Abstract
Objectives
This feasibility study investigated the effect of combined upper and lower limb functional electrical stimulation (FES) to reduce bradykinesia in Parkinson’s disease (PD).
Method
Eleven people with PD and Hoehn and Yahr score 2–3 used FES to assist dorsiflexion and hand opening or fine hand movements for 2 weeks. Outcome measures were the nine-hole peg test, box and block test, 10 m walking test, Tinetti balance scale, modified Parkinson’s disease quality of life questionnaire (PDQL), SPES/SCOPA scale, and compliance. All tests were carried out without FES. Comparisons were tested using the Student paired t-test.
Results
Two participants dropped out due to difficulty in using the equipment. Mean walking speed increased by 0.29 m s−1 (p = 0.002), step length by 0.09 m (p = 0.007), and cadence by 19.8 steps min−1 (p = 0.045). Tinetti balance score increased by 2.9 (p = 0.006). There was an increase in the box and block test of 5.1 (p = 0.025). The PD symptoms score of the PDQL improved by 4.9 (p = 0.013) and a reduction in SPES/SCOPA score of 5.7 (p = 0.005) indicated a reduced impact of PD.
Conclusions
FES produced clinically meaningful improvements in gait and upper limb function. Some participants found using both interventions challenging and we would recommend that their introduction be staggered.
Keywords: Parkinson’s disease, functional electrical stimulation, bradykinesia
Introduction
Functional electrical stimulation (FES) is a means of producing an active muscle contraction controlled in such a way to provide functional movement to assist everyday tasks. It is most frequently used for correction of dropped foot for individuals who have brain or spinal cord damage with preserved nerve and muscle integrity.1–10 Electrical stimulation is applied to the common peroneal nerve using skin electrodes placed over the head of the fibula bone and the tibialis anterior muscle. The stimulation is timed to the gait cycle using a foot switch placed in the shoe, causing the foot to be lifted when the foot is taken from the ground. The practical assistance this produces increases the safety of gait,1 improves walking speed,11 and is associated with an improvement in quality of life measures.2 Further, longitudinal studies have shown that FES users with non-progressive conditions can also receive a training effect, demonstrated by increased walking speed without FES after several months of FES use.11–13
Parkinsonian gait is characterized by bradykinesia, hypokinesia, and akinesia. This can severely restrict mobility and increase the risk of falls. Mann et al. hypothesized that FES, when used to produce dorsiflexion may be a useful intervention to assist the initiation of stepping,14 overcoming freezing in gait. In a feasibility study, seven people with Parkinson’s disease (PD) who exhibited freezing in gait used an FES device for a period of 2 months. The participants chosen for the study did not have dropped foot, therefore allowing the effect on freezing to be studied independently. The study showed that FES use was associated with reduced episodes of freezing, increased gait speed, increase stride length and reduced incidence of trips and falls. Further, it was found that there was a training effect, demonstrated by improved gait parameters, four weeks after FES was withdrawn.
More recently, Popa et al. have attempted to exploit the effect on bradykinesia and hypokinesia seen in the lower limb (LL) to improve dexterity and speed of movement in the upper limb (UL).15,16 Electrical stimulation was used to train the finger, thumb and wrist extensors for 30 minutes per day for 10 days on the most affected UL. Improvements were seen in the nine-hole peg test (9-HPT) and in the vertical tapping test (VTT), demonstrating that dexterity was bilaterally improved; this may be due to an interhemispheric transfer called “cross education.”15–17 Initial experience with UL stimulation has used stimulation to open the hand. This was done because hand opening is required at the initiation of a movement sequence when acquiring an object. However, we have observed that people with PD often have difficulty in producing fine movements of the hand involved in a palmar grasp where the tips of the index and middle fingers are brought together with the thumb. This movement can be produced by stimulation of the median nerve at the wrist and of the ulnar nerve supplying the lumbricals on the dorsal face of the hand.
The evidence to date indicates that people with PD may benefit from both upper and lower limb stimulation but to our knowledge, there are no reports of both interventions being used in combination. The aim of this feasibility study was to explore the practicality of providing, in a standard clinical setting, combined upper and lower limb FES treatment in PD, using a pragmatic approach to selection of stimulation targets, determined by the symptom presentation of the person with PD.
Method
The study was a prospective interventional clinical feasibility study. The study was reviewed by the institutions ethical review process and was conducted following the principles outlined in the “Declaration of Helsinki.”
Participants were identified by the hospital’s PD specialist nurse and invited to contact the researchers if they wished to take part in the study. Volunteers then attended an assessment clinic where they were assessed using the following criteria. Inclusion criteria: aged 18 years or above; Hoehn and Yahr stages I–III under medication; difficulty with gait; medically stable; able to understand and comply with the protocol and give informed consent. Exclusion criteria: other treatment than standard oral drug therapy (deep brain stimulation, Duodopa, apomorphine); atypical or secondary parkinsonism; parkinsonism related to other neurodegenerative diseases; pyramidal and/or extrapyramidal systems injuries; untreated or refractory epilepsy; pregnancy; cardiac pacemaker; radial, median, ulnar or common peroneal nerve injuries; osteoarticular pathology that involves the forearm or leg bones or wrist, elbow, knee, and tibiotarsal joint; malignancy or dermatological conditions in the area of the electrodes; severe cardiovascular disease; major cognitive impairment or dementia.
Suitable participants were asked to return to the clinic on two consecutive days to begin the study. On the first day the outcome measures were performed. On the following day participants were taught how to use the FES device and followed up by telephone 2 days later. If participants were experiencing difficulties an extra clinic appointment was offered in the first week of treatment. Final assessments were made 14 days after beginning treatment. The 2 week treatment period was chosen because clinically meaningful results were seen after 10 days in our previous upper limb study.15,16 Participants were allocated to one of two groups based on clinical observation of hand function. If the functional deficit was predominantly hand opening they were allocated to the radial nerve stimulation group (group 1), while if the deficit was predominantly related to the manipulation of objects they were allocated to the median/ulnar nerve group (group 2). In each case the most affected upper and lower limbs were treated. Group 1 received stimulation of wrist finger and thumb extensors and group 2 received stimulation of the intrinsic muscles of the hand (Figure 1). The stimulation parameters were pulse width 180 μs, frequency 40 Hz, on time 5 s, off time 5 s, ramp time 2 s. The current intensity was adjusted until a strong but comfortable muscle contraction was produced. Participants were asked to use the stimulator twice a day starting with sessions of 10 min on the first day, 15 min on the second day, and 20 min on the third to 14th day. Both groups received common peroneal stimulation in the swing phase of gait. Stimulation parameters were: pulse width 180 μs, frequency 40 Hz with the current increased until dorsiflexion with mild eversion were produced. Other stimulation parameters (rising and falling ramps, extension time an output waveform) were adjusted to suit each individual in accordance with standard clinical practice. Participants were asked to build up use of LL stimulation over the two week study, starting with short walks of 5–10 min, building up to unlimited use by 2 weeks.
Figure 1.
Electrode positions.
Stimulation was delivered using an ODFS® Pace V1.2 using its cyclic exercise mode for the upper limb and foot switch control for the lower limb (Odstock Medical Ltd, Salisbury, UK).18 For upper limb exercises, 30 mm × 50 mm Pals Plus electrodes were used while 50 mm × 50 mm Pals Platinum Blue electrodes were used in the lower limb (Axelgaard, CA, USA).
Both assessments were made at the same time of day to ensure the same presentation of PD symptoms. Assessments were made using the following:
Hoehn and Yahr (H-Y):19 the severity of the disease (0 = no symptoms, 5 = incapacitated state).
Modified Parkinson’s disease quality of life questionnaire (PDQL):20 37 items, scored with a five-point scale covering four domains, a higher score indicating better quality of life.
SPES/SCOPA scale (short Parkinson’s evaluation scale/scales for outcomes in Parkinson’s disease) section A:21 a motor evaluation scale with 10 items each with a range from 0 to 3. A lower total score indicates better function.
Box and block test (BBT):22 a measure of gross manual dexterity. The total number of blocks transferred from one box to another in 1 min was recorded.
9-HPT:23 a measure of fine manual dexterity. The time taken to fill and then empty nine-hole peg board with pegs was recorded.
Tinetti balance evaluation (TBE):24 a nine-item scale, with a range from 0 to 1 or 0 to 2 each (the total score: 16 = no symptoms, 0 = major balance problems).
10-meter walk test (10-MWT):25 the volunteer was asked to cover a 10 m course over smooth flooring in an open environment without FES. A single instruction, “walk briskly but safely” was given. Two measurements were taken and the mean walking speed, stride length and cadence were calculated.
Treatment compliance: device usage was recorded using the ODFS® Pace data logger.
Comparisons were tested using the Student’s paired t-test.
Results
Eleven people were recruited to the project (see demographic details in Table 1). However one participant was unable to use upper limb stimulation due to an arm injury. Two people dropped out, both because they were unable to successfully use the equipment. Table 1 also gives the participations opinions of the treatment. Five people wanted to continue to use FES following the trial.
Table 1.
Demographic data and participant opinions.
| No. | Age years (SD) | Gender | Time since diagnosis years (SD) | Hand dominance | Most effected side | Hoehn and Yahr | Participant opinion lower limb FES | Participant opinion upper limb FES | Want to continue FES post study? |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 72 | m | 13 | r | l | 3 | dropped out | ||
| 2 | 78 | f | 6 | l | l | 2.5 | not useful | fairly useful | no |
| 3 | 72 | m | 4 | r | r | 3 | useful | useful | yes |
| 4 | 74 | m | 6 | r | r | 2 | fairly useful | fairly useful | no |
| 5 | 80 | m | 7 | r | r | 3 | very useful | useful | yes |
| 6 | 70 | m | 3 | r | r | 2 | yes | no | yes |
| 7 | 66 | f | 4 | r | r | 2.5 | not useful | not useful | no |
| 8 | 74 | m | 9 | r | r | 2.5 | very useful | no | |
| 9 | 77 | m | 3 | l | r | 2.5 | very useful | very useful | yes |
| 10 | 63 | f | 2 | r | r | 2 | useful | not useful | yes |
| 11 | 80 | m | 4 | r | l | 2 | dropped out | ||
| 73.3 (5.5) | 8 m 3 f | 5.5 (3.2) | 9 right 2 left | 8 right 3 left | HY2: 4 HY2.5: 4 HY3: 3 | not useful: 2 fairly useful: 1 useful: 2 very useful: 3 dropped out: 2 | not useful : 2 fairly useful: 2 useful: 2 very useful: 1 dropped out:2 | Yes: 5 No: 4 dropped out: 2 |
The results for the upper limb are shown in Table 2. Eight participants were allocated to group 1 (radial nerve), while two were allocated to group 2 (medial and ulna nerves). Both groups’ responded similarly to treatment so the results were pooled. Statistically significant increases in Box and Block score were seen on the treated side with a strong trend seen in the reduction in the time to perform the 9-HPT. There was no change seen in the non-treated UL.
Table 2.
Upper limb results.
| Treated hand pre FES | Treated hand post FES | Treated hand mean difference | p | Non treated hand post FES | Non treated hand pre FES | Non treated hand mean difference | p | |
|---|---|---|---|---|---|---|---|---|
| nine-hole peg test (s) | 33.2 (5.9) | 29.7 (5.1) | −3.5 (4.2) | 0.072 | 33.2 (10.3) | 30.6 (5.0) | 2.6 (5.6) | 0.269 |
| Box and block (n) | 41.6 (6.7) | 46.7 (7.7) | 5.1 (4.6) | 0.025 | 49.7 (9.9) | 47.3 (6.6) | 2.4 (4.7) | 0.219 |
Results of the lower limb tests are shown in Table 3. Statistically significant changes were seen in walking speed, cadence, steps taken over 10 m, and stride length indicating a training effect. A significant reduction was also recorded in the SPES/SCOPA score indicating that PD specific motor function symptoms were reduced. Similarly, a significant improvement was recorded in the Tinetti Balance score. Table 3 also gives the results of the PDQL. There were statistically significant improvements in the Systemic Symptoms domain with a strong trend to statistical significance in the Social Function and Total scores, indicating that the combined upper and lower limb treatment had a positive impact on quality of life.
Table 3.
Lower limb and quality of life results.
| Pre FES mean (SD) | Post FES mean (SD) | Mean difference mean (SD) | p | |
|---|---|---|---|---|
| Steps in 10 m (n) | 24.4 (8.7) | 20.5 (8.5) | −3.9 (4.3) | 0.025 |
| 10 m walking speed (m s−1) | 0.82 (0.44) | 1.11 (0.43) | 0.29 (0.19) | 0.002 |
| Stride length (m) | 0.90 (0.26) | 1.08 (0.32) | 0.18 (0.14) | 0.007 |
| Cadence (steps/minute) | 100.8 (33.0) | 121.2 (18) | 19.8 (25.2) | 0.045 |
| SPES/SCOPA | 12.1 (3.8) | 6.4 (5.1) | −5.7 (2.8) | 0.005 |
| Tinetti balance score | 10.8 (3.5) | 13.7 (3.3) | 2.9 (2.3) | 0.006 |
| PDQL: PD symptoms | 46.7 (10.6) | 51.0 (7.9) | 4.3 (10.1) | 0.236 |
| PDQL: Systemic symptoms | 20.7 (4.4) | 25.6 (4.0) | 4.9 (4.6) | 0.013 |
| PDQL: Social function | 22.7 (5.4) | 26.0 (3.8) | 3.3 (4.4) | 0.053 |
| PDQL: Emotional function | 32.4 (5.5) | 34.6 (5.4) | 2.1 (7.8) | 0.439 |
| PDQL: Total | 122.4 (94) | 137.1 (111) | 14.7 (22.6) | 0.087 |
Table 4 shows the stimulator usage. It appeared that most participants did not use the stimulator all the time but used it for a set walk each day. This was in part because we asked participants to build up walking gradually over the 2 week period. Hence the number of walks each day is relatively low averaging about five. A mean of 2350 steps were taken each day. The exercise time was less than expected (9 hours) at around 5 hours.
Table 4.
Adherence and dosage.
| Steps,a n (SD) | Walks,b n (SD) | Walk time,c hours (SD) | Exercise sessions,d n (SD) | Exercise time,e hours (SD) |
|---|---|---|---|---|
| 32,868 (9373) | 76 (49) | 8.6 (2.4) | 42.9 (28.6) | 4.9 (2.6) |
Steps is the number of steps taken with FES.
Walks is the number of times the stimulator is put into active mode to begin walking.
Walk time is the total stimulation time, i.e. the swing phase of gait only.
Exercise sessions is the number of times the stimulation is switched on in exercise mode.
Exercise time is the total time of spent in exercise mode.
Discussion
In this study modest improvements in upper limb function were recorded. However, in contrast to our previous study, we did not observe an effect on the non-stimulated upper limb.15,16 This may be due to differences between the protocols. In the earlier study,15 electrical stimulation was used for longer each day, 30 minutes, while in this study compliance with UL stimulation was less than expected, averaging 21 min a day. It is possible that the combination of UL FES with FES with walking may have effected UL treatment compliance. Further, the mean age of participants in the present study at 73.5 years is older than either previous study (66.4 and 67.3 years).15,16 The first study was also significantly larger with 26 participants.
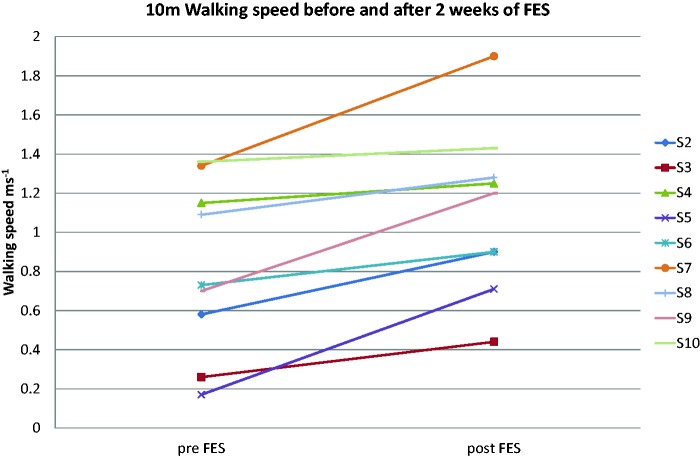
Changes seen in this study were more marked in the lower limb, with a substantial clinically meaningful change in walking speed (>0.1 m s−1) being achieved by all but one of the participants who completed the protocol (Figure 2). Further, all five participants whose initial walking speed was sufficiently reduced so that they could be categorized as household walking only (<0.4 m s−1), most limited community walking (0.4–0.58 m s−1), or least limited community walking (0.59–0.79 m s−1) all changed their functional walking category.13,18 Four of the five participants who wanted to continue FES use were in this group, suggesting the intervention has greatest impact on the slower walkers. Statistically significant increases were also seen in stride length and cadence indicating improvements in both hypokinesia and bradykinesia.
Figure 2.
10 m walking speed of each participant.
In contrast to the UL, LL electrical stimulation was applied synchronized to the gait cycle and therefore in direct association with the function it was intended to improve. LL stimulation had a direct orthotic effect, assisting gait by increasing dorsiflexion and stabilizing the ankle in early stance. The mean stimulation time was also greater than received in the UL. A possible confounding effect in understanding the mechanism behind the reported gait changes may be the increased amount of walking that was reported by some participants in the study period, compared to their normal levels of activity. The protocol required that the time spent walking was increased through the intervention period. However, it is possible that FES use also enabled greater activity. Several studies have investigated the use of physiotherapy interventions aimed at increasing activity and walking speed changes have been reported.26–30 A weighted mean gain in walking speed in these studies was calculated to be 0.11 m s−1, less than half the gain reported in this study, suggesting that FES may have an additional effect.
In EMG studies by Cioni et al. it was identified that there was a reduced activation of tibialis anterior (TA) during the early stance and in the early and late swing phase of the gait in people with PD who were in the “off-medication” stage of their condition.31, 32 Stimulation to correct dropped foot directly assists TA function while the stimulation is active. However, our results indicate a training or carry-over effect after stimulation was removed. This may relate to increased TA and other muscles strength due to hypertrophy but may also indicate improved motor recruitment. In our earlier study, we measured the excitability of the motor cortex using transcranial magnetic stimulation (TMS) in 10 people with PD, who used electrical stimulation of the forearm extensors for 30 minutes a day for 10 days.16 A statistically significant increase in cortical excitability was found, indicating that FES may improve recruitment of voluntary muscles. However, it is also possible that FES may have an impact at the spinal level. It has been reported that there is a reduction in reciprocal inhibition in PD associated with rigidity.33 Crone et al. demonstrated disynaptic reciprocal inhibition in 74 neurologically intact subjects by showing the H reflexes induced in the soleus muscle were inhibited by stimulation of the common peroneal nerve.34 The effect was greatly reduced in 39 patients who had spasticity except for four people who had multiple sclerosis and were regular dropped foot stimulator users. This suggests that regular stimulation of the common peroneal nerve may preserve this reflex and possibly increase the excitability of the agonist alpha motor neuron.
The current work was conceived as a feasibility study, so it presents some limitations. The study did not have a control group, so it is possible that the changes seen may be, at least in part due to a placebo or Hawthorne effect. The intervention period was shorter than might be envisaged in clinical practice possibly reducing the effect of the treatment. The outcome measures were non-blinded. Also, there may be some question over the accuracy of the observed changes, as all but one of the second assessments were completed by a different assessor to the first. Two participants dropped out because of difficulties using the equipment. This is a greater dropout rate than stroke or MS in our experience.1–3,13 Setting up and teaching the participant UL and LL stimulation at the same clinic appointment was found to be demanding for some participants. In clinical practice, it may be more effective to spend more time introducing the treatment and to stagger the introduction of UL and LL interventions by several weeks.
Several of the more able participants while noticing a difference from the FES did not think it was sufficient to be worthwhile with their current presentation of PD, but thought it might helpful in the future. The least able participant did not get a consistent response to FES and it was often not possible to overcome freezing. An association of FES therapy with additional cueing techniques might overcome this difficulty.
A significant limitation was that the sample size was small, limiting the power of the study. A sample size calculation has been made based on data from this and the previous observational study,14 based on the repeated measures analysis of covariance (rmANCOVA) method. The power calculation was set up to estimate the sample size required to have a 90% chance of detecting a clinically meaningful change in walking speed of 0.1 m s−1 relative to a control group. The between subject standard deviation (SD) for walking speed was 0.295 m s−1 with an upper confidence limit of 0.36 m s−1. The following assumptions were made; alpha = 0.05 (2 sides), power = 0.9, control group mean 0.65 m s−1, intervention group mean 0.75 m s−1, between subject SD (walking speed) = 0.36 m s−1, equal numbers in the treatment and control group, and two pre- and two post-intervention measurements. Analysis from the previous studies showed a correlation between pre- and post-intervention measures and between follow up measures of r > 0.85. Conservatively we assume a correlation between follow up measurements of r = 0.75, between base line measurements of r = 0.60 and between base line and follow up measurements of 0.75. This gives a sample size of 94 (47 participants per group). This estimate makes no allowance for drop outs from the study. (STATA v.12, Statcorp, USA).
Conclusion
This study confirms the findings of previous studies that FES can cause a clinically meaningful training effect on bradykinesia in both upper and lower limb function in people with PD and indicate that this is associated with improvements in health related quality of life. Its application is feasible for most people with PD but compliance may be improved by a staggered introduction of each modality. More experience is needed to determine the best clinical methods, dosage and an appropriately powered study is needed to demonstrate the clinical effectiveness of the intervention.
Acknowledgment
FES devices were loaned by Odstock Medical Limited. We would like to thank movement disorder nurse specialist James Lee for assistance with recruitment and medical statistician Paul Strike for the power calculation. We would also like to thank the research participants who took part.
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Paul Taylor is employed by Salisbury NHS Foundation Trust who are the majority owner of Odstock Medical Ltd which supplied the medical devices used in this study. Fifty percent of his time is seconded to Odstock Medical Ltd to provide clinical and R&D services. He also holds a number of stocks in Odstock Medical.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by European Union Program POSDRU/88/1.5/S/58965, co-funded by the Social European Fund through the Operational Sector-Specific Human Resources Development Program 2007–2013.
References
- 1.Esnouf JE, Taylor PN, Mann GE, et al. Impact on falls and activities of daily living of use of a functional electrical stimulation (FES) device for correction dropped foot in people with multiple sclerosis. Mult Scler 2010; 16: 1141–1147. [DOI] [PubMed] [Google Scholar]
- 2.Barrett CL, Taylor PN. The effects of the Odstock drop foot stimulator on perceived quality of life for people with stroke and multiple sclerosis. Neuromodulation 2010; 13: 58–64. [DOI] [PubMed] [Google Scholar]
- 3.Barrett CL, Mann GE, Taylor PN, et al. A randomized trial to investigate the effects of functional electrical stimulation and therapeutic exercise on walking performance for people with multiple sclerosis. Mult Scler 2009; 15: 493–504. [DOI] [PubMed] [Google Scholar]
- 4.Sheffler LR, Hennessey MT, Knutson JS, et al. Neuroprosthetic effect of peroneal nerve stimulation in multiple sclerosis: a preliminary study. Arch Phys Med Rehabil 2009; 90: 362–365. [DOI] [PubMed] [Google Scholar]
- 5.Van der Linden ML, Hazlewood E, Hillman SJ, et al. Functional electrical stimulation to the dorsiflexors and quadriceps in children with cerebral palsy. Pediatr Phys Ther 2008; 20: 23–29. [DOI] [PubMed] [Google Scholar]
- 6.Sheffler LR, Hennessey MT, Naples GG, et al. Improvement in functional ambulation as a therapeutic effect of peroneal nerve stimulation in hemiplegia: two case reports. Neurorehabil Neural Repair 2007; 21: 366–369. [DOI] [PubMed] [Google Scholar]
- 7.Burridge JH, Elessi K, Pickering RM, et al. Walking on an uneven surface: the effect of common peroneal nerve stimulation on gait parameters and relationship between perceived and measured benefits in a sample of participants with a drop foot. Neuromodulation 2007; 10: 59–67. [DOI] [PubMed] [Google Scholar]
- 8.Sheffler LR, Hennessey MT, Naples GG, et al. Peroneal nerve stimulation versus an ankle foot orthosis for correction of footdrop in stroke: impact on functional ambulation. Neurorehabil Neural Repair 2006; 20: 355–360. [DOI] [PubMed] [Google Scholar]
- 9.Taylor PN. The use of electrical stimulation for correction of dropped foot in subjects with upper motor neuron lesions. Adv Clin Neurosci Rehab 2002; 2: 16–18. [Google Scholar]
- 10.Burridge JH, McLellan DL. Relation between abnormal patterns of muscle activation and response to common peroneal nerve stimulation in paraplegia. J Neurol Neurosurg Psychiatry 2000; 69: 353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taylor PN, Burridge JH, Wood DE, et al. Clinical use of the Odstock drop foot stimulator its effect on the speed and effort of walking. Arch Phys Med Rehab 1999; 80: 1577–1583. [DOI] [PubMed] [Google Scholar]
- 12.Stein RB, Everaert DG, Thompson AK, et al. Long-term therapeutic and orthotic effects of a foot drop stimulator on walking performance in progressive and nonprogressive neurological disorders. Neurorehabil Neural Repair 2010; 24: 152–167. [DOI] [PubMed] [Google Scholar]
- 13.Taylor P, Humphreys L, Swain I. The long term cost-effectiveness of the use of functional electrical stimulation for the correction of dropped foot due to upper motor neuron lesion. J Rehabil Med 2013; 45: 154–160. [DOI] [PubMed] [Google Scholar]
- 14.Mann GE, Finn SM, Taylor PN. A pilot study to investigate the feasibility of electrical stimulation to assist gait in Parkinson’s disease. Neuromodulation 2008; 11: 143–149. [DOI] [PubMed] [Google Scholar]
- 15.Popa L, Alexa D, Rotar A, et al. Functional electrical stimulation effect on the motor performances in parkinsonian patients. Rev Med Chur Soc Med Nat Iasi 2011; 116: 436–441. [PubMed] [Google Scholar]
- 16.Popa L, Constantinescu A, Mureşanu DF, et al. Clinical improvement and cortical adaptations after functional electrical stimulation in Parkinson’s disease patients. CNS Neurolo Disorders Drug Targets 2013; 12: 265–273. [DOI] [PubMed] [Google Scholar]
- 17.Popa L, Bolbocean O, Ignat B, et al. Transcranial magnetic stimulation assessment of functional electrical stimulation effect on Parkinson’s disease patients. Eur J Neurol 2012; 19(Suppl. 1): S679. [Google Scholar]
- 18.Perera S, Moody S, Woodman RC, et al. meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc 2006; 54: 743–749. [DOI] [PubMed] [Google Scholar]
- 19.Hoehn M, Yahr M. Parkinsonism: onset, progression and mortality. Neurology 1967; 17: 427–442. [DOI] [PubMed] [Google Scholar]
- 20.De Boer AG, Wijker W, Speelman JD, et al. Quality of life in patients with Parkinson's disease: development of a questionnaire. J Neurol Neurosurg Psychiatry 1996; 61: 70–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marinus J, Visser M, Stigglebout AM, et al. A short scale for the assessment of motor impairments and disabilities in Parkinson’s disease: the SPES/SCOPA. J Neurol Neurosurg Psychiatry 2004; 75: 388–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holser P, Fuchs E. Box and nlock test. In: Cromwell FS. (ed). Occupational therapists manual for basic skills assessment: primary prevocational evaluation, Pasadena, CA: Fair Oaks Printing Company, 1960, pp. 29–31. [Google Scholar]
- 23.Kellor M, Frost J, Silberberg N, et al. Hand strength and dexterity. Am J Occup Ther 1971; 25: 77–83. [PubMed] [Google Scholar]
- 24.Tinetti ME. Performance-oriented assessment of mobility problems in elderly patients. J Am Geriatr Soc 1986; 34: 119–126. [DOI] [PubMed] [Google Scholar]
- 25.Bohannon RW, Andrews AW, Thomas MW. Walking speed: reference values and correlates for older adults. J Orthop Sports Phys Ther 1996; 24: 86–90. [DOI] [PubMed] [Google Scholar]
- 26.Chandler C, Plant R. A targeted physiotherapy service for people with Parkinson’s disease from diagnosis to end stage: a pilot study. In: Percival R, Hobson P. (eds). Parkinson’s disease: studies in psychological and social care, BPS Books, 1999, pp. 256–269. [Google Scholar]
- 27.Ellis T, De Goede CJ, Feldman RG, et al. Efficacy of a physical therapy program in patients with Parkinson’s disease: a randomized controlled trial. Arch Phys Med Rehabil 2005; 86: 626–632. [DOI] [PubMed] [Google Scholar]
- 28.Fisher BE, Wu AD, Salem GJ, et al. The effect of exercise training in improving motor performance and corticomotor excitability in people with early Parkinson’s disease. Arch Phys Med 2008; 89: 1221–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thaut MH, McIntosh GC, Rice RR, et al. Rhythmic auditory stimulation in gait training for Parkinson’s disease patients. Mov Disorder 1996; 11: 193–200. [DOI] [PubMed] [Google Scholar]
- 30.Canning CG, Allen NE, Dean CM, et al. Home-based treadmill training for individuals with Parkinson’s disease: a randomized controlled pilot trial. Clin Rehabil 2012; 26: 817–826. [DOI] [PubMed] [Google Scholar]
- 31.Cioni M, Richards CL, Malouin F, et al. Characteristics of the electromyographic patterns of lower limbs muscles during gait in patients with Parkinson’s disease when OFF and ON L-Dopa treatment. Ital J Neurol Sci 1997; 18: 195–208. [DOI] [PubMed] [Google Scholar]
- 32.Caliandro P, Ferrarin M, Cioni M, et al. Levodopa effect on electromyographic activation patterns of tibialis anterior muscle during walking in Parkinson’s disease. Gait Posture 2011; 33: 436–441. [DOI] [PubMed] [Google Scholar]
- 33.Lelli S, Panizza M, Hallett M. Spinal cord inhibitory mechanism in Parkinson’s disease. Neurology 1991; 41: 553–556. [DOI] [PubMed] [Google Scholar]
- 34.Crone C, Nielsen J, Petersen N, et al. Disynaptic reciprocal inhibition of ankle extensors in spastic patients. Brain 1994; 117(Pt 5): 1161–1168. [DOI] [PubMed] [Google Scholar]