Abstract
Neurologic symptoms in Wilson disease (WD) appear at an older age compared to hepatic symptoms and manifest in patients with misdiagnosed liver disease, in patients when the hepatic stage is clinically silent, in the case of non-compliance with anti-copper treatment, or with treatment failure. Neurologic symptoms in WD are caused by nervous tissue damage that is primarily a consequence of extrahepatic copper toxicity. Copper levels in brain tissues as well as cerebrospinal fluid (CSF) are diffusely increased by a factor of 10 and its toxicity involves various mechanisms such as mitochondrial toxicity, oxidative stress, cell membrane damage, crosslinking of DNA, and inhibition of enzymes. Excess copper is initially taken-up and buffered by astrocytes and oligodendrocytes but ultimately causes dysfunction of blood-brain-barrier and demyelination. Most severe neuropathologic abnormalities, including tissue rarefaction, reactive astrogliosis, myelin palor, and presence of iron-laden macrophages, are typically present in the putamen while other basal ganglia, thalami, and brainstem are usually less affected. The most common neurologic symptoms of WD are movement disorders including tremor, dystonia, parkinsonism, ataxia and chorea which are associated with dysphagia, dysarthria and drooling. Patients usually manifest with various combinations of these symptoms while purely monosymptomatic presentation is rare. Neurologic symptoms are largely reversible with anti-copper treatment, but a significant number of patients are left with residual impairment. The approach for symptomatic treatment in WD is based on guidelines for management of common movement disorders. The vast majority of WD patients with neurologic symptoms have abnormalities on brain magnetic resonance imaging (MRI). Pathologic MRI changes include T2 hyperintensities in the basal ganglia, thalami and white matter, T2 hypointensities in the basal ganglia, and atrophy. Most importantly, brain damage and neurologic symptoms can be prevented with an early initiation of anti-copper treatment. Introducing population WD screening, e.g., by exome sequencing genetic methods, would allow early treatment and decrease the neurologic burden of WD.
Keywords: Wilson disease (WD), copper toxicity, neuropathology, neuroradiology, magnetic resonance imaging (MRI), clinical scales
Introduction
Historically, the neurologic symptoms of Wilson disease (WD) were first noted in 1912 by Wilson who provided detailed accounts of the clinical presentation of 12 patients with WD, including descriptions of various movement disorders, drooling, dysarthria and psychiatric symptoms (1). The disease was initially coined ‘hepatolenticular degeneration’. Only later it became clear that neurologic symptoms are typically preceded by liver involvement and are not inevitable when proper treatment is initiated in the early phase of the disease (2,3). Approximately 50% of patients with neurologic symptoms have liver cirrhosis at the time of diagnosis. It is not clear whether there are WD patients with neurologic symptoms without any liver involvement (4). The first symptoms of WD are usually hepatic. Later in the natural course of untreated disease, in the case of poor compliance with anti-copper treatment or treatment failure, neurologic and other WD symptoms usually occur. Neurologic symptoms in WD are, thus, considered as a manifestation of more advanced disease stage that typically occurs in patients with misdiagnosed liver disease or when the hepatic stage is clinically silent (5). The term neuro-WD was introduced to emphasize the significance of neurologic symptoms and their dominant influence on disability in some WD patients. Although this paper is focused on neurologic symptoms, it is important to note that brain damage in WD frequently leads also to psychiatric impairment (6). Neuro-WD is thus a representative neuropsychiatric disorder.
Pathophysiology
Although ATP7B is expressed in the human central nervous system (CNS) (7), its dysfunction in the brain apparently does not lead to overt clinical symptoms. According to the current paradigm, nervous tissue damage leading to neurologic and psychiatric symptoms in WD is primarily a consequence of extrahepatic copper toxicity. This paradigm is supported by several observations: (I) copper concentration in the brain tissue and cerebrospinal fluid (CSF) is increased by a factor of 10 in WD compared to healthy subjects (8,9) and (II) anti-copper treatment leads to an improvement of neurologic symptoms that is paralleled with disappearance of magnetic resonance imaging (MRI) abnormalities and lowering of copper concentrations in the CSF (10,11). Excess copper is toxic to the brain tissue. It may cause cell injury and inflammation via various mechanisms including mitochondrial toxicity, oxidative stress, cell membrane damage, crosslinking of DNA, and inhibition of enzymes as has been shown in in vitro, animal, and post mortem human studies (12-16). Astrocytes, being part of the blood-brain barrier, can partially buffer toxic effects of “loosely bound” blood copper. It has been documented that astrocytes can upregulate synthesis of metallothionein and glutathione, which are peptides with the capacity to detoxify copper (17,18). Upon chronic copper intoxication, astrocytes increase in numbers and undergo morphological changes, but their copper storage capacity is ultimately exhausted. Among brain cells, oligodendrocytes appear to be particularly sensitive to copper toxicity; hydropic swelling of myelin sheaths and demyelination can be one of the earliest consequences of cerebral copper overload (19). Accordingly, MRI studies in de novo WD patients provide indirect evidence of cytotoxic edema and myelin damage (20,21). Another factor contributing to cerebral dysfunction in WD is hepatic encephalopathy which occurs in patients with severe liver damage and portal hypertension with portosystemic shunting. Neurologic symptoms in hepatic encephalopathy are likely caused by accumulation of neurotoxic substances that are normally cleared from blood by the liver, e.g., ammonia and manganese (22,23).
Neuropathologic findings
Pathologic changes in WD are typically observed in the central grey matter nuclei and white matter tracts in the brainstem. The underlying cause of high susceptibility of these brain regions to copper toxicity is unknown (24,25). Macroscopically, most severe abnormalities are present in the putamen, which is typically shrunken, soft, and brown-yellowish discolored. In the most severe cases, there is a putaminal necrosis with iron-laden macrophages surrounding the necrotic cavity (26). Cavitation can be infrequently found also in the thalamus, dentate nucleus or white matter. The latter was more common before anti-copper treatment became available and is only rarely described in treated patients (27,28).
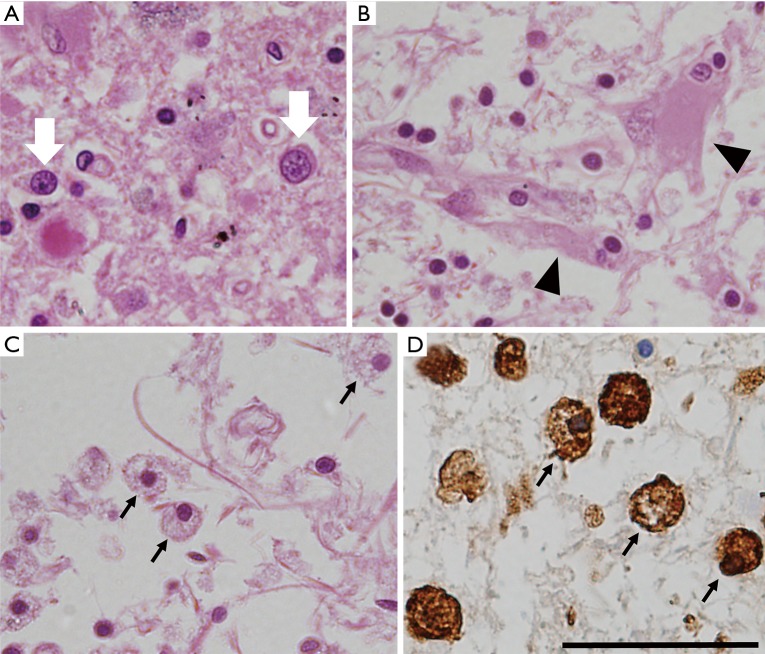
Upon microscopic examination, tissue rarefaction of various severity, astrocytes with abnormal morphologies, loss of myelination, and iron-laden macrophages are found predominantly in the central grey matter (Figure 1). Specific type of astrocytes, referred to as Alzheimer type II glia, with swollen pale nuclei and little cytoplasm are frequently found in the basal ganglia and less commonly also in the cortex. These astrocytic population is not specific for WD, it has been associated with hepatic encephalopathy (25). Reactive astrocytes, also referred to as Alzheimer type I glia, are enlarged cells with cytoplasm immunoreactive for glial fibrillary acidic protein (GFAP) and metallothionein and with histochemical positivity for copper deposits (18). Opalski cells are large cells of ambiguous origin with foamy cytoplasm, which are considered to be specific for WD. It is not clear whether these cells are derived from the astrocytic or histiocytic cell line (29). In addition to astrocytes, cells with oligodendrocyte morphology were also shown to accumulate copper (30). Oligodendrocyte rarefaction is typically present in the bundles passing through lentiform nucleus, the entire dentato-rubro-thalamic pathway, and fibers of the pontocerebellar pathway. Demyelination of pontine fibers may lead to pathology resembling central pontine myelinolysis (29). Neuronal dysfunction usually follows glial abnormalities and, when present, is pathologically manifested by axonal swelling and spheroid formation. Neuronal loss is observed in more severe cases (31).
Figure 1.
Neuropathological abnormalities in WD. (A) Hematoxylin-eosin (HE) staining showing disorganization of tissue with Alzheimer type 2 glia (white arrows); (B) severe tissue rarefaction with reactive gliosis (black arrowheads show swollen reactive astrocytes) (HE staining); (C) severe tissue rarefaction and macrophages (black arrows) (HE staining); (D) ferritin immunostaining showing cells with macrophage morphology strongly positive for ferritin (black arrowheads). All images are from the putamen, scale bar represents 50 µm. WD, Wilson disease.
Neurologic symptoms and their treatment
Neurologic symptoms of WD typically occur between the ages 20–40 years, however the range is very large (32-34). The youngest WD patient with neurologic symptoms was aged 6, and the oldest 72 years (32,35,36). The most common neurologic symptoms of WD are movement disorders including tremor, dystonia, parkinsonism, and ataxia, which are frequently associated with dysphagia, dysarthria and drooling (33,34). In most cases these symptoms overlap, fluctuate and can be aggravated by several factors (e.g., emotions, stress, general health conditions, concomitant disorders as well as drugs). All these factors cause difficulties with symptom classifications as well as with implementation and using neurological scoring systems. Nevertheless, there are several propositions of neurologic phenotypes classifications (37-42) (Table 1) and a few scales quantifying severity of neurologic symptoms (43,44). WD belongs to the group of treatable neurometabolic disorders. In most cases, neurologic symptoms diminish or even disappear during the correct anti-copper treatment (32,45). However, symptoms with varying severity persist in about 40–50% patients even after long-term anti-copper treatment leading to decreased quality of life and requirement for symptomatic treatment (32,45,46). It should be emphasized that no controlled clinical trials have been performed, which document the efficacy of symptomatic treatment of neurologic symptoms. Only several naturalistic studies and clinical experience of expert centers indicate that such treatment may partially improve disabling neurologic symptoms of WD (46,47). It is also important to note that unless neurologic symptoms are severely disabling upon diagnosis, it is preferable not to start symptomatic drugs together with anti-copper therapy as symptomatic drugs may obscure its effects.
Table 1. Neurologic symptoms in Wilson disease—historically proposed classifications.
| The first author (year of proposition) | Classification |
|---|---|
| Hall et al., 1921 (37) | • Classic form (progressive rigidity and tremor) with domination of rigidity and bad prognosis |
| • Tremor form (Strumpell-Westphal, pseudosclerotic form) occurring in adults with a relatively good prognosis | |
| • Torsion spasms (currently dystonic form) | |
| Konovalov et al., 1960 (42) | • Arrhythmic-hyperkinetic form (hyperkinesias and dystonia) |
| • Tremulous form (ataxia and postural tremor) | |
| • Tremulous-rigid form (rigidity and resting tremor—currently parkinsonian) | |
| Denny-Brown et al., 1964 (38) | • Juvenile form (onset before second decade of life, hyperkinetic/dystonic symptoms with putaminal lesions) |
| • Pseudosclerotic form (dominant intention and postural tremors with lesions in the cerebral cortex, thalamus, subthalamic regions, nucleus dentatus) | |
| Marsden et al., 1987 (39) | • Hyperkinetic-dystonic form (dystonia and choreoathetosis) |
| • Ataxic form (postural and intentional tremor, ataxia) | |
| • Parkinsonian form (rigidity, rest tremor, hypokinesia) | |
| Oder et al., 1993 (41) | • Dyskinetic form (dystonia and choreoathetosis) |
| • Pseudosclerotic (ataxia and postural tremor) | |
| • Pseudoparkinsonian form (rigidity, resting tremor, abnormal cognition) | |
| Czlonkowska et al., 1996 (40) | • Dystonic form |
| • Tremor form | |
| • Rigidity-tremor form | |
| • Rigidity form |
In carefully selected patients, deep brain stimulation (DBS) may be a last resort treatment of neurologic symptoms that are refractory to pharmacotherapy (48). However, DBS should not be considered sooner than 2–3 years after treatment initiation, while positive effects of anti-copper treatment may be expected. Below, we present a clinical description of the most common neurologic symptoms of WD as well as possible options for their symptomatic treatment (Table 2).
Table 2. The options for symptomatic treatment of WD neurologic symptoms.
| WD neurologic symptoms | Possible therapeutic interventions |
|---|---|
| Tremor (the treatment depends mostly on the type of tremor—described in the text) | • Beta-blockers (propranolol) |
| • Barbiturates (primidone) | |
| • Benzodiazepines (most commonly—clonazepam) | |
| • Anticholinergics (trihexyphenidyl or biperiden) | |
| • Presynaptic gamma-aminobutyric acid agonist (baclofen) | |
| • BTX injections | |
| • Neurosurgical treatment—Vim DBS or thalamotomy | |
| Dystonia (the treatment depends mostly on type of dystonia—described in the text) | • Anticholinergics (as in WD tremor treatment) |
| • Presynaptic gamma-aminobutyric acid agonist (baclofen) | |
| • Benzodiazepines (as in WD tremor treatment) | |
| • Levodopa or dopamine agonists (e.g., ropinirole or pramipexole) | |
| • Dopamine depleting drugs | |
| • Antiepileptics drugs (mostly used carbamazepine, oxcarbazepine or gabapentin); | |
| • BTX injections | |
| • Neurosurgical treatment—DBS of GPi, pallidotomy or thalamotomy | |
| Parkinsonism (therapeutic test with levodopa suggested to establish dopaminergic responsiveness) | • Levodopa |
| • Dopamine receptors agonists | |
| • Neurosurgical treatment—DBS or neuroablative lesions of GPi or STN | |
| Chorea | • Dopamine depletion agents (tetrabenazine) |
| Dysphagia | • Behavioral therapy |
| • Dietary modifications | |
| • Stopping the drugs influencing arousal | |
| • Neuromuscular electrical stimulation | |
| • Tube feeding (percutaneous gastrostomy) if needed | |
| Dysarthria | • Speech therapy |
| • Augmentative communications devices if needed | |
| Drooling | • Non-Pharmacological methods |
| • Anticholinergics | |
| • Adrenergic alfa-2 receptor agonists | |
| • BTX (injection in parotid and submandibular glands) |
BTX, botulinum toxin; DBS, deep brain stimulation; GPi, globus pallidus internus; STN, subthalamic nucleus; Vim, ventral intermediate nucleus of the thalamus; WD, Wilson disease.
Tremor
The most characteristic neurologic symptom of neuro-WD is tremor. It occurs in up to 55% of patients as the first neurologic symptom and in almost 90% patients during the course of the disease (33,34,49). Tremor in WD may be resting, postural (with “wing beating” features), action or intention. It may have features of dystonic, rubral, parkinsonian, and essential tremor, or have a mixed presentation. The phenotype of tremor can also change during the disease progression, especially in untreated patients. Usually, it first affects distal upper extremities, as unilateral or bilateral asymmetric tremor. During WD progression, the head, legs, as well as whole body may be affected by tremor. The symptomatic treatment depends on the particular phenotype of tremor (47). In the case of essential tremor-like phenotype, i.e., postural, and kinetic tremor affecting predominantly hands, the treatment with non-selective beta-blockers (preferentially propranolol) is the first option to alleviate the symptoms. Further possibilities of treatment include barbiturates (primidone), benzodiazepines (BZD) or neurosurgical treatment—DBS of ventral intermediate nucleus of the thalamus (Vim DBS) or thalamotomy (46,47). Dystonic tremor can be treated additionally with anticholinergics or botulinum toxin (BTX) injections.
Dystonia
Dystonia has been reported in 11–65% of neuro-WD patients in different cohorts and is the most severe and refractory symptom (33,34,49,50). Dystonia can involve different body parts and can be focal (e.g., “risus sardonicus”, cervical dystonia, “starfish” hand), segmental (trunk dystonia—“Pisa” sign), multifocal, or even generalized (status dystonicus). However, the most common presentation of dystonia in WD is abnormal face expression, which can present as: (I) “risus sardonicus” (due to dystonic spasm of the risorius muscle) or (II) “vacuous smile” (due to dystonic dropped jaw). In untreated WD, focal dystonia usually spreads to other body regions during disease progression, eventually affecting axial muscles and leading to abnormal posture and gait disturbances. Dystonic muscle activity may interfere with eating, speech, and various physical activities leading to immobilization and increased mortality (49,50). The options for symptomatic treatment of dystonia in WD is not different from other etiologies: BTX, anticholinergics, benzodiazepines, baclofen, dopamine agonists, antiepileptic drugs (oxcarbazepine or gabapentin), or neurosurgical treatment—DBS of globus pallidus internus (GPi) or pallidotomy. The latter is reserved for generalized dystonia or status dystonicus not responding to pharmacotherapy. BTX injection directly into affected muscles is the first-line treatment for focal dystonia. Pharmacotherapy is recommended for multifocal and generalized dystonia. In the case of severe disabling dystonia, all these treatments, even in combination, should be considered (46,47,50).
Parkinsonism
Parkinsonism is a clinical syndrome, which consists of bradykinesia, rigidity, resting tremor, along with postural imbalance. Drooling, hypomimia, dysarthria, micrographia, and shuffling gait are manifestations of these symptoms in specific muscle groups. Parkinsonism has been reported in 19–62% of WD patients (33,34,47). As for other causes of parkinsonism, levodopa or dopamine agonists should be tried in patients with disabling symptoms. Further, in cases with severe symptoms, DBS or neuroablative procedures of the subthalamic nucleus (STN) or GPi could be considered (46,47).
Cerebellar ataxia
Cerebellar ataxia has been reported in almost 30% of neuro-WD patients, mostly in combination with other neurologic symptoms (33,46,47). Symptoms of impaired cerebellar function can be distinguished as: (I) ataxic gait (wide stance and wide-based gait with impaired tandem walking); (II) intentional tremor; (III) dysdiadochokinesis; (IV) impaired coordination of fine hand movements; (V) ataxic speech (described below). There is no proven effective pharmacotherapy for the symptomatic treatment of ataxia. Physiotherapy targeted at gait and balance control, as well a speech therapy could be of some benefit (46,47).
Chorea
Chorea as well as athetosis occur relatively rarely in WD, in approximately 6–16% of neurologic patients. They occur very sporadically as an isolated neurologic symptom of WD, more commonly in young patients. Data about the efficacy of symptomatic treatment of choreoathetosis in WD are very limited; presynaptic monoamine depletory drug, tetrabenazine, could be considered in cases with severe chorea (46,47).
Dysarthria
Dysarthria is apparently the most frequent neurologic symptoms of WD. It occurs in almost every neurologic patient. Speech disturbances as a clinical symptom of WD are caused by the damage of basal ganglia (leading to dystonic and parkinsonian features), cerebellar nuclei and their tracts (leading to cerebellar features) and possibly also cortico-bulbar tracts (leading to pseudobulbar features) (34,47,51). Based on the predominant characteristic of speech abnormality, several types of dysarthria in WD can be distinguished including: (I) mixed unclassified (due to involvement of several brain structures); (II) ataxic (cerebellar); (III) dystonic (hyperkinetic); and (IV) hypokinetic. Treatment of dysarthria in WD is not specific but is based on general rules for dysarthria management and the approach depends mostly on the specific type of speech disturbance. Treatment involves relaxation techniques in the case of dystonic dysarthria, techniques improving speech rate in the case of cerebellar ataxia, and loudness and articulation in the case of hypokinetic dysarthria. In patients with severe dysarthria or anarthria, alternative and augmentative communication devices such as tablets or smartphone application can be recommended (47).
Dysphagia
Dysphagia, defined as difficulty in any phase of swallowing, occurs in about 18% of WD patients and in 50% of patients with neurologic symptoms (33,34,52). Any phase of the swallowing act can be affected including oral, preparation/chewing, oral transit, and swallowing itself. Dysphagia may emerge due to impairment of muscle tone (e.g., in oro-facial dystonia), incoordination, slowness and weakness of deglutition muscles. As dysphagia may lead to aspiration, pneumonia, and malnutrition, the assessment of neuro-WD patients should always include examination of swallowing and nutritional status using questionnaires, body weight measurement, and biochemical markers. In the case of persistent or progressive severe dysphagia, feeding via percutaneous gastrostomy (PEG) should be considered (46,47).
Drooling
Drooling, along with dysarthria and “wing beating” tremor, belongs to the most prominent and characteristic symptoms of WD (32-34,53). Defined as involuntary flow of saliva from the mouth, drooling affects approximately 70% of neuro-WD patients. Very often it is the consequence of dysphagia and/or the inability to retain saliva within the mouth due to orofacial dystonia. This typically occurs in patients with “open mouth smile”. Medical interventions that may reduce drooling are as follows: (I) non-pharmacological, such as chewing gum or sucking on hard candies, which reduce hypersalivation by triggering automatic swallowing; (II) pharmacological, such as anticholinergic drugs or BTX injection in parotid and submandibular glands to decrease saliva production (46,47).
Gait and posture disturbances
Gait and posture disturbances have been reported in 44–75% of neuro-WD patients (33,34,54). Gait disturbances are consequent to cerebellar dysfunction which leads to ataxia and incoordination and to movement disorders including dystonia, parkinsonism and chorea. Gait disturbances occur especially when motor control of legs and axial muscles are affected. The symptomatic treatment is based on pharmacotherapy for specific movement disorders as well as on rehabilitation of postural control and gait (46,47).
Other neurologic symptoms
Additionally to the typical neurologic symptoms of WD described above, other neurologic symptoms may occur in the course of WD including myoclonus (55,56), tics, headache, taste and olfactory dysfunction (57-59), neuropathies (60), epilepsy (61,62), restless leg syndrome (63), sleep disturbances (64-66), and other abnormalities (34). However, the frequency of these symptoms, except of epilepsy which has 10-fold greater frequency in WD compared to general population, is rare and their presentation is not specific for WD (32,34,45).
Neuroimaging
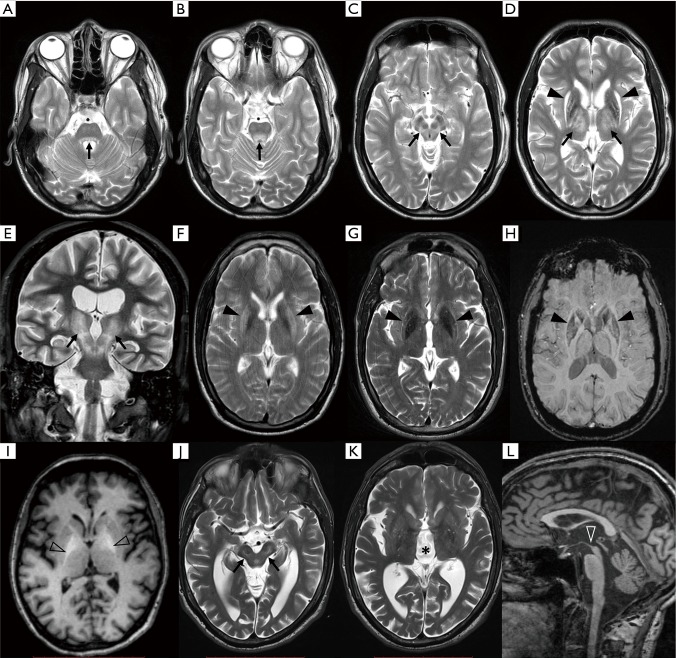
Brain MRI is nowadays the most widely used neuroimaging method in the differential diagnosis of neurological WD. More than 90% WD patients with neurological symptoms have pathology on brain MRI (67,68) which, when fully developed, is reasonably specific for WD (Figure 2). Negative or inconspicuous MR finding alone should however not exclude WD in a patient with neurologic symptoms. Neuroimaging abnormalities are also present in approximately 40–70% of hepatic, and even in 20% of presymptomatic WD cases (68,69). The most prominent findings in untreated patients are symmetric hyperintensities in T2-weighted image in the deep grey matter (DGM) nuclei and mesencephalic and pontine white matter (70-76). The prevalence of T2 lesions in brain structures varies across studies but the most commonly lesioned site appears to be putamen (45–85%), followed by caudate nucleus (30–60%), anterolateral thalamic nuclei (30–60%), pons (10–80%) and mesencephalon (20–70%) (67,77-84). Importantly, tectal plate hyperintensity and central pontine myelinolysis-like lesions as well as concurrent involvement of the brainstem, basal ganglia, and/or thalamus are highly suggestive of WD (77). These T2 hyperintense lesions are partly reversible upon anti-copper treatment (11,85-88) and presumably reflect edema and demyelination caused by copper toxicity. Presence of cytotoxic edema as an early WD pathology was supported by findings on diffusion-weighted imaging (DWI) showing restricted diffusion in areas affected by T2 hyperintensities in some patients (83,89,90). In the case of more severe tissue pathology, T2 hyperintensity corresponds to gliosis or rarefaction that may, particularly in the putamen, progress to necrosis manifesting as cavitation on MRI (91,92). The latter changes are typically visible also as hypointensities on T1-weighted images. Structural disorganization of the DGM in WD was further documented by a novel diffusion-weighted imaging (DWI) technique, neurite orientation dispersion and density imaging (NODDI). Using this method, WD patients were shown to have lower intracellular compartment, lower orientation dispersion index and higher isotropic volume fraction as well as higher mean diffusivity throughout DGM nuclei compared to healthy subjects (93). These results indicate increased extracellular volume and reduced neurite density, i.e., rarefaction of the nervous tissue in WD.
Figure 2.
MRI findings in WD. (A-E) T2 hyperintensities at different levels of the dentato-rubro-thalamic pathway (black arrows) in a single WD patient; (A,B) at the level of tectal plate; (C) in the white matter fibers surrounding red nucleus, and (D) in the anterolateral group of thalamic nuclei; (D) shows also severe damage to the putamina with atrophy and mixed, hyperintense and hypointense, signal abnormalities; (E) coronal slice of the same patient showing continuous affliction of the continuous nervous pathway. (F) T2w image acquired at 1.5 Tesla scanner showing profound T2 hyperintensity in the putamen; (G) same patient examined at 3 Tesla scanner showing mixed T2 signal in the putamen; (H) SWI in the same patient showing definite hypointensity in the striatum and globus pallidus (black arrowheads); (I) T1 hyperintense signal in the globi pallidi (empty arrowheads); (J-L) typical pattern of brain atrophy in WD, severe mesencephalic atrophy is shown in (J) (black arrows) along with (K) 3rd ventricle enlargement (asterisk); (L) mesencephalic atrophy is best assessed on the mid-sagittal slice (white arrowhead). MRI, magnetic resonance imaging; SWI, susceptibility weighted imaging; WD, Wilson disease.
From the early disease stages, hypointensities may be seen along with T2 hyperintensities in the DGM on T2-weighted images (94-99). These hypointensities are better visualized with T2*-weighted (i.e., dependent on effective T2 relaxation time) or susceptibility weighted imaging (SWI). As validated in a post mortem MRI histopathology correlation study, these hypointense lesions are caused by abnormal iron accumulation (26) and may even progress after anti-copper treatment initiation (100,101). When using scanners with higher magnetic field and imaging techniques sensitive to paramagnetic species such as T2*-weighted or SWI, hypointense lesions become prominent and may overshadow T2 hyperintensities. The clinical significance of cerebral iron accumulation in WD is not clear.
There is a wide range of reported prevalence of demyelinating changes in the hemispheric white matter (5–60%). Demyelination in neuro-WD may affect splenium of the corpus callosum (102), internal capsule (103), and cerebellar white matter including superior and middle peduncles containing dentato-rubro-thalamic and ponto-cerebellar tracts respectively (79,103-105). Continuous or patchy lesions in the superior cerebellar peduncle, caudal mesencephalic tectum, rostral mesecephalic tegmentum (surrounding nuclei ruber) and anterolateral thalami likely represent lesions in the entire dentato-rubro-thalamic tract (106-108). Interestingly, quantitative analysis of diffusion tensor imaging (DTI) parameters showed markers of altered tissue microstructure, i.e., increased mean diffusivity and decreased fractional anisotropy, even in normal appearing lobar white matter (21,109,110) as well as in the thalamus with normal signal on T2-weighted images (111); this DTI metric was shown to improve with treatment (112). These results suggest widespread reversible pathology of the white matter beyond lesions visible on T2-weighted images that is consistent with impaired myelination. Importantly, white matter lesions visible on T2-weighted images were also reported to improve on anti-copper therapy (113). The above-mentioned signal changes are typically symmetrical. In the early stages, however, signal changes may sometimes be asymmetrical in correlation with clinical symptoms (114). WD patients may rarely exhibit extensive, sometimes asymmetric, cortico-subcortical lesions, typically affecting the frontal lobe (61,115), which are associated with an unfavorable prognosis.
Atrophy is the ultimate and mostly irreversible consequence of degenerative changes induced by copper toxicity. However, improvement of atrophy has been also reported in few patients (11). Atrophy is present in approximately 30–45% of newly diagnosed neurological WD patients and it appears to be more prevalent in men compared to women (68,87). Tissue loss may progress on treatment and some degree of atrophic changes can be eventually observed in up to 70–90% of chronically treated patients (103,116,117). A specific pattern of atrophy is present in a subgroup of WD patients with predominant involvement of putamina and other DGM structures leading to profound enlargement of 3rd ventricle along with shrinkage of mesencephalon. The degree of mesencephalon atrophy in WD is comparable to findings commonly seen in progressive supranuclear palsy (118,119). Non-specific atrophy of the cerebellum and frontoparietal cortex is also common in neuro-WD.
As in other liver disorders with cirrhosis and portosystemic shunt, WD patients with severe hepatic manifestation may present with symmetrical hyperintense lesions in the globus pallidus in T1-weighted images. These lesions are likely caused by manganese deposits and indicate hepatic encephalopathy (80). With improvement of liver function, these changes typically disappear (120,121).
In addition to MRI markers, several independent studies have shown that transcranial sonography (TCS) consistently shows hyperechogenicity of lenticular nuclei in neuro-WD (122-125). TCS could be thus a cheap screening test for the differential diagnosis of WD and other neurodegenerative disorders (123). A longitudinal case study showed that TCS hyperechogenicity does not change with anti-copper therapy and is likely not suited for treatment monitoring (100).
Several studies have examined dopamine transporter (DAT) and D2 dopamine-receptor single photon emission computed tomography (SPECT) with the result that parkinsonism in WD is associated with both, pre- and post-synaptic lesions in the nigrostriatal pathway (126-128) and that it may improve with therapy (129). Therefore, DAT-SPECT cannot be reliably used in differential diagnosis between WD and Parkinson’s disease. Reduction in DAT binding was significantly associated with the degree of midbrain atrophy in WD (119). Perfusion SPECT and fluorodeoxyglucose positron emission tomography (PET) have been also used to study brain metabolism and perfusion in WD to find decreased cerebral blood flow and glucose consumption in DGM, cerebellum and cortex (130,131). The latter abnormalities may improve on treatment (132-134).
Scales for neurologic and imaging severity
Clinical rating scales designed and validated for assessment of severity of specific diseases are helpful in routine clinical monitoring of patients and are necessary for clinical trials with novel drugs. In addition to clinical scales, (semi)quantitative analysis of MR images may serve as surrogate marker of the type, reversibility, and extent of brain damage in de novo patients before anti-copper treatment initiation. Such MRI markers will be also helpful as outcome measures in clinical trials. While universal disability scales such as Schwab and England Activities of daily living score can be used for WD clinical scoring (11), standardized quantitative assessment of disease severity and monitoring of treatment effects is hampered by the large clinical variability of WD. Therefore, scales designed for assessment of specific syndromes such as tremor, ataxia, parkinsonism or dystonia are not capable of capturing the distinctive and complex spectrum of WD symptoms (135). Several scales were created to score neurologic WD severity in studies comparing clinical symptoms with results of paraclinical examinations (136). The first scale specifically developed and validated to assess the whole spectrum of neurologic clinical symptoms in WD was the unified Wilson disease rating scale (UWDRS) consisting of three parts: consciousness, historical review of activities of daily living adapted from the Barthel index, and neurological examination (44). The majority of items in the latter was taken from established scales focused on specific syndromes: parkinsonism [unified Parkinson’s disease rating scale (UPDRS)] (137), dystonia [Burke-Fahn-Marsden dystonia rating scale (BFMDRS)] (138), Huntington disease [unified Huntington disease rating scale (UHDRS)] (139), tremor [clinical rating scale for tremor (CRST)] (140), and ataxia [International Cooperative Ataxia Rating Scale (ICARS)] (141). The severity of neurologic impairment was shown to correlate with the degree of disability in activities of daily living as assessed by the UWDRS (135). Hepatic and psychiatric subscales were later added to the scale. All UWDRS items show excellent inter-rater agreement in validation studies (44,142). A second WD specific scale is the global assessment scale (GAS) for WD; it has a two-tier design with tier 1 being a global disability measure of the disease burden across hepatic, psychiatric, motor and osseo-muscular systems, and tier 2 being neurological assessment (43). Except of the hepatic subscore, all items from tier 1 were shown to correlate with the severity of neurologic impairment. The neurologic assessment in GAS for WD is considerably shorter and focused more on disability compared to the one included in UWDRS, which is reflected in higher interrater variability of the latter scale. Direct comparison of these scales confirmed excellent correlation between neurologic UWDRS and the GAS for WD tier 2 sub-scores (143). In this study, the “minimal UWDRS” score consisting in nine items from the historical review of activities of daily living was suggested for routine clinical monitoring of WD patients (143). It is however not clear whether this score, which is based only on the patient’s history, has any advantage over simpler universal scales, e.g., modified Rankin scale, and whether it would have enough sensitivity to pick up subtle clinical changes occurring after initiation of anti-copper treatment.
It has been suggested that brain MRI could be helpful not only in the WD diagnosis but also in treatment monitoring and outcome prediction. In order to quantify MRI severity and monitor treatment effects on brain parenchyma, several scales with variable complexity were developed. All scales are represented by a total severity score based on the sum of pathology in predefined structures, typically basal ganglia, thalami, and brainstem. The simplest score considers only the presence or absence of T2 hyperintensities in six regions and represents the number of affected areas (144). Other authors suggested adding atrophy as another item and assessing the overall grade of the severity of change in signal intensity on the scale 0 (normal) to 3 (severe) in addition to the extent of the pathology (79). In another scale, grading was done separately for each assessed structure taking into account signal change and associated atrophy as follows: “0” = no abnormality, “1” = change in signal intensity with no atrophy, “2” = change in signal intensity with mild or moderate atrophy, and “3” = change in signal intensity with severe atrophy (103). Yet, a more complex scale considers the presence of T2 hyperintensities, T2 hypointensities, T1 hyperintensities (only in the globus pallidus), and a semi-quantitative assessment of global atrophy classified as absent (“0”), slight (“1”) or severe (“2”) (117,123). Development of a reliable and valid MR severity scale is hampered by several factors: (I) there are no MRI histopathology correlation studies to accurately determine the pathological basis of T2 hyperintensities, (II) pathology not visible on routine MRI scans may significantly contribute to clinical disability, (III) it is not clear whether and how specific pathological findings on MRI contribute to disability. It can be assumed that while mild T2 hyperintensities represent changes reversible with treatment, T1 hypointensities and atrophy as markers of tissue loss and T2 hypointensities as a marker of iron depositions represent irreversible changes likely associated with worse prognosis. These assumptions should be first validated and, if confirmed, they should be taken into account in future MR severity scales.
Concluding remarks and outlook
Neurologic symptoms in WD are largely reversible with anti-copper treatment but most patients have at least minor residual neuropsychiatric impairment and approximately 20% of patients have unfavorable outcome with severe disability or death (145,146). The prognosis of WD is much better when treatment is started before neurologic symptoms develop. Thus, population screening for WD is well justified but there are no biochemical markers with sufficient sensitivity/specificity and acceptable costs (147). With improving reliability and decreasing costs of next generation sequencing, it is likely that newborn genetic screening of treatable metabolic disorders including WD will be feasible in the upcoming years (148). Gene therapy has been recently tested in animal WD models with promising results (149). Combination of genetic newborn screening and gene therapy would be the ultimate solution for WD.
Development of novel anti-copper drugs with a lower risk of neurologic worsening is also desirable. Tetrathiomolybdate has shown promising results with respect to neurological complications in pilot studies (150-152) and a randomized controlled trial with this compound is currently being performed. For future clinical studies, it would be advantageous if there was one universally accepted and well-validated scale for the assessment of clinical severity (153). Also, identification of MRI markers related to the degree of CNS damage would help to define outcome measures for WD clinical trials.
Acknowledgements
Funding: This work was supported by the Czech Ministry of Health (15-25602A).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Wilson SAK. Progressive Lenticular Degeneration: A Familial Nervous Disease Associated with Cirrhosis of the Liver. Brain 1912;34:295-507. 10.1093/brain/34.4.295 [DOI] [PubMed] [Google Scholar]
- 2.Dziezyc K, Karlinski M, Litwin T, et al. Compliant treatment with anti-copper agents prevents clinically overt Wilson's disease in pre-symptomatic patients. Eur J Neurol 2014;21:332-7. 10.1111/ene.12320 [DOI] [PubMed] [Google Scholar]
- 3.Sternlieb I, Scheinberg IH. Prevention of Wilson's disease in asymptomatic patients. N Engl J Med 1968;278:352-9. 10.1056/NEJM196802152780702 [DOI] [PubMed] [Google Scholar]
- 4.Przybylkowski A, Gromadzka G, Chabik G, et al. Liver cirrhosis in patients newly diagnosed with neurological phenotype of Wilson's disease. Funct Neurol 2014;29:23-9. [PMC free article] [PubMed] [Google Scholar]
- 5.Ferenci P. Pathophysiology and clinical features of Wilson disease. Metab Brain Dis 2004;19:229-39. 10.1023/B:MEBR.0000043973.10494.85 [DOI] [PubMed] [Google Scholar]
- 6.Litwin T, Dusek P, Szafranski T, et al. Psychiatric manifestations in Wilson's disease: possibilities and difficulties for treatment. Ther Adv Psychopharmacol 2018;8:199-211. 10.1177/2045125318759461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davies KM, Hare DJ, Cottam V, et al. Localization of copper and copper transporters in the human brain. Metallomics 2013;5:43-51. 10.1039/C2MT20151H [DOI] [PubMed] [Google Scholar]
- 8.Litwin T, Gromadzka G, Szpak GM, et al. Brain metal accumulation in Wilson's disease. J Neurol Sci 2013;329:55-8. 10.1016/j.jns.2013.03.021 [DOI] [PubMed] [Google Scholar]
- 9.Weisner B, Hartard C, Dieu C. CSF copper concentration: a new parameter for diagnosis and monitoring therapy of Wilson's disease with cerebral manifestation. J Neurol Sci 1987;79:229-37. 10.1016/0022-510X(87)90275-9 [DOI] [PubMed] [Google Scholar]
- 10.Stuerenburg HJ. CSF copper concentrations, blood-brain barrier function, and coeruloplasmin synthesis during the treatment of Wilson's disease. J Neural Transm (Vienna) 2000;107:321-9. 10.1007/s007020050026 [DOI] [PubMed] [Google Scholar]
- 11.Sinha S, Taly AB, Prashanth LK, et al. Sequential MRI changes in Wilson's disease with de-coppering therapy: a study of 50 patients. Br J Radiol 2007;80:744-9. 10.1259/bjr/48911350 [DOI] [PubMed] [Google Scholar]
- 12.Brewer GJ, Yuzbasiyan-Gurkan V. Wilson disease. Medicine (Baltimore) 1992;71:139-64. 10.1097/00005792-199205000-00004 [DOI] [PubMed] [Google Scholar]
- 13.Verity MA, Gambell JK. Studies of copper ion-induced mitochondrial swelling in vitro. Biochem J 1968;108:289-95. 10.1042/bj1080289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scheiber IF, Bruha R, Dusek P. Pathogenesis of Wilson disease. Handb Clin Neurol 2017;142:43-55. 10.1016/B978-0-444-63625-6.00005-7 [DOI] [PubMed] [Google Scholar]
- 15.Reddy PV, Rao KV, Norenberg MD. The mitochondrial permeability transition, and oxidative and nitrosative stress in the mechanism of copper toxicity in cultured neurons and astrocytes. Lab Invest 2008;88:816-30. 10.1038/labinvest.2008.49 [DOI] [PubMed] [Google Scholar]
- 16.Letelier ME, Lepe AM, Faundez M, et al. Possible mechanisms underlying copper-induced damage in biological membranes leading to cellular toxicity. Chem Biol Interact 2005;151:71-82. 10.1016/j.cbi.2004.12.004 [DOI] [PubMed] [Google Scholar]
- 17.Scheiber IF, Dringen R. Copper-treatment increases the cellular GSH content and accelerates GSH export from cultured rat astrocytes. Neurosci Lett 2011;498:42-6. 10.1016/j.neulet.2011.04.058 [DOI] [PubMed] [Google Scholar]
- 18.Bertrand E, Lewandowska E, Szpak GM, et al. Neuropathological analysis of pathological forms of astroglia in Wilson's disease. Folia Neuropathol 2001;39:73-9. [PubMed] [Google Scholar]
- 19.Vogel FS, Evans JW. Morphologic alterations produced by copper in neural tissues with consideration of the role of the metal in the pathogenesis of Wilson's disease. J Exp Med 1961;113:997-1004. 10.1084/jem.113.6.997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sener RN. Diffusion MR imaging changes associated with Wilson disease. AJNR Am J Neuroradiol 2003;24:965-7. [PMC free article] [PubMed] [Google Scholar]
- 21.Jadav R, Saini J, Sinha S, et al. Diffusion tensor imaging (DTI) and its clinical correlates in drug naive Wilson's disease. Metab Brain Dis 2013;28:455-62. 10.1007/s11011-013-9407-1 [DOI] [PubMed] [Google Scholar]
- 22.Finlayson MH, Superville B. Distribution of cerebral lesions in acquired hepatocerebral degeneration. Brain 1981;104:79-95. 10.1093/brain/104.1.79 [DOI] [PubMed] [Google Scholar]
- 23.Klos KJ, Ahlskog JE, Kumar N, et al. Brain metal concentrations in chronic liver failure patients with pallidal T1 MRI hyperintensity. Neurology 2006;67:1984-9. 10.1212/01.wnl.0000247037.37807.76 [DOI] [PubMed] [Google Scholar]
- 24.Poujois A, Mikol J, Woimant F. Wilson disease: brain pathology. Handb Clin Neurol 2017;142:77-89. 10.1016/B978-0-444-63625-6.00008-2 [DOI] [PubMed] [Google Scholar]
- 25.Horoupian DS, Sternlieb I, Scheinberg IH. Neuropathological findings in penicillamine-treated patients with Wilson's disease. Clin Neuropathol 1988;7:62-7. [PubMed] [Google Scholar]
- 26.Dusek P, Bahn E, Litwin T, et al. Brain iron accumulation in Wilson disease: a post mortem 7 Tesla MRI - histopathological study. Neuropathol Appl Neurobiol 2017;43:514-32. 10.1111/nan.12341 [DOI] [PubMed] [Google Scholar]
- 27.Richter R. The pallial component in hepato-lenticular degeneration. J Neuropathol Exp Neurol 1948;7:1-18. 10.1097/00005072-194801000-00001 [DOI] [PubMed] [Google Scholar]
- 28.Mikol J, Vital C, Wassef M, et al. Extensive cortico-subcortical lesions in Wilson's disease: clinico-pathological study of two cases. Acta Neuropathol 2005;110:451-8. 10.1007/s00401-005-1061-1 [DOI] [PubMed] [Google Scholar]
- 29.Meenakshi-Sundaram S, Mahadevan A, Taly AB, et al. Wilson's disease: a clinico-neuropathological autopsy study. J Clin Neurosci 2008;15:409-17. 10.1016/j.jocn.2006.07.017 [DOI] [PubMed] [Google Scholar]
- 30.Nishimuta M, Masui K, Yamamoto T, et al. Copper deposition in oligodendroglial cells in an autopsied case of hepatolenticular degeneration. Neuropathology 2018;38:321-8. 10.1111/neup.12456 [DOI] [PubMed] [Google Scholar]
- 31.Anzil AP, Herrlinger H, Blinzinger K, et al. Ultrastructure of brain and nerve biopsy tissue in Wilson disease. Arch Neurol 1974;31:94-100. 10.1001/archneur.1974.00490380042004 [DOI] [PubMed] [Google Scholar]
- 32.European Association for Study of L. EASL Clinical Practice Guidelines : Wilson's disease. J Hepatol 2012;56:671-85. [DOI] [PubMed] [Google Scholar]
- 33.Lorincz MT. Neurologic Wilson's disease. Ann N Y Acad Sci 2010;1184:173-87. 10.1111/j.1749-6632.2009.05109.x [DOI] [PubMed] [Google Scholar]
- 34.Czlonkowska A, Litwin T, Chabik G. Wilson disease: neurologic features. Handb Clin Neurol 2017;142:101-19. 10.1016/B978-0-444-63625-6.00010-0 [DOI] [PubMed] [Google Scholar]
- 35.Ferenci P, Czlonkowska A, Merle U, et al. Late-onset Wilson's disease. Gastroenterology 2007;132:1294-8. 10.1053/j.gastro.2007.02.057 [DOI] [PubMed] [Google Scholar]
- 36.Ala A, Borjigin J, Rochwarger A, et al. Wilson disease in septuagenarian siblings: Raising the bar for diagnosis. Hepatology 2005;41:668-70. 10.1002/hep.20601 [DOI] [PubMed] [Google Scholar]
- 37.Hall HC. La dégénérescence hépato-lenticulaire: maladie de Wilson, pseudo-sclérose. Paris: Masson, 1921. [Google Scholar]
- 38.Denny-Brown D. Hepatolenticular Degeneration (Wilson's Disease). Two Different Components. N Engl J Med 1964;270:1149-56. 10.1056/NEJM196405282702203 [DOI] [PubMed] [Google Scholar]
- 39.Marsden CD. Wilson's disease. Q J Med 1987;65:959-66. [PubMed] [Google Scholar]
- 40.Czlonkowska A, Gajda J, Rodo M. Effects of long-term treatment in Wilson's disease with D-penicillamine and zinc sulphate. J Neurol 1996;243:269-73. 10.1007/BF00868525 [DOI] [PubMed] [Google Scholar]
- 41.Oder W, Prayer L, Grimm G, et al. Wilson's disease: evidence of subgroups derived from clinical findings and brain lesions. Neurology 1993;43:120-4. 10.1212/WNL.43.1_Part_1.120 [DOI] [PubMed] [Google Scholar]
- 42.Konovalov NV. Gepato-tserebral'naia distrofiia. Moscow: Medgiz, 1960. [Google Scholar]
- 43.Aggarwal A, Aggarwal N, Nagral A, et al. A novel Global Assessment Scale for Wilson's Disease (GAS for WD). Mov Disord 2009;24:509-18. 10.1002/mds.22231 [DOI] [PubMed] [Google Scholar]
- 44.Czlonkowska A, Tarnacka B, Moller JC, et al. Unified Wilson's Disease Rating Scale - a proposal for the neurological scoring of Wilson's disease patients. Neurol Neurochir Pol 2007;41:1-12. [PubMed] [Google Scholar]
- 45.Czlonkowska A, Litwin T, Dusek P, et al. Wilson disease. Nat Rev Dis Primers 2018;4:21. 10.1038/s41572-018-0018-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Litwin T, Dusek P, Czlonkowska A. Neurological manifestations in Wilson’s disease –possible treatment options for symptoms. Expert Opinion on Orphan Drugs 2016;4:719-28. 10.1080/21678707.2016.1188003 [DOI] [Google Scholar]
- 47.Litwin T, Dusek P, Czlonkowska A. Symptomatic treatment of neurologic symptoms in Wilson disease. Handb Clin Neurol 2017;142:211-23. 10.1016/B978-0-444-63625-6.00018-5 [DOI] [PubMed] [Google Scholar]
- 48.Hedera P. Treatment of Wilson's disease motor complications with deep brain stimulation. Ann N Y Acad Sci 2014;1315:16-23. 10.1111/nyas.12372 [DOI] [PubMed] [Google Scholar]
- 49.Prashanth LK, Taly AB, Sinha S, et al. Prognostic factors in patients presenting with severe neurological forms of Wilson's disease. QJM 2005;98:557-63. 10.1093/qjmed/hci095 [DOI] [PubMed] [Google Scholar]
- 50.Svetel M, Kozic D, Stefanova E, et al. Dystonia in Wilson's disease. Mov Disord 2001;16:719-23. 10.1002/mds.1118 [DOI] [PubMed] [Google Scholar]
- 51.Berry WR, Darley FL, Aronson AE. Dysarthria in Wilson's disease. J Speech Hear Res 1974;17:169-83. 10.1044/jshr.1702.169 [DOI] [PubMed] [Google Scholar]
- 52.da Silva-Junior FP, Carrasco AE, da Silva Mendes AM, et al. Swallowing dysfunction in Wilson's disease: a scintigraphic study. Neurogastroenterol Motil 2008;20:285-90. 10.1111/j.1365-2982.2007.01036.x [DOI] [PubMed] [Google Scholar]
- 53.Trocello JM, Osmani K, Pernon M, et al. Hypersialorrhea in Wilson's Disease. Dysphagia 2015;30:489-95. 10.1007/s00455-015-9627-0 [DOI] [PubMed] [Google Scholar]
- 54.Dziezyc K, Litwin T, Chabik G, et al. Frequencies of initial gait disturbances and falls in 100 Wilson's disease patients. Gait Posture 2015;42:601-3. 10.1016/j.gaitpost.2015.09.005 [DOI] [PubMed] [Google Scholar]
- 55.Verma R, Holla VV, Pandey S, et al. Multifocal myoclonus as a heralding manifestation of Wilson disease. J Pediatr Neurosci 2016;11:358-60. 10.4103/1817-1745.199468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barbosa ER, Silveira-Moriyama L, Machado AC, et al. Wilson's disease with myoclonus and white matter lesions. Parkinsonism Relat Disord 2007;13:185-8. 10.1016/j.parkreldis.2006.03.012 [DOI] [PubMed] [Google Scholar]
- 57.Henkin RI, Keiser HR, Jafee IA, et al. Decreased taste sensitivity after D-penicillamine reversed by copper administration. Lancet 1967;2:1268-71. 10.1016/S0140-6736(67)90388-1 [DOI] [PubMed] [Google Scholar]
- 58.Degirmenci N, Veyseller B, Hanagasi H, et al. Olfactory function and olfactory bulb volume in Wilson's disease. Eur Arch Otorhinolaryngol 2019;276:139-42. 10.1007/s00405-018-5216-9 [DOI] [PubMed] [Google Scholar]
- 59.Mueller A, Reuner U, Landis B, et al. Extrapyramidal symptoms in Wilson's disease are associated with olfactory dysfunction. Mov Disord 2006;21:1311-6. 10.1002/mds.20989 [DOI] [PubMed] [Google Scholar]
- 60.Jung KH, Ahn TB, Jeon BS. Wilson disease with an initial manifestation of polyneuropathy. Arch Neurol 2005;62:1628-31. 10.1001/archneur.62.10.1628 [DOI] [PubMed] [Google Scholar]
- 61.Prashanth LK, Sinha S, Taly AB, et al. Spectrum of epilepsy in Wilson's disease with electroencephalographic, MR imaging and pathological correlates. J Neurol Sci 2010;291:44-51. 10.1016/j.jns.2010.01.007 [DOI] [PubMed] [Google Scholar]
- 62.Dening TR, Berrios GE, Walshe JM. Wilson's disease and epilepsy. Brain 1988;111:1139-55. 10.1093/brain/111.5.1139 [DOI] [PubMed] [Google Scholar]
- 63.Trindade MC, Bittencourt T, Lorenzi-Filho G, et al. Restless legs syndrome in Wilson's disease: frequency, characteristics, and mimics. Acta Neurol Scand 2017;135:211-8. 10.1111/ane.12585 [DOI] [PubMed] [Google Scholar]
- 64.Cochen De Cock V, Girardot-Tinant N, Woimant F, et al. Sleep Abnormalities in Wilson's Disease. Curr Treat Options Neurol 2018;20:46. 10.1007/s11940-018-0531-4 [DOI] [PubMed] [Google Scholar]
- 65.Tribl GG, Trindade MC, Bittencourt T, et al. Wilson's disease with and without rapid eye movement sleep behavior disorder compared to healthy matched controls. Sleep Med 2016;17:179-85. 10.1016/j.sleep.2015.09.003 [DOI] [PubMed] [Google Scholar]
- 66.Nevsimalova S, Buskova J, Bruha R, et al. Sleep disorders in Wilson's disease. Eur J Neurol 2011;18:184-90. 10.1111/j.1468-1331.2010.03106.x [DOI] [PubMed] [Google Scholar]
- 67.Zhong W, Huang Z, Tang X. A study of brain MRI characteristics and clinical features in 76 cases of Wilson's disease. J Clin Neurosci 2019;59:167-74. 10.1016/j.jocn.2018.10.096 [DOI] [PubMed] [Google Scholar]
- 68.Litwin T, Gromadzka G, Czlonkowska A, et al. The effect of gender on brain MRI pathology in Wilson's disease. Metab Brain Dis 2013;28:69-75. 10.1007/s11011-013-9378-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van Wassenaer-van Hall HN, van den Heuvel AG, Algra A, et al. Wilson disease: findings at MR imaging and CT of the brain with clinical correlation. Radiology 1996;198:531-6. 10.1148/radiology.198.2.8596862 [DOI] [PubMed] [Google Scholar]
- 70.Hitoshi S, Iwata M, Yoshikawa K. Mid-brain pathology of Wilson's disease: MRI analysis of three cases. J Neurol Neurosurg Psychiatry 1991;54:624-6. 10.1136/jnnp.54.7.624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nazer H, Brismar J, al-Kawi MZ, et al. Magnetic resonance imaging of the brain in Wilson's disease. Neuroradiology 1993;35:130-3. 10.1007/BF00593969 [DOI] [PubMed] [Google Scholar]
- 72.Aisen AM, Martel W, Gabrielsen TO, et al. Wilson disease of the brain: MR imaging. Radiology 1985;157:137-41. 10.1148/radiology.157.1.4034959 [DOI] [PubMed] [Google Scholar]
- 73.Magalhaes AC, Caramelli P, Menezes JR, et al. Wilson's disease: MRI with clinical correlation. Neuroradiology 1994;36:97-100. 10.1007/BF00588068 [DOI] [PubMed] [Google Scholar]
- 74.Sener RN. The claustrum on MRI: normal anatomy, and the bright claustrum as a new sign in Wilson's disease. Pediatr Radiol 1993;23:594-6. 10.1007/BF02014975 [DOI] [PubMed] [Google Scholar]
- 75.Lawler GA, Pennock JM, Steiner RE, et al. Nuclear magnetic resonance (NMR) imaging in Wilson disease. J Comput Assist Tomogr 1983;7:1-8. 10.1097/00004728-198302000-00001 [DOI] [PubMed] [Google Scholar]
- 76.Hegde S, Sinha S, Rao SL, et al. Cognitive profile and structural findings in Wilson's disease: a neuropsychological and MRI-based study. Neurol India 2010;58:708-13. 10.4103/0028-3886.72172 [DOI] [PubMed] [Google Scholar]
- 77.Prashanth LK, Sinha S, Taly AB, et al. Do MRI features distinguish Wilson's disease from other early onset extrapyramidal disorders? An analysis of 100 cases. Mov Disord 2010;25:672-8. 10.1002/mds.22689 [DOI] [PubMed] [Google Scholar]
- 78.Sinha S, Taly AB, Ravishankar S, et al. Central pontine signal changes in Wilson's disease: distinct MRI morphology and sequential changes with de-coppering therapy. J Neuroimaging 2007;17:286-91. 10.1111/j.1552-6569.2007.00120.x [DOI] [PubMed] [Google Scholar]
- 79.King AD, Walshe JM, Kendall BE, et al. Cranial MR imaging in Wilson's disease. AJR Am J Roentgenol 1996;167:1579-84. 10.2214/ajr.167.6.8956601 [DOI] [PubMed] [Google Scholar]
- 80.Saatci I, Topcu M, Baltaoglu FF, et al. Cranial MR findings in Wilson's disease. Acta Radiol 1997;38:250-8. 10.1080/02841859709172059 [DOI] [PubMed] [Google Scholar]
- 81.Li X, Feng Z, Tang W, et al. Sex Differences in Clinical Characteristics and Brain MRI Change in Patients With Wilson's Disease in a Chinese Population. Front Physiol 2018;9:1429. 10.3389/fphys.2018.01429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Starosta-Rubinstein S, Young AB, Kluin K, et al. Clinical assessment of 31 patients with Wilson's disease. Correlations with structural changes on magnetic resonance imaging. Arch Neurol 1987;44:365-70. 10.1001/archneur.1987.00520160007005 [DOI] [PubMed] [Google Scholar]
- 83.Ranjan A, Kalita J, Kumar S, et al. A study of MRI changes in Wilson disease and its correlation with clinical features and outcome. Clin Neurol Neurosurg 2015;138:31-6. 10.1016/j.clineuro.2015.07.013 [DOI] [PubMed] [Google Scholar]
- 84.Yu XE, Gao S, Yang RM, et al. MR Imaging of the Brain in Neurologic Wilson Disease. AJNR Am J Neuroradiol 2019;40:178-83. 10.3174/ajnr.A5936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kozic DB, Petrovic I, Svetel M, et al. Reversible lesions in the brain parenchyma in Wilson's disease confirmed by magnetic resonance imaging: earlier administration of chelating therapy can reduce the damage to the brain. Neural Regen Res 2014;9:1912-6. 10.4103/1673-5374.145360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kim TJ, Kim IO, Kim WS, et al. MR imaging of the brain in Wilson disease of childhood: findings before and after treatment with clinical correlation. AJNR Am J Neuroradiol 2006;27:1373-8. [PMC free article] [PubMed] [Google Scholar]
- 87.da Costa Mdo D, Spitz M, Bacheschi LA, et al. Wilson's disease: two treatment modalities. Correlations to pretreatment and posttreatment brain MRI. Neuroradiology 2009;51:627-33. 10.1007/s00234-009-0536-5 [DOI] [PubMed] [Google Scholar]
- 88.Thuomas KA, Aquilonius SM, Bergstrom K, et al. Magnetic resonance imaging of the brain in Wilson's disease. Neuroradiology 1993;35:134-41. 10.1007/BF00593970 [DOI] [PubMed] [Google Scholar]
- 89.Sener RN. Diffusion MRI findings in Wilson's disease. Comput Med Imaging Graph 2003;27:17-21. 10.1016/S0895-6111(02)00047-2 [DOI] [PubMed] [Google Scholar]
- 90.Kawamura N, Ohyagi Y, Kawajiri M, et al. Serial diffusion-weighted MRI in a case of Wilson's disease with acute onset hemichorea. J Neurol 2004;251:1413-4. 10.1007/s00415-004-0555-4 [DOI] [PubMed] [Google Scholar]
- 91.Litwin T, Karlinski M, Skowronska M, et al. MR image mimicking the "eye of the tiger" sign in Wilson's disease. J Neurol 2014;261:1025-7. 10.1007/s00415-014-7322-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Juan CJ, Chen CY, Liu YJ, et al. Acute putaminal necrosis and white matter demyelination in a child with subnormal copper metabolism in Wilson disease: MR imaging and spectroscopic findings. Neuroradiology 2005;47:401-5. 10.1007/s00234-004-1306-z [DOI] [PubMed] [Google Scholar]
- 93.Song YK, Li XB, Huang XL, et al. A study of neurite orientation dispersion and density imaging in wilson's disease. J Magn Reson Imaging 2018;48:423-30. 10.1002/jmri.25930 [DOI] [PubMed] [Google Scholar]
- 94.Yang J, Li X, Yang R, et al. Susceptibility-Weighted Imaging Manifestations in the Brain of Wilson's Disease Patients. PLoS One 2015;10:e0125100. 10.1371/journal.pone.0125100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fritzsch D, Reiss-Zimmermann M, Trampel R, et al. Seven-tesla magnetic resonance imaging in Wilson disease using quantitative susceptibility mapping for measurement of copper accumulation. Invest Radiol 2014;49:299-306. 10.1097/RLI.0000000000000010 [DOI] [PubMed] [Google Scholar]
- 96.Skowronska M, Litwin T, Dziezyc K, et al. Does brain degeneration in Wilson disease involve not only copper but also iron accumulation? Neurol Neurochir Pol 2013;47:542-6. 10.5114/ninp.2013.39071 [DOI] [PubMed] [Google Scholar]
- 97.Sudmeyer M, Saleh A, Wojtecki L, et al. Wilson's disease tremor is associated with magnetic resonance imaging lesions in basal ganglia structures. Mov Disord 2006;21:2134-9. 10.1002/mds.21136 [DOI] [PubMed] [Google Scholar]
- 98.Brugieres P, Combes C, Ricolfi F, et al. Atypical MR presentation of Wilson disease: a possible consequence of paramagnetic effect of copper? Neuroradiology 1992;34:222-4. 10.1007/BF00596341 [DOI] [PubMed] [Google Scholar]
- 99.Mironov A. Decreased signal intensity of the putamen and the caudate nucleus in Wilson disease of the brain. Neuroradiology 1993;35:166. 10.1007/BF00593979 [DOI] [PubMed] [Google Scholar]
- 100.Dusek P, Skoloudik D, Maskova J, et al. Brain iron accumulation in Wilson's disease: A longitudinal imaging case study during anticopper treatment using 7.0T MRI and transcranial sonography. J Magn Reson Imaging 2018;47:282-5. 10.1002/jmri.25702 [DOI] [PubMed] [Google Scholar]
- 101.Engelbrecht V, Schlaug G, Hefter H, et al. MRI of the brain in Wilson disease: T2 signal loss under therapy. J Comput Assist Tomogr 1995;19:635-8. 10.1097/00004728-199507000-00026 [DOI] [PubMed] [Google Scholar]
- 102.Trocello JM, Guichard JP, Leyendecker A, et al. Corpus callosum abnormalities in Wilson's disease. J Neurol Neurosurg Psychiatry 2011;82:1119-21. 10.1136/jnnp.2009.204651 [DOI] [PubMed] [Google Scholar]
- 103.Sinha S, Taly AB, Ravishankar S, et al. Wilson's disease: cranial MRI observations and clinical correlation. Neuroradiology 2006;48:613-21. 10.1007/s00234-006-0101-4 [DOI] [PubMed] [Google Scholar]
- 104.van Wassenaer-van Hall HN, van den Heuvel AG, Jansen GH, et al. Cranial MR in Wilson disease: abnormal white matter in extrapyramidal and pyramidal tracts. AJNR Am J Neuroradiol 1995;16:2021-7. [PMC free article] [PubMed] [Google Scholar]
- 105.Das M, Misra UK, Kalita J. A study of clinical, MRI and multimodality evoked potentials in neurologic Wilson disease. Eur J Neurol 2007;14:498-504. 10.1111/j.1468-1331.2006.01676.x [DOI] [PubMed] [Google Scholar]
- 106.Yousaf M, Kumar M, Ramakrishnaiah R, et al. Atypical MRI features involving the brain in Wilson's disease. Radiol Case Rep 2015;4:312. 10.2484/rcr.v4i3.312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Martinez-Fernandez R, Caballol N, Gomez-Choco M. MRI and transcranial sonography findings in Wilson's disease. Mov Disord 2013;28:740. 10.1002/mds.25492 [DOI] [PubMed] [Google Scholar]
- 108.van Wassenaer-van Hall HN. Neuroimaging in Wilson disease. Metab Brain Dis 1997;12:1-19. 10.1007/BF02676350 [DOI] [PubMed] [Google Scholar]
- 109.Favrole P, Chabriat H, Guichard JP, et al. Clinical correlates of cerebral water diffusion in Wilson disease. Neurology 2006;66:384-9. 10.1212/01.wnl.0000196482.71636.7d [DOI] [PubMed] [Google Scholar]
- 110.Wang A, Wu H, Xu C, et al. Study on Lesion Assessment of Cerebello-Thalamo-Cortical Network in Wilson's Disease with Diffusion Tensor Imaging. Neural Plast 2017;2017:7323121. 10.1155/2017/7323121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li G, Zhou X, Xu P, et al. Microstructure assessment of the thalamus in Wilson's disease using diffusion tensor imaging. Clin Radiol 2014;69:294-8. 10.1016/j.crad.2013.10.016 [DOI] [PubMed] [Google Scholar]
- 112.Lawrence A, Saini J, Sinha S, et al. Improvement of Diffusion Tensor Imaging (DTI) Parameters with Decoppering Treatment in Wilson's Disease. JIMD Rep 2016;25:31-7. 10.1007/8904_2015_466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chung EJ, Kim EG, Kim SJ, et al. Wilson's disease with cognitive impairment and without extrapyramidal signs: improvement of neuropsychological performance and reduction of MRI abnormalities with trientine treatment. Neurocase 2016;22:40-4. 10.1080/13554794.2015.1032977 [DOI] [PubMed] [Google Scholar]
- 114.Zhou XX, Li XH, Chen DB, et al. The asymmetry of neural symptoms in Wilson's disease patients detecting by diffusion tensor imaging, resting-state functional MRI, and susceptibility-weighted imaging. Brain Behav 2018;8:e00930. 10.1002/brb3.930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Trocello JM, Woimant F, El Balkhi S, et al. Extensive striatal, cortical, and white matter brain MRI abnormalities in Wilson disease. Neurology 2013;81:1557. 10.1212/WNL.0b013e3182a95883 [DOI] [PubMed] [Google Scholar]
- 116.Roh JK, Lee TG, Wie BA, et al. Initial and follow-up brain MRI findings and correlation with the clinical course in Wilson's disease. Neurology 1994;44:1064-8. 10.1212/WNL.44.6.1064 [DOI] [PubMed] [Google Scholar]
- 117.Frota NA, Barbosa ER, Porto CS, et al. Cognitive impairment and magnetic resonance imaging correlations in Wilson's disease. Acta Neurol Scand 2013;127:391-8. 10.1111/ane.12037 [DOI] [PubMed] [Google Scholar]
- 118.Semnic R, Svetel M, Dragasevic N, et al. Magnetic resonance imaging morphometry of the midbrain in patients with Wilson disease. J Comput Assist Tomogr 2005;29:880-3. 10.1097/01.rct.0000181723.61974.51 [DOI] [PubMed] [Google Scholar]
- 119.Strecker K, Schneider JP, Barthel H, et al. Profound midbrain atrophy in patients with Wilson's disease and neurological symptoms? J Neurol 2006;253:1024-9. 10.1007/s00415-006-0151-x [DOI] [PubMed] [Google Scholar]
- 120.Kozic D, Svetel M, Petrovic B, et al. MR imaging of the brain in patients with hepatic form of Wilson's disease. Eur J Neurol 2003;10:587-92. 10.1046/j.1468-1331.2003.00661.x [DOI] [PubMed] [Google Scholar]
- 121.Mochizuki H, Kamakura K, Masaki T, et al. Atypical MRI features of Wilson's disease: high signal in globus pallidus on T1-weighted images. Neuroradiology 1997;39:171-4. 10.1007/s002340050386 [DOI] [PubMed] [Google Scholar]
- 122.Tribl GG, Trindade MC, Almeida KJ, et al. Quantitative transcranial sonography in Wilson's disease and healthy controls: Cut-off values and functional correlates. J Neurol Sci 2018;385:69-74. 10.1016/j.jns.2017.11.026 [DOI] [PubMed] [Google Scholar]
- 123.Maskova J, Skoloudik D, Burgetova A, et al. Comparison of transcranial sonography-magnetic resonance fusion imaging in Wilson's and early-onset Parkinson's diseases. Parkinsonism Relat Disord 2016;28:87-93. 10.1016/j.parkreldis.2016.04.031 [DOI] [PubMed] [Google Scholar]
- 124.Svetel M, Mijajlovic M, Tomic A, et al. Transcranial sonography in Wilson's disease. Parkinsonism Relat Disord 2012;18:234-8. 10.1016/j.parkreldis.2011.10.007 [DOI] [PubMed] [Google Scholar]
- 125.Walter U, Krolikowski K, Tarnacka B, et al. Sonographic detection of basal ganglia lesions in asymptomatic and symptomatic Wilson disease. Neurology 2005;64:1726-32. 10.1212/01.WNL.0000161847.46465.B9 [DOI] [PubMed] [Google Scholar]
- 126.Oertel WH, Tatsch K, Schwarz J, et al. Decrease of D2 receptors indicated by 123I-iodobenzamide single-photon emission computed tomography relates to neurological deficit in treated Wilson's disease. Ann Neurol 1992;32:743-8. 10.1002/ana.410320607 [DOI] [PubMed] [Google Scholar]
- 127.Jeon B, Kim JM, Jeong JM, et al. Dopamine transporter imaging with [123I]-beta-CIT demonstrates presynaptic nigrostriatal dopaminergic damage in Wilson's disease. J Neurol Neurosurg Psychiatry 1998;65:60-4. 10.1136/jnnp.65.1.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tatsch K, Schwarz J, Oertel WH, et al. SPECT imaging of dopamine D2 receptors with 123I-IBZM: initial experience in controls and patients with Parkinson's syndrome and Wilson's disease. Nucl Med Commun 1991;12:699-707. 10.1097/00006231-199108000-00005 [DOI] [PubMed] [Google Scholar]
- 129.Schlaug G, Hefter H, Engelbrecht V, et al. Neurological impairment and recovery in Wilson's disease: evidence from PET and MRI. J Neurol Sci 1996;136:129-39. 10.1016/0022-510X(95)00293-B [DOI] [PubMed] [Google Scholar]
- 130.Piga M, Murru A, Satta L, et al. Brain MRI and SPECT in the diagnosis of early neurological involvement in Wilson's disease. Eur J Nucl Med Mol Imaging 2008;35:716-24. 10.1007/s00259-007-0681-1 [DOI] [PubMed] [Google Scholar]
- 131.Giagheddu M, Tamburini G, Piga M, et al. Comparison of MRI, EEG, EPs and ECD-SPECT in Wilson's disease. Acta Neurol Scand 2001;103:71-81. 10.1034/j.1600-0404.2001.103002071.x [DOI] [PubMed] [Google Scholar]
- 132.Ishida S, Doi Y, Yamane K, et al. Resolution of cranial MRI and SPECT abnormalities in a patient with Wilson's disease following oral zinc monotherapy. Intern Med 2012;51:1759-63. 10.2169/internalmedicine.51.7341 [DOI] [PubMed] [Google Scholar]
- 133.Cordato DJ, Fulham MJ, Yiannikas C. Pretreatment and posttreatment positron emission tomographic scan imaging in a 20-year-old patient with Wilson's disease. Mov Disord 1998;13:162-6. 10.1002/mds.870130131 [DOI] [PubMed] [Google Scholar]
- 134.De Volder A, Sindic CJ, Goffinet AM. Effect of D-penicillamine treatment on brain metabolism in Wilson's disease: a case study. J Neurol Neurosurg Psychiatry 1988;51:947-9. 10.1136/jnnp.51.7.947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Czlonkowska A, Litwin T, Dziezyc K, et al. Characteristics of a newly diagnosed Polish cohort of patients with neurological manifestations of Wilson disease evaluated with the Unified Wilson's Disease Rating Scale. BMC Neurol 2018;18:34. 10.1186/s12883-018-1039-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Meenakshi-Sundaram S, Taly AB, Kamath V, et al. Autonomic dysfunction in Wilson's disease -- a clinical and electrophysiological study. Clin Auton Res 2002;12:185-9. 10.1007/s10286-002-0038-6 [DOI] [PubMed] [Google Scholar]
- 137.Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord 2008;23:2129-70. 10.1002/mds.22340 [DOI] [PubMed] [Google Scholar]
- 138.Comella CL, Leurgans S, Wuu J, et al. Rating scales for dystonia: a multicenter assessment. Mov Disord 2003;18:303-12. 10.1002/mds.10377 [DOI] [PubMed] [Google Scholar]
- 139.Unified Huntington's Disease Rating Scale: reliability and consistency. Huntington Study Group. Mov Disord 1996;11:136-42. 10.1002/mds.870110204 [DOI] [PubMed] [Google Scholar]
- 140.Fahn S, Tolosa E, Marin C. Clinical rating scale for tremor. In: Jankovic J, Tolosa E, editors. Parkinson’s Disease and Movement Disorders. Baltimore-Munich: Urban & Schwarzenberg; 1988. p. 225-34. [Google Scholar]
- 141.Trouillas P, Takayanagi T, Hallett M, et al. International Cooperative Ataxia Rating Scale for pharmacological assessment of the cerebellar syndrome. The Ataxia Neuropharmacology Committee of the World Federation of Neurology. J Neurol Sci 1997;145:205-11. 10.1016/S0022-510X(96)00231-6 [DOI] [PubMed] [Google Scholar]
- 142.Leinweber B, Moller JC, Scherag A, et al. Evaluation of the Unified Wilson's Disease Rating Scale (UWDRS) in German patients with treated Wilson's disease. Mov Disord 2008;23:54-62. 10.1002/mds.21761 [DOI] [PubMed] [Google Scholar]
- 143.Volpert HM, Pfeiffenberger J, Groner JB, et al. Comparative assessment of clinical rating scales in Wilson's disease. BMC Neurol 2017;17:140. 10.1186/s12883-017-0921-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Poujois A, Trocello JM, Djebrani-Oussedik N, et al. Exchangeable copper: a reflection of the neurological severity in Wilson's disease. Eur J Neurol 2017;24:154-60. 10.1111/ene.13171 [DOI] [PubMed] [Google Scholar]
- 145.Litwin T, Dziezyc K, Karlinski M, et al. Early neurological worsening in patients with Wilson's disease. J Neurol Sci 2015;355:162-7. 10.1016/j.jns.2015.06.010 [DOI] [PubMed] [Google Scholar]
- 146.Kalita J, Kumar V, Chandra S, et al. Worsening of Wilson disease following penicillamine therapy. Eur Neurol 2014;71:126-31. 10.1159/000355276 [DOI] [PubMed] [Google Scholar]
- 147.Hahn SH. Population screening for Wilson's disease. Ann N Y Acad Sci 2014;1315:64-9. 10.1111/nyas.12423 [DOI] [PubMed] [Google Scholar]
- 148.Poulsen JB, Lescai F, Grove J, et al. High-Quality Exome Sequencing of Whole-Genome Amplified Neonatal Dried Blood Spot DNA. PLoS One 2016;11:e0153253. 10.1371/journal.pone.0153253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Murillo O, Luqui DM, Gazquez C, et al. Long-term metabolic correction of Wilson's disease in a murine model by gene therapy. J Hepatol 2016;64:419-26. 10.1016/j.jhep.2015.09.014 [DOI] [PubMed] [Google Scholar]
- 150.Weiss KH, Askari FK, Czlonkowska A, et al. Bis-choline tetrathiomolybdate in patients with Wilson's disease: an open-label, multicentre, phase 2 study. Lancet Gastroenterol Hepatol 2017;2:869-76. 10.1016/S2468-1253(17)30293-5 [DOI] [PubMed] [Google Scholar]
- 151.Stremmel W. Bis-choline Tetrathiomolybdate as Old Drug in a New Design for Wilson's Disease: Good for Brain and Liver? Hepatology 2019;69:901-3. 10.1002/hep.30130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Brewer GJ, Askari F, Lorincz MT, et al. Treatment of Wilson disease with ammonium tetrathiomolybdate: IV. Comparison of tetrathiomolybdate and trientine in a double-blind study of treatment of the neurologic presentation of Wilson disease. Arch Neurol 2006;63:521-7. 10.1001/archneur.63.4.521 [DOI] [PubMed] [Google Scholar]
- 153.Hobart JC, Cano SJ, Zajicek JP, et al. Rating scales as outcome measures for clinical trials in neurology: problems, solutions, and recommendations. Lancet Neurol 2007;6:1094-105. 10.1016/S1474-4422(07)70290-9 [DOI] [PubMed] [Google Scholar]