Abstract
Our awareness and appreciation of the many regulatory roles of RNA have dramatically increased in the past decade. This understanding, in addition to the impact of RNA in many disease states, has renewed interest in developing selective RNA-targeted small molecule probes. However, the fundamental guiding principles in RNA molecular recognition that could accelerate these efforts remain elusive. While high-resolution structural characterization can provide invaluable insight, examples of well-characterized RNA structures, not to mention small molecule:RNA complexes, remain limited. This Perspective provides an overview of the current techniques used to understand RNA molecular recognition when high-resolution structural information is unavailable. We will place particular emphasis on a new method, pattern recognition of RNA with small molecules (PRRSM), that provides rapid insight into critical components of RNA recognition and differentiation by small molecules as well as into RNA structural features.
Graphical Abstract
The need to understand RNA molecular recognition has become increasingly pressing given the newly discovered roles for RNA in multiple disease states.1–7 These studies imply that selective RNA targeting could be a potential treatment method, yet nearly all medicinal, Food and Drug Administration (FDA)-approved compounds target proteins.8–11 Distinct from proteins, which are largely recognized through defined ligand binding pockets, identifying selective ligands for RNA is challenging due to the ability of RNA to sample multiple energetically stable conformations, the minimal chemical property differences among the four nucleobases, and the negatively charged backbone.11–18 In part because of these challenges, we are only beginning to discover the guiding principles behind the differential affinity and selectivity of small molecules for RNA motifs, including the topological and sequence dependence of small molecule:-RNA interactions. It is clear that the use of several complementary methods will be needed to elucidate principles of RNA molecular recognition, which will in turn yield insight into the fundamental biology, disease relevance, and therapeutic targeting of RNA.
Numerous experimental approaches have been developed to examine the interactions of RNA with small molecules as well as biomacromolecules.13,19–22 Relevant methods can focus on RNA structure and dynamics as well as biophysical measurements of RNA interactions. High-resolution structural characterization, generally through nuclear magnetic resonance (NMR) spectroscopy or X-ray diffraction, can be highly informative but also difficult and occasionally impossible for some RNA molecules and complexes.23–27 Several additional techniques have been employed to probe RNA structure and recognition. These include, for example, two-dimensional (2D) and three-dimensional (3D) structure prediction that can be enhanced via chemical probing.28–33 Small-angle X-ray scattering (SAXS) and cryo-electron microscopy (cryo-EM) can experimentally characterize RNA structural ensembles.34–37 RNA dynamics have been investigated with techniques such as NMR spectroscopy and Förster resonance energy transfer (FRET).23,38–41 Methods for directly investigating RNA interactions are numerous and varied, ranging from large-scale screening methods to those that allow careful calculation of binding constants along with kinetic and thermodynamic parameters. A summary of select methods that have been used to investigate recognition and select references can be found in Table 1. While often highly successful in determining specific RNA binding partners, these techniques do not typically provide binding profiles for multiple RNA sequences and small molecules simultaneously, which would aid more rapid elucidation of general binding principles.
Table 1.
Methods for Assessing Small Molecule:RNA Interactions
| method | obtainable data | example refs |
|---|---|---|
| isothermal titration calorimetry (ITC) | binding constants, thermodynamic binding parameters, stoichiometry | 149–152 |
| thermal denaturing studies | small molecule stabilization of RNA structure | 153, 154 |
| surface plasmon resonance (SPR) | binding constants, kinetic parameters | 155–159 |
| Förster resonance energy transfer (FRET) | ligand-induced conformational change, binding constants | 20, 38, 103 |
| second harmonic generation (SHG) | ligand-induced conformational changes, binding constants | 160, 161 |
| selective 2′-hydroxyl acylation analyzed by primer extension (SHAPE) | site-specific ligand-induced conformational changes | 162–165 |
| nuclear magnetic resonance (NMR) | secondary and tertiary structures of RNA, insight into potential binding sites and conformational changes, binding constants | 23, 40, 100, 101, 110, 111, 118, 147, 166–169 |
| X-ray diffraction | secondary and tertiary RNA structures, small molecule binding sites, insight into targetable structures within the RNA | 26, 27, 49, 170–174 |
| fluorophore incorporation | site-specific binding interactions, binding constants | 175–180 |
| indicator displacement assay (IDA) | binding constants | 181–185 |
| intrinsic ligand fluorescence | binding constants | 159, 186–188 |
| mass spectroscopy | stoichiometry, binding constants | 161, 189–191 |
| microscale thermophoresis | binding constants | 192 |
| small molecule microarray (SMM) | specific small molecule ligands | 164, 193, 194 |
| two-dimensional combinatorial screening (2DCS) | specific small molecule RNA binders, insight into RNA-targeted small molecule chemical space | 12, 138, 195–200 |
Our research in this area has included the implementation of a molecular-scale pattern recognition technique to reveal small molecule:RNA interaction principles, which we termed pattern recognition of RNA with small molecules (PRRSM).42,43 General pattern recognition illuminates complex binding properties through the use of differentially binding receptors that interact with a range of analytes of interest.44–47 Advantageously, selective receptor:analyte binding is not required, and commercially available or easily synthesized small molecule receptors can be utilized. In this context, our laboratory demonstrated that RNA binding small molecules acting as “receptors” could differentially sense RNA secondary structures as “analytes” (Figure 1). Additionally, PRRSM can provide insights into both small molecule properties privileged for RNA binding and the components of RNA topology that influence the molecular recognition of RNA. In addition to the focus on discerning the guiding principles of RNA recognition, PRRSM can provide RNA structural information. The work to date suggests a wide range of applications and areas of expansion for the PRRSM method moving forward.
Figure 1.
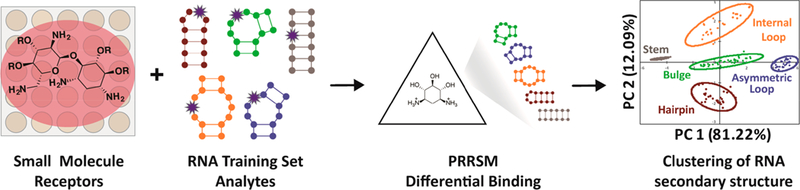
Pattern recognition of RNA by small molecules (PRRSM). An array of small molecule receptors is titrated with RNA secondary structure analytes. Utilizing the small molecule differential binding and an unbiased statistical method allows for clustering based on the RNA structural motifs.
In this Perspective, we discuss the current techniques used to gain insight into RNA structure and dynamics as well as small molecule:RNA interactions and how PRRSM can be used as a complementary technique to investigate both RNA structure and molecular recognition. Given the difficulty of high-resolution 3D characterization for RNA and previous reviews on the subject,23,24,26,27,48–51 we will focus on alternative methods. Additionally, we will discuss future opportunities for understanding and harnessing small molecule:RNA interactions as well as the potential role of PRRSM in elucidating guiding principles of RNA recognition.
STRUCTURAL AND DYNAMICS CONSIDERATIONS FOR RNA RECOGNITION
RNA sequence, structure, and dynamics play important roles in RNA recognition. While high-throughput sequencing techniques have revolutionized the determination of RNA sequence, elucidation of RNA structure and dynamics can be more difficult. RNA folding is generally hierarchical, where the primary sequence forms secondary structure motifs based on Watson–Crick base pairs, which can form tertiary interactions in the presence of magnesium, leading to increasingly complex structures.52–55 RNA dynamic flexibility affects the structural complexity and increases the potential structural diversity for individual RNA sequences.56 In addition, post-transcriptional modifications have recently been revealed to impact RNA structure, dynamics, and recognition.57–61
For RNA secondary structure, advances in computational methods are constantly increasing the length of RNA sequence for which the structure can be accurately predicted.62–64 For example, 700 nucleotide RNA structures have been predicted with 73% accuracy.65 Computational predictions are typically informed through structural constraints derived from chemical probing techniques, such as selective 2′-hydroxyl acylation analyzed by primer extension and mutational profiling (SHAPE-MaP).66–68 SHAPE reagents acylate more solvent-exposed 2′-OH groups at higher rates compared to less exposed 2′-OH groups, i.e., base-paired nucleotides (Figure 2).31,69,70 The modified RNA is then analyzed through error prone reverse transcription, where acylated nucleotides are more prone to mutations. These data are added to computational prediction programs to provide experimentally constrained structure predictions, thus enabling higher accuracy and elucidation of an impressive range of RNA structures.68,71–,77 Several other chemical probing techniques also provide valuable insight, including dimethyl sulfate (DMS),78,79 terbium-induced cleavage,80,81 light-activated structural examination of RNA (LASER),82 and in-line probing techniques.83,84 Each technique mentioned utilizes different patterns of reactivity toward RNA structure, and the data can be combined to provide additional constraints for RNA computational predictions.64 At the same time, in vivo RNA secondary structure determination techniques have been developed to understand important structural motifs in biological systems.31,68,85,86 Current research has been focused on merging 2D probing and tertiary predictions to contribute insight into RNA global structure and interactions.65,87
Figure 2.

Overview of SHAPE reactivity. 2′-Hydroxyl acylation reactivity is dependent on the local environment, where constrained base pair nucleotides are unreactive compared to flexible nucleotides within secondary structure motifs. SHAPE reactivity based on nucleotide position provides additional constraints for structure prediction models. Adapted with permission from ref 70.
The accuracy of computational predictions for three-dimensional RNA structure is currently limited due to a number of factors, including (1) the computational cost of probing the wide range of potential conformations and tertiary interactions within a given sequence, (2) the paucity of high-resolution structures from which to train the algorithms, and (3) the need for improved force fields for RNA.50,88–90 Nonetheless, great progress has been made in this area. For example, 3D Motif Atlas can correctly predict the bacterial 16S rRNA subunit 3D structure and highlight the differences between the bacterial and eukaryotic 16S rRNA subunits.91 Future computational progress is expected to include analysis of conformationally flexible and electronically equivalent RNA structures. Experimentally, RNA interaction groups by mutational profiling (RING-MaP) can help identify potential tertiary contacts using data from DMS probing and single-molecule sequencing experiments.92 Specifically, if two or more sites have statistically similar mutation frequencies within an individual RNA molecule and are not predicted to form secondary structures, a through-space interaction is predicted and thus provides insights into RNA tertiary interactions and global folding of RNA structures. Mutate-and-map readout by next-generation sequencing (M2-Seq) is a complementary technique developed to analyze RNA structure in a one-pot experiment.93 To achieve a low false positive rate for helix identification, a neural network-inspired algorithm termed M2-net is used to recognize helix-specific signatures. These current techniques are useful for in vitro RNA tertiary structure prediction, and future work will be focused on examining in vivo RNA tertiary structures with minimally biologically perturbing experiments.94
RNA dynamics and conformational sampling are also critical to RNA molecular recognition.23,95–98 Techniques such as NMR spectroscopy, FRET, and molecular dynamics simulations have been invaluable in elucidating these processes.38–40,56,97,99––113 For example, residual dipolar coupling (RDC), heteronuclear single-quantum coherence (HSQC), and nuclear Overhauser effect (NOE) NMR investigations, combined with computational simulations, have revealed that despite the general flexibility of RNA, RNA structures sample only a specific subset of the potential conformations.104,114–117 Nucleotide identity, buffer composition, and magnesium concentration were each shown to alter this conformational sampling,97,118 and it has been proposed that both charge and topological constraints play important roles.109,119 These constrained dynamics can also impact binding interactions, including those with small molecules. Indeed, recent work has suggested that small molecules can bind to specific but minor RNA conformers, implying a conformational selection in small molecule:RNA interactions.97,120–122
Recently, dynamics of more than 1000 RNA two-way junctions were interrogated by combining tectoRNA, a method for organizing RNA in 3D space using tetraloop-based tertiary contacts, and a high-throughput method termed quantitative analysis of RNA on a massively parallel array (RNA-MaP).123–125 In this application, an array of immobilized tectoRNA pieces were generated that included a comprehensive set of three-nucleotide two-way junctions (bulges and internal loops) along with others derived from X-ray diffraction structures. The binding equilibria between these sequences and another tectoRNA piece in solution were measured via FRET and were dependent upon the conformational dynamics of each junction. Through this method, “thermodynamic fingerprints” were generated that reveal patterns in two-way junction dynamics, including the general finding that the number and arrangement of unpaired residues primarily determine the conformations sampled while sequence identity plays a secondary role. These determinants also varied by secondary structure. Bulge conformations were found to be relatively independent of sequence identity, while mismatches (i.e., internal loops) were strongly dependent on nucleobase identity. At the same time, unbiased clustering revealed that traditional secondary structure classes do not completely define the conformational landscape, with select junctions having conformational ensembles more similar to those of other classes. These data could further be used to predict tertiary folding energetics and, in the future, may reveal insights into the impact of small molecule binding and other factors on the conformational dynamics of large sets of RNA sequences.
Small Molecule:RNA Recognition.
In addition to a detailed understanding of RNA structure and dynamics, it is important to understand the specific interactions RNA utilizes in binding and selectivity. In general, small molecule:RNA recognition is achieved through a combination of noncovalent interactions such as electrostatics, π-stacking, and hydrogen bonding. Other biomacromolecules, including proteins, also utilize these noncovalent interactions to bind RNA, and multiple reviews that discuss these in greater detail are available.126–136
One approach toward a better understanding of small molecule:RNA recognition is to analyze the properties of small molecules that have achieved selective recognition.11,16,137,138 To this end, we have examined the differences in the cheminformatic parameters of bioactive RNA-targeted small molecules, which we have consolidated into the RNA-targeted Bioactive ligaNd Database (R-BIND), as compared to FDA-approved small molecules, which are thought to largely target proteins.16 Although many “drug-like” properties were consistent across both libraries, bioactive RNA-targeted small molecules had statistically significant differences in structural and spatial properties. These included increased nitrogen counts compared to oxygen counts as well as an increase in the number of aromatic and heteroatom-containing rings. These and other differences underscore the importance of hydrogen bonding, stacking, and complementarity of both electrostatics and shape in small molecule:RNA recognition.
Computational and experimental techniques have been developed to analyze selective small molecule:RNA interactions. As computational docking studies have typically modeled a single biomacromolecule conformation with likely binding partners, the ability of RNA to adopt multiple conformations has hindered such investigations.106,139––146 To address this issue, the Al-Hashimi laboratory constructed an ensemble of RNA structures derived from NMR and molecular dynamics (Figure 3).98,101,122,147,148 Specifically, NMR studies are used to determine the structural constraints of RNA in solution, which are then utilized to select appropriate conformations found through molecular dynamics simulations of the RNA structure. Docking studies with this ensemble of conformations thus offer improved predictive power because small molecules are virtually screened against RNA structures that better resemble those present in solution. These studies have successfully identified small molecule leads for HIV-1 trans-activation response element (TAR) RNA and are being continually refined via comparison with experimental screens.122,148
Figure 3.
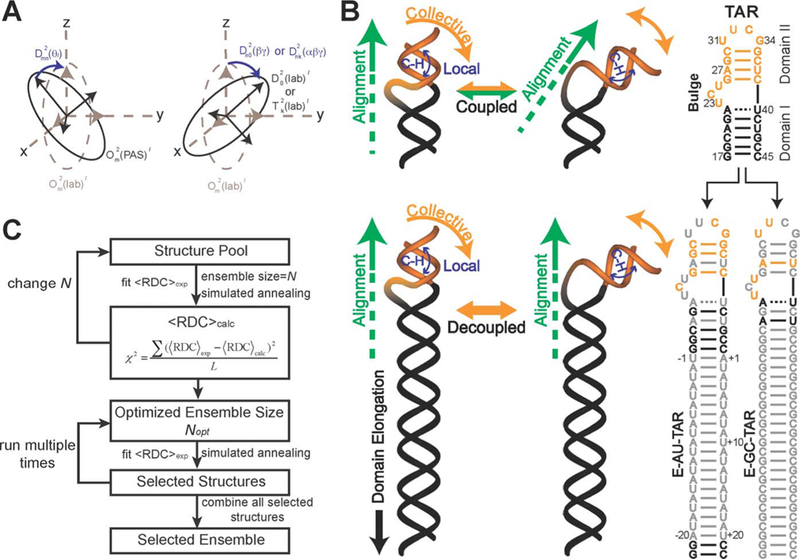
Ensemble determination of the trans-activation response element (TAR) utilizing NMR residual dipolar couplings (RDCs) and molecular dynamics. (A) Rotational and molecular observation and the impact on RDCs. (B) Elongation of TAR allows decoupling of alignment vs local structural changes, allowing for RDC structural analysis. (C) Utilizing RDC from panel B along with molecular dynamics to select a structural ensemble of most likely native structures. Reproduced from ref 147. Copyright 2012 The Royal Society of Chemistry.
A range of experimental assays have also been used to elucidate small molecule:RNA interactions, including assays that compare multiple small molecule:RNA binding events as well as structural studies of complexes when possible. While certainly not exhaustive, a detailed list of select methods and references that have been used to investigate recognition can be found in Table 1. Importantly, each of these methods has been employed to study multiple RNA constructs, and each can yield different biophysical insights. For example, ITC employs the heat of binding to calculate thermodynamic parameters for small molecule interactions, allowing for determination of dissociation constants. Thermal denaturation studies analyze the shift in RNA melting temperatures (Tm) in the absence and presence of a small molecule, which are presumed to reflect changes in the stability of the RNA structures. SPR experiments can be utilized to examine the kinetic parameters that influence small molecule:RNA interactions. Conformational changes are often investigated via FRET through the incorporation of paired fluorophores at RNA locations sensitive to such changes to reveal if the RNA structure is altered upon small molecule binding. FRET can also be employed within a two-body system where the FRET fluorophore pairs are covalently attached to the RNA and the small molecule to monitor ligand binding. Recently, conformational changes and their relationship to selectivity have also been elucidated via second harmonic generation (SHG). Additionally, chemical probing techniques such as SHAPE can be used to globally interrogate site-specific conformational changes in complex RNAs. NMR studies can determine the potential site specificity of small molecules for RNA secondary structures as well as small molecule-induced conformational changes. Recently, band-selective optimized flip-angle short transient heteronuclear multiple quantum coherence (SO-FAST-HMQC) has been used to rapidly identify small molecule-induced perturbations based on 13C and 1H peak shifts, suggesting potential binding sites of small molecules as well as the specificity of interactions. We note that high-resolution NMR and X-ray diffraction have also been used to reveal important interactions in small molecule:RNA complexes, including a recent example of time-resolved crystallographic measurements, though limited examples are available outside of native riboswitch ligands.
Numerous assays have been used to rapidly generate binding constants and investigate the associated selectivity. Traditional optical methods include (1) site-specific incorporation of a fluorophore into the RNA sequence, e.g., 2-aminopurine or other longer wavelength base mimics, which report on the base solvent exposure; (2) indicator displacement assays (IDAs), which utilize indicators that differentially fluoresce when bound to RNA, allowing for monitoring of small molecule binding without covalent labeling of either binding partner (Figure 4); (3) monitoring of intrinsic ligand fluorescence; and (4) FRET, mentioned above. Other valuable approaches include mass spectrometry and microscale thermophoresis.
Figure 4.

Indicator displacement assay utilizing a peptide indicator with a covalent fluorophore, which, upon binding to RNA, can fluoresce. The displacement of the fluorophore peptide by λN protein, inducing quenching of the peptide fluorophore. Adapted from ref 182.
Higher-throughput methods that investigate multiple small molecule:RNA interactions can yield valuable information regarding selectivity. In small molecule microarrays (SMMs), several thousand small molecules can be covalently attached to a glass microscope slide surface and then incubated with a fluorescently tagged RNA to rapidly assess binding interactions. Because the same small molecule set can be screened against multiple RNA targets, specificity can be readily determined and promiscuous small molecules identified. A related approach, two-dimensional combinatorial screening (2DCS), generally utilizes a smaller number of small molecules immobilized in agarose and incubation with RNA secondary structure libraries of randomized sequences. Bound sequences are isolated, cloned, and sequenced to reveal the most tightly bound RNA motifs for each small molecule. Several additional methods have been used for ligand discovery and recently reviewed elsewhere.8,11,15
The invaluable techniques listed above are often combined to provide insight into small molecule:RNA binding modes, thermodynamic properties, and binding constants. With the exception of 2DCS, these techniques have examined individual RNAs and/or small molecules rather than elucidating general guiding principles of small molecule:RNA interactions. As discussed below, our laboratory has applied pattern recognition protocols to this question for the first time via PRRSM. This method can rapidly elucidate the ability of multiple small molecules to differentiate a range of RNA structures. We have also used PRRSM to evaluate the influence of environmental factors on this recognition and to determine RNA structural characteristics.
Pattern Recognition of RNA by Small Molecules.
Pattern-based sensing is a technique used for the determination of complex recognition properties in which a suite of receptors with reasonable affinity and selectivity can classify analytes of interest through differential interactions (Figure 5).44–47,201,202 Similar to the olfactory and gustatory senses that differentially sense an expansive number of stimuli,203 pattern-based sensing assays can discriminate among highly similar analytes, including nitrated explosives,204 ions in aqueous solutions,205 tannins in red wines,206 and normal, cancerous, or metastatic cells.207,208 While traditional detection methods utilize lock-and-key receptor design to achieve highly specific binding of a single analyte, receptors for pattern-based sensing need only show differential binding and thus can often be purchased commercially or designed with simple and rapid synthetic schemes. Our work has focused on extending pattern recognition research to classify RNA secondary structure and understand the fundamental properties that are important in small molecule:RNA recognition.
Figure 5.
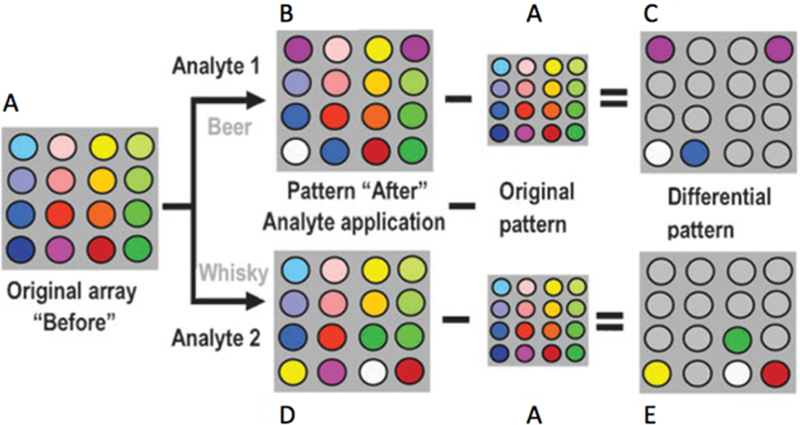
Example of a pattern recognition assay. (A) An array of receptors is tested for background interference. (B) An analyte (beer in this example) is examined with the receptor array, and the background signal is removed. (C) The resulting pattern is used to classify potential beer analytes. (D) Utilizing the same receptor array, additional analytes can be analyzed, such as whisky, for example. (E) After the assay is repeated and the background removed, a differential pattern will form for whisky as compared to beer. Reproduced from ref 202. Copyright 2010 The Royal Society of Chemistry.
Initial experiments examined the ability of well-characterized RNA ligands, specifically aminoglycosides, to differentiate canonical RNA secondary structure motifs.42 The canonical secondary structure motifs of RNA have been shown computationally to have a wide range of conformations that are dependent on sequence and the number of nucleotides within the motif. We proposed that PRRSM could test whether the computationally derived topological distinctions were important for differential binding of small molecules. As a training set, we identified a set of 16 RNA sequences with a range of well-predicted secondary structure motifs and both variable sequence and nucleotide length (excluding the internal loops and stems, which maintain the same number of nucleotides within the secondary structures) (Figure 6A). To measure binding of this set, we developed a 384-well plate assay utilizing the solvatochromic chemosensor benzofuranyluridine (BFU).209 The BFU fluorophore mimics uridine in an RNA structure, allowing for replacement of a native uridine base with a minimal change in base pairing. Each RNA construct was thus chemically synthesized, and a BFU was inserted at a computationally predicted flexible site in the hopes of achieving maximal signal changes upon small molecule binding. The fluorescence was measured upon exposure to 11 aminoglycosides at various concentrations, and the data were used as input for principal component analysis (PCA), which revealed an unbiased clustering of each secondary structure class (Figure 6B). Additionally, the clusters were quantitatively analyzed by leave-one-out cross validation (LOOCV).210 Specifically, an R-script was designed utilizing a naiv̈e Bayes algorithm to remove randomly selected data sets, and then the ability of the remaining experimental data to recapitulate the PRRSM clustering was examined iteratively. Through LOOCV, PRRSM could predict secondary structure motifs with 100% accuracy. In addition, some separation of individual sequences was observed (Figure 7). These results suggest that aminoglycosides, while often characterized as promiscuous RNA ligands, can sense topological differences among these RNA structures.
Figure 6.
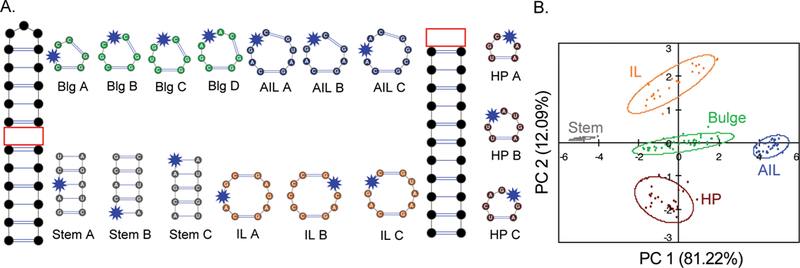
(A) Secondary structure of 16 RNA analytes determined computationally, with the BFU chemosensor shown in each structure (blue star). The remaining sequences outside the variable secondary structures were kept constant to allow consistent comparisons. Abbreviations: Blg, bulge; AIL, asymmetrical internal loop; IL, internal loop; HP, hairpin. (B) PCA plot of the RNA secondary structure motifs based on aminoglycoside differential binding. The predictive power of the assay was determined to be 100%.42
Figure 7.

All 16 RNA training set sequences, stems, bulges (Blg), the internal loop (IL), the asymmetrical internal loop (AIL), and hairpins (HP). PC 1 correlated to the increasing motif size (from stem to AIL), while PC 2 correlated to the purine:pyrimidine ratio, which is dependent on the sequence of the RNA (HP to IL).42
To understand why the RNA structures were clustered differently, we examined the small molecule and RNA structures. One of the most widely used small molecule structural comparison techniques is Tanimoto coefficient comparison, in which path-based fingerprints are compared between small molecules.211 On the basis of literature standards, structures with a Tanimoto coefficient of >0.85 were deemed to be significantly similar, any structure with values from 0.55 to 0.85 was not significantly similar, and any structure with a Tanimoto coefficient of <0.55 was seen to be structurally different (Figure 8). Because our receptors are within the same small molecule class, i.e., aminoglycosides, it is unsurprising that many of the receptors were structurally similar with major differences seen in a small subset of receptors (sisomicin and 2-deoxystreptamine). We then compared the Tanimoto coefficients to the loading factors of the principal components, which can be used to interpret the differential binding propensities of the receptors for the RNA motifs. In some cases, significantly similar structures revealed highly similar binding tendencies (kanamycin, neamine, and neomycin), but in other cases (guanidino-kanamycin and guanidino-paromomycin), similar structures displayed somewhat different binding tendencies. In addition, little correlation between loading factors and standard physicochemical properties (total charge, molecular weight, etc.) was identified. These results are in line with suggestions that aminoglycoside recognition depends on the three-dimensional properties of the ligand.
Figure 8.

(A) Comparison of the Tanimoto coefficients of the aminoglycoside receptors. Significantly similar structures are colored dark green (>0.85), and structurally different structures light green (0–0.55). All other aminoglycosides were moderately similar based on the Tanimoto coefficients (0.55–0.85). (B) Loading factors of the 11 aminoglycoside receptors for the first three principal components. Abbreviations: 2DOS, 2-deoxystreptamine; Amik, amikacin; Apra, apramycin; D-Strep, dihydrostreptomycin; G-Kana, guanidino-kanamycin; G-Paro, guanidino-paromomycin; Kana, kanamycin; Neam, neamine; Neom, neomycin; Siso, sisomicin.42
Insights into the RNA properties that allow for small molecule differentiation could also be gained through this initial PRRSM study. In general, differential RNA recognition is achieved by proteins through a combination of sequence- and topology-dependent binding, which allows for high selectivity and binding affinity.112,130,134,212 We used PRRSM to evaluate the importance of sequence and topology in small molecule differentiation of RNA, where far fewer interactions can be utilized to achieve affinity and specificity. By examining the PRRSM data for the 16 individual sequences, we identified rough correlations among the principal component axes for both sequence and motif size (Figure 7). The largest amount of variance within the data correlated with the motif size of the RNA secondary structures (PC 1, 81.22%), while sequence dependence was second in importance (PC 2, 12.09%). These data, along with motif-based clustering, support previous work showing that small molecule:RNA differentiation, at least among aminoglycosides, is primarily dependent on the topology of the RNA structure.104
These initial findings inspired a range of other research directions, including how modulation of RNA topology impacts differentiation by small molecules. One way to modulate RNA topology is by changing buffer conditions.213 For example, it was previously established that the stability of RNA secondary and tertiary structure can be altered through binding of monovalent and divalent cations, the presence of molecular crowders, and variations in temperature.214,215 We thus evaluated the impact of environmental factors on RNA molecular recognition by employing the PRRSM assay under a range of different buffer conditions (Table 2).
Table 2.
Predictive Power of the RNA Training Set under Varying Conditions
| buffer | predictive power (%) |
|---|---|
| standard | 59.5 |
| pH 5a | 19.2 |
| pH 6a | 50.8 |
| pH 8a | 49.8 |
| 140 mM Naa | 10.5 |
| 0 mM Mga | 47.9 |
| 10 mM Mga | 29.2 |
| Trisb | 57.1 |
| 37 Ca | 39.6 |
| PEGc | 65 |
| PEG/37 Cc | 71 |
| PEG no stemc | 85 |
| PEG/37 C no stemc | 92 |
In 10 mM NaH2PO4, 25 mM NaCl, 4 mM MgCl2, and 0.5 mM EDTA at pH 7.3 and 25 °C unless otherwise indicated.
In 10 mM Tris, 25 mM NaCl, 4 mM MgCl2, and 0.5 mM EDTA at pH 7.3.
In 10 mM NaH2PO4, 25 mM NaCl, 4 mM MgCl2, 0.5 mM EDTA, and 8 mM polyethylene glycol (PEG) 12000 at pH 7.3 and 25 or 37 °C.
Using predictive power as an indicator of RNA differentiation, we evaluated a set of common buffer conditions utilized in small molecule:RNA assays, biologically relevant conditions, and conditions expected to ablate aminoglycoside binding. Unsurprisingly, a high sodium concentration (140 mM Na) and a low pH (pH 5) were found to significantly decrease the level of differentiation as high salt screens electrostatic interactions and lower pH changes the protonation states of the aminoglycosides. Relative to the original conditions, removal of magnesium, a relatively neutral pH, and changing the buffer composition (phosphate vs Tris) had a minimal effect on differentiation. On the other hand, addition of polyethylene glycol (PEG) and increasing the temperature from 25 to 37 °C increased the level of differentiation. This result was surprising as PEG and an increased temperature are known to destabilize secondary structure motifs.216 Conversely, a decreased level of differentiation was observed with an increased magnesium concentration, which would be expected to stabilize secondary structure, though an increased level of competition between the magnesium ions and the aminoglycosides for RNA binding cannot be ruled out.217,218 Taken together, this study suggested that conformationally dynamic RNA secondary structures are better differentiated by small molecules. In the context of RNA molecular recognition, these results imply that a conformational selection or induced fit mechanism is valuable for differential recognition of RNA structural motifs by small molecules.161,192,219 Furthermore, these findings are consistent with previous work suggesting that dynamic RNA structures sample a defined set of available conformations and that these defined sets can be unique for a given RNA structure, thus allowing differentiation.104,116,161,220
In addition to providing a better understanding of RNA molecular recognition, we evaluated whether the classification power of PRRSM could generate insight into site-specific RNA structure. We analyzed biologically relevant RNA constructs of known structure with multiple secondary structure motifs and/ or inducible conformational changes.221 We fluorescently labeled each construct with BFU at nucleotide positions of interest. To begin, we examined a truncated version of the HIV-1 TAR RNA consisting of a three-nucleotide bulge and a six-nucleotide hairpin. Second, we assessed the prequeuosine-1 riboswitch (PreQ1) and fluoride riboswitch (FR), which undergo conformational changes in the presence of the respective analyte.102,107,108,113,222,223
The TAR, PreQ1, and FR RNA constructs were synthesized with the BFU fluorophore at different, biologically important sites and were studied with the PRRSM assay (Figure 9). Within the RNA sequences, TAR was modified at a single position in two respective constructs, and both riboswitches were modified at three different positions within three respective RNA constructs. Of the eight RNA constructs analyzed, six of the constructs were classified as expected on the basis of the known ligand-bound structures. The two constructs that did not cluster as expected were the U6- and U11-modified fluoride riboswitches. In the folded state, the U6-modified fluoride riboswitch showed no folding via PRRSM, which was consistent with previous work showing that a U6–C6 mutation prevented proper folding and was confirmed via NMR.113 The U11–fluoride riboswitch construct did not cluster with any known secondary structure classes, which could again indicate incomplete folding given the proximity of the modification to the binding site or perhaps a limitation of the training set used. The first possibility was supported by a PRRSM experiment using mixtures of RNA sequences closely representing the two states as a mimic of incomplete folding that demonstrated similar results, and this was also confirmed via NMR. On the basis of these results, we determined that PRRSM can classify a range of RNA structures, including folded and unfolded states, and provide insight into sites that are critical for these structural changes. Overall, PRRSM has provided a new understanding of RNA molecular recognition, evidence of differential binding of small molecules based on RNA topology, and an orthogonal technique for analyzing RNA secondary structure.
Figure 9.
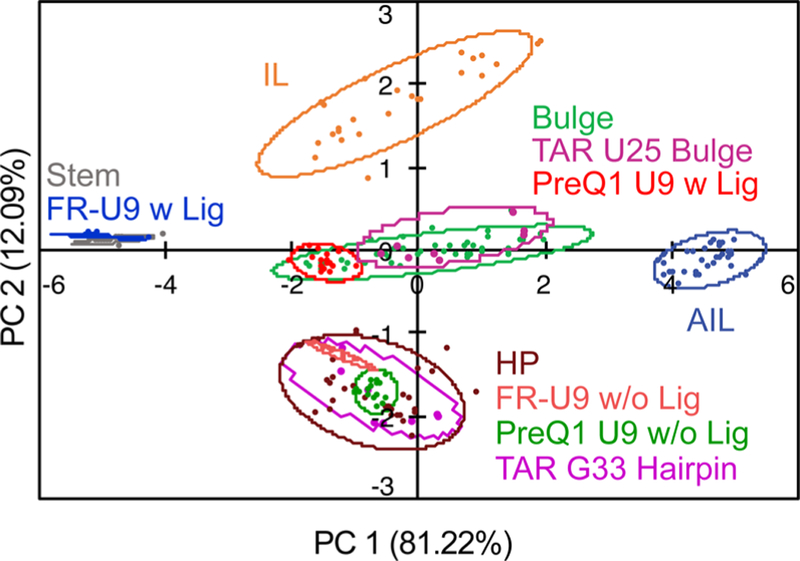
PCA plot of PreQ1 U9 and FR U9 BFU constructs with and without a ligand. Also, U25 and G33 TAR BFU constructs cluster for bulge and hairpin secondary structures, respectively.221
Future Work.
The insights gained via PRRSM to date and the robustness of the assay suggest a wide range of future applications for this technique. To date, we have utilized only aminoglycoside receptors, and although an important class of RNA binding small molecules, these receptors can be more difficult to tune for specificity and favorable biological activity relative to more traditional “drug-like” small molecules. Our next steps toward understanding general guiding principles for non-aminoglycoside small molecule recognition will utilize a new, chemically diverse set of receptors. For example, PRRSM has the potential to reveal the impact of the binding modes of aromatic, aliphatic, and/or charged ligands on RNA differentiation. Additionally, diverse receptors are likely to have greater differential binding, potentially creating more spatially separated clustering and allowing more complex structures to be analyzed. As well as aiding in understanding recognition differences, PRRSM can determine the environmental conditions that are important for modulation of RNA binding with more diverse chemical structures.
PRRSM is currently based on a fluorescent plate reader assay utilizing the covalently attached solvatochromic fluorophore BFU, which may impact tertiary interactions and must be incorporated through solid phase synthesis. For applications in which the label may interrupt native structure, one potential solution is an indicator displacement assay, allowing for classification of RNA secondary structure with unmodified RNA. For applications involving large RNAs that cannot be chemically synthesized, small molecule microarrays may be employed. These arrays can be used to visualize hundreds to thousands of small molecules binding to RNA structures, though with a single RNA at a time, via enzymatic modification of the 3′-end. For applications in which site-specific information about a large RNA is critical, ligation of a chemically synthesized fragment is under investigation.224–226
PRRSM could also evolve to inform the recognition of RNA tertiary motifs. A wide range of tertiary structures is formed through secondary structure motif interactions, including pseudoknots, kissing loops, and G-quadruplexes. Similar to that of RNA secondary structure, utilization of a training set of previously characterized RNA tertiary structures with an array of differentially binding small molecule receptors would allow clustering based on distinctive interactions with these tertiary structures. After an initial RNA training set is designed and tested, RNAs with unknown tertiary structures can be analyzed. In addition to classification, recognition principles for RNA tertiary structures could be probed and explored, allowing an improved understanding of important interactions that can be exploited for RNA-targeted small molecules.
We further envision the implementation of PRRSM to classify RNA function. An overwhelming number of RNA sequences thought to be important in regulating both normal and diseased states lack a known molecular function, in part due to the time-consuming nature of this process. Because PRRSM can classify RNA structure, and RNA structure is often related to RNA function, we propose that small molecules can be used to directly classify function. Such an assay will likely require a large set of diverse small molecule receptors such as those that can be displayed on a microarray, which will also allow for rapid analysis of binding. Again, on the basis of a known training set, this one step assay could be used to narrow the set of likely functions for a particular RNA of interest, significantly focusing more in-depth analysis of molecular function.
In conclusion, the field of RNA molecular recognition has multiple tools for examining single small molecule:RNA interactions. Our technique, PRRSM, can discretely assess a wide range of RNA secondary structures and small molecule receptors that together provide a better understanding of the guiding principles of these interactions. Our research, along with that of others, has shown that RNA topology as well as sequence is important for distinguishing small molecule binding, and modulating the RNA topology has a strong effect on the small molecule binding propensities. On the basis of initial data classifying biologically relevant RNA structure, PRRSM is a promising technique for examining tertiary motifs as well as for classifying the most likely function for new RNA structures. The future of RNA molecular recognition and RNA targeting is dependent on understanding small molecule binding in the context of RNA three-dimensional structure and dynamics, and PRRSM is uniquely positioned to contribute to this understanding.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the members of the Hargrove lab for stimulating discussion and input.
Funding
A.E.H. acknowledges the financial support for this work from Duke University, the National Institutes of Health (U54GM103297), and the Research Corporation for Science Advancement Cottrell Scholar Award. C.S.E. was supported in part through a U.S. Department of Education GAANN Fellowship (P200A150114).
Footnotes
The authors declare no competing financial interest.
REFERENCES
- (1).Esteller M (2011) Non-coding RNAs in human disease. Nat. Rev. Genet 12, 861. [DOI] [PubMed] [Google Scholar]
- (2).Diederichs S (2012) Non-coding RNA and disease. RNA Biol 9, 701–702. [DOI] [PubMed] [Google Scholar]
- (3).Lee TI, and Young RA (2013) Transcriptional Regulation and Its Misregulation in Disease. Cell 152, 1237–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Ling H, Fabbri M, and Calin GA (2013) MicroRNAs and other non-coding RNAs as targets for anticancer drug development. Nat. Rev. Drug Discovery 12, 847–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Cech TR, and Steitz JA (2014) The noncoding RNA revolution-trashing old rules to forge new ones. Cell 157, 77–94. [DOI] [PubMed] [Google Scholar]
- (6).Morris KV, and Mattick JS (2014) The rise of regulatory RNA. Nat. Rev. Genet 15, 423–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Schmitt AM, and Chang HY (2016) Long Noncoding RNAs in Cancer Pathways. Cancer Cell 29, 452–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Connelly CM, Moon MH, and Schneekloth JS (2016) The Emerging Role of RNA as a Therapeutic Target for Small Molecules. Cell Chem. Biol 23, 1077–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Santos R, Ursu O, Gaulton A, Bento AP, Donadi RS, Bologa CG, Karlsson A, Al-Lazikani B, Hersey A, Oprea TI, and Overington JP (2017) A comprehensive map of molecular drug targets. Nat. Rev. Drug Discovery 16, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Matsui M, and Corey DR (2017) Non-coding RNAs as drug targets. Nat. Rev. Drug Discovery 16, 167–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Morgan BS, Forte JE, and Hargrove AE (2018) Insights into the development of chemical probes for RNA. Nucleic Acids Res 46, 8025–8037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Disney MD, Yildirim I, and Childs-Disney JL (2014) Methods to enable the design of bioactive small molecules targeting RNA. Org. Biomol. Chem 12, 1029–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Shortridge MD, and Varani G (2015) Structure based approaches for targeting non-coding RNAs with small molecules. Curr. Opin. Struct. Biol 30, 79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Garbaccio RM, and Parmee ER (2016) The Impact of Chemical Probes in Drug Discovery: A Pharmaceutical Industry Perspective. Cell Chem. Biol 23, 10–17. [DOI] [PubMed] [Google Scholar]
- (15).Hermann T (2016) Small molecules targeting viral RNA. Wiley Interdiscip Rev. RNA 7, 726–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Morgan BS, Forte JE, Culver RN, Zhang Y, and Hargrove AE (2017) Discovery of Key Physicochemical, Structural, and Spatial Properties of RNA-Targeted Bioactive Ligands. Angew. Chem., Int. Ed 56, 13498–13502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Donlic A, and Hargrove AE (2018) Targeting RNA in mammalian systems with small molecules. Wiley Inter. Rev. RNA 9, No. e1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Warner KD, Hajdin CE, and Weeks KM (2018) Principles for targeting RNA with drug-like small molecules. Nat. Rev. Drug Discovery 17, 547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Marz M, and Stadler PF (2011) RNA interactions. Adv. Exp. Med. Biol 722, 20–38. [DOI] [PubMed] [Google Scholar]
- (20).Blakeley BD, DePorter SM, Mohan U, Burai R, Tolbert BS, and McNaughton BR (2012) Methods for identifying and characterizing interactions involving RNA. Tetrahedron 68, 8837–8855. [Google Scholar]
- (21).McHugh CA, Russell P, and Guttman M (2014) Methods for comprehensive experimental identification of RNA-protein interactions. Gen. Biol 15, 203–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).McFadden EJ, and Hargrove AE (2016) Biochemical Methods To Investigate lncRNA and the Influence of lncRNA:Protein Complexes on Chromatin. Biochemistry 55, 1615–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Al-Hashimi HM (2005) Dynamics-Based Amplification of RNA Function and Its Characterization by Using NMR Spectroscopy. ChemBioChem 6, 1506–1519. [DOI] [PubMed] [Google Scholar]
- (24).Latham MP, Brown DJ, McCallum SA, and Pardi A (2005) NMR Methods for Studying the Structure and Dynamics of RNA. ChemBioChem 6, 1492–1505. [DOI] [PubMed] [Google Scholar]
- (25).Patel DJ, Shapiro L, and Hare D (1987) DNA and RNA: NMR studies of conformations and dynamics in solution. Q. Rev. Biophys 20, 35–112. [DOI] [PubMed] [Google Scholar]
- (26).Reyes FE, Garst AD, and Batey RT (2009) Chapter 6 - Strategies in RNA Crystallography. In Methods in Enzymology, pp 119–139, Academic Press. [DOI] [PubMed] [Google Scholar]
- (27).Westhof E (2015) Twenty years of RNA crystallography. RNA 21, 486–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Andronescu M, Bereg V, Hoos HH, and Condon A (2008) RNA STRAND: the RNA secondary structure and statistical analysis database. BMC Bioinf 9, 340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Weeks KM, and Mauger DM (2011) Exploring RNA structural codes with SHAPE chemistry. Acc. Chem. Res 44, 1280–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Moss WN (2013) Chapter One - Computational Prediction of RNA Secondary Structure. In Methods in Enzymology (Lorsch J, Ed.) pp 3–65, Academic Press. [DOI] [PubMed] [Google Scholar]
- (31).Spitale RC, Flynn RA, Torre EA, Kool ET, and Chang HY (2014) RNA structural analysis by evolving SHAPE chemistry. Wiley Interdiscip. Rev.: RNA 5, 867–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Cheng CY, Chou FC, and Das R (2015) Modeling complex RNA tertiary folds with Rosetta. Methods Enzymol 553, 35–64. [DOI] [PubMed] [Google Scholar]
- (33).Sloma MF, and Mathews DH (2015) Improving RNA secondary structure prediction with structure mapping data. Methods Enzymol 553, 91–114. [DOI] [PubMed] [Google Scholar]
- (34).Yang S, Parisien M, Major F, and Roux B (2010) RNA structure determination using SAXS data. J. Phys. Chem. B 114, 10039–10048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Cheng Y (2015) Single-Particle Cryo-EM at Crystallographic Resolution. Cell 161, 450–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Nogales E, and Scheres SHW (2015) Cryo-EM: A Unique Tool for the Visualization of Macromolecular Complexity. Mol. Cell 58, 677–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Chen Y, and Pollack L (2016) SAXS Studies of RNA: structures, dynamics, and interactions with partners. Wiley Interdiscip. Rev.: RNA 7, 512–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Walter NG (2002) Probing RNA Structural Dynamics and Function by Fluorescence Resonance Energy Transfer (FRET). Curr. Protoc. Nucleic Acid Chem 11, 11.10.1–11.10.23. [DOI] [PubMed] [Google Scholar]
- (39).Hengesbach M, Kobitski A, Voigts-Hoffmann F, Frauer C, Nienhaus GU, and Helm M (2008) RNA intramolecular dynamics by single-molecule FRET. Current Protocols in Nucleic Acid Chemistry, Chapter 11, Unit 11.12, Wiley. [DOI] [PubMed] [Google Scholar]
- (40).Rinnenthal J, Buck J, Ferner J, Wacker A, Furtig B, and Schwalbe H (2011) Mapping the Landscape of RNA Dynamics with NMR Spectroscopy. Acc. Chem. Res 44, 1292–1301. [DOI] [PubMed] [Google Scholar]
- (41).Stephenson JD, Kenyon JC, Symmons MF, and Lever AML (2016) Characterizing 3D RNA structure by single molecule FRET. Methods 103, 57–67. [DOI] [PubMed] [Google Scholar]
- (42).Eubanks CS, Forte JE, Kapral GJ, and Hargrove AE (2017) Small Molecule-Based Pattern Recognition To Classify RNA Structure. J. Am. Chem. Soc 139, 409–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Eubanks CS, and Hargrove AE (2017) Sensing the impact of environment on small molecule differentiation of RNA sequences. Chem. Commun. (Cambridge, U. K.) 53, 13363–13366. [DOI] [PubMed] [Google Scholar]
- (44).Lavigne JJ, and Anslyn EV (2001) Sensing a paradigm shift in the field of molecular recognition: from selective to differential receptors. Angew. Chem., Int. Ed 40, 3118–3130. [DOI] [PubMed] [Google Scholar]
- (45).Wright AT, Griffin MJ, Zhong Z, McCleskey SC, Anslyn EV, and McDevitt JT (2005) Differential receptors create patterns that distinguish various proteins. Angew. Chem., Int. Ed 44, 6375–6378. [DOI] [PubMed] [Google Scholar]
- (46).Kitamura M, Shabbir SH, and Anslyn EV (2009) Guidelines for pattern recognition using differential receptors and indicator displacement assays. J. Org. Chem 74, 4479–4489. [DOI] [PubMed] [Google Scholar]
- (47).Umali AP, and Anslyn EV (2010) A general approach to differential sensing using synthetic molecular receptors. Curr. Opin. Chem. Biol 14, 685–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Dayie KT (2008) Key labeling technologies to tackle sizeable problems in RNA structural biology. Int. J. Mol. Sci 9, 1214–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Espinosa S, Zhang L, Li X, and Zhao R (2017) Understanding pre-mRNA splicing through crystallography. Methods 125, 55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Miao Z, and Westhof E (2017) RNA Structure: Advances and Assessment of 3D Structure Prediction. Annu. Rev. Biophys 46, 483–503. [DOI] [PubMed] [Google Scholar]
- (51).Marchanka A, Kreutz C, and Carlomagno T (2018) Isotope labeling for studying RNA by solid-state NMR spectroscopy. J. Biomol. NMR 71, 151–164. [DOI] [PubMed] [Google Scholar]
- (52).Brion P, and Westhof E (1997) HIERARCHY AND DYNAMICS OF RNA FOLDING. Annu. Rev. Biophys. Biomol. Struct 26, 113. [DOI] [PubMed] [Google Scholar]
- (53).Tinoco I, and Bustamante C (1999) How RNA folds. J. Mol. Biol 293, 271–281. [DOI] [PubMed] [Google Scholar]
- (54).Greenleaf WJ, Frieda KL, Foster DAN, Woodside MT, and Block SM (2008) Direct Observation of Hierarchical Folding in Single Riboswitch Aptamers. Science 319, 630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Sattin BD, Zhao W, Travers K, Chu S, and Herschlag D (2008) Direct Measurement of Tertiary Contact Cooperativity in RNA Folding. J. Am. Chem. Soc 130, 6085–6087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Al-Hashimi HM, and Walter NG (2008) RNA dynamics: it is about time. Curr. Opin. Struct. Biol 18, 321–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Roost C, Lynch SR, Batista PJ, Qu K, Chang HY, and Kool ET (2015) Structure and thermodynamics of N6-methyladenosine in RNA: A spring-loaded base modification. J. Am. Chem. Soc 137, 2107–2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Liu N, Zhou KI, Parisien M, Dai Q, Diatchenko L, and Pan T (2017) N6-methyladenosine alters RNA structure to regulate binding of a low-complexity protein. Nucleic Acids Res 45, 6051–6063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Preethi SP, Sharma P, and Mitra A (2017) Higher order structures involving post transcriptionally modified nucleobases in RNA. RSC Adv 7, 35694–35703. [Google Scholar]
- (60).Seelam PP, Sharma P, and Mitra A (2017) Structural landscape of base pairs containing post-transcriptional modifications in RNA. RNA 23, 847–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Liu B, Merriman DK, Choi SH, Schumacher MA, Plangger R, Kreutz C, Horner SM, Meyer KD, and Al-Hashimi HM (2018) A potentially abundant junctional RNA motif stabilized by m6A and Mg2+. Nat. Commun 9, 2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Mathews DH, Disney MD, Childs JL, Schroeder SJ, Zuker M, and Turner DH (2004) Incorporating chemical modification constraints into a dynamic programming algorithm for prediction of RNA secondary structure. Proc. Natl. Acad. Sci. U. S. A 101, 7287–7292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Reuter JS, and Mathews DH (2010) RNAstructure: software for RNA secondary structure prediction and analysis. BMC Bioinf 11, 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Sloma MF, and Mathews DH (2015) Chapter Four - Improving RNA Secondary Structure Prediction with Structure Mapping Data. In Methods in Enzymology (Chen S-J, and Burke-Aguero DH, Eds.) pp 91–114, Academic Press. [DOI] [PubMed] [Google Scholar]
- (65).Seetin MG, and Mathews DH (2012) RNA structure prediction: An overview of methods. Methods Mol. Biol. (N. Y., NY, U. S.) 905, 99–122. [DOI] [PubMed] [Google Scholar]
- (66).Siegfried NA, Busan S, Rice GM, Nelson JAE, and Weeks KM (2014) RNA motif discovery by SHAPE and mutational profiling (SHAPE-MaP). Nat. Methods 11, 959–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Weeks KM (2015) Review toward all RNA structures, concisely. Biopolymers 103, 438–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Smola MJ, Christy TW, Inoue K, Nicholson CO, Friedersdorf M, Keene JD, Lee DM, Calabrese JM, and Weeks KM (2016) SHAPE reveals transcript-wide interactions, complex structural domains, and protein interactions across the Xist lncRNA in living cells. Proc. Natl. Acad. Sci. U. S. A 113, 10322–10327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Merino EJ, Wilkinson KA, Coughlan JL, and Weeks KM (2005) RNA Structure Analysis at Single Nucleotide Resolution by Selective 2’-Hydroxyl Acylation and Primer Extension (SHAPE). J. Am. Chem. Soc 127, 4223–4231. [DOI] [PubMed] [Google Scholar]
- (70).Gherghe CM, Shajani Z, Wilkinson KA, Varani G, and Weeks KM (2008) Strong Correlation between SHAPE Chemistry and the Generalized NMR Order Parameter (S2) in RNA. J. Am. Chem. Soc 130, 12244–12245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).Novikova IV, Hennelly SP, and Sanbonmatsu KY (2012) Structural architecture of the human long non-coding RNA, steroid receptor RNA activator. Nucleic Acids Res 40, 5034–5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).Hajdin CE, Bellaousov S, Huggins W, Leonard CW, Mathews DH, and Weeks KM (2013) Accurate SHAPE-directed RNA secondary structure modeling, including pseudoknots. Proc. Natl. Acad. Sci. U. S. A 110, 5498–5503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (73).Novikova IV, Dharap A, Hennelly SP, and Sanbonmatsu KY (2013) 3S: Shotgun secondary structure determination of long non-coding RNAs. Methods 63, 170–177. [DOI] [PubMed] [Google Scholar]
- (74).Lavender CA, Gorelick RJ, and Weeks KM (2015) Structure-Based Alignment and Consensus Secondary Structures for Three HIV-Related RNA Genomes. PLoS Comput. Biol 11, e1004230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (75).Hawkes EJ, Hennelly SP, Novikova IV, Irwin JA, Dean C, and Sanbonmatsu KY (2016) COOLAIR Antisense RNAs Form Evolutionarily Conserved Elaborate Secondary Structures. Cell Rep 16, 3087–3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (76).Xue Z, Hennelly S, Doyle B, Gulati AA, Novikova IV, Sanbonmatsu KY, and Boyer LA (2016) A G-Rich Motif in the lncRNA Braveheart Interacts with a Zinc-Finger Transcription Factor to Specify the Cardiovascular Lineage. Mol. Cell 64, 37–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (77).Liu F, Somarowthu S, and Pyle AM (2017) Visualizing the secondary and tertiary architectural domains of lncRNA RepA. Nat. Chem. Biol 13, 282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (78).Tijerina P, Mohr S, and Russell R (2007) DMS Footprinting of Structured RNAs and RNA-Protein Complexes. Nat. Protoc 2, 2608–2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (79).Cordero P, Kladwang W, VanLang CC, and Das R (2012) Quantitative Dimethyl Sulfate Mapping for Automated RNA Secondary Structure Inference. Biochemistry 51, 7037–7039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (80).Harris D, Todd A, Gabrielle C, and Nils GW (2014) Terbium(III) Footprinting as a Probe of RNA Structure and Metal Binding Sites. In Handbook of RNA Biochemistry (Hartmann RK, Bindereif AS, and Westhof E, Eds.) 2nd ed., Wiley-VCH Verlag GmbH & Co. KGaA. [Google Scholar]
- (81).Somarowthu S, Legiewicz M, Chillon I, Marcia M, Liu F, and Pyle AM (2015) HOTAIR Forms an Intricate and Modular Secondary Structure. Mol. Cell 58, 353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (82).Feng C, Chan D, Joseph J, Muuronen M, Coldren WH, Dai N, Correa IR Jr, Furche F, Hadad CM, and Spitale RC (2018) Light-activated chemical probing of nucleobase solvent accessibility inside cells. Nat. Chem. Biol 14, 276–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (83).Regulski E, and Breaker R (2008) line Probing Analysis of Riboswitches. Methods Mol. Biol 419 (419), 53–67. [DOI] [PubMed] [Google Scholar]
- (84).Strauss B, Nierth A, Singer M, and Jaschke A (2012) Direct structural analysis of modified RNA by fluorescent in-line probing. Nucleic Acids Res 40, 861–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (85).Spitale RC, Crisalli P, Flynn RA, Torre EA, Kool ET, and Chang HY (2013) RNA SHAPE analysis in living cells. Nat. Chem. Biol 9, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (86).Tian S, and Das R (2016) RNA structure through multidimensional chemical mapping. Q. Rev. Biophys 49, e7. [DOI] [PubMed] [Google Scholar]
- (87).Cheng CY, Chou FC, Kladwang W, Tian S, Cordero P, and Das R (2015) Consistent global structures of complex RNA states through multidimensional chemical mapping. eLife 4, No. e07600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (88).Shapiro BA, Yingling YG, Kasprzak W, and Bindewald E (2007) Bridging the gap in RNA structure prediction. Curr. Opin. Struct. Biol 17, 157–165. [DOI] [PubMed] [Google Scholar]
- (89).Das R, Karanicolas J, and Baker D (2010) Atomic accuracy in predicting and designing noncanonical RNA structure. Nat. Methods 7, 291–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (90).Kladwang W, VanLang CC, Cordero P, and Das R (2011) Understanding the errors of SHAPE-directed RNA structure modeling. Biochemistry 50, 8049–8056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (91).Parlea LG, Sweeney BA, Hosseini-Asanjan M, Zirbel CL, and Leontis NB (2016) The RNA 3D Motif Atlas: Computational methods for extraction, organization and evaluation of RNA motifs. Methods 103, 99–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (92).Homan PJ, Favorov OV, Lavender CA, Kursun O, Ge X, Busan S, Dokholyan NV, and Weeks KM (2014) Single-molecule correlated chemical probing of RNA. Proc. Natl. Acad. Sci. U. S. A 111, 13858–13863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (93).Cheng CY, Kladwang W, Yesselman JD, and Das R (2017) RNA structure inference through chemical mapping after accidental or intentional mutations. Proc. Natl. Acad. Sci. U. S. A 114, 9876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (94).Lu Z, Zhang QC, Lee B, Flynn RA, Smith MA, Robinson JT, Davidovich C, Gooding AR, Goodrich KJ, Mattick JS, Mesirov JP, Cech TR, and Chang HY (2016) RNA Duplex Map in Living Cells Reveals Higher-Order Transcriptome Structure. Cell 165, 1267–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (95).Simon AE, and Gehrke L (2009) RNA conformational changes in the life cycles of RNA viruses, viroids, and virus-associated RNAs. Biochim. Biophys. Acta, Gene Regul. Mech 1789, 571–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (96).Haller A, Soulier̀e MF, and Micura R (2011) The Dynamic Nature of RNA as Key to Understanding Riboswitch Mechanisms. Acc. Chem. Res 44, 1339–1348. [DOI] [PubMed] [Google Scholar]
- (97).Dethoff EA, Chugh J, Mustoe AM, and Al-Hashimi HM (2012) Functional complexity and regulation through RNA dynamics. Nature 482, 322–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (98).Salmon L, Yang S, and Al-Hashimi HM (2014) Advances in the Determination of Nucleic Acid Conformational Ensembles. Annu. Rev. Phys. Chem 65, 293–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (99).Klostermeier D, and Millar DP (2001) RNA Conformation and Folding Studied with Fluorescence Resonance Energy Transfer. Methods 23, 240–254. [DOI] [PubMed] [Google Scholar]
- (100).Buck J, Furtig B, Noeske J, Wohnert J, and Schwalbe H (2009) Time-resolved NMR spectroscopy: ligand-induced refolding of riboswitches. Methods Mol. Biol 540, 161–171. [DOI] [PubMed] [Google Scholar]
- (101).Frank AT, Stelzer AC, Al-Hashimi HM, and Andricioaei I (2009) Constructing RNA dynamical ensembles by combining MD and motionally decoupled NMR RDCs: new insights into RNA dynamics and adaptive ligand recognition. Nucleic Acids Res 37, 3670–3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (102).Kang M, Peterson R, and Feigon J (2009) Structural Insights into riboswitch control of the biosynthesis of queuosine, a modified nucleotide found in the anticodon of tRNA. Mol. Cell 33, 784–790. [DOI] [PubMed] [Google Scholar]
- (103).Xie Y, Dix AV, and Tor Y (2009) FRET Enabled Real Time Detection of RNA-Small Molecule Binding. J. Am. Chem. Soc 131, 17605–17614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (104).Bailor MH, Sun X, and Al-Hashimi HM (2010) Topology links RNA secondary structure with global conformation, dynamics, and adaptation. Science 327, 202–206. [DOI] [PubMed] [Google Scholar]
- (105).Duchardt-Ferner E, Weigand JE, Ohlenschlager O, Schmidtke SR, Suess B, and Wohnert J (2010) Highly modular structure and ligand binding by conformational capture in a minimalistic riboswitch. Angew. Chem., Int. Ed 49, 6216–6219. [DOI] [PubMed] [Google Scholar]
- (106).Fulle S, and Gohlke H (2010) Molecular recognition of RNA: challenges for modelling interactions and plasticity. J. Mol. Recognit 23, 220–231. [DOI] [PubMed] [Google Scholar]
- (107).Ren A, Rajashankar KR, and Patel DJ (2012) Fluoride ion encapsulation by Mg2+ ions and phosphates in a fluoride riboswitch. Nature 486, 85–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (108).Serganov A, and Patel DJ (2012) Metabolite Recognition Principles and Molecular Mechanisms Underlying Riboswitch Function. Annu. Rev. Biophys 41, 343–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (109).Mustoe AM, Brooks CL 3rd, and Al-Hashimi HM (2014) Topological constraints are major determinants of tRNA tertiary structure and dynamics and provide basis for tertiary folding cooperativity. Nucleic Acids Res 42, 11792–11804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (110).Zhao B, Hansen AL, and Zhang Q (2014) Characterizing Slow Chemical Exchange in Nucleic Acids by Carbon CEST and Low Spin-Lock Field R1ρ NMR Spectroscopy. J. Am. Chem. Soc 136, 20–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (111).Ren A, Xue Y, Peselis A, Serganov A, Al-Hashimi HM, and Patel DJ (2015) Structural and Dynamic Basis for Low-Affinity, High-Selectivity Binding of L-Glutamine by the Glutamine Riboswitch. Cell Rep 13, 1800–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (112).Schlundt A, Tants J-N, and Sattler M (2017) Integrated structural biology to unravel molecular mechanisms of protein-RNA recognition. Methods 118–119, 119–136. [DOI] [PubMed]
- (113).Zhao B, Guffy SL, Williams B, and Zhang Q (2017) An excited state underlies gene regulation of a transcriptional riboswitch. Nat. Chem. Biol 13, 968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (114).Friederich MW, Vacano E, and Hagerman PJ (1998) Global Flexibility of Tertiary Structure in RNA: Yeast tRNAPhe as a Model System. Proc. Natl. Acad. Sci. U. S. A 95, 3572–3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (115).Hansen AL, and Al-Hashimi HM (2006) Insight into the CSA tensors of nucleobase carbons in RNA polynucleotides from solution measurements of residual CSA: Towards new long-range orientational constraints. J. Magn. Reson 179, 299–307. [DOI] [PubMed] [Google Scholar]
- (116).Mustoe AM, Bailor MH, Teixeira RM, Brooks CL 3rd, and Al-Hashimi HM (2012) New insights into the fundamental role of topological constraints as a determinant of two-way junction conformation. Nucleic Acids Res 40, 892–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (117).Merriman DK, Xue Y, Yang S, Kimsey IJ, Shakya A, Clay M, and Al-Hashimi HM (2016) Shortening the HIV-1 TAR RNA Bulge by a Single Nucleotide Preserves Motional Modes over a Broad Range of Time Scales. Biochemistry 55, 4445–4456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (118).Getz M, Sun X, Casiano-Negroni A, Zhang Q, and Al-Hashimi HM (2007) Review NMR studies of RNA dynamics and structural plasticity using NMR residual dipolar couplings. Biopolymers 86, 384–402. [DOI] [PubMed] [Google Scholar]
- (119).Mustoe AM, Al-Hashimi HM, and Brooks CL 3rd (2014) Coarse grained models reveal essential contributions of topological constraints to the conformational free energy of RNA bulges. J. Phys. Chem. B 118, 2615–2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (120).Hermann T (2002) Rational ligand design for RNA: the role of static structure and conformational flexibility in target recognition. Biochimie 84, 869–875. [DOI] [PubMed] [Google Scholar]
- (121).Hermann T, and Tor Y (2005) RNA as a target for small-molecule therapeutics. Expert Opin. Ther. Pat 15, 49–62. [Google Scholar]
- (122).Stelzer AC, Frank AT, Kratz JD, Swanson MD, Gonzalez-Hernandez MJ, Lee J, Andricioaei I, Markovitz DM, and Al-Hashimi HM (2011) Discovery of selective bioactive small molecules by targeting an RNA dynamic ensemble. Nat. Chem. Biol 7, 553–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (123).Jaeger L, and Leontis NB (2000) Tecto-RNA: One-dimensional self-assembly through tertiary interactions. Angew. Chem., Int. Ed 39, 2521–2524. [DOI] [PubMed] [Google Scholar]
- (124).Buenrostro JD, Araya CL, Chircus LM, Layton CJ, Chang HY, Snyder MP, and Greenleaf WJ (2014) Quantitative analysis of RNA-protein interactions on a massively parallel array reveals biophysical and evolutionary landscapes. Nat. Biotechnol 32, 562–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (125).Denny SK, Bisaria N, Yesselman JD, Das R, Herschlag D, and Greenleaf WJ (2018) High-Throughput Investigation of Diverse Junction Elements in RNA Tertiary Folding. Cell 174, 377–390.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (126).Williamson JR (2000) Induced fit in RNA-protein recognition. Nat. Struct. Biol 7, 834–837. [DOI] [PubMed] [Google Scholar]
- (127).Higgs PG (2000) RNA secondary structure: physical and computational aspects. Q. Rev. Biophys 33, 199–253. [DOI] [PubMed] [Google Scholar]
- (128).Kim H, Jeong E, Lee SW, and Han K (2003) Computational analysis of hydrogen bonds in protein-RNA complexes for interaction patterns. FEBS Lett 552, 231–239. [DOI] [PubMed] [Google Scholar]
- (129).Zaratiegui M, Irvine DV, and Martienssen RA (2007) Noncoding RNAs and gene silencing. Cell 128, 763–776. [DOI] [PubMed] [Google Scholar]
- (130).Butcher S, and Pyle A (2011) The Molecular Interactions that Stabilize RNA Tertiary Structure: RNA Motifs, Patterns, and Networks. Acc. Chem. Res 44, 1302–1311. [DOI] [PubMed] [Google Scholar]
- (131).Gupta A, and Gribskov M (2011) The role of RNA sequence and structure in RNA–protein interactions. J. Mol. Biol 409, 574–587. [DOI] [PubMed] [Google Scholar]
- (132).Lewis BA, Walia RR, Terribilini M, Ferguson J, Zheng C, Honavar V, and Dobbs D (2011) PRIDB: a protein–RNA interface database. Nucleic Acids Res 39, D277–D282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (133).Thapar R, Denmon AP, and Nikonowicz EP (2014) Recognition modes of RNA tetraloops and tetraloop-like motifs by RNA-binding proteins. Wiley Interdiscip. Rev.: RNA 5, 49–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (134).Achsel T, and Bagni C (2016) Cooperativity in RNA-protein interactions: the complex is more than the sum of its partners. Curr. Opin. Neurobiol 39, 146–151. [DOI] [PubMed] [Google Scholar]
- (135).Li Y, Syed J, and Sugiyama H (2016) RNA-DNA Triplex Formation by Long Noncoding RNAs. Cell Chem. Biol 23, 1325–1333. [DOI] [PubMed] [Google Scholar]
- (136).Barra J, and Leucci E (2017) Probing Long Non-coding RNA-Protein Interactions. Front. Mol. Biosci 4, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (137).Aboul-ela F (2010) Strategies for the design of RNA-binding small molecules. Future Med. Chem 2, 93–119. [DOI] [PubMed] [Google Scholar]
- (138).Disney MD, and Angelbello AJ (2016) Rational Design of Small Molecules Targeting Oncogenic Noncoding RNAs from Sequence. Acc. Chem. Res 49, 2698–2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (139).McDowell SE, Špačková N. a., Šponer J, and Walter NG (2007) Molecular Dynamics Simulations of RNA: An In Silico Single Molecule Approach. Biopolymers 85, 169–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (140).Barbault F, Ren B, Rebehmed J, Teixeira C, Luo Y, Smila-Castro O, Maurel F, Fan B, Zhang L, and Zhang L (2008) Flexible computational docking studies of new aminoglycosides targeting RNA 16S bacterial ribosome site. Eur. J. Med. Chem 43, 1648–1656. [DOI] [PubMed] [Google Scholar]
- (141).Guilbert C, and James TL (2008) Docking to RNA via Root-Mean-Square-Deviation-Driven Energy Minimization with Flexible Ligands and Flexible Targets. J. Chem. Inf. Model 48, 1257–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (142).Fulle S, and Gohlke H (2009) Molecular recognition of RNA: challenges for modelling interactions and plasticity. J. Mol. Recognit 23, 220–231. [DOI] [PubMed] [Google Scholar]
- (143).Amaro RE, and Li WW (2010) Emerging methods for ensemble-based virtual screening. Curr. Top. Med. Chem 10, 3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (144).Puton T, Kozlowski L, Tuszynska I, Rother K, and Bujnicki JM (2012) Computational methods for prediction of protein-RNA interactions. J. Struct. Biol 179, 261–268. [DOI] [PubMed] [Google Scholar]
- (145).Philips A, Milanowska K, Lach G, and Bujnicki JM (2013) LigandRNA: computational predictor of RNA-ligand interactions. RNA 19, 1605–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (146).Feixas F, Lindert S, Sinko W, and McCammon JA (2014) Exploring the role of receptor flexibility in structure-based drug discovery. Biophys. Chem 186, 31–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (147).Eichhorn CD, Yang S, and Al-Hashimi HM (2012) Chapter 9: Characterising RNA Dynamics using NMR Residual Dipolar Couplings. In Recent Developments in Biomolecular NMR, pp 184–215, The Royal Society of Chemistry. [Google Scholar]
- (148).Ganser LR, Lee J, Rangadurai A, Merriman DK, Kelly ML, Kansal AD, Sathyamoorthy B, and Al-Hashimi HM (2018) High-performance virtual screening by targeting a high-resolution RNA dynamic ensemble. Nat. Struct. Mol. Biol 25, 425–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (149).Gilbert SD, Mediatore SJ, and Batey RT (2006) Modified Pyrimidines Specifically Bind the Purine Riboswitch. J. Am. Chem. Soc 128, 14214–14215. [DOI] [PubMed] [Google Scholar]
- (150).Kumar GS, and Basu A (2016) The use of calorimetry in the biophysical characterization of small molecule alkaloids binding to RNA structures. Biochim. Biophys. Acta, Gen. Subj 1860, 930–944. [DOI] [PubMed] [Google Scholar]
- (151).Vogel M, and Suess B (2016) Label-Free Determination of the Dissociation Constant of Small Molecule-Aptamer Interaction by Isothermal Titration Calorimetry. Methods Mol. Biol 1380, 113–125. [DOI] [PubMed] [Google Scholar]
- (152).Polaski JT, Webster SM, Johnson JE Jr., and Batey RT (2017) Cobalamin riboswitches exhibit a broad range of ability to discriminate between methylcobalamin and adenosylcobalamin. J. Biol. Chem 292, 11650–11658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (153).Mergny J-L, and Lacroix L (2003) Analysis of Thermal Melting Curves. Oligonucleotides 13, 515–537. [DOI] [PubMed] [Google Scholar]
- (154).Felden B (2007) RNA structure: experimental analysis. Curr. Opin. Microbiol 10, 286–291. [DOI] [PubMed] [Google Scholar]
- (155).Hendrix M, Priestley ES, Joyce GF, and Wong C-H (1997) Direct Observation of Aminoglycoside–RNA Interactions by Surface Plasmon Resonance. J. Am. Chem. Soc 119, 3641–3648. [DOI] [PubMed] [Google Scholar]
- (156).Frolov L, Dix A, Tor Y, Tesler AB, Chaikin Y, Vaskevich A, and Rubinstein I (2013) Direct Observation of Aminoglycoside–RNA Binding by Localized Surface Plasmon Resonance Spectroscopy. Anal. Chem 85, 2200–2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (157).Chang AL, McKeague M, Liang JC, and Smolke CD (2014) Kinetic and Equilibrium Binding Characterization of Aptamers to Small Molecules using a Label-Free, Sensitive, and Scalable Platform. Anal. Chem 86, 3273–3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (158).Fukuzumi T, Murata A, Aikawa H, Harada Y, and Nakatani K (2015) Exploratory Study on the RNA-Binding Structural Motifs by Library Screening Targeting pre-miRNA-29 a. Chem. - Eur. J 21, 16859–16867. [DOI] [PubMed] [Google Scholar]
- (159).Hilimire TA, Bennett RP, Stewart RA, Garcia-Miranda P, Blume A, Becker J, Sherer N, Helms ED, Butcher SE, Smith HC, and Miller BL (2016) N-Methylation as a Strategy for Enhancing the Affinity and Selectivity of RNA-binding Peptides: Application to the HIV-1 Frameshift-Stimulating RNA. ACS Chem. Biol 11, 88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (160).Butko MT, Moree B, Mortensen RB, and Salafsky J (2016) Detection of Ligand-Induced Conformational Changes in Oligonucleotides by Second-Harmonic Generation at a Supported Lipid Bilayer Interface. Anal. Chem 88, 10482–10489. [DOI] [PubMed] [Google Scholar]
- (161).Rizvi NF, Howe JA, Nahvi A, Klein DJ, Fischmann TO, Kim H-Y, McCoy MA, Walker SS, Hruza A, Richards MP, Chamberlin C, Saradjian P, Butko MT, Mercado G, Burchard J, Strickland C, Dandliker PJ, Smith GF, and Nickbarg EB (2018) Discovery of Selective RNA-Binding Small Molecules by Affinity-Selection Mass Spectrometry. ACS Chem. Biol 13, 820–831. [DOI] [PubMed] [Google Scholar]
- (162).Wang B, Wilkinson KA, and Weeks KM (2008) Complex ligand-induced conformational changes in tRN(Asp) revealed by single-nucleotide resolution SHAPE chemistry. Biochemistry 47, 3454–3461. [DOI] [PubMed] [Google Scholar]
- (163).Rice GM, Busan S, Karabiber F, Favorov OV, and Weeks KM (2014) Chapter Eight - SHAPE Analysis of Small RNAs and Riboswitches. In Methods in Enzymology (Burke-Aguero DH, Ed.) pp 165–187, Academic Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (164).Sztuba-Solinska J, Shenoy SR, Gareiss P, Krumpe LR, Le Grice SF, O’Keefe BR, and Schneekloth JS Jr. (2014) Identification of biologically active, HIV TAR RNA-binding small molecules using small molecule microarrays. J. Am. Chem. Soc 136, 8402–8410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (165).Dai Y, Wynn JE, Peralta AN, Sherpa C, Jayaraman B, Li H, Verma A, Frankel AD, Le Grice SF, and Santos WL (2018) Discovery of a Branched Peptide That Recognizes the Rev Response Element (RRE) RNA and Blocks HIV-1 Replication. J. Med. Chem 61, 9611–9620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (166).Mayer M, and James TL (2005) Discovery of ligands by a combination of computational and NMR-based screening: RNA as an example target. Methods Enzymol 394, 571–587. [DOI] [PubMed] [Google Scholar]
- (167).Moumné R, Catala M, Larue V, Micouin L, and Tisné C (2012) Fragment-based design of small RNA binders: Promising developments and contribution of NMR. Biochimie 94, 1607–1619. [DOI] [PubMed] [Google Scholar]
- (168).Sathyamoorthy B, Lee J, Kimsey I, Ganser LR, and Al-Hashimi H (2014) Development and application of aromatic [13C, 1H] SOFAST-HMQC NMR experiment for nucleic acids. J. Biomol. NMR 60, 77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (169).Patwardhan NN, Ganser LR, Kapral GJ, Eubanks CS, Lee J, Sathyamoorthy B, Al-Hashimi H, and Hargrove AE (2017) Amiloride as a new RNA-binding scaffold with activity against HIV-1 TAR. MedChemComm 8, 1022–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (170).Adamiak DA, Rypniewski WR, Milecki J, and Adamiak RW (2001) The 1.19 Å X-ray structure of 2′-O-Me(CGCGCG)2 duplex shows dehydrated RNA with 2-methyl-2,4-pentanediol in the minor groove. Nucleic Acids Res 29, 4144–4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (171).Edwards TE, and Ferre-D’Amare AR (2006) Crystal structures of the thi-box riboswitch bound to thiamine pyrophosphate analogs reveal adaptive RNA-small molecule recognition. Structure 14, 1459–1468. [DOI] [PubMed] [Google Scholar]
- (172).Ennifar E, Paillart J-C, Bodlenner A, Walter P, Weibel J-M, Aubertin A-M, Pale P, Dumas P, and Marquet R (2006) Targeting the dimerization initiation site of HIV-1 RNA with aminoglycosides: from crystal to cell. Nucleic Acids Res 34, 2328–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (173).Ippolito JA, Kanyo ZF, Wang D, Franceschi FJ, Moore PB, Steitz TA, and Duffy EM (2008) Crystal Structure of the Oxazolidinone Antibiotic Linezolid Bound to the 50S Ribosomal Subunit. J. Med. Chem 51, 3353–3356. [DOI] [PubMed] [Google Scholar]
- (174).Stagno JR, Liu Y, Bhandari YR, Conrad CE, Panja S, Swain M, Fan L, Nelson G, Li C, Wendel DR, White TA, Coe JD, Wiedorn MO, Knoska J, Oberthuer D, Tuckey RA, Yu P, Dyba M, Tarasov SG, Weierstall U, Grant TD, Schwieters CD, Zhang J, Ferre-D’Amare AR, Fromme P, Draper DE, Liang M, Hunter MS, Boutet S, Tan K, Zuo X, Ji X, Barty A, Zatsepin NA, Chapman HN, Spence JCH, Woodson SA, and Wang YX (2017) Structures of riboswitch RNA reaction states by mix-and-inject XFEL serial crystallography. Nature 541, 242–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (175).Bradrick TD, and Marino JP (2004) Ligand-induced changes in 2-aminopurine fluorescence as a probe for small molecule binding to HIV-1 TAR RNA. RNA 10, 1459–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (176).Kaul M, Barbieri CM, and Pilch DS (2004) Fluorescence-Based Approach for Detecting and Characterizing Antibiotic-Induced Conformational Changes in Ribosomal RNA: Comparing Aminoglycoside Binding to Prokaryotic and Eukaryotic Ribosomal RNA Sequences. J. Am. Chem. Soc 126, 3447–3453. [DOI] [PubMed] [Google Scholar]
- (177).Shandrick S, Zhao Q, Han Q, Ayida BK, Takahashi M, Winters GC, Simonsen KB, Vourloumis D, and Hermann T (2004) Monitoring Molecular Recognition of the Ribosomal Decoding Site. Angew. Chem., Int. Ed 43, 3177–3182. [DOI] [PubMed] [Google Scholar]
- (178).Tanpure AA, and Srivatsan SG (2015) Conformation-sensitive nucleoside analogues as topology-specific fluorescence turn-on probes for DNA and RNA G-quadruplexes. Nucleic Acids Res 43, e149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (179).Abulwerdi FA, Shortridge MD, Sztuba-Solinska J, Wilson R, Le Grice SF, Varani G, and Schneekloth JS Jr. (2016) Development of Small Molecules with a Noncanonical Binding Mode to HIV-1 Trans Activation Response (TAR) RNA. J. Med. Chem 59, 11148–11160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (180).Manna S, Panse CH, Sontakke VA, Sangamesh S, and Srivatsan SG (2017) Probing Human Telomeric DNA and RNA Topology and Ligand Binding in a Cellular Model by Using Responsive Fluorescent Nucleoside Probes. ChemBioChem 18, 1604–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (181).Zhang J, Umemoto S, and Nakatani K (2010) Fluorescent Indicator Displacement Assay for Ligand–RNA Interactions. J. Am. Chem. Soc 132, 3660–3661. [DOI] [PubMed] [Google Scholar]
- (182).Asare-Okai PN, and Chow CS (2011) A modified fluorescent intercalator displacement assay for RNA ligand discovery. Anal. Biochem 408, 269–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (183).Watkins D, Norris FA, Kumar S, and Arya DP (2013) A fluorescence-based screen for ribosome binding antibiotics. Anal. Biochem 434, 300–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (184).Hickey SF, and Hammond MC (2014) Structure-guided design of fluorescent S-adenosylmethionine analogs for a high-throughput screen to target SAM-I riboswitch RNAs. Chem. Biol 21, 345–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (185).Patwardhan NN, Cai Z, Newson CN, and Hargrove AE (2018) Fluorescent peptide displacement as a general assay for screening small molecule libraries against RNA. Org. Biomol. Chem, DOI: 10.1039/C8OB02467G. [DOI] [PMC free article] [PubMed]
- (186).Ratmeyer L, Zapp M, Green M, Vinayak R, Kumar A, Boykin DW, and Wilson WD (1996) Inhibition of HIV-1 Rev-RRE Interaction by Diphenylfuran Derivatives. Biochemistry 35, 13689–13696. [DOI] [PubMed] [Google Scholar]
- (187).Pushechnikov A, Lee MM, Childs-Disney JL, Sobczak K, French JM, Thornton CA, and Disney MD (2009) Rational design of ligands targeting triplet repeating transcripts that cause RNA dominant disease: application to myotonic muscular dystrophy type 1 and spinocerebellar ataxia type 3. J. Am. Chem. Soc 131, 9767–9779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (188).Donlic A, Morgan BS, Xu JL, Liu A, Roble C Jr., and Hargrove AE (2018) Discovery of small molecule ligands for MALAT1 by tuning an RNA-binding scaffold. Angew. Chem., Int. Ed 57, 13242–13247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (189).Sannes-Lowery KA, Griffey RH, and Hofstadler SA (2000) Measuring Dissociation Constants of RNA and Aminoglycoside Antibiotics by Electrospray Ionization Mass Spectrometry. Anal. Biochem 280, 264–271. [DOI] [PubMed] [Google Scholar]
- (190).Seth PP, Miyaji A, Jefferson EA, Sannes-Lowery KA, Osgood SA, Propp SS, Ranken R, Massire C, Sampath R, Ecker DJ, Swayze EE, and Griffey RH (2005) SAR by MS: Discovery of a New Class of RNA-Binding Small Molecules for the Hepatitis C Virus: Internal Ribosome Entry Site IIA Subdomain. J. Med. Chem 48, 7099–7102. [DOI] [PubMed] [Google Scholar]
- (191).Rosu F, De Pauw E, and Gabelica V (2008) Electrospray mass spectrometry to study drug-nucleic acids interactions. Biochimie 90, 1074–1087. [DOI] [PubMed] [Google Scholar]
- (192).Moon MH, Hilimire TA, Sanders AM, and Schneekloth JS (2018) Measuring RNA–Ligand Interactions with Microscale Thermophoresis. Biochemistry 57, 4638–4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (193).Connelly CM, Abulwerdi FA, and Schneekloth JS Jr (2017) Discovery of RNA Binding Small Molecules Using Small Molecule Microarrays. Methods Mol. Biol 1518, 157–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (194).Connelly CM, Boer RE, Moon MH, Gareiss P, and Schneekloth JS Jr. (2017) Discovery of Inhibitors of MicroRNA-21 Processing Using Small Molecule Microarrays. ACS Chem. Biol 12, 435–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (195).Tran T, and Disney MD (2010) Two-Dimensional Combinatorial Screening of a Bacterial rRNA A-Site-like Motif Library: Defining Privileged Asymmetric Internal Loops That Bind Aminoglycosides. Biochemistry 49, 1833–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (196).Velagapudi SP, Seedhouse SJ, and Disney MD (2010) Structure-activity relationships through sequencing (StARTS) defines optimal and suboptimal RNA motif targets for small molecules. Angew. Chem., Int. Ed 49, 3816–3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (197).Velagapudi SP, Pushechnikov A, Labuda LP, French JM, and Disney MD (2012) Probing a 2-Aminobenzimidazole Library for Binding to RNA Internal Loops via Two-Dimensional Combinatorial Screening. ACS Chem. Biol 7, 1902–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (198).Velagapudi SP, and Disney MD (2013) Defining RNA motif-aminoglycoside interactions via two-dimensional combinatorial screening and structure-activity relationships through sequencing. Bioorg. Med. Chem 21, 6132–6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (199).Velagapudi SP, and Disney MD (2014) Two-dimensional combinatorial screening enables the bottom-up design of a micro-RNA-10b inhibitor. Chem. Commun. (Cambridge, U. K.) 50, 3027–3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (200).Childs-Disney JL, Tran T, Vummidi BR, Velagapudi SP, Haniff HS, Matsumoto Y, Crynen G, Southern MR, Biswas A, Wang ZF, Tellinghuisen TL, and Disney MD (2018) A Massively Parallel Selection of Small Molecule-RNA Motif Binding Partners Informs Design of an Antiviral from Sequence. Chem 4, 2384–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (201).Folmer-Andersen JF, Kitamura M, and Anslyn EV (2006) Pattern-based discrimination of enantiomeric and structurally similar amino acids: an optical mimic of the mammalian taste response. J. Am. Chem. Soc 128, 5652–5653. [DOI] [PubMed] [Google Scholar]
- (202).Anzenbacher P Jr., Lubal P, Bucek P, Palacios MA, and Kozelkova ME (2010) A practical approach to optical cross-reactive sensor arrays. Chem. Soc. Rev 39, 3954–3979. [DOI] [PubMed] [Google Scholar]
- (203).Bushdid C, Magnasco MO, Vosshall LB, and Keller A (2014) Humans Can Discriminate More than 1 Trillion Olfactory Stimuli. Science 343, 1370–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (204).Hughes AD, Glenn IC, Patrick AD, Ellington A, and Anslyn EV (2008) A pattern recognition based fluorescence quenching assay for the detection and identification of nitrated explosive analytes. Chem. - Eur. J 14, 1822–1827. [DOI] [PubMed] [Google Scholar]
- (205).Palacios MA, Nishiyabu R, Marquez M, and Anzenbacher P Jr (2007) Supramolecular Chemistry Approach to the Design of a High-Resolution Sensor Array for Multianion Detection in Water. J. Am. Chem. Soc 129, 7538–7544. [DOI] [PubMed] [Google Scholar]
- (206).Umali AP, LeBoeuf SE, Newberry RW, Kim S, Tran L, Rome WA, Tian T, Taing D, Hong J, Kwan M, Heymann H, and Anslyn EV (2011) Discrimination of flavonoids and red wine varietals by arrays of differential peptidic sensors. Chem. Sci 2, 439–445. [Google Scholar]
- (207).Bajaj A, Miranda OR, Kim I-B, Phillips RL, Jerry DJ, Bunz UHF, and Rotello VM (2009) Detection and differentiation of normal, cancerous, and metastatic cells using nanoparticle-polymer sensor arrays. Proc. Natl. Acad. Sci. U. S. A 106, 10912–10916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (208).Bajaj A, Miranda OR, Phillips R, Kim I-B, Jerry DJ, Bunz UHF, and Rotello VM (2010) Array-Based Sensing of Normal, Cancerous, and Metastatic Cells Using Conjugated Fluorescent Polymers. J. Am. Chem. Soc 132, 1018–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (209).Tanpure AA, and Srivatsan SG (2012) Synthesis and Photophysical Characterisation of a Fluorescent Nucleoside Analogue that Signals the Presence of an Abasic Site in RNA. ChemBioChem 13, 2392–2399. [DOI] [PubMed] [Google Scholar]
- (210).Borra S, and Di Ciaccio A (2010) Measuring the prediction error. A comparison of cross-validation, bootstrap and covariance penalty methods. Computational Statistics & Data Analysis 54, 2976–2989. [Google Scholar]
- (211).Koutsoukas A, Paricharak S, Galloway WRJD, Spring DR, Ijzerman AP, Glen RC, Marcus D, and Bender A (2014) How Diverse Are Diversity Assessment Methods? A Comparative Analysis and Benchmarking of Molecular Descriptor Space. J. Chem. Inf. Model 54, 230–242. [DOI] [PubMed] [Google Scholar]
- (212).Cooper TA, Wan L, and Dreyfuss G (2009) RNA and Disease. Cell 136, 777–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (213).Buchmueller KL, and Weeks KM (2004) Tris-borate is a poor counterion for RNA: a cautionary tale for RNA folding studies. Nucleic Acids Res 32, e184–e184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (214).Lambert D, and Draper DE (2007) Effects of Osmolytes on RNA Secondary and Tertiary Structure Stabilities and RNA-Mg2+ Interactions. J. Mol. Biol 370, 993–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (215).Leamy KA, Yennawar NH, and Bevilacqua PC (2017) Cooperative RNA Folding under Cellular Conditions Arises From Both Tertiary Structure Stabilization and Secondary Structure Destabilization. Biochemistry 56, 3422–3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (216).Tyrrell J, Weeks KM, and Pielak GJ (2015) Challenge of mimicking the influences of the cellular environment on RNA structure by PEG-induced macromolecular crowding. Biochemistry 54, 6447–6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (217).Clouet-d’Orval B, Stage TK, and Uhlenbeck OC (1995) Neomycin Inhibition of the Hammerhead Ribozyme Involves Ionic Interactions. Biochemistry 34, 11186–11190. [DOI] [PubMed] [Google Scholar]
- (218).Hermann T (2000) Strategies for the design of drugs targeting RNA and RNA - Protein complexes. Angew. Chem., Int. Ed 39, 1890–1904. [DOI] [PubMed] [Google Scholar]
- (219).Basu P, and Suresh Kumar G (2017) Small molecule–RNA recognition: Binding of the benzophenanthridine alkaloids sanguinarine and chelerythrine to single stranded polyribonucleotides. J. Photochem. Photobiol., B 174, 173–181. [DOI] [PubMed] [Google Scholar]
- (220).Bailor MH, Mustoe AM, Brooks CL 3rd, and Al-Hashimi HM (2011) Topological constraints: using RNA secondary structure to model 3D conformation, folding pathways, and dynamic adaptation. Curr. Opin. Struct. Biol 21, 296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (221).Eubanks C, Zhao B, Patwardhan N, Thompson R, Zhang Q, and Hargrove A Visualizing RNA conformational changes via Pattern Recognition of RNA by Small Molecules. Manuscript under review [DOI] [PMC free article] [PubMed]
- (222).Jenkins JL, Krucinska J, McCarty RM, Bandarian V, and Wedekind JE (2011) Comparison of a preQ1 riboswitch aptamer in metabolite-bound and free states with implications for gene regulation. J. Biol. Chem 286, 24626–24637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (223).Baker JL, Sudarsan N, Weinberg Z, Roth A, Stockbridge RB, and Breaker RR (2012) Widespread genetic switches and toxicity resistance proteins for fluoride. Science 335, 233–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (224).Zhao C, and Marino JP (2007) Synthesis of HIV-1 Ψ-site RNA sequences with site specific incorporation of the fluorescent base analog 2-aminopurine. Tetrahedron 63, 3575–3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (225).Frommer J, Hieronymus R, Selvi Arunachalam T, Heeren S, Jenckel M, Strahl A, Appel B, and Müller S (2014) Preparation of modified long-mer RNAs and analysis of FMN binding to the ypaA aptamer from B. subtilis. RNA Biol 11, 609–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (226).Keyhani S, Goldau T, Blümler A, Heckel A, and Schwalbe H (2018) Chemo-Enzymatic Synthesis of Position-Specifically Modified RNA for Biophysical Studies including Light Control and NMR Spectroscopy. Angew. Chem., Int. Ed 57, 12017–12021. [DOI] [PubMed] [Google Scholar]


