Abstract
Background
Large-cell neuroendocrine carcinoma (LCNEC) and small cell lung cancer (SCLC) are categorized as high-grade neuroendocrine carcinoma (HGNEC). We analyzed the efficacy of perioperative chemotherapy for HGNEC and the prognostic factors.
Methods
We retrospectively reviewed the medical records of patients who underwent tumor resection and were diagnosed with HGNEC between January 2001 and December 2014. The overall survival (OS) was estimated by the Kaplan-Meier method. Propensity score matching was performed to compare the OS between the treatment groups. Multivariate analyses using a Cox proportional hazards model were performed to search for prognostic factors for HGNEC.
Results
We analyzed 146 HGNEC patients (LCNEC n=92, SCLC n=54) without synchronous multiple cancers, who underwent complete resection. Seventy patients (LCNEC n=31, SCLC n=32) received perioperative chemotherapy and all of them received a platinum-based anticancer drug. Perioperative chemotherapy significantly improved the 5-year OS rates of HGNEC patients (all stages: 74.5% vs. 34.7%, P<0.01, stage I: 88.5% vs. 40.0%, P<0.01). The efficacy of perioperative chemotherapy was similar between LCNEC and SCLC patients [LCNEC all stages: hazard ratio (HR) 0.27, P<0.01, LCNEC stage I: HR 0.27, P=0.01; SCLC all stages: HR 0.38, P=0.02, SCLC stage I: HR 0.34, P=0.06]. The survival benefit of perioperative chemotherapy for HGNEC patients was confirmed by propensity score matching analysis (HR 0.31, P<0.01). The multivariate analysis revealed that perioperative chemotherapy (HR 0.29, P<0.01), sublobar resection (HR 2.11, P=0.04), and lymph node metastasis (HR 3.34, P<0.01) were independently associated with survival.
Conclusions
Surgical resection combined with perioperative chemotherapy was considered to be effective even for stage I HGNEC patients. Sublobar resection might increase the risk of death in HGNEC patients.
Keywords: High-grade neuroendocrine carcinoma (HGNEC), chemotherapy, lung cancer, surgery
Introduction
Lung neuroendocrine tumors, including large-cell neuroendocrine carcinoma (LCNEC) and small cell lung cancer (SCLC) are originate from neuroendocrine cells and represent 20% of all lung cancers (1). The patients with these tumors are reported to have similar backgrounds and prognoses, and to show a similar response to chemotherapy (2-4). The biological similarities between LCNEC and SCLC have been also reported (5-7). Jones et al. analyzed the gene expression profiles of surgically resected samples of LCNEC and SCLC cell lines using a microarray, and were unable to distinguish LCNEC from SCLC (5). The Clinical Lung Cancer Genome Project (CLCGP) and Network Genomic Medicine (NGM) identified important genetic similarities between LCNEC and SCLC with regard to the transcriptome, the amplified and deleted regions and the mutated genes (6). These clinical and biological similarities make it challenging to differentiate between LCNEC and SCLC in some cases. LCNEC and SCLC were categorized into high-grade neuroendocrine carcinoma (HGNEC) in the 4th edition of the World Health Organization (WHO) Classification of Lung Tumors (8). In this situation, data showing the efficacy of therapy for HGNEC as a single category is required in order to develop practical therapeutic strategies, however, there are few data available (4,9).
In this study, we retrospectively reviewed the medical records and evaluated the clinical benefits of perioperative chemotherapy for HGNEC, and searched for factors predicting the prognosis of patients with HGNEC.
Methods
We retrospectively reviewed the medical records of patients who underwent surgery and who were diagnosed with LCNEC or SCLC at Kobe University Hospital (Kobe, Japan) and Hyogo Cancer Center (Akashi, Japan) between January 2001 and December 2014. We collected data on the patients’ characteristics (age, gender), clinical stage, pathological diagnosis (tumor size, lymph node metastasis, pleural invasion, pulmonary metastasis, vascular invasion, lymphatic permeation), therapeutic method (perioperative chemotherapy, surgical procedure), and prognosis. Patient’s comorbidities were assessed using the Charlson Comorbidity Index (CCI) score (10). Patients with stage 4 disease, an incompletely resected tumor, synchronous multiple cancers, performance of concurrent radiotherapy and insufficient medical records were excluded. Surgery was performed for patients with an Eastern Cooperative Oncology Group Performance Status Scale (ECOG PS) of 0 or 1.
For staging, the TNM classification was determined according to the 7th edition of the American Joint Committee on Cancer Staging Manual and the Revised International System for staging lung cancer. Contrast-enhanced chest and abdominal computed tomography (CT), positron emission tomography (PET)-CT, and brain magnetic resonance imaging (MRI) were performed for preoperative staging. Patients were evaluated postoperatively at 3-month intervals for 2 years, at 6-month intervals for the subsequent 3 years, and once yearly thereafter. Follow-up examinations included chest radiography, contrast-enhanced CT, brain MRI, PET-CT or bone scintigraphy, and hematologic and biochemical analyses, including the measurement of the tumor marker levels.
The study was approved by institutional ethics board of each hospital (Kobe University Hospital: No. 170042. Hyogo Cancer Center: No. R504), and written informed consent was obtained from all patients.
Statistical analyses
All statistical analyses were performed using the JMP 13 software program (SAS Institute, Cary, NC, USA). Student’s t-test and the chi-squared test were performed to assess the significance of differences in age, sex, surgical procedure, clinical stage, and pathological invasion between the two patient groups. Survival was calculated according to the Kaplan-Meier method, and differences in distribution were evaluated using the log-rank test. The Cox proportional hazards model, with calculation of the hazard ratio (HR) and 95% confidence interval (CI), was used to evaluate the associations between prognostic factors and OS after pulmonary resection. P values of <0.05 were considered to indicate statistical significance. Propensity score matching was performed to compare overall survival between the treatment groups. Patients in this analysis were matched for age, gender, CCI, histology, surgical procedure, pathological tumor size, lymph node metastasis, pleural invasion, vascular invasion, and lymphatic permeation.
Results
We identified 197 patients who underwent surgery and who were diagnosed with HGNEC. Fifty-one patients were excluded for the following reasons: pathological stage 4 or incomplete resection (n=25), synchronous multiple cancers (n=14), concurrent radiation therapy (n=7) and insufficient medical records (n=5). The 146 remaining patients were analyzed (LCNEC n=92, SCLC n=54, Figure 1). Among them, 63 patients underwent surgery plus perioperative chemotherapy, and 83 patients underwent surgery alone. The median follow-up period was 34.5 months (surgery plus chemotherapy group, 43.9 months; surgery alone group, 27.9 months). The patients’ clinicopathological characteristics are summarized in Table 1. The surgery plus chemotherapy group included significantly younger patients (P<0.01), more SCLC patients (P<0.01), their extent of resection was more frequently lobectomy or more (P=0.02), and included more patients with pathological lymph node metastasis (P=0.02). The chemotherapy regimens that were used in the treatment of the patients in the surgery plus chemotherapy group are shown in Table 2. All of them were received a platinum-based anticancer drug, and 81% of them received combination treatment with either irinotecan or etoposide (n=51). Postoperative radiotherapy for the mediastinal lymph node was performed for 1 patient in the surgery alone group. Seven patients who took tegafur-uracil (UFT) orally after surgery were included in the surgery alone group.
Figure 1.
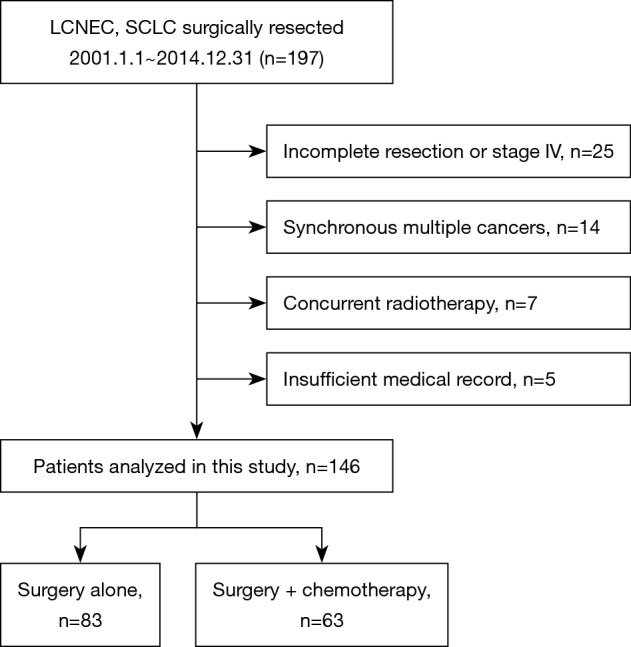
A flowchart of the patient selection. LCNEC, large-cell neuroendocrine carcinoma; SCLC, small cell lung cancer.
Table 1. The clinicopathological characteristics of the patients.
| Factors | Surgery + chemotherapy (n=63) | Surgery alone (n=83) | P value |
|---|---|---|---|
| Age, mean [range] (years) | 63.2 [30–78] | 72.3 [54–84] | <0.01 |
| Gender, n [%] | 0.98 | ||
| Male | 54 [86] | 71 [86] | |
| Female | 9 [14] | 12 [14] | |
| CCI, n [%] | 0.69 | ||
| 0–1 | 43 [68] | 54 [65] | |
| ≥2 | 20 [32] | 29 [35] | |
| Histology, n [%] | <0.01 | ||
| LCNEC | 31 [49] | 61 [73] | |
| SCLC | 32 [51] | 22 [27] | |
| Operation procedure, n [%] | 0.02 | ||
| Lobectomy or more | 58 [92] | 65 [78] | |
| Sublobar resection | 5 [8] | 18 [22] | |
| Stage, n [%] | 0.19 | ||
| IA | 22 [35] | 41 [49] | |
| IB | 14 [22] | 20 [24] | |
| IIA | 14 [22] | 10 [12] | |
| IIB | 4 [6] | 6 [7] | |
| IIIA | 9 [14] | 5 [6] | |
| IIIB | 0 [0] | 1 [1] | |
| Tumor size, mean [range] (mm) | 36.5 [0–80] | 33.5 [10–110] | 0.34 |
| Lymph node metastasis, n [%] | 0.02 | ||
| pN 0 | 35 [56] | 61 [73] | |
| pN 1–2 | 28 [44] | 22 [27] | |
| Pleural invasion, n [%] | 0.42 | ||
| pl 0 | 33 [52] | 49 [59] | |
| pl 1–2 | 30 [48] | 34 [41] | |
| Vascular invasion, n [%] | 0.37 | ||
| v 0 | 12 [19] | 21 [25] | |
| v 1–2 | 51 [81] | 62 [75] | |
| Lymphatic permeation, n [%] | 0.27 | ||
| ly 0 | 18 [29] | 31 [37] | |
| ly 1–2 | 45 [71] | 52 [63] |
LCNEC, large-cell neuroendocrine carcinoma; SCLC, small cell lung cancer; CCI, Charlson Comorbidity Index.
Table 2. The chemotherapy regimens (n=63).
| Regimen | LCNEC (n=31) | SCLC (n=32) |
|---|---|---|
| Neoadjuvant setting (n=5) | ||
| CDDP + CPT-11 | 0 | 1 |
| CDDP + VP-16 | 0 | 3 |
| CBDCA + VP-16 | 0 | 1 |
| Adjuvant setting (n=58) | ||
| CDDP + CPT-11 | 11 | 7 |
| CDDP + VP-16 | 6 | 14 |
| CBDCA + PTX | 6 | 0 |
| CDDP + VNR | 5 | 1 |
| CBDCA + VP-16 | 3 | 4 |
| CBDCA + CPT-11 | 0 | 1 |
CDDP, cisplatin; CPT-11, irinotecan; CBDCA, carboplatin; VNR, vinorelbine; PTX, paclitaxel; VP16, etoposide.
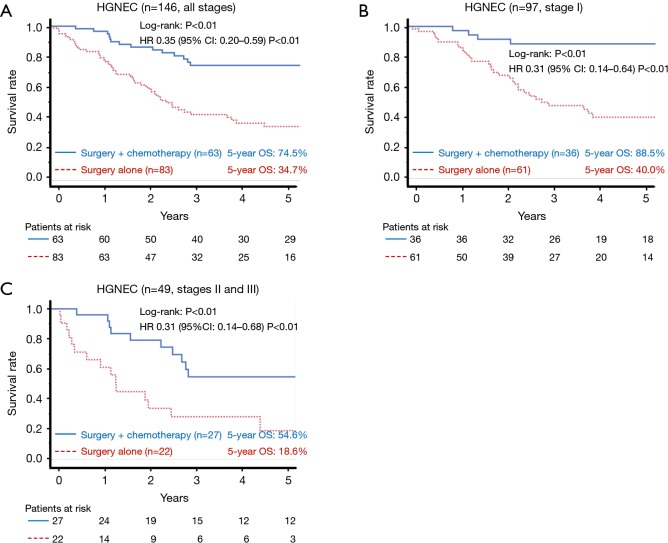
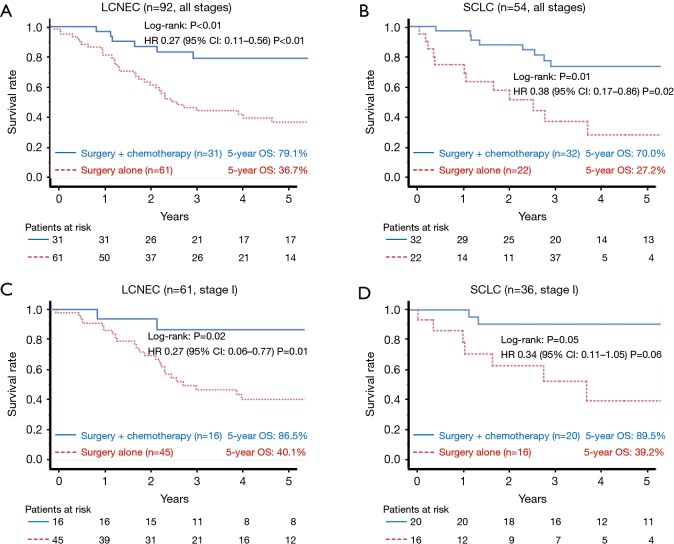
The 5-year OS rates of the surgery plus chemotherapy and surgery alone groups were 74.5% vs. 34.7% in all patients (log-rank: P<0.01, Figure 2A), 88.5% vs. 40.0% in stage I patients (log-rank: P<0.01, Figure 2B), and 54.6% vs. 18.6% in stage II and III patients (log-rank: P<0.01, Figure 2C). The HR for death in the surgery plus chemotherapy group in comparison to the surgery alone group was 0.35 (95% CI: 0.20–0.59; P<0.01) in all patients, 0.31 (95% CI: 0.14–0.64; P<0.01) in stage I patients, and 0.31 (95% CI: 0.14–0.68; P<0.01) in stage II and III patients. Efficacy of perioperative chemotherapy was similar between LCNEC and SCLC patients (Figure 3A,B; LCNEC: HR 0.27, 95% CI: 0.11–0.56, P<0.01; SCLC: HR 0.38, 95% CI: 0.17–0.86; P=0.02), and this tendency was also observed even in stage I patients (Figure 3C,D; LCNEC: HR 0.27, 95% CI: 0.06–0.77, P=0.01; SCLC: HR 0.34, 95% CI: 0.11–1.05, P=0.06).
Figure 2.
A comparison of the overall survival according to the treatment group in all-stage patients (A), stage I patients (B) and stage II and III patients (C). A significant difference in the survival was observed between the groups (log-rank: A: P<0.01, B: P<0.01, C: P<0.01). HGNEC, high-grade neuroendocrine carcinoma; OS, overall survival; HR, hazard ratio; CI, confidence interval.
Figure 3.
A comparison of the overall survival among the treatment groups, analyzed separately according to the histological type [all stages: LCNEC (A), SCLC (B); stage I: LCNEC (C), SCLC (D)]. Perioperative chemotherapy showed similar efficacy against LCNEC and SCLC. LCNEC, large-cell neuroendocrine carcinoma; SCLC, small cell lung cancer; OS, overall survival; HR, hazard ratio; CI, confidence interval.
A propensity score matching analysis was performed to compare the OS between surgery plus chemotherapy and surgery alone groups. The background characteristics of the patients were well balanced between these two groups after adjusting the propensity score for the performance of perioperative chemotherapy (n=76, Table 3). After adjustment, the 5-year OS rates of the surgery plus chemotherapy group were found to be significantly higher in comparison to the surgery alone group (77.0% and 33.9%, respectively; log-rank: P<0.01; Figure 4). The HR for death in the surgery plus chemotherapy group was 0.31 (95% CI: 0.14–0.64; P<0.01) in comparison to the surgery alone group.
Table 3. The patient characteristics after propensity score matching.
| Factors | Surgery + chemotherapy (n=38) | Surgery alone (n=38) | P value |
|---|---|---|---|
| Age, mean [range] (years) | 64.7 [30–78] | 68.6 [54–80] | 0.06 |
| Gender, n [%] | 1.00 | ||
| Male | 31 [82] | 31 [82] | |
| Female | 7 [18] | 7 [18] | |
| CCI, n [%] | 0.80 | ||
| 0–1 | 27 [71] | 26 [68] | |
| ≥2 | 11 [29] | 12 [32] | |
| Histology, n [%] | 0.64 | ||
| LCNEC | 22 [58] | 20 [53] | |
| SCLC | 16 [42] | 18 [47] | |
| Operation procedure, n [%] | 0.72 | ||
| Lobectomy or more | 34 [89] | 33 [87] | |
| Sublobar resection | 4 [11] | 5 [13] | |
| Stage, n [%] | 0.45 | ||
| IA | 14 [37] | 18 [47] | |
| IB | 10 [26] | 8 [21] | |
| IIA | 8 [21] | 5 [13] | |
| IIB | 1 [3] | 4 [11] | |
| IIIA | 5 [13] | 3 [8] | |
| IIIB | 0 [0] | 0 [0] | |
| Tumor size, mean [range] (mm) | 33.0 [0–80] | 38.2 [10–110] | 0.29 |
| Lymph node metastasis, n [%] | 0.81 | ||
| pN 0 | 24 [63] | 25 [66] | |
| pN 1–2 | 14 [37] | 13 [34] | |
| Pleural invasion, n [%] | 1.00 | ||
| pl 0 | 21 [55] | 21 [55] | |
| pl 1–2 | 17 [45] | 17 [45] | |
| Vascular invasion, n [%] | 1.00 | ||
| v 0 | 7 [18] | 7 [18] | |
| v 1–2 | 31 [82] | 31 [82] | |
| Lymphatic permeation, n [%] | 1.00 | ||
| ly 0 | 11 [29] | 11 [29] | |
| ly 1–2 | 27 [71] | 27 [71] |
LCNEC, large-cell neuroendocrine carcinoma; SCLC, small cell lung cancer; CCI, Charlson Comorbidity Index.
Figure 4.
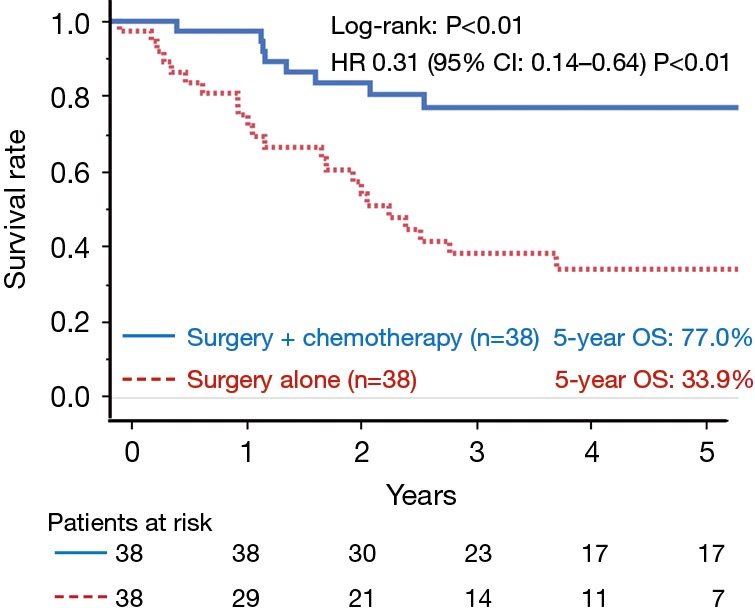
A comparison of the overall survival (n=76) according to the treatment group after propensity score matching. A significant difference was observed in the survival of the two groups (log-rank: P<0.01). OS, overall survival; HR, hazard ratio; CI, confidence interval.
Table 4 shows the univariate and multivariate analyses of the factors associated with OS in the HGNEC patients. The univariate analysis included 11 clinical parameters (age, gender, CCI, histological type, perioperative chemotherapy, surgical procedure, tumor size, lymph node metastasis, pleural invasion, vascular invasion, and lymphatic permeation). Perioperative chemotherapy and lymph node metastasis were significant factors for survival. The histological type was not a significant factor (HR 1.08, 95% CI: 0.66–1.79, P=0.74). Parameters with P values of <0.20 were included in the multivariate model (age, perioperative chemotherapy, surgical procedure, lymph node metastasis, lymphatic permeation). The multivariate analysis revealed that perioperative chemotherapy (HR 0.29, 95% CI: 0.16–0.52, P<0.01), surgical procedure (sublobar resection) (HR 2.11, 95% CI: 1.03–4.08, P=0.04), and lymph node metastasis (HR 3.34, 95% CI: 1.88–5.94, P<0.01) were significant independent predictors of survival.
Table 4. The univariate and multivariate analyses of overall survival (Cox proportional hazards model).
| Variables | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| Age (>70 years) | 1.48 | 0.91–2.43 | 0.11 | 1.11 | 0.64–1.95 | 0.71 | |
| Gender (male) | 0.89 | 0.48–1.86 | 0.74 | − | − | − | |
| CCI (≥2) | 1.2 | 0.71–2.00 | 0.5 | − | − | − | |
| Histological type (SCLC) | 1.08 | 0.66–1.79 | 0.74 | − | − | − | |
| Perioperative chemotherapy (performed) | 0.35 | 0.20–0.60 | <0.01 | 0.29 | 0.16–0.52 | <0.01 | |
| Surgical procedure (sublobar resection) | 1.79 | 0.99–3.25 | 0.06 | 2.11 | 1.03–4.08 | 0.04 | |
| Tumor size (≥3 cm) | 0.87 | 0.53–1.41 | 0.58 | − | − | − | |
| Lymph node metastasis (present) | 2.13 | 1.30–3.48 | <0.01 | 3.34 | 1.88–5.94 | <0.01 | |
| Pleural invasion (present) | 0.96 | 0.59–1.58 | 0.89 | − | − | − | |
| Vascular invasion (present) | 0.82 | 0.48–1.43 | 0.5 | − | − | − | |
| Lymphatic permeation (present) | 1.49 | 0.86–2.57 | 0.14 | 1.33 | 0.72−2.55 | 0.37 | |
SCLC, small cell lung cancer; CCI, Charlson Comorbidity Index; HR, hazard ratio; CI, confidence interval.
Discussion
In this present study, we demonstrated the importance of perioperative chemotherapy for HGNEC patients using a propensity score matching analysis. Our data showed that perioperative chemotherapy significantly improved the 5-year survival rate even in stage I HGNEC patients, and this result was confirmed by a multivariate analysis (HR 0.29, 95% CI: 0.16–0.52, P<0.01). Actually, in our study, there were no significant differences between LCNEC and SCLC with regard to the prognosis (HR 1.08, 95% CI: 0.66–1.79, P=0.74) or sensitivity to chemotherapy (Figure 3). This might reflect the similarity in the genetic background between LCNEC and SCLC, and suggest that it might be better to make treatment guidelines as a single category of HGNEC. Several studies have suggested that perioperative platinum-based chemotherapy for LCNEC (11-17) and SCLC (18-20) remarkably improved the 5-year survival rate by 20–45%, however, there was only one retrospective study that evaluated the efficacy of perioperative chemotherapy in a single category of HGNEC. In the study, Abedallaa et al. reported that the hazard radio for death in patients who underwent perioperative chemotherapy was 0.48 (95% CI: 0.24–0.99; P=0.04) in comparison to those who were treated by surgery alone (4). The efficacy of perioperative chemotherapy in the HGNEC patients of our study was higher than that of the previous study. This might be all because of the patients in the surgery plus chemotherapy group of our study received platinum-based chemotherapy, while only 75% of those received platinum-based chemotherapy in the previous study. This difference might have affected the results.
We additionally revealed that the performance of sublobar resection for HGNEC was an independent negative prognostic factor for survival (HR 2.11, 95% CI: 1.03–4.08; P=0.04). As for LCNEC, though two retrospective studies reported that sublobar resection did not affect the survival of the LCNEC patients (13,21), the data were not sufficient to draw any definitive conclusions. As for SCLC, Brock et al. revealed that the performance of sublobar resection for SCLC was an independent negative prognostic factor for survival in the retrospective study (18). Schreiber et al. analyzed the data from the Surveillance, Epidemiology, and End Results (SEER) Program of the National Cancer Institute and reported that the prognosis of the patients who underwent lobectomy was better than that of those who underwent sublobar resection and the authors recommended lobectomy for SCLC patients (22). No studies have evaluated sublobar resection for HGNEC as a single category, and the present study is the first to show that sublobar resection might increase the risk of death in HGNEC patients. Sublobar resection is recognized as an alternative to lobectomy for patients with small-sized peripheral NSCLC (23,24). Based on our results, if HGNEC is suspected before surgery, it would be better to avoid sublobar resection. Moreover, the preoperative diagnosis might change to HGNEC after surgery due to intra-tumor heterogeneity. Actually, out of 146 HGNEC patients in our study, 13 (8.9%), 13 (8.9%), and 15 (10.3%) patients were preoperatively diagnosed with adenocarcinoma, squamous cell carcinoma, and non-small cell lung cancer, respectively. Thus, when we perform sublobar resection for patients, we have to keep in mind the possibility that the preoperative diagnosis can be change to HGNEC and these patients might as well undergo completion lobectomy.
The present study is associated with some limitations. Firstly, as this study was a retrospective, non-randomized study, it potentially has a strong selection bias. The criteria with regard to the performance of chemotherapy and sublobar resection were not clarified in this study. Thus, we performed a propensity score matching analysis and a multivariate analysis for adjustment. Despite the adjustments, there is a possibility that some biases still remain. Secondly the perioperative chemotherapy regimens were not standardized in the present study. In 2005, Rossi et al. reported that LCNEC responded better to an SCLC-targeting regimen (platinum-etoposide) than to an NSCLC-targeting regimen (platinum-vinorelbine) (11). SCLC-targeting regimens are now considered to be the best option for the treatment of LCNEC patients (17,25). We therefore have changed the perioperative chemotherapy regimen for LCNEC patients from an NSCLC-targeting regimen (platinum-vinorelbine or paclitaxel) to an SCLC-targeting regimen (platinum-etoposide or irinotecan) during the study period. This might have affected the results of our study to some extent. The combination of irinotecan plus cisplatin was recently shown to be acceptable as adjuvant chemotherapy for completely resected HGNEC (9). Subsequently, a randomized phase III trial was initiated (and is currently ongoing) in Japan to compare this combination to the conventional combination of cisplatin and etoposide, for completely resected HGNEC (Japan Clinical Oncology Group 1205/1206) (26). This study will provide a great deal of information on perioperative chemotherapy for HGNEC.
In conclusion, our results suggested that surgical resection combined with perioperative chemotherapy was considered to be optimum treatment for HGNEC patients. If the general condition of the patient permits, the extent of resection should be lobectomy or more.
Acknowledgements
None.
Ethical Statement: The study was approved by institutional ethics board of each hospital (Kobe University Hospital: No. 170042. Hyogo Cancer Center: No. R504), and written informed consent was obtained from all patients.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Gustafsson BI, Kidd M, Chan A, et al. Bronchopulmonary neuroendocrine tumors. Cancer 2008;113:5-21. 10.1002/cncr.23542 [DOI] [PubMed] [Google Scholar]
- 2.Asamura H, Kameya T, Matsuno Y, et al. Neuroendocrine neoplasms of the lung: a prognostic spectrum. J Clin Oncol 2006;24:70-6. 10.1200/JCO.2005.04.1202 [DOI] [PubMed] [Google Scholar]
- 3.Sun JM, Ahn MJ, Ahn JS, et al. Chemotherapy for pulmonary large cell neuroendocrine carcinoma: similar to that for small cell lung cancer or non-small cell lung cancer? Lung Cancer 2012;77:365-70. 10.1016/j.lungcan.2012.04.009 [DOI] [PubMed] [Google Scholar]
- 4.Abedallaa N, Tremblay L, Baey C, et al. Effect of chemotherapy in patients with resected small-cell or large-cell neuroendocrine carcinoma. J Thorac Oncol 2012;7:1179-83. 10.1097/JTO.0b013e3182572ead [DOI] [PubMed] [Google Scholar]
- 5.Jones MH, Virtanen C, Honjoh D, et al. Two prognostically significant subtypes of high-grade lung neuroendocrine tumours independent of small-cell and large-cell neuroendocrine carcinomas identified by gene expression profiles. Lancet 2004;363:775-81. 10.1016/S0140-6736(04)15693-6 [DOI] [PubMed] [Google Scholar]
- 6.Clinical Lung Cancer Genome Project (CLCGP) Network Genomic Medicine (NGM) A genomics-based classification of human lung tumors. Sci Transl Med 2013;5:209ra153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miyoshi T, Umemura S, Matsumura Y, et al. Genomic Profiling of Large-Cell Neuroendocrine Carcinoma of the Lung. Clin Cancer Res 2017;23:757-65. 10.1158/1078-0432.CCR-16-0355 [DOI] [PubMed] [Google Scholar]
- 8.Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol 2015;10:1243-60. 10.1097/JTO.0000000000000630 [DOI] [PubMed] [Google Scholar]
- 9.Kenmotsu H, Niho S, Ito T, et al. A pilot study of adjuvant chemotherapy with irinotecan and cisplatin for completely resected high-grade pulmonary neuroendocrine carcinoma (large cell neuroendocrine carcinoma and small cell lung cancer). Lung Cancer 2014;84:254-8. 10.1016/j.lungcan.2014.03.007 [DOI] [PubMed] [Google Scholar]
- 10.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373-83. 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 11.Rossi G, Cavazza A, Marchioni A, et al. Role of chemotherapy and the receptor tyrosine kinases KIT, PDGFRalpha, PDGFRbeta, and Met in large-cell neuroendocrine carcinoma of the lung. J Clin Oncol 2005;23:8774-85. 10.1200/JCO.2005.02.8233 [DOI] [PubMed] [Google Scholar]
- 12.Iyoda A, Hiroshima K, Moriya Y, et al. Prospective study of adjuvant chemotherapy for pulmonary large cell neuroendocrine carcinoma. Ann Thorac Surg 2006;82:1802-7. 10.1016/j.athoracsur.2006.05.109 [DOI] [PubMed] [Google Scholar]
- 13.Veronesi G, Morandi U, Alloisio M, et al. Large cell neuroendocrine carcinoma of the lung: a retrospective analysis of 144 surgical cases. Lung Cancer 2006;53:111-5. 10.1016/j.lungcan.2006.03.007 [DOI] [PubMed] [Google Scholar]
- 14.Iyoda A, Hiroshima K, Moriya Y, et al. Postoperative recurrence and the role of adjuvant chemotherapy in patients with pulmonary large-cell neuroendocrine carcinoma. J Thorac Cardiovasc Surg 2009;138:446-53. 10.1016/j.jtcvs.2008.12.037 [DOI] [PubMed] [Google Scholar]
- 15.Saji H, Tsuboi M, Matsubayashi J, et al. Clinical response of large cell neuroendocrine carcinoma of the lung to perioperative adjuvant chemotherapy. Anticancer Drugs 2010;21:89-93. 10.1097/CAD.0b013e328330fd79 [DOI] [PubMed] [Google Scholar]
- 16.Tanaka Y, Ogawa H, Uchino K, et al. Immunohistochemical studies of pulmonary large cell neuroendocrine carcinoma: a possible association between staining patterns with neuroendocrine markers and tumor response to chemotherapy. J Thorac Cardiovasc Surg 2013;145:839-46. 10.1016/j.jtcvs.2012.03.036 [DOI] [PubMed] [Google Scholar]
- 17.Iyoda A, Makino T, Koezuka S, et al. Treatment options for patients with large cell neuroendocrine carcinoma of the lung. Gen Thorac Cardiovasc Surg 2014;62:351-6. 10.1007/s11748-014-0379-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brock MV, Hooker CM, Syphard JE, et al. Surgical resection of limited disease small cell lung cancer in the new era of platinum chemotherapy: Its time has come. J Thorac Cardiovasc Surg 2005;129:64-72. 10.1016/j.jtcvs.2004.08.022 [DOI] [PubMed] [Google Scholar]
- 19.Combs SE, Hancock JG, Boffa DJ, et al. Bolstering the case for lobectomy in stages I, II, and IIIA small-cell lung cancer using the National Cancer Data Base. J Thorac Oncol 2015;10:316-23. 10.1097/JTO.0000000000000402 [DOI] [PubMed] [Google Scholar]
- 20.Bunn PA, Jr, Minna JD, Augustyn A, et al. Small Cell Lung Cancer: Can Recent Advances in Biology and Molecular Biology Be Translated into Improved Outcomes? J Thorac Oncol 2016;11:453-74. 10.1016/j.jtho.2016.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Filosso PL, Ferolla P, Guerrera F, et al. Multidisciplinary management of advanced lung neuroendocrine tumors. J Thorac Dis 2015;7:S163-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schreiber D, Rineer J, Weedon J, et al. Survival Outcomes With the Use of Surgery in Limited-Stage Small Cell Lung Cancer Should Its Role Be Re-Evaluated? Cancer 2010;116:1350-7. 10.1002/cncr.24853 [DOI] [PubMed] [Google Scholar]
- 23.Okada M, Nishio W, Sakamoto T, et al. Effect of tumor size on prognosis in patients with non-small cell lung cancer: the role of segmentectomy as a type of lesser resection. J Thorac Cardiovasc Surg 2005;129:87-93. 10.1016/j.jtcvs.2004.04.030 [DOI] [PubMed] [Google Scholar]
- 24.Nakamura K, Saji H, Nakajima R, et al. A phase III randomized trial of lobectomy versus limited resection for small-sized peripheral non-small cell lung cancer (JCOG0802/WJOG4607L). Jpn J Clin Oncol 2010;40:271-4. 10.1093/jjco/hyp156 [DOI] [PubMed] [Google Scholar]
- 25.Fasano M, Della Corte CM, Papaccio F, et al. Pulmonary Large-Cell Neuroendocrine Carcinoma: From Epidemiology to Therapy. J Thorac Oncol 2015;10:1133-41. 10.1097/JTO.0000000000000589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eba J, Kenmotsu H, Tsuboi M, et al. A Phase III trial comparing irinotecan and cisplatin with etoposide and cisplatin in adjuvant chemotherapy for completely resected pulmonary high-grade neuroendocrine carcinoma (JCOG1205/1206). Jpn J Clin Oncol 2014;44:379-82. 10.1093/jjco/hyt233 [DOI] [PubMed] [Google Scholar]