Abstract
Aims
Previous data suggest ventricular high rate episodes (VHREs) on pacemakers are frequent and not associated with overall mortality on short term follow up. We sought to determine whether VHREs are associated with mortality, device upgrade, or change in ejection fraction on long term follow up.
Methods
A single center, retrospective study was performed on 542 patients with permanent pacemakers followed between 2011 and 2013. Follow-up was extended to 2017 for determination of long term outcomes. “True” VHREs were defined as episodes adjudicated to be due to non-sustained ventricular tachycardia on review of electrograms and “false” VHREs were defined as supraventricular arrhythmias or noise.
Results
VHRE occurred in 202(37.2%)/542 included patients. True VHRE was detected in 148(27.3%) while 54(10%) had false VHRE. The mean age of the population was 72 ± 15 years and 46% were women. Mean follow-up was 3.3 ± 1.4 years. The baseline characteristics of the true, false and no VHRE patients were similar. There was no difference in all-cause mortality between groups (27% mortality in true VHRE, 33% in false VHRE and 29% in no VHRE). Furthermore, there was no difference between groups with regards to any device upgrade (5% any upgrades in the VHRE, 9% in false VHRE and 5% in no VHRE.) On follow up, EF declined in all groups: −4% vs −2.4% vs −3.5% for true, false and no VHRE.
Conclusion
VHRE are frequently encountered on remote monitoring of pacemakers and not associated with increased risk of mortality or need for downstream device upgrade.
Keywords: Non-sustained ventricular tachycardia, Pacemakers, Remote monitoring, Mortality
1. Introduction
The use of pacemakers has been steadily growing with 2.9 million patients having received pacemakers between 1993 and 2009 and approximately 200,000 pacemakers implanted annually in the United States [1,2]. In addition to offering pacing therapy, these devices may also be used to detect the frequency, time of onset, duration, and rate characteristics of both atrial and ventricular arrhythmias [3]. These high quality electrograms have the potential to provide robust data which could improve the clinical outcomes in these patients [4].
Remote monitoring of pacemakers allows the detection of both high rate atrial episodes and ventricular episodes earlier. While high rate atrial episodes are important to recognize because atrial fibrillation is a prominent risk factor for stroke [[5], [6], [7]], ventricular high rate events (VHRE) may also occur and identify patients with non-sustained ventricular tachycardia (NSVT) or sustained ventricular tachycardia. The prognostic significance of NSVT, however, has been shown to be variable depending on the clinical setting. NSVT is associated with an increase in mortality in patient with structural heart disease but is generally considered to be benign in patients with a normal left ventricular function [8,9]. However, the apparent clinical significance of NSVT has been suggested mostly on the basis of external ambulatory monitoring while the significance of NSVT when detected on routine remote monitoring using intracardiac devices and whether they are associated with any differences in long-term outcomes or mortality is not explicit.
Thus, we performed a study to ascertain the prevalence, and impact of VHR on mortality, and on clinical outcomes such as device upgrade, change in ventricular function and need for a change in management.
2. Material and methods
2.1. Study population and data extraction
We retrospectively screened the electronic medical records of 542 patients who had Medtronic pacemaker implants (Medtronic, Minneapolis, MN, USA) or pack changes between July 2011 and November 2013 and followed them up to assess long term effects. The study was approved by the Mayo Clinic Institutional Review Board. Patients who were undergoing remote monitoring via CareLink by Medtronic and were receiving follow up care at our center were included. The inclusion criteria comprised of age >18 years, presence of a permanent pacemaker, follow up of device with remote monitoring and the presence of research authorization.
Data from the device interrogations for routine pacemaker monitoring were extracted and reviewed. VHRE was defined as ventricular high rate events that were detected by the pacemaker software based on the Medtronic detection algorithm. Among these, “true VHRE” was defined as ventricular high rate events due to non-sustained ventricular tachycardia as per review of available intracardiac tracings by device nurses and physicians. However, if the detected VHR episode was deemed to be due to rapidly conducted atrial tachycardia or atrial fibrillation, these were designated as “false VHRE”. Care was taken to include only NSVT as true VHRE and any VHRE which could not be specified on the basis of pacemaker data was categorized as false VHRE (Fig. 1). Recognizing that patients could have both true and false VHRE, the true VHRE group comprised of patients with at least one true VHRE. The false VHRE group comprised of patients with only false VHRE.
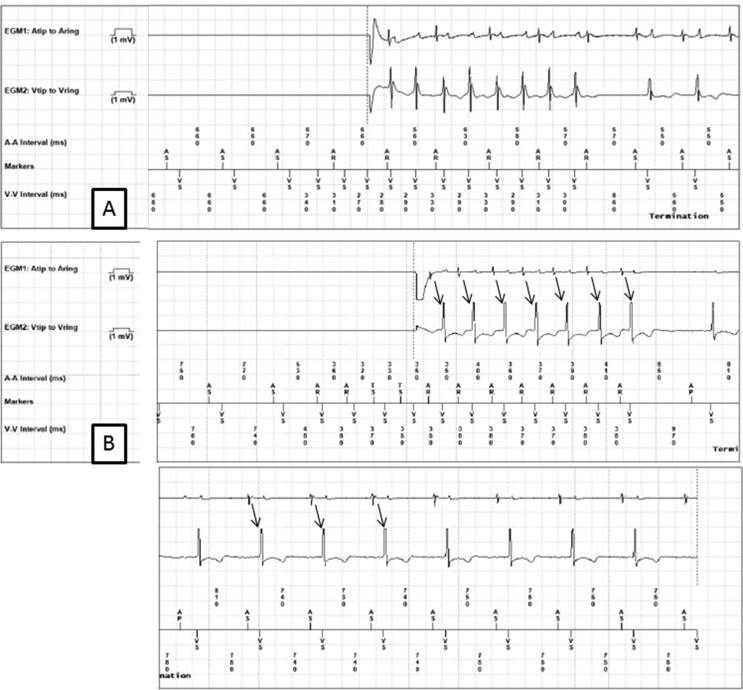
Fig. 1.
Panel A: The ventricular channel (Vtip to Vring) shows ventricular sensing at a cycle length of 290 ms, and is dissociated from atrial activity on the atrial channel (Atip to Aring). Far field ventricular activation is visible on the atrial channel. This was classified as a true VHR episode. Panel B: The ventricular channel shows ventricular sensing at a cycle length of 360 ms. The atrial channel shows 1:1 atrioventricular activity, which persists after termination of the episode (thin black arrows). This was classified as a false VHR episode.
Characteristics of the VHREs including number of VHRE transmissions, rate (beats per minute), and longest duration of VHR were extracted from device interrogation reports. Medical records were reviewed for demographic data, comorbidities, echocardiographic data, medication profiles, anti-coagulation status, electrocardiogram data and indication for permanent pacemaker implantation. Outcome data included all-cause mortality, changes in management following VHREs, device upgrade and change in ejection fraction (EF).
2.2. Statistical methods
Descriptive statistics were used to describe the demographic and clinical characteristics of the cohort. Categorical variables are presented as percentages and continuous variables as mean ± standard deviation (SD). Comparisons of continuous variables between groups were performed with t tests and of categorical variables by the Chi square test. Subgroup analysis was performed in patients with low EF and without presence of LBBB. For the outcomes of death and device upgrade, we constructed Kaplan-Meier survival curves and used the Log-Rank test to assess differences between the groups. A p-value less than 0.05 was considered significant. Statistical analysis was performed using JMP version 9.0.1 (SAS Institute Inc., Cary, North Carolina).
3. Results
3.1. Patient characteristics
A total of 542 patients were included and ventricular high rate events occurred in 202 (37.2%) of these patients. Amongst these, true VHRE was detected in 148 (27.3%) patients while 54 (10%) had false VHRE as per defined criteria.
The mean age of the population was 72 ± 15 years and 46% were women. The baseline clinical and ECG characteristics in the study cohort have been summarized in Table 1. The true VHRE and no VHRE groups were similar with respect to age, sex and comorbidities except for frequency of LBBB. The false VHRE group had lower frequency of dilated cardiomyopathy (0 vs 5%, p = 0.02) and diabetes mellitus (12 vs 30%, p = 0.005) compared to the no VHRE group. The false VHRE group had lower amiodarone (0 vs 6%, p = 0.02) and other antiarrhythmic drug use (1 vs 10%, p = 0.019) compared to the no VHRE group. The baseline characteristics of the true, false and no VHRE patients were similar in other aspects.
Table 1.
Baseline characteristics of the entire cohort, and the true, false, any and no VHRE groups.
| Entire cohort (N = 542) | No VHRE (N = 340) | True VHRE (N = 148) |
False VHRE (N = 54) |
Any VHRE (N = 202) |
||||
|---|---|---|---|---|---|---|---|---|
| p-valuea | p-valuea | p-valuea | ||||||
| Age | 73 ± 14.97 | 73.5 ± 14.7 | 71.9 ± 16.41 | 0.15 | 72.7 ± 11.96 | 0.34 | 72.1 ± 15.4 | 0.29 |
| Gender(male) | 295 (54%) | 177(52%) | 85(57%) | 0.26 | 33(61%) | 0.21 | 117(58%) | 0.15 |
| Total Sends | 4.2 ± 3.7 | 3.9 ± 4.2 | 4.7 ± 2.8 | 0.99 | 3.9 ± 2.54 | 0.38 | 4.5 ± 2.8 | 0.96 |
| Ejection fraction | 57.3 ± 11 | 57.8 ± 11 | 56.5 ± 11 | 0.14 | 56.1 ± 10.3 | 0.15 | 56.4 ± 11 | 0.09 |
| Atrial fibrillation | 337(62%) | 215(63%) | 83(56%) | 0.15 | 39(72%) | 0.18 | 122(60%) | 0.58 |
| Coronary artery disease | 245(45%) | 157(46%) | 66(44%) | 0.77 | 22(40%) | 0.47 | 55(43.7%) | 0.60 |
| Heart Failure | 234(43%) | 149(43%) | 66(44%) | 0.85 | 19(35%) | 0.24 | 85(42%) | 0.75 |
| Dilated cardiomyopathy | 28(5%) | 18(5%) | 10(6.7%) | 0.52 | 0 | 0.020a | 10(4.9%) | 0.88 |
| Diabetes Mellitus | 152(27%) | 104(30%) | 41(27%) | 0.53 | 7(12%) | 0.005a | 48(23.8%) | 0.09 |
| Hypertension | 423(77%) | 274(80%) | 108(72%) | 0.07 | 41(75%) | 0.46 | 149(74.1%) | 0.09 |
| Hyperlipidemia | 385(70%) | 247(72%) | 99(66%) | 0.22 | 39(72%) | 0.97 | 138(68.6%) | 0.35 |
| Ischemic cardiomyopathy | 25(4%) | 12(5%) | 4(2.7%) | 0.19 | 3(5%) | 0.93 | 7(3.4%) | 0.32 |
| Betablocker | 275 (50%) | 177(52%) | 70(47%) | 0.33 | 28(51%) | 0.98 | 98(48.7%) | 0.46 |
| ACEI/ARB | 272(50%) | 169(49%) | 76(51%) | 0.74 | 27(50%) | 0.97 | 102(50.7%) | 0.81 |
| Aspirin | 311(57%) | 189(55%) | 90(60%) | 0.28 | 32(59%) | 0.61 | 121(60.1%) | 0.29 |
| Statins | 290(53%) | 183(53%) | 82(55%) | 0.75 | 25(46%) | 0.30 | 106(52.7%) | 0.81 |
| Diuretic | 256(47%) | 163(47%) | 72(48%) | 0.89 | 21(38%) | 0.21 | 93(46.2%) | 0.71 |
| Amiodarone | 26(5%) | 22(6%) | 4(2.7%) | 0.06 | 0 | 0.010a | 26(4.8%) | 0.020a |
| Digoxin | 39(7%) | 26(7.6%) | 8(5%) | 0.36 | 5(9%) | 0.69 | 12(6.4%) | 0.61 |
| Other antiarrhythmics | 44(8%) | 35(10%) | 8(5.4%) | 0.07 | 1(1%) | 0.02 | 44(8.1%) | 0.01 |
| Calcium channel blocker | 67(12%) | 37(10%) | 23(15%) | 0.16 | 7(12%) | 0.66 | 30(14.9%) | 0.17 |
| Anticoagulants | 213(39%) | 134(39%) | 56(37%) | 0.15 | 23(42%) | 0.45 | 78(38.8%) | 0.09 |
| QRS | 114.14 ± 30 | 112.5 ± 30 | 118.1 ± 30.5 | 0.96 | 113.4 ± 31.6 | 0.57 | 116.8 ± 30.8 | 0.93 |
| LBBB | 55(11%) | 25(8.4%) | 26(21%) | 0.001a | 4(9%) | 0.95 | 55(11%) | 0.003a |
p value compared with no VHRE.
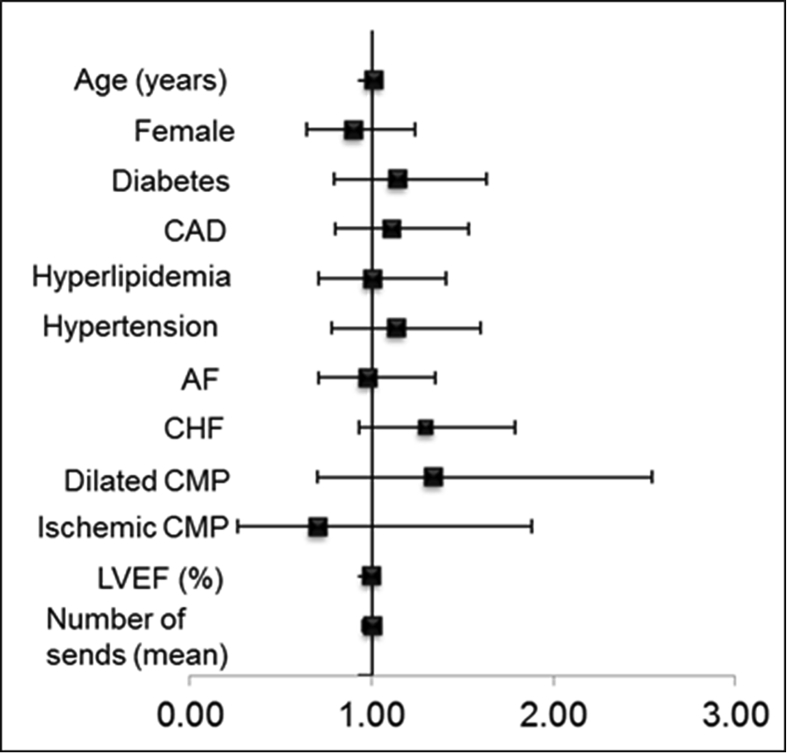
Baseline echocardiography for the entire cohort showed mean 57 ± 10%, with no difference between the true, false and no VHR groups. On univariate analysis, none of the baseline characteristics were associated with the outcome of true VHR (Fig. 2). Baseline ECG for the entire cohort showed mean QRS duration 114 ± 30 ms, and 11% patients had LBBB. Left bundle branch block was more frequent in the true VHRE group in comparison to no VHRE group (21% vs 8.4%, p = 0.001).
Fig. 2.
Forest plot depicting hazard ratios (HR), for the outcome of true VHRE (Univariate analysis). Horizontal bars, 95% confidence intervals (CI), all p > 0.05.
3.2. Device characteristics
The most common indication for permanent pacemaker implantation was sinus node dysfunction (46%) followed by atrioventricular block (AVB) (39%). The groups were similar in terms of indication for device implant, with the exception of lower frequency of sinus node dysfunction in the true VHRE group compared to the no VHRE group (41% vs 48%, p = 0.01).
There were a mean of 4.2 ± 3.7 device transmissions for the entire cohort, with no differences between the groups. Among patients with true VHRE, the mean heart rate was 194 ± 21 bpm. The longest episodes were 5.4 ± 4.2 s in duration, with an average of 11 ± 6 beats. Episodes were brief and non-sustained, and only 3.6% patients had episodes longer than 10 s (Table 2).
Table 2.
Characteristics of VHRE transmissions.
| Variable | Overall (N = 542) |
|---|---|
| VHRE, N(%) | 202 (37%) |
| Mean Number of VHR Sends (SD) | 4.5 ± 2.8 |
| True VHRE, n (%) |
148 (27%) |
| ⁃Mean Number of True VHR (SD) | 4.7 ± 2.8 |
| ⁃Longest Duration of VHR in seconds (SD) | 5.4 ± 4.2 |
| ⁃Number of beats (SD) | 11 ± 5.6 |
| ⁃Rate of VHRE (beats per minute) (SD) |
194 ± 21.3 |
| False VHR, n (%) | 54 (10%) |
| ⁃Mean Number of False VHRE Sends (SD) | 3.9 ± 2.5 |
The average right ventricular pacing percentage for the entire cohort was 53%, with no difference between the groups (p = 0.059). Fifty-five percent patients had >40% right ventricular pacing, and there was no difference in the frequency of true VHR among patients with <40 versus >40% right ventricular pacing (p = 0.73).
3.3. Outcomes
The mean follow-up for the entire cohort was 3.3 ± 1.4 years and median was 4 years (interquartile range: 2.7–4.2years). There was no difference between the groups in terms of follow up duration.
3.3.1. Mortality
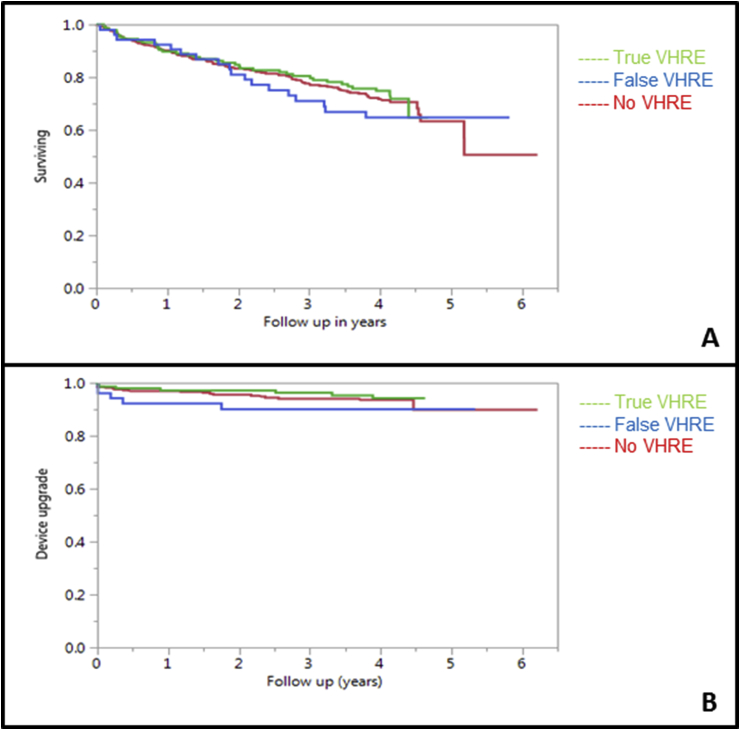
The overall mortality for the entire cohort was 29%, with 27% mortality in the true VHRE group, 33% in false VHRE and 29% in the no VHRE group. There was no difference in all-cause mortality between the groups. The Kaplan Meier curves are depicted in Fig. 3.
Fig. 3.
Panel A: Kaplan Meier Curve for survival analysis, depicting all-cause mortality for the true, false and no VHRE groups. p = 0.56 (log rank test) Panel B: Kaplan Meier Curve depicting any device upgrade for the true, false and no VHRE groups. p = 0.46 (log rank test).
There was no association of true VHR rates >200 bpm with all-cause mortality (p = 0.38), and no association of number of VHR episodes with all-cause mortality (p = 0.16).
3.3.2. Device upgrade
Any device upgrade occurred in 5% of the entire cohort, with 5% any upgrades in the true VHRE group, 9% in false VHRE group and 5% in the no VHRE group. There was no difference between the groups with regards to any device upgrade.
3.3.3. Ejection fraction
On follow up, EF declined in all groups: −4% vs −2.4% vs −3.5% for true, false and no VHRE respectively (p = NS for true vs no VHRE & false vs no VHRE) (Table 3).
Table 3.
Outcomes.
| Entire cohort | No VHRE | True VHRE | False VHRE | Any VHRE | |
|---|---|---|---|---|---|
| Death (N) | 155(29%) | 98(29%) | 39(27%) | 18(33%) | 57(28%) |
| Upgrade | 32(5%) | 20(5%) | 7(5%) | 5(9%) | 12(6%) |
| Upgrade ICD | 5(1%) | 3(1%) | 1(1%) | 1(2%) | 2(1%) |
| Upgrade CRT-P | 16(3%) | 11(3%) | 2(1%) | 3(5%) | 5(2.5%) |
| Upgrade CRT-D | 12(2%) | 7(2%) | 4(3%) | 1(2%) | 5(2.4%) |
| Follow up EF | 53.3 ± 12 | 53.6 ± 11.5 | 52.5 ± 13.2 | 53.7 ± 11 | 53(12.5%) |
3.3.4. Management
In response to detection of true VHRE episodes, there was no change in management in 91% of the patients. 7(4.7%) patients underwent a clinic visit and 5 (3.4%) underwent investigations such as Holter monitoring or echocardiography or had a change in management based on the presence of VHR such as addition of a beta-blocker. There was no significant difference in mortality between patients that underwent additional diagnostic testing or change in management and those who did not (15% vs 21%, p = 0.17).
3.4. Subgroup analysis
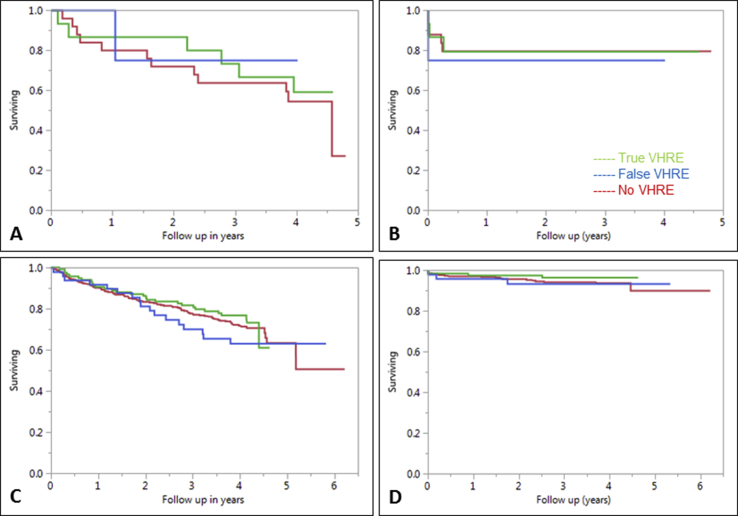
As patients with reduced EF are at greater risk of sudden cardiac death (SCD), we performed a subgroup analysis among patients with EF<40%. The all-cause mortality was greater in the low EF groups compared to the entire cohort, but there was no difference in the all-cause mortality rate between the true VHRE (40%) and no VHRE (44%) groups (Supplemental Fig. 1). The device upgrade rates were more frequent in the low EF group compared to the entire cohort, with no difference between the true VHRE (20%) and no VHRE (21%) groups (Table 4).
Table 4.
Outcomes in subgroup of patients with EF ≤ 40%.
| Subgroup analysis on patients with low EF<=40 | True VHR | No VHR |
|---|---|---|
| Death | 6(40%) | 11(44%) |
| Upgrade | 3(20%) | 5(21%) |
| Upgrade ICD | 1(6.6%) | 0 |
| Upgrade CRT-P | 0 | 3(12%) |
| Upgrade CRT-D | 2(12%) | 2(8%) |
Since the frequency of LBBB was high in the true VHRE groups, there was concern that these patients may represent false VHRE due to supraventricular tachycardias with a wide QRS. Hence, we performed a sensitivity analysis by excluding all patients with LBBB on ECG. The all-cause mortality rate for the no LBBB-true VHRE group was 24% (Supplemental Fig. 1). There was no difference between the no LBBB-true VHRE group and no VHRE group. The device upgrade rate for the no LBBB-true VHRE group was 3%. There was no difference between the no LBBB-true VHRE group and no VHRE group.
4. Discussion
The primary finding of our study is that VHRE alerts is a common discovery (prevalence of 37.2%) among patients through routine remote monitoring of pacemakers and does not seem to affect survival or lead to the requirement of a device upgrade on long term follow up.
The prognostic significance of NSVT which has been defined as true VHRE in this study with relation to various cardiovascular diseases has been extensively studied. NSVT has been associated with increased mortality in patients with left ventricular hypertrophy and following myocardial infarction [10,11]. However, in otherwise normal patients with no structural heart disease, their significance remains unknown [12,13].
Our results demonstrating no relationship of true VHRE to mortality are, however, in concordance with previous studies which showed that NSVT was not associated with increased risk of death in patients without ischemia or structural heart disease [14]. Kennedy et al. demonstrated that asymptomatic healthy individuals had no increased risk of death on comparison to a healthy U.S. population on long term follow up for 10 years [15]. Singh et al. indicated that NSVT is frequently seen in patients with heart failure but is not an independent risk factor for all-cause mortality or sudden death [16].
Further on remote monitoring of pacemakers, Faber et al. demonstrated 25.7% prevalence of NSVT in 210 pacemaker implanted patients [17]. In 1125 patients with implanted pacemakers, Seth et al. verified a similar 20% incidence of NSVT with no association with mortality on long term follow up [18]. Gabriels et al. also implied the same in a cohort of 262 patients showing no relationship of NSVT to mortality [19].
We further assessed if a patient having episodes of NSVT will require a device upgrade in the future to an Implantable Cardioverter Defibrillator (ICD), cardiac resynchronization therapy - pacemaker (CRT-P) or a cardiac resynchronization therapy - defibrillator (CRT-D) due to worsening morbidity and risk of SCD. There was no significant difference in the need for a device upgrade in comparison to patients with no episodes of ventricular high rate events thus further consolidating the benign nature of the episodes. To the best of our knowledge, this is the first study to evaluate the downstream requirement of device upgrades in patients with NSVT.
A sensitivity analysis was further done as the frequency of LBBB was high in the true VHRE groups (21% vs. 8.4%, p = 0.001) which led to a concern that number of NSVTs were actually being contaminated by wide complex tachycardias from an underlying LBBB. However, there was no significant difference in mortality or device upgrade in no LBBB-true VHRE group and the no VHRE group.
Both true VHRE and no VHRE groups of patients had a decline in follow up EF but there was no significant difference among them. The decline in EF in both groups may be due to the effects of right ventricular pacing on ventricular function and dyssynchrony [20]. Jacobsson et al. demonstrated that VHREs predict increased mortality in heart failure patients treated with cardiac resynchronization therapy [21]. This could be explained by the fact that patients with CRT generally have lower EF, which could be responsible for the increased mortality. Our population was limited to pacemaker patients, who generally have a higher EF (mean of 57 ± 11%). However, our subgroup analysis in patients with low EF did not show a significant difference in mortality, nor a requirement of a device upgrade in future, between the patients with and without VHRE.
The vast majority of patients (91%) with NSVT did not undergo any change in management such as additional diagnostic testing or addition of a medication such as a beta blocker. The absence of any difference in mortality between the groups that underwent change in management and those who did not, demonstrates the low yield of diagnostic testing in these patients. Notably, beta blockers have been shown to be effective for the treatment of NSVT in patients with a history of heart failure or myocardial infarction [22]. It is important to point out that 50% of the entire cohort was already on beta blocker therapy with no difference between the groups.
On further analysis of the epidemiological data, no clinical predictors were found to be associated with the development of VHREs, including NSVT; and the number and characteristics of VHRE episodes did not differ between groups.
5. Limitations
The retrospective design along with the single institution nature of our study is the primary limitation. Cardiac vs non-cardiac causes of death for all subjects could not be determined for all subjects. All the pacemakers that were analyzed were Medtronic which could incorporate selection bias of one particular device or device detection algorithm. Further, our definition of NSVT is limited by the detection algorithms of Medtronic and it is plausible that a lower detection rate could produce different results.
Also, as our study included patients with NSVT on routine monitoring of pacemakers, it may not be applicable to patients with NSVT in other settings. It is significant to note that most episodes of VHR were relatively brief. It is uncertain whether our findings apply to patients with significantly longer episodes. Finally, we included patients with either first time device implantation or pack changes. It is possible that survival bias could affect results among patients included after pack changes.
6. Conclusion
Ventricular high rate events which are frequently encountered on routine remote monitoring of pacemakers are not associated with increased risk of mortality. The presence of VHRE also did not result an increased need for device upgrade in the population, supporting the notion that these are benign findings. Diagnostic testing following the detection of NSVT on routine remote monitoring of pacemakers is of low yield. Further prospective studies are required to account for the limitations and shed light on the true effect of VHRE on mortality.
Footnotes
Peer review under responsibility of Indian Heart Rhythm Society.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ipej.2018.12.002.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Supplemental Fig. 1.
Panel A: Kaplan Meier survival curves depicting all cause mortality in the subgroup of patients with ejection fraction ≤40%, p = 0.73 (log-rank test for all comparisons). Panel B: Kaplan Meier curves depicting device upgrades in the subgroup of patients with ejection fraction ≤40%, p = 0.73. Panel C: Kaplan Meier survival curves depicting all cause mortality in the subgroup of patients with left bundle branch block, p = 0.50. Panel D: Kaplan Meier curves depicting device upgrades in the subgroup of patients with left bundle branch block, p = 0.57.
References
- 1.Greenspon A.J., Patel J.D., Lau E., Ochoa J.A., Frisch D.R., Ho R.T., Pavri B.B., Kurtz S.M. Trends in permanent pacemaker implantation in the United States from 1993 to 2009: increasing complexity of patients and procedures. J Am Coll Cardiol. 2012;60(16):1540–1545. doi: 10.1016/j.jacc.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 2.Mond H.G., Proclemer A. The 11th world survey of cardiac pacing and implantable cardioverter-defibrillators: calendar year 2009–A world society of Arrhythmia's project. Pacing Clin Electrophysiol. 2011;34(8):1013–1027. doi: 10.1111/j.1540-8159.2011.03150.x. [DOI] [PubMed] [Google Scholar]
- 3.Crossley G.H., Chen J., Choucair W., Cohen T.J., Gohn D.C., Johnson W.B., Kennedy E.E., Mongeon L.R., Serwer G.A., Qiao H. Clinical benefits of remote versus transtelephonic monitoring of implanted pacemakers. J Am Coll Cardiol. 2009;54(22):2012–2019. doi: 10.1016/j.jacc.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Hindricks G., Taborsky M., Glikson M., Heinrich U., Schumacher B., Katz A., Brachmann J., Lewalter T., Goette A., Block M. Implant-based multiparameter telemonitoring of patients with heart failure (IN-TIME): a randomised controlled trial. Lancet. 2014;384(9943):583–590. doi: 10.1016/S0140-6736(14)61176-4. [DOI] [PubMed] [Google Scholar]
- 5.Crossley G.H., Boyle A., Vitense H., Chang Y., Mead R.H. The CONNECT (Clinical Evaluation of Remote Notification to Reduce Time to Clinical Decision) trial: the value of wireless remote monitoring with automatic clinician alerts. J Am Coll Cardiol. 2011;57(10):1181–1189. doi: 10.1016/j.jacc.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 6.Varma N., Epstein A.E., Irimpen A., Schweikert R., Love C. Efficacy and safety of automatic remote monitoring for implantable cardioverter-defibrillator follow-up: the Lumos-T Safely Reduces Routine Office Device Follow-up (TRUST) trial. Circulation. 2010;122(4):325–332. doi: 10.1161/CIRCULATIONAHA.110.937409. [DOI] [PubMed] [Google Scholar]
- 7.Wolf P.A., Abbott R.D., Kannel W.B. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22(8):983–988. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 8.Katritsis D.G., Zareba W., Camm A.J. Nonsustained ventricular tachycardia. J Am Coll Cardiol. 2012;60(20):1993–2004. doi: 10.1016/j.jacc.2011.12.063. [DOI] [PubMed] [Google Scholar]
- 9.Katritsis D.G., Camm A.J. Nonsustained ventricular tachycardia: where do we stand? Eur Heart J. 2004;25(13):1093–1099. doi: 10.1016/j.ehj.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 10.Anderson K.P., DeCamilla J., Moss A.J. Clinical significance of ventricular tachycardia (3 beats or longer) detected during ambulatory monitoring after myocardial infarction. Circulation. 1978;57(5):890–897. doi: 10.1161/01.cir.57.5.890. [DOI] [PubMed] [Google Scholar]
- 11.Cheema A.N., Sheu K., Parker M., Kadish A.H., Goldberger J.J. Nonsustained ventricular tachycardia in the setting of acute myocardial infarction: tachycardia characteristics and their prognostic implications. Circulation. 1998;98(19):2030–2036. doi: 10.1161/01.cir.98.19.2030. [DOI] [PubMed] [Google Scholar]
- 12.Monserrat L., Elliott P.M., Gimeno J.R., Sharma S., Penas-Lado M., McKenna W.J. Non-sustained ventricular tachycardia in hypertrophic cardiomyopathy: an independent marker of sudden death risk in young patients. J Am Coll Cardiol. 2003;42(5):873–879. doi: 10.1016/s0735-1097(03)00827-1. [DOI] [PubMed] [Google Scholar]
- 13.Spirito P., Rapezzi C., Autore C., Bruzzi P., Bellone P., Ortolani P., Fragola P.V., Chiarella F., Zoni-Berisso M., Branzi A. Prognosis of asymptomatic patients with hypertrophic cardiomyopathy and nonsustained ventricular tachycardia. Circulation. 1994;90(6):2743–2747. doi: 10.1161/01.cir.90.6.2743. [DOI] [PubMed] [Google Scholar]
- 14.Montague TJ, McPherson DD, MacKenzie BR, Spencer CA, Nanton MA, Horacek BM, Rigby SM, Black SA: Frequent ventricular ectopic activity without underlying cardiac disease: analysis of 45 subjects. Am J Cardiol, 52(8):980-984. [DOI] [PubMed]
- 15.Kennedy H.L., Whitlock J.A., Sprague M.K., Kennedy L.J., Buckingham T.A., Goldberg R.J. Long-term follow-up of asymptomatic healthy subjects with frequent and complex ventricular ectopy. N Engl J Med. 1985;312(4):193–197. doi: 10.1056/NEJM198501243120401. [DOI] [PubMed] [Google Scholar]
- 16.Singh S.N., Fisher S.G., Carson P.E., Fletcher R.D. Prevalence and significance of nonsustained ventricular tachycardia in patients with premature ventricular contractions and heart failure treated with vasodilator therapy. Department of Veterans Affairs CHF STAT Investigators. J Am Coll Cardiol. 1998;32(4):942–947. doi: 10.1016/s0735-1097(98)00338-6. [DOI] [PubMed] [Google Scholar]
- 17.Faber T.S., Gradinger R., Treusch S., Morkel C., Brachmann J., Bode C., Zehender M. Incidence of ventricular tachyarrhythmias during permanent pacemaker therapy in low-risk patients results from the German multicentre EVENTS study. Eur Heart J. 2007;28(18):2238–2242. doi: 10.1093/eurheartj/ehm242. [DOI] [PubMed] [Google Scholar]
- 18.Seth N., Kaplan R., Bustamante E., Kulkarni C., Subacius H., Rosenthal J.E., Passman R. Clinical significance of nonsustained ventricular tachycardia on routine monitoring of pacemaker patients. Pacing Clin Electrophysiol : PACE. 2015;38(8):980–988. doi: 10.1111/pace.12632. [DOI] [PubMed] [Google Scholar]
- 19.Gabriels J., Wu M., Rosen L., Patel A., Goldner B. Clinical significance of nonsustained ventricular tachycardia on stored electrograms in permanent pacemaker patients. Pacing Clin Electrophysiol: PACE. 2016;39(12):1335–1339. doi: 10.1111/pace.12968. [DOI] [PubMed] [Google Scholar]
- 20.Tops L.F., Schalij M.J., Bax J.J. The effects of right ventricular apical pacing on ventricular function and dyssynchrony: implications for therapy. J Am Coll Cardiol. 2009;54(9):764–776. doi: 10.1016/j.jacc.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 21.Jacobsson J., Reitan C., Platonov P.G., Borgquist R. Ventricular high-rate episodes predict increased mortality in heart failure patients treated with cardiac resynchronization therapy. Scand Cardiovasc J. 2015;49(1):20–26. doi: 10.3109/14017431.2015.1006245. [DOI] [PubMed] [Google Scholar]
- 22.Friedman L.M., Byington R.P., Capone R.J., Furberg C.D., Goldstein S., Lichstein E. Effect of propranolol in patients with myocardial infarction and ventricular arrhythmia. J Am Coll Cardiol. 1986;7(1):1–8. doi: 10.1016/s0735-1097(86)80250-9. [DOI] [PubMed] [Google Scholar]