Abstract
Background
Vitamin C has shown several beneficial effects on sepsis in preclinical studies. However, clinical data supporting these reports are scarce. This study aimed to evaluate whether adjunctive intravenous vitamin C therapy could reduce hospital mortality in patients with severe sepsis or septic shock requiring mechanical ventilation.
Methods
For this retrospective cohort study, consecutive medical ICU patients with severe sepsis or septic shock requiring mechanical ventilation were included. The study patients were classified into the vitamin C or control groups depending on the administration of intravenous vitamin C (2 g every 8 hours). The primary outcome was hospital mortality.
Results
Thirty-five patients in the vitamin C group and 40 patients in the control group were included. The two groups were comparable in regards to the baseline characteristics at ICU admission. The hospital mortality was 46% (16 of 35 patients) in the vitamin C group and 40% (16 of 40 patients) in the control group, showing a statistically nonsignificant difference (P=0.62). The mortality at 90 days after ICU admission (60% vs. 48%) did not significantly differ between groups. The median time to shock reversal was 3 days [interquartile range (IQR), 2 to 5 days] in both groups. The changes in the Sepsis-related Organ Failure Assessment (SOFA) scores during the first 4 ICU days were −1.4±3.3 and −1.4±3.0 in the vitamin C and control groups, respectively.
Conclusions
Adjunctive intravenous vitamin C therapy alone did not reduce hospital mortality in mechanically ventilated patients with severe sepsis or septic shock.
Keywords: Sepsis, septic shock, vitamin C, mortality
Introduction
More than 30 million people suffer from sepsis worldwide annually, with 5–6 million deaths (1). Although the incidence of sepsis has increased, mortality from severe sepsis and septic shock remains as high as 18–51% (2-5). Despite numerous clinical trials, no drugs other than antibiotics have improved the outcome of sepsis (6).
Vitamin C has shown several beneficial effects on pathophysiologic changes in sepsis in preclinical studies. It improved arteriolar responsiveness to vasopressors (7-9), enhanced endothelial and epithelial barrier function (10-12), and increased microvascular blood flow (13-15). In addition, it acts as a cofactor for dopamine β-hydroxylase and for peptidylglycine α-amidating monooxygenase, enzymes required in norepinephrine synthesis and in postprocessing of pre-pro-vasopressin respectively (16). Plasma concentrations of vitamin C were decreased in patients with critical illnesses including sepsis, and there was a study suggesting the relationship between such a decrease and the development of multiple organ failure (17-19). Therefore, the administration of sufficient vitamin C to critically ill patients with sepsis may lead to an earlier resolution of shock, prevent further development of organ failure, and reduce mortality. Marik et al. reported that combination therapy of vitamin C, hydrocortisone, and thiamine decreased hospital mortality among patients with severe sepsis and septic shock by approximately 32% (20).
However, clinical data demonstrating favorable outcomes with vitamin C alone in sepsis patients are scarce. To date, there have been only 2 clinical studies investigating the effect of vitamin C alone in patients with sepsis. One was small phase I study, which showed reduction in Sepsis-related Organ Failure Assessment (SOFA) score and plasma levels of C-reactive protein, procalcitonin, and thrombomodulin in severe sepsis patients treated with intravenous vitamin C (19). The other small randomized, placebo-controlled study showed that intravenous vitamin C therapy reduced the requirement for norepinephrine and improved 28-day survival in surgical patients with septic shock (21). It is unknown whether vitamin C may be effective against sepsis in monotherapy or in combination with hydrocortisone. The purpose of this retrospective cohort study is to test the hypothesis that adjunctive intravenous vitamin C therapy reduces hospital mortality in mechanically ventilated patients with severe sepsis or septic shock.
Methods
Study patients
For this retrospectively study, among all patients admitted to the medical ICU of a tertiary referral teaching hospital from January to July 2017, those who met the criteria for severe sepsis or septic shock and required mechanical ventilation within 24 hours from ICU admission were included. The diagnosis of severe sepsis and septic shock was made according to the American College of Chest Physician/Society of Critical Care Medicine Consensus definitions (22). The exclusion criteria were age less than 18 years, pregnancy, death within 24 hours from ICU admission, do not resuscitate or limitation of care order, and organ transplantation during ICU stay. Episodes other than the first admission episode during the study period were also excluded. Included patients were classified into the vitamin C or control groups depending on the administration of intravenous vitamin C. One of 4 attending physicians in our medical ICU has had a strong preference for vitamin C therapy in sepsis since 2015. The use of vitamin C was based on its favorable historical safety profile, its potential clinical efficacy from previous studies, and personal experience of successful treatment. From 2017, vitamin C has been administered in all sepsis patients who were assigned to certain attending physician and the therapy was started on the first working day after ICU admission regardless of the severity of a patient. This study protocol was approved by the institutional review board of Asan Medical Center (IRB No. 2018-0412). Informed consent was waived by the board due to the retrospective nature of the study.
Sepsis management
Except for the administration of vitamin C, all study patients were treated with the same treatment protocol based on the Surviving Sepsis Guidelines (23). Specifically, empirical broad-spectrum antimicrobials against possible pathogens were administered immediately after microbiologic studies; fluid resuscitation using crystalloids was performed in case of sepsis-induced hypotension or significant lactic acidosis; if hypotension persisted, norepinephrine followed by vasopressin and epinephrine was given to maintain a mean arterial pressure (MAP) of 65 mmHg; the lactate levels were serially followed up to guide further resuscitation; established sources of infection were controlled with intervention or surgery as soon as possible; a lung-protective ventilation strategy was applied; unnecessary use of sedatives was minimized to keep patients on light sedation; low-molecular weight heparin was administered for the prevention of deep vein thrombosis in the absence of contraindications; patients with risk factors for gastrointestinal bleeding received stress ulcer prophylaxis; blood glucose levels were maintained between 100 and 180 mg/dL; and when a MAP of 65 mmHg could not be achieved despite fluid resuscitation and use of vasopressors, hydrocortisone was infused intravenously at a rate of 10 mg/hr. In addition, patients in the vitamin C group received vitamin C therapy. Two grams of vitamin C mixed in 50 mL of 5% dextrose solution or normal saline was administered intravenously over 30 minutes every 8 hours until ICU discharge. Each dose of vitamin C was prepared immediately before administration and was infused with covered with a shading bag.
Data collection
The ICU admission and treatment data of the study patients were obtained from hospital electronic medical records. The information included age, sex, body weight, hospital and ICU admission date, time zero of sepsis (defined as the time when the patient was triaged in the emergency room or defined as the first medical record time that satisfied the criteria of severe sepsis or septic shock in the patient already hospitalized) (24), ICU admission diagnosis, site of admission, comorbidities, presence of immunosuppression (immunodeficiency diseases, on chemotherapy for malignancy, use of prednisolone ≥10 mg/day or equivalent doses of other systemic corticosteroids, use of immunosuppressants, or hematologic diseases affecting immune system), types and duration of vasopressors used, dose of each vasopressor during the first 4 ICU days, time to shock reversal (defined as maintaining a MAP of 65 mmHg or more for at least 24 hours without vasopressor support) (25), duration of mechanical ventilation, development of and recovery from acute kidney injury (AKI; defined according to the Kidney Disease Improving Global Outcomes definition) (26), use of renal replacement therapy (RRT), hospital and ICU discharge date, date of death, levels of serum lactate and procalcitonin on the day of ICU admission, results of cultures from blood or infection sources, acute physiology and chronic health evaluation (APACHE) II scores (27), and SOFA scores during the first 4 ICU days (28). If a patient survived to hospital discharge before 90 days from ICU admission, the survival status of the patient was followed up to day 90 through national health insurance service data.
The primary outcome was hospital mortality. The secondary outcomes included ICU mortality, 90-day mortality, time to shock reversal, doses of vasopressors used during the first 4 ICU days, duration of initial mechanical ventilation, changes in SOFA scores and PaO2/FiO2 ratio during the first 4 ICU days, use of RRT in patients with AKI, and ICU and hospital lengths of stay.
Statistical analysis
The baseline characteristics and treatment outcomes were compared between the vitamin C and control groups. Continuous variables were analyzed using Student’s t or Mann-Whitney U tests and categorical variables were compared using chi-squared or Fisher’s exact tests. The cumulative survival rate to day 90 was compared by log-rank test. Time to shock reversal was analyzed in the function of the cumulative incidence by considering death as a competing risk (29). The daily total vasopressor requirement was calculated in a norepinephrine equivalent dose using the following formula: [norepinephrine (mcg/min)] + [dopamine (mcg/kg/min) ÷ 2] + [epinephrine (mcg/min)] + [phenylephrine (mcg/min) ÷ 10] + [vasopressin (units/h) × 8.33] (30,31). Repeated measures analysis of variance was used to analyze the effect of vitamin C on the daily total vasopressor requirement or SOFA scores over the first 4 ICU days. As a prespecified subgroup analysis, the primary outcome in the patients who received intravenous hydrocortisone therapy was compared between the two groups. A two-sided P value of <0.05 was considered statistically significant. The analyses were performed using IBM SPSS Statistics version 21 (IBM Corp., Armonk, NY, USA) or R 3.5.1.
Results
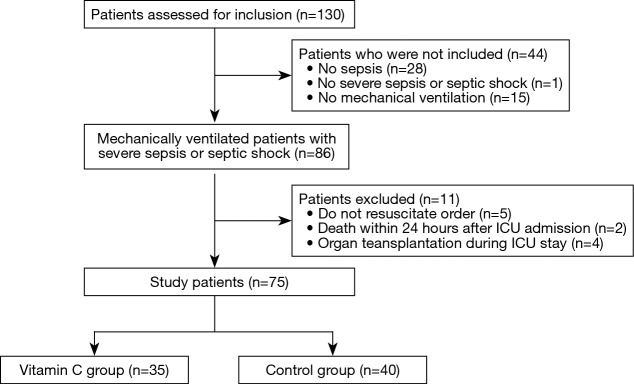
Between January and July 2017, 86 patients were included in the study (Figure 1). After excluding 11 patients, the remaining 75 mechanically ventilated patients with severe sepsis or septic shock were grouped into the vitamin C (n=35) or control (n=40) groups. There were no significant differences between the two groups at ICU admission (Table 1). Sixteen patients (46%) in the vitamin C group and 11 (28%) patients in the control group were admitted from the general ward to the ICU (P=0.1). And their median pre-ICU hospital lengths of stay were 3 days (IQR, 1–18 days) and 9 days (IQR, 1–24 days), respectively (P=0.53). The median time from time zero to ICU admission was 4 hours (IQR, 2–9 hours) in the vitamin C group and 4 hours (IQR, 3–6 hours) in the control group (P=0.65). In the vitamin C group, vitamin C therapy was started a median of 7 hours after ICU admission (IQR, 4–20 hours) and continued for a median of 9 days (IQR, 5–19 days).
Figure 1.
A flowchart illustrating the inclusion and exclusion of the study patients
Table 1. Baseline demographic and clinical characteristics of the study patients.
| Characteristics | Total (n=75) | Vitamin C group (n=35) | Control group (n=40) | P value |
|---|---|---|---|---|
| Age, mean ± SD, years | 65.6 ±14.0 | 67.8±12.1 | 63.6±15.3 | 0.19 |
| Sex, male, n [%] | 53 [71] | 24 [69] | 29 [73] | 0.71 |
| Types of infection, n [%] | 0.97 | |||
| Community-acquired | 58 [77] | 27 [77] | 31 [78] | |
| Hospital-acquired | 17 [23] | 8 [23] | 9 [23] | |
| Types of patients, n [%] | 0.21 | |||
| Medical | 73 [97] | 33 [94] | 40 [100] | |
| Surgical (elective) | 2 [3] | 2 [6] | 0 [0] | |
| Comorbidities, n [%] | ||||
| None | 6 [8] | 2 [6] | 4 [10] | 0.68 |
| Diabetes | 26 [35] | 13 [37] | 13 [33] | 0.67 |
| Hypertension | 23 [31] | 12 [34] | 11 [28] | 0.53 |
| Heart failure | 2 [3] | 1 [3] | 1 [2] | >0.99 |
| Chronic kidney disease | 10 [13] | 4 [11] | 6 [15] | 0.74 |
| Chronic lung disease | 13 [17] | 8 [23] | 5 [13] | 0.24 |
| Liver cirrhosis | 5 [7] | 1 [3] | 4 [10] | 0.36 |
| Stroke | 5 [7] | 3 [9] | 2 [5] | 0.66 |
| Malignancy | 31 [41] | 15 [43] | 16 [40] | 0.8 |
| Immunocompromised | 29 [39] | 14 [40] | 15 [38] | 0.82 |
| Others | 17 [23] | 9 [26] | 8 [20] | 0.56 |
| Admission diagnosis, n [%] | 0.32 | |||
| Pneumonia | 53 [71] | 27 [77] | 26 [65] | |
| Hepatobiliary/intra-abdominal | 14 [19] | 4 [11] | 10 [25] | |
| Others | 8 [11] | 4 [11] | 4 [10] | |
| Positive culture, n [%] | 0.21 | |||
| Gram-positive | 22 [29] | 9 [26] | 13 [33] | |
| Gram-negative | 17 [23] | 5 [14] | 12 [30] | |
| Polymicrobial | 6 [8] | 4 [11] | 2 [5] | |
| Creatinine, mean ± SD, mg/dL | 1.96±1.81 | 1.74±1.81 | 2.15±1.81 | 0.33 |
| Lactate, mean ± SD, mmol/L | 5.68±4.09 | 5.95±4.2 | 5.44±4.03 | 0.60 |
| Procalcitonin, mean ± SD, ng/mL | 25.06±48.62 | 17.88±36.5 | 31.51±57.08 | 0.23 |
| PaO2/FiO2 ratio, mean ± SD | 151±82 | 144±85 | 156±81 | 0.53 |
| Acute kidney injury, n [%] | 49 [65] | 21 [62] | 28 [74] | 0.28 |
| Vasopressors, n [%] | 73 [97] | 33 [94] | 40 [100] | 0.21 |
| Hydrocortisone, n [%] | 43 [57] | 16 [46] | 27 [68] | 0.06 |
| APACHE II score, mean ± SD | 24.6±6.0 | 25.2±6.1 | 24.1±6.0 | 0.47 |
| Day 1 SOFA score, mean ± SD | 10.8±3.2 | 10.3±3.3 | 11.3±3.1 | 0.20 |
SD, standard deviation; APACHE, acute physiology and chronic health evaluation; SOFA, Sepsis-related Organ Failure Assessment.
The hospital mortality was 46% (16 of 35 patients) in the vitamin C group and 40% (16 of 40 patients) in the control group, showing a difference without statistical significance (P=0.62). In the prespecified subgroup analysis of patients who received hydrocortisone therapy, the hospital mortality in the vitamin C group (8 of 16 patients, 50%) was not significantly different from that in the control group (12 of 27 patients, 44%; P=0.72). The secondary outcomes are shown in Tables 2,3. The two groups had comparable cumulative survival rates up to 90 days after ICU admission (Figure 2, P=0.2).
Table 2. Treatment outcomes of the study patients based on the administration of adjunctive intravenous vitamin C therapy.
| Outcomes | Vitamin C group (n=35) | Control group (n=40) | P value |
|---|---|---|---|
| 28-day mortality, n [%] | 12 [34] | 8 [20] | 0.16 |
| 90-day mortality, n [%] | 21 [60] | 19 [48] | 0.28 |
| ICU mortality, n [%] | 12 [34] | 12 [30] | 0.69 |
| Hospital mortality, n [%] | 16 [46] | 16 [40] | 0.62 |
| Time to shock reversal, median [IQR], days | 3 [2–5] | 3 [2–5] | 0.28 |
| Duration of mechanical ventilation, median [IQR], days | 8 [5–17] | 8 [4–19] | 0.30 |
| ICU length of stay, median [IQR], days | 10 [6–19] | 11 [7–35] | 0.13 |
| Hospital length of stay, median [IQR], days | 23 [11–40] | 41 [19–63] | 0.23 |
| Changes in the SOFA score, mean ± SD* | −1.4±3.3 | −1.4±3 | 0.96 |
| Changes in PaO2/FiO2 ratio, mean ± SD* | 46.3±81.7 | 49.6±65.1 | 0.85 |
*, during the first 4 ICU days. IQR, interquartile range; SOFA, Sepsis-related Organ Failure Assessment; SD, standard deviation.
Table 3. Renal outcomes depending on the vitamin C therapy in the study patients who developed acute kidney injury.
| Outcomes | Vitamin C group (n=28) | Control group (n=31) | P value |
|---|---|---|---|
| Use of RRT for acute kidney injury, n [%] | 14 [50] | 19 [61] | 0.38 |
| Recovery from acute kidney injury, n [%] | 13 [46] | 16 [52] | 0.69 |
RRT, renal replacement therapy.
Figure 2.
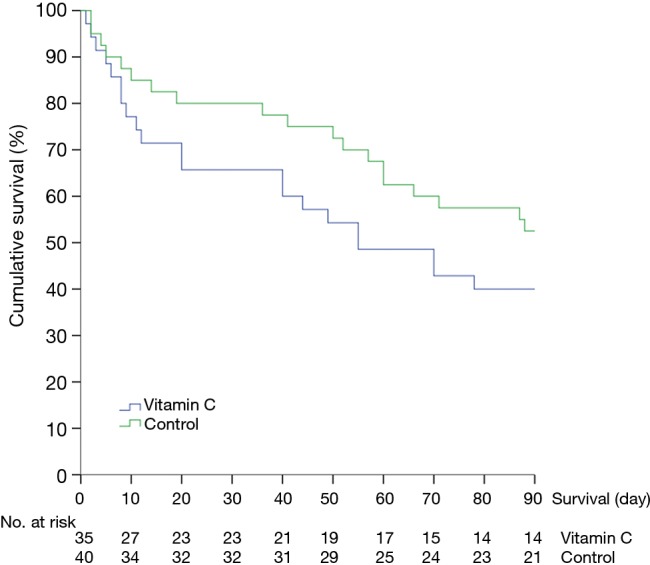
The Kaplan-Meier curves for cumulative survival of the study patients depending on the administration of adjunctive intravenous vitamin C therapy. P=0.2 between the vitamin C and control groups.
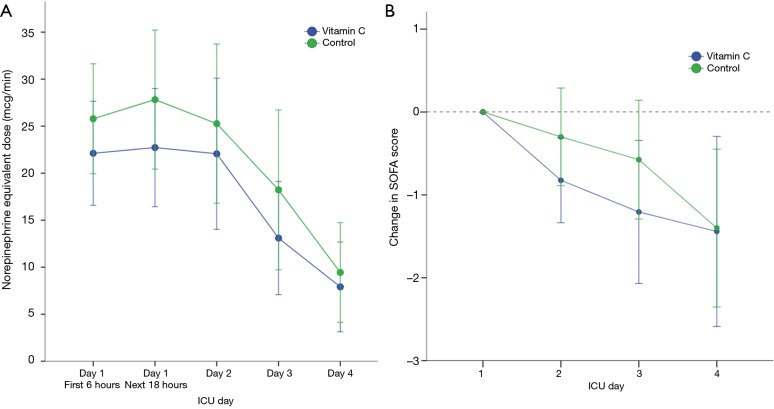
The median time to shock reversal was 3 days (IQR, 2–5 days) in both groups (P=0.28). The cumulative incidence functions of shock reversal are plotted in Figure 3 (P=0.81). In addition, there was no significant difference in daily vasopressor requirement between the two groups during the first 4 ICU days (Figure 4A, P=0.82). The changes in SOFA scores from ICU day 1 to day 4 were −1.4±3.3 and −1.4±3.0 in the vitamin C and control groups, respectively (P=0.96). Over the first 4 ICU days, the reduction in SOFA scores did not differ significantly between the two groups (Figure 4B, P=0.46). Twenty-eight patients (80%) in the vitamin C group and 31 patients (78%) in the control group developed AKI at or during ICU admission. Among those, 14 (50%) and 19 patients (61%) received RRT, respectively (P=0.38). The fluid balance during the first 24 hours was 1.7±1.8 L in the vitamin C group and 1.9±1.7 L in the control group (P=0.49). The recovery rates from AKI until hospital discharge did not differ significantly (46% in the vitamin C group vs. 52% in the control group, P=0.69).
Figure 3.
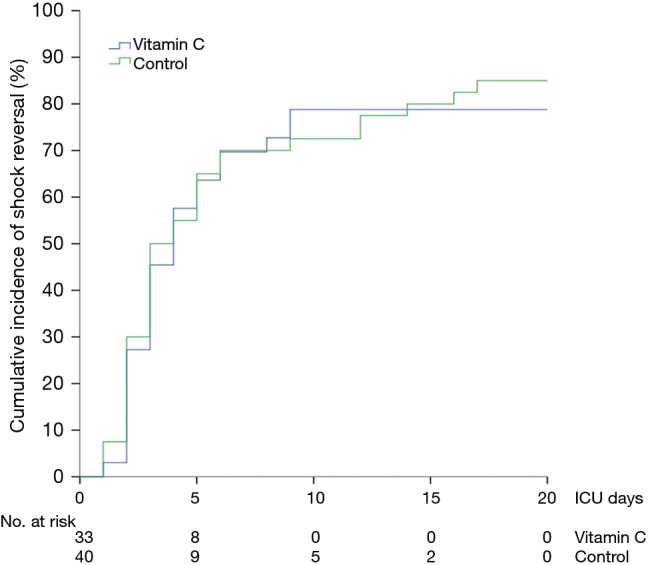
The cumulative incidence function plot of time to shock reversal in the vitamin C and control groups. P=0.81 for comparison between the two groups by treating death as a competing risk.
Figure 4.
The effect of intravenous vitamin C on vasopressor requirement and the SOFA score during the first 4 ICU days. (A) Daily vasopressor requirement in the vitamin C and control groups, calculated in norepinephrine equivalent doses. P=0.82 for intergroup difference in dose over time; (B) serial changes in SOFA scores from ICU day 1 according to treatment group. P=0.46 for intergroup difference in the score change over time. SOFA, Sepsis-related Organ Failure Assessment.
Discussion
This retrospective cohort study evaluated the therapeutic efficacy of adjunctive intravenous vitamin C in mechanically ventilated patients with severe sepsis or septic shock. The intravenous administration of 6 g/day vitamin C did not result in a decrease in hospital and 90-day mortalities. This finding was similar in the subgroup of patients who received hydrocortisone therapy. In addition, there were no significant differences in the time to shock reversal and change in SOFA scores between the two groups. These results may suggest that intravenous vitamin C alone cannot improve clinical outcomes in severe sepsis and septic shock.
Many preclinical studies suggested the beneficial effects and associated mechanisms of vitamin C against sepsis and some of these demonstrated increased survival in septic mouse models (8,11,13,15). However, there have been few published reports which evaluated the effects of intravenous vitamin C on critically ill patients with sepsis. In a phase I study investigating the safety of intravenous vitamin C in medical ICU patients with severe sepsis, Fowler et al. reported a significantly greater reduction in the daily SOFA score over 96 hours in the high-dose vitamin C group compared to that in the placebo group (19). Because of the small number of study patients (8 patients per group), the study could not demonstrate the effect of vitamin C on 28-day mortality with statistical significance. Zabet et al. conducted a double-blinded randomized trial for surgical ICU patients with septic shock, showing a significantly lower mean norepinephrine dose and shorter norepinephrine duration in the vitamin C group than those in the placebo group (21). In that study, 28-day mortality in the vitamin C group was also significantly lower than that in the placebo group (14.3% vs. 64.3%, P=0.009). However, considering the lower baseline APACHE II score and smaller norepinephrine requirement during the first 24 hours in the vitamin C group and the small number of study participants without statistical sample size calculation (14 patients per group), the final difference in 28-day mortality might be attributed to the lower severity in the vitamin C group at study enrollment. A recent retrospective before-after study by Marik et al. reported that combination therapy of intravenous vitamin C, hydrocortisone, and thiamine reduced hospital mortality in ICU patients with severe sepsis or septic shock (20). Their study was different from ours in that combination therapy including vitamin C was used. Marik et al. suggested that multiple and overlapping effects of these medications had a synergistic impact on the natural course of sepsis, which cannot be achieved with each drug alone.
There are three possible explanations for the negative results in the current study. First, the pathophysiologic alterations caused by sepsis were too severe for vitamin C to affect the natural course of sepsis. In our study, all study patients were on mechanical ventilation (51% in the study of Marik et al.), 97% of the patients required vasopressors (47% in the study of Marik et al.), and 44% of the patients received RRT (15% in the study of Marik et al.) (20). In addition, 39% of our study patients were in the immunocompromised state (13% in the study of Marik et al.). Vitamin C may not be effective in immunocompromised patients or in patients with established multiple organ failure. Secondly, the timing of vitamin C administration was relatively late. It took a median of 7 hours (IQR, 4–20 hours) and 16 hours (IQR, 6–29 hours) for the vitamin C group to receive vitamin C therapy from ICU admission and from time zero, respectively. In contrast to the study by Marik et al. (20), vitamin C was started after 24 hours from ICU admission in 8 of 35 patients (23%) in our vitamin C group. An earlier administration of intravenous vitamin C before fully-blown clinical manifestations of sepsis may be necessary to improve outcomes. Thirdly, vitamin C alone was insufficient to ameliorate pathophysiologic derangement resulting from sepsis. In an in-vitro sepsis model, Barabutis et al. suggested that the significant beneficial effects of vitamin C and hydrocortisone were observed only in combination, not in each alone (32). In clinical data, studies showed that hydrocortisone alone did not improve the outcome of severe sepsis or septic shock, while the combination of vitamin C, hydrocortisone, and thiamine reduced hospital mortality in these cases (20,33,34). Because there are no data on the ineffectiveness of vitamin C monotherapy, our results could serve as a logical clue to support vitamin C and hydrocortisone combination therapy for sepsis.
Our study has several limitations. First, the plasma levels of vitamin C were not measured. Although the intravenous administration of 3 g/day vitamin C to critically ill patients can restore plasma vitamin C levels to normal or supranormal levels (19,35,36), how much vitamin C levels were reduced initially and how quickly these were recovered in our patients are unknown. Second, the administration of vitamin C was determined based on the assignment of the patient to certain attending physician. Our study may have a bias resulting from possible differences in practice between physicians. Lastly, the retrospective study design with a small sample size makes the results less persuasive.
Conclusions
In conclusion, adjunctive intravenous vitamin C therapy alone did not reduce hospital mortality in mechanically ventilated patients with severe sepsis or septic shock in this study. Our findings should be interpreted with caution because the negative results in difference do not mean that there are no differences. A well-controlled randomized trial is necessary to determine the therapeutic efficacy of vitamin C for sepsis in alone or in combination with hydrocortisone and thiamine.
Acknowledgements
None.
Ethical Statement: This study protocol was approved by Asan Medical Center Institutional Review Board (approval number, 2018-0412). Informed consent was waived by the board due to the retrospective nature of the study.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Fleischmann C, Scherag A, Adhikari NK, et al. Assessment of global incidence and mortality of hospital-treated sepsis. Current Estimates and Limitations. Am J Respir Crit Care Med 2016;193:259-72. [DOI] [PubMed] [Google Scholar]
- 2.Gaieski DF, Edwards JM, Kallan MJ, et al. Benchmarking the incidence and mortality of severe sepsis in the United States. Crit Care Med 2013;41:1167-74. [DOI] [PubMed] [Google Scholar]
- 3.Kadri SS, Rhee C, Strich JR, et al. Estimating ten-year trends in septic shock incidence and mortality in United States Academic Medical Centers using clinical data. Chest 2017;151:278-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee CC, Yo CH, Lee MG, et al. Adult sepsis—a nationwide study of trends and outcomes in a population of 23 million people. J Infect 2017;75:409-19. [DOI] [PubMed] [Google Scholar]
- 5.Kaukonen KM, Bailey M, Suzuki S, et al. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000-2012. JAMA 2014;311:1308-16. [DOI] [PubMed] [Google Scholar]
- 6.Cohen J, Vincent J-L, Adhikari NKJ, et al. Sepsis: a roadmap for future research. Lancet Infect Dis 2015;15:581-614. [DOI] [PubMed] [Google Scholar]
- 7.Armour J, Tyml K, Lidington D, et al. Ascorbate prevents microvascular dysfunction in the skeletal muscle of the septic rat. J Appl Physiol 2001;90:795-803. [DOI] [PubMed] [Google Scholar]
- 8.Wu F, Wilson JX, Tyml K. Ascorbate protects against impaired arteriolar constriction in sepsis by inhibiting inducible nitric oxide synthase expression. Free Radic Biol Med 2004;37:1282-9. [DOI] [PubMed] [Google Scholar]
- 9.McKinnon RL, Lidington D, Tyml K. Ascorbate inhibits reduced arteriolar conducted vasoconstriction in septic mouse cremaster muscle. Microcirculation 2007;14:697-707. [DOI] [PubMed] [Google Scholar]
- 10.Han M, Pendem S, Teh SL, et al. Ascorbate protects endothelial barrier function during septic insult: role of protein phosphatase type 2A. Free Radic Biol Med 2010;48:128-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fisher BJ, Kraskauskas D, Martin EJ, et al. Mechanisms of attenuation of abdominal sepsis induced acute lung injury by ascorbic acid. Am J Physiol Lung Cell Mol Physiol 2012;303:L20-32. [DOI] [PubMed] [Google Scholar]
- 12.Zhou G, Kamenos G, Pendem S, et al. Ascorbate protects against vascular leakage in cecal ligation and puncture-induced septic peritonitis. Am J Physiol Regul Integr Comp Physiol 2012;302:R409-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tyml K, Li F, Wilson JX. Septic impairment of capillary blood flow requires nicotinamide adenine dinucleotide phosphate oxidase but not nitric oxide synthase and is rapidly reversed by ascorbate through an endothelial nitric oxide synthase-dependent mechanism. Crit Care Med 2008;36:2355-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Secor D, Li F, Ellis CG, et al. Impaired microvascular perfusion in sepsis requires activated coagulation and P-selectin-mediated platelet adhesion in capillaries. Intensive Care Med 2010;36:1928-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fisher BJ, Seropian IM, Kraskauskas D, et al. Ascorbic acid attenuates lipopolysaccharide-induced acute lung injury. Crit Care Med 2011;39:1454-60. [DOI] [PubMed] [Google Scholar]
- 16.Carr AC, Shaw GM, Fowler AA, et al. Ascorbate-dependent vasopressor synthesis: a rationale for vitamin C administration in severe sepsis and septic shock? Crit Care 2015;19:418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borrelli E, Roux-Lombard P, Grau GE, et al. Plasma concentrations of cytokines, their soluble receptors, and antioxidant vitamins can predict the development of multiple organ failure in patients at risk. Crit Care Med 1996;24:392-7. [DOI] [PubMed] [Google Scholar]
- 18.Schorah CJ, Downing C, Piripitsi A, et al. Total vitamin C, ascorbic acid, and dehydroascorbic acid concentrations in plasma of critically ill patients. Am J Clin Nutr 1996;63:760-5. [DOI] [PubMed] [Google Scholar]
- 19.Fowler AA, Syed AA, Knowlson S, et al. Phase I safety trial of intravenous ascorbic acid in patients with severe sepsis. J Transl Med 2014;12:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marik PE, Khangoora V, Rivera R, et al. Hydrocortisone, vitamin C, and thiamine for the treatment of severe sepsis and septic shock: a retrospective before-after study. Chest 2017;151:1229-38. [DOI] [PubMed] [Google Scholar]
- 21.Zabet MH, Mohammadi M, Ramezani M, et al. Effect of high-dose ascorbic acid on vasopressor's requirement in septic shock. J Res Pharm Pract 2016;5:94-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med 1992;20:864-74. [PubMed] [Google Scholar]
- 23.Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Crit Care Med 2017;45:486-552. [DOI] [PubMed] [Google Scholar]
- 24.Levy MM, Evans LE, Rhodes A. The surviving sepsis campaign bundle: 2018 update. Intensive Care Med 2018;44:925-8. [DOI] [PubMed] [Google Scholar]
- 25.Myburgh JA, Higgins A, Jovanovska A, et al. A comparison of epinephrine and norepinephrine in critically ill patients. Intensive Care Med 2008;34:2226-34. [DOI] [PubMed] [Google Scholar]
- 26.Kellum JA, Lameire N, Aspelin P, et al. Kidney disease: improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2012;2:1-138. [Google Scholar]
- 27.Knaus WA, Draper EA, Wagner DP, et al. APACHE II: a severity of disease classification system. Crit Care Med 1985;13:818-29. [PubMed] [Google Scholar]
- 28.Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the working group on sepsis-related problems of the european society of intensive care medicine. Intensive Care Med 1996;22:707-10. [DOI] [PubMed] [Google Scholar]
- 29.Brock GN, Barnes C, Ramirez JA, et al. How to handle mortality when investigating length of hospital stay and time to clinical stability. BMC Med Res Methodol 2011;11:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Russell JA, Walley KR, Singer J, et al. Vasopressin versus norepinephrine infusion in patients with septic shock. N Engl J Med 2008;358:877-87. [DOI] [PubMed] [Google Scholar]
- 31.Ensor CR, Sabo RT, Voils SA. Impact of early postoperative hydrocortisone administration in cardiac surgical patients after cardiopulmonary bypass. Ann Pharmacother 2011;45:189-94. [DOI] [PubMed] [Google Scholar]
- 32.Barabutis N, Khangoora V, Marik PE, et al. Hydrocortisone and ascorbic acid synergistically prevent and repair lipopolysaccharide-induced pulmonary endothelial barrier dysfunction. Chest 2017;152:954-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keh D, Trips E, Marx G, et al. Effect of hydrocortisone on development of shock among patients with severe sepsis: the HYPRESS randomized clinical trial. JAMA 2016;316:1775-85. [DOI] [PubMed] [Google Scholar]
- 34.Venkatesh B, Finfer S, Cohen J, et al. Adjunctive glucocorticoid therapy in patients with septic shock. N Engl J Med 2018;378:797-808. [DOI] [PubMed] [Google Scholar]
- 35.Nathens AB, Neff MJ, Jurkovich GJ, et al. Randomized, prospective trial of antioxidant supplementation in critically ill surgical patients. Ann Surg 2002;236:814-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Long CL, Maull KI, Krishnan RS, et al. Ascorbic acid dynamics in the seriously ill and injured. J Surg Res 2003;109:144-8. [DOI] [PubMed] [Google Scholar]