Abstract
Background
Air leakage is a common complication after pulmonary wedge resection. The aim of this study was to evaluate the effect of staple line reinforcement in reducing air leakage after pulmonary wedge resection.
Methods
A retrospective analysis was performed on patients who underwent pulmonary wedge resection. The patients were classified into 2 groups; the Stapler with polyglycolic acid sheet was used for the reinforced and the Stapler without polyglycolic acid sheet was used for the non-reinforced group. The patients were matched one-to-one based on a propensity score that comprised several patient characteristics. A propensity score-matched analysis was performed to compare patient outcomes.
Results
A total of 291 patients who met the inclusion criteria were investigated. There were 165 in the reinforced group and 126 patients in the non-reinforced group. Propensity score analysis generated 104 matched pairs of patients in both the reinforced and the non-reinforced groups. The rate of non-placement of chest tube was significantly higher in the reinforced group than in the non-reinforced group (61.5% vs. 36.5%; P<0.001). The rate of postoperative air leakage was higher in the non-reinforced group than in the reinforced group (13.5% vs. 1.9%, P<0.001). On logistic regression analysis, not using the reinforcement device was one of the independent factors related to pulmonary air leakage after pulmonary wedge resection (OR: 8.58, P<0.001).
Conclusions
The use of the Stapler with polyglycolic acid sheet during pulmonary wedge resection increased the rate of intraoperative chest tube removal and reduced the rate of postoperative air leakage.
Keywords: Chest tubes, surgical fixation devices, propensity score, wedge resection
Introduction
Pulmonary wedge resection is practiced widely for metastatic lung cancer, benign lung tumors, lung biopsy (e.g., interstitial pneumonia) and pneumothorax. With the advancements in automatic suturing devices, pulmonary wedge resection has become a relatively safe method. Air leakage from the staple line is a major complication after pulmonary wedge resection (1). Since this problem cannot be avoided completely during resection of the lung parenchyma, prolonged air leakage causes long-term chest tube placement, which causes persistent pain and an extended hospital stay for patients (2,3). Furthermore, pleurodesis (4,5) or re-operation may be necessary if air leakage cannot be controlled. Therefore, surgeons must strive to reduce air leakage as much as possible.
Several materials have been commonly used for staple line reinforcement and include polyglycolic acid (PGA) felt, excised pleura, or bovine pericardium; these are then are fixed with fibrin glue on the staple line (6-11). Reinforcement with PGA felt, polytetrafluoroethylene felt, or excised pleura alone is often insufficient. Harvesting excised pleura takes extra time and is associated with a risk of bleeding. The use of a stapling device covered with various reinforcement sheets, such as PGA felt and polytetrafluoroethylene felt, are associated with some problems, including the extra time and effort needed to prepare the automatic suturing device with the reinforcement sheets and the fact that these reinforcement sheets could easily fall out of the cartridge upon a slight impact. On the other hand, the use of fibrin glue to fix these materials more strongly makes reoperation difficult, in case the patient suffers relapse or develops other diseases or extensive intrathoracic adhesions. Moreover, fibrin glue is a blood product that is commonly used for young patients with spontaneous pneumothorax and the use of blood products should be limited as much as possible.
In 2014, the Stapler with PGA sheet (Endo GIATM Reinforced Reload with Tri-StapleTM technology, Medtronic, Minneapolis, MN) was developed and made available for use. This device is preloaded with a fixed PGA felt [(NEOVEIL (GUNZE MEDICAL, Osaka, Japan)] on a cartridge with a thread. Therefore, preparation of the automatic suturing device does not take time and effort and the felt does not fall out of the cartridge upon a large impact. Although this technology is generally recognized to be useful in preventing air leakage after pulmonary resection, there had been no study that verified its effectiveness. The randomized trial would have been much better to compare older against newer staplers for wedge resection. However, it is hard to form because the general recognition that newer stapler might be useful than older. Since we decided to perform a propensity score matching to compensate retrospectively. In this retrospective observation study, we evaluated the usefulness of the Stapler with PGA sheet for preventing postoperative air leakage.
Methods
Patients selection
This retrospective cohort study reviewed the medical records of all patients who underwent pulmonary wedge resection between January 2013 and December 2017 at the Department of Thoracic Surgery of Iwate Medical University. This study was approved by the Iwate Medical University institutional review board, and informed consent was waived. We began to use the Stapler with PGA sheet (Endo GIATM Reinforced Reload with Tri-StapleTM technology) from February 2015. Data were compared between patients in whom the Stapler with PGA sheet was used (reinforced group) and patients in whom other device [Echelon (Johnson & Johnson, New Brunswick, NJ)] were used (non-reinforced group).
Patients who underwent either pulmonary lobectomy or segmentectomy were excluded because they required creation of interlobar fissures using tools other than the automatic suturing device. And patients with pneumothorax were excluded because pneumothorax, especially secondary spontaneous pneumothorax is a disease with air leakage from the beginning of surgery due to severe emphysema change. Cases in which fibrin glue or PGA sheet was used were also excluded. Air leakage was defined as prolonged when it lasted for 7 days or more (12). In cases of malignant disease, such as metastatic pulmonary tumor or primary lung cancer, the chest computed tomography (CT) was carefully examined before the operation to determine if the location of nodule could be identified at the time of thoracoscopy. Based on previous experience, nodules that were located deeper than 1 cm below the pleural surface or those that were less than 1 cm in size were assumed to be visually undetectable or non-palpable during exploratory thoracoscopy. In such cases, preoperative localization [GUIDING-MARKER SYSTEM® (Hakko Medical, Nagano, Japan)] was performed under CT guidance.
Surgical procedures
Thoracoscopic surgery was performed under general anesthesia with a double-lumen endotracheal tube for single-lung ventilation. The affected lung was deflated as soon as the pleural space was opened, and deflation was maintained throughout most of the operative period. For patients who have developed pneumothorax during preoperative localization, the side of the pneumothorax is blocked by single-lung ventilation immediately after intubation in order to avoid the tension pneumothorax. Thereafter, the patient was placed in the lateral decubitus position with the trocar placed in the posterior axillary line in the 6th intercostal space. General exploratory thoracoscopy was performed to determine the location of the target lesion. An additional intercostal incision was made under direct vision on the anterior or posterior line. Once the target lesion was identified, thoracoscopic wedge resection was performed. After resection, a sealing test was performed before the wound was closed. The sealing test was confirmed by re-inflating the lung on the affected side and with the use of a chest tube [16F thoracic UK catheter (Nipro, Osaka, Japan)] and a chest drain bag. For cases in which pulmonary fistula was completely denied, chest tube placement was avoided; otherwise, a 19F chest tube [Blake® silicon drain (Johnson & Johnson)] was placed from the additional 5th intercostal trocar into the apex.
Postoperative management
In general, patients were extubated at the end of the operation and were transferred to the ward after a brief stay in the recovery area. In cases that needed chest tube placement, a −5 cmH2O suction was added and then changed to water seal on the morning of postoperative day 1. A chest X-ray was obtained daily. The criteria for chest tube removal were (I) the absence of air leakage through the chest tube at the time of evaluation; (II) pleural fluid drainage was less than 200 mL for 24 hours; and (III) postoperative chest X-ray showed no pneumothorax. In the morning after chest tube removal, a chest X-ray was performed to rule out the occurrence of pneumothorax. The patients were discharged upon stabilization and if no complications occurred during the postoperative period.
Routine postoperative pain management was performed in all patients of both groups. Briefly, oral analgesia was started 6 hours after surgery and typically comprised loxoprofen 60 mg at 3 times per day, sometimes with diclofenac 25 mg suppository 1 to 2 times per day, as needed.
Statistical analysis
JMP 12.2.0 (SAS Institute, Cary, NC, USA) statistical software was used for statistical analysis. Groups were compared using the Pearson chi-square test. To control for the potential differences in the preoperative characteristics between patients in the 2 groups, a propensity score matching method was used. The propensity scores were generated using logistic regression based on clinically relevant preoperative variables, such as age, gender, height, pack years smoked, ECOG (Eastern Cooperative Oncology Group) performance status, and severity of COPD (chronic obstructive pulmonary disease), that were considered possible confounders due to their potential association with the outcome of interest, based on clinical knowledge. We have defined COPD as less than 70% of FEV1%. Patients were matched 1:1 by nearest neighbor matching (caliper width: 0.2) without replacement. Comparisons between the matched groups were performed with the McNemar’s test for categorical variables and paired t-test or Wilcoxon’s rank-sum test for continuous variables. The standardized difference was used to measure covariate balance, where an absolute standardized difference above 0.1 represented meaningful imbalance.
The multivariate predictors were evaluated using logistic regression analysis, and the odds ratios (ORs) and 95% confidence intervals (CIs) were estimated. On logistic regression analysis, the conventional receiver-operating characteristic (ROC) curve was used to determine the cut-off value for each variable that gave the highest sensitivity and specificity in predicting postoperative prolonged air leakage. Differences between groups were considered significant at P<0.05. Continuous data were expressed as mean ± SD. Categorical data were expressed as counts and proportions.
Results
A total of 512 patients who underwent pulmonary wedge resection were included in this study (Figure 1). The excluded patients included 180 in whom underwent surgery for pneumothorax, 1 in whom fibrin glue was used, and 40 in whom fibrin glue and PGA sheet were used. Finally, 291 patients who met the inclusion criteria were investigated; there were 165 patients in the reinforced group and 126 patients in the non-reinforced group. The patient characteristics are summarized in Table 1. Propensity score analysis generated 104 matched pairs of patients in both groups (Table 2), 63.0% (104/165) in the reinforced group and 82.5% (104/126) in the non-reinforced group. After matching, there were no significant differences in the preoperative variables, such as age, gender, height, Brinkman index, pulmonary function, population of COPD, performance status, and tumor size between the groups. The balance of each sample size was assessed by standardized differences, and the values of preoperative variables were almost under 0.1.
Figure 1.
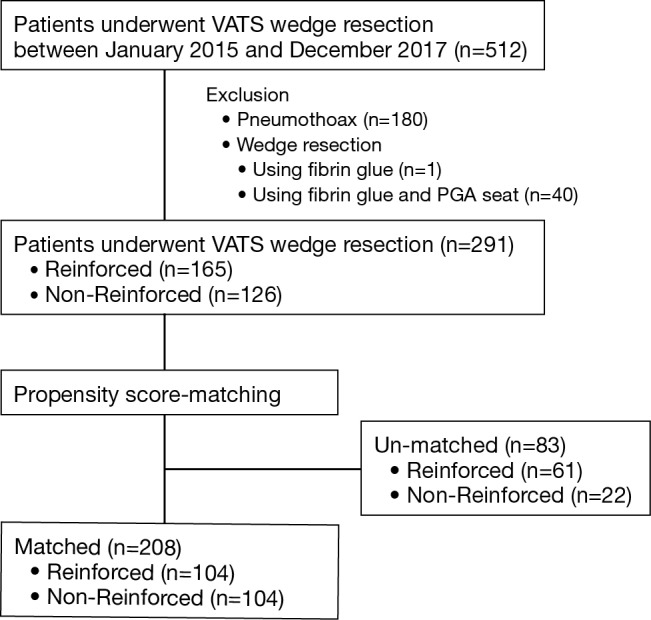
Flowchart of the study population inclusion. From January 2015 to December 2017, 512 patients underwent pulmonary wedge resection at the Department of Thoracic Surgery, Iwate Medical University; of these 208 patients were analyzed and matched by propensity score. PGA, polyglycolic acid.
Table 1. Characteristics and outcomes of this study.
| Characteristics | All patients | P | |
|---|---|---|---|
| Stapler with PGA sheet (n=165) | Stapler (n=126) | ||
| Age (years) | 64.8±13.3 | 65.9±10.5 | 0.964 |
| Gender, n (%) | 0.834 | ||
| Male | 87 (52.7) | 68 (54.0) | |
| Female | 78 (47.3) | 58 (46.0) | |
| Height (cm) | 160.0±9.5 | 159.3±8.7 | 0.493 |
| Brinkman Index | 317.6±455.4 | 377.9±479.2 | 0.374 |
| Population of never smoker, n (%) | 86 (52.1) | 65 (51.6) | 0.531 |
| Performance status, n (%) | 0.458 | ||
| 0 | 162 (98.2) | 125 (99.2) | |
| 1 | 3 (1.8) | 1 (0.8) | |
| Preoperative pulmonary function | |||
| VC (mL) | 3,184.0±810.9 | 3,263.7±845.0 | 0.567 |
| FEV1 (mL) | 2,362.6±631.9 | 2,472.8±760.2 | 0.332 |
| FEV1% (%) | 75.4±9.9 | 76.6±10.6 | 0.501 |
| %DLCO (%) | 108.7±24.6 | 114.2±21.8 | 0.051 |
| GOLD classification, n (%) | 0.370 | ||
| 1 | 23 (13.9) | 11 (8.7) | |
| 2 | 12 (7.3) | 9 (7.1) | |
| 3 | 2 (1.2) | 2 (1.6) | |
| Tumor size (mm) | 14.0±8.6 | 11.4 ±5.1 | 0.008 |
| Operation time (min) | 73.3±39.5 | 66.1±32.3 | 0.026 |
| Blood loss (mL) | 8.6±49.7 | 4.7±10.0 | 0.095 |
| Non-placement of chest tube, n (%) | 101 (61.2) | 47 (37.3) | <0.001 |
| Period of chest tube placement (days) | 0.7±1.1 | 1.0±1.2 | 0.002 |
| Post-operative stay (days) | 4.0±3.4 | 4.2±2.9 | 0.320 |
| Postoperative air leakage, n (%) | 3 (1.8) | 15 (11.9) | <0.001 |
| Prolonged air leakage (>7 days) | 0 (0) | 1 (0.8) | 0.253 |
FEV1, forced expiratory volume in one second; FEV1%, FEV1/VC ratio; GODL, global initiative for chronic obstructive lung disease; VC, vital capacity; %DLCO, carbon monoxide diffusing capacity.
Table 2. Characteristics and outcomes after propensity score matching.
| Characteristics | After propensity score matching | Standardized difference | P | |
|---|---|---|---|---|
| Stapler with PGA sheet (n=104) | Stapler (n=104) | |||
| Age (years) | 65.6±12.3 | 65.3±10.6 | 0.026 | 0.439 |
| Gender | 0.488 | |||
| Male | 51 (49.0) | 56 (53.8) | 0.096 | |
| Female | 53 (51.0) | 48 (46.2) | ||
| Height (cm) | 159.7±10.4 | 159.8±8.6 | 0.010 | 0.993 |
| Brinkman Index | 372.2±506.5 | 351.4±461.8 | 0.043 | 0.955 |
| Population of never smoker (%) | 50 (48.0) | 50 (48.0) | 0.000 | 1.000 |
| Performance status, n (%) | 1.000 | |||
| 0 | 103 (99.0) | 103 (99.0) | 0.000 | |
| 1 | 1 (1.0) | 1 (1.0) | ||
| Preoperative pulmonary function | ||||
| VC (mL) | 3,106.7±827.7 | 3,312.3±862.2 | 0.243 | 0.118 |
| FEV1 (mL) | 2,313.7±641.5 | 2,494.3±777.7 | 0.253 | 0.123 |
| FEV1% (%) | 75.6±9.4 | 75.9±9.9 | 0.031 | 0.794 |
| %DLCO (%) | 107.7±24.8 | 113.6±21.8 | 0.253 | 0.063 |
| GOLD classification, n (%) | 0.122 | 0.738 | ||
| 1 | 13 (12.5) | 9 (8.7) | ||
| 2 | 7 (6.7) | 8 (7.7) | ||
| 3 | 2 (1.9) | 2 (1.9) | ||
| Tumor size (mm) | 11.7±5.0 | 11.9 ±5.2 | 0.039 | 0.870 |
| Operation time (min) | 72.9±39.5 | 65.7±30.6 | 0.204 | 0.158 |
| Blood loss (mL) | 3.3±4.8 | 4.2±9.9 | 0.116 | 0.281 |
| Non-placement of chest tube, n (%) | 64 (61.5) | 38 (36.5) | 0.516 | <0.001 |
| Period of chest tube placement (days) | 0.7±1.0 | 1.1±1.3 | 0.345 | 0.002 |
| Post-operative stay (days) | 3.6±2.1 | 4.3±3.0 | 0.270 | 0.115 |
| Postoperative air leakage, n (%) | 2 (1.9) | 14 (13.5) | 0.444 | 0.002 |
| Prolonged air leakage (>7 days) | 0 (0) | 1 (0.9) | 0.139 | 0.316 |
FEV1, forced expiratory volume in one second; FEV1%, FEV1/VC ratio; GODL, global initiative for chronic obstructive lung disease; VC, vital capacity; %DLCO, carbon monoxide diffusing capacity.
Compared with the non-reinforced group, the reinforced group had significantly higher rate of intraoperative chest tube removal (61.5% vs. 36.5%; P<0.001); significantly shorter duration of indwelling chest tube (0.7±1.0 vs. 1.1±1.3 days; P=0.002); and significantly lower rate of postoperative air leakage (1.9% vs. 13.5%; P=0.002). The operation time and blood loss were not significantly different between the groups after matching. Multivariate logistic regression analysis (Table 3) showed that the significant predictive factors for prolonged air leakage after surgery was non-reinforcement (OR 8.58, P<0.001).
Table 3. Predictive factors for postoperative air leakage.
| Variable | After propensity score matching | ||
|---|---|---|---|
| OR | 95% CI | P | |
| Age (≥70 years) | 0.74 | 0.19–2.51 | 0.639 |
| Gender (male) | 2.04 | 0.45–9.76 | 0.353 |
| Height (≥160 cm) | 1.77 | 0.11–1.87 | 0.281 |
| Brinkman Index (≥400) | 0.47 | 0.11–1.87 | 0.281 |
| COPD (presence) | 2.86 | 0.73–10.43 | 0.128 |
| Performance status (≥1) | 1.20 | 0.01–4.43 | 0.538 |
| Tumor size (≥15 mm) | 0.86 | 0.25–3.16 | 0.808 |
| Stapler | 8.58 | 2.25–56.50 | <0.001 |
OR, odds ratio; CI, confidence interval; COPD, chronic obstructive pulmonary disease.
Discussion
Postoperative pain can predispose to several medical complications, such as pneumonia, deep vein thrombosis, infection, chronic pain, and depression (13). For thoracic surgery, postoperative pain is more often due to chest tube placement rather than skin incision (3,14). In many cases that undergo pulmonary surgery, a chest tube is routinely placed in order to obtain information about massive bleeding and massive air leakage, and for drainage of blood and air leakage. In general, intraoperative removal of the chest tube greatly contributes to the reduction of postoperative pain (2,15,16). For cases of primary lung cancer, a 19F blake® silicon drain was routinely placed during radical surgery, such as pneumonectomy, pulmonary lobectomy, and pulmonary segmentectomy. On the other hand, a chest tube was not routinely placed for cases of pulmonary wedge resection if the intraoperative sealing test confirmed the absence of air leakage. In 2018 Park et al. described prospective randomized trial that by removing chest tube intraoperative against spontaneous pneumothorax, it resulted in reduction of postoperative pain and shortening hospital stay (17). In 2016 Holbek et al. described prospective observational study that pulmonary wedge resection without postoperative chest tube is safe (18). In this study, the chest tube was removed intraoperatively in 148 of 291 (50.9%) patients. Only one patient (0.7%) had a collapsed lung on postoperative chest X-ray and needed chest tube reinsertion before the effect of anesthesia weaned off; this patient was included in the non-reinforced group and was not counted as non-placement of chest tube. There were no patients who need drainage for massive pleural effusion during the observation period. No patient developed any other complications by non-placement of a chest tube. In other words, routinely placing a chest tube may mean that unnecessary tube were placed in 147 patients (99.3%) who remove chest tube intraoperative. Therefore, intraoperative chest tube removal was considered safe only if the patient passed a sealing test. This study showed that the use of the Stapler with PGA sheet during pulmonary wedge resection may increase the rate of intraoperative chest tube removal, which may contribute greatly in reducing postoperative pain (2). In addition, a low rate of chest tube placement may help reduce the risks of self-removal or accidental removal and wound infection, as well as the adverse effects of rehabilitation. In this study, the reinforced group had shortened period of chest tube placement, but there was no shortening of postoperative hospital stay. In previous studies, a shorter duration of chest tube placement resulted in a shorter hospital stay (19,20). We supposed that our medical area (Iwate prefecture in Japan) is inconvenient for public transportation, family comes to pick up patients after 2–4 days from the day of allowing to leave hospital. The same operation time and blood loss between the groups can be explained by the fact that the use of the Stapler with PGA sheet did not require a complicated preparation for the surgeons and the operating room staff, compared with that of the other devices (21,22). Therefore, the use of such a device did not cause any physical burden on the patients due to a long operative time.
This study had some limitations. First of all, this study was retrospective study. Therefore, we could not mention the degree of pain, difference in cost, comparison with equipment with more similar stapler. I hope to consider these matters in a prospective study in the future. Next, even if we used propensity score matching, that not all of the preoperative differences between groups were compensated for by the propensity score matching despite real efforts to control possible biases. Even so, propensity score matching is one of the useful methods that is effective when prediction is already attached to results to some extent and it is difficult to form a random comparison test ethically. And further this study was a single hospital study, and the number of patients included is small. Therefore, a multicenter prospective study is required to validate our results.
In conclusion, this study demonstrated that the use of the Stapler with PGA sheet during pulmonary wedge resection reduced the rate of postoperative air leakage.
Acknowledgements
None.
Ethical Statement: This study was approved by the Iwate Medical University institutional review board as number H29-88. The patient’s personal data have been secured.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Brunelli A, Varela G, Refai M, et al. A scoring system to predict the risk of prolonged air leak after lobectomy. Ann Thorac Surg 2010;90:204-9. 10.1016/j.athoracsur.2010.02.054 [DOI] [PubMed] [Google Scholar]
- 2.Nadlonek NA, Acker SN, Deterding RR, et al. Intraoperative chest tube removal following thoracoscopic lung biopsy results in improved outcomes. J Pediatr Surg 2014;49:1573-6. 10.1016/j.jpedsurg.2014.04.008 [DOI] [PubMed] [Google Scholar]
- 3.Miyazaki T, Sakai T, Yamasaki N, et al. Chest tube insertion is one important factor leading to intercostal nerve impairment in thoracic surgery. Gen Thorac Cardiovasc Surg 2014;62:58-63. 10.1007/s11748-013-0328-z [DOI] [PubMed] [Google Scholar]
- 4.Okereke I, Murthy SC, Alster JM, et al. Characterization and importance of air leak after lobectomy. Ann Thorac Surg 2005;79:1167-73. 10.1016/j.athoracsur.2004.08.069 [DOI] [PubMed] [Google Scholar]
- 5.Liberman M, Muzikansky A, Wright CD, et al. Incidence and risk factors of persistent air leak after major pulmonary resection and use of chemical pleurodesis. Ann Thorac Surg 2010;89:891-7; discussion 897-8. 10.1016/j.athoracsur.2009.12.012 [DOI] [PubMed] [Google Scholar]
- 6.Vaughn CC, Vaughn PL, Vaughn CC, 3rd, et al. Tissue response to biomaterials used for staple-line reinforcement in lung resection: a comparison between expanded polytetrafluoroethylene and bovine pericardium. Eur J Cardiothorac Surg 1998;13:259-65. 10.1016/S1010-7940(98)00012-8 [DOI] [PubMed] [Google Scholar]
- 7.Whitlark JD, Hsu HK. Technique to reduce air leaks after the resection of emphysematous lung. Ann Thorac Surg 1994;58:1560. 10.1016/0003-4975(94)91966-6 [DOI] [PubMed] [Google Scholar]
- 8.Moser C, Opitz I, Zhai W, et al. Autologous fibrin sealant reduces the incidence of prolonged air leak and duration of chest tube drainage after lung volume reduction surgery: a prospective randomized blinded study. J Thorac Cardiovasc Surg 2008;136:843-9. 10.1016/j.jtcvs.2008.02.079 [DOI] [PubMed] [Google Scholar]
- 9.Kawamura M, Kase K, Sawafuji M, et al. Staple-line reinforcement with a new type of polyglycolic acid felt. Surg Laparosc Endosc Percutan Tech 2001;11:43-6. 10.1097/00129689-200102000-00012 [DOI] [PubMed] [Google Scholar]
- 10.Hashimoto A, Kuwabara M, Hirasaki Y, et al. Reduction of air leaks in a canine model of pulmonary resection with a new staple-line buttress. J Thorac Cardiovasc Surg 2011;142:366-71. 10.1016/j.jtcvs.2011.05.001 [DOI] [PubMed] [Google Scholar]
- 11.Miller JI, Jr, Landreneau RJ, Wright CE, et al. A comparative study of buttressed versus nonbuttressed staple line in pulmonary resections. Ann Thorac Surg 2001;71:319-22; discussion 323. 10.1016/S0003-4975(00)02203-7 [DOI] [PubMed] [Google Scholar]
- 12.Rivera C, Bernard A, Falcoz PE, et al. Characterization and prediction of prolonged air leak after pulmonary resection: a nationwide study setting up the index of prolonged air leak. Ann Thorac Surg 2011;92:1062-8; discussion 1068. 10.1016/j.athoracsur.2011.04.033 [DOI] [PubMed] [Google Scholar]
- 13.Meissner W, Coluzzi F, Fletcher D, et al. Improving the management of post-operative acute pain: priorities for change. Curr Med Res Opin 2015;31:2131-43. 10.1185/03007995.2015.1092122 [DOI] [PubMed] [Google Scholar]
- 14.Refai M, Brunelli A, Salati M, et al. The impact of chest tube removal on pain and pulmonary function after pulmonary resection. Eur J Cardiothorac Surg 2012;41:820-2; discussion 823. 10.1093/ejcts/ezr126 [DOI] [PubMed] [Google Scholar]
- 15.Ueda K, Hayashi M, Tanaka T, et al. Omitting chest tube drainage after thoracoscopic major lung resection. Eur J Cardiothorac Surg 2013;44:225-9; discussion 229. 10.1093/ejcts/ezs679 [DOI] [PubMed] [Google Scholar]
- 16.Deng B, Qian K, Zhou JH, et al. Optimization of Chest Tube Management to Expedite Rehabilitation of Lung Cancer Patients After Video-Assisted Thoracic Surgery: A Meta-Analysis and Systematic Review. World J Surg 2017;41:2039-45. 10.1007/s00268-017-3975-x [DOI] [PubMed] [Google Scholar]
- 17.Park JB, Hwang JJ, Lee WS, et al. Postoperative chest tube placement after thoracoscopic wedge resection of lung for primary spontaneous pneumothorax: is it mandatory? J Thorac Dis 2018;10:4812-8. 10.21037/jtd.2018.07.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holbek BL, Hansen HJ, Kehlet H, et al. Thoracoscopic pulmonary wedge resection without post-operative chest drain: an observational study. Gen Thorac Cardiovasc Surg 2016;64:612-7. 10.1007/s11748-016-0692-6 [DOI] [PubMed] [Google Scholar]
- 19.Pompili C, Detterbeck F, Papagiannopoulos K, et al. Multicenter international randomized comparison of objective and subjective outcomes between electronic and traditional chest drainage systems. Ann Thorac Surg 2014;98:490-6; discussion 496-7. 10.1016/j.athoracsur.2014.03.043 [DOI] [PubMed] [Google Scholar]
- 20.Brunelli A, Salati M, Refai M, et al. Evaluation of a new chest tube removal protocol using digital air leak monitoring after lobectomy: a prospective randomised trial. Eur J Cardiothorac Surg 2010;37:56-60. 10.1016/j.ejcts.2009.05.006 [DOI] [PubMed] [Google Scholar]
- 21.Catanzarite T, Saha S, Pilecki MA, et al. Longer Operative Time During Benign Laparoscopic and Robotic Hysterectomy Is Associated With Increased 30-Day Perioperative Complications. J Minim Invasive Gynecol 2015;22:1049-58. 10.1016/j.jmig.2015.05.022 [DOI] [PubMed] [Google Scholar]
- 22.Ando M, Takahashi Y, Kikuchi T. Short operation time: an important element to reduce operative invasiveness in pediatric cardiac surgery. Ann Thorac Surg 2005;80:631-5. 10.1016/j.athoracsur.2005.02.087 [DOI] [PubMed] [Google Scholar]


