Abstract
Background
Preoperative identification of non-small cell lung cancer (NSCLC) patients at risk for disease recurrence has proven unreliable. The extraction of quantitative metrics from imaging based on tumor intensity and texture may enhanced disease characterization. This study evaluated tumor-specific 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography/computerized tomography (PET/CT) uptake patterns and their association with disease recurrence in early-stage NSCLC.
Methods
Sixty-four stage I/II NSCLC patients who underwent anatomic resection between 2001 and 2014 were examined. Pathologically or radiographic confirmed disease recurrence within 5 years of resection comprised the study group. Quantitative imaging metrics were extracted within the primary tumor volume. Squamous cell carcinoma (SCC) (N=27) and adenocarcinoma (AC) (N=41) patients were compared using a Wilcoxon signed-rank test. Associations between imaging and clinical variables with 5-year disease-free survival (DFS) and overall survival (OS) were evaluated by Cox proportional-hazards regression.
Results
Clinical and pathologic characteristics were similar between recurrence (N=34) and patients achieving 5-year DFS (N=30). Standardized uptake value (SUV)max and SUVmean varied significantly by histology, with SCC demonstrating higher uptake intensity and heterogeneity patterns. Entropy-grey-level co-occurrence matrix (GLCM) was a significant univariate predictor of DFS (HR =0.72, P=0.04) and OS (HR =0.65, P=0.007) independent of histology. Texture features showed higher predictive ability for DFS in SCC than AC. Pathologic node status and staging classification were the strongest clinical predictors of DFS, independent of histology.
Conclusions
Several imaging metrics correlate with increased risk for disease recurrence in early-stage NSCLC. The predictive ability of imaging was strongest when patients are stratified by histology. The incorporation of 18F-FDG PET/CT texture features with preoperative risk factors and tumor characteristics may improve identification of high-risk patients.
Keywords: Positron emission tomography (PET), quantitative imaging, early-stage non-small cell lung cancer (early-stage NSCLC), surgical resection, clinical outcome
Introduction
Non-small cell lung cancer (NSCLC) remains the number one cause of cancer-associated mortality in the world (1). Patients with early-stage disease (stages I and II) are at higher risk for recurrence compared to other solid organ malignancies of similar stage (2,3). Accurate preoperative staging has a significant impact on surgical outcome, and the incorporation of 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography/computerized tomography (PET/CT) imaging has been integral to define NSCLC subgroups best managed by primary resection. Despite these advancements, including NCCN guidelines that recommend routine use of 18F-FDG PET/CT imaging, 5-year survival for stage I and II NSCLC is only 80–85% and 50%, respectively (4).
Risk-stratification utilizing quantitative PET/CT has demonstrated prognostic significance, particularly in advanced stages of NSCLC, identifying elevated standardized uptake value (SUV)max as a marker for reduced 5-year survival and metabolic tumor volume (MTV) as a marker for decreased survival (5-7). However, commonly used SUVmax has not been identified as independent predictors of overall survival (OS) in early stage (I/II) patients (8,9). FDG uptake varies by histologic subtype, confounding the ability for imaging metrics to predict risk for recurrence. For example, differences in SUVmax for adenocarcinoma (AC) and squamous cell carcinoma (SCC) have been reported (8,10,11), with notably higher SUV uptake in SCC lesions reflecting a high proliferative index of this cell type (12).
Recently there have been efforts to assess the prognostic significance of FDG heterogeneity within a tumor (13). These higher-order quantitative PET features correlate with several prognostic factors, including survival, risk for recurrence, and resistance to chemotherapy in advanced disease (14,15). Several works have evaluated the prognostic ability of radiomics features in various stages and treatments of lung cancer, including early stage NSCLC (16,17). Complementary work has shown variation in heterogeneity features across NSCLC subtypes (18) and that clustering of radiomics-based features from FDG PET/CT can distinguish AC from SCC tumors (19).
The purpose of this study is to test the ability of quantitative metrics, including texture-based metrics describing heterogeneity, in 18F-FDG PET/CT imaging to predict recurrence following surgical resection of early-stage NSCLC. We further aim to assess the impact of histology-specific FDG PET/CT patterns on correlation to outcome.
Methods
Patient population
This retrospective case-controlled study examined NSCLC resections performed at a single university medical center between 2001 and 2014. The use of STS data for eligible patients was approved by the institutional Internal Review Board. All patients with a new primary diagnosis of NSCLC and preoperative 18F-FDG PET/CT PET imaging that underwent anatomic lung resection for Stage I and II disease (7th edition AJCC/UCII staging system) were evaluated. To ensure quantitative accuracy of this analysis, additional imaging exclusions were applied. A complete list of study inclusion and exclusion criteria are listed (Table 1).
Table 1. Patient selection criteria.
| Inclusion criteria | Exclusion criteria |
|---|---|
| Clinicopathologic considerations | Clinicopathologic considerations |
| 1. Pathologic stage I or II NSCLC | 1. Wedge resection |
| 2. Surgical procedures performed with intent to cure | 2. Stage IIIA disease or greater |
| 3. Performance of lobectomy or pneumonectomy | 3. Treatment with neoadjuvant chemotherapy or chemoradiation therapy |
| 4. Performance of mediastinal lymph node sampling or dissection | 4. The presence of synchronous lung masses |
| 5. Follow-up clinic and imaging data for 5 years postoperatively | 5. Lost to follow-up without retrievable records or from which recurrence data could not be determined |
| 6. Adenocarcinoma in situ or more than one tumor in the specimen | |
| 7. Recurrence within 3 months of resection | |
| Imaging considerations | Imaging considerations |
| 1. PET/CT scans performed at the study institution | 1. Tumors less than 2 cm |
| 2. Surgical procedures performed within 90 days of imaging study | 2. Greater than 90 days between imaging and surgical procedure |
| 3. Imaging performed on one of three dedicated clinical scanners | 3. Use of outside or non-dedicated clinical scanner |
NSCLC, non-small cell lung cancer; PET/CT, positron emission tomography/computerized tomography.
Preoperative clinic and inpatient medical records were reviewed to obtain baseline characteristics. Date of death was confirmed from the social security death index, including patients who were lost to clinical follow-up. As standard practice, endobronchial ultrasound-guided fine needle aspiration (EBUS-FNA) and mediastinoscopy are selectively used in this patient population, as clinically indicated. The type of resection performed was based on patient and tumor characteristics, as well as surgeon judgment. Patients are followed with physical exam and non-contrast CT scan every 6 months for the first 2 years, then yearly for life.
Disease recurrence in ipsilateral hemithorax, mediastinum, or a distant site was evaluated within 5 years of resection and determined by either biopsy proven NSCLC or radiographic evidence of disease without a contralateral lung lesion if biopsy was not feasible. In cases where only radiographic data was available, the designation of recurrence, rather than a new primary lung cancer, was based on consensus evaluation at a multi-disciplinary lung tumor board. Controls were defined as patients without disease recurrence and 5-year follow-up who otherwise met inclusion criteria.
Image acquisition and analysis
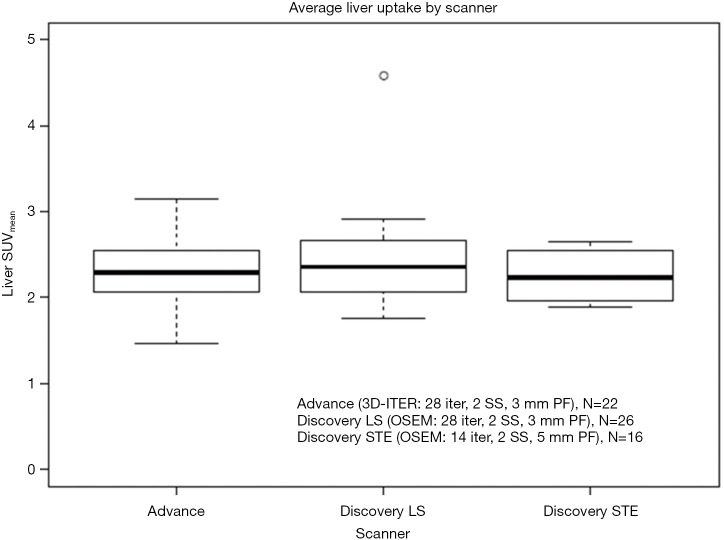
PET imaging was acquired on one of GE Advance (N=26), GE Discovery LS (N=28), and GE Discovery STE (N=20) scanners (Waukesha, WI, USA). Patients were injected with 10 mCi (range, 9–20 mCi) of 18F-FDG and scanned 60 min (±9.2 min) post-injection. All scans were performed to allow acquisition of seven to eight bed positions to cover skull to mid-thigh. After reconstruction, images were normalized to the body mass SUV. Following PERCIST recommendations, normal reference tissue values in a 3-cm-diameter region of interest in the liver to evaluate image quality across the three scanners (20). Acquisition and reconstruction parameters were as follows: Advance (2 min/bp; 3D-ITER reconstruction with 28 iterations, 2 subsets, 3 mm post-filter for final grid size 128×128×205 of 4.3×4.3×4.25 mm3), Discovery LS (3 min/bp; OSEM reconstruction with 28 iterations, 2 subsets, 3 mm post-filter for final grid size 128×128×205 of 3.9×3.9×4.25 mm3), Discovery STE (3 min/bp; OSEM reconstruction with 14 iterations, 2 subsets, 5 mm post-filter for final grid size 128×128×311 of 5.5×5.5×3.27 mm3). Comparison of liver uptake can be found in Figure S1, no significant differences in liver uptake was found across the three scanners.
Figure S1.
Average liver uptake for each patient, calculated from a 3 cm diameter sphere. Patients were separated by which scanner their FDG PET/CT was acquired on, no significant differences in uptake patterns were noted.
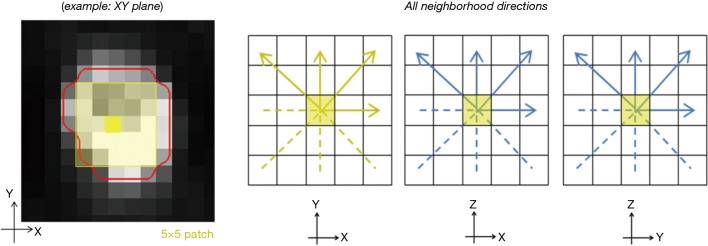
Tumors were automatically segmented on reconstructed PET scans using an institutional algorithm (21). After segmentation, tumor regions of interest (ROIs) were reviewed and manually adjusted if necessary, by a staff nuclear medicine physician. MTV was reported for each patient. All quantitative analyses and feature extraction were performed in MATLAB (MathWorks 2016) (Table 2). SUV metrics were calculated over the entire tumor volume, including metrics describing tracer uptake avidity (20). Additionally, three texture-based metrics representing local heterogeneity derived from the grey-level co-occurrence matrix (GLCM) were extracted (22,23). These metrics have shown prognostic potential in prior works (17) and variability due to image acquisition has been characterized. The methodology for 3D voxel-based feature extraction follows the works of Galavis et al. (24,25) and other published works (26), as shown in Figure 1. SUV metrics are quantized into a number of gray tones for analysis, in this case 256 grey levels, by resampling to the maximum uptake within the ROI (i.e., relative discretization) (25). Next, local neighbors surrounding each voxel contained within the lesion ROI, called a patch, are identified. These patches are defined for XY, XZ, and YZ planes of 5×5 neighbors (i.e., 2 voxel distance) surrounding each voxel within the ROI (26). Texture values are then calculated in each of the planes, averaged for each voxel, and finally averaged across the entire ROI.
Table 2. Quantitative metrics extracted from lesion ROIs on PET images. Radiomics-based metrics described are second-order metrics from gray-level co-occurrence matrix (GLCM) (13).
| Metric | Description |
|---|---|
| MTV | Volume of ROI |
| SUVmax | Maximum uptake within tumor ROI |
| SUVpeak | 1 cm sphere surrounding SUVmax |
| SUVmean | Mean of all uptake values within tumor ROI |
| TLG | Product of SUVmean and ROI volume |
| SUVstdev | Standard deviation of uptake values within tumor ROI |
| Homogeneity | Measure of closeness of elements along the diagonal of GLCM, inversely correlated with Entropy-GLCM |
| Entropy-GLCM | Measure of randomness in GLCM, thought to provide measure of heterogeneity |
| Dissimilarity | Measure of variation in pairs of GLCM |
ROI, region of interest; PET, positron emission tomography; MTV, metabolic tumor volume; SUV, standardized uptake value; TLG, total lesion glycolysis.
Figure 1.
In voxel-based extraction of texture features, neighborhoods are built around a single voxel in regional patches (example 5×5 patches on left). This is computed for XY, XZ, and YZ planes surrounding each voxel. Four directions are considered for each plane.
Statistical analysis
Wilcoxon’s rank sum test was performed to investigate differences in SUV metrics across various pathologic characteristics. Spearman’s correlation was performed to investigate the correlation of imaging metrics with pathological tumor size and MTV. The primary endpoint was 5-year disease-free survival (DFS), measured from the date of surgical resection to the date of first evidence of tumor recurrence, including locoregional recurrence, distant metastasis, or death. Univariate and multivariate Cox proportional hazard regression analyses were conducted to evaluate associations between clinical and imaging metrics with clinical outcomes (DFS and OS). A multivariate Cox proportional hazard model was created utilizing forward selection, considering univariate predictors of level P<0.2, and backward selection using Bayesian information criteria. The Kaplan-Meier method was used to estimate DFS and OS survival, and compared between groups using the log-rank test. Statistical analysis was performed with R version 3.1.1 (R, The R Foundation). Significance was determined at the 0.05 level and all tests were two-sided.
Results
Sixty-four patients met clinical and imaging criteria for inclusion in this study. The median time between preoperative imaging and surgical resection was 23 days (range, 2–84 days). Thirty-four patients demonstrated recurrent disease within 5 years of resection, with a median DFS interval of 14.9 months (range, 3.6–57.6 months). Patient characteristics and associations with clinical outcomes are listed in Table 3. No differences in DFS across clinical stage, pathological tumor stage, or histology were noted. Patients with pathologic node-positive, pN1, disease demonstrated shortened DFS compared to pN(−) (HR =3.24, 95% CI, 1.49–7.02, P=0.003). Patient-specific clinical factors such as history of hypertension and decreased diffusing capacity of the lungs for carbon monoxide (DLCO) were also significantly associated with non-favorable DFS interval. At the time of data collection, 26 of 64 patients had expired. Pathological node status remained the most significant clinical factor, where pN1 disease was significantly associated with shorter survival interval (HR =4.16, 95% CI, 1.70–11, P=0.002).
Table 3. Clinical patient characteristics and associations with disease-free and overall survival from Cox proportional hazards regression.
| Patient characteristics | Category | Number | DFS (Nevents=34) | OS (Nevents=26) | |||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||||
| Age (years) | Mean [range] | 69.2 [48–88] | 1.06 (0.76–1.47) | 0.74 | 1.06 (0.72–1.55) | 0.78 | |
| Sex | M | 32 | Ref | – | |||
| F | 32 | 0.91 (0.91–1.78) | 0.77 | 1.13 (0.52–2.45) | 0.75 | ||
| Smoking history | Never | 3 | Ref | – | |||
| Former | 59 | 0.85 (0.2–3.58) | 0.83 | ||||
| Current | 2 | 4.8 (0.64–32) | 0.13 | ||||
| Pack years | Mean [range] | 38.8 [2–100] | 1.07 (0.77–1.49) | 0.68 | 1.28 (0.87–1.89) | 0.21 | |
| Clinical stage | IA | 34 | Ref | – | |||
| IB | 14 | 0.54 (0.2–1.46) | 0.23 | 0.22 (0.05–0.96) | 0.04 | ||
| IIA | 10 | 1.22 (0.49–3.06) | 0.67 | 1.01 (0.37–2.75) | 0.98 | ||
| IIB | 6 | 1.25 (0.43–3.68) | 0.68 | 0.55 (0.13–2.45) | 0.44 | ||
| Pathological size | Mean (median) | 3.88 (3.05) | 1.06 (0.76–1.47) | 0.74 | 0.90 (0.61–1.35) | 0.62 | |
| Pathological node status | pN0 | 54 | Ref | – | |||
| pN1 | 10 | 3.24 (1.49–7.02) | 0.003 | 4.16 (1.70–11) | 0.002 | ||
| Pre-Op mediastinal staging | No | 34 | Ref | – | |||
| Yes | 30 | 2.06 (1.04–4.06) | 0.04 | 2.04 (0.93–4.47) | 0.08 | ||
| Histology | Adenocarcinoma | 33 | Ref | – | |||
| Squamous cell carcinoma | 26 | 1.04 (0.51–2.11) | 0.92 | 0.80 (0.36–1.79) | 0.58 | ||
| Mixed | 5 | 1.33 (0.39–4.54) | 0.65 | 0.38 (0.05–2.92) | 0.36 | ||
| Tumor grade | Well | 8 | Ref | – | |||
| Mod | 45 | 3.12 (0.74–13) | 0.12 | 0.93 (0.32–2.72) | 0.89 | ||
| Poor | 11 | 3.55 (0.74–17) | 0.11 | 0.93 (0.23–3.73) | 0.92 | ||
| Adjuvant chemotherapy | No | 52 | Ref | – | |||
| Yes | 12 | 2.49 (1.18–5.23) | 0.02 | 3.04 (1.33–6.96) | 0.01 | ||
| History of hypertension | No | 27 | Ref | – | |||
| Yes | 37 | 2.81 (1.31–6.03) | 0.008 | 2.74 (1.13–6.67) | 0.03 | ||
| History of diabetes | No | 52 | Ref | – | |||
| Yes | 12 | 0.89 (0.37–2.16) | 0.8 | 0.87 (0.32–2.33) | 0.78 | ||
| DLCO | Mean (median) | 76.6 (75.0) | 0.63 (0.43–0.92) | 0.02 | 0.55 (0.35–0.87) | 0.01 | |
| Hemoglobin | Mean (median) | 12.8 (12.9) | 0.74 (0.52–1.04) | 0.08 | 0.80 (0.54–1.18) | 0.26 | |
| Creatinine | Mean (median) | 0.9 (1.0) | 0.70 (0.47–1.04) | 0.08 | 0.87 (0.59–1.27) | 0.46 | |
| Recurrence location | Local | 10 | – | – | |||
| Contralateral | 14 | ||||||
| Distant | 10 | ||||||
| Extent of surgery | Lobectomy | 63 | – | – | |||
| Pneumonectomy | 1 | ||||||
DLCO, diffusing capacity of the lungs for carbon monoxide.
Quantitative imaging characteristics
Tumors of larger pathological dimension exhibited higher TLG (P=0.86, P<0.001), while Homogeneity and Dissimilarity were inversely associated with tumor size. Using Spearman’s correlation, first-order features, such as SUVmax, exhibited stronger correlations to tumor size (P>0.5) than texture features (Table S1). No significant difference in tumor volume was noted between histologies (P=0.26, Table 4). However, all standard SUV metrics and two radiomics-based metrics were significantly different between AC and SCC patients. SCC tumors had higher metrics compared to AC tumors, with the exception of Entropy-GLCM, which was higher in AC (P=0.01).
Table S1. Imaging correlation with tumor size from pathology (pathological size) and imaging (MTV).
| Metric | Path. size | MTV | |||
|---|---|---|---|---|---|
| ρ | P value | ρ | P value | ||
| MTV | 0.85 | <0.001 | – | – | |
| SUVmax | 0.61 | <0.001 | 0.59 | <0.0001 | |
| SUVpeak | 0.63 | <0.001 | 0.62 | <0.0001 | |
| SUVmean | 0.55 | <0.001 | 0.53 | <0.0001 | |
| TLG | 0.85 | <0.001 | 0.94 | <0.0001 | |
| Homogeneity | −0.29 | 0.01 | −0.38 | 0.002 | |
| Entropy-GLCM | 0.32 | 0.01 | 0.4 | 0.001 | |
| Dissimilarity | −0.36 | 0.004 | −0.38 | 0.002 | |
SUV, standardized uptake value; TLG, total lesion glycolysis; GLCM, grey-level co-occurrence matrix.
Table 4. Histology-specific associations with imaging metrics.
| Metric | AC (N=33), median (SD) | SCC (N=26), median (SD) | P value |
|---|---|---|---|
| MTV | 12.8 (25.7) | 15.4 (55.1) | 0.26 |
| SUVmax | 8.79 (5.59) | 17.0 (7.05) | 0.002 |
| SUVpeak | 5.08 (4.66) | 12.69 (5.40) | 0.001 |
| SUVmean | 3.73 (2.48) | 7.05 (2.92) | 0.0007 |
| TLG | 41.9 (167.6) | 67.6 (481) | 0.05 |
| Homogeneity | 0.015 (0.0003) | 0.01 (0.0005) | 0.03 |
| Entropy-GLCM | 4.24 (0.01) | 4.23 (0.02) | 0.01 |
| Dissimilarity | 39.1 (3.3) | 39.5 (3.28) | 0.7 |
MTV, metabolic tumor volume; SUV, standardized uptake value; TLG, total lesion glycolysis; GLCM, grey-level co-occurrence matrix; AC, adenocarcinoma; SCC, squamous cell carcinoma.
Entropy-GLCM was the only imaging metric to demonstrate a significant relationship with DFS (Table 5). In a multivariate model combining clinical and imaging features (Table S2), Entropy-GLCM remained significant (HR =0.65, 95% CI, 0.49–0.87, P=0.004), combining with hemoglobin levels (HR =0.76, 95% CI, 0.61–0.94, P=0.01), DLCO (HR =0.95, 95% CI, 0.93–0.98, P=0.005), history of hypertension (HR =2.83, 95% CI, 1.29–6.22, P=0.01), and tumor grade (HR =8.9, 95% CI, 1.9–41, P=0.005).
Table 5. Imaging associations with disease-free and overall survival from Cox proportional hazards regression.
| Metric | DFS (Nevents=34) | OS (Nevents=26) | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| MTV | 1.06 (0.79–1.41) | 0.71 | 0.91 (0.61–1.35) | 0.63 | |
| SUVmax | 1.31 (0.89–1.93) | 0.18 | 0.84 (0.52–1.36) | 0.48 | |
| SUVpeak | 1.32 (0.89–1.93) | 0.16 | 0.84 (0.53–1.35) | 0.47 | |
| SUVmean | 1.38 (0.94–2.03) | 0.10 | 0.81 (0.49–1.34) | 0.41 | |
| TLG | 1.10 (0.83–1.46) | 0.50 | 0.89 (0.59–1.32) | 0.55 | |
| Homogeneity | 1.35 (0.97–1.86) | 0.07 | 1.45 (1.05–2.01) | 0.03 | |
| Entropy-GLCM | 0.72 (0.52–0.98) | 0.04 | 0.65 (0.48–0.89) | 0.007 | |
| Dissimilarity | 1.19 (0.87–1.64) | 0.28 | 1.38 (0.95–2.01) | 0.09 | |
MTV, metabolic tumor volume; SUV, standardized uptake value; TLG, total lesion glycolysis; GLCM, grey-level co-occurrence matrix; DFS, disease-free survival; OS, overall survival.
Table S2. Final multivariate model after backwards selection using BIC criteria. Variables considered: clinical stage, smoking status, tumor grade, mediastinal staging, pathologic node status, hemoglobin levels, DLCO, history of hypertension, use of adjuvant chemotherapy, SUVmax, SUVpeak, SUVmean, Homogeneity, Entropy-GLCM.
| Metric | DFS (Nevents=34) | OS (Nevents=26) | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Hemoglobin | 0.76 (0.61–0.94) | 0.01 | |||
| DLCO | 0.95 (0.93–0.98) | 0.005 | 0.96 (0.94–0.99) | 0.005 | |
| Hypertension | 2.83 (1.29–6.22) | 0.01 | 2.97 (1.17–7.52) | 0.02 | |
| Tumor grade (mod/poor) | 8.9 (1.9–41) | 0.005 | |||
| Entropy-GLCM | 0.65 (0.49–0.87) | 0.004 | 0.60 (0.45–0.81) | 0.0007 | |
| Adjuvant chemotherapy | 1.19 (0.87–1.64) | 0.28 | 4.79 (1.9–12) | 0.09 | |
DLCO, diffusing capacity of the lungs for carbon monoxide; GLCM, grey-level co-occurrence matrix.
Similar to DFS, no standard SUV metrics were associated with OS, though Entropy-GLCM and Homogeneity showed significant associations (Table 5). In a multivariate model (Table S2), Entropy-GLCM remained an independent predictor of OS (HR =0.60, 95% CI, 0.45–0.81, P=0.0007) along with DLCO (HR =0.96, 95% CI, 0.94–0.99, P=0.005), history of hypertension (HR =2.97, 95% CI, 1.17–7.52, P=0.02), and use of adjuvant chemotherapy (HR =4.79, 95% CI, 1.9–12, P=0.09).
Impact of histology-specific imaging characteristics on outcome
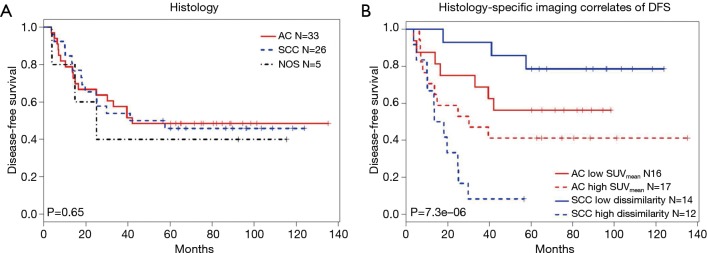
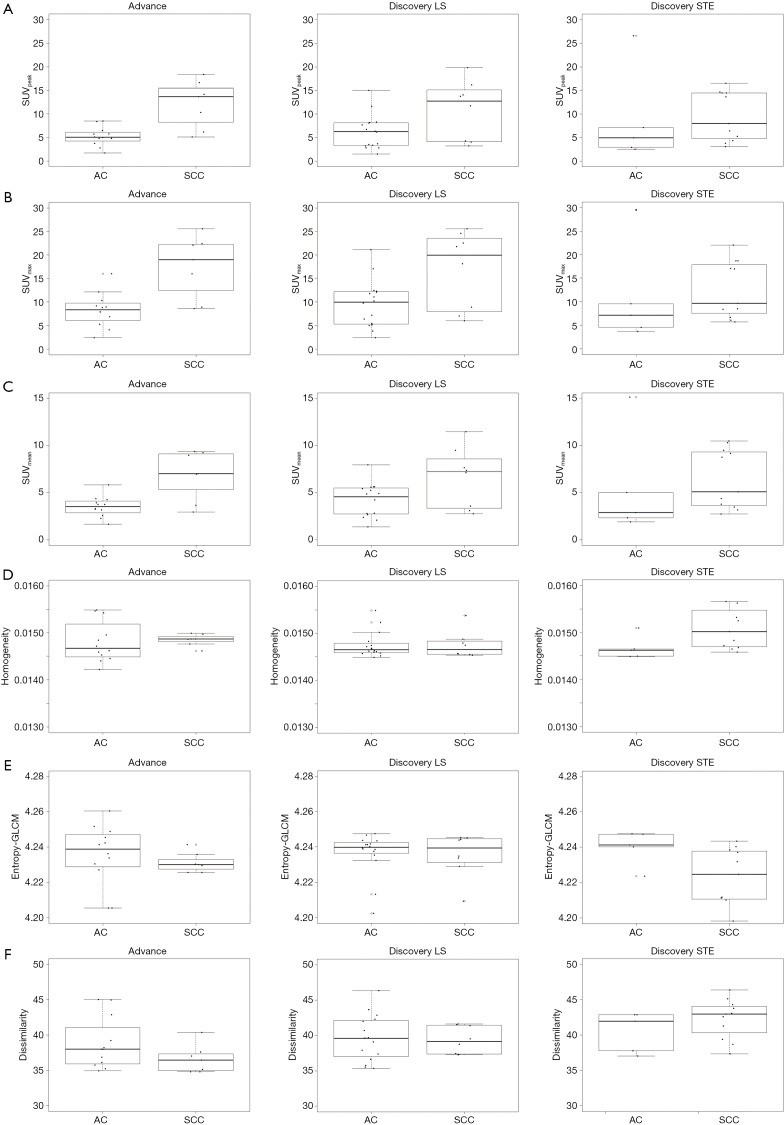
AC and SCC patients were evaluated separately to identify imaging characteristics associated with DFS for each subgroup (Table 6). In SCC patients, radiomics-based metrics (Homogeneity, Entropy-GLCM, and Dissimilarity) were significant predictors of DFS. No radiomics-based imaging characteristics were independent predictors for AC patients. However, SUVmean and TLG showed significant association with DFS. Patients were classified based on histology and histology-specific imaging correlates (Dissimilarity for SCC, SUVmean for AC) using median values reported in Table 6 for dichotomizing patients. Significant differences in DFS were noted for each group (log-rank P<0.001) when imaging correlates were included in the model (Figure 2). The low number of observed survival events did not allow for this analysis in relation to OS in this population. Quantitative imaging characteristics summarized by histology and scanner type are presented in Table S3 and Figure S2.
Table 6. Histology-specific univariate associations with disease-free survival for AC (N=33) and SCC (N=26) patients.
| Metric | AC (N=33, Nevents=17) | SCC (N=26, Nevents=14) | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| MTV | 1.31 (0.90–1.90) | 0.16 | 0.95 (0.56–1.60) | 0.84 | |
| SUVmax | 1.45 (0.92–2.28) | 0.11 | 0.74 (0.43–1.26) | 0.26 | |
| SUVpeak | 1.48 (0.94–2.33) | 0.09 | 0.73 (0.42–1.27) | 0.27 | |
| SUVmean | 1.58 (1.02–2.44) | 0.04 | 0.74 (0.43–1.27) | 0.28 | |
| TLG | 1.50 (1.01–2.22) | 0.04 | 0.89 (0.51–1.55) | 0.69 | |
| Homogeneity | 1.08 (0.67–1.76) | 0.75 | 1.83 (1.12–2.98) | 0.02 | |
| Entropy-GLCM | 0.80 (0.5–1.29) | 0.36 | 0.56 (0.34–0.92) | 0.02 | |
| Dissimilarity | 0.88 (0.55–1.40) | 0.58 | 2.16 (1.30–3.59) | 0.003 | |
MTV, metabolic tumor volume; SUV, standardized uptake value; TLG, total lesion glycolysis; GLCM, grey-level co-occurrence matrix; AC, adenocarcinoma; SCC, squamous cell carcinoma.
Figure 2.
Kaplan-Meier survival analysis. (A) Histology was not shown to be a prognostic factor for 5-year disease-free survival. (B) Patients were classified into four separate groups based on histology and histology-specific correlates (Dissimilarity for SCC and SUVmean for AC). Here, histology-specific median values reported in Table 4 were used for dichotomizing patients into low (−) or high (+) groups. SCC, squamous cell carcinoma; SUV, standardized uptake value; AC, adenocarcinoma.
Table S3. Histologic summary by scanner type, with DFS and OS events reported as (# DFS events/# OS events).
| Scanner | AC | SCC | Other |
|---|---|---|---|
| Advance | 12 (5/5) | 7 (2/1) | 3 (1/0) |
| Dis LS | 16 (9/8) | 8 (4/3) | 2 (2/1) |
| Dis STE | 5 (3/2) | 11 (8/6) | 0 |
AC, adenocarcinoma; SCC, squamous cell carcinoma; DFS, disease-free survival; OS, overall survival.
Figure S2.
Imaging metrics vs. histology for each scanner model: (A) SUVpeak, (B) SUVmax, (C) SUVmean, (D) Homogeneity, (E) Entropy-GLCM, (F) Dissimilarity.
Discussion
The standard of care for the treatment of early-stage NSCLC (stage I/II) is lobectomy and mediastinal lymph node dissection. Even after an R0 resection with pathologically negative lymph nodes, risk for recurrence is approximately 2% to 5% per year, with 5-year survival rates as low as 50% in patients with stage II disease (27). Both the 2009 International Association for the Study of Lung Cancer (IASLC) staging system and NCCN guidelines highlight 18F-FDG PET/CT scanning as a diagnostic aid (28,29). However, current FDG PET/CT use is largely limited to qualitative review. Tumors, even of the same histology, often demonstrate variable and distinct imaging characteristics (30). This study aimed to determine if quantitative metrics, including texture-based metrics describing heterogeneity, in 18F-FDG PET/CT imaging might enhance the clinical prediction of disease recurrence following surgical resection.
The extraction of quantitative features from PET data (Radiomics) allows for a more comprehensive analysis of imaging characteristics that may be predictive of the biologic potential of a lung cancer (31,32). This assessment may be clinically relevant to define independent prognostic variables associated with survival in early-stage disease. Variable associations between texture features, including Entropy-GLCM and Dissimilarity, and treatment-related outcomes have been previously reported in NSCLC patients treated with SBRT (33,34). In our analysis, Entropy-GLCM was significantly associated with clinical endpoints in all patients, and was the only imaging metric to remain significant in a multivariate model of DFS and OS. Our results also demonstrate a significant association between imaging metrics and histology-specific DFS and OS.
In this study we demonstrate that histology specific differences in FDG heterogeneity may predict risk for recurrent disease. While differences in PET avidity between AC and SCC have been previously reported in both standard (8,10,11,35) and radiomics-based metrics (18,19). Their association with risk for recurrence was previously unknown. In this study, despite no significant differences in clinical endpoints, each histology showed unique imaging variables associated with DFS (Figure 2). Texture features, including Dissimilarity and Entropy-GLCM, were significant correlates of DFS in SCC patients. More commonly used SUV metrics describing tracer avidity, such as SUVmean, demonstrated a moderately significant association with DFS. There is wide molecular variability observed within the AC histology (e.g., EGFR or ALK mutations), which may impact our quantitative analysis (36,37).
Established clinical metrics were significantly associated with DFS in this study. Pathological nodal status was the most predictive univariate variable for OS and 5-year DFS. However, pathological node status did not remain significantly independent in multivariate analysis. Other clinical metrics associated with survival in univariate and multivariate analyses included the use of adjuvant chemotherapy, pre-surgical hemoglobin, hypertension, and DLCO; all established clinical risk factors that impact survival (29). Within this study, no stage-specific differences of 5-year DFS were observed, likely due to the small number of patients. Commonly used FDG metric SUVmax was not strongly associated with OS, 5-year DFS, or risk for recurrence. These study conclusions are consistent with other modern series in which preoperative SUVmax is a poor independent prognostic factor due to its strong association with tumor size and stage (35,38).
There are several limitations that impact the translation of our study and require further validation. Foremost is the small sample size, which prevented extending our multivariate analysis to each histologic subtype and requires further exploration in a larger patient cohort matched for clinical characteristics and image acquisition across histologies. FDG texture features have been shown to be highly sensitive to reconstruction variation and MTV measurements (39,40). We have attempted to address these concerns by including robust and repeatable heterogeneity features characterized in prior work (24). Additionally, only tumors with metabolic volumes >2 cm3 were evaluated in this study to avoid sensitivity to very small tumor volumes, including lack of reproducible segmentations and texture extraction. Partial volume effects and respiratory motion were not addressed within this study. Future prospective studies that include harmonization of PET/CT scans from multiple institutions will allow for a broader application of these study conclusions.
Higher-order extraction of FDG PET/CT features show promise in evaluating associations between imaging characteristics and molecular drivers of disease. Linking these imaging features with tumor histology, stage, and potentially serum-based biomarkers may provide a platform for improved classification of early-stage NSCLC (41). This multifactorial diagnostic algorithm may be particularly helpful in identifying patient subsets at high risk for recurrence. This work motivates future studies to validate the role of quantitative FDG PET measures for predicting surgical outcomes in early-stage disease, where particular evaluation of histology-specific imaging signatures may be helpful to define high-risk patients.
Acknowledgements
Funding: Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Number T32CA009206. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Ethical Statement: The Institutional Review Board at the University of Wisconsin approved this study including a waiver for patient consent due to the retrospective and de-identified nature of the data (IRB #2015-0266).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Malvezzi M, Bertuccio P, Levi F, et al. European cancer mortality predictions for the year 2014. Ann Oncol 2014;25:1650-6. 10.1093/annonc/mdu138 [DOI] [PubMed] [Google Scholar]
- 2.Hung JJ, Yeh YC, Jeng WJ, et al. Predictive Value of the International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society Classification of Lung Adenocarcinoma in Tumor Recurrence and Patient Survival. J Clin Oncol 2014;32:2357-64. 10.1200/JCO.2013.50.1049 [DOI] [PubMed] [Google Scholar]
- 3.El-Sherif A, Gooding WE, Santos R, et al. Outcomes of sublobar resection versus lobectomy for stage I non-small cell lung cancer: a 13-year analysis. Ann Thorac Surg 2006;82:408-15; discussion 415-6. 10.1016/j.athoracsur.2006.02.029 [DOI] [PubMed] [Google Scholar]
- 4.Reich J, Asaph J. Natural history of stage I lung cancer. Chest 2007;132:2062; author reply 2062-3. 10.1378/chest.07-2032 [DOI] [PubMed] [Google Scholar]
- 5.Berghmans T, Dusart M, Paesmans M, et al. Primary tumor standardized uptake value (SUVmax) measured on fluorodeoxyglucose positron emission tomography (FDG-PET) is of prognostic value for survival in non-small cell lung cancer (NSCLC): a systematic review and meta-analysis (MA) by the European Lung Cancer Working Party for the IASLC Lung Cancer Staging Project. J Thorac Oncol 2008;3:6-12. 10.1097/JTO.0b013e31815e6d6b [DOI] [PubMed] [Google Scholar]
- 6.Paesmans M, Berghmans T, Dusart M, et al. Primary tumor standardized uptake value measured on fluorodeoxyglucose positron emission tomography is of prognostic value for survival in non-small cell lung cancer: update of a systematic review and meta-analysis by the European Lung Cancer Working Party for the International Association for the Study of Lung Cancer Staging Project. J Thorac Oncol 2010;5:612-9. 10.1097/JTO.0b013e3181d0a4f5 [DOI] [PubMed] [Google Scholar]
- 7.Bazan JG, Duan F, Snyder BS, et al. Metabolic tumor volume predicts overall survival and local control in patients with stage III non-small cell lung cancer treated in ACRIN 6668/RTOG 0235. Eur J Nucl Med Mol Imaging 2017;44:17-24. 10.1007/s00259-016-3520-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agarwal M, Brahmanday G, Bajaj SK, et al. Revisiting the prognostic value of preoperative (18)F-fluoro-2-deoxyglucose ((18)F-FDG) positron emission tomography (PET) in early-stage (I & II) non-small cell lung cancers (NSCLC). Eur J Nucl Med Mol Imaging 2010;37:691-8. 10.1007/s00259-009-1291-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hyun SH, Choi JY, Kim K, et al. Volume-based parameters of (18)F-fluorodeoxyglucose positron emission tomography/computed tomography improve outcome prediction in early-stage non-small cell lung cancer after surgical resection. Ann Surg 2013;257:364-70. 10.1097/SLA.0b013e318262a6ec [DOI] [PubMed] [Google Scholar]
- 10.Jeong HJ, Min JJ, Park JM, et al. Determination of the prognostic value of [(18)F]fluorodeoxyglucose uptake by using positron emission tomography in patients with non-small cell lung cancer. Nucl Med Commun 2002;23:865-70. 10.1097/00006231-200209000-00010 [DOI] [PubMed] [Google Scholar]
- 11.Downey RJ, Akhurst T, Gonen M, et al. Preoperative F-18 fluorodeoxyglucose-positron emission tomography maximal standardized uptake value predicts survival after lung cancer resection. J Clin Oncol 2004;22:3255-60. 10.1200/JCO.2004.11.109 [DOI] [PubMed] [Google Scholar]
- 12.Mangili F, Cigala C, Arrigoni G, et al. Cell loss and proliferation in non-small cell lung carcinoma: correlation with histological subtype. Eur J Histochem 1998;42:287-95. [PubMed] [Google Scholar]
- 13.El Naqa I, Grigsby P, Apte A, et al. Exploring feature-based approaches in PET images for predicting cancer treatment outcomes. Pattern Recognit 2009;42:1162-71. 10.1016/j.patcog.2008.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hatt M, Visvikis D, Albarghach NM, et al. Prognostic value of 18F-FDG PET image-based parameters in oesophageal cancer and impact of tumour delineation methodology. Eur J Nucl Med Mol Imaging 2011;38:1191-202. 10.1007/s00259-011-1755-7 [DOI] [PubMed] [Google Scholar]
- 15.Miles KA, Ganeshan B, Griffiths MR, et al. Colorectal cancer: texture analysis of portal phase hepatic CT images as a potential marker of survival. Radiology 2009;250:444-52. 10.1148/radiol.2502071879 [DOI] [PubMed] [Google Scholar]
- 16.Nakajo M, Jinguji M, Shinaji T, et al. A Pilot Study of Texture Analysis of Primary Tumor [18F]FDG Uptake to Predict Recurrence in Surgically Treated Patients with Non-small Cell Lung Cancer. Mol Imaging Biol 2018. [Epub ahead of print]. [DOI] [PubMed]
- 17.Han S, Woo S, Suh CH, et al. A systematic review of the prognostic value of texture analysis in. Ann Nucl Med 2018;32:602-10. 10.1007/s12149-018-1281-9 [DOI] [PubMed] [Google Scholar]
- 18.Orlhac F, Soussan M, Chouahnia K, et al. 18F-FDG PET-Derived Textural Indices Reflect Tissue-Specific Uptake Pattern in Non-Small Cell Lung Cancer. PLoS One 2015;10:e0145063. 10.1371/journal.pone.0145063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ha S, Choi H, Cheon GJ, et al. Autoclustering of Non-small Cell Lung Carcinoma Subtypes on (18)F-FDG PET Using Texture Analysis: A Preliminary Result. Nucl Med Mol Imaging 2014;48:278-86. 10.1007/s13139-014-0283-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wahl RL, Jacene H, Kasamon Y, et al. From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J Nucl Med 2009;50 Suppl 1:122S-50S. 10.2967/jnumed.108.057307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galavis PE, Paliwal B, Holden J, et al. System and method for gradient assisted non-connected automatic region (GANAR) analysis. Google Patents, 2016. [Google Scholar]
- 22.Haralick RM, Shanmugam K, Dinstein I. Textural features for image classification. IEEE Trans Syst Man Cybernet 1973;SMC-3:610-21. 10.1109/TSMC.1973.4309314 [DOI] [Google Scholar]
- 23.Tang X. Texture information in run-length matrices. IEEE Trans Image Process 1998;7:1602-9. 10.1109/83.725367 [DOI] [PubMed] [Google Scholar]
- 24.Galavis PE, Hollensen C, Jallow N, et al. Variability of textural features in FDG PET images due to different acquisition modes and reconstruction parameters. Acta Oncol 2010;49:1012-6. 10.3109/0284186X.2010.498437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galavis PE. Robust tumor segmentation for radiotherapy target definition. Madison, WI: University of Wisconsin, Madison, 2013. [Google Scholar]
- 26.Maani R, Yang YH, Kalra S. Voxel-based texture analysis of the brain. PLoS One 2015;10:e0117759. 10.1371/journal.pone.0117759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jemal A, Murray T, Ward E, et al. Cancer statistics, 2005. CA Cancer J Clin 2005;55:10-30. 10.3322/canjclin.55.1.10 [DOI] [PubMed] [Google Scholar]
- 28.Mountain CF. A new international staging system for lung cancer. 1986. Chest 2009;136:e25. [DOI] [PubMed] [Google Scholar]
- 29.Ettinger DS, Akerley W, Borghaei H, et al. Non-small cell lung cancer, version 2.2013. J Natl Compr Canc Netw 2013;11:645-53; quiz 653. 10.6004/jnccn.2013.0084 [DOI] [PubMed] [Google Scholar]
- 30.Rees JH, Smirniotopoulos JG, Jones RV, et al. Glioblastoma multiforme: radiologic-pathologic correlation. Radiographics 1996;16:1413-38; quiz 1462-3. 10.1148/radiographics.16.6.8946545 [DOI] [PubMed] [Google Scholar]
- 31.Lambin P, Rios-Velazquez E, Leijenaar R, et al. Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer 2012;48:441-6. 10.1016/j.ejca.2011.11.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kumar V, Gu Y, Basu S, et al. Radiomics: the process and the challenges. Magn Reson Imaging 2012;30:1234-48. 10.1016/j.mri.2012.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pyka T, Bundschuh RA, Andratschke N, et al. Textural features in pre-treatment [F18]-FDG-PET/CT are correlated with risk of local recurrence and disease-specific survival in early stage NSCLC patients receiving primary stereotactic radiation therapy. Radiat Oncol 2015;10:100. 10.1186/s13014-015-0407-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lovinfosse P, Janvary ZL, Coucke P, et al. FDG PET/CT texture analysis for predicting the outcome of lung cancer treated by stereotactic body radiation therapy. Eur J Nucl Med Mol Imaging 2016;43:1453-60. 10.1007/s00259-016-3314-8 [DOI] [PubMed] [Google Scholar]
- 35.Vesselle H, Turcotte E, Wiens L, et al. Relationship between non-small cell lung cancer fluorodeoxyglucose uptake at positron emission tomography and surgical stage with relevance to patient prognosis. Clin Cancer Res 2004;10:4709-16. 10.1158/1078-0432.CCR-03-0773 [DOI] [PubMed] [Google Scholar]
- 36.Choi H, Paeng JC, Kim DW, et al. Metabolic and metastatic characteristics of ALK-rearranged lung adenocarcinoma on FDG PET/CT. Lung Cancer 2013;79:242-7. 10.1016/j.lungcan.2012.11.021 [DOI] [PubMed] [Google Scholar]
- 37.Yip SS, Kim J, Coroller T, et al. Associations between somatic mutations and metabolic imaging phenotypes in non-small cell lung cancer. J Nucl Med 2017;58:569-76. 10.2967/jnumed.116.181826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vesselle H, Freeman JD, Wiens L, et al. Fluorodeoxyglucose uptake of primary non-small cell lung cancer at positron emission tomography: new contrary data on prognostic role. Clin Cancer Res 2007;13:3255-63. 10.1158/1078-0432.CCR-06-1128 [DOI] [PubMed] [Google Scholar]
- 39.Brooks FJ, Grigsby PW. The effect of small tumor volumes on studies of intratumoral heterogeneity of tracer uptake. J Nucl Med 2014;55:37-42. 10.2967/jnumed.112.116715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Traverso A, Wee L, Dekker A, et al. Repeatability and Reproducibility of Radiomic Features: A Systematic Review. Int J Radiat Oncol Biol Phys 2018;102:1143-58. 10.1016/j.ijrobp.2018.05.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rinewalt D, Shersher DD, Daly S, et al. Development of a serum biomarker panel predicting recurrence in stage I non-small cell lung cancer patients. J Thorac Cardiovasc Surg 2012;144:1344-50; discussion 1350-1. 10.1016/j.jtcvs.2012.08.033 [DOI] [PubMed] [Google Scholar]