Abstract
Background
Alpha-1-antitrypsin (A1AT) deficiency (A1ATD) is characterized by accelerated degradation of lung function. We examined our experience with lung transplantation for chronic obstructive pulmonary disease (COPD) with and without A1ATD to compare survival and rates of postoperative surgical complications.
Methods
Patients with A1ATD and non-A1ATD COPD undergoing lung transplantation from 1988−2015 at our institution were analyzed. Complications were categorized into non-gastroenteritis gastrointestinal (GI), wound, airway, and reoperation for bleeding. Overall and complication-free survival were evaluated using Kaplan-Meier curves and Cox proportional hazards models.
Results
Three hundred and eighty-five patients underwent lung transplant for COPD (98 A1ATD). For A1ATD, 56.1% underwent single lung transplantation (80.6% for COPD). Early overall and complication-free survival was worse for A1ATD, but this trend reversed at longer follow up. Unadjusted estimated survival showed advantage for COPD at 90 days and 1 year, which attenuated by 5 years and reversed at 10 years (P<0.001). On adjusted analysis, A1ATD was associated with a trend toward lower complication-free survival at 90 days and 1 year, due partly to increased rates of post-transplant GI pathology, particularly in the era of the lung allocation score (LAS).
Conclusions
A1ATD lung recipients had worse short-term complication-free survival but improved long-term survival compared to COPD patients. A1ATD was associated with greater risk of new GI pathology after transplant. Close monitoring of A1ATD patients with timely evaluation of GI complaints after transplant is warranted.
Keywords: Lung transplantation, alpha-1-antitrypsin deficiency (A1ATD), pulmonary disease, chronic obstructive pulmonary disease (COPD)
Introduction
Lung transplantation is an important therapy for patients with end-stage chronic obstructive pulmonary disease (COPD), including those with alpha-1-antitrypsin (A1AT) deficiency (A1ATD). Since 2004, 28.5% of lung transplants been performed for A1AT-replete (non-A1ATD) COPD, making this the second-most common indication for lung transplantation. Transplantation for A1ATD-related COPD is less common, constituting only 3.6% of transplants during this same time (1). Prior work has demonstrated superior long-term survival for A1ATD lung recipients compared to those with A1AT-replete COPD, although A1ATD patients may suffer greater short-term morbidity and mortality (2-4).
Gastrointestinal (GI) complications occur in up to 50% of recipients following lung transplantation (5-18). Compared to other transplant indications, A1ATD is a risk factor for early post-transplant laparotomy (5). Whether A1ATD patients suffer a greater number of overall GI complications (compared to those with A1AT-replete COPD) and the associated survival effect, along with the comparative incidence of other major complications (e.g., airway, wound, reoperation for bleeding) is unclear.
We used a large single-center cohort of lung transplant recipients for end-stage COPD to: (I) compare the short- and long-term survival of patients who underwent lung transplantation for A1ATD vs. A1AT-replete COPD; (II) compare the relative incidence of GI and other major postoperative surgical complications; and (III) explore the role of other major demographic elements on overall and complication-free survival.
Methods
Study design
We retrospectively reviewed our institutional database for all patients who underwent lung transplantation for COPD, with or without A1ATD, from 1988−2015. Patients requiring re-transplantation were excluded. The study was approved by institutional review board of the University of Minnesota (study number 1006M83333, approval date 11/29/2016).
Definitions
COPD caused by A1ATD is referred to as “A1ATD” and A1A-replete COPD is referred to as “COPD”. All patients undergoing lung transplantation after the institution of the lung allocation score (LAS) in May 2005 are referred to as the “LAS cohort”. Postoperative complications were reviewed and classified as non-gastroenteritis GI (henceforth “GI”), airway, wound, or reoperation for bleeding. GI complications included any documented pathologic diagnosis related to the hepatopancreatobiliary (HPB) complex (e.g., cirrhosis, steatosis, ascites, gallstone disease, cholangitis, pancreatitis) or mechanical alimentary function (e.g., bowel obstruction, diverticulitis). GI bleeding from any source and sequelae of peptic ulcer disease were also included. Gastroenteritis of any etiology was excluded. Any bronchial or tracheal anastomotic stricture or dehiscence was considered an airway complication. Any wound infection or dehiscence was considered a wound complication. Any patient who required operative exploration for bleeding in the first 48 hours after surgery was included in the reoperation for bleeding category.
Clinical considerations
The standard pre-transplant GI workup at our institution includes a standard hepatic laboratory panel with additional imaging and liver biopsy as indicated. Upper endoscopy and esophageal manometry are standard, as is colonoscopy for all potential recipients over 50 years of age. Cardiopulmonary bypass (CPB) is used for all bilateral lung transplants at our institution.
Induction immunosuppression (IS) is not used at our institution. Following transplant, standard IS consists of a calcineurin inhibitor (tacrolimus or cyclosporine if tacrolimus is not tolerated), a cell cycle inhibitor [mycophenolate mofetil (MMF) or azathioprine if MMF is not tolerated], and prednisone. These agents are used in all recipients, regardless of indication for transplant (e.g., A1ATD vs. COPD). There have been no changes to this protocol since these drugs became available. Our IS protocol is similar to reported contemporary practice (19). Post-transplant A1AT repletion for A1ATD is not used at our institution and has not been used historically.
Statistical methods
Descriptive statistics were tabulated overall and by diagnosis, including the mean and standard deviation for continuous variables and frequency with percentage for categorical variables. Unadjusted survival curves were based on Kaplan-Meier estimates. Adjusted analyses were summarized with hazard ratios (HR) based on Cox proportional hazards regression models and differences in proportions based on pseudo-observations (20,21). Survival analyses included endpoints of overall survival at 90 days, 1 year, 5 years and 10 years and complication-free and GI complication-free survival at 90 days, 1 year, and 5 years. Adjusted analysis for each timeframe used separate regression models. Analyses of complication rates were confined to transplants performed in 2002−2015 due to lack of historical detail in our database; these analyses were also repeated for the LAS cohort. The incidence of complications was evaluated using cumulative incidence functions to account for the competing risk of death, with comparisons based on the subdistribution between groups (22). The adjusted competing risks analyses used the Fine-Gray proportional subdistribution hazards regression model (23).
In addition to COPD/A1ATD status, all adjusted analyses accounted for recipient age, donor age, bilateral vs. single transplantation (henceforth “laterality”), recipient sex, and LAS (LAS era vs. pre-LAS) when appropriate. These were chosen a priori based on established prominent risk factors, availability of the patient information across our database, and the available sample size, which limited the number of variables included in regression models. Robust variance estimation was used throughout for confidence intervals and P values. All analyses were performed using R v3.2.4 (24).
Results
Study population
Between 1988 and 2015, a total of 886 lung transplants were performed. The most common indications were COPD/A1ATD (n=403 total, n=385 after exclusion of pediatric transplants and re-transplants), interstitial lung disease (ILD)/idiopathic pulmonary fibrosis (IPF) (n=195), cystic fibrosis (CF, n=121), and pulmonary arterial hypertension (PAH, mean pulmonary artery pressure >40 mmHg, n=67). Of the 385 transplants for COPD or A1ATD, 129 (33.5%) were performed following the implementation of the LAS in May 2005 (LAS cohort, Table 1). Compared to those without A1ATD, recipients with A1ATD were more likely to be male (53.1% vs. 43.2%), younger (51.7±8.0 vs. 58.1±6.1 years), and less likely to receive a single lung transplant (56.1% vs. 80.6%). The groups were approximately evenly matched for ethnicity, donor age, and recipient and donor smoking status. Fewer transplants for A1ATD were performed in the LAS era than for COPD (20.4% vs. 38.1%). These differences in demographic and clinical characteristics between COPD and A1ATD recipients were similar in the 2002−2015 and LAS cohorts (Table 1). Rates of pre-transplant chronic GI pathology (most commonly gastroesophageal reflux disease and a history of colon polyps) were also similar between diagnosis groups in both analysis cohorts (Table S1).
Table 1. Cohort by diagnosis.
| Cohort | Overall | A1ATD | COPD | P value |
|---|---|---|---|---|
| Entire cohort | n=385 | n=98 | n=287 | |
| Demographics | ||||
| Recipient male (n, %) | 176 (45.7) | 52 (53.1) | 124 (43.2) | 0.116 |
| Caucasian (n, %) | 378 (98.2) | 98 (100.0) | 280 (97.6) | 0.296 |
| Black (n, %) | 5 (1.3) | 0 (0.0) | 5 (1.7) | − |
| Other (n, %) | 2 (0.5) | 0 (0.0) | 2 (0.7) | − |
| Age | ||||
| Recipient (mean ± SD) | 56.5±7.2 | 51.7±8.0 | 58.1±6.1 | <0.001 |
| Donor (mean ± SD) | 35.9±15.0 | 34.4±14.7 | 36.5±15.1 | 0.225 |
| Pulmonary Status | ||||
| Recipient smoker (n, %) | 196 (50.9) | 47 (48.0) | 149 (51.9) | 0.576 |
| Donor smoker (n, %) | 42 (10.9) | 8 (8.2) | 34 (11.8) | 0.411 |
| Transplant | ||||
| LAS era transplant (n, %) | 129 (33.5) | 20 (20.4) | 109 (38.1) | 0.002 |
| Single lung transplant (n, %) | 286 (74.3) | 55 (56.1) | 231 (80.6) | <0.001 |
| Transplant LOS >14 days (n, %) | 170 (44.2%) | 48 (49.0%) | 122 (42.7) | 0.346 |
| LAS cohort | n=129 | n=20 | n=109 | |
| Demographics | ||||
| Recipient male (n, %) | 60 (46.5) | 10 (50.0) | 50 (45.9) | 0.923 |
| Caucasian (n, %) | 127 (98.4) | 20 (100.0) | 107 (99.0) | 0.830 |
| Black (n, %) | 1 (0.8) | 0 (0.0) | 1 (0.9) | − |
| Other (n, %) | 1 (0.8) | 0 (0.0) | 1 (0.9) | − |
| Age | ||||
| Recipient (mean ± SD) | 59.8±5.3 | 57.9±7.1 | 60.1±4.8 | 0.090 |
| Donor (mean ± SD) | 39.6±15.8 | 42.8±14.0 | 39.1±16.1 | 0.336 |
| Pulmonary Status | ||||
| Recipient smoker (n, %) | 126 (97.7) | 20 (100.0) | 106 (97.2) | >0.999 |
| Donor smoker (n, %) | 42 (32.6) | 8 (40.0) | 34 (31.2) | 0.608 |
| Transplant | ||||
| Single lung transplant (n, %) | 87 (67.4) | 12 (60.0) | 75 (68.8) | 0.608 |
| Lung allocation score (mean ± SD) | 37.8±14.7 | 33.7±2.84 | 38.5±15.8 | 0.189 |
| Longest ischemic time (mins, mean ± SD) | 306±147 | 266±160 | 314±144 | 0.228 |
| Transplant LOS >14 days (n, %) | 63 (48.8) | 13 (65.0) | 50 (45.9) | 0.196 |
“Longest ischemic time” refers to the ischemic time of the second lung implanted during BLTx or to the ischemic time of the only implanted lung in SLTx. A1ATD, alpha-1-antitrypsin deficiency; COPD, chronic obstructive pulmonary disease; LAS, lung allocation score; LOS, length of stay; BLTx, bilateral lung transplant; SLTx, single lung transplant.
Table S1. 2002−2015 cohort.
| 2002−2015 cohort | Overall (n=191) | A1ATD (n=42) | COPD (n=149) | P value |
|---|---|---|---|---|
| Demographics | ||||
| Recipient male (n, %) | 92 (48.2) | 25 (59.5) | 67 (45.0) | 0.135 |
| Caucasian (n, %) | 188 (98.4) | 42 (100.0) | 146 (98.0) | 0.651 |
| Black (n, %) | 2 (1.0) | 0 (0.0) | 2 (1.3) | − |
| Other (n, %) | 1 (0.5) | 0 (0.0) | 1 (0.7) | − |
| Age | ||||
| Recipient (mean ± SD) | 58.8±5.7 | 55.9±6.82 | 59.6±5.09 | <0.001 |
| Donor (mean ± SD) | 36.9±15.8 | 36.2±16.4 | 37.1±15.6 | 0.758 |
| Pulmonary status | ||||
| Recipient smoker (n, %) | 158 (82.7) | 33 (78.6) | 125 (83.9) | 0.566 |
| Donor smoker (n, %) | 42 (22.0) | 8 (19.0) | 34 (22.8) | 0.756 |
| Transplant | ||||
| LAS era transplant (n, %) | 129 (67.5) | 20 (47.6) | 109 (73.2) | 0.003 |
| Single lung transplant (n, %) | 134 (70.2) | 23 (54.8) | 111 (74.5) | 0.023 |
| Longest ischemic time (mins, mean ± SD) | 306 (146.7) | 266 (159.6) | 314 (143.6) | 0.228 |
| Transplant LOS >14 days (n, %) | 91 (47.6) | 25 (59.5) | 66 (44.3) | 0.125 |
“Longest ischemic time” refers to the ischemic time of the second lung implanted during BLTx or to the ischemic time of the only implanted lung in SLTx. A1ATD, alpha-1-antitrypsin deficiency; COPD, chronic obstructive pulmonary disease; LAS, lung allocation score; LOS, length of stay; BLTx, bilateral lung transplant; SLTx, single lung transplant.
Unadjusted outcomes
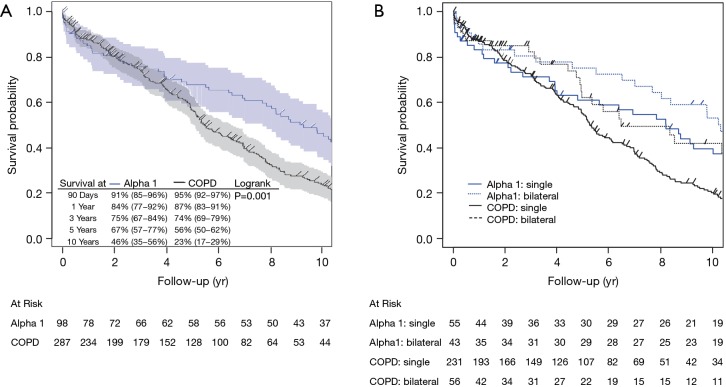
In unadjusted Kaplan-Meier estimates, A1ATD patients demonstrated slightly increased early mortality compared to COPD patients at 90 days [91% (95% CI: 0.85−0.96) vs. 95% (95% CI: 0.92−0.97)] and 1 year [84% (95% CI: 0.77−0.92 vs. 87% (95% CI: 0.83−0.91)] despite decreased long-term mortality: 46% (95% CI: 0.35−0.56) of A1ATD patients were alive at 10 years compared to only 23% (95% CI: 0.17−0.29) of patients with COPD. Survival was similar in both groups at 3 years [A1ATD: 75% (95% CI: 0.67−0.84), COPD: 74% (95% CI: 0.69−0.79)], but was superior in A1ATD at 5 years [67% (95% CI: 0.57−0.77) vs. 56% (95% CI: 0.50−0.62)] compared to COPD (log-rank P=0.001, Figure 1). Unadjusted estimates for the non-COPD/A1ATD cohort (e.g., recipients transplanted for ILD/IPF, CF, PAH) were similar at 3 years [69% (95% CI: 0.64−0.74)] and 5 years [58% (95% CI: 0.53−0.63)].
Figure 1.
Unadjusted overall survival by diagnosis for entire cohort (A) by diagnosis alone and (B) by diagnosis, stratified by single vs. bilateral lung transplantation. Confidence limits excluded from (B) for readability. COPD, chronic obstructive pulmonary disease; yr, year.
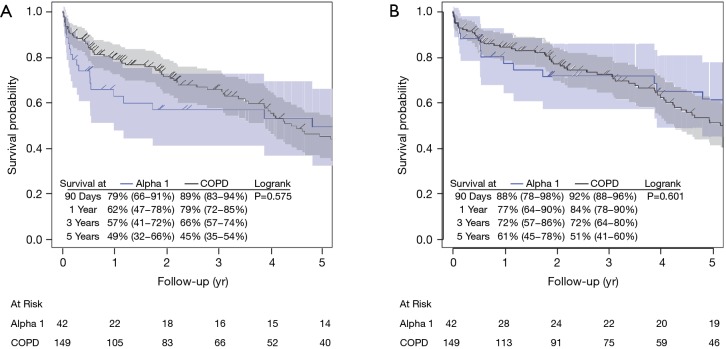
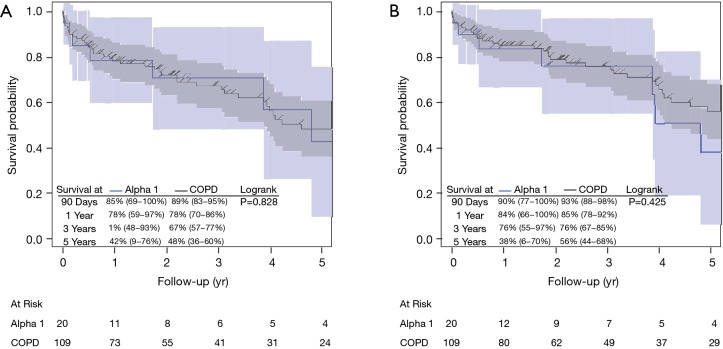
Complication-free and GI complication-free survival during the first three years were superior for COPD patient compared with A1ATD, but this trend attenuated and even reversed around years 3 and 4 (Figures 2,S1). Cumulative incidence estimates of postoperative complications was higher in the A1ATD group at 90 days (17.02% vs. 12.16%), 1 year (27.65% vs. 18.53%), and 5 years (34.65% vs. 26.26%) (Table 2). The majority of these were airway (P=0.048) and GI complications, which were more common in the A1ATD patients at each time point, attributable to greater rates of GI bleeding, diverticulitis, gallstone disease, ascites, and other HPB disease. The overall cumulative incidence of wound complications was low in both groups (Table 2). Among the entire cohort, only 4 patients (1.4%, all COPD) required reoperation for bleeding.
Figure 2.
Unadjusted (A) complication-free survival and (B) GI complication-free survival by diagnosis in 2002−2015 cohort. COPD, chronic obstructive pulmonary disease; yr, year; GI, gastrointestinal.
Figure S1.
Unadjusted (A) complication-free survival and (B) GI complication-free survival by diagnosis in LAS cohort. COPD, chronic obstructive pulmonary disease; yr, year; GI, gastrointestinal.
Table 2. Cumulative incidence estimates for complications by diagnosis (%), 2002−2015.
| Complication type | Time | Overall (n=191) | A1ATD (n=42) | COPD (n=149) | P value |
|---|---|---|---|---|---|
| All | 90 days | 12.16 | 17.02 | 10.81 | 0.139 |
| 1 year | 18.53 | 27.65 | 15.99 | ||
| 5 years | 26.26 | 34.65 | 24 | ||
| Airway | 90 days | 3.71 | 7.32 | 2.71 | 0.048 |
| 1 year | 4.79 | 12.27 | 2.71 | ||
| 5 years | 6.03 | 12.27 | 4.28 | ||
| Wound | 90 days | 1.06 | 2.44 | 0.68 | 0.649 |
| 1 year | 3.36 | 2.44 | 3.60 | ||
| 5 years | 4.09 | 2.44 | 4.52 | ||
| All GI | 90 days | 5.81 | 4.88 | 6.08 | 0.556 |
| 1 year | 9.30 | 10.37 | 9.01 | ||
| 5 years | 12.98 | 16.78 | 11.90 | ||
| GI bleed | 90 days | 2.64 | 2.44 | 2.70 | 0.839 |
| 1 year | 2.64 | 2.44 | 2.70 | ||
| 5 years | 4.67 | 5.48 | 4.50 | ||
| All alimentary | 90 days | 2.64 | 2.38 | 2.70 | 0.705 |
| 1 year | 2.64 | 2.38 | 2.70 | ||
| 5 years | 7.33 | 5.82 | 7.78 | ||
| Diverticulitis | 90 days | 0.52 | 2.38 | 0 | 0.357 |
| 1 year | 0.52 | 2.38 | 0 | ||
| 5 years | 1.34 | 2.38 | 1.07 | ||
| Small bowel obstruction | 90 days | 0 | 0 | 0 | 0.333 |
| 1 year | 0 | 0 | 0 | ||
| 5 years | 2.24 | 0 | 2.94 | ||
| Other alimentary | 90 days | 2.12 | 0 | 2.70 | 0.718 |
| 1 year | 2.12 | 0 | 2.70 | ||
| 5 years | 3.75 | 3.39 | 3.78 | ||
| All HPB | 90 days | 3.70 | 2.44 | 4.05 | 0.452 |
| 1 year | 7.72 | 10.37 | 6.98 | ||
| 5 years | 10.10 | 13.70 | 9.04 | ||
| Acute pancreatitis | 90 days | 2.12 | 2.44 | 2.03 | 0.751 |
| 1 year | 2.71 | 2.44 | 2.79 | ||
| 5 years | 3.47 | 2.44 | 3.78 | ||
| Ascites | 90 days | 0.53 | 0 | 0.68 | 0.326 |
| 1 year | 1.09 | 2.65 | 0.68 | ||
| 5 years | 1.09 | 2.65 | 0.68 | ||
| Gallstone disease | 90 days | 1.06 | 0 | 1.35 | 0.381 |
| 1 year | 4.52 | 8.13 | 3.56 | ||
| 5 years | 5.33 | 8.13 | 4.62 | ||
| Cirrhosis | 90 days | 0 | 0 | 0 | N/A |
| 1 year | 0 | 0 | 0 | ||
| 5 years | 0 | 0 | 0 | ||
| Other HPB | 90 days | 0 | 0 | 0 | 0.175 |
| 1 year | 1.68 | 2.44 | 1.44 | ||
| 5 years | 2.46 | 5.72 | 1.44 |
“Gallstone disease” includes cholelithiasis, cholecystitis, and choledocholithiasis. “Other alimentary” includes gastric bezoar [1] for A1ATD and colon perforation [3], abdominal compartment syndrome [1], perforated duodenal ulcer [1], and unspecified GI perforation requiring ileostomy [1] for COPD. “Other HPB” includes gastroesophageal varices [1], ruptured hepatic artery aneurysm [1], cholangitis [1], portal hypertension [1], pancreatic duct stricture [1] for A1ATD and non-alcoholic steatohepatitis [1], cholestatic hepatitis [1], and pancreatocolonic fistula [1] for COPD. GI, gastrointestinal; HPB, hepatopancreatobiliary; A1ATD, Alpha-1-antitrypsin deficiency; COPD, chronic obstructive pulmonary disease; N/A, not applicable.
Adjusted outcomes
After adjustment for transplant laterality, donor age, recipient age and sex, and LAS era, A1ATD had a negative effect on 90-day (HR 1.78, 95% CI: 0.52−6.05, P=0.356) and 1-year (HR 1.40, 95% CI: 0.67−2.94, P=0.369) overall survival compared to COPD (Table 3). Despite this relatively high risk of early mortality, the point estimate for the hazard ratio for overall survival for A1ATD patients actually becomes superior to that of COPD patients at 5 years (HR 0.97, 95% CI: 0.59−1.57, P=0.894). This change persists at 10 years (HR 0.76, 95% CI: 0.51−1.13, P=0.169) and the difference becomes more dramatic and statistically significant with the use of a time-varying HR over 5−10 years (HR 0.52, 95% CI: 0.30−0.91, P=0.021). Additionally, restricted mean survival estimates revealed a 16% (95% CI: 0.02−0.30, P=0.022) survival advantage in A1ATD at 10 years compared to COPD (Table 4). Notably, survival for the entire cohort was significantly improved in the LAS era.
Table 3. Overall, rejection-free, and BOS-free survival.
| Covariate | Entire cohort: overall survival | LAS-era cohort: five-year outcomes | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 90-day survival | 1-year survival | 5-year survival | 10-year survival | 10-year restricted mean survival | Overall Survival | Rejection-free survival | BOS-free survival | ||||||||||||||||||
| HR, 95% CI | P value | HR, 95% CI | P value | HR, 95% CI | P value | HR, 95% CI | P value | Time-varying HR, 95% CI | P value | Estimate, 95% CI | P value | HR, 95% CI | P value | HR, 95% CI | P value | HR, 95% CI | P value | ||||||||
| A1ATD (vs. COPD, ≤5 years) | 1.78 (0.52−6.05) | 0.356 | 1.40 (0.67−2.94) | 0.369 | 0.97 (0.59− 1.57) | 0.894 | 0.76 (0.51−1.13) | 0.169 | 0.98 (0.63−1.52) | 0.919 | − | − | − | − | − | − | − | − | |||||||
| A1ATD (vs. COPD, >5 years) | − | − | − | − | − | − | − | − | 0.52 (0.30−0.91) | 0.021 | − | − | − | − | − | − | − | − | |||||||
| A1ATD (vs. COPD) | − | − | − | − | − | − | − | − | − | − | 0.16 (0.02−0.30) | 0.022 | 1.02 (0.40–2.56) | 0.970 | 0.94 (0.50–1.75) | 0.845 | 1.43 (0.70−2.95) | 0.327 | |||||||
| Recipient age (per 10 years) | 1.43 (0.67−3.04) | 0.358 | 1.66 (0.97−2.86) | 0.065 | 1.43 (1.02−2.00) | 0.039 | 1.33 (1.03−1.72) | 0.026 | 1.33 (1.03−1.72) | 0.031 | −0.09 (−0.18–0.00) | 0.055 | 1.96 (0.93–4.13) | 0.076 | 1.50 (0.89–2.52) | 0.128 | 1.31 (0.81−2.10) | 0.269 | |||||||
| Donor age (per 10 years) | 1.22 (0.91−1.64) | 0.193 | 1.28 (1.05−1.56) | 0.015 | 1.11 (0.98−1.25) | 0.099 | 1.10 (1.00−1.21) | 0.042 | 1.10 (1.00−1.21) | 0.047 | −0.03 (−0.06–0.00) | 0.074 | 0.84 (0.67–1.07) | 0.160 | 1.03 (0.89–1.20) | 0.663 | 1.01 (0.87−1.17) | 0.913 | |||||||
| Male (vs. female) | 1.03 (0.44−2.39) | 0.952 | 1.25 (0.71−2.21) | 0.442 | 1.07 (0.76−1.50) | 0.716 | 1.11 (0.85−1.45) | 0.441 | 1.11 (0.85−1.46) | 0.436 | −0.06 (−0.16−0.04) | 0.217 | 2.00 (0.95−4.19) | 0.066 | 1.57 (0.94−2.60) | 0.082 | 1.87 (1.13−3.09) | 0.014 | |||||||
| SLTx (vs. BLTx) | 0.65 (0.21−1.96) | 0.442 | 0.67 (0.30−1.46) | 0.312 | 1.11 (0.68−1.82) | 0.68 | 1.31 (0.89−1.94) | 0.177 | 1.31 (0.88−1.94) | 0.183 | −0.13 (−0.26−0.01) | 0.06 | 0.79 (0.35−1.77) | 0.570 | 1.09 (0.65−1.83) | 0.747 | 1.49 (0.83−2.70) | 0.185 | |||||||
| LAS era (vs. pre-LAS) | 0.14 (0.03−0.60) | 0.008 | 0.25 (0.12−0.55) | <0.001 | 0.56 (0.37−0.86) | 0.008 | 0.66 (0.47−0.92) | 0.015 | 0.66 (0.46−0.95) | 0.024 | 0.07 (−0.03−0.18) | 0.187 | − | − | − | − | − | − | |||||||
All HRs based on adjusted Cox proportional hazards analysis. 90-day and 1-year survival estimates are excluded from LAS-only analysis due to a paucity of events in the A1ATD group. Analysis of 10-year restricted mean survival performed using pseudo-observations. Estimates are multiplicative changes in restricted mean survival time. BOS, bronchiolitis obliterans syndrome; LAS, lung allocation score; HR, hazard ratio; A1ATD, alpha-1-antitrypsin deficiency; COPD: chronic obstructive pulmonary disease; SLTx, single lung transplant; BLTx, bilateral lung transplant.
Table 4. Post-transplant complications- adjusted cox proportional hazards.
| Covariate | 90-day survival | 1-year survival | 5-year survival | |||||
|---|---|---|---|---|---|---|---|---|
| HR, 95% CI | P value | HR, 95% CI | P value | HR, 95% CI | P value | |||
| Complication-free survival | ||||||||
| 2002−2015 cohort | ||||||||
| A1ATD (vs. COPD) | 1.30 (0.48−3.51) | 0.611 | 1.15 (0.54−2.48) | 0.714 | 0.89 (0.46−1.71) | 0.726 | ||
| Recipient age (per 10 years) | 1.01 (0.52−1.98) | 0.976 | 1.12 (0.63−1.99) | 0.693 | 1.27 (0.80−2.00) | 0.310 | ||
| Donor age (per 10 years) | 0.88 (0.66−1.18) | 0.407 | 0.90 (0.73−1.11) | 0.320 | 0.98 (0.84−1.15) | 0.847 | ||
| SLTx (vs. BLTx) | 0.43 (0.16−1.15) | 0.092 | 0.32 (0.15−0.69) | 0.004 | 0.44 (0.25−0.79) | 0.005 | ||
| Male (vs. female) | 1.78 (0.76−4.21) | 0.187 | 2.06 (1.09−3.87) | 0.025 | 1.40 (0.89−2.19) | 0.142 | ||
| LAS era (vs. pre-LAS) | 0.72 (0.24−2.17) | 0.553 | 0.64 (0.28−1.49) | 0.303 | 0.70 (0.39−1.23) | 0.210 | ||
| LAS cohort | ||||||||
| A1ATD (vs. COPD) | 1.50 (0.44−5.08) | 0.515 | 1.01 (0.32−3.16) | 0.991 | 1.03 (0.44−2.41) | 0.941 | ||
| Recipient age (per 10 years) | 0.86 (0.43−1.71) | 0.662 | 1.01 (0.50−2.01) | 0.988 | 0.94 (0.57−1.55) | 0.815 | ||
| Donor age (per 10 years) | 0.76 (0.54−1.08) | 0.122 | 0.76 (0.60−0.98) | 0.031 | 0.82 (0.69−0.99) | 0.035 | ||
| SLTx (vs. BLTx) | 0.67 (0.23−1.91) | 0.451 | 0.43 (0.19−0.99) | 0.048 | 0.52 (0.27−1.00) | 0.048 | ||
| Male (vs. female) | 3.27 (1.02−10.43) | 0.046 | 1.70 (0.95−3.05) | 0.072 | 1.70 (0.95−3.05) | 0.072 | ||
| GI complication-free survival | ||||||||
| 2002−2015 cohort | ||||||||
| A1ATD (vs. COPD) | 0.94 (0.24−3.67) | 0.925 | 0.88 (0.32−2.38) | 0.797 | 0.76 (0.36−1.60) | 0.464 | ||
| Recipient age (per 10 years) | 0.98 (0.42−2.27) | 0.963 | 1.23 (0.58−2.60) | 0.584 | 1.34 (0.77−2.31) | 0.300 | ||
| Donor age (per 10 years) | 0.83 (0.58−1.19) | 0.321 | 0.91 (0.71−1.16) | 0.433 | 0.97 (0.82−1.16) | 0.767 | ||
| SLTx (vs. BLTx) | 0.36 (0.11−1.21) | 0.098 | 0.34 (0.13−0.88) | 0.026 | 0.64 (0.34−1.23) | 0.185 | ||
| Male (vs. female) | 1.36 (0.50−3.74) | 0.548 | 1.87 (0.89−3.93) | 0.100 | 1.15 (0.71−1.86) | 0.577 | ||
| LAS era (vs. pre-LAS) | 0.69 (0.17−2.83) | 0.609 | 0.63 (0.22−1.78) | 0.380 | 0.77 (0.41−1.45) | 0.425 | ||
| LAS cohort | ||||||||
| A1ATD (vs. COPD) | 1.65 (0.36−7.62) | 0.519 | 1.14 (0.30−4.28) | 0.851 | 1.64 (0.71−3.82) | 0.249 | ||
| Recipient age (per 10 years) | 0.93 (0.35−2.47) | 0.890 | 1.38 (0.49−3.85) | 0.543 | 1.18 (0.63−2.21) | 0.601 | ||
| Donor age (per 10 years) | 0.63 (0.40−1.00) | 0.049 | 0.70 (0.52−0.93) | 0.016 | 0.74 (0.60−0.92) | 0.007 | ||
| SLTx (vs. BLTx) | 0.59 (0.17−2.06) | 0.407 | 0.41 (0.15−1.10) | 0.076 | 0.67 (0.33−1.34) | 0.259 | ||
| Male (vs. female) | 2.64 (0.65−10.65) | 0.172 | 1.50 (0.80−2.83) | 0.206 | 1.50 (0.80−2.83) | 0.206 | ||
| Airway complication-free survival | ||||||||
| 2002−2015 cohort | ||||||||
| A1ATD (vs. COPD) | 1.84 (0.42−8.02) | 0.419 | 1.48 (0.57−3.89) | 0.422 | 0.93 (0.47−1.84) | 0.841 | ||
| Recipient age (per 10 years) | 0.93 (0.39−2.21) | 0.874 | 1.46 (0.73−2.94) | 0.288 | 1.46 (0.87−2.46) | 0.151 | ||
| Donor age (per 10 years) | 1.11 (0.74−1.66) | 0.628 | 1.18 (0.90−1.56) | 0.225 | 1.15 (0.95−1.38) | 0.151 | ||
| SLTx (vs. BLTx) | 0.40 (0.10−1.65) | 0.205 | 0.30 (0.11−0.85) | 0.024 | 0.69 (0.36−1.31) | 0.253 | ||
| Male (vs. female) | 1.29 (0.35−4.73) | 0.702 | 2.32 (0.90−6.02) | 0.082 | 1.76 (1.03−3.00) | 0.038 | ||
| LAS era (vs. pre-LAS) | 0.41 (0.11−1.46) | 0.167 | 0.30 (0.12−0.80) | 0.016 | 0.55 (0.30−1.03) | 0.062 | ||
| LAS cohort | ||||||||
| A1ATD (vs. COPD) | 1.02 (0.12−8.69) | 0.982 | 0.46 (0.05−4.19) | 0.49 | 0.73 (0.29−1.87) | 0.516 | ||
| Recipient age (per 10 years) | 1.19 (0.45−3.17) | 0.726 | 2.05 (0.75−5.61) | 0.161 | 1.55 (0.83−2.92) | 0.172 | ||
| Donor age (per 10 years) | 0.92 (0.53−1.59) | 0.762 | 0.96 (0.69−1.33) | 0.817 | 0.91 (0.74−1.12) | 0.371 | ||
| SLTx (vs. BLTx) | 0.51 (0.08−3.11) | 0.464 | 0.40 (0.11−1.42) | 0.155 | 0.76 (0.36−1.61) | 0.473 | ||
| Male (vs. female) | 2.12 (0.33−13.48) | 0.425 | 2.65 (1.28−5.49) | 0.009 | 2.65 (1.28−5.49) | 0.009 | ||
HR, hazard ratio; A1ATD, alpha-1-antitrypsin deficiency; COPD, chronic obstructive pulmonary disease; SLTx, single lung transplant; BLTx, bilateral lung transplant; LAS, lung allocation score; GI, gastrointestinal.
As a secondary analysis, adjusted overall survival analysis of the LAS cohort demonstrated a mortality risk at 5 years after transplant that was similar between A1ATD and COPD (HR 1.02, 95% CI: 0.40−2.56, P=0.970), though this was restricted by the available sample size, evidenced by the wider confidence interval. A paucity of events prohibited analysis of earlier (<5 years) intervals. Bilateral lung transplantation (BLTx) was more common for A1ATD in both the overall and LAS cohorts. BLTx recipients as a group were subject to greater early (90-day and 1-year) morbidity and mortality compared to those undergoing single lung transplantation (SLTx) but enjoyed improved long-term (5-year and 10-year) survival (Tables 1,3, Figure 1).
Regarding complications, A1ATD demonstrated a trend toward worse complication-free survival at 90 days (HR 1.30, 95% CI: 0.48−3.51, P=0.611) and 1 year (HR 1.15, 95% CI: 0.54−2.48, P=0.714), adjusting for laterality, donor age, recipient age and sex, and LAS era (vs. pre-LAS) in the 2002−2015 cohort. Similar to overall survival, the HR between A1ATD and COPD attenuates at longer follow-up. These findings are replicated in the LAS cohort (Table 4). Due to limitations in completeness of complication data over extended follow-up, comparisons were not calculated past the first 5 years. Single lung transplant was associated with improved complication-free survival in the 2002−2015 and LAS cohorts despite worse overall long-term survival (Table 4).
A1ATD was associated with a trend towards worse GI complication-free survival at 90 days (HR 1.65, 95% CI: 0.36−7.62, P=0.519), 1 year (HR 1.14, 95% CI: 0.30−4.28, P=0.851), and 5 years (HR 1.64, 95% CI: 0.71−3.82, P=0.249) in the LAS cohort (Table 4). Increased recipient age and BLTx (vs. SLTx) were associated with worse 1- and 5-year GI complication-free survival. Fine-Gray model results revealed that, after accounting for the competing risk of death, A1ATD patients demonstrated a trend toward greater risk of GI complications at 1 year [subdistribution hazard ratio (SHR) 1.24, 95% CI: 0.28−5.54, P=0.780] and 5 years (SHR 1.87, 95% CI: 0.56−6.19, P=0.310) in the LAS cohort adjusting for recipient and donor age, laterality, and sex (Table 5). The attenuation over time of the A1ATD effect on GI complications is no longer observed when accounting for death as a competing risk. In fact, the trend is reversed. In both the 2002−2015 cohort and the LAS cohort, A1ATD demonstrates lower risk of GI complications over 90 days, similar risk of GI complications over 1 year, and greater risk over 5 years (Table 5).
Table 5. Fine-Gray model of adjusted competing risks analysis.
| Covariate | 90-day survival | 1-year survival | 5-year survival | |||||
|---|---|---|---|---|---|---|---|---|
| SHR, 95% CI | P value | SHR, 95% CI | P value | SHR, 95% CI | P value | |||
| GI complications | ||||||||
| 2002−2015 cohort | ||||||||
| A1ATD (vs. COPD) | 0.71 (0.12−4.20) | 0.700 | 0.97 (0.26−3.65) | 0.970 | 1.46 (0.46−4.63) | 0.520 | ||
| Recipient age (per 10 years) | 1.18 (0.44−3.18) | 0.750 | 1.01 (0.38−2.64) | 0.990 | 0.84 (0.39−1.79) | 0.640 | ||
| Donor age (per 10 years) | 0.66 (0.41−1.07) | 0.089 | 0.63 (0.43−0.93) | 0.018 | 0.70 (0.51−0.94) | 0.019 | ||
| SLTx (vs. BLTx) | 0.47 (0.10−2.29) | 0.350 | 0.46 (0.12−1.80) | 0.260 | 0.52 (0.18−1.56) | 0.250 | ||
| Male (vs. female) | 1.19 (0.35−4.02) | 0.780 | 1.11 (0.42−2.89) | 0.830 | 0.84 (0.36−1.96) | 0.680 | ||
| LAS era (vs. pre-LAS) | 1.35 (0.21−8.44) | 0.750 | 1.41 (0.34−5.94) | 0.640 | 2.54 (0.71−9.06) | 0.150 | ||
| LAS cohort | ||||||||
| A1ATD (vs. COPD) | 0.87 (0.12−6.41) | 0.890 | 1.24 (0.28−5.54) | 0.780 | 1.87 (0.56−6.19) | 0.310 | ||
| Recipient age (per 10 years) | 0.97 (0.36−2.61) | 0.950 | 0.96 (0.32−2.89) | 0.940 | 0.79 (0.34−1.82) | 0.580 | ||
| Donor age (per 10 years) | 0.67 (0.42−1.06) | 0.090 | 0.64 (0.43−0.93) | 0.021 | 0.70 (0.51−0.97) | 0.030 | ||
| SLTx (vs. BLTx) | 0.98 (0.24−3.94) | 0.980 | 0.77 (0.21−2.87) | 0.700 | 0.71 (0.23−2.16) | 0.540 | ||
| Male (vs. female) | 3.37 (0.76−14.94) | 0.110 | 1.53 (0.51−4.58) | 0.450 | 0.97 (0.38−2.49) | 0.950 | ||
| Airway complications | ||||||||
| 2002−2015 cohort | ||||||||
| A1ATD (vs. COPD) | 1.74 (0.31−9.85) | 0.530 | 2.31 (0.49−10.95) | 0.290 | 1.89 (0.56−6.36) | 0.300 | ||
| Recipient age (per 10 years) | 0.95 (0.30−3.02) | 0.930 | 1.25 (0.39−4.02) | 0.710 | 0.96 (0.38−2.45) | 0.940 | ||
| Donor age (per 10 years) | 1.05 (0.58−1.90) | 0.870 | 0.98 (0.57−1.67) | 0.930 | 1.02 (0.67−1.54) | 0.930 | ||
| SLTx (vs. BLTx) | 0.38 (0.06−2.45) | 0.310 | 0.23 (0.04−1.54) | 0.130 | 0.44 (0.10−1.86) | 0.260 | ||
| Male (vs. female) | 2.12 (0.35−12.98) | 0.420 | 2.49 (0.46−13.58) | 0.290 | 3.84 (0.74−20.08) | 0.110 | ||
| LAS era (vs. pre-LAS) | 0.73 (0.10−5.21) | 0.750 | 0.43 (0.06−2.93) | 0.390 | 0.73 (0.17−3.14) | 0.680 | ||
Fine-Gray analysis not performed for airway complications in LAS cohort due to a paucity of events. Death is the sole competing risk in all analyses shown. SHR, subdistribution hazard ratio; GI, gastrointestinal; A1ATD, alpha-1-antitrypsin deficiency; COPD, chronic obstructive pulmonary disease; SLTx, single lung transplant; BLTx, bilateral lung transplant; LAS, lung allocation score.
Finally, there was an association between A1ATD and worse airway complication-free survival in the 2002−2015 cohort, particularly in the first 90 days (HR 1.84, 95% CI: 0.42−8.02, P=0.419) and 1 year (HR 1.48, 95% CI: 0.57−3.89, P=0.422) after transplant, but this effect appeared to dissipate by 5 years (HR 0.93, 95% CI: 0.47−1.84, P=0.841). This is concordant with the corresponding cumulative incidence estimates in Table 2. Notably, this effect was not observed in the LAS cohort (Table 4). Fine-Gray analysis re-demonstrates greater risk of airway complications in A1ATD patients in the 2002−2015 cohort but such analysis could not be performed in the LAS cohort due to a paucity of events (Table 5).
Discussion
We present a large cohort of patients who underwent lung transplantation for COPD and A1ATD and find that A1ATD was associated with lower complication-free and GI complication-free survival over the first five years following lung transplantation compared to COPD. While the effect of primary diagnosis on overall survival was not statistically significant based on the hazard ratio, A1ATD patients demonstrated a notable trend toward increased early mortality (HR 1.78 at 90 days and HR 1.40 at 1 year) after transplant but equal or improved survival over the long-term compared to those with COPD. This is also reflected in the crossing survival curves from the unadjusted analyses and suggests that the A1ATD patients have better survival (compared to COPD) at prolonged follow up. Indeed, adjusted analysis evaluating survival beyond 5 years showed the hazard for the A1ATD group was significantly smaller compared to the COPD group. The increased early morbidity and mortality for A1ATD patients in our cohort is consistent with prior reports on this subject (3,4,25-27).
The loss of A1AT in A1ATD patients results in the unregulated activity of neutrophil elastase, which has the potential to impair wound healing and anastomotic integrity (28,29). Although the rates of wound complication did not differ significantly by diagnosis and the global rate of reoperation for bleeding was low, there was an increased rate of airway complications (AC) in the short and long term in A1ATD recipients compared to those with COPD. Our findings are concurrent with those of Gulack et al., who reported an increased rate of airway dehiscence in A1ATD patients in an analysis of UNOS data (30). The rate of AC in the general lung transplant population is 10−15%, which is consistent with our data (31). Reported risk factors include anastomotic ischemia and technique, allograft infection (usually fungal), rejection, and need for tracheostomy after transplant (31-35). The use of a short donor bronchus (divided at or near the lobar carina) and an “end-to-end” (vs. telescoping) anastomotic technique both appear to be protective against AC (34,35). For over two decades, the practice at the University of Minnesota has been to trim the donor bronchus two rings from the lobar carina and to perform an end-to-end anastomosis with a single running 4-0 polypropylene suture. Given this and the absence of historical detail about the factors listed above prior to the LAS era in our database, the cause of the decreased rate of airway complications in the LAS era is not apparent from our data.
Our findings suggest that A1ATD patients suffer a greater comparative incidence of post-transplant GI pathology, especially GI bleeding, diverticulitis, and HPB disease. High rates of GI complications have been reported in lung recipients, but A1ATD patients may have a uniquely increased risk (5,9,11,35-37). Bredahl et al. demonstrated that A1ATD was an independent predictor of early post-transplant laparotomy, most commonly segmental colectomy (5). Similarly, we showed an increased incidence of overall mechanical complications in A1ATD vs. COPD at 1 year (5% vs. 2%) due to an increased burden of diverticulitis in A1ATD patients (Table 2).
The increased rates of HPB pathology in the A1ATD cohort appear to be based on increased rates of gallstone disease and ascites. Ongoing polymerization of Z-type A1AT causes chronic hepatocyte injury in A1ATD, promoting progressive fibrosis (38). Although there were no new cirrhosis diagnoses after transplant, patients with such chronic liver disease have increased rates of cholelithiasis, increasing the risk of gallstone-related disease (39). The cumulative burden of these factors, plus perioperative stress, immunosuppression, and the natural course of A1ATD, likely associated with some level of subclinical liver dysfunction, may be responsible for these findings after lung transplantation (40). Our data also suggest that greater recipient age and BLTx may exacerbate this risk (Table 4).
Regarding GI bleeding, lung recipients may experience low-flow states associated with CPB and/or hemodynamic instability. A1AT is protective against reperfusion injury in experimental settings (41-43). Yang et al. demonstrated improved mucosal integrity in a murine model of small bowel transplantation when grafts were preserved using a A1AT-containing solution, implying a protective role of A1AT in splanchnic ischemia-reperfusion injury (41). The greater demonstrated incidence of postop GI bleeding suggests this protection may be impaired in A1ATD recipients.
At this time, there is not a clear benefit to post-transplant A1A supplementation. At our institution, only a few patients were receiving supplementation prior to transplant but this was discontinued after transplant. This practice is based on a lack of substantial evidence that supplementation changes outcomes or is cost-effective after transplant. The American Thoracic Society/European Respiratory Society guidelines for management of A1ATD do not recommend supplementation after transplant (44). Although published in 2003 (pre-LAS), there have been no new studies since that time addressing the utility of supplementation after transplant. Notably, listing for lung transplantation was actually an exclusion criterion in the largest existing randomized controlled trial of A1AT supplementation (45).
Despite this, there may be some utility to A1AT supplementation in the peri- and/ or post-transplant period. There have been small studies that demonstrate elevated levels of neutrophil elastase in bronchoalveolar lavage samples of A1ATD lung transplant recipients during severe lower respiratory tract inflammatory conditions, which argues for potential use of this therapy during episodes of acute rejection or infection (29,46). Improvement in wound healing with A1AT supplementation in an affected patient has also been reported (47).
How can we use our findings to improve care? We found that the burden of wound and early bleeding complications following transplant for A1ATD is not greater than that for COPD. However, our data suggest a relationship between A1ATD and GI pathology after transplant. Aggressive workup of post-transplant abdominal complaints, particularly in A1ATD patients should be considered; this is supported by prior reports on the subject (5,7,9,11,37,48). Paul et al. presented a standardized approach to all lung transplant patients, including preoperative abdominal ultrasound with post-transplant cholecystectomy (when appropriate) and mandatory abdominal computed tomography with general surgery consultation for postoperative abdominal symptoms (9). More rigorous, protocolized screening for GI pathology before or after transplant in this population is intuitively appealing but further study is required to determine the ideal regimen.
Our findings also suggest a need for investigation of the potential benefits of perioperative and/or postoperative A1AT supplementation. von Schönfeld et al. demonstrated impaired hepatic metabolism despite normal laboratory values in patients with A1ATD-related emphysema using indocynanine green (ICG) clearance testing (40). Oxidative stress, common in major surgery, contributes to liver damage in animal models of A1ATD (49). Perioperative A1AT supplementation could potentially mitigate perioperative splanchnic insult, especially in patients with impaired ICG clearance (50,51). There may also be a benefit to wound healing, as above. However, demonstrating a benefit to supplementation aimed at reduction of perioperative morbidity would require long follow-up in a large cohort or serial ICG clearance testing.
Limitations
Limitations of our study include its single-center, retrospective nature, the possibility of inconsistent documentation of complication data over the life of our database, and the sample size. Small sample size and subsequently few GI events in the LAS era prohibits conclusions regarding this subpopulation. Greater sample size would improve estimates of the effect of transplant diagnosis on overall outcomes, partly by allowing more robust adjusted analyses. A1ATD lung transplant recipients are a rare subgroup and, although many of our survival estimates fall shy of statistical significance, we believe their magnitude, trend over the time points presented, and overall composite outcome analysis is notable and important to describe in this unique cohort. A1ATD genotype was not captured by our database. Sufficient analyzable data regarding CPB times are not available in our database; the same is true of spirometry.
Conclusions
Patients undergoing lung transplantation for A1ATD suffer greater short-term post-transplant morbidity and mortality compared to their COPD counterparts despite improved long-term survival. This may be related to an increased early incidence of GI pathology after transplant. Absent these complications, A1ATD lung recipients may not suffer the relative decrement in early post-transplant survival and would continue to have superior long-term survival compared to lung recipients for COPD. Aggressive evaluation of abdominal complaints following lung transplantation, especially in A1ATD patients, is warranted.
Acknowledgements
Funding: This work was partly supported by National Center for Advancing Translational Sciences (NCATS) Award #UL1TR000114.
Ethical Statement: The study was approved by institutional review board of the University of Minnesota (study number 1006M83333, approval date 11/29/2016).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Chambers DC, Cherikh WS, Goldfarb SB, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-fifth adult lung and heart-lung transplant report-2018; Focus theme: Multiorgan Transplantation. J Heart Lung Transplant 2018;37:1169-83. 10.1016/j.healun.2018.07.020 [DOI] [PubMed] [Google Scholar]
- 2.Tanash HA, Riise GC, Hansson L, et al. Survival benefit of lung transplantation in individuals with severe α1-anti-trypsin deficiency (PiZZ) and emphysema. J Heart Lung Transplant 2011;30:1342-7. 10.1016/j.healun.2011.07.003 [DOI] [PubMed] [Google Scholar]
- 3.Chatterji S, Parmar J. 623: Survival of Patients with Alpha 1 Antitrypsin Deficiency Receiving Lung Transplantation for Emphysema–A Review of 20 Years’ Experience at a Major UK Transplant Centre. J Heart Lung Transplant 2009;28:S282 10.1016/j.healun.2008.11.630 [DOI] [Google Scholar]
- 4.Gulack BC, Ganapathi AM, Speicher PJ, et al. Survival After Lung Transplant in Alpha-1-Antitrypsin Deficiency Recipients Compared to Other Forms of Chronic Obstructive Pulmonary Disease. J Heart Lung Transplant 2015;34:S243-4. 10.1016/j.healun.2015.01.676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bredahl P, Zemtsovski M, Perch M, et al. Early laparotomy after lung transplantation: increased incidence for patients with α1-anti-trypsin deficiency. J Heart Lung Transplant 2014;33:727-33. 10.1016/j.healun.2014.02.025 [DOI] [PubMed] [Google Scholar]
- 6.Timrott K, Vondran FW, Jaeger MD, et al. Incidence and outcome of abdominal surgical interventions following lung transplantation--a single center experience. Langenbecks Arch Surg 2011;396:1231-7. 10.1007/s00423-011-0754-2 [DOI] [PubMed] [Google Scholar]
- 7.Leonardi MJ, Jamil KG, Hiscox B, et al. Abdominal surgery after lung transplantation. Am Surg 2010;76:1130-4. [PubMed] [Google Scholar]
- 8.Morton JR, Ansari N, Glanville AR, et al. Distal intestinal obstruction syndrome (DIOS) in patients with cystic fibrosis after lung transplantation. J Gastrointest Surg 2009;13:1448-53. 10.1007/s11605-009-0924-5 [DOI] [PubMed] [Google Scholar]
- 9.Paul S, Escareno CE, Clancy K, et al. Gastrointestinal complications after lung transplantation. J Heart Lung Transplant 2009;28:475-9. 10.1016/j.healun.2009.02.011 [DOI] [PubMed] [Google Scholar]
- 10.Goldberg HJ, Hertz MI, Ricciardi R, et al. Colon and rectal complications after heart and lung transplantation. J Am Coll Surg 2006;202:55-61. 10.1016/j.jamcollsurg.2005.08.025 [DOI] [PubMed] [Google Scholar]
- 11.Miller CB, Malaisrie SC, Patel J, et al. Intraabdominal complications after lung transplantation. J Am Coll Surg 2006;203:653-60. 10.1016/j.jamcollsurg.2006.07.024 [DOI] [PubMed] [Google Scholar]
- 12.Andersson B, Nilsson J, Brandt J, et al. Gastrointestinal complications after cardiac surgery. Br J Surg 2005;92:326-33. 10.1002/bjs.4823 [DOI] [PubMed] [Google Scholar]
- 13.Qasabian RA, Meagher AP, Lee R, et al. Severe diverticulitis after heart, lung, and heart-lung transplantation. J Heart Lung Transplant 2004;23:845-9. 10.1016/j.healun.2003.07.019 [DOI] [PubMed] [Google Scholar]
- 14.Gilljam M, Chaparro C, Tullis E, et al. GI complications after lung transplantation in patients with cystic fibrosis. Chest 2003;123:37-41. 10.1378/chest.123.1.37 [DOI] [PubMed] [Google Scholar]
- 15.Maurer JR. The spectrum of colonic complications in a lung transplant population. Ann Transplant 2000;5:54-7. [PubMed] [Google Scholar]
- 16.Pollard TR, Schwesinger WH, Sako EY, et al. Abdominal operations after lung transplantation. Indications and outcome. Arch Surg 1997;132:714-7; discussion 717-8. 10.1001/archsurg.1997.01430310028004 [DOI] [PubMed] [Google Scholar]
- 17.Gemma V, Mistrot D, Row D, et al. Pneumatosis intestinalis in solid organ transplant recipients. J Thorac Dis 2018;10:1984-97. 10.21037/jtd.2018.02.52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mistrot DP, Gemma V, Gagliano RA, Jr, et al. A 54-Year Old Man Presenting With an Abnormal Abdominal CT Scan 8 Months After Double Lung Transplant. Chest 2016;149:e151-5. 10.1016/j.chest.2015.12.031 [DOI] [PubMed] [Google Scholar]
- 19.Scheffert JL, Raza K. Immunosuppression in lung transplantation. J Thorac Dis 2014;6:1039-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klein JP, Logan B, Harhoff M, et al. Analyzing survival curves at a fixed point in time. Stat Med 2007;26:4505-19. 10.1002/sim.2864 [DOI] [PubMed] [Google Scholar]
- 21.Klein JP, Gerster M, Andersen PK, et al. SAS and R functions to compute pseudo-values for censored data regression. Comput Methods Programs Biomed 2008;89:289-300. 10.1016/j.cmpb.2007.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gray R. A class of K-sample tests for comparing the cumulative incidence of competing risk. Ann Stat 1988;16:1141-54. 10.1214/aos/1176350951 [DOI] [Google Scholar]
- 23.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. J Am Stat Assoc 1999;94:496-509. 10.1080/01621459.1999.10474144 [DOI] [Google Scholar]
- 24.R Core Team (2014). R: A language and environment for statistical computing. The R Project for Statistical Computing. Vienna, Austria. Available online: http://www.r-project.org/
- 25.de Perrot M, Chaparro C, McRae K, et al. Twenty-year experience of lung transplantation at a single center: Influence of recipient diagnosis on long-term survival. J Thorac Cardiovasc Surg 2004;127:1493-501. 10.1016/j.jtcvs.2003.11.047 [DOI] [PubMed] [Google Scholar]
- 26.Banga A, Gildea T, Rajeswaran J, et al. The natural history of lung function after lung transplantation for α(1)-antitrypsin deficiency. Am J Respir Crit Care Med 2014;190:274-81. [DOI] [PubMed] [Google Scholar]
- 27.Yusen RD, Edwards LB, Kucheryavaya AY, et al. The registry of the International Society for Heart and Lung Transplantation: thirty-first adult lung and heart-lung transplant report--2014; focus theme: retransplantation. J Heart Lung Transplant 2014;33:1009-24. 10.1016/j.healun.2014.08.004 [DOI] [PubMed] [Google Scholar]
- 28.Takagi N, Kawakami K, Kanno E, et al. IL-17A promotes neutrophilic inflammation and disturbs acute wound healing in skin. Exp Dermatol 2017;26:137-44. 10.1111/exd.13115 [DOI] [PubMed] [Google Scholar]
- 29.Meyer KC, Nunley DR, Dauber JH, et al. Neutrophils, unopposed neutrophil elastase, and alpha1-antiprotease defenses following human lung transplantation. Am J Respir Crit Care Med 2001;164:97-102. 10.1164/ajrccm.164.1.2006096 [DOI] [PubMed] [Google Scholar]
- 30.Gulack BC, Mulvihill MS, Ganapathi AM, et al. Survival after lung transplantation in recipients with alpha-1-antitrypsin deficiency compared to other forms of chronic obstructive pulmonary disease: a national cohort study. Transpl Int 2018;31:45-55. 10.1111/tri.13038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Machuzak M, Santacruz JF, Gildea T, et al. Airway Complications After Lung Transplantation. Thorac Surg Clin 2015;25:55-75. 10.1016/j.thorsurg.2014.09.008 [DOI] [PubMed] [Google Scholar]
- 32.Shofer SL, Wahidi MM, Davis WA, et al. Significance of and Risk Factors for the Development of Central Airway Stenosis After Lung Transplantation. Am J Transplant 2013;13:383-9. 10.1111/ajt.12017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mahajan AK, Folch E, Khandhar SJ, et al. The Diagnosis and Management of Airway Complications Following Lung Transplantation. Chest 2017;152:627-38. 10.1016/j.chest.2017.02.021 [DOI] [PubMed] [Google Scholar]
- 34.van Berkel V, Guthrie TJ, Puri V, et al. Impact of anastomotic techniques on airway complications after lung transplant. Ann Thorac Surg 2011;92:316-20; discussion 320-1. 10.1016/j.athoracsur.2011.03.031 [DOI] [PubMed] [Google Scholar]
- 35.Panchabhai TS, Chaddha U, McCurry JR, et al. Historical perspectives of lung transplantation: connecting the dots. J Thorac Dis 2018;10:4516-31. 10.21037/jtd.2018.07.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lahon B, Mordant P, Thabut G, et al. Early severe digestive complications after lung transplantation. Eur J Cardiothorac Surg 2011;40:1419-24. [DOI] [PubMed] [Google Scholar]
- 37.Hoekstra HJ, Hawkins K, de Boer WJ, et al. Gastrointestinal complications in lung transplant survivors that require surgical intervention. Br J Surg 2001;88:433-8. 10.1046/j.1365-2168.2001.01693.x [DOI] [PubMed] [Google Scholar]
- 38.Fregonese L, Stolk J. Hereditary alpha-1-antitrypsin deficiency and its clinical consequences. Orphanet J Rare Dis 2008;3:16. 10.1186/1750-1172-3-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elzouki AN, Nilsson S, Nilsson P, et al. The prevalence of gallstones in chronic liver disease is related to degree of liver dysfunction. Hepatogastroenterology 1999;46:2946-50. [PubMed] [Google Scholar]
- 40.von Schönfeld J, Breuer N, Zotz R, et al. Liver function in patients with pulmonary emphysema due to severe alpha-1-antitrypsin deficiency (PiZZ). Digestion 1996;57:165-9. 10.1159/000201331 [DOI] [PubMed] [Google Scholar]
- 41.Yang R, Liu Q, Collins MH, et al. Alpha-1-proteinase inhibitor prolongs small intestinal graft preservation and survival. J Pediatr Surg 1996;31:1052-55. 10.1016/S0022-3468(96)90085-8 [DOI] [PubMed] [Google Scholar]
- 42.Gao W, Zhao J, Kim H, et al. α1-Antitrypsin inhibits ischemia reperfusion-induced lung injury by reducing inflammatory response and cell death. J Heart Lung Transplant 2014;33:309-15. 10.1016/j.healun.2013.10.031 [DOI] [PubMed] [Google Scholar]
- 43.Daemen MARC, Heemskerk VH, van ’t Veer C, et al. Functional Protection by Acute Phase Proteins α1-Acid Glycoprotein and α1-Antitrypsin Against Ischemia/Reperfusion Injury by Preventing Apoptosis and Inflammation. Circulation 2000;102:1420-26. 10.1161/01.CIR.102.12.1420 [DOI] [PubMed] [Google Scholar]
- 44.American Thoracic Society, European Respiratory Society American Thoracic Society/European Respiratory Society Statement. Am J Respir Crit Care Med 2003;168:818-900. 10.1164/rccm.168.7.818 [DOI] [PubMed] [Google Scholar]
- 45.Chapman KR, Burdon JGW, Piitulainen E, et al. Intravenous augmentation treatment and lung density in severe α1 antitrypsin deficiency (RAPID): a randomised, double-blind, placebo-controlled trial. Lancet (London, England) 2015;386:360-8. 10.1016/S0140-6736(15)60860-1 [DOI] [PubMed] [Google Scholar]
- 46.King MB, Campbell EJ, Gray BH, et al. The proteinase-antiproteinase balance in alpha-1-proteinase inhibitor-deficient lung transplant recipients. Am J Respir Crit Care Med 1994;149:966-71. 10.1164/ajrccm.149.4.8143063 [DOI] [PubMed] [Google Scholar]
- 47.Cathomas M, Schüller A, Candinas D, et al. Severe postoperative wound healing disturbance in a patient with alpha-1-antitrypsin deficiency: the impact of augmentation therapy. Int Wound J 2015;12:601-4. 10.1111/iwj.12419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith PC, Slaughter MS, Petty MG, et al. Abdominal complications after lung transplantation. J Heart Lung Transplant 1995;14:44-51. [PubMed] [Google Scholar]
- 49.Marcus NY, Blomenkamp K, Ahmad M, et al. Oxidative stress contributes to liver damage in a murine model of alpha-1-antitrypsin deficiency. Exp Biol Med (Maywood) 2012;237:1163-72. 10.1258/ebm.2012.012106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haegele S, Reiter S, Wanek D, et al. Perioperative Non-Invasive Indocyanine Green-Clearance Testing to Predict Postoperative Outcome after Liver Resection. Gruttadauria S, ed. PLoS One 2016;11:e0165481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chotirmall SH, Al-Alawi M, McEnery T, et al. Alpha-1 proteinase inhibitors for the treatment of alpha-1 antitrypsin deficiency: safety, tolerability, and patient outcomes. Ther Clin Risk Manag 2015;11:143-51. 10.2147/TCRM.S51474 [DOI] [PMC free article] [PubMed] [Google Scholar]