Abstract
Background
Although immune checkpoint inhibitors (ICIs) for non-small cell lung cancer (NSCLC) have been established as one of standard therapy, the prognostic factors of ICIs remain unclear, aside from the programed cell death-ligand 1 (PD-L1) expression of tumor cells. The aim of this study was to determine the prognostic factors of ICIs.
Methods
We analyzed the clinicopathological data of 44 cases of advanced NSCLC targeted with ICIs in our hospital, between February 2016 and February 2018, in order to determine the prognostic factors of ICIs. We also reviewed the literature regarding ICIs.
Result
We retrospectively analyzed the 44 cases (26 nivolumab and 18 pembrolizumab cases). These patients were 38 men and 6 women, comprising 13 cases of adenocarcinoma, 29 squamous cell carcinoma and 2 unclassified types. Seven patients were using first-line therapy and while the others were using second-line therapy or later. Epidermal growth factor receptor (EGFR) mutation and anaplastic lymphoma kinase (ALK) mutations were negative in all the cases. The response rate and disease control rate were 20.5% and 51.3%, respectively. The median progression-free survival time and median survival time were 146 days and 257 days, respectively. We observed five severe adverse effects (AEs) (three cases of interstitial pneumonia, one of liver dysfunction and one of adrenal failure), that were resolved by steroid pulse therapy. In multivariate analyses, the Eastern Cooperative Oncology Group performance status (ECOG PS), pathological type, standardized uptake value (SUV) on positron emission tomography (PET), white blood cell (WBC) count, neutrophil, neutrophil-to-lymphocyte ratio (NLR), lactate dehydrogenase (LDH) and albumin were independently prognostic factors. There were no significant differences in the prognosis between nivolumab and pembrolizumab.
Conclusions
ICIs were effective in 44 treated NSCLC cases. Our analysis suggests that while ICIs are effective in treating patients, candidates must be carefully selected and cautiously observed.
Keywords: Programed cell death protein 1 (PD-1), lung cancer, immune check inhibitor (ICI), tumor immunology
Introduction
Non-small cell carcinoma (NSCLC) is the leading cause of death from cancer worldwide (1,2). The development of immunotherapy has allowed for therapies directed against programed cell death protein 1 (PD-1) signaling, which have shown promising effects among patients with advanced NSCLC and have produced superior survival outcomes compared with cytotoxic chemotherapies in patients with metastatic NSCLC (1-6).
PD-1 is expressed on the surface of T cells and regulates excessive autoimmune responses. Programmed cell death-ligand 1 (PD-L1), an immune-modulating ligand of PD-1, is expressed on antigen-presenting cells as well as tumor cells. Recently, immune checkpoint inhibitors (ICIs) were administered to patients with NSCLC, and marked effects were obtained even for chemotherapy-resistant patients (7,8). ICIs block the immune checkpoint molecules expressed on tumor cells, thereby blocking the inhibitory signals from the ligand and thus prolonging the activation of T cells. T cells are thus induced to attack tumor cells. The PD-L1 expression of tumor cells is an important factor in the immune response against tumor cells.
Although ICIs for NSCLC have been established as a standard therapy, the prognostic factors of ICIs are still unclear aside from the PD-L1 expression of tumor cells. The aim of this study was to identify prognostic factors of ICIs by analyzing the clinicopathological data of 44 cases of advanced or postoperative recurrent NSCLC targeted with ICIs in our hospital.
Methods
Patients
We retrospectively reviewed the clinicopathological data of 44 cases of advanced or postoperative recurrent NSCLC targeted with ICIs in our hospital, between February 2016 and February 2018, in order to determine the prognostic factors and reviewed the literature regarding ICIs. Nivolumab and pembrolizumab were administered at the standard dose of 3 mg/kg every 2 weeks and 200 mg/body every 3 weeks, respectively. The PD-L1 expression was assessed in formalin-fixed tumor samples using immunohistochemistry antibody 22C3 (Dako, USA) (9,10) in advance of the administration of pembrolizumab. The administration criteria of pembrolizumab were a PD-L1 expression of the tumor cells ≥50% in the first-line therapy and ≥1% in the second-line therapy or later. Electronic medical records and pharmacy databases were collected to obtain patient-specific information. This information included the following at the time of initiating ICIs, data concerning the patient demographics, Eastern Cooperative Oncology Group Performance Status (ECOG PS), smoking history, pathology, advanced or postoperative recurrent NSCLC, bevacizumab administration, molecular profiling for epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase (ALK) and c-ros oncogene 1 (ROS-1), PD-L1 status, metastatic sites, treatment history, blood data [including the neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR)], frequency of nivolumab administration and response status as well as the adverse effects (AEs) and date of progression as determined by a review of the clinician’s progress notes and radiology reports, and data on death or last follow-up. Tumors were classified according to the current World Health Organization (WHO)/International Association for the Study of Lung Cancer (IASLC) criteria (11). The overall response rate (ORR) was assessed utilizing the Response Evaluation Criteria in Solid Tumors, version 1.1 (RECIST v1.1) (12). Toxicities and their severity were assessed using the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 4.
The present study was approved by the Ethics Committee of University of Occupational and Environmental Health, Japan.
Statistical analyses
The survival rates were calculated by the Kaplan–Meier method and the significance of the differences between the prognosis and clinicopathological factors was evaluated by Cox proportional hazard model using the Stata software program version 14.1 (Stata, College Station, USA) by a specialized statistician. All tests were two-sided, and statistical significance was defined as P<0.05.
Results
The patients’ characteristics are presented in Table 1. The cohort comprised of 38 men (86.4%) and 6 women (13.6%) with a median age at presentation of 71 years (range, 42–91 years). The pathological types included 29 (65.9%) squamous cell carcinoma, 13 (29.5%) adenocarcinoma and 2 (4.5%) unclassified NSCLC among the patients. Nivolumab was administered in 26 cases and pembrolizumab in 18 cases. Seven patients were using the first line therapy and the others were using second line therapy or later. EGFR, ALK and ROS-1 mutations were negative in all non-squamous cell carcinoma cases. The median follow-up period was 145 days.
Table 1. Characteristics of the immune check inhibitor cases (n=44).
| Characteristics | Classifications | No. (%) |
|---|---|---|
| Sex | Male | 38 (86.4) |
| Female | 6 (13.6) | |
| Age [42−91 (median: 71)] | <60 | 6 (13.6) |
| 60−69 | 13 (29.5) | |
| 70−79 | 19 (43.2) | |
| >80 | 6 (13.6) | |
| Pathology | Squamous cell carcinoma | 29 (65.9) |
| Adenocarcinoma | 13 (29.5) | |
| Unclassified | 2 (4.5) | |
| Line* | 1st | 7 (15.9) |
| 2nd | 18 (40.9) | |
| 3rd | 10 (22.7) | |
| 4th | 5 (11.4) | |
| 5th | 4 (9.1) | |
| 6th | 1 (2.3) | |
| 7th | 1 (2.3) | |
| Frequency of administration [1−25 (median: 4)] | <5 | 29 (65.9) |
| 5−10 | 9 (20.5) | |
| >10 | 6 (13.6) | |
| PS grade (ECOG) | 0 | 12 (27.3) |
| 1 | 20 (45.5) | |
| 2 | 6 (13.6) | |
| 3 | 5 (11.4) | |
| 4 | 1 (2.3) | |
| Smoking history | Smoker | 8 (18.2) |
| Non-smoker | 36 (81.8) | |
| Metastatic sites* | Brain | 9 (20.5) |
| Lung | 20 (45.5) | |
| Liver | 4 (9.1) | |
| Bone | 9 (20.5) | |
| Adrenal grand | 12 (27.3) | |
| Pleural dissemination | 2 (4.5) | |
| Lymph nodes | 16 (36.4) |
*, overlapped cases were included. PS, performance status; ECOG, Eastern Cooperative Oncology Group.
The response rate and disease control rate were 20.5% and 51.3%, respectively. The median progressive-free survival time and median survival time were 146 and 257 days, respectively (Figure 1). We observed five severe AEs (three cases of interstitial pneumonia, one of liver dysfunction and one of adrenal failure) that were resolved by steroid pulse therapy (Table 2).
Figure 1.
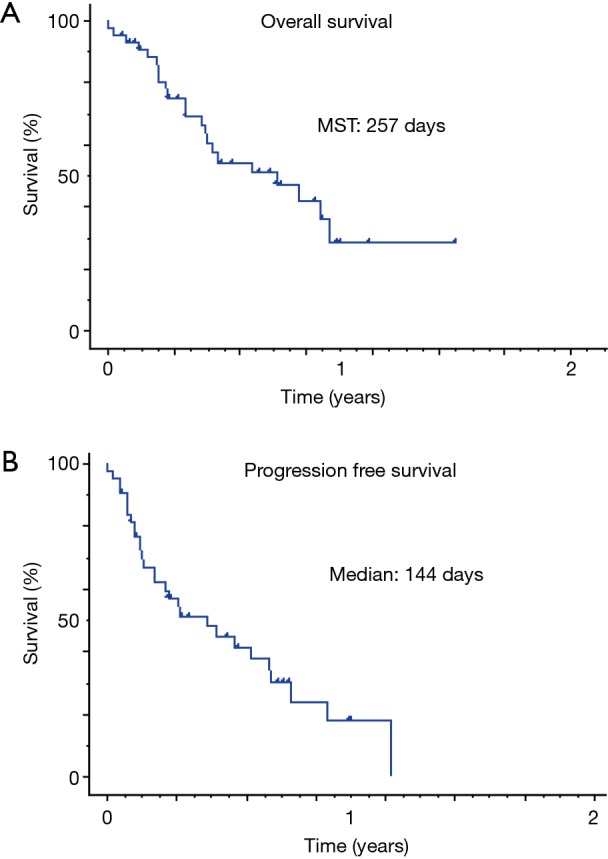
Survival curve of the immune check inhibitor cases. (A) It shows overall survival curve of non-small cell lung cancer patients receiving ICIs; (B) it shows progression free survival curve of patients receiving ICIs. Hash marks represent censored patients. MST, median survival time; ICIs, patients receiving immune check point inhibitors.
Table 2. Outcomes of the immune check inhibitor cases (n=44).
| Outcomes | No. (%) |
|---|---|
| Response* | |
| CR | 0 (0.0) |
| PR | 9 (20.5) |
| SD | 11 (25.0) |
| PD | 19 (43.2) |
| NE | 5 (11.4) |
| Adverse event** | |
| Liver dysfunction | |
| Grade 4 | 1 (2.3) |
| Grade 1 | 1 (2.3) |
| Interstitial lung disease | |
| Grade 4 | |
| Grade 3 | 2 (4.5) |
| Grade 2 | 1 (2.3) |
| Pyrexia | |
| Grade 1 | 1 (2.3) |
| Hypothyroidism | |
| Grade 2 | 2 (4.5) |
| Grade 1 | 2 (4.5) |
| Adrenal failure | |
| Grade 3 | 1 (2.3) |
| Hematuria | |
| Grade 1 | 1 (2.3) |
| Thrombopenia | |
| Grade 1 | 1 (2.3) |
*, objective response rate: 20.5%, disease control rate: 45.5%; **, overlapped cases were included. CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; NE, not evaluable.
In univariate analyses, the ECOG PS (PS 0.1 vs. 2.4), pathological type (adenocarcinoma vs. squamous cell carcinoma), standardized uptake value (SUV) of positron emission tomography (PET), white blood cell (WBC) count, neutrophil count, lactate dehydrogenase (LDH) level and albumin level were independently prognostic factors (Table 3).
Table 3. Univariate analysis of overall survival (n=44).
| Clinical pathological factor | HR | 95% CI | P value |
|---|---|---|---|
| Agent (Pembro vs. Nivo) | 0.30 | 0.09–1.01 | 0.052 |
| Pathological type (Ad vs. Sq) | 5.60 | 1.03–5.83 | 0.043 |
| PS (0.1 vs. 2) | 4.29 | 1.80 –10.25 | 0.001 |
| PET (SUV) (SD) | 1.98 | 1.61−3.37 | 0.012 |
| WBC (SD) | 2.38 | 1.39–4.00 | 0.002 |
| Neutro (SD) | 2.49 | 1.44–4.30 | 0.001 |
| NLR (SD) | 2.07 | 1.24–3.45 | 0.050 |
| LDH (SD) | 2.22 | 1.41−3.47 | 10 |
| Alb (SD) | 0.41 | 0.25–0.65 | 0.0 |
Pembro, pembrolizumab; Nivo, nivolumab; Ad, adenocarcinoma; Sq, squamous cell carcinoma; PS, performance status; PET, positron emission tomography; SUV, standardized uptake value; SD, standard deviation; WBC, white blood cell; Neutro, neutrophil; NLR, neutrophil-to-lymphocyte ratio; LDH, lactate dehydrogenase; alb, albumin.
In multivariate analyses, the ECOG PS, pathological type, SUV of PET, WBC count, neutrophil count, NLR, LDH level and albumin level were independently prognostic factors. There was no significant difference between patients with nivolumab and pembrolizumab (Table 4).
Table 4. Multivariable analysis of overall survival (n=44).
| Clinical pathological factor | HR | 95% CI | P value |
|---|---|---|---|
| Agent | 0.42 | 0.12–1.48 | 0.178 |
| Pathological type | 5.60 | 1.86–16.89 | 0.020 |
| PS | 4.44 | 1.43–13.77 | 0.010 |
| PET (SUV) | 1.89 | 1.06−3.37 | 0.030 |
| WBC | 3.03 | 1.54–5.95 | 0.001 |
| Neutro | 3.20 | 1.63–6.30 | 0.001 |
| NLR | 3.02 | 1.49–6.13 | 0.002 |
| LDH | 1.90 | 1.05−3.45 | 0.035 |
| Alb | 0.58 | 0.36–0.94 | 0.028 |
Adjusted by agent, pathological type, PS, performance status; PET, positron emission tomography; SUV, standardized uptake value; WBC, white blood cell; Neutro, neutrophil; NLR, neutrophil-to-lymphocyte ratio; LDH, lactate dehydrogenase; alb, albumin; PS, performance status.
Discussion
Anti-PD-1 antibodies block the interaction of PD-1 on T-cells and its ligand PD-L1 on tumor cells. Three large phase III trials showed the superiority of the two anti-PD-1 antibodies nivolumab and pembrolizumab with regards to the ORR, overall survival (OS) and, variably, also the progression free survival (PFS) compared with docetaxel as the standard second-line chemotherapy (6-8). However, this treatment is expensive and the identification of the relatively small subset of <20% of patients likely to enjoy with long-term benefit from these agents is currently not possible, as reliable predictive and prognostic markers for the response and survival are lacking. Furthermore, pembrolizumab showed a longer PFS and OS and fewer AEs than platinum-based combination chemotherapy in patients with previously untreated advanced NSCLC with at least 50% PD-L1 expression (4). We can therefore apply pembrolizumab to cases advanced NSCLC with at least 50% PD-L1 expression as a first-line therapy. In the present study, we reviewed the clinicopathological data of 44 cases of advanced or postoperative recurrent NSCLC targeted with ICIs.
NSCLC patients with driver gene mutations like EGFR, ALK and ROS-1 reportedly tend to be resistant to ICIs (3,4,6-8), so we did not administer ICIs for patients with driver gene mutations. This limited our study with regard to the low number of cases and the inherent selection bias. The response rate and disease control rate were 20.5% and 51.3%, respectively. The median progressive-free survival time and median survival time were 146 and 257 days, respectively. The effects of ICIs were relatively fair. We observed five severe AEs (three cases of interstitial pneumonia, one of liver dysfunction and one of adrenal failure), that were resolved by steroid pulse therapy. The rate of AEs and severity were also acceptable. In multivariate analyses, the ECOG PS, pathological type, SUV of PET, WBC count, neutrophil count, NLR, LDH levels and albumin levels were independently prognostic factors. There were no significant differences between the patients with nivolumab and pembrolizumab.
The general condition, including the nutrition status, might be ensuring the efficacy of ICIs because the immune reaction is strongly affected by the host status. Kato et al. reported that the albumin level after the first cycle of nivolumab was associated with the response to the treatment (13). Our data also showed that the ECOG PS and albumin level before treatment were independent prognostic factors in multivariate analyses. Early intervention using ICIs might be essential for activating the immune responses completely before cachexia occurs.
The SUV of PET and LDH level before treatment were also independent prognostic factors in this study. Machtay et al. reported that the SUV of PET was associated with a poor prognosis in stage III NSCLC (14). Wulaningsih et al. revealed that a high serum LDH level before treatment was strongly associated with the overall and cancer-specific death in 5,799 cancer patients (15). Aggressive cancers, such as those with a high SUV of PET and high LDH level, might not be sensitive to ICIs.
A significant difference was noted in the pathological types among our patients. Though our data showed that squamous cell carcinoma was a poor prognostic factor, the mechanism underlying this association remains unclear. The phase III trial data of pembrolizumab showed no significant difference among pathological types (4,6). Nivolumab was separately analyzed in squamous cell carcinoma and non-squamous cell carcinoma patients (7,8).
To evaluate the effect of surgery on the outcome, we compared advanced NSCLC without surgery with postoperative recurrence, but noted no significant differences between them. Lymph-node dissection (LND) is performed to remove metastasis of the lymph nodes, but can also eliminate tumor-specific cytotoxic T lymphocyte (CTL) and antigen-presenting cells, as tumor-specific CTL is believed to accumulate in the regional lymph nodes in order to kill tumor cells (16,17). In this study, we were unable to determine the benefits and drawbacks of LND for ICI-treated NSCLC as there were no significant differences between LND-treated cases and others (data not shown). An additional analysis in a larger cohort is required to draw a firm conclusion on this point.
Bagley et al. reported that the pretreatment NLR before treatment is independently associated with the PFS and OS in patients with advanced NSCLC treated with nivolumab (18). Diem et al. reported that high pre-treatment NLR and PLR values before treatment are simple prognostic markers strongly correlating with a poor survival in NSCLC patients treated with the anti-PD1 antibody nivolumab. The NLR and PLR are cheap and readily available biomarkers providing additional prognostic information to identify patients likely to benefit from treatment with nivolumab (19). Our data also showed that not only the NLR but also elevated WBC and neutrophil counts were significant prognostic factors. Whether the NLR is predictive or prognostic is unclear at present, and further studies are warranted to determine the utility of the NLR in the context of other biomarkers of PD-1 therapy. However, the PLR was not an independent prognostic factor in our study.
It was reported that bevacizumab depletes circulating regulatory T cells, expands B and T cell compartments, favors the differentiation of dendritic cells and facilitates tumor infiltration by lymphocytes (20). While we also analyzed the relationship of bevacizumab administration and the prognosis, no significant relationship was noted (data not shown).
In this study, there was no significant difference in the prognosis between nivolumab and pembrolizumab. Pembrolizumab was able to be applied to chemotherapy-naive patients with PD-L1-high-expressing NSCLC. Reck et al. reported that pembrolizumab had significantly longer PFS and OS in patients with PD-L1-high-expressing NSCLC as the first-line therapy (21). Seven PD-L1 high expressed-NSCLC patients using pembrolizumab as the first-line therapy did not show a significant better prognosis than patients using ICIs as the second-line therapy or later. Lisberg et al. reported that AEs predicted for improved clinical outcomes in 97 NSCLC patients with pembrolizumab (22). However, AEs were not a predictive factor of the favor clinical responses in our data. One limitation associated with our study might be the limited number of cases.
In conclusion, ICIs were effective in 44 treated NSCLC cases. These findings suggest that while ICIs are effective in treating patients, candidates must be carefully selected and cautiously observed.
Acknowledgements
None.
Ethical Statement: The study was approved by the Ethics Committee of University of Occupational and Environmental Health, Japan (No. H30-017).
Footnotes
Conflicts of Interest: F Tanaka has received lecture fees and received funds Chugai Pharmaceutical Co. Ltd., AstraZeneca Co. Ltd., Taiho Pharmaceutical Co. Ltd., MSD Co. Ltd. The other authors have no conflicts of interest to declare.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. 10.3322/caac.21332 [DOI] [PubMed] [Google Scholar]
- 2.Didkowska J, Wojciechowska U, Mańczuk M, et al. Lung cancer epidemiology: contemporary and future challenges worldwide. Ann Transl Med 2016;4:150. 10.21037/atm.2016.03.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372:2018-28. 10.1056/NEJMoa1501824 [DOI] [PubMed] [Google Scholar]
- 4.Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. 10.1056/NEJMoa1606774 [DOI] [PubMed] [Google Scholar]
- 5.Gettinger SN, Horn L, Gandhi L, et al. Overall Survival and Long-Term Safety of Nivolumab (Anti-Programmed Death 1 Antibody, BMS-936558, ONO-4538) in Patients With Previously Treated Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2015;33:2004-12. 10.1200/JCO.2014.58.3708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. 10.1016/S0140-6736(15)01281-7 [DOI] [PubMed] [Google Scholar]
- 7.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. 10.1056/NEJMoa1504627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. 10.1056/NEJMoa1507643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.PD-L1 IHC 22C3 pharmDx. Carpinteria, CA: Dako North America. (2015) (pakage insert). Available online: https://www.agilent.com/ja-jp/product/pharmdx/pd-l1-ihc-22c3-pharmdx
- 10.Roach C, Zhang N, Corigliano E, et al. Development of a Companion Diagnostic PD-L1 Immunohistochemistry Assay for Pembrolizumab Therapy in Non-Small-cell Lung Cancer. Appl Immunohistochem Mol Morphol 2016;24:392-7. 10.1097/PAI.0000000000000408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.The Japan Lung Cancer Society. General rule for clinical and pathological record of lung cancer. The 8th ed. Available online: https://www.kanehara-shuppan.co.jp/_data/ebooks/20366T/FLASH/
- 12.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 13.Kato R, Yamasaki M, Urakawa S, et al. Increased Tim-3+ T cells in PBMCs during nivolumab therapy correlate with responses and prognosis of advanced esophageal squamous cell carcinoma patients. Cancer Immunol Immunother 2018;67:1673-83. 10.1007/s00262-018-2225-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Machtay M, Duan F, Siegel BA, et al. Prediction of survival by [18F]fluorodeoxyglucose positron emission tomography in patients with locally advanced non-small-cell lung cancer undergoing definitive chemoradiation therapy: results of the ACRIN 6668/RTOG 0235 trial. J Clin Oncol 2013;31:3823-30. 10.1200/JCO.2012.47.5947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wulaningsih W, Holmberg L, Garmo H, et al. Serum lactate dehydrogenase and survival following cancer diagnosis. Br J Cancer 2015;113:1389-96. 10.1038/bjc.2015.361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ichiki Y, Takenoyama M, Mizukami M, et al. Simultaneous cellular and humoral immune response against mutated p53 in a patient with lung cancer. J Immunol 2004;172:4844-50. 10.4049/jimmunol.172.8.4844 [DOI] [PubMed] [Google Scholar]
- 17.Sugaya M, Takenoyama M, Osaki T, et al. Establishment of 15 cancer cell lines from patients with lung cancer and the potential tools for immunotherapy. Chest 2002;122:282-8. 10.1378/chest.122.1.282 [DOI] [PubMed] [Google Scholar]
- 18.Bagley SJ, Kothari S, Aggarwal C, et al. Pretreatment neutrophil-to-lymphocyte ratio as a marker of outcomes in nivolumab-treated patients with advanced non-small-cell lung cancer. Lung Cancer 2017;106:1-7. 10.1016/j.lungcan.2017.01.013 [DOI] [PubMed] [Google Scholar]
- 19.Diem S, Schmid S, Krapf M, et al. Neutrophil-to-Lymphocyte ratio (NLR) and Platelet-to-Lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer 2017;111:176-81. 10.1016/j.lungcan.2017.07.024 [DOI] [PubMed] [Google Scholar]
- 20.Galluzzi L, Senovilla L, Zitvogel L, et al. The secret ally: immunostimulation by anticancer drugs. Nat Rev Drug Discov 2012;11:215-33. 10.1038/nrd3626 [DOI] [PubMed] [Google Scholar]
- 21.Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. 10.1056/NEJMoa1606774 [DOI] [PubMed] [Google Scholar]
- 22.Lisberg A, Tucker DA, Goldman JW, et al. Treatment-Related Adverse Events Predict Improved Clinical Outcome in NSCLC Patients on KEYNOTE-001 at a Single Center. Cancer Immunol Res 2018. [Epub ahead of print]. 10.1158/2326-6066.CIR-17-0063 [DOI] [PMC free article] [PubMed] [Google Scholar]


