomavirus infection and related diseases in Israel. Vaccine. 2013;22(31):108. doi: 10.1016/j.vaccine.2013.05.108. [DOI] [PubMed] [Google Scholar]
- 32.Singh S, Zhou Q, Yu Y, Xu X, Huang X, Zhao J, et al. Distribution of HPV genotypes in Shanghai women. Int J Clin Exp Pathol. 2015;8(9):11901–11908. [PMC free article] [PubMed] [Google Scholar]
- 33.Smith JS, Melendy A, Rana RK, Pimenta JM. Age-specific prevalence of infection with human papillomavirus in females: a global review. J Adolesc Health. 2008;43(4 Suppl):009. doi: 10.1016/j.jadohealth.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 34.Ting J, Kruzikas DT, Smith JS. A global review of age-specific and overall prevalence of cervical lesions. Int J Gynecol Cancer. 2010;20(7):1244–1249. doi: 10.1111/IGC.0b013e3181f16c5f. [DOI] [PubMed] [Google Scholar]
- 35.Zhai L, Tumban E. Gardasil-9: a global survey of projected efficacy. Antivir Res. 2016;130:101–109. doi: 10.1016/j.antiviral.2016.03.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.