Abstract
Background
Occult nodal metastasis results in a poor prognosis for lung cancer patients. The aim of this study was to develop an efficient approach for predicting occult nodal metastasis in peripheral clinical stage I lung adenocarcinoma.
Methods
Data for 237 peripheral clinical stage I lung adenocarcinoma patients who underwent complete resection were retrospectively reviewed. Univariate and multivariate analyses were performed to investigate predictors of occult nodal metastasis. Kaplan-Meier analysis was performed for survival.
Results
Occult nodal metastasis was detected in 26/237 (11.0%) patients. Nodule type, tumor SUVmax, whole tumor size, solid tumor size, and preoperative serum carcinoembryonic antigen (CEA) were identified as preoperative predictors of occult nodal metastasis (all P<0.05). Solid tumor size (P<0.001) and preoperative serum CEA (P=0.004) were identified as independent predictors on multivariate analysis. A prediction model was established using the independent predictors. The occult nodal metastasis rate was 2.4% with solid tumor size ≤2.3 cm (low-risk group), 17.0% with solid tumor size >2.3 cm and CEA ≤5 ng/mL (moderate-risk group), and 56.0% with solid tumor size >2.3 cm and CEA >5 ng/mL (high-risk group). The occult nodal metastasis rate was significantly higher in papillary-predominant (11.0%) and solid-predominant subtypes (28.6%; P=0.001). Patients having a micropapillary component had a significantly higher occult nodal metastasis rate (24.2%) compared with no micropapillary component (P=0.007). Histological subtype with micropapillary component and all preoperative predictors were significant prognostic factors affecting disease-free survival (DFS) (all P<0.05).
Conclusions
A novel approach to predict occult nodal metastasis was developed for peripheral clinical stage I lung adenocarcinoma. It would be helpful for selecting candidates for stereotactic ablative radiotherapy (SABR) or wedge resection and mediastinoscopy or endobronchial ultrasound transbronchial needle aspiration (EBUS-TBNA). Complete nodal dissection should be performed for moderate to high-risk patients or patients with poor histologic subtypes.
Keywords: Lung adenocarcinoma, occult nodal metastasis, solid tumor size, preoperative serum carcinoembryonic antigen (preoperative serum CEA), histologic subtype
Introduction
Anatomic resection and systematic nodal dissection are recognized as the standard approach to treatment for stage I non-small cell lung cancer (NSCLC) patients. However, almost 25% of patients are not amenable to standard surgery because they refuse or for medical reasons (1); in these circumstances, wedge resection and stereotactic ablative radiotherapy (SABR) are considered alternative treatments (2,3). For the extent of nodal dissection, systematic sampling and lobe-specific selective nodal dissection have currently been performed in surgery for early stage lung cancer patients. In these approaches, no systematic nodal dissection is the clear difference from standard surgical treatment. However, one cannot ignore the risk that there may be undetected microscopic hilar or mediastinal lymph node metastases, which would be expected to progress and result in a poor prognosis. In addition, whether preoperative mediastinoscopy or endobronchial ultrasound transbronchial needle aspiration (EBUS-TBNA) should be routinely performed for clinical stage I NSCLC patients and the standard criteria for candidates remain controversial (4). Therefore, it is important to predict occult lymph node metastasis as accurately as possible, which will help us to formulate individualized treatment strategies.
The main methods used to predict lymph node metastasis in NSCLC in current clinical practice include high-resolution computed tomography (HRCT), integrated 18F-fluoro-2-deoxy-d-glucose positron emission tomography/computed tomography (FDG-PET/CT), and mediastinoscopy or EBUS-TBNA. Preoperative mediastinoscopy or EBUS-TBNA is often used to classify N stage with reliable pathological diagnosis before surgery. However, as an invasive diagnostic method, due to issues of cost-effectiveness and possible complications, mediastinoscopy and EBUS-TBNA are not performed routinely, especially for early-stage NSCLC patients. During the last 10-20 years, FDG-PET/CT as a noninvasive examination has been gradually used to stage NSCLC instead of CT. FDG-PET/CT has better accuracy for clinical N stage than CT, because the functional scans obtained with FDG-PET/CT are not only complementary to those obtained with conventional modalities, but they may also be more sensitive to alterations in tissue metabolism that generally precede anatomic changes (5). FDG-PET/CT was found to provide a sensitivity of 79–85% and a specificity of 87–92% for identifying lymph node metastasis in NSCLC (6,7). However, with sensitivity of 79–85%, occult nodal metastasis still occurs in 15–21% of cases, which cannot be ignored.
In the present study, occult nodal metastasis was analyzed in peripheral clinical stage I lung adenocarcinoma patients. Independent predictors for occult nodal metastasis were identified. A concise and effective prediction model for predicting occult nodal metastasis in peripheral clinical stage I lung adenocarcinoma was established. The relationship between histologic subtypes of lung adenocarcinoma and occult nodal metastasis was also analyzed.
Methods
Patient inclusion criteria
A total of 237 lung adenocarcinoma patients who underwent anatomical resection (including 18 segmentectomies and 219 lobectomies; wedge resection was excluded) and systematic regional lymph node dissection (ND2a-1 or greater according to the criterion of The Japan Lung Cancer Society) in Hirosaki University Hospital from January 2008 to December 2017 were included (Figure 1). Clinical staging was performed based on the HRCT and FDG-PET/CT reports. The selection criteria were as follows: (I) clinical stage I lung adenocarcinoma patients according to the TNM Classification of Malignant Tumors (8th Edition) (8); (II) incompletely resected tumors, R1 or R2, were excluded; (III) patients who underwent preoperative neoadjuvant chemotherapy or radiotherapy were excluded; (IV) both HRCT and FDG-PET/CT were performed for patients and were limited to within 3 months prior to surgery; and (V) patients with a centrally located tumor were excluded. No patient underwent preoperative mediastinoscopy. Preoperative EBUS-TBNA was performed for 2 patients after FDG-PET/CT before surgery, and both results were negative.
Figure 1.
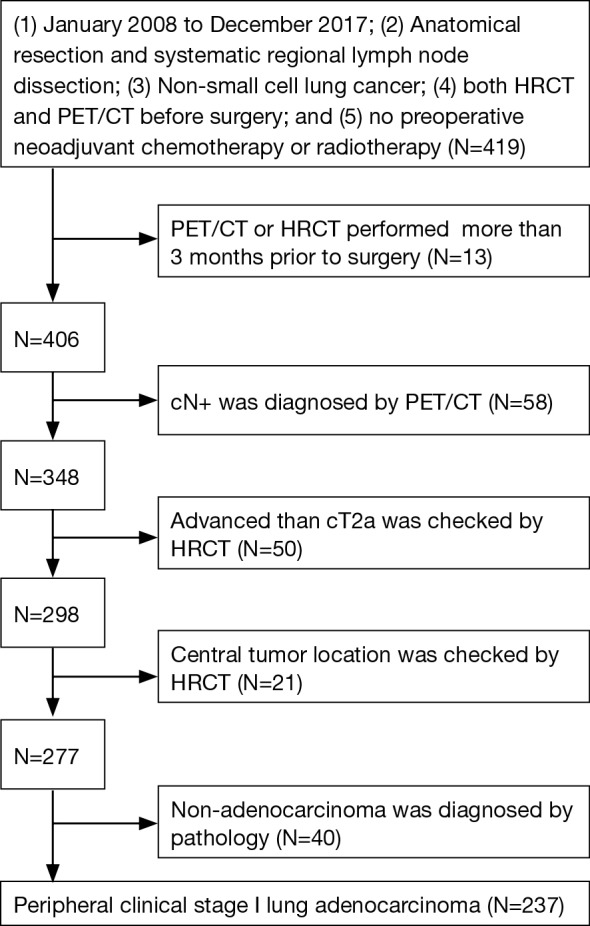
Patient selection flow chart.
HRCT
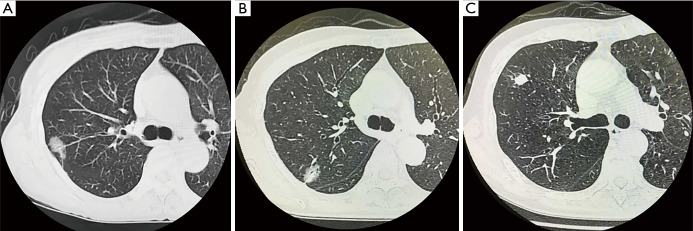
HRCT scans or contrast-enhanced HRCT scans were performed for each patient within 3 months prior to surgery. Each HRCT report was reviewed retrospectively. Whole tumor size was defined as the maximum dimension of the lung windows, and solid tumor size was defined as excluding the ground-glass opacity (GGO) area (Figure 2) (9). The primary tumor nodule type was divided into three groups: pure GGO type (with no solid area), partial solid type (with GGO area and solid area), and solid type (only solid area) (9). Central tumor was defined as a tumor whose center was in the inner 1/3 of the lung parenchyma (adjacent to the mediastinum); peripheral tumor was defined as a tumor whose center was in the outer 2/3 of the lung parenchyma on transverse CT scans (10). The results of whole tumor size, solid tumor size, nodule type, and tumor location were collected from the HRCT reports. Clinical T stage was re-evaluated by two thoracic surgeons (CY Song and D Kimura) from the results of HRCT reports, because the criteria for T staging were updated in TNM Classification of Malignant Tumors (8th Edition).
Figure 2.
Examples of whole and solid tumor sizes on high-resolution computed tomography. (A) Pure GGO type: whole tumor size 2.2 cm and solid tumor size 0 cm; (B) partial solid type: whole tumor size 2.1 cm and solid tumor size 1.1 cm; (C) solid type: whole tumor size 1.5 cm and solid tumor size 1.5 cm. GGO, ground-glass opacity.
FDG-PET/CT
All patients were assessed using FDG-PET/CT within 3 months of surgery. Each FDG-PET/CT examination was diagnosed by two experienced nuclear medicine physicians when the examination was done. SUVmax was normalized to the weight of each patient and measured by region of interest (ROI). Irrespective of the size, hilar and mediastinal lymph nodes were staged as cN0 if lymph nodes had SUVmax ≤2.5. Clinical N+ was diagnosed based on the experience of the nuclear medicine physicians.
Evaluation of pathologic findings
All intraoperative and postoperative specimens were examined by a pathologist. All pathology reports were retrospectively reviewed. Pathologic nodal stage was classified as pN0, pN1, or pN2 for all patients. For pathological subtype, 199 patients were diagnosed by new standard criteria according to the International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society in 2011 (11). The remaining 38 cases diagnosed by WHO (3th Edition) were not analyzed for pathologic subtype.
Follow-up
Each patient was followed-up by HRCT or FDG-PET/CT every year for at least 5 years after surgery. The deadline for follow-up was December 2017. Whenever recurrence was suspected, the interval of follow-up was shortened, and recurrence was determined by pathological or unequivocal radiological results. Disease-free survival (DFS) was defined as the time from the date of operation to recurrence.
Statistical analysis
Statistical analyses were carried out using the Statistical Package for the Social Sciences (SPSS) (version 22, IBM, Armonk, NY, USA). The optimal cut-off values for SUVmax, whole tumor size, and solid tumor size were assessed for predicting occult nodal metastasis by receiver operating characteristic (ROC) curve analysis. Univariate data analysis was conducted by Fisher’s exact test or Pearson’s chi-squared (χ2) test. Multivariate analysis was conducted by the logistic regression method (Forward: LR). DFS was analyzed by the Kaplan-Meier method and the log-rank test. Significance was accepted as a P value less than 0.05 for all analyses.
Results
Patients’ characteristics
A total of 237 patients (107 males, 130 females) were analyzed in the present study, including 15 pure GGO, 99 partial solid, and 123 solid cases. The patients’ ages ranged from 32 to 81 years (median 67 years). Median whole tumor size was 2.3 cm (range. 0.8–5.4 cm), and median solid tumor size was 1.8 cm (range. 0–4.0 cm). The median values of primary tumor SUVmax and preoperative serum CEA were 2.8 and 2.7 ng/mL, respectively. A total of 26 (11.0%) cases had occult nodal metastasis, including 11 (4.6%) pN1 and 15 (6.3%) pN2. The number of resected lymph nodes was 20.2±10.8 in all cases, 19.9±10.8 in the pN0 group, 23.4±10.5 in the pN+ group, and 21.5±10.9 in the pN2 group, with no significant difference (Table 1, P>0.05).
Table 1. Univariate analysis for occult pathological N upstaging and occult pathological N2 upstaging.
| Variable | Occult nodal metastasis | Occult mediastinal nodal metastasis | |||||
|---|---|---|---|---|---|---|---|
| pN0 (n=211) (%) | pN+ (n=26) (%) | P | pN0 + pN1 (n=222) (%) | pN2 (n=15) (%) | P | ||
| Age (years) | 0.515 | 0.406 | |||||
| <65 | 73 (86.9) | 11 (13.1) | 77 (91.7) | 7 (8.3) | |||
| ≥65 | 138 (90.2) | 15 (9.8) | 145 (94.8) | 8 (5.2) | |||
| Sex | 0.300 | 0.792 | |||||
| Male | 98 (91.6) | 9 (8.4) | 101 (94.4) | 6 (5.6) | |||
| Female | 113 (86.9) | 17 (13.1) | 121 (93.1) | 9 (6.9) | |||
| Smoking index | 1.000 | 0.790 | |||||
| <400 | 122 (89.1) | 15 (10.9) | 129 (94.2) | 8 (5.8) | |||
| ≥400 | 89 (89.0) | 11 (11.0) | 93 (93.0) | 7 (7.0) | |||
| Tumor side | 0.673 | 1.000 | |||||
| Left | 86 (90.5) | 9 (9.5) | 89 (93.7) | 6 (6.3) | |||
| Right | 125 (88.0) | 17 (12.0) | 133 (93.7) | 9 (6.3) | |||
| Tumor localization | 0.927 | 0.610 | |||||
| Upper lobe | 127 (88.8) | 16 (11.2) | 133 (93.0) | 10 (7.0) | |||
| Middle lobe | 12 (92.3) | 1 (7.7) | 13 (100.0) | 0 (0.0) | |||
| Lower lobe | 72 (88.9) | 9 (11.1) | 76 (93.8) | 5 (6.2) | |||
| Nodule type | 0.002 | 0.072 | |||||
| Pure GGO | 15 (100.0) | 0 (0.0) | 15 (100.0) | 0 (0.0) | |||
| Partial solid | 95 (96.0) | 4 (4.0) | 96 (97.0) | 3 (3.0) | |||
| Solid | 101 (82.1) | 22 (17.9) | 111 (90.2) | 12 (9.8) | |||
| Tumor SUVmax | <0.001 | 0.002 | |||||
| ≤3.4 | 136 (97.1) | 4 (2.9) | 137 (97.9) | 3 (2.1) | |||
| >3.4 | 75 (77.3) | 22 (22.7) | 85 (87.6) | 12 (12.4) | |||
| Whole tumor size (cm) | <0.001 | 0.003 | |||||
| ≤2.3 | 118 (97.5) | 3 (2.5) | 119 (98.3) | 2 (1.7) | |||
| >2.3 | 93 (80.2) | 23 (19.8) | 103 (88.8) | 13 (11.2) | |||
| Solid tumor size (cm) | <0.001 | <0.001 | |||||
| ≤2.3 | 161 (97.6) | 4 (2.4) | 162 (98.2) | 3 (1.8) | |||
| >2.3 | 50 (69.4) | 22 (30.6) | 60 (83.3) | 12 (16.7) | |||
| CEA (ng/mL) | <0.001 | 0.014 | |||||
| ≤5 | 178 (93.3) | 12 (6.7) | 182 (95.8) | 8 (4.2) | |||
| >5 | 33 (70.2) | 14 (29.8) | 40 (85.1) | 7 (14.9) | |||
| Lymph nodes resected, No. | 19.9±10.8 | 23.4±10.5 | 0.120 | 20.2±10.8 | 21.5±10.9 | 0.670 | |
Pure GGO, pure ground-glass opacity; SUVmax, maximal standardized uptake value; CEA, carcinoembryonic antigen; smoking index, the average root number per day multiplied by smoking years.
Optimal cutoff points for predicting occult nodal metastasis
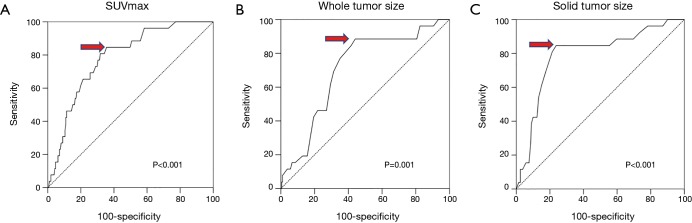
The ROC curves for primary tumor SUVmax, whole tumor size, and solid tumor size are shown in Figure 3A,B,C. The area under the ROC curve (AUC) values for SUVmax, whole tumor size, and solid tumor size were 0.783 [95% confidence interval (CI): 0.702–0.865], 0.707 (95% CI: 0.609–0.805), and 0.791 (95% CI: 0.695–0.887), respectively. The optimal cutoff points were 3.45 for SUVmax, 2.35 cm for whole tumor size, and 2.35 cm for solid tumor size. The patients were divided into two groups according to the optimal cutoff points for further analysis.
Figure 3.
Receiver operating characteristic curves illustrating the ability of (A) SUVmax, (B) whole tumor size, and (C) solid tumor size to predict occult lymph node metastasis in patients with peripheral clinical stage I lung adenocarcinoma. The areas under the curves for SUVmax, whole tumor size, and solid tumor size are (A) 0.783 (95% CI: 0.702–0.865), (B) 0.707 (95% CI: 0.609–0.805), and (C) 0.791 (95% CI: 0.695–0.887), respectively. The optimal cutoff points are: (A) 3.45 for SUVmax, (B) 2.35 cm for whole tumor size, and (C) 2.35 cm for solid tumor size. SUVmax, maximal standardized uptake value; AUC, area under the curve; CI, confidence interval.
Preoperative predictors for occult nodal metastasis and occult mediastinal nodal metastasis
Univariate analysis was performed to identify potential predictors of occult nodal metastasis and occult mediastinal nodal metastasis. The results showed that nodule type (P=0.002), primary tumor SUVmax (P<0.001), whole tumor size (P<0.001), solid tumor size (P<0.001), and preoperative serum CEA (P<0.001) were the predictors for occult nodal metastasis; primary tumor SUVmax (P=0.002), whole tumor size (P=0.003), solid tumor size (P<0.001), and preoperative serum CEA (P=0.014) were the predictors for occult mediastinal nodal metastasis (Table 1).
Independent predictors for occult nodal metastasis and occult mediastinal nodal metastasis
Multivariate analysis was performed to identify independent predictors for occult nodal metastasis and occult mediastinal nodal metastasis. The results showed that solid tumor size (P<0.001) and preoperative serum CEA (P=0.004) were the independent predictors for occult nodal metastasis, while solid tumor size was the independent predictor for occult mediastinal nodal metastasis (Table 2, P<0.001).
Table 2. Multivariate analysis for occult pathological N upstaging and occult pathological N2 upstaging.
| Variables | For occult pathologic N upstaging | For occult pathologic N2 upstaging | |||
|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | ||
| Age | N/A | 0.124 | N/A | 0.224 | |
| Sex | N/A | 0.144 | N/A | 0.525 | |
| Smoking index | N/A | 0.322 | N/A | 0.927 | |
| Tumor side | N/A | 0.875 | N/A | 0.746 | |
| Tumor localization | N/A | 0.691 | N/A | 0.515 | |
| Nodule type | N/A | 0.722 | N/A | 0.947 | |
| Tumor SUVmax | N/A | 0.243 | N/A | 0.262 | |
| CEA | 3.981 (1.548–10.24) | 0.004 | N/A | 0.132 | |
| Whole tumor size | N/A | 0.766 | N/A | 0.792 | |
| Solid tumor size | 14.02 (4.517–43.50) | <0.001 | 10.80(2.945-39.60) | <0.001 | |
SUVmax, maximal standardized uptake value; CEA, carcinoembryonic antigen; smoking index, the average root number per day multiplied by smoking years; CI, confidence interval.
Establishment of a novel prediction model by independent predictors
Four subgroups were divided according to solid tumor size and preoperative serum CEA and analyzed (Table 3). The occult nodal metastasis rate was 2.8% (4/143) in the solid tumor size ≤2.3 cm and CEA ≤5.0 ng/mL group; 0% (0/22) in the solid tumor size ≤2.3 cm and CEA >5.0 ng/mL group; 17.0% (8/47) in the solid tumor size >2.3 cm and CEA ≤5.0 ng/mL group; and 56.0% (14/25) in the solid tumor size >2.3 cm and CEA >5.0 ng/mL group. The occult mediastinal nodal metastasis rate was 2.1% (3/143) in the solid tumor size ≤2.3 cm and CEA ≤5.0 ng/mL group; 0% (0/22) in the solid tumor size ≤2.3 cm and CEA >5.0 ng/mL group; 10.6% (5/47) in the solid tumor size >2.3 cm and CEA ≤5.0 ng/mL group; and 28.0% (7/25) in the solid tumor size >2.3 cm and CEA >5.0 ng/mL group. Accordingly, solid tumor size ≤2.3 cm and CEA ≤5 ng/mL or CEA >5 ng/mL was defined as the low-risk group (2.4% occult nodal metastasis), solid tumor size >2.3 cm and CEA ≤5.0 ng/mL was defined as the moderate-risk group, and solid tumor size >2.3 cm and CEA >5.0 ng/mL was defined as the high-risk group.
Table 3. Prediction model for occult nodal metastasis and occult mediastinal nodal metastasis CEA: carcinoembryonic antigen.
| Risk definition | Predictor | Total | Occult nodal metastasis | Occult mediastinal nodal metastasis | |||||
|---|---|---|---|---|---|---|---|---|---|
| pN0 (%) | pN+ (%) | P | pN0 + pN1 (%) | pN2 (%) | P | ||||
| Low risk | Solid tumor size ≤2.3 cm; CEA ≤5.0 ng/mL | 143 | 139 (97.2) | 4 (2.8) | <0.001 | 140 (97.9) | 3 (2.1) | <0.001 | |
| Solid tumor size ≤2.3 cm; CEA >5.0 ng/mL | 22 | 22 (100) | 0 (0) | 22 (100) | 0 (0) | ||||
| Moderate risk | Solid tumor size >2.3 cm; CEA ≤5.0 ng/mL | 47 | 39 (83.0) | 8 (17.0) | 42 (89.4) | 5 (10.6) | |||
| High risk | Solid tumor size >2.3 cm; CEA >5.0 ng/mL | 25 | 11 (44.0) | 14 (56.0) | 18 (72.0) | 7 (28.0) | |||
CEA, carcinoembryonic antigen.
Association of pathological subtypes with occult nodal metastasis and occult mediastinal nodal metastasis
No occult nodal metastasis was found in adenocarcinoma in situ (0/4), minimally invasive (0/8), invasive mucinous (0/4), enteric (0/2), lepidic-predominant (0/12), and acinar-predominant (0/7) subtypes. Compared with these subtypes, the occult nodal metastasis rate was higher in the papillary-predominant subtype (17/154, 11.0%) and in the solid-predominant subtype (2/7, 28.6%) (P=0.001, Table 4). One micropapillary-predominant case was found to have occult nodal metastasis in the present study. The solid component-positive group did not have significantly more occult nodal metastases (P=0.273, Table 4). Compared with the no micropapillary component, having a micropapillary component had a significantly higher occult nodal metastasis rate (24.2%) (P=0.007) and occult mediastinal nodal metastasis rate (15.2%) (P=0.013, Table 4).
Table 4. Association of pathological subtypes with occult nodal metastasis and occult mediastinal nodal metastasis.
| Histologic subtype | Total | Occult nodal metastasis | Occult mediastinal nodal metastasis | |||||
|---|---|---|---|---|---|---|---|---|
| pN0 (%) | pN+ (%) | P | pN0 + pN1 (%) | pN2 (%) | P | |||
| Subtype [1] | 0.001 | 0.017 | ||||||
| Papillary-predominant | 154 | 137 (89.0) | 17 (11.0) | 146 (94.8) | 8 (5.2) | |||
| Solid-predominant | 7 | 5 (71.4) | 2 (28.6) | 5 (71.4) | 2 (28.6) | |||
| Micropapillary-predominant | 1 | 0 (0) | 1 (100.0) | 1 (100.0) | 0 (0) | |||
| Other subtypes | 37 | 37 (100.0) | 0 (0) | 37 (100.0) | 0 (0) | |||
| Subtype [2] | 0.273 | 0.105 | ||||||
| Solid component (+) | 24 | 20 (83.3) | 4 (16.7) | 21 (87.5) | 3 (12.5) | |||
| Solid component (−) | 175 | 159 (90.9) | 16 (9.1) | 168 (96.0) | 7 (4.0) | |||
| Subtype [3] | 0.007 | 0.013 | ||||||
| Micropapillary component (+) | 33 | 25 (75.8) | 8 (24.2) | 28 (84.8) | 5 (15.2) | |||
| Micropapillary component (−) | 166 | 154 (92.8) | 12 (7.2) | 161 (97.0) | 5 (3.0) | |||
Other subtypes (37 cases) included adenocarcinoma in situ (4 cases), minimally invasive (8 cases), invasive mucinous (4 cases), enteric (2 cases), lepidic predominant (12 cases) and acinar predominant (7 cases).
Association of clinicopathological factors with DFS
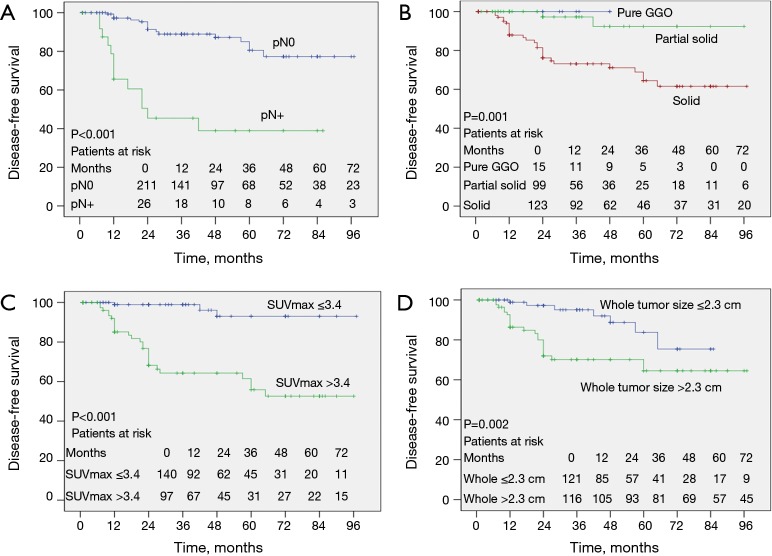
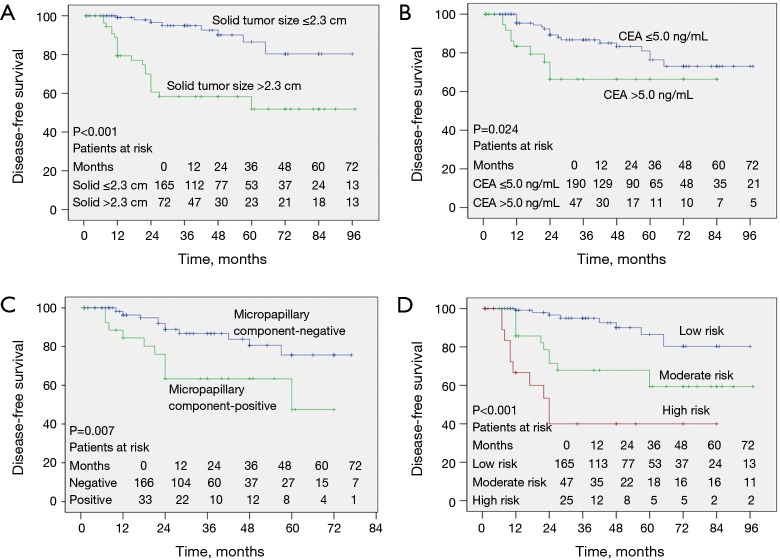
Kaplan-Meier analysis showed that occult nodal metastasis (Figure 4A, P<0.001), solid tumor type (Figure 4B, P=0.001), primary tumor SUVmax >3.4 (Figure 4C, P<0.001), whole tumor size >2.3 cm (Figure 4D, P=0.002), solid tumor size >2.3 cm (Figure 5A, P<0.001), preoperative CEA >5.0 ng/mL (Figure 5B, P=0.024), and having a micropapillary component (Figure 5C, P=0.007) predicted a worse DFS. In addition, the prognosis became gradually worse from low to moderate to high-risk groups of the preoperative prediction model (Figure 5D, P<0.001).
Figure 4.
Kaplan-Meier curves comparing disease-free survival time between groups according to (A) pathological N stage, (B) nodule type, (C) SUVmax of primary tumor, (D) whole tumor size. Pure GGO, pure ground-glass opacity; SUVmax, maximal standardized uptake value.
Figure 5.
Kaplan-Meier curves comparing disease-free survival time between groups according to (A) solid tumor size, (B) preoperative CEA, (C) micropapillary component, and (D) risk groups of the prediction model. CEA, carcinoembryonic antigen.
Discussion
Occult nodal metastasis was analyzed in 237 peripheral clinical stage I lung adenocarcinoma patients in the present study. Clinical stage was classified by HRCT and FDG-PET/CT. Occult nodal metastasis was detected in 11.0% (26/237) of peripheral clinical stage I lung adenocarcinoma patients. Nodule type, primary tumor SUVmax, whole tumor size, solid tumor size, and preoperative serum CEA were identified as preoperative predictors, while solid tumor size and preoperative serum CEA were identified as independent preoperative predictors. A concise prediction model for occult nodal metastasis was established with solid tumor size and preoperative serum CEA, which defined cases with low, moderate, and high risks. For histological subtype, the occult nodal metastasis rate was higher in papillary-predominant, solid-predominant, and micropapillary component-positive subtypes, being especially higher in solid-predominant and micropapillary component-positive subtypes than in other subtypes. Kaplan-Meier analysis showed that occult nodal metastasis had a significant negative effect on recurrence in stage I adenocarcinoma patients. Histological subtype with micropapillary component, solid tumor type, primary tumor SUVmax >3.4, whole tumor size >2.3 cm, solid tumor size >2.3 cm, preoperative CEA >5.0 ng/mL, and the moderate to high-risk groups of the prediction model predicted worse DFS.
The solid area of the primary tumor has been considered more important for predicting occult nodal metastasis than whole tumor size (12). According to the Union for International Cancer Control 8th Edition (UICC 8th Edition), the maximum dimension of the solid component, but not the whole component, is used to assign the T category in NSCLC. That means, compared with whole tumor size, solid tumor size plays a more important role in determining the malignancy of NSCLC. Solid tumor size was reported to have greater predictive value than whole tumor size for high-grade malignancy and prognosis in clinical stage IA lung adenocarcinoma (13). Tsutani et al. (14) reported that preoperative solid tumor size on HRCT was useful for predicting pN0 in clinical stage IA lung adenocarcinoma, which may be helpful for avoiding systematic lymphadenectomy. Maeyashiki et al. (12) reported that the maximum dimension of consolidation on thin-section CT was a significant factor for predicting nodal metastasis in clinical stage IA lung cancer patients. In the present study, although both whole tumor size and solid tumor size were predictors for occult nodal metastases, solid tumor size, but not whole tumor size, was identified as an independent predictor. Therefore, solid tumor size had higher efficiency than whole tumor size for predicting occult lymph node metastasis in peripheral clinical stage I lung adenocarcinoma.
CEA is the most commonly used tumor marker in clinical practice. Inoue et al. (15) found that a high preoperative serum CEA level was related to a higher rate of lymph node metastasis and predicted a worse prognosis in small-sized peripheral NSCLC. In the present study, preoperative serum CEA was identified as an independent predictor of occult node metastasis for peripheral clinical stage I lung adenocarcinoma. Based on the results of the prediction model, CEA showed high efficiency for predicting occult nodal metastasis when solid tumor size was greater than 2.3 cm. The preoperative serum CEA level was routinely checked for lung cancer patients, and it should be used to estimate the risk of occult nodal metastasis for early-stage lung adenocarcinoma.
Anatomical lobectomy and systemic nodal dissection are recognized as a standard approach to treatment for stage I lung adenocarcinoma patients. Anatomical segmentectomy is an option in some cases of early-stage peripheral pulmonary cancer according to the National Comprehensive Cancer Network guidelines. However, not all patients are amenable to standard surgery due to medical reasons or personal refusal. SABR and wedge resection are considered optional treatments for such patients (16,17), which could spare lung parenchyma and be well tolerated. However, there might be undetected microscopic hilar or mediastinal lymph node metastasis, which would be expected to progress and result in a worse prognosis (18). Better selection of candidates would enhance the reliability of the treatment effect of SABR and wedge resection. According to the prediction model established, SABR and wedge resection are good alternative treatments to anatomical resection for low-risk patients. However, moderate and high-risk patients are not good candidates for SABR or wedge resection because of the high incidence of occult nodal metastasis. For high-risk patients, if SABR or wedge resection was performed as a reluctant choice, preventive chemotherapy and close follow-up should be performed. For moderate-risk patients, preventive chemotherapy or close follow-up with special attention to the lymph nodes should be performed after wedge resection or SABR.
Mediastinoscopy and EBUS-TBNA are used to assess mediastinal lymph node metastasis in NSCLC patients. However, the standard criteria for candidates are controversial, especially for early-stage patients. Postoperative N upstaging in early-stage NSCLC was found to strongly predict a poor prognosis (19), and patients might benefit from neoadjuvant therapy followed by surgery if mediastinal node metastasis is identified before surgery (20,21). However, due to issues of cost-effectiveness and possible complications, mediastinoscopy and EBUS-TBNA were not routinely performed for early-stage NSCLC patients. According to the prediction model, high-risk patients might benefit from mediastinoscopy or EBUS-TBNA because of the high possibility of occult mediastinal lymph node metastasis. However, moderate and low-risk patients are not good candidates because of the costs and complications.
Histologic subtype in lung adenocarcinoma has been shown to have a strong association with occult nodal metastasis (9,22-25). Dai et al. (22) reported that presence of a micropapillary component was an independent predictor of a higher possibility of micrometastasis. In the study of Hung et al. (23), micropapillary or solid pattern component was a predictor of occult mediastinal lymph node metastasis in tumors ≤3 cm. In the present study, occult nodal metastasis was more frequently observed in micropapillary component-positive or solid-predominant subtypes. A positive micropapillary component predicted significantly worse DFS. In addition, in papillary-predominant subtype, occult nodal metastasis was observed in 11.0% of cases, which should not be ignored either. In clinical practice, it was sometimes hard to diagnose pathological subtype and the component of lung adenocarcinoma by preoperative biopsy because of limited tissue. In such cases, pathological subtype and component could not be used as a preoperative predictor. However, pathological subtype and component could be diagnosed by frozen section during operation in most cases, which was meaningful for the choice of the extent of lymph node dissection. Combined with the preoperative predictors, complete nodal dissection should be performed for patients with moderate to high occult nodal metastasis risk evaluated before operation, or for patients with solid-predominant, papillary-predominant, or micropapillary component-positive subtype diagnosed by intraoperative frozen section.
This study has several limitations: (I) potential selection bias, given the nature of a retrospective study; (II) although FDG-PET/CT and HRCT were limited to within 3 months before surgery, tumor might progress during that period, which might cause bias; and (III) the limited number of cases might cause potential bias, especially for the analysis of histologic subtype.
In conclusion, a novel approach to predict occult nodal metastasis was developed for peripheral clinical stage I lung adenocarcinoma patients. It would be helpful in selecting candidates for SABR or wedge resection and mediastinoscopy or EBUS-TBNA. Complete nodal dissection should be performed for moderate to high-risk patients identified before operation or patients with poor histologic subtype diagnosed by intraoperative frozen section.
Acknowledgements
None.
Ethical Statement: This study was approved by The Committee of Medical Ethics of Hirosaki University Graduate School of Medicine, Hirosaki, Japan (approval number 2018-1063).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Li L, Ren S, Zhang Y, et al. Risk factors for predicting the occult nodal metastasis in T1-2N0M0 NSCLC patients staged by PET/CT: potential value in the clinic. Lung Cancer 2013;81:213-7. 10.1016/j.lungcan.2013.04.012 [DOI] [PubMed] [Google Scholar]
- 2.Landreneau RJ, Sugarbaker DJ, Mack MJ, et al. Wedge resection versus lobectomy for stage I (T1 N0 M0) non-small-cell lung cancer. J Thorac Cardiovasc Surg 1997;113:691-8; discussion 698-700. 10.1016/S0022-5223(97)70226-5 [DOI] [PubMed] [Google Scholar]
- 3.Ezer N, Veluswamy RR, Mhango G, et al. Outcomes after Stereotactic Body Radiotherapy versus Limited Resection in Older Patients with Early-Stage Lung Cancer. J Thorac Oncol 2015;10:1201-6. 10.1097/JTO.0000000000000600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meyers BF, Haddad F, Siegel BA, et al. Cost-effectiveness of routine mediastinoscopy in computed tomography- and positron emission tomography-screened patients with stage I lung cancer. J Thorac Cardiovasc Surg 2006;131:822-9; discussion 829. 10.1016/j.jtcvs.2005.10.045 [DOI] [PubMed] [Google Scholar]
- 5.Gupta NC, Tamim WJ, Graeber GG, et al. Mediastinal lymph node sampling following positron emission tomography with fluorodeoxyglucose imaging in lung cancer staging. Chest 2001;120:521-7. 10.1378/chest.120.2.521 [DOI] [PubMed] [Google Scholar]
- 6.Birim O, Kappetein AP, Stijnen T, et al. Meta-analysis of positron emission tomographic and computed tomographic imaging in detecting mediastinal lymph node metastases in nonsmall cell lung cancer. Ann Thorac Surg 2005;79:375-82. 10.1016/j.athoracsur.2004.06.041 [DOI] [PubMed] [Google Scholar]
- 7.Toloza EM, Harpole L, McCrory DC. Noninvasive staging of non-small cell lung cancer: a review of the current evidence. Chest 2003;123:137S-46S. 10.1378/chest.123.1_suppl.137S [DOI] [PubMed] [Google Scholar]
- 8.Detterbeck FC, Boffa DJ, Kim AW, et al. The Eighth Edition Lung Cancer Stage Classification. Chest 2017;151:193-203. [DOI] [PubMed] [Google Scholar]
- 9.Travis WD, Asamura H, Bankier AA, et al. The IASLC Lung Cancer Staging Project: Proposals for Coding T Categories for Subsolid Nodules and Assessment of Tumor Size in Part-Solid Tumors in the Forthcoming Eighth Edition of the TNM Classification of Lung Cancer. J Thorac Oncol 2016;11:1204-23. [DOI] [PubMed] [Google Scholar]
- 10.Silvestri GA, Gould MK, Margolis ML, et al. Noninvasive staging of non-small cell lung cancer: ACCP evidenced-based clinical practice guidelines (2nd edition). Chest 2007;132:178S-201S. [DOI] [PubMed] [Google Scholar]
- 11.Travis WD, Brambilla E, Noguchi M, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society International Multidisciplinary Classification of Lung Adenocarcinoma. J Thorac Oncol 2011;6:244-85. 10.1097/JTO.0b013e318206a221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maeyashiki T, Suzuki K, Hattori A, et al. The size of consolidation on thin-section computed tomography is a better predictor of survival than the maximum tumour dimension in resectable lung cancer. Eur J Cardiothorac Surg 2013;43:915-8. 10.1093/ejcts/ezs516 [DOI] [PubMed] [Google Scholar]
- 13.Tsutani Y, Miyata Y, Nakayama H, et al. Prognostic significance of using solid versus whole tumor size on high-resolution computed tomography for predicting pathologic malignant grade of tumors in clinical stage IA lung adenocarcinoma: a multicenter study. J Thorac Cardiovasc Surg 2012;143:607-12. 10.1016/j.jtcvs.2011.10.037 [DOI] [PubMed] [Google Scholar]
- 14.Tsutani Y, Miyata Y, Nakayama H, et al. Prediction of pathologic node-negative clinical stage IA lung adenocarcinoma for optimal candidates undergoing sublobar resection. J Thorac Cardiovasc Surg 2012;144:1365-71. 10.1016/j.jtcvs.2012.07.012 [DOI] [PubMed] [Google Scholar]
- 15.Inoue M, Minami M, Shiono H, et al. Clinicopathologic study of resected, peripheral, small-sized, non-small cell lung cancer tumors of 2 cm or less in diameter: pleural invasion and increase of serum carcinoembryonic antigen level as predictors of nodal involvement. J Thorac Cardiovasc Surg 2006;131:988-93. 10.1016/j.jtcvs.2005.12.035 [DOI] [PubMed] [Google Scholar]
- 16.Grills IS, Mangona VS, Welsh R, et al. Outcomes after stereotactic lung radiotherapy or wedge resection for stage I non-small-cell lung cancer. J Clin Oncol 2010;28:928-35. 10.1200/JCO.2009.25.0928 [DOI] [PubMed] [Google Scholar]
- 17.Ricardi U, Filippi AR, Guarneri A, et al. Stereotactic body radiation therapy for early stage non-small cell lung cancer: results of a prospective trial. Lung Cancer 2010;68:72-7. 10.1016/j.lungcan.2009.05.007 [DOI] [PubMed] [Google Scholar]
- 18.Burdick MJ, Stephans KL, Reddy CA, et al. Maximum standardized uptake value from staging FDG-PET/CT does not predict treatment outcome for early-stage non-small-cell lung cancer treated with stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys 2010;78:1033-9. 10.1016/j.ijrobp.2009.09.081 [DOI] [PubMed] [Google Scholar]
- 19.Koike T, Tsuchiya R, Goya T, et al. Prognostic factors in 3315 completely resected cases of clinical stage I non-small cell lung cancer in Japan. J Thorac Oncol 2007;2:408-13. 10.1097/01.JTO.0000268674.02744.f9 [DOI] [PubMed] [Google Scholar]
- 20.Uy KL, Darling G, Xu W, et al. Improved results of induction chemoradiation before surgical intervention for selected patients with stage IIIA-N2 non-small cell lung cancer. J Thorac Cardiovasc Surg 2007;134:188-93. 10.1016/j.jtcvs.2007.01.078 [DOI] [PubMed] [Google Scholar]
- 21.Scagliotti GV, Pastorino U, Vansteenkiste JF, et al. Randomized phase III study of surgery alone or surgery plus preoperative cisplatin and gemcitabine in stages IB to IIIA non-small-cell lung cancer. J Clin Oncol 2012;30:172-8. 10.1200/JCO.2010.33.7089 [DOI] [PubMed] [Google Scholar]
- 22.Dai CY, Xie HK, Kadeer X, et al. Relationship of Lymph Node Micrometastasis and Micropapillary Component and Their Joint Influence on Prognosis of Patients With Stage I Lung Adenocarcinoma. Am J Surg Pathol 2017;41:1212-20. 10.1097/PAS.0000000000000901 [DOI] [PubMed] [Google Scholar]
- 23.Hung JJ, Yeh YC, Jeng WJ, et al. Factors predicting occult lymph node metastasis in completely resected lung adenocarcinoma of 3 cm or smaller. Eur J Cardiothorac Surg 2016;50:329-36. 10.1093/ejcts/ezv485 [DOI] [PubMed] [Google Scholar]
- 24.Haruki T, Wakahara M, Matsuoka Y, et al. Clinicopathological Characteristics of Lung Adenocarcinoma with Unexpected Lymph Node Metastasis. Ann Thorac Cardiovasc Surg 2017;23:181-7. 10.5761/atcs.oa.16-00309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ye B, Cheng M, Li W, et al. Predictive Factors for Lymph Node Metastasis in Clinical Stage IA Lung Adenocarcinoma. Ann Thorac Surg 2014;98:217-23. 10.1016/j.athoracsur.2014.03.005 [DOI] [PubMed] [Google Scholar]