Abstract
Background
Comparative studies of robotic lung resection are limited. Our study aims to compare short-term and long-term outcomes of anatomic lung resection by robotic or video-assisted thoracoscopic surgery (VATS) from a single surgeon experienced in both approaches.
Methods
A retrospective analysis of consecutive anatomic lung resections by robot or VATS was performed to compare perioperative characteristics and long-term survival.
Results
From December 2010 to June 2015, 61 patients underwent robotic surgery, and 105 patients underwent VATS. Patient demographics were similar except that the VATS group had higher percentage of diabetic patients (robotic 14.75% vs. VATS 30.48%, P=0.0258) and a slightly lower percentage of patients with previous cancer history (robotic 57.38% vs. VATS 40.95%, P=0.0409). The robotic group had a higher rate of prolonged air leak ≥7 d (robotic 14.75% vs. VATS 3.81%; P=0.0161), and a modestly longer length of hospital stay (robotic median of 4.0 days vs. VATS median of 3.0 days, P=0.0123). Other postoperative complications, mortality, nodal upstaging and conversion rate were similar. Disease-free survival was not different. The robotic group appeared to have slightly better overall survival, however, this observation was confounded by a lower percentage of diabetic patients in this group. Further analysis has demonstrated that in non-diabetic patients who underwent either surgery, the overall survival remained similar. The same observation was also made in diabetic patients.
Conclusions
Robotic anatomic lung resection appears to be associated with a higher rate of prolonged air leak (≥7 d), and resulting slightly longer length of hospital stay than VATS. Within the same follow-up period, both the disease-free survival and the overall survival are similar.
Keywords: Robotic, video-assisted thoracoscopy, lobectomy, segmentectomy
Introduction
Video-assisted thoracoscopic surgery (VATS) has become accepted as a safe and effective procedure in early-stage non-small cell lung cancer (NSCLC) patients (1-4). In recent years, robotic-assisted technologies have been applied rapidly to general thoracic surgery. Both minimally invasive approaches are believed to provide advantages including decreased postoperative pain, fewer complications and more rapid recovery to preoperative activity when compared with thoracotomy. The perceived benefits of using a robot including more intuitive wristed movements, greater flexibility and high definition three-dimensional vision (5). In addition, some feel that surgeons may be more easily trained to perform robotic minimally invasive lung resection. While several studies demonstrate that robotic lobectomy and segmentectomy are feasible and safe (6-14), direct comparisons between robotic and VATS approaches for perioperative characteristics and long-term survival benefits remain limited. Previous studies do not control for institution and surgeon expertise, which opens the possibility that the observed advantages of robot or VATS are merely due to surgeon or institution factors rather than the approach itself. To minimize this confounding and compare the safety and oncologic efficacy of anatomic lung resection by robot and VATS, we performed a retrospective analysis of data of consecutive robotic/VATS anatomic lung resections by a single surgeon experienced in both approaches at a single institution. Importantly, because of limited robot availability, patients were not chosen for either procedure, minimizing selection bias.
Methods
This study evaluated patients who underwent anatomic lung resection by video-assisted thoracic surgery (VATS) or by robotic-assisted surgery from December 2010 to June 2015. It was approved by the Institutional Review Board of Duke University. All surgical procedures were performed by one surgeon at Duke University Medical Center. All patients received staging CT of the chest and upper abdomen and/or integrated PET/CT. Before the current study period, the surgeon had substantial experience with VATS lung resection, the results of which have been published previously (1,2). Because Duke Hospital has two robots that are shared between services, the robot has only been available for approximately half of the surgeon’s operating room (OR) time. Cancer patients are booked for surgery as expeditiously as possible. If a robot was available and other operations for which the robot is highly beneficial (mediastinal masses, for example) were not scheduled, the lobectomy was performed robotically. Otherwise, the procedure was performed via thoracoscopy.
VATS and robotic approaches were both performed using an anterior-to-posterior approach and a “fissureless” technique. VATS resection was performed without any rib spreading with the thoracoscope placed in the 8th intercostal space in the midaxillary line and a 3–4 cm anterior utility incision in the 5th intercostal space and a 1 cm posterior incision in the 9th intercostal space in the line of the scapular tip. When performing robotic resection (Si robot), a 1cm incision was made in the 8th intercostal space anterior to the posterior axillary line, and dissection was carried into the chest. A balloon port was placed, and insufflation was begun with CO2 with pressure and flow at 7 cm. A handbreadth above and anterior to the camera incision, an 8 or 5 mm robot port was placed. A handbreadth posterior and inferior to the camera incision, a second 8 or 5 mm robotic port was placed. A handbreadth posterior to this in the same intercostal space, a third 8 or 5 mm robot port was placed. Finally, a 12 mm step port was placed just above the diaphragm between the camera and above ports. An epidural catheter for postoperative pain relief was routinely offered to all patients regardless of planned operative approach. One chest tube was used for postoperative drainage in all patients. For both groups, chest tubes were removed when air leak was absent and the volume of serosanguinous drainage was <400 mL in a 24-hour time period.
Patient demographics, intraoperative data, and postoperative data were collected from our prospective thoracic database. Chart review was utilized as necessary to complete data collection. All postoperative events that prolonged or otherwise complicated the postoperative course were recorded. Postoperative mortality was defined as any death occurred within 30 days after operation or those occurred later but during the same hospitalization. In determining the length of hospital stay, the day of the surgical procedure was considered as day 0 and the day of discharge was considered as 1 day of stay. Once a patient was discharged, hospital stay during readmission was not counted into the length of hospital stay during original operation. Patient clinical follow-up was performed approximately 2 weeks after surgery, every 3 months for the first year, then every 6 months for year 2, and then yearly from years 3 to 5. Chest CT scan was performed for each 6-month follow-up in the first 2 years and yearly thereafter. Disease-free survival was defined as the time from surgery to the time of first recurrence or death. Pathologic confirmation of recurrent disease was not mandatory in cases where clinical and radiographic evaluation was clearly consistent with recurrence. Overall survival was defined as the time from surgery to the time of death. Patients alive and without recurrent disease were censored at the time their last known follow-up. Staging was performed according to the Lung Cancer Staging Manual (7th edition) by the American Joint Committee on Cancer.
Categorical variables were presented as number with the corresponding percentage, and comparisons between groups were made using chi-square test or Fisher’s exact test when the expected values in any of the cells of a contingency table are below 5, or below 10 when there is only one degree of freedom. Continuous variables were presented as median with interquartile range and compared using Mann-Whitney U test or Student’s t-test where appropriate. All tests were two-tailed and P<0.05 was considered to be statistically significant. Survival was estimated using Kaplan-Meier method and compared using log-rank test. All statistical analyses were performed using Graphpad Prism 6.
Results
From December 2010 to June 2015, 61 patients underwent robotic-assisted anatomic lung resection and 105 patients underwent VATS anatomic lung resection. These two groups were similar in terms of baseline characteristics (Table 1). No significant differences between groups were identified in age, sex, smoking status, pre-operative pulmonary function, and tumor attributes. The VATS group had higher percentage of patients with diabetes mellitus (robotic 14.75% vs. VATS 30.48%, P=0.0258) and a slightly lower percentage of patients with previous cancer history (robotic 57.38% vs. VATS 40.95%, P=0.0409). Other comorbidities remained the same in both groups. Table 2 shows the lobes or segments of lungs resected by either robotic or VATS approach. Both approaches were effective in resecting all lobes. As presented in Table 3, no operative death was reported in either group. In the robotic group, 4 patients (6.56%) required conversion to thoracotomy: 2 were to perform right upper lobe sleeve resection, and the other 2 were due to difficult dissection. In the VATS group, 2 conversions (1.90%) were observed: 1 was to resect chest wall, and the other for left pneumonectomy. All conversions were performed with hemodynamic stability and no further sequelae after conversion. No difference was noted in conversion rate between two groups. These patients converted to an open procedure remained in their original groups during data analysis, following the intent to treat paradigm.
Table 1. Patient demographics.
| Patient demographics | Robotic lobectomy (n=61) | VATS lobectomy (n=105) | P value |
|---|---|---|---|
| Age at time of surgery, median (IQR) | 67 (14.0) range, [31–85] | 67 (13.0) range, [40–91] | 0.7439 |
| Sex (male) | 44.3% | 55.2% | 0.1726 |
| Smoking statusa | 0.2322 | ||
| Ever | 52 (85.25%) | 81 (77.14%) | – |
| Never | 9 (14.75%) | 24 (22.86%) | − |
| Predicted FEV1, median (IQR) | b71 (26.75%) | c74 (29.0%) | 0.7033 |
| Predicted DLCO, median (IQR) | d73 (33.75%) | e75.5 (33.75%) | 0.7455 |
| Primary lung cancer | 51 (83.61%) | 82 (78.10%) | 0.3910 |
| Adenocarcinoma | 28 (45.90%) | 46 (43.81%) | |
| Squamous cell | 14 (22.95%) | 28 (26.67%) | |
| Large cell | 4 (6.56%) | 3 (2.86%) | |
| Mixed squamous cell and adenocarcinoma | 0 (0.00%) | 1 (0.95%) | |
| Mixed large cell and adenocarcinoma | 1 (1.64%) | 0 (0.00%) | |
| Typical carcinoid | 3 (4.92%) | 2 (1.90%) | |
| Atypical carcinoid | 1 (1.64%) | 1 (0.95%) | |
| Sarcomatoid | 0 (0.00%) | 1 (0.95%) | |
| Pre-operative induction therapy received | 12 (19.67%) | 22 (20.95%) | 0.8438 |
| Comorbidities | |||
| Cardiovascular diseases | 20 (32.79%) | 41 (39.05%) | 0.4199 |
| Hypertension | 33 (54.10%) | 56 (53.33%) | 0.9241 |
| Diabetes a | 9 (14.75%) | 32 (30.48%) | 0.0258 |
| Chronic pulmonary disease | 22 (36.07%) | 31 (29.52%) | 0.3834 |
| Chronic renal insufficiency/failurea | 4 (6.56%) | 9 (8.57%) | 0.7698 |
| Previous cancer history | 35 (57.38%) | 43 (40.95%) | 0.0409 |
Continuous variables were reported as median (interquartile range) and categorical variables were reported as number (%). Cardiovascular diseases include coronary artery disease, atrial fibrillation, myocardial infarction, valve diseases, congestive heart failure and cardiomyopathy. a, categorical variables were analyzed using chi square test, unless indicated Fisher’s exact test; b, missing data of predicted FEV1 in robotic group (7 patients); c, missing data of predicted FEV1 in VATS group (10 patients); d, missing data of predicted DLCO in robotic group (7 patients); e, missing data of predicted DLCO in VATS group (11 patients). VATS, video-assisted thoracoscopic surgery; FEV1, forced expiratory volume in 1 second; DLCO, diffusing capacity of the lung for carbon monoxide.
Table 2. Anatomic lung resection by robotic or VATS approach.
| Anatomic lung resection | Robotic (n=61) | VATS (n=105) | P value |
|---|---|---|---|
| Left | 27 (44.26%) | 56 (53.33%) | 0.2598 |
| Upper lobe | 11 | 26a | |
| Lingular | 1 | 3 | |
| Lingular-sparing upper lobe | 5 | 8 | |
| Lower lobe | 9 | 15 | |
| Lower lobe segment | 1 | 2 | |
| Pneumonectomy | 0 | 2 | |
| Right | 34 (55.74%) | 49 (46.67%) | |
| Upper lobe | 16 | 23 | |
| Middle lobe | 4 | 9 | |
| Lower lobe | 4 | 12 | |
| Lower lobe superior segment | 2 | 2 | |
| Lower lobe basilar segments | 1 | 0 | |
| Upper/middle bilobe | 2 | 1 | |
| Middle/lower bilobe | 2 | 2 | |
| Pneumonectomy | 1 | 0 | |
| Upper lobe sleeve resectionb | 2 | 0 |
Categorical variables were reported as number (%). a, one left upper lobectomy was converted to thoracotomy for chest wall resection; b, procedures were converted to thoracotomy for upper lobe sleeve resection. VATS, video-assisted thoracoscopic surgery.
Table 3. Operative characteristics.
| Characteristics | Robotic (n=61) | VATS (n=105) | P value |
|---|---|---|---|
| Operative mortalitya | 0 (0.00%) | 0 (0.00%) | 1.0000 |
| Conversion to thoracotomya | 4 (6.56%) | 2 (1.90%) | 0.1937 |
Categorical variables were reported as number (%). a, Fisher’s exact test. VATS, video-assisted thoracoscopic surgery.
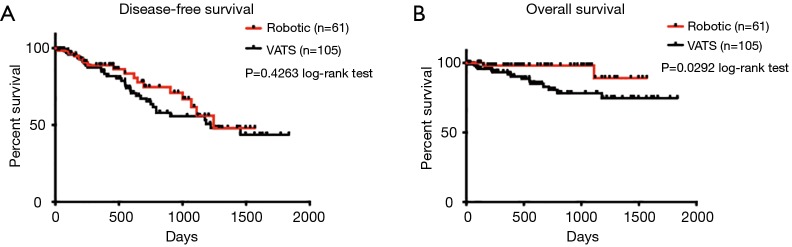
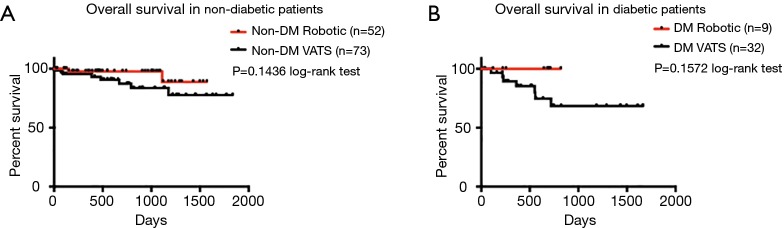
Postoperative complications and outcomes are summarized in Table 4. The two groups were similar, with the exception of a higher rate of prolonged air leak ≥7 d in the robotic group (14.75% for robotic vs. 3.81% for VATS group; P=0.0161). In addition, the robotic group had longer length of hospital stay (median of 4.0 days) than the VATS group (median of 3.0 days) (P=0.0123). Number of N1 lymph nodes sampled and examined by pathology was similar between the two groups (median =3.0 for robotic vs. median =3.0 for VATS, P=0.2684). No differences were found in other postoperative complications and outcomes, including the specific types of complication, proportion without complications and postoperative mortality. In patients with clinical N0 lung cancer who did not receive induction therapy prior to surgery, pathologic nodal upstaging was similar between the two groups (Table 5), at 17.78% for robotic and 17.46% for VATS (Robotic: 11.11% N1 and 6.67% N2 upstaging; VATS: 11.11% N1 and 6.35% N2 upstaging). The median follow-up time was 546 days in robotic group and 510 days in VATS group (P=0.8759). As shown in Figure 1A, in patients from December 2010 to June 2015, disease-free survival was similar in both groups (median survival: 1,245 days in robotic and 1,223 days in VATS, P=0.4263 log-rank test). The robotic group had slightly better Kaplan-Meier overall survival than the VATS group as shown in Figure 1B (survival proportion: 89.16% in robotic vs. 74.67% in VATS, P=0.0292 log-rank test. Median survival is not defined because both groups have more than 50% of the subjects alive at the end of study). However, the VATS group had significantly higher percentage of diabetic patients (robotic 14.75% vs. VATS 30.48%, P=0.0258). Diabetes often leads to microvascular diseases that impair wound healing and overall health, therefore, it may be a confounding factor that causes the difference in overall survival. Stratified analysis was performed. As shown in Figure 2A, in non-diabetic patients who underwent either surgery, the overall survival remained similar [survival proportion: 88.84% in robotic (n=52) vs. 77.67% in VATS (n=73), P=0.1436 log-rank test. Median survival is not defined because both groups have more than 50% of the subjects alive at the end of study]. The same observation was also made in diabetic patients [survival proportion: 100% in robotic (n=9) vs. 68.42% in VATS (n=32), P=0.1572 log-rank test. Median survival is not defined because both groups have more than 50% of the subjects alive at the end of study].
Table 4. Postoperative complications and outcomes.
| Complications/outcomes | Robotic (n=61) | VATS (n=105) | P value |
|---|---|---|---|
| Atrial fibrillation | 6 (9.84%) | 7 (6.67%) | 0.5522 |
| Prolonged air leak, ≥7 d | 9 (14.75%) | 4 (3.81%) | 0.0161 |
| Postoperative bleeding requiring reoperation | 1 (1.64%) | 1 (0.95%) | 1.0000 |
| Pneumonia | 3 (4.92%) | 3 (2.86%) | 0.6702 |
| Pulmonary edema | 1 (1.64%) | 0 (0.00%) | 0.3675 |
| Pulmonary embolism | 0 (0.00%) | 1 (0.95%) | 1.0000 |
| Atelectasis requiring bronchoscopy | 6 (9.84%) | 3 (2.86%) | 0.0762 |
| Chylothorax | 0 (0.00%) | 1 (0.95%) | 1.0000 |
| Cardiorespiratory failure | 1 (1.64%) | 3 (2.86%) | 1.0000 |
| Wound infection | 0 (0.0%) | 1 (0.95%) | 1.0000 |
| Renal insufficiency | 2 (3.28%) | 3 (2.86%) | 1.0000 |
| Clostridium difficile | 1 (1.64%) | 1 (0.95%) | 1.0000 |
| GI bleeding | 1 (1.64%) | 0 (0.0%) | 0.3675 |
| 30-day readmission | 4 (6.56%) | 5 (4.76%) | 0.7262 |
| 30-day reoperation | 1 (1.64%) | 3 (2.86%) | 1.0000 |
| Postoperative mortality | 1 (1.64%) | 3 (2.86%) | 1.0000 |
| Patient without complications | 42 (68.85%) | 87 (82.86%) | 0.0522 |
| Chest tube duration, d median (interquartile range) | 2.0 (2.0) | 2.0 (1.0) | 0.1308 |
| Length of hospital stay, d median (interquartile range) | 4.0 (4.0) | 3.0 (2.0) | 0.0123 |
| N1 lymph nodes resected median (interquartile range) | 3.0 (4.0) | 3.0 (3.0) | 0.2684 |
Categorical variables were reported as number (%), and analyzed using Fisher’s exact test. Continuous variables were reported as median (interquartile range) and analyzed using Mann-Whitney U test. VATS, video-assisted thoracoscopic surgery; GI, gastrointestinal bleeding.
Table 5. Distribution of pathological nodal staging in clinical N0 lung cancer patients.
| Pathologic nodal stage | cN0 in robotic (n=45) | cN0 in VATS (n=63) | P Value |
|---|---|---|---|
| pN0 | 37 (82.22%) | 52 (82.54%) | 1.0000 |
| pN1 | 5 (11.11%) | 7 (11.11%) | 1.0000 |
| pN2 | 3 (6.67%) | 4 (6.35%) | 1.0000 |
| Total nodal upstaging (pN1 + pN2) | 8 (17.78%) | 11 (17.46%) | 1.0000 |
Only patients with clinical N0 stage of primary lung cancer were included. Patients who received induction therapy prior to surgery were also excluded from this analysis. Categorical variables were reported as number (%) and analyzed using Fisher’s exact test. pN, pathological nodal staging; cN0, clinical N0; VATS, video-assisted thoracoscopic surgery.
Figure 1.
Survival of patients receiving surgery from December 2010 to June 2015 (robotic vs. VATS lobectomy). (A) Disease-free survival (P=0.4263); (B) overall survival (P=0.0292). Curves were analyzed using log-rank test. VATS, video-assisted thoracoscopic surgery.
Figure 2.
Overall survival in non-diabetic and diabetic patients (Robotic vs. VATS lobectomy). (A) Overall survival in non-diabetic patients (P=0.1436); (B) overall survival in diabetic patients (P=0.1572). Curves were analyzed using log-rank test. DM, diabetes mellitus; VATS, video-assisted thoracoscopic surgery.
Discussion
VATS has been accepted as a safe, minimally invasive alternative to thoracotomy (“open” procedure) for anatomic lung resection to avoid rib spreading and division of major thoracic muscles. In comparison to thoracotomy for early-stage NSCLC, VATS was associated with significantly shorter chest tube duration, shorter length of hospital stay, and better survival at 4 years (4). Additionally, VATS is shown to reduce postoperative pain, need for blood transfusion and postoperative complications, as well as to improve aesthetic and functional outcomes leading to better quality of life (15). However, VATS has drawbacks that impede its widespread adoption, including counter-intuitive hand movements, instrument fulcrum effect and tremor amplification. Therefore, VATS may have a steep learning curve that requires substantial training. Robotic surgery may overcome some of the limitations of VATS, and it provides more intuitive movements, greater wrist flexibility and high definition three-dimensional vision. Because of these potential advantages, many feel that a surgeon may be trained more easily to perform minimally invasive robotic lung resection. Robotic surgery is gaining popularity nationwide, although the absence of haptic feedback and high running costs of robotic systems still challenge its application. While robotic resection has been shown to have lower morbidity and mortality, significantly better mental quality of life and shorter hospital stay in comparison to thoracotomy (6,16), studies comparing robotic to VATS resection are limited. While some studies demonstrate that robotic surgery has similar postoperative outcomes and survival as VATS cases (7,9,17), a population-based analysis of a national database suggests that robotic lobectomy does not offer any substantial benefit over VATS lobectomy and may increase operative risk due to a higher rate of intraoperative injury and bleeding (10). However, all these studies fail to control for institution and surgeon expertise, which opens the possibility that the observed benefits and risks are simply due to the expertise of the surgeon and the institution rather than the approaches themselves. In fact, the latter national database study has shown that the robotic lobectomies were performed at smaller hospitals and less frequently at teaching institutions (10). Recognizing these limitations, we sought to compare robotic lung resection with VATS, assessing both short- and long-term outcomes, and control for surgeon and institution experience. Patients were assigned to either approach based on the availability of surgeon and equipment, which eliminates selection bias and the surgeon’s personal preference. This is reflected by the similarities in patient demographics and tumor characteristics between the two groups.
Our study found no intraoperative mortality in either group, and the conversion rate was similar in both groups. The robotic approach was associated with a higher rate of prolonged air leak (≥7 d), and slightly longer length of hospital stay than VATS resection. In general, each operation was performed in a “fissureless” manner: taking the fissure last with the stapler. No obvious reason for the prolonged air leaks in the robotic group is apparent, but three potential explanations make sense. First, lack of haptic feedbacks may lead to parenchymal injury during retraction. Second, enhanced vision may have led to more parenchymal division with the monopolar cautery during dissection. Lastly, a learning curve with the robot is possible but unlikely as the prolonged leaks were not clustered at the beginning of the experience.
No differences in other postoperative complications and mortality were identified. Number of N1 lymph nodes sampled during surgery was similar between two groups, which is consistent with previous studies (9,17) and indicates that the robotic approach achieves similar oncological radicality to that achieved by VATS. In patients with clinical N0 lung cancer who did not receive induction therapy prior to surgery, pathologic nodal upstaging was similar between the two groups. This serves as another indirect indicator of similar oncological radicality. These findings are expected given the similar upstaging rate found in surgeons who perform the majority of lobectomies minimally-invasively (18). Within the same follow-up period, disease-free survival was similar in both groups. The robotic group appeared to have slightly better overall survival, however, this observation was confounded by a significantly lower percentage of diabetic patients in this group. When this confounder is controlled, stratified analysis demonstrated that the overall survival remained similar in both groups.
This study possesses several limitations, including the small number of patients, the absence of data on costs, narcotic use, and possible confounding factor related to the surgeon’s learning curve with robotic surgery. The strengths of this study include the review of data from a prospective database, the non-biased patient selection for either surgical approach, data from an experienced surgeon who has no bias towards the use of either approach, and the concurrent use of both approaches during the study period. Taken together, our data suggest that after adjusting for surgeon expertise, robotic lung resection is feasible and safe, and has generally comparable outcomes to VATS resection. More single-surgeon studies are needed to confirm our findings. A large multi-institution randomized trial involving experienced thoracic surgeons should be considered before concluding any one approach to be superior.
Acknowledgements
This study was supported by The American Association for Thoracic Surgery’s Summer Intern Scholarship, and the Dean’s fellowship of Washington University School of Medicine in St. Louis.
Ethical Statement: The study was approved by the Institutional Review Board of Duke University (No. Pro00055346).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Onaitis MW, Petersen RP, Balderson SS, et al. Thoracoscopic lobectomy is a safe and versatile procedure: experience with 500 consecutive patients. Ann Surg 2006;244:420-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daniels LJ, Balderson SS, Onaitis MW, et al. Thoracoscopic lobectomy: a safe and effective strategy for patients with stage I lung cancer. Ann Thorac Surg 2002;74:860-4. 10.1016/S0003-4975(02)03764-5 [DOI] [PubMed] [Google Scholar]
- 3.Paul S, Altorki NK, Sheng S, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg 2010;139:366-78. 10.1016/j.jtcvs.2009.08.026 [DOI] [PubMed] [Google Scholar]
- 4.Whitson BA, Groth SS, Duval SJ, et al. Surgery for early-stage non-small cell lung cancer: a systematic review of the video-assisted thoracoscopic surgery versus thoracotomy approaches to lobectomy. Ann Thorac Surg 2008;86:2008-16; discussion 2016-8. [DOI] [PubMed]
- 5.Veronesi G. Robotic lobectomy and segmentectomy for lung cancer: results and operating technique. J Thorac Dis 2015;7:S122-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Veronesi G, Galetta D, Maisonneuve P, et al. Four-arm robotic lobectomy for the treatment of early-stage lung cancer. J Thorac Cardiovasc Surg 2010;140:19-25. 10.1016/j.jtcvs.2009.10.025 [DOI] [PubMed] [Google Scholar]
- 7.Park BJ, Melfi F, Mussi A, et al. Robotic lobectomy for non-small cell lung cancer (NSCLC): long-term oncologic results. J Thorac Cardiovasc Surg 2012;143:383-9. 10.1016/j.jtcvs.2011.10.055 [DOI] [PubMed] [Google Scholar]
- 8.Gharagozloo F, Margolis M, Tempesta B. Robot-assisted thoracoscopic lobectomy for early-stage lung cancer. Ann Thorac Surg 2008;85:1880-5; discussion 1885-6. [DOI] [PubMed]
- 9.Louie BE, Farivar AS, Aye RW, Vallières E. Early experience with robotic lung resection results in similar operative outcomes and morbidity when compared with matched video-assisted thoracoscopic surgery cases. Ann Thorac Surg 2012;93:1598-604; discussion 1604-5. 10.1016/j.athoracsur.2012.01.067 [DOI] [PubMed] [Google Scholar]
- 10.Paul S, Jalbert J, Isaacs AJ, et al. Comparative effectiveness of robotic-assisted vs. thoracoscopic lobectomy Chest 2014;146:1505-12. 10.1378/chest.13-3032 [DOI] [PubMed] [Google Scholar]
- 11.Pardolesi A, Park B, Petrella F, et al. Robotic anatomic segmentectomy of the lung: technical aspects and initial results. Ann Thorac Surg 2012;94:929-34. 10.1016/j.athoracsur.2012.04.086 [DOI] [PubMed] [Google Scholar]
- 12.Pardolesi A, Veronesi G. Robot-assisted lung anatomic segmentectomy: technical aspects. Thorac Surg Clin 2014;24:163-8, vi. 10.1016/j.thorsurg.2014.02.008 [DOI] [PubMed] [Google Scholar]
- 13.Toker A, Ayalp K, Uyumaz E, et al. Robotic lung segmentectomy for malignant and benign lesions. J Thorac Dis 2014;6:937-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schuchert MJ, Pettiford BL, Pennathur A, et al. Anatomic segmentectomy for stage I non-small-cell lung cancer: comparison of video-assisted thoracic surgery versus open approach. J Thorac Cardiovasc Surg 2009;138:1318-25.e1. 10.1016/j.jtcvs.2009.08.028 [DOI] [PubMed] [Google Scholar]
- 15.Cheng D, Downey RJ, Kernstine K, et al. Video-assisted thoracic surgery in lung cancer resection: a meta-analysis and systematic review of controlled trials. Innovations (Phila) 2007;2:261-92. [DOI] [PubMed] [Google Scholar]
- 16.Cerfolio RJ, Bryant AS, Skylizard L, et al. Initial consecutive experience of completely portal robotic pulmonary resection with 4 arms. J Thorac Cardiovasc Surg 2011;142:740-6. 10.1016/j.jtcvs.2011.07.022 [DOI] [PubMed] [Google Scholar]
- 17.Jang HJ, Lee HS, Park SY, et al. Comparison of the early robot-assisted lobectomy experience to video-assisted thoracic surgery lobectomy for lung cancer: a single-institution case series matching study. Innovations (Phila) 2011;6:305-10. [DOI] [PubMed] [Google Scholar]
- 18.Boffa DJ, Kosinski AS, Paul S, et al. Lymph Node Evaluation by Open or Video-Assisted Approaches in 11,500 Anatomic Lung Cancer Resections. Ann Thorac Surg 2012;94:347-53; discussion 353. 10.1016/j.athoracsur.2012.04.059 [DOI] [PubMed] [Google Scholar]