Abstract
This article reviews important features for improving the diagnosis and management of fetal arrhythmias. The normal fetal heart rate ranges between 110 and 160 beats per minute. A fetal heart rate is considered abnormal if the heart rate is beyond the normal ranges or the rhythm is irregular. The rate, duration, and origin of the rhythm and degree of irregularity usually determine the potential for hemodynamic consequences. Most of the fetal rhythm disturbances are the result of premature atrial contractions (PACs) and are of little clinical significance. Other arrhythmias include tachyarrhythmias (heart rate in excess of 160 beats/min) such as atrioventricular (AV) reentry tachycardia, atrial flutter, and ventricular tachycardia, and bradyarrhythmias (heart rate <110 beats/min) such as sinus node dysfunction, complete heart block (CHB) and long QT syndrome (which is associated with sinus bradycardia and pseudo-heart block).
1. Introduction
The normal fetal heart rate ranges between 110 and 160 beats per minute. A fetal heart rate is considered abnormal if the heart rate is beyond the normal ranges or the rhythm is irregular. The rate, duration, and origin of the rhythm and degree of irregularity usually determine the potential for hemodynamic consequences. Most of the fetal rhythm disturbances are the result of premature atrial contractions (PACs) and are of little clinical significance. Other arrhythmias include tachyarrhythmias (heart rate in excess of 160 beats/min) such as atrioventricular (AV) reentry tachycardia, atrial flutter, and ventricular tachycardia, and bradyarrhythmias (heart rate <110 beats/min) such as sinus node dysfunction, complete heart block (CHB) and long QT syndrome (which is associated with sinus bradycardia and pseudo-heart block). Fetal arrhythmias can be detected in approximately 1% of all fetuses and up to 49% of all referrals for fetal echocardiography [1]. In approximately 10% of pregnancies complicated by fetal arrhythmias, the arrhythmia may be life-threatening [2].
1.1. Clinical presentation
Fetal arrhythmias are usually detected during routine auscultation of the fetal heart or during an obstetric scan [3]. The pregnancy is otherwise unremarkable. If the arrhythmia is sustained, there is a greater risk of fetal hemodynamic compromise leading to hydrops fetalis and fetal demise. Intermittent tachycardias can also be associated with hydrops. Hence, patients with hydrops and a normal heart rate warrant repeat assessment of the fetal heart rate to detect intermittent arrhythmias. Earlier onset in gestation of a tachyarrhythmia and a higher ventricular rate are other risk factors associated with a greater risk for development of hydrops fetalis [4].
In fetuses with bradycardia, a slow ventricular escape rate of less than 55 beats/minute appears to be poor prognostic factor [5]. Fetuses of mothers suffering from a connective tissue disease (commonly Sjogren's syndrome or Systemic Lupus Erythematosus) are at risk for developing isolated complete heart block [6]. Both fetal brady and tachyarrhythmias can be associated with structural heart disease and warrant a thorough echocardiogram and evaluation by a pediatric cardiologist. The combination of a sustained arrhythmia, structural heart disease and hydrops fetalis is an ominous sign carrying a poor prognosis [5].
2. Pathophysiology
2.1. Non-sustained arrhythmias
PACs represent by far the single most common arrhythmia in fetuses referred for an arrhythmia. PACs are usually clinically insignificant though about 1% of fetuses with PACs will have significant structural heart disease, and 0.5% will develop supraventricular tachycardia [7]. Isolated premature ventricular contractions (PVCs) are far less common compared with PACs. The prevalence of fetal PACs to PVCs has been estimated at around 10:1. PVCs may suggest a dilated or dysfunctional ventricle, intracardiac rhabdomyomas, or other anatomic abnormalities. While premature contractions can be easily identified using Doppler or M-mode echocardiography, in-utero PVCs are difficult to distinguish from PACs.
2.2. Sustained tachycardia
A pathological tachycardia in the fetus is described as a sustained heart rate of over 180 beats per minute. Sustained tachyarrhythmias are much more common than sustained bradyarrhythmias [8]. Atrial tachyarrhythmias are much more common than ventricular tachyarrhythmias. The most common form of atrial tachyarrhythmias involves a reentry mechanism either within the atrium (atrial flutter) or between the atrium and the ventricle via an accessory pathway (Atrio-Ventricular reentrant tachycardia).
2.3. Sustained bradycardia
Fetal bradycardia that is non-sustained may be secondary to an exaggerated variability of the sinus rhythm. Sinus bradycardia per se is well tolerated but may be secondary to fetal distress, sinus node dysfunction (anti-Ro antibody related, left isomerism), and LQTS (KCNQ1 mutations) [8]. Sustained fetal bradycardia is most commonly secondary to congenital CHB. The incidence of CHB at birth has been reported to be approximately 1 in 20,000. In patients presenting with fetal CHB, complex structural heart defects have been reported in up to 53% of the patients [9]. The combination of CHB and structural heart disease is usually an ominous sign with a high likelihood of hydrops leading to fetal or neonatal death [10]. Isolated CHB in the absence of structural heart disease is usually well tolerated in utero and does not lead to hemodynamic consequences unless the heart rates are consistently less than 60 beats per minute. There is a high association of isolated CHB with maternal lupus, and all gravid mothers with fetal CHB should undergo testing for autoantibodies. The presence of maternal anti SS-A/Ro or anti SS-B/La antibodies has been associated with an increased risk for fetal CHB [11]. Autoantibody-associated CHB is not coincident with major structural abnormalities, is most often identified in the late second trimester, carries a higher mortality, and frequently requires a pacemaker in the neonatal period [12]. The recurrence rate of CHB is at least two to three times higher than that of the first affected pregnancy, supporting the need for close echocardiographic monitoring in all subsequent pregnancies, with heightened surveillance between 18 and 24 weeks of gestation.
The fetus with repeated heart rates of less than third percentile of gestational age may have Long QT Syndrome (LQTS) [13]. In utero 2nd degree AV block with or without intermittent Torsades de pointes (TdP) can be attributed to LQTS. The LQTS AV block occurs not because of conduction system disease per se, but because ventricular repolarization is so prolonged that the atrium is activated before the ventricle completely repolarizes. Early recognition and appropriate treatment can be life saving for the fetus and unsuspecting LQTS family members.
3. Diagnosis
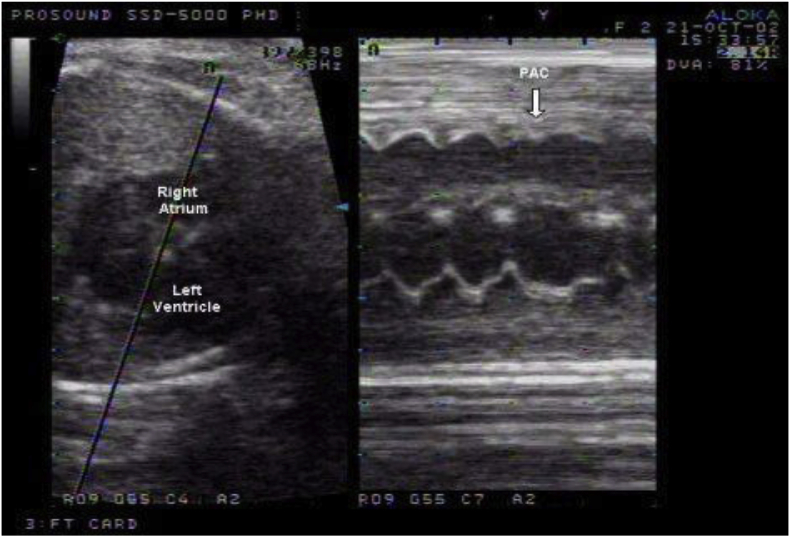
The diagnosis of fetal arrhythmias remains challenging. Routine obstetrical evaluation relies on auscultation and Doppler detection of pulsatile flow within the fetal heart. These techniques allow determination of the ventricular rate but are incapable of evaluating the AV relationship or the origin of the rhythm. M-mode echocardiography with simultaneous recording of the atrial and ventricular contractions is the primary modality for determining the atrial and ventricular relationship and rates (Fig. 1).
Fig. 1.
M-mode echocardiography with simultaneous recording of the atrial and ventricular contractions showing a premature atrial contraction (PAC) that did not conduct to the ventricle.
Blocked atrial bigeminy can produce fetal heart rates between 75 and 90 bpm when conduction is in a 2:1 AV pattern [14]. This condition can be mistaken for second-degree AV block. Atrial flutter usually has atrial rates between 300 and 500 beats per minute with variable conduction to the ventricle leading to ventricular rates between 200 and 250 beats per minute. In AV reentry tachycardia, the 1:1 AV conduction leads to faster ventricular rates about 250–350 beats per minute. Episodes of abrupt onset and termination of tachycardia frequently help confirm the diagnosis of a reentry tachycardia. Sustained tachycardia can lead to hydrops. M-mode echocardiography can help in differentiating between atrial flutter (variable AV conduction), AV reentry tachycardia (1:1 AV conduction) and ventricular tachycardia (complete AV dissociation). Fetal bradycardia can be determined by routine Doppler auscultation. The diagnosis of heart block can be made by fetal echocardiography.
Periods of 2nd degree block that are isolated or occur with episodes of TdP can be seen in fetal LQTS [15,16]. The timeline of LQTS rhythms seems related to gestational age. A slow sinus rate can be seen as early as 14 weeks, 2nd degree AV block has been typically described at 18–40 weeks, and TdP generally does not occur until after 29 weeks [17,18]. Fetal rhythm phenotype and postnatal QTc can predict postnatal rhythm and suggest genotype: bradycardic fetuses usually have KCNQ1 mutation, while those with TdP and/or a postnatal QTc more than 500 ms have SCN5A, KCNH2 or uncharacterized mutations [19].
Fetal magnetocardiography is the magnetic analog of fetal electrocardiogram (ECG) and currently is the most effective means of assessing fetal arrhythmias [7]. It is done using external leads affixed to the maternal abdomen and detects magnetic fields caused by the external excitation of the fetal heart. These magnetic fields are plotted against time resulting in information equivalent to the surface ECG. This technique has provided usefulness in the detection of fetal arrhythmias, particularly those not detectable by other diagnostic tools such in fetuses with long QT intervals [7]. However, fetal magnetocardiography is not routinely available for clinical use.
4. Treatment
A precise assessment of the fetal arrhythmia is essential in order to administer the appropriate therapy. Prenatal therapy to treat the fetus can be administered through the maternal or direct fetal route. Therapy using the direct fetal route needs special expertise and is outside the expertise of most centers. Initiation of antiarrhythmics in the mother should be done in an inpatient setting. The mother should be examined with a 12-lead electrocardiogram to exclude maternal diseases such as Wolff-Parkinson-White Syndrome (WPW), prolonged QT interval or myocarditis that may contraindicate certain antiarrhythmic therapies. Serial ECG monitoring for possible side effects to the mother is indicated. Serum drug concentrations may also be helpful. High concentrations may reflect potential for drug toxicity, and low levels may indicate sub therapeutic treatment. An understanding of the half-life for each medication is essential as it is important to allow for enough time for the drug to reach therapeutic levels before deciding to switch to or add another agent. Concentrations of the antiarrhythmic drugs measured in maternal blood may differ from that in the fetal blood. The transplacental transfer of each drug varies and is further influenced by fluid status of the fetus, gestational age, development of the villous placenta and other physiological changes.
4.1. Treatment of premature beats
In the setting of a structurally normal heart, both PACs and PVCs are benign, require no specific therapy and usually resolve spontaneously before or shortly after birth. A complete fetal echocardiogram and Doppler assessment is indicated in these patients to rule out the associated risk of congenital heart disease, evaluate ventricular function, and to look for other sustained arrhythmias. In rare cases, frequent “blocked” PACs with non-conducted beats and present as a bradycardic rhythm called blocked atrial bigeminy. These patients require close monitoring with weekly visits. We are unaware of isolated premature beats leading to hemodynamic compromise or hydrops and hence do not recommend any medical therapy or premature delivery for these patients.
4.2. Treatment of tachyarrhythmias
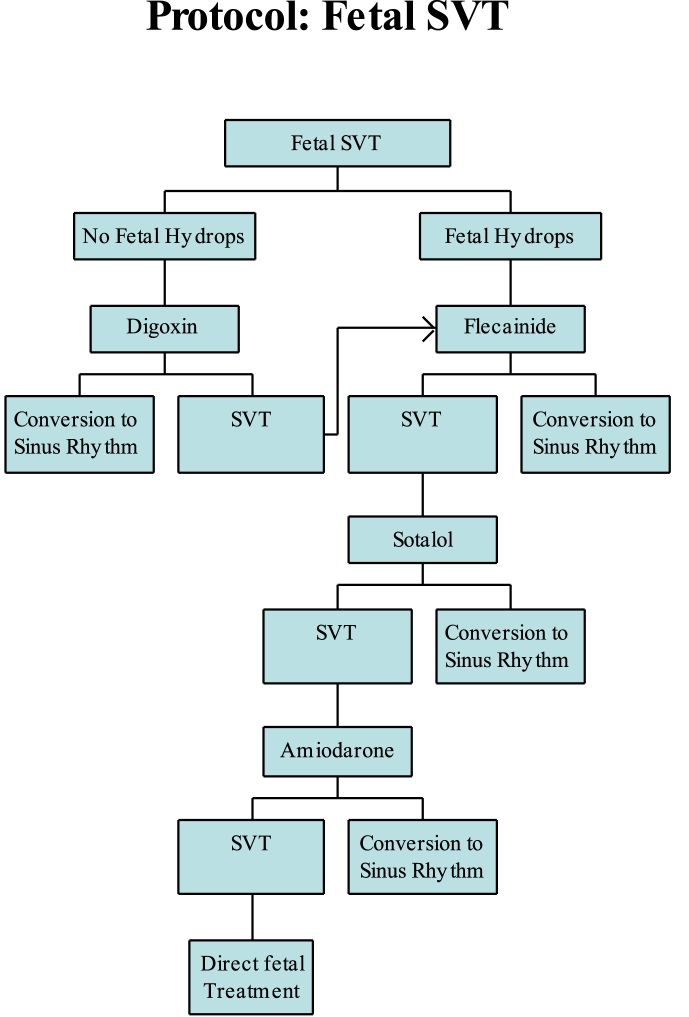
Intermittent fetal tachycardias may require only close observation of the fetal heart rates during the remainder of the pregnancy. Sustained fetal tachycardias however, usually require treatment. Different centers may have somewhat different approaches to which antiarrhythmics to use but most centers will start off with Digoxin followed by flecainide or sotalol as the next drug of choice. We have summarized our treatment protocol in Fig. 2. The efficacy, side effects and monitoring parameters for each antiarrhythmic are summarized in Table 1.
Fig. 2.
Protocol for management of SVT.
Table 1.
| Drug | Class | Use | Dose | Metabolism | Half life | Therap range | Side effects, maternal | Side effects, fetal |
|---|---|---|---|---|---|---|---|---|
| Flecainide | 1C | SVT AF VT |
PO: 100–400 mg bid |
Hepatic excretion 67%, renal excretion 33% | 13–19 h | <1 μg/mL | proarrhythmia, vertigo, nausea, headache, disturbed vision, parasthesia | negative inotrope, proarrhythmia |
| Amiodarone | III | SVT AF VT |
IV: 5 mg/kg over 20 min; 500–1000 mg over 24 h, PO: 1200–1600 mg/d for 7–14 days (loading), then 200–400 mg/day (maint) | hepatic metabolism; renal excretion | 25–110 days | 1.0–2.5 μg/mL | proarrhythmia risk | proarrhythmic, hypothyroid |
| Sotalol | III | SVT AF VT |
PO: 80–160 mg q 12h; increase to 160 mg q 8h | renal excretion | 15–17 h | 1.5–2.5 μg/mL | proarrhythmia risk | negative inotrope, proarrhythmia |
| Adenosine | IV | SVT | IV: 100–200 Ug/kg (into umbilical vein) | throughout body | 10–30 s | NA | useful for acute termination of SVT | proarrhythmia |
| Digoxin | cardiac glyco-side | SVT AF |
IV: 1 mg divided over 24 h, PO: 0.5–1.0 mg daily in 2 divided doses. | renal excretion | 36 h | 1–2 ng/mL | proarrhythmia, AV block, nausea, anorexia, vomiting |
SVT = supraventricular tachycardia, AF = atrial flutter, VT = ventricular tachycardia, AV = atrio-ventricular.
4.3. Digoxin
Digoxin remains the initial drug treatment of choice for fetal tachycardia due to its safety profile and long track record. It acts by inhibiting the sodium-potassium ATPase and is classified as an inotrope rather than an antiarrhythmic drug. Simpson et al. in a review of 127 consecutive fetuses showed that digoxin monotherapy converted most (62%) of the treated non-hydropic fetuses with atrial tachycardias, and 96% survived through the neonatal period [20]. However, the response rates to digoxin in the hydropic fetus were only 20%, suggesting that it is a poor choice in this setting. Serum levels in the fetus range from 70% to 100% of the maternal serum levels. The target maternal digoxin serum levels should be relatively high, between 2.0 and 2.5 ng/mL, mostly because of the higher glomerular filtration rate near term. The measurement of digoxin levels should be performed 6–8 h after the last dose of digoxin. Digoxin therapy is usually initiated with a loading dose either intravenously (over 48–72 h) or orally (over 6–7 days). This is followed by maintenance dosing. Lower doses are indicated in the presence of maternal renal failure.
4.4. Flecainide
When digoxin fails to restore sinus rhythm or in a hydropic fetus with SVT, flecainide is a good next drug of choice. Flecainide can be used in conjunction with Digoxin. It has been shown to successfully convert up to 92% of the non-hydropic and 59% of the hydropic fetuses to sinus rhythm [21,22]. It is a class I C antiarrhythmic agent and acts by blocking the fast sodium channel and slowing conduction velocity in cardiac pathways [23]. Its therapeutic range for serum drug levels lies between 200 ng/mL and 1000 ng/mL. Flecainide has an excellent bioavailability with 95% of the maternal plasma levels achieved in the non-hydropic fetus and 80% of the maternal plasma levels achieved in the hydropic fetus [24]. Therapeutic serum levels are achieved after about 3 days. Hence, conversion into sinus rhythm can be expected 72 h after initiation of therapy but may take up to 14 days. Therapy should be continued beyond 72 h, especially when an initial decrease of fetal heart rate is observed which may represent an early therapeutic response. Paradoxical proarrhythmic effects can be seen with this drug. Initial studies reported proarrhythmic effects with this drug in adults with myocardial infarction [25] and in children with underlying congenital heart defects [26]. However, contrary to adult studies, there is no difference in the incidence of adverse events between patients with normal hearts and patients with CHD and Flecainide is a safe and effective antiarrhythmic medication, even for children with underlying CHD [27].
4.5. Sotalol
Just as with flecainide, sotalol is a consideration for therapy when digoxin fails to restore sinus rhythm or in a hydropic fetus with SVT and can be used in conjunction with Digoxin [28]. However, at least one study with sotalol reported good success rates in fetuses with atrial flutter but a relatively high mortality in the fetus with SVT [29]. These results may support initiating therapy with flecainide before sotalol. Sotalol is a class III antiarrhythmic drug with an additional β-adrenergic blocking effect. It acts on the potassium channels by prolonging the action potential. Its bioavailability and placental passage are excellent with fetal serum levels approaching 70%–100% of maternal levels within 48–72 h of initiation of therapy. It is exclusively excreted renally and adjustment of dosage is necessary in the presence of maternal renal failure. This drug is associated with a proarrhythmic risk. It prolongs the QT interval, which puts the mother at risk for life threatening ventricular arrhythmias such as TdP. Serial ECGs to monitor the QT intervals should be performed before and at regular intervals (daily until therapeutic levels are reached) after initiating therapy with sotalol.
4.6. Amiodarone
When therapy with digoxin, sotalol or flecainide fails to restore sinus rhythm in hydropic fetuses with SVT, amiodarone therapy should be considered as it allows a substantial number of these fetuses to be converted prenatally [30]. Amiodarone is a class III antiarrhythmic drug and acts on the potassium channels resulting in prolongation of the action potential and repolarization. It has a very long half-life of 1–3 months and has an active hepatic metabolite desethylamiodarone. Amiodarone has relatively low fetal bioavailability. It is administered to the mother as a loading dose of 1200 mg/day through the intravenous or oral route followed by a maintenance dose of 600–900 mg/day. The transplacental transfer is poor with fetomaternal ratios for amiodarone between 10 and 40%, with even lower ratios in hydropic fetuses. Side effects of amiodarone therapy in the fetus that have been reported include hypothyroidism [31]. The hypothyroidism is usually transient and resolves after a few months of discontinuing therapy. Monitoring thyroid function of the mother and of the newborn are therefore necessary when administering amiodarone.
4.7. Adenosine
Adenosine given directly to the fetus is an option that can only be used n highly specialized centers with expertise in this technique. Adenosine is an endogenous purine nucleoside with a very short-lasting effect mediated by A1-purine receptors. Intravenous application of 100–200Ug/kg estimated fetal weight resulted in cardioversion within 15–30 s [32]. Adenosine acts by blocking conduction across the AV node and hence terminates SVT that utilize the AV node but fails to terminate atrial tachycardias, atrial flutter and ventricular tachycardias. However, the failure to terminate these arrhythmias may aid in narrowing the differential diagnosis for the tachyarrhythmia.
4.8. Treatment of bradyarrhythmias
Fetal bradycardia rarely warrants intervention. Fetuses with heart rates above 60 beats per minute generally do well and do not require premature delivery or medical intervention in the neonatal period. On the other hand, sustained fetal heart rates of 55 beats per minute, in association with complex congenital heart disease or hydrops may warrant early delivery and immediate intervention in the neonatal period. This intervention may be medical, using drugs that have a positive chronotropic effect such as isoproterenol, or surgical with placement of a pacemaker.
Intervention with medications for treatment of fetal bradycardia is controversial. Therapy with corticosteroids for treatment of immune mediated fetal CHB have been reported in isolated case reports but the evidence favoring this therapy is far from conclusive [5]. Dexamethasone crosses the placenta well and has been associated with the resolution of fetal hydrops. Reported benefits of dexamethasone (4–8 mg/d) include reduction of inflammation, reversal or stabilization of incomplete block, and improvement or resolution of hydrops or endocardial fibroelastosis [33]. Copel et al. evaluated the response of oral dexamethasone 4 mg daily administered to mothers [34]. They reported improvement from complete heart block to second degree heart block or sinus rhythm in 5 cases, resolution of second degree heart block in two patients, and no response in 2 patients. A subsequent larger series incorporating pregnancies reported to the National Lupus Registry failed to show any change in the degree of heart block among those fetuses exposed to steroids but did show a tendency towards improvement in fetal hydrops [35].
Maternal administration of sympathomimetic therapy with terbutaline, ritodrine, isoprenaline, or albuterol has also been advocated [36]. Although these drugs tend to increase the fetal heart rates, their effect on fetal mortality appears to be unchanged. A micro-pacemaker to treat severe fetal bradycardia with comorbid hydrops fetalis is currently undergoing design and testing and may become an option in the future [37].
4.9. Treatment of long QT syndrome
The asymptomatic LQTS fetus with a heart rate of less than third percentile or 2nd degree AV block needs only to be observed. The premature fetus with LQTS and TdP and hydrops need not be delivered emergently as case reports have described transplacental intravenous magnesium and a continuous infusion of lidocaine restoring sinus rhythm and resolving hydrops in premature fetuses [[38], [39], [40]]. Transplacental beta-adrenergic blocking agents such as propranolol have not successfully restored sinus rhythm in TdP and hydrops and have been only partially effective in maintaining normal rhythm, most likely because the transplacental transfer rate is only 25–30% [41]. They can be considered in the fetus with intermittent, very short-lived runs of TdP and no heart failure. Other ways to optimize the outcome of fetal LQTS include withholding QTc-prolonging agents commonly used during pregnancy such as pitocin (used to augment labor), ondansetron (an antiemetic), and erythromycin, and optimizing maternal magnesium, vitamin D and calcium levels in the hope of maintaining sinus rhythm. If it is known that the fetus has LQTS, premature delivery can be postponed if there is 2nd degree AV block or a nonreactive fetal heart rate tracing. Anticipatory postnatal care can improve pre and postnatal outcome.
5. Summary
It is essential to have a thorough understanding of the mechanism of arrhythmia, treatment options, knowledge of drugs being used and awareness of side effects from these drugs for mother and fetus. Paroxysmal tachycardias such as PACs and PVCs are common and do not warrant any therapy. Sustained SVT in the non-hydropic fetus can be treated initially with digoxin. In cases where digoxin fails to treat the tachycardia, flecainide and sotalol can be used and if these drugs are not successful, amiodarone can be used. Digoxin is rarely successful in a hydropic fetus. Fetuses with complete heart block generally carry a good prognosis especially in the absence of structural heart disease or hydrops. Intervention is rarely indicated. Therapy with steroids, sympathomimetic agents and plasmapheresis has been described in small case series of fetuses with heart block and hydrops, but verification with larger series of patients has not been done. Long QT syndrome can be diagnosed prenatally with a careful review of the preceding fetal heart rhythm and rate as most fetuses with TdP will have antecedent sinus bradycardia and frequently episodes of 2nd degree AV block. The risks of premature delivery should be weighed against those of transplacental therapy when treating fetal arrhythmias.
Footnotes
Peer review under responsibility of Indian Heart Rhythm Society.
References
- 1.Ferrer P.L. Fetal arrhythmias. In: Deal B., Wolff G.S., Gelband H., editors. Current concepts in diagnosis and treatment of arrhythmias in infants and children. Futura Publishing Company, Inc; Armonk, NY: 1998. p. 17. [Google Scholar]
- 2.Wacker-Gussmann A., Strasburger J.F., Cuneo B.F., Wakai R.T. Diagnosis and treatment of fetal arrhythmia. Am J Perinatol. 2014;31:617–628. doi: 10.1055/s-0034-1372430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bravo-Valenzuela N.J., Rocha L.A., Machado Nardozza L.M., Júnior E.A. Fetal cardiac arrhythmias: current evidence. Ann Pediatr Cardiol. 2018;11:148–163. doi: 10.4103/apc.APC_134_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krapp M., Kohl T., Simpson J.M. Review of diagnosis, treatment, and outcome of fetal atrial flutter compared with supraventricular tachycardia. Heart. 2003;89:913–917. doi: 10.1136/heart.89.8.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eliasson H., Sonesson S.E., Sharland G. Isolated atrioventricular block in the fetus: a retrospective, multinational, multicenter study of 175 patients. Circulation. 2011;124:1919–1926. doi: 10.1161/CIRCULATIONAHA.111.041970. [DOI] [PubMed] [Google Scholar]
- 6.Izmirly P.M., Saxena A., Kim M.Y. Maternal and fetal factors associated with mortality and morbidity in a multi-racial/ethnic registry of anti-SSA/Ro-associated cardiac neonatal lupus. Circulation. 2011;124:1927–1935. doi: 10.1161/CIRCULATIONAHA.111.033894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donofrio M.T., Moon-Grady A.J., Hornberger L.K. Diagnosis and treatment of fetal cardiac disease: a scientific statement from the American Heart Association. Circulation. 2014;129:2183–2242. doi: 10.1161/01.cir.0000437597.44550.5d. [DOI] [PubMed] [Google Scholar]
- 8.Jaeggi E, Ohman A. Fetal and neonatal arrhythmias. Clin Perinatol. 43;1: 99-112. [DOI] [PubMed]
- 9.Schmidt K.G., Ulmer H.E., Silverman N.H., Kleinman C.S., Copel J.A. Perinatal outcome of fetal complete atrioventricular block: a multicenter experience. J Am Coll Cardiol. 1991;17:1360. doi: 10.1016/s0735-1097(10)80148-2. [DOI] [PubMed] [Google Scholar]
- 10.Jaeggi E.T., Hornberger L.K., Smallhorn J.F. Prenatal diagnosis of complete atrioventricular block associated with structural heart disease: combined experience of two tertiary care centers and review of the literature. Ultrasound Obstet Gynecol. 2005;26:16–21. doi: 10.1002/uog.1919. [DOI] [PubMed] [Google Scholar]
- 11.Izmirly P.M., Saxena A., Kim M.Y. Maternal and fetal factors associated with mortality and morbidity in a multi-racial/ethnic registry of anti-SSA/Ro-associated cardiac neonatal lupus. Circulation. 2011;124:1927–1935. doi: 10.1161/CIRCULATIONAHA.111.033894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jaeggi E.T., Hamilton R.M., Silverman E.D. Outcome of children with fetal, neonatal or childhood diagnosis of isolated congenital atrioventricular block. A single institution's experience of 30 years. J Am Coll Cardiol. 2002;39:130–137. doi: 10.1016/s0735-1097(01)01697-7. [DOI] [PubMed] [Google Scholar]
- 13.Mitchell M., Cuneo B., Etheridge S. Fetal heart rate predictors of LQTS. Circulation. 2012;126:2688–2695. doi: 10.1161/CIRCULATIONAHA.112.114132. [DOI] [PubMed] [Google Scholar]
- 14.Eliasson H., Wahren-Herlenius M., Sonesson S.E. Mechanisms in fetal bradyarrhythmia: 65 cases in a single center analyzed by Doppler flow echocardiographic techniques. Ultrasound Obstet Gynecol. 2011;37:172–178. doi: 10.1002/uog.8866. [DOI] [PubMed] [Google Scholar]
- 15.Cuneo B., Etheridge S., Horigome H. Arrhythmia phenotype during fetal life suggests LQTS genotype: risk stratification of perinatal long QT syndrome. Circ Arrhythm Electrophysiol. 2013;6:946–951. doi: 10.1161/CIRCEP.113.000618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horigome H., Nagashima M., Sumitomo N. Clinical characteristics and genetic background of congenital long QT syndrome diagnosed in fetal, neonatal and infantile life. A nation-wide questionnaire survey in Japan. Circ Arrhythm Electrophysiol. 2010;3:10–17. doi: 10.1161/CIRCEP.109.882159. [DOI] [PubMed] [Google Scholar]
- 17.Simpson J.M., Maxwell D., Rosenthal E. Fetal ventricular tachycardia secondary to long QT syndrome treated with maternal intravenous magnesium: case report and review of the literature. Ultrasound Obstet Gynecol. 2009;34:475–480. doi: 10.1002/uog.6433. [DOI] [PubMed] [Google Scholar]
- 18.Greene A.E., Berul C.I., Donorio M.T. Prenatal diagnosis of long QT syndrome: implications for delivery room and neonatal management. Cardiol Young. 2012;23:141–145. doi: 10.1017/S1047951112000583. [DOI] [PubMed] [Google Scholar]
- 19.Cuneo B.F., Etheridge S.P., Horigome H. Arrhythmia phenotype during fetal life suggests long-QT syndrome genotype: risk stratification of perinatal long-QT syndrome. Circ Arrhythm Electrophysiol. 2013;6:946–951. doi: 10.1161/CIRCEP.113.000618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simpson J.M., Sharland G.K. Fetal tachycardias: management and outcome of 127 consecutive cases. Heart. 1998;79:576–581. [PMC free article] [PubMed] [Google Scholar]
- 21.Ebenroth E.S., Cordes T.M., Darragh R.K. Second-line treatment of fetal supraventricular tachycardia using flecainide acetate. Pediatr Cardiol. 2001;22:483–487. doi: 10.1007/s002460010279. [DOI] [PubMed] [Google Scholar]
- 22.Krapp M., Baschat A.A., Gembruch U. Flecainide in the intrauterine treatment of fetal supraventricular tachycardia. Ultrasound Obstet Gynecol. 2002;19:158–164. doi: 10.1046/j.0960-7692.2001.00562.x. [DOI] [PubMed] [Google Scholar]
- 23.Wacker-Gussmann A., Strasburger J.F., Cuneo B.F., Wakai R.T. Diagnosis and treatment of fetal arrhythmia. Am J Perinatol. 2014;31:617–628. doi: 10.1055/s-0034-1372430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jaeggi E., Fouron J.C., Drblik S.P. Atrial flutter in the fetal period: diagnosis, clinical features, treatment and outcome. J Pediatr. 1998;132:335–339. doi: 10.1016/s0022-3476(98)70455-x. [DOI] [PubMed] [Google Scholar]
- 25.Echt D.S., Liebson P.R., Mitchell L.B. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N Engl J Med. 1991;324:781–788. doi: 10.1056/NEJM199103213241201. [DOI] [PubMed] [Google Scholar]
- 26.Fish F.A., Gillette P.C., Benson D.W., Jr. Proarrhythmia, cardiac arrest and death in young patients receiving encainide and flecainide. The Pediatric Electrophysiology Group. J Am Coll Cardiol. 1991;18:356–365. doi: 10.1016/0735-1097(91)90586-x. [DOI] [PubMed] [Google Scholar]
- 27.Cunningham T., Uzun O., Morris R. The safety and effectiveness of flecainide in children in the current era. Pediatr Cardiol. 2017;38:1633–1638. doi: 10.1007/s00246-017-1707-5. [DOI] [PubMed] [Google Scholar]
- 28.Miyoshi T., Maeno Y., Sago H Japan, Fetal Arrhythmia Group Antenatal antiarrhythmic treatment for fetal tachyarrhythmias: a study protocol for a prospective multicentre trial BMJ. Open. 2017;7 doi: 10.1136/bmjopen-2017-016597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oudijk M.A., Michon M.M., Kleinman C.S. Sotalol in the treatment of fetal dysrhythmias. Circulation. 2000;101:2721–2726. doi: 10.1161/01.cir.101.23.2721. [DOI] [PubMed] [Google Scholar]
- 30.Jouannic J.M., Delahaye S., Fermont L. Fetal supraventricular tachycardia: a role for amiodarone as second-line therapy? Prenat Diagn. 2003;23:152–156. doi: 10.1002/pd.542. [DOI] [PubMed] [Google Scholar]
- 31.Matsumura L.K., Born D., Kunii I.S. Outcome of thyroid function in newborns from mothers treated with amiodarone. Thyroid. 1992;2:279–281. doi: 10.1089/thy.1992.2.279. [DOI] [PubMed] [Google Scholar]
- 32.Kohl T., Tercanli S., Kececioglu D. Direct fetal administration of adenosine for the termination of incessant supraventricular tachycardia. Obstet Gynecol. 1995;85:873–874. doi: 10.1016/0029-7844(94)00314-4. [DOI] [PubMed] [Google Scholar]
- 33.Trucco S.M., Jaeggi E., Cuneo B. Use of intravenous gamma globulin and corticosteroids in the treatment of maternal autoantibody-mediated cardiomyopathy. J Am Coll Cardiol. 2011;57:715. doi: 10.1016/j.jacc.2010.09.044. [DOI] [PubMed] [Google Scholar]
- 34.Copel J.A., Buyon J.P., Kleinman C.S. Successful in utero therapy of fetal heart block. Am J Obstet Gynecol. 1995;173:1384. doi: 10.1016/0002-9378(95)90621-5. [DOI] [PubMed] [Google Scholar]
- 35.Saleeb S., Copel J., Friedman D. Comparison of treatment with fluorinated glucocorticoids to the natural history of autoantibody associated complete heart block: retrospective review of the Research Registry for Neonatal Lupus. Arthritis Rheum. 1999;42:2335. doi: 10.1002/1529-0131(199911)42:11<2335::AID-ANR12>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 36.Groves A.M., Allan L.D., Rosenthal E. Therapeutic trial of sympathomimetics in three cases of complete heart block in the fetus. Circulation. 1995;92:3394–3396. doi: 10.1161/01.cir.92.12.3394. [DOI] [PubMed] [Google Scholar]
- 37.Vest A.N., Zhou L., Huang X. Design and testing of a transcutaneous RF recharging system for a fetal micropacemaker. IEEE Trans Biomed Circuits Syst. 2017;11:336–346. doi: 10.1109/TBCAS.2016.2620805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simpson J.M., Maxwell D., Rosenthal E. Fetal ventricular tachycardia secondary to long QT syndrome treated with maternal intravenous magnesium: case report and review of the literature. Ultrasound Obstet Gynecol. 2009;34:475–480. doi: 10.1002/uog.6433. [DOI] [PubMed] [Google Scholar]
- 39.Cuneo B., Ovadia M., Strasburger J. Prenatal diagnosis and in utero treatment of torsades de pointes associated with long QT syndrome. Am J Cardiol. 2003;91:1395–1398. doi: 10.1016/s0002-9149(03)00343-6. [DOI] [PubMed] [Google Scholar]
- 40.Mitchell J., Cuneo B., Etheridge S.P., Horigome H., Weng H., Benson W. Fetal heart rate predictors of long QT syndrome. Circulation. 2012;126:2688–2695. doi: 10.1161/CIRCULATIONAHA.112.114132. [DOI] [PubMed] [Google Scholar]
- 41.Schneider H., Proegler M. Placental transfer of beta-adrenergic antagonists studied in an in vitro perfusion system of human placental tissue. Am J Obstet Gynecol. 1988;159:42–47. doi: 10.1016/0002-9378(88)90491-7. [DOI] [PubMed] [Google Scholar]