Abstract
Introduction
The glucagon-like peptide-1 receptor agonist (GLP-1 RA) class is evolving and expanding. This retrospective database study evaluated recent real-world treatment and dosing patterns of patients with type 2 diabetes (T2D) initiating GLP-1 RAs in Belgium (BE), France (FR), Germany (DE), Italy (IT), the Netherlands (NL), and Canada (CA).
Methods
Adult T2D patients initiating GLP-1 RA therapy (dulaglutide [DULA], exenatide twice daily [exBID], exenatide once weekly [exQW], liraglutide [LIRA], or lixisenatide [LIXI]) from 2015 to 2016 were identified using the IQVIA (IQVIA, Durham, NC, and Danbury, CT, USA) Real-World Data Adjudicated Pharmacy Claims. The therapy initiation date was termed the ‘index date.’ Eligible patients had ≥ 180 days pre-index and ≥ 360 days post-index. Persistence (until discontinuation or switch) was evaluated over the variable follow-up using Kaplan–Meier (KM) survival analysis. Average daily dose (ADD) was calculated until discontinuation or switch.
Results
A total of 34,649 DULA, 3616 exBID, 11,138 exQW, 48,317 LIRA, and 2,204 LIXI patients were included in the analysis (34.9–63.2% female; median age range 53–62 years; median follow-up 16–30 months). Proportion persistent at 1-year post-index was 36.8–67.2% for DULA, 5.9–44.4% for exBID, 24.7–44.2% for exQW, 22.2–57.5% for LIRA, and 15.5–40.0% for LIXI. Median time persistent (days) was 245–381 for DULA, 62–243 for exBID, 121–319 for exQW, 103–507 for LIRA, and 99–203 for LIXI. Mean ADD was 13.21–20.43 µg for exBID, 1.44–1.68 mg for LIRA, and 19.88–20.54 µg for LIXI. Mean average weekly dose (AWD) ranged from 2.03 to 2.14 mg for exQW. Mean AWD for DULA was 1.25 mg in Canada and ranged from 1.43 to 1.53 mg in the other countries.
Conclusion
Across six countries, persistence was highest among DULA patients and generally lowest among exBID patients. ADD/AWD for all GLP-1 RAs was in line with the recommended label. Longer-term data would be useful to obtain a better understanding of GLP-1 RA treatment patterns over time.
Funding
Eli Lilly and Company, Indianapolis, IN, USA.
Electronic Supplementary Material
The online version of this article (10.1007/s13300-019-0615-5) contains supplementary material, which is available to authorized users.
Keywords: Dulaglutide, Exenatide BID, Exenatide QW, Glucagon-like peptide-1 receptor agonists (GLP-1 RAs), Liraglutide, Lixisenatide, Persistence, Type 2 diabetes
Introduction
Globally, an estimated 425 million adults (8.8% of the global adult population) had diabetes in 2017 [1]. In high-income countries, 87–91% of all people with diabetes have type 2 diabetes (T2D). Diabetes is associated with a substantial economic burden, with approximately US$727 billion (€671 billion)—or 12.5% of the total global health expenditure—attributed to diabetes in 2017. In Europe and Canada, the prevalence of diabetes among adults was 8.8% and 9.6% in 2017, with total diabetes-related health expenditures of US$207 and US$15 billion, respectively.
Most patients with T2D will require drug therapy with an antihyperglycemic agent to assist in regulating glucose control [2]. Metformin is the preferred option for initial monotherapy in T2D [3]. However, due to the progressive nature of the disease, combination therapy is typically needed after several years, and for many patients, injectable glucose-lowering therapies will become necessary within 5–10 years of diagnosis [3]. Advancements in treatment options for T2D have led to the development of a new class of drugs: glucagon-like peptide-1 receptor agonists (GLP-1 RAs) [4]. GLP-1 RA therapies represent an important development in the management of T2D, offering practical treatment options because they are associated with high glucose-lowering efficacy and weight loss in addition to a low risk of hypoglycemia [3, 5]. Some GLP-1 RAs may also provide cardiovascular and renal benefits [3].
The 2018 consensus report recently published by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) places GLP-1 RAs as a possible second-line therapy in a dual therapy regimen with metformin [3]. The selection of the medication to be added to metformin should be individualized with careful consideration of patient factors such as atherosclerotic cardiovascular disease (ASCVD), heart failure, chronic kidney disease, weight, and patient preferences. For instance, among patients with T2D who have established ASCVD, a GLP-1 RA or sodium–glucose cotransporter-2 inhibitor (SGLT2i) with proven cardiovascular benefit is recommended as part of glycemic management in the second-line treatment. Among patients with a compelling need to minimize weight gain or promote weight loss, a GLP-1 RA with high efficacy for weight loss, or an SGLT2i if the estimated glomerular filtration rate is adequate, is recommended. GLP-1 RAs are generally the preferred choice as the first injectable medication rather than insulin. Treatment guidelines from Diabetes Canada similarly recommend the addition of GLP-1 RAs in second-line treatment after metformin monotherapy [6].
The GLP-1 RA class is rapidly evolving and expanding. Six GLP-1 RAs have been approved in Europe and Canada through December 2017 (the end of our study period). In Europe, exenatide twice daily (BID; Byetta®, AstraZeneca) was first in class and approved by the European Medicines Agency in 2006, followed by liraglutide (Victoza®, Novo Nordisk) in 2009 and exenatide once weekly (QW; Bydureon®, AstraZeneca) in 2011 [7–9]. Subsequent approvals include lixisenatide (Lyxumia®, Sanofi) in 2013 and dulaglutide (Trulicity®, Eli Lilly) in 2014 [10, 11]. These therapies have also been approved by Health Canada in Canada [12–16]. More recently, dulaglutide was approved in November 2015 while lixisenatide (Adlyxine™) was approved in May 2017 [15, 16]. Of note, albiglutide (Eperzan®, GlaxoSmithKline) was approved in Europe in 2014 and in Canada in 2015; however, its commercial sale was discontinued worldwide in 2018 [17–19].
GLP-1 RAs vary in their effect on hemoglobin A1c (HbA1c) and weight reductions, as well as their adverse event profiles [20–22]. Additionally, frequency of injection varies. Exenatide BID is taken twice daily, while liraglutide and lixisenatide are taken once daily. Dulaglutide and exenatide QW are taken once weekly. Dosing is also variable for dulaglutide, exenatide BID, and liraglutide. In Europe, the recommended dose of dulaglutide is 0.75 milligrams (mg) once weekly when used as monotherapy and 1.5 mg when used as add-on therapy [11]. However, for potentially vulnerable populations (e.g., patients 75 years or older), 0.75 mg can be considered a starting dose. In Canada, the recommended initiating dose of dulaglutide is 0.75 mg, which may be increased to 1.5 mg for additional glycemic control [23]. The initial dose of exenatide BID is 5 micrograms (μg) twice daily, within 60 min before the two main meals of the day [7, 24]. The dose can then be increased to 10 µg twice daily after 1 month to further improve glycemic control. Liraglutide should be initiated with a dose of 0.6 mg once daily [8, 25]. After at least 1 week, the dose should be increased to 1.2 mg. Based on the clinical response, the dose can be increased to 1.8 mg after at least 1 week to further improve glycemic control. Dosing is fixed for exenatide QW, which is administered once weekly at 2 mg [9, 26]. Lixisenatide is administered once daily within 60 min prior to a meal with a starting dose of 10 μg in the first 2 weeks following initiation. After the first 2 weeks, lixisenatide has a fixed maintenance dose of 20 μg [10, 27]. In Canada, if the 20 μg dose is not tolerated, the dose can be temporarily reduced to 10 μg. Increasing the dose to 20 μg should be considered within 4 weeks.
Persistence with antihyperglycemic therapy plays a critical role in the improvement of glycemic control and is also associated with improved clinical and economic outcomes [28, 31]. However, patient persistence with T2D therapy is not optimal, and this issue is well documented in the literature [32–34]. Given the relatively recent approvals of lixisenatide and dulaglutide, there is limited information available on current real-world treatment patterns among GLP-1 RA therapy users. Only a few published studies have examined persistence or dosing among these newer therapies in Europe and the US [35–39].
The primary objective of this real-world analysis was to evaluate current persistence and treatment patterns among patients with T2D newly initiated on the GLP-1 RA therapy class using longitudinal prescription data from Belgium, France, Germany, Italy, the Netherlands, and Canada. Secondary objectives included evaluating the average daily dose (ADD) or average weekly dose (AWD) of the GLP-1 RA therapy. This study is an update of prior multi-country European analyses from the authors, incorporating more recent data and, specifically, the inclusion of patients treated with dulaglutide [35, 40]. Recent analyses have also been completed by the authors in Italy and Germany, although with varied selection windows and follow-up periods [37, 39].
Methods
A retrospective cohort analysis was conducted using IQVIA’s Real-World Data (RWD) Adjudicated Pharmacy Claims in Belgium, France, Germany, Italy, the Netherlands, and Canada. Data from these European countries were utilized in prior analyses by the authors, and these countries were selected to provide a broad representation of several countries in Europe [35, 37, 39–41]. The current analysis follows previously published methods [35, 40]. Further details on the methods and definitions utilized for this analysis may be found in these publications.
IQVIA’s RWD Adjudicated Pharmacy Claims provides longitudinal retail pharmacy data. The database includes prescription data (EphMRA Anatomical Classification code in Europe, quantity dispensed, prescriber specialty [unavailable in Italy], etc.) and limited demographic data (e.g., age [unavailable for analysis in Belgium; only 5-year age bands available in Italy] and gender [partially available in Germany]). Payer type is also available in Canada. This study involved a retrospective cohort analysis using six databases, and the analysis does not contain new studies with human or animal subjects performed by any of the authors. Ethics approval was not required for the use of the de-identified prescription data. Patient consent was not required for a study of this nature.
Retail prescription coverage based on population and pharmacy coverage during the study period is as follows: 30% Belgium (i.e., 30% of all retail prescriptions in Belgium are captured by the database), 35% France, 60% Germany, 90% reimbursed retail coverage and 73% direct primary care retail channel coverage in Italy, 75% Netherlands, and 71% in Canada. Retail pharmacy coverage is as follows: ~ 1400 pharmacies in Belgium, ~ 7500 pharmacies in France, ~ 12,300 pharmacies in Germany, ~ 17,800 pharmacies in Italy, ~ 1390 pharmacies in the Netherlands, and ~ 6700 pharmacies in Italy. The data are representative of both large and small cities, and most if not all regions.
Patient Selection
Patients were first identified based on a prescription for the GLP-1 RA therapy of interest (dulaglutide, exenatide BID, exenatide QW, liraglutide, or lixisenatide) within the selection window of January 1, 2015 to December 31, 2016. Albiglutide (Eperzan®) was not included given its global discontinuation.
This analysis focused on single GLP-1 RA molecules that are indicated for the treatment of T2D. Of note, liraglutide (Saxenda®, Novo Nordisk), indicated for weight management for adults who are obese or overweight, was not included as a therapy of interest [42, 43]. Neither were the fixed-dose insulin/GLP-1 RA combinations insulin degludec/liraglutide (Xultophy®, Novo Nordisk) or insulin glargine/lixisenatide (Suliqua®, Sanofi) [44, 47].
The first prescription for a therapy of interest within the selection window was termed the index therapy, and the date was termed the index date. Patients were followed through the end of continuous eligibility (CE, i.e., visibility [composite of patient activity in the database and stability of pharmacy reporting]) up to the end of the study period (December 31, 2017).
Patients were identified as eligible if they met the following inclusion/exclusion criteria: (1) ≥ 18 years on the index date (exceptions for Belgium [age unavailable for analysis] and Italy [only 5-year age bands available, thus patients ≥ 20 years were included]); (2) ≥ 1 oral antihyperglycemic medication class used in the 180-day pre-index period (as evidence of T2D, given the lack of diagnosis codes); (3) ≥ 180 days CE pre-index (the 6-month pre-index or baseline period); (4) ≥ 360 days CE post-index (a post-index or follow-up period of at least 1 year); (5) naïve to the GLP-1 RA therapy class with no prescription for any GLP-1 RA in the 180-day pre-index period (including albiglutide, liraglutide [Saxenda®], and fixed-dose insulin/GLP-1 RA combinations); (6) not initiating any other injectable antihyperglycemic therapy on the index date other than the index therapy; and (7) no invalid, poor, or missing prescription data.
Measures and Analysis
Patient age and gender, follow-up time, and prescriber specialty and payer type (Canada only) associated with the index therapy were reported. Non-index antihyperglycemic therapy use in the pre-index period and concomitant antihyperglycemic therapy use on the index date were assessed. A non-index antihyperglycemic therapy class was considered concomitant if the time between prescriptions in the pre- and post-index was less than 120 days, or if the therapy class was dispensed on the index date.
First treatment modifications of the index GLP-1 RA therapy were assessed over the 1-year post-index period. Discontinuation, switch, augmentation, off-label dose increase, and off-label dose decrease were evaluated. Discontinuation was defined as a gap following an index therapy prescription ≥ 2 × the expected duration of that prescription . A switch was defined as a new non-index antihyperglycemic prescription within 30 days before or after discontinuation of the patient’s index treatment. A new non-index antihyperglycemic prescription could be for a non-index GLP-1 RA or a new antihyperglycemic therapy class, including fixed-dose insulin/GLP-1 RA combinations. A new non-index antihyperglycemic prescription started more than 30 days before the end of follow-up or the index discontinuation date defined augmentation.
The data lack reliable prescribed dose information. Therefore, dose changes for exenatide BID and liraglutide were identified based on calculated ADD, given the per-label dose-increase schedule. An off-label dose increase was defined as a dose increase outside of label recommendations (daily dose > 20 µg for exenatide BID; two consecutive prescriptions with daily dose > 1.8 mg for liraglutide). An off-label dose decrease was defined as two consecutive prescriptions with doses lower than the index dose.
On-label dose increase was assessed as a separate outcome and was not considered a treatment modification. For exenatide BID and liraglutide, this was defined as any dose increase based on label recommendations (two consecutive prescriptions with ADD of 20 µg for exenatide BID; two consecutive prescriptions with ADD ≥ 1.2 mg up to 1.8 mg for liraglutide). On-label dose increase was assessed among dulaglutide patients with an index dose of 0.75 mg based on observed prescriptions (given the availability of recorded dose/strength). On-label dose increase was defined as a subsequent prescription for dulaglutide with a 1.5 mg dose. Off-label dose changes were not assessed for dulaglutide patients; it was assumed that a patient would only use one single-dose pen (providing a 7-day supply) in a week.
Persistence (i.e., continuation of the index therapy) was evaluated over the variable follow-up using Kaplan–Meier (KM) survival analysis. Patients were considered persistent until evidence of discontinuation or switch (whichever came first). Proportion persistent at 1 year and median persistence over the variable follow-up were reported, along with 95% confidence intervals.
The ADD of the index therapy was assessed for all patients while persistent (i.e., until index therapy discontinuation or switch). ADD was calculated because the data lack a reliable prescribed dose field. Daily dose was calculated as the total amount or units of drug prescribed divided by the number of days between two consecutive prescriptions. ADD was evaluated by calendar month among patients with an index therapy prescription during that month. Average ADD by calendar month was summarized to provide yearly ADD and overall ADD. AWD was calculated as ADD × 7 for exenatide QW and dulaglutide. AWD was further reported by index dose (0.75 or 1.5 mg at initiation) for dulaglutide given the different label posologies in Europe versus Canada [11, 23].
Data were described descriptively. Categorical variables were reported using frequency and percentage distributions. Continuous and count variables were reported using the mean, standard deviation, and median. In Canada, data are suppressed where the patient count is less than 5, as well as for associated cells, in line with Canadian patient confidentiality.
No formal statistical tests were performed to compare outcomes between index therapy cohorts. Analyses were performed using SAS version 9.2 or above (Cary, NC, USA).
Results
Patient Sample
The final sample meeting the eligibility criteria consisted of 34,649 dulaglutide patients (389 Belgium, 3464 France, 23,039 Germany, 5795 Italy, 180 Netherlands, 1782 Canada); 3616 exenatide BID patients (68 Belgium, 487 France, 2579 Germany, 393 Italy, 18 Netherlands, 71 Canada); 11,138 exenatide QW patients (1058 Belgium, 3111 France, 2181 Germany, 4346 Italy, 86 Netherlands, 356 Canada); 48,317 liraglutide patients (997 Belgium, 8012 France, 15,792 Germany, 9557 Italy, 1225 Netherlands, 12,734 Canada); and 2204 lixisenatide patients (407 Belgium, 1772 Italy, 25 Netherlands).
All results reported using ranges represent the range across countries and cohorts, unless otherwise specified. See Table 1 for baseline demographic characteristics and Tables S1A and S1B in the Electronic supplementary material (ESM) for antihyperglycemic therapy use of the study sample. Median age at index ranged from 53 to 62 years, and 34.9–63.2% were female. Median follow-up ranged from 16 to 30 months. Median follow-up was shorter for dulaglutide (16–21 months) due to its more recent approval. On average, patients used 1.4–3.0 antihyperglycemic therapy classes in the 180 days pre-index (with a median of two classes for most therapy cohorts except in Canada, where the median was three). In general, biguanides (i.e., metformin) were the most common antihyperglycemic therapy class used in the 180 days pre-index across cohorts (52.9–89.5%). In general, sulphonylureas were the second most common antihyperglycemic therapy class used in the 180 days pre-index (10.2–88.9%), although some variation was observed in Germany (frequent insulin and dipeptidyl peptidase-4/biguanide use) and Canada (frequent insulin and SGLT2i use). Mean number of concomitant antidiabetic therapy classes used on the index date ranged from 1.0 to 2.2 classes. Across countries, biguanides were most frequently used in a concomitant manner (43.5–80.4%).
Table 1.
Demographic characteristics
| Country | Cohort | Median age (in years) at indexa | Female genderb | Mean/median follow-up (months) |
|---|---|---|---|---|
| BE | DULA N = 389 | 42.2% | 16.2/16 | |
| exBID N = 68 | 63.2% | 25.1/27 | ||
| exQW N = 1058 | 40.5% | 23.7/23 | ||
| LIRA N = 997 | 43.9% | 24.1/25 | ||
| LIXI N = 407 | 49.9% | 25.6/27 | ||
| FR | DULA N = 3464 | 62 | 44.0% | 16.7/17 |
| exBID N = 487 | 61 | 46.0% | 26.8/28 | |
| exQW N = 3111 | 62 | 42.9% | 22.1/23 | |
| LIRA N = 8012 | 61 | 45.1% | 25.0/25 | |
| DE | DULA N = 23,039 | 60 | 39.4% | 22.0/21 |
| exBID N = 2579 | 59 | 36.5% | 27.2/29 | |
| exQW N = 2181 | 59 | 38.4% | 26.0/27 | |
| LIRA N = 15,792 | 59 | 34.9% | 24.9/26 | |
| IT | DULA N = 5795 | 42.8% | 16.7/17 | |
| exBID N = 393 | 51.1% | 27.4/29 | ||
| exQW N = 4346 | 44.0% | 25.1/26 | ||
| LIRA N = 9557 | 45.8% | 24.5/25 | ||
| LIXI N = 1772 | 45.7% | 26.8/28 | ||
| NL | DULA N = 180 | 57 | 54.4% | 21.1/21 |
| exBID N = 18 | 62 | 50.0% | 28.0/30 | |
| exQW N = 86 | 59 | 44.2% | 24.8/27 | |
| LIRA N = 1225 | 57 | 56.0% | 25.2/26 | |
| LIXI N = 25 | 53 | 60.0% | 27.5/30 | |
| CA | DULA N = 1782 | 60 | 48.1% | 16.0/16 |
| exBID N = 71 | 61 | 53.5% | 28.2/30 | |
| exQW N = 356 | 60 | 44.4% | 16.7/17 | |
| LIRA N = 12,734 | 60 | 49.3% | 23.9/24 |
BE Belgium, CA Canada, DULA dulaglutide, exBID exenatide twice daily, exQW exenatide once weekly, FR France, DE Germany, IT Italy, LIRA liraglutide, LIXI lixisenatide, NL Netherlands
aAge unavailable continuously in Belgium or Italy due to privacy regulations
bMissing gender possible
Across countries and index therapies, a general practitioner or internist was the most common prescribing physician specialty associated with the index prescription (28.7–100.0%), with a few exceptions. In Belgium, an endocrinologist was associated with the index prescription for 62.7% of dulaglutide patients, 42.3% of liraglutide patients, and 45.0% of lixisenatide patients. In France, the index prescription was prescribed by a physician in a public hospital setting for 40.2% of dulaglutide patients and 37.6% of liraglutide patients. In Canada, an endocrinology/metabolism specialist was associated with the index prescription for 33.5% of dulaglutide patients and 29.2% of exenatide QW patients.
Payer type associated with the index therapy was available in Canada only. A private payer (i.e., private health insurance) was most commonly associated with the index therapy, ranging from 68.3% (liraglutide) to 85.4% (exenatide QW). Patient out-of-pocket payment ranged from 10.3% (dulaglutide) to 21.1% (exenatide BID). Government payment was associated with the index therapy for 11.5% of dulaglutide patients and 14.4% of liraglutide patients.
Treatment Patterns
Treatment modifications at 1 year post-index can be found in Table 2 by index therapy cohort. Apart from dulaglutide patients in the Netherlands, more than half of patients experienced a treatment modification by 1 year post-index: 38.3–67.1% of dulaglutide patients, 85.6–97.1% of exenatide BID patients, 58.1–79.8% of exenatide QW patients, 60.7–86.2% of liraglutide patients, and 64.0–87.5% of lixisenatide patients. Across cohorts, discontinuation was the most common first treatment modification type (22.8–67.6%).
Table 2.
First treatment modification at 1 year post-index
| Country | Cohort | No treatment modification | With a treatment modification | First treatment modification—type | ||||
|---|---|---|---|---|---|---|---|---|
| Discontinuation | Switch | Augmentation | Off-label dose increase | Off-label dose decrease | ||||
| BE | DULA N = 389 | 32.9% | 67.1% | 49.4% | 10.5% | 7.2% | ||
| exBID N = 68 | 2.9% | 97.1% | 67.6% | 10.3% | 7.4% | 11.8% | 0.0% | |
| exQW N = 1058 | 25.8% | 74.2% | 53.8% | 15.2% | 5.2% | |||
| LIRA N = 997 | 13.8% | 86.2% | 65.2% | 6.4% | 4.6% | 1.3% | 8.6% | |
| LIXI N = 407 | 12.5% | 87.5% | 65.6% | 13.8% | 8.1% | |||
| FR | DULA N = 3464 | 45.3% | 54.7% | 36.2% | 10.6% | 7.9% | ||
| exBID N = 487 | 14.4% | 85.6% | 50.5% | 20.7% | 4.9% | 3.7% | 5.7% | |
| exQW N = 3111 | 31.7% | 68.3% | 38.1% | 23.9% | 6.3% | |||
| LIRA N = 8012 | 20.2% | 79.8% | 43.9% | 11.0% | 5.9% | 7.8% | 11.2% | |
| DE | DULA N = 23,039 | 38.7% | 61.3% | 43.5% | 3.9% | 13.9% | ||
| exBID N = 2579 | 13.3% | 86.7% | 55.8% | 6.4% | 10.4% | 14.1% | 0.0% | |
| exQW N = 2181 | 27.5% | 72.5% | 53.3% | 9.1% | 10.1% | |||
| LIRA N = 15,792 | 21.7% | 78.3% | 38.6% | 4.5% | 12.8% | 1.2% | 21.3% | |
| IT | DULA N = 5795 | 43.8% | 56.2% | 39.9% | 6.7% | 9.7% | ||
| exBID N = 393 | 6.1% | 93.9% | 64.6% | 12.5% | 5.9% | 6.9% | 4.1% | |
| exQW N = 4346 | 31.0% | 69.0% | 51.2% | 10.1% | 7.8% | |||
| LIRA N = 9557 | 24.2% | 75.8% | 47.0% | 6.3% | 8.9% | 3.4% | 10.3% | |
| LIXI N = 1772 | 23.0% | 77.0% | 52.1% | 15.0% | 9.9% | |||
| NL | DULA N = 180 | 61.7% | 38.3% | 22.8% | 7.8% | 7.8% | ||
| exBID N = 18 | 5.6% | 94.4% | 33.3% | 11.1% | 22.2% | 22.2% | 5.6% | |
| exQW N = 86 | 41.9% | 58.1% | 38.4% | 14.0% | 5.8% | |||
| LIRA N = 1225 | 39.3% | 60.7% | 30.1% | 7.5% | 7.9% | 6.0% | 9.1% | |
| LIXI N = 25 | 36.0% | 64.0% | 40.0% | 12.0% | 12.0% | |||
| CA | DULA N = 1782 | 40.0% | 60.0% | 35.4% | 9.2% | 15.4% | ||
| exBID N = 71 | s | s | 66.2% | s | 9.9% | s | s | |
| exQW N = 356 | 20.2% | 79.8% | 54.2% | 15.4% | 10.1% | |||
| LIRA N = 12,734 | 25.6% | 74.4% | 40.1% | 7.8% | 12.4% | 6.0% | 8.2% | |
s indicates that data were suppressed in Canada when the patient count was less than 5 (and for associated cells), in compliance with Canadian privacy legislation
BE Belgium, CA Canada, DULA dulaglutide, exBID exenatide twice daily, exQW exenatide once weekly, FR France, DE Germany, IT Italy, LIRA liraglutide, LIXI lixisenatide, NL Netherlands
On-label dose increase by 1 year post-index occurred among 7.0–19.9% of exenatide BID patients and 38.9–65.6% of liraglutide patients (Table 3). In Canada, most dulaglutide patients had an index dose of 0.75 mg (N = 1229, 69.0%). Of these patients, half (54.3%) had an on-label dose increase. In the European countries, most dulaglutide patients had an index dose of 1.5 mg (ranging from 74.7% in Germany to 93.2% in Italy). Among patients with an index dose of 0.75 mg (6.8% in Italy to 25.3% in Germany), on-label dose increase occurred among 36.7% in the Netherlands to 65.2% in Germany.
Table 3.
On-label dose increase at 1 year post-index
| Country | Cohort | On-label dose increase (%) |
|---|---|---|
| BE | DULA 0.75 mg N = 54 | 40.7 |
| exBID N = 68 | 7.4 | |
| LIRA N = 997 | 38.9 | |
| FR | DULA 0.75 mg N = 433 | 47.3 |
| exBID N = 487 | 9.9 | |
| LIRA N = 8012 | 59.1 | |
| DE | DULA 0.75 mg N = 5837 | 65.2 |
| exBID N = 2579 | 19.9 | |
| LIRA N = 15,792 | 44.1 | |
| IT | DULA 0.75 mg N = 394 | 38.3 |
| exBID N = 393 | 11.2 | |
| LIRA N = 9557 | 55.1 | |
| NL | DULA 0.75 mg N = 30 | 36.7 |
| exBID N = 18 | 11.1 | |
| LIRA N = 1225 | 65.6 | |
| CA | DULA 0.75 mg N = 1229 | 54.3 |
| exBID N = 71 | 7.0 | |
| LIRA N = 12,734 | 58.5 |
BE Belgium, CA Canada, DULA dulaglutide, exBID exenatide twice daily, FR France, DE Germany, IT Italy, LIRA liraglutide, mg milligrams, NL Netherlands
Persistence
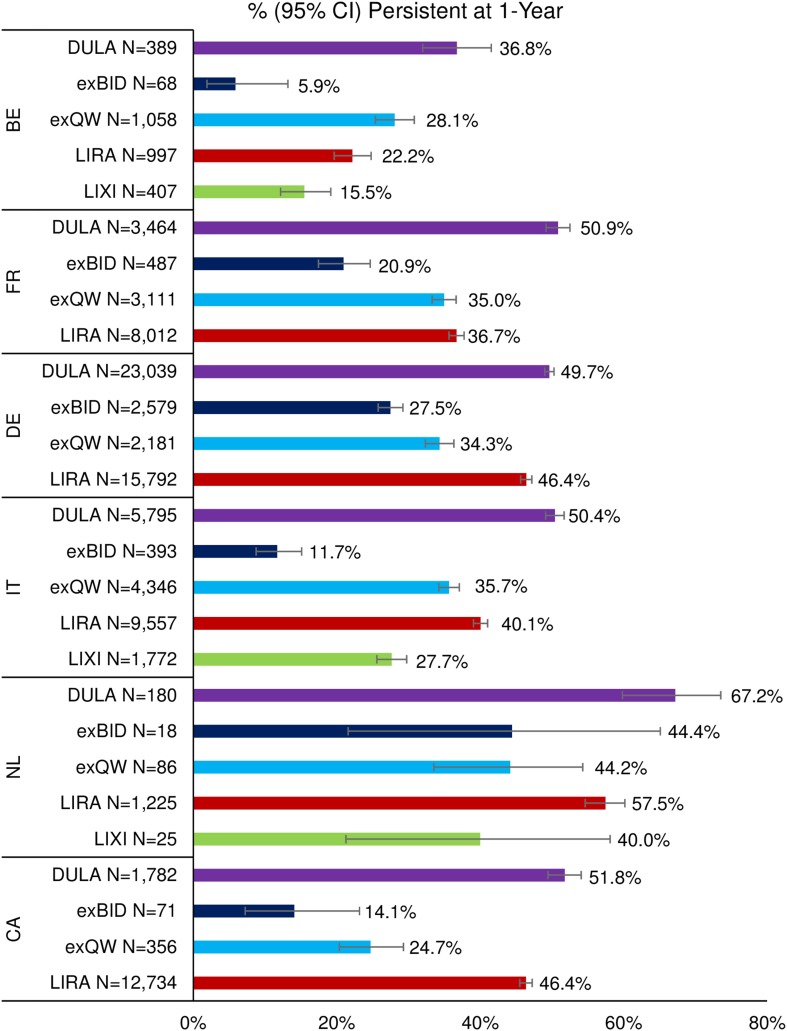
Across countries, the proportion persistent at 1 year post-index (Fig. 1) was highest among dulaglutide patients and lowest among exenatide BID patients (except for lixisenatide patients in the Netherlands [N = 25]). The proportion persistent at 1 year was 36.8–67.2% for dulaglutide patients, 5.9–44.4% for exenatide BID patients, 24.7–44.2% for exenatide QW patients, 22.2–57.5% for liraglutide patients, and 15.5–40.0% for lixisenatide patients.
Fig. 1.
Persistence at 1 year post-index. As an example, 36.8% of dulaglutide patients in Belgium were persistent at 1 year. BE Belgium, CA Canada, DULA dulaglutide, exBID exenatide twice daily, exQW exenatide once weekly, FR France, DE Germany, IT Italy, LIRA liraglutide, LIXI lixisenatide, NL Netherlands
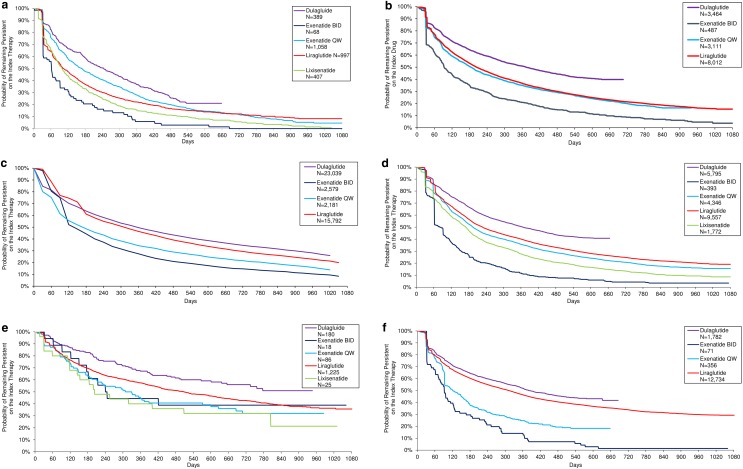
KM results for time persistent (until discontinuation or switch) on the index therapy over the variable follow-up can be found by country in Fig. 2a–f. Across countries, for most time points, the probability of persisting with therapy was highest among dulaglutide patients. The probability of persisting with therapy was lowest among exenatide BID patients, again apart from lixisenatide patients in the Netherlands.
Fig. 2.
Kaplan–Meier analyses for time persistent over the variable follow-up: a Belgium, b France, c Germany, d Italy, e Netherlands, f Canada
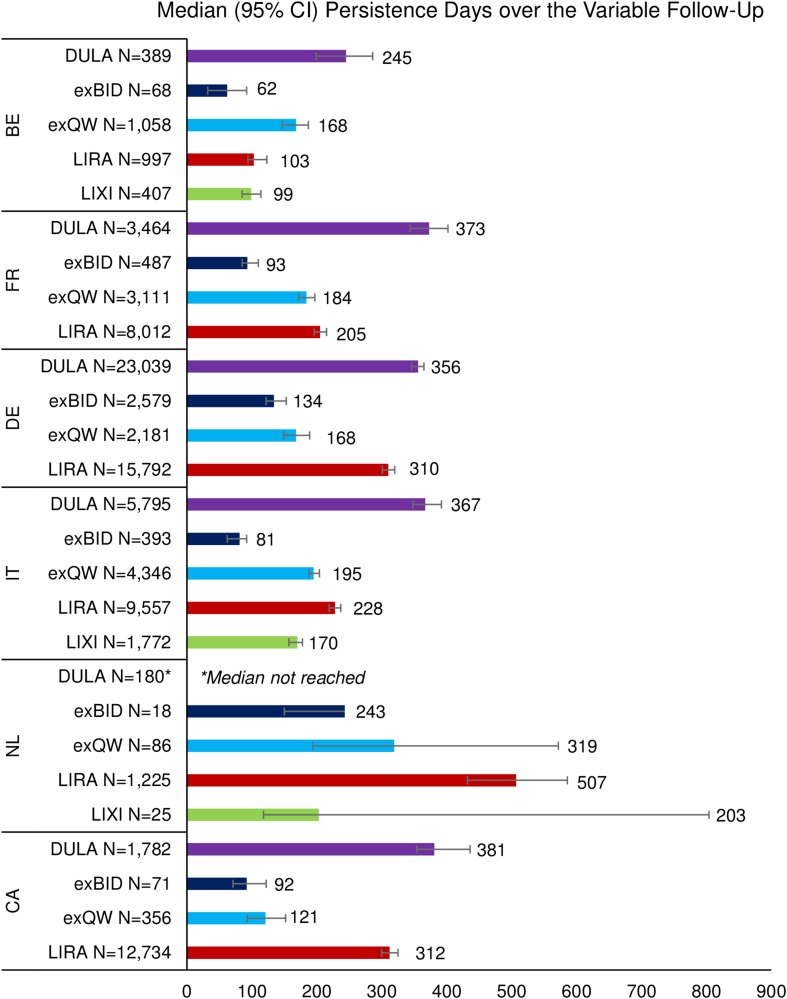
For all countries, median persistence (in days) over the variable follow-up (Fig. 3) was highest among dulaglutide patients. Median persistence was lowest among exenatide BID patients, except for lixisenatide patients in the Netherlands. It is important to note that in the Netherlands, median persistence was not yet reached for dulaglutide patients at the end of the available follow-up (i.e., more than 50% of the dulaglutide patients were still persisting with therapy at the end of follow-up). At 946 days, the last point estimate for dulaglutide, 50.9% of patients were persistent. In comparison, liraglutide patients were second most persistent in the Netherlands and had a median persistence of 507 days. Across countries, median persistence over the variable follow-up was 245–381 days for dulaglutide patients, 62–243 days for exenatide BID patients, 121–319 days for exenatide QW patients, 103–507 days for liraglutide patients, and 99–203 days for lixisenatide patients.
Fig. 3.
Median persistence over the variable follow-up. As an example, 50% of dulaglutide patients in Belgium were persistent at 245 days post-index. BE Belgium, CA Canada, CI confidence intervals, DULA dulaglutide, exBID exenatide twice daily, exQW exenatide once weekly, FR France, DE Germany, IT Italy, LIRA liraglutide, LIXI lixisenatide, NL Netherlands. *More than half of the dulaglutide patients in the Netherlands were still persisting with therapy at the end of the variable follow-up
Average Daily and Weekly Dose
ADD overall, and by year, can be found in Table 4 for exenatide BID, liraglutide, and lixisenatide. Mean (standard deviation [SD]) overall ADD for exenatide BID was generally at the higher end of the approved doses and ranged from 13.21 (0.91) μg in France to 20.43 (5.43) μg in Italy. Mean overall ADD for liraglutide was generally in the middle of the indicated doses: from 1.44 (0.16) mg in Belgium to 1.68 (0.30) mg in the Netherlands. An increase by year was observed across countries. Mean overall ADD for lixisenatide was close to the maintenance dose of 20 μg and ranged from 19.88 (1.88) μg in Belgium to 20.54 (4.19) μg in the Netherlands.
Table 4.
Yearly and overall ADD
| Therapy | Cohort | ADD | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | 2015 | 2016 | 2017 | ||||||||||
| Mean | SD | Median | Mean | SD | Median | Mean | SD | Median | Mean | SD | Median | ||
| exBID | BE N = 68 | 17.03 | 3.32 | 16.71 | 18.32 | 3.44 | 17.83 | 16.08 | 2.66 | 16.56 | 16.62 | 3.94 | 15.03 |
| FR N = 487 | 13.21 | 0.91 | 13.03 | 12.65 | 0.72 | 12.48 | 12.83 | 0.36 | 12.92 | 14.23 | 0.67 | 13.97 | |
| DE N = 2579 | 18.55 | 2.43 | 17.90 | 17.61 | 0.51 | 17.58 | 17.74 | 0.71 | 17.83 | 20.45 | 3.67 | 19.47 | |
| IT N = 393 | 20.43 | 5.43 | 19.48 | 18.68 | 0.81 | 18.75 | 19.17 | 1.35 | 19.60 | 23.44 | 8.74 | 21.38 | |
| NL N = 18 | 19.31 | 4.76 | 18.93 | 16.75 | 4.52 | 14.91 | 20.42 | 5.48 | 18.93 | 20.76 | 3.36 | 21.12 | |
| CA N = 71 | 14.22 | 3.00 | 14.31 | 13.48 | 1.86 | 13.97 | 13.35 | 2.79 | 13.72 | s | |||
| LIRA | BE N = 997 | 1.44 | 0.16 | 1.42 | 1.35 | 0.09 | 1.31 | 1.41 | 0.08 | 1.39 | 1.58 | 0.19 | 1.53 |
| FR N = 8012 | 1.51 | 0.14 | 1.49 | 1.41 | 0.04 | 1.42 | 1.49 | 0.03 | 1.49 | 1.64 | 0.16 | 1.59 | |
| DE N = 15,792 | 1.61 | 0.41 | 1.48 | 1.41 | 0.04 | 1.41 | 1.48 | 0.04 | 1.48 | 1.93 | 0.60 | 1.61 | |
| IT N = 9,557 | 1.54 | 0.17 | 1.51 | 1.42 | 0.04 | 1.42 | 1.50 | 0.03 | 1.50 | 1.70 | 0.21 | 1.62 | |
| NL N = 1225 | 1.68 | 0.30 | 1.61 | 1.49 | 0.08 | 1.50 | 1.62 | 0.05 | 1.61 | 1.92 | 0.42 | 1.76 | |
| CA N = 12,734 | 1.61 | 0.18 | 1.61 | 1.43 | 0.10 | 1.45 | 1.60 | 0.03 | 1.61 | 1.80 | 0.15 | 1.79 | |
| LIXI | BE N = 407 | 19.88 | 1.88 | 19.68 | 19.54 | 1.64 | 19.54 | 19.66 | 1.04 | 19.82 | 20.43 | 2.64 | 20.78 |
| IT N = 1772 | 20.07 | 2.31 | 19.76 | 18.92 | 0.53 | 19.04 | 19.90 | 0.27 | 19.93 | 21.39 | 3.65 | 20.34 | |
| NL N = 25 | 20.54 | 4.19 | 19.99 | 20.51 | 5.33 | 18.78 | 20.21 | 4.31 | 19.59 | 21.02 | 2.55 | 20.36 | |
ADD reported for exBID (µg), LIRA (mg), and LIXI (µg)
ADD presented represents a summary of monthly ADD for eligible prescription records. Patient N changes over time, and patients reflect a mix of new initiators and prevalent users over time
s indicates that data were suppressed in Canada when the patient count was less than 5, in compliance with Canadian privacy legislation
BE Belgium, CA Canada, exBID exenatide twice daily, FR France, DE Germany, IT Italy, LIRA liraglutide, LIXI lixisenatide, µg micrograms, mg milligrams, NL Netherlands
AWD overall, and by year, for dulaglutide and exenatide QW, and further stratified by index dose for dulaglutide, can be found in Table 5. Mean overall AWD for dulaglutide was at the higher end of the approved doses. The mean overall AWD in Canada was 1.25 (0.13) mg; in the other countries, it ranged from 1.43 (0.08) mg in France to 1.53 (0.13) mg in Italy and 1.53 (0.10) mg in the Netherlands. An increase by year was observed in most countries. Among patients with an index dose of 0.75 mg, overall AWD ranged from 0.76 (0.04) mg in France to 1.36 (0.27) mg in the Netherlands. Among patients with an index dose of 1.5 mg, overall AWD ranged from 1.53 (0.09) mg in France and 1.53 (0.13) mg in Canada to 1.59 (0.21) mg in Germany. Mean overall AWD for exenatide QW was close to the expected 2.0 mg, and ranged from 2.03 (0.12) mg in France to 2.14 (0.14) mg in Belgium.
Table 5.
Yearly and overall AWD
| Therapy | Cohort | AWD | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | 2015 | 2016 | 2017 | ||||||||||
| Mean | SD | Median | Mean | SD | Median | Mean | SD | Median | Mean | SD | Median | ||
| DULA | BE N = 389 | 1.52 | 0.18 | 1.48 | 1.47 | 0.06 | 1.48 | 1.56 | 0.23 | 1.50 | |||
| BE 0.75 mg N = 54 | 1.13 | 0.23 | 1.14 | 0.97 | 0.19 | 1.01 | 1.28 | 0.15 | 1.28 | ||||
| BE 1.5 mg N = 335 | 1.58 | 0.17 | 1.54 | 1.56 | 0.06 | 1.55 | 1.60 | 0.23 | 1.53 | ||||
| FR N = 3464 | 1.43 | 0.08 | 1.42 | 1.44 | 0.03 | 1.43 | 1.43 | 0.11 | 1.40 | ||||
| FR 0.75 mg N = 433 | 0.76 | 0.04 | 0.75 | 0.76 | 0.02 | 0.76 | 0.77 | 0.05 | 0.75 | ||||
| FR 1.5 mg N = 3031 | 1.53 | 0.09 | 1.51 | 1.53 | 0.04 | 1.53 | 1.53 | 0.13 | 1.49 | ||||
| DE N = 23,039 | 1.49 | 0.13 | 1.45 | 1.45 | 0.03 | 1.46 | 1.44 | 0.02 | 1.45 | 1.57 | 0.21 | 1.47 | |
| DE 0.75 mg N = 5,837 | 1.21 | 0.16 | 1.19 | 1.05 | 0.12 | 1.11 | 1.19 | 0.02 | 1.18 | 1.37 | 0.11 | 1.33 | |
| DE 1.5 mg N = 17,202 | 1.59 | 0.21 | 1.54 | 1.55 | 0.06 | 1.57 | 1.53 | 0.03 | 1.54 | 1.69 | 0.34 | 1.52 | |
| IT N = 5795 | 1.53 | 0.13 | 1.51 | 1.50 | 0.12 | 1.52 | 1.56 | 0.15 | 1.51 | ||||
| IT 0.75 mg N = 394 | 1.09 | 0.20 | 1.09 | 0.97 | 0.07 | 0.97 | 1.20 | 0.22 | 1.14 | ||||
| IT 1.5 mg N = 5401 | 1.56 | 0.14 | 1.55 | 1.53 | 0.13 | 1.56 | 1.59 | 0.14 | 1.54 | ||||
| NL N = 180 | 1.53 | 0.10 | 1.51 | 1.56 | 0.13 | 1.52 | 1.50 | 0.07 | 1.51 | 1.53 | 0.11 | 1.51 | |
| NL 0.75 mg N = 30 | 1.36 | 0.27 | 1.41 | 1.19 | 0.31 | 1.02 | 1.31 | 0.31 | 1.23 | 1.52 | 0.04 | 1.50 | |
| NL 1.5 mg N = 150 | 1.55 | 0.10 | 1.53 | 1.59 | 0.12 | 1.55 | 1.54 | 0.04 | 1.54 | 1.53 | 0.11 | 1.51 | |
| CA N = 1782 | 1.25 | 0.13 | 1.26 | 1.15 | 0.08 | 1.18 | 1.35 | 0.09 | 1.33 | ||||
| CA 0.75 mg N = 1229 | 1.15 | 0.17 | 1.18 | 1.00 | 0.07 | 1.02 | 1.29 | 0.10 | 1.27 | ||||
| CA 1.5 mg N = 553 | 1.53 | 0.13 | 1.49 | 1.55 | 0.15 | 1.51 | 1.51 | 0.11 | 1.48 | ||||
| exQW | BE N = 1058 | 2.14 | 0.14 | 2.12 | 2.17 | 0.07 | 2.19 | 2.11 | 0.07 | 2.09 | 2.14 | 0.22 | 2.09 |
| FR N = 3111 | 2.03 | 0.12 | 2.00 | 2.05 | 0.06 | 2.02 | 2.00 | 0.02 | 2.00 | 2.06 | 0.19 | 2.00 | |
| DE N = 2181 | 2.08 | 0.13 | 2.04 | 2.07 | 0.05 | 2.08 | 2.02 | 0.05 | 2.04 | 2.15 | 0.19 | 2.04 | |
| IT N = 4346 | 2.09 | 0.12 | 2.07 | 2.10 | 0.05 | 2.09 | 2.06 | 0.03 | 2.06 | 2.12 | 0.20 | 2.06 | |
| NL N = 86 | 2.07 | 0.17 | 2.06 | 2.08 | 0.18 | 2.06 | 2.03 | 0.13 | 2.06 | 2.09 | 0.21 | 2.07 | |
| CA N = 356 | 2.09 | 0.10 | 2.08 | 2.11 | 0.14 | 2.11 | 2.08 | 0.06 | 2.07 | ||||
AWD presented represents a summary of monthly AWD for eligible prescription records. Patient N changes over time, and patients reflect a mix of new initiators and prevalent users over time
AWD reported for ExQW (mg) and DULA (mg). AWD calculated as ADD × 7
BE Belgium, CA Canada, DULA dulaglutide, exQW exenatide once weekly, FR France, DE Germany, IT Italy, mg milligrams, NL Netherlands
Discussion
In this real-world analysis of T2D patients initiating GLP-1 RAs in five European countries and Canada, we consistently observed that dulaglutide patients were most persistent with therapy and least likely to modify therapy. For the most part, liraglutide patients were the second most persistent, while exenatide BID patients were least persistent and most likely to modify therapy. The overall ADD and AWD of GLP-1 RAs were generally within the indicated label ranges. Initiating dose, AWD, and on-label dose increase for dulaglutide in Europe versus Canada were consistent with the respective label posologies. ADD for liraglutide was generally in the middle of the indicated maintenance doses (1.2 or 1.8 mg), and increased by year from initiation, suggesting that many patients are using the higher dose to maintain or achieve glycemic control. Longer-term data is needed to evaluate how treatment patterns change over time, given the relatively recent introduction of dulaglutide.
Only a few studies have examined treatment patterns among the newer GLP-1 RA therapies (lixisenatide and dulaglutide) [35–39]. These have been conducted in the US and Europe, with none identified in Canada. In the prior analysis conducted by the authors of patients initiating GLP-1 RAs in 2013 (before the launch of dulaglutide) in several European countries, liraglutide patients were most persistent across countries [35]. Our findings suggest that GLP-1 RA treatment patterns are changing following the introduction of dulaglutide. In our current multi-country analysis, after dulaglutide had become available, dulaglutide patients were most persistent across countries while liraglutide patients were generally second most persistent. In both the current and prior analyses, we observed that exenatide BID patients were, in general, least persistent and most likely to modify therapy. The few studies which have included dulaglutide initiators have similarly found persistence to be highest among those treated with dulaglutide. Alatorre et al. evaluated treatment patterns among US patients initiating dulaglutide, exenatide QW, and liraglutide over a 6-month follow-up period [36]. In matched cohort comparisons, significantly fewer dulaglutide patients discontinued therapy compared to exenatide QW patients (26% vs. 48%, p < 0.0001) and liraglutide patients (28% vs. 36%, p < 0.0001) at 6 months. Federici et al. evaluated treatment patterns among Italian patients initiating GLP-1 RAs between March and July 2016 with a minimum 6-month follow-up period using the same database employed in this study [37]. At 6 months, persistence was highest among dulaglutide patients (62%) followed by liraglutide patients (50%), and was lowest among exenatide BID patients (35%). We found comparable results from the current analysis in Italy: at 6 months post-index (using KM analysis), persistence was highest among dulaglutide patients (67%) followed by liraglutide patients (57%), and was lowest among exenatide BID patients (27%). Otto et al. evaluated treatment patterns among German patients initiating GLP-1 RAs between February 2015 and January 2016 with a minimum 12-month follow-up period, also using the same database [39]. At 1 year post-index, persistence was highest among dulaglutide patients (51%) followed by liraglutide patients (48%), and lowest for exenatide BID patients (27%). These findings closely mirror the results from the current analysis in Germany: dulaglutide patients had the highest persistence at 1 year (50%) followed by liraglutide patients (46%), while exenatide BID patients had the lowest persistence (27%). In both studies, dulaglutide patients had the highest probability of remaining persistent over time. With these study comparisons, it is important to note the differences in study design, such as the patient selection windows and follow-up periods.
Previous research suggests an association between higher persistence with GLP-1 RAs and greater reductions in HbA1c and lower medical costs [28, 30]. Future research with longer-term data and additional data sources is needed to assess whether the greater persistence observed with dulaglutide is associated with improved clinical and economic outcomes. A recent real-world study conducted in the US found that the use of dulaglutide was associated with a significant decrease in HbA1c levels 6 months after treatment initiation [38]. Patients who were persistent with therapy experienced greater decreases in HbA1c levels. The pharmacy claims data utilized in the current study lack the clinical detail to further investigate the reasons for the greater persistence among dulaglutide patients.
It is possible that the higher persistence observed with dulaglutide may be related to dulaglutide’s convenient dosing schedule and ready-to-use single dose pen. Dosing frequency, ease of preparation, and delivery system have been identified as key drivers of patient preference for GLP-1 RAs [48–50]. Patient preference studies conducted in the UK and Japan have suggested an overall preference for dulaglutide compared to liraglutide [50, 51]. The 2018 ADA/EASD consensus report recognizes that patient preference is a major factor driving the choice of medication, and that patient preferences regarding treatment attributes (such as route of administration or injection devices) may prevent their use by some individuals [3].
The overall ADDs and AWDs of GLP-1 RAs were generally within the indicated label ranges. The overall ADD for exenatide BID was generally at the higher end of the indicated maintenance doses (10 μg or 20 μg). The overall ADD for liraglutide was generally in the middle of the range of indicated maintenance doses (1.2 mg or 1.8 mg) and increased by year from initiation. The mean overall AWD for dulaglutide was at the higher end of the approved doses. The mean overall AWD for dulaglutide was 1.25 mg in Canada, and ranged from 1.43 mg to 1.53 mg in the European countries. This is consistent with the different label posologies in Europe and Canada (Europe: 0.75 mg once weekly as monotherapy and 1.5 mg as add-on therapy; Canada: initiating dose of 0.75 mg once weekly) [11, 23]. The majority of dulaglutide patients in Canada initiated the 0.75 mg dose, while in Europe, the majority of dulaglutide patients initiated the 1.5 mg dose. In Canada, 54% of the dulaglutide patients who initiated the 0.75 mg dose experienced an on-label dose increase by 1 year post-index.
In our prior analysis of GLP-1 RA initiators in several European countries, ADD and AWD were also within indicated label ranges, and were generally similar to the ADD and AWD observed in the current study [35]. A difference, however, was the reporting of trimmed ADD and AWD in the prior analysis, whereby calendar months with fewer than 30 patients were excluded from the calculations. In the prior analysis, the mean overall ADD for exenatide BID was 18.55 μg in Germany and 18.69 μg in France, whereas it was 18.55 μg in Germany and 13.21 μg in France in the current analysis. The difference in values observed for France could be related to a shorter duration of persistence in the current analysis (44% and 21% persisted for at least 1 year in the prior versus the current analysis; i.e., there was less opportunity for patients to increase or remain on the higher dose while they were persisting with treatment). In the prior analysis, the mean overall AWD for exenatide QW ranged from 2.03 mg in Germany to 2.10 mg in the Netherlands, and in the current analysis it ranged from 2.03 mg in France to 2.14 mg in Belgium. In the prior analysis, the mean overall ADD for liraglutide ranged from 1.41 mg in Belgium to 1.68 mg in the Netherlands, and it ranged from 1.44 mg in Belgium to 1.68 mg in Netherlands in the current analysis. In both analyses, liraglutide ADD increased by year. An understanding of the dosing associated with GLP-1 RA therapy may be important from the payer perspective. Variable dosing may result in a less predictable budget impact.
Limitations
There are a few limitations to note regarding retrospective database studies in general and the data utilized. Results from retrospective database studies must be interpreted with caution, and in context with results from other studies, because they can only establish associations and not cause-and-effect relationships. Patients included in IQVIA’s RWD Adjudicated Pharmacy Claims databases may not be fully representative of all patients in the respective country, as data are collected only from participating pharmacies. The data do not provide any insight into prescriptions purchased outside of the participating pharmacies. Additionally, while a prescription may be prescribed or filled, real world consumption patterns may differ. ADD and AWD were calculated based on dispensed prescriptions, given the lack of a reliable dose field. Thus, patient behaviors (e.g., medication stockpiling or picking up prescriptions early) could lead to overestimation. The data lack diagnosis codes or clinical detail. Therefore, we were unable to confirm the presence of T2D through medical diagnosis codes, to identify mortality, or to investigate reasons for treatment modifications or non-persistence (e.g., lack of effectiveness, adverse events, etc.). We were also unable to adjust for any potential treatment selection bias. We did not conduct any statistical analyses because we could not adequately adjust for baseline confounders. Our sample may be biased towards a healthier population due to our continuous eligibility requirements, which were necessary to ensure adequate visibility of the patients’ clinical history and prescribed therapies; this may be less of an issue among patients with chronic diseases such as diabetes. Further, small sample sizes were observed for several therapy cohorts, which can limit the interpretation of study results. Follow-up was limited for dulaglutide, which is related to its more recent approval.
Conclusion
The GLP-1 RA class is evolving and expanding, with several new approvals in recent years. This real-world study is the first to comprehensively examine recent treatment and dosing patterns of GLP-1 RA therapies, including dulaglutide, across several European countries and Canada. Across the countries considered, persistence was highest among dulaglutide patients, and generally lowest among exenatide BID patients. ADD was within indicated label ranges. Longer-term data, particularly for dulaglutide, would be useful to improve our understanding of treatment patterns of GLP-1 RAs over time.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
This study and article processing charges were financially supported by Eli Lilly and Company, Indianapolis, IN, USA. Only IQVIA had full access to the raw de-identified data. All authors had access to the aggregated results of this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published.
Medical Writing, Editorial, and Other Assistance
The authors thank Estella Yi Wang, employee of IQVIA, who was involved in data analysis for Belgium, the Netherlands, and Italy; Hartmut Richter, employee of IQVIA, who was involved in data analysis for Germany; Xavier Ansolabehere and Julie De Nascimento, employees of IQVIA who were involved in data analysis in France; and Sarah Power and Oliver Sang, employees of IQVIA who were involved in data analysis in Canada. This study, including the data analysis, was financially supported by Eli Lilly and Company, Indianapolis, IN, USA.
Disclosures
Kirsi Norrbacka is an employee of and a minor shareholder in Eli Lilly. Kristina S. Boye is an employee of and a minor shareholder in Eli Lilly. Jeremie Lebrec is employed as a consultant for Eli Lilly. Victoria Divino is an employee of IQVIA. Mitch DeKoven is an employee of IQVIA. IQVIA received consulting fees from Eli Lilly for this study.
Compliance with Ethics Guidelines
This study involved a retrospective cohort analysis using six databases, and the analysis does not contain new studies with human or animal subjects performed by any of the authors. Ethics approval was not required for the use of the de-identified prescription data. Patient consent was not required for a study of this nature.
Data Availability
The original de-identified data used in this analysis were obtained from and are the property of IQVIA. IQVIA has restrictions prohibiting the authors from making the data set publicly available. Interested researchers may contact IQVIA to apply to gain access to the study’s data in the same way the authors obtained the data (see https://www.iqvia.com/contact/sf).
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced Digital Features
To view enhanced digital features for this article go to 10.6084/m9.figshare.7965122.
References
- 1.International Diabetes Federation. IDF diabetes atlas. 8th edn. Brussels: International Diabetes Federation; 2017. http://www.idf.org/diabetesatlas. Accessed Sept 21, 2018.
- 2.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38:140–149. doi: 10.2337/dc14-2441. [DOI] [PubMed] [Google Scholar]
- 3.Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycaemia in type 2 diabetes. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetologia. 2018;2018(41):2669–2701. doi: 10.2337/dci18-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garber Alan J. Incretin Therapy – Present and Future. The Review of Diabetic Studies. 2011;8(3):307–322. doi: 10.1900/RDS.2011.8.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trujillo JM, Nuffer W, Ellis SL. GLP-1 receptor agonists: a review of head-to-head clinical studies. Ther Adv Endocrinol Metab. 2015;6:19–28. doi: 10.1177/2042018814559725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diabetes Canada Clinical Practice Guidelines Expert Committee, Lipscombe L, Booth G, et al. Pharmacologic glycemic management of type 2 diabetes in adults. Can J Diabetes. 2018;42(Suppl 1):S88-S103. [DOI] [PubMed]
- 7.European Medicines Agency. Byetta: EPAR. 2018. https://www.ema.europa.eu/documents/product-information/byetta-epar-product-information_en.pdf. Accessed Nov 16, 2018.
- 8.European Medicines Agency. Victoza: EPAR. 2018. https://www.ema.europa.eu/documents/product-information/victoza-epar-product-information_en.pdf. Accessed Nov 16, 2018.
- 9.European Medicines Agency. Bydureon: EPAR. 2018. https://www.ema.europa.eu/documents/product-information/byetta-epar-product-information_en.pdf. Accessed Nov 16, 2018.
- 10.European Medicines Agency. Lyxumia: EPAR. 2017. https://www.ema.europa.eu/documents/product-information/lyxumia-epar-product-information_en.pdf. Accessed Nov 16, 2018.
- 11.European Medicines Agency. Trulicity: EPAR. 2018. https://www.ema.europa.eu/documents/product-information/trulicity-epar-product-information_en.pdf. Accessed November 16, 2018.
- 12.Health Canada. Notice of compliance information—Byetta. https://health-products.canada.ca/noc-ac/info.do?lang=en&no=11997. Accessed June 29, 2018.
- 13.Health Canada. Notice of compliance information—Victoza. https://health-products.canada.ca/noc-ac/info.do?lang=en&no=11407. Accessed June 29, 2018.
- 14.Health Canada. Notice of compliance information—Bydureon. https://health-products.canada.ca/noc-ac/info.do?lang=en&no=17427. Accessed June 29, 2018.
- 15.Health Canada. Notice of compliance information—Trulicity. https://health-products.canada.ca/noc-ac/info.do?lang=en&no=17461. Accessed June 29, 2018.
- 16.Health Canada. Notice of compliance information—Adlyxine. https://health-products.canada.ca/noc-ac/info.do?lang=en&no=19339. Accessed June 29, 2018.
- 17.European Medicines Agency. Eperzan: EPAR. 2017. https://www.ema.europa.eu/documents/product-information/eperzan-epar-product-information_en.pdf. Accessed Nov 16, 2018.
- 18.Health Canada. Notice of compliance information—Eperzan. https://health-products.canada.ca/noc-ac/info.do?lang=en&no=17080. Accessed June 29, 2018.
- 19.US Food and Drug Administration. FDA drug shortages. https://www.accessdata.fda.gov/scripts/drugshortages/dsp_ActiveIngredientDetails.cfm?AI=Albiglutide+%28Tanzeum%29+Injection&st=d&tab=tabs-2. Accessed Oct 12, 2018.
- 20.Gentilella Raffaella, Pechtner Valeria, Corcos Antonella, Consoli Agostino. Glucagon-like peptide-1 receptor agonists in type 2 diabetes treatment: are they all the same? Diabetes/Metabolism Research and Reviews. 2018;35(1):e3070. doi: 10.1002/dmrr.3070. [DOI] [PubMed] [Google Scholar]
- 21.Htike Zin Z., Zaccardi Francesco, Papamargaritis Dimitris, Webb David R., Khunti Kamlesh, Davies Melanie J. Efficacy and safety of glucagon-like peptide-1 receptor agonists in type 2 diabetes: A systematic review and mixed-treatment comparison analysis. Diabetes, Obesity and Metabolism. 2017;19(4):524–536. doi: 10.1111/dom.12849. [DOI] [PubMed] [Google Scholar]
- 22.Madsbad S. Review of head-to-head comparisons of glucagon-like peptide-1 receptor agonists. Diabetes Obes Metab. 2016;18:317–332. doi: 10.1111/dom.12596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eli Lilly Canada Inc. Trulicity product monograph. 2017. https://pdf.hres.ca/dpd_pm/00042736.PDF. Accessed June 29, 2018.
- 24.AstraZeneca Canada Inc. Byetta product monograph 2014. https://pdf.hres.ca/dpd_pm/00025660.PDF. Accessed November 16, 2018.
- 25.Novo Nordisk Canada Inc. Victoza product monograph 2017. https://pdf.hres.ca/dpd_pm/00042188.PDF. Accessed November 16, 2018.
- 26.AstraZeneca Canada Inc. Bydureon product monograph 2018. https://pdf.hres.ca/dpd_pm/00046236.PDF. Accessed November 16, 2018.
- 27.Sanofi–Aventis Canada Inc. Adlyxine product monograph 2017. https://pdf.hres.ca/dpd_pm/00039567.PDF. Accessed Nov 16, 2018.
- 28.Lin J, Lingohr-Smith M, Fan T. Real-world medication persistence and outcomes associated with basal insulin and glucagon-like peptide 1 receptor agonist free-dose combination therapy in patients with type 2 diabetes in the US. Clinicoecon Outcomes Res. 2017;9:19–29. doi: 10.2147/CEOR.S117200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wei W, Pan C, Xie L, Baser O. Real-world insulin treatment persistence among patients with type 2 diabetes. Endocr Pract. 2014;20:52–61. doi: 10.4158/EP13159.OR. [DOI] [PubMed] [Google Scholar]
- 30.Buysman EK, Liu F, Hammer M, et al. Impact of medication adherence and persistence on clinical and economic outcomes in patients with type 2 diabetes treated with liraglutide: a retrospective cohort study. Adv Ther. 2015;32:341–355. doi: 10.1007/s12325-015-0199-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stuart B. C., Simoni-Wastila L., Zhao L., Lloyd J. T., Doshi J. A. Increased Persistency in Medication Use by U.S. Medicare Beneficiaries With Diabetes Is Associated With Lower Hospitalization Rates and Cost Savings. Diabetes Care. 2009;32(4):647–649. doi: 10.2337/dc08-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McGovern A, Tippu Z, Hinton W, et al. Comparison of medication adherence and persistence in type 2 diabetes: a systematic review and meta-analysis. Diabetes Obes Metab. 2018;20:1040–1043. doi: 10.1111/dom.13160. [DOI] [PubMed] [Google Scholar]
- 33.Wei Wenhui, Buysman Erin, Grabner Michael, Xie Lin, Brekke Lee, Ke Xuehua, Chu James W., Levin Philip A. A real-world study of treatment patterns and outcomes in US managed-care patients with type 2 Diabetes initiating injectable therapies. Diabetes, Obesity and Metabolism. 2017;19(3):375–386. doi: 10.1111/dom.12828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kennedy-Martin T, Boye KS, Peng X. Cost of medication adherence and persistence in type 2 diabetes mellitus: a literature review. Patient Prefer Adherence. 2017;11:1103–1117. doi: 10.2147/PPA.S136639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Divino Victoria, DeKoven Mitch, Khan Farhad Ali, Boye Kristina S., Sapin Hélène, Norrbacka Kirsi. GLP-1 RA Treatment Patterns Among Type 2 Diabetes Patients in Five European Countries. Diabetes Therapy. 2017;8(1):115–128. doi: 10.1007/s13300-016-0224-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alatorre C, Fernández Landó L, Yu M, et al. Treatment patterns in patients with type 2 diabetes mellitus treated with glucagon-like peptide-1 receptor agonists: higher adherence and persistence with dulaglutide compared with once-weekly exenatide and liraglutide. Diabetes Obes Metab. 2017;19(7):953–961. doi: 10.1111/dom.12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Federici Marco Orsini, McQuillan Janette, Biricolti Giovanni, Losi Serena, Lebrec Jeremie, Richards Catrina, Miglio Cristiana, Norrbacka Kirsi. Utilization Patterns of Glucagon-Like Peptide-1 Receptor Agonists in Patients with Type 2 Diabetes Mellitus in Italy: A Retrospective Cohort Study. Diabetes Therapy. 2018;9(2):789–801. doi: 10.1007/s13300-018-0396-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mody R, Grabner M, Yu M, et al. Real-world effectiveness, adherence and persistence among patients with type 2 diabetes mellitus initiating dulaglutide treatment. Curr Med Res Opin. 2018;34(6):995–1003. doi: 10.1080/03007995.2017.1421146. [DOI] [PubMed] [Google Scholar]
- 39.Otto Thorsten, Myland Melissa, Jung Heike, Lebrec Jeremie, Richter Hartmut, Norrbacka Kirsi. Utilization patterns of glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes mellitus in Germany: a retrospective cohort study. Current Medical Research and Opinion. 2018;35(5):893–901. doi: 10.1080/03007995.2018.1538011. [DOI] [PubMed] [Google Scholar]
- 40.Divino Victoria, DeKoven Mitch, Hallinan Shawn, Varol Nebibe, Wirta Sara Bruce, Lee Won Chan, Reaney Matthew. Glucagon-Like Peptide-1 Receptor Agonist Treatment Patterns Among Type 2 Diabetes Patients in Six European Countries. Diabetes Therapy. 2014;5(2):499–520. doi: 10.1007/s13300-014-0087-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miller LA, et al. Exenatide BID and liraglutide QD treatment patterns among type 2 diabetes patients in Germany. J Med Econ. 2012;15(4):746–757. doi: 10.3111/13696998.2012.679756. [DOI] [PubMed] [Google Scholar]
- 42.European Medicines Agency. Saxenda: EPAR. 2018. https://www.ema.europa.eu/documents/product-information/saxenda-epar-product-information_en.pdf. Accessed Nov 16, 2018.
- 43.Novo Nordisk Canada Inc. Saxenda product monograph. 2017. https://pdf.hres.ca/dpd_pm/00040084.PDF. Accessed Nov 16, 2018.
- 44.European Medicines Agency. Xultophy: EPAR. 2018. https://www.ema.europa.eu/documents/product-information/xultophy-epar-product-information_en.pdf. Accessed Nov 16, 2018.
- 45.Novo Nordisk Canada Inc. Xultophy product monograph. 2018. https://pdf.hres.ca/dpd_pm/00044749.PDF. Accessed Nov 16, 2018.
- 46.European Medicines Agency. Suliqua: EPAR. 2017. https://www.ema.europa.eu/documents/product-information/suliqua-epar-product-information_en.pdf. Accessed Nov 16, 2018.
- 47.Sanofi–Aventis Canada Inc. Soliqua product monograph. 2018. https://pdf.hres.ca/dpd_pm/00046262.PDF. Accessed Nov 16, 2018.
- 48.Qin L, Chen S, Flood E, et al. Glucagon-like peptide-1 receptor agonist treatment attributes important to injection-naïve patients with type 2 diabetes mellitus: a multinational preference study. Diabetes Ther. 2017;8:321–334. doi: 10.1007/s13300-017-0230-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qin Lei, Chen Stephanie, Flood Emuella, Shaunik Alka, Romero Beverly, de la Cruz Marie, Alvarez Cynthia, Grandy Susan. Glucagon-Like Peptide-1 Receptor Agonist Treatment Attributes Important to Injection-Experienced Patients with Type 2 Diabetes Mellitus: A Preference Study in Germany and the United Kingdom. Diabetes Therapy. 2017;8(2):335–353. doi: 10.1007/s13300-017-0237-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gelhorn HL, Poon JL, Davies EW, et al. Evaluating preferences for profiles of GLP-1 receptor agonists among injection-naïve type 2 diabetes patients in the UK. Patient Prefer Adherence. 2015;9:1611–1622. doi: 10.2147/PPA.S90842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gelhorn HL, Bacci ED, Poon JL. Evaluating preferences for profiles of glucagon-like peptide-1 receptor agonists among injection-naive type 2 diabetes patients in Japan. Patient Prefer Adherence. 2016;10:1337–1348. doi: 10.2147/PPA.S109289. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original de-identified data used in this analysis were obtained from and are the property of IQVIA. IQVIA has restrictions prohibiting the authors from making the data set publicly available. Interested researchers may contact IQVIA to apply to gain access to the study’s data in the same way the authors obtained the data (see https://www.iqvia.com/contact/sf).