Abstract
Introduction: Ambulation can be used to monitor the healing of lower extremity fractures. However, the ambulatory behavior of tibia fracture patients remains unknown due to an inability to continuously quantify ambulation outside of the clinic. The goal of this study was to design and validate an algorithm to assess ambulation in tibia fracture patients using the ambulatory tibial load analysis system during recovery, outside of the clinic.
Methods
Data were collected from a cyclic tester, 14 healthy volunteers performing a 2-min walk test on the treadmill, and 10 tibia fracture patients who wore the ambulatory tibial load analysis system during recovery.
Results
The algorithm accurately detected 2000/2000 steps from simulated ambulatory data. During the 2-min walk test, step counts derived from the algorithm and treadmill showed a strong correlation (r2>0.98) to the visual (“actual”) step count. Applying the algorithm to continuous data from tibia fracture patients revealed qualitative differences in gait between the initial and later stages of recovery. Additionally, a relatively large standard deviation (≤3000 steps) in the daily average step count indicated a variety of patient ambulatory behaviors.
Conclusion
The algorithm reported in this study can assess the ambulatory activity of tibia fracture patients during the recovery period.
Keywords: Biomedical devices, community ambulation, continuous fracture care, gait, patient behaviour monitoring devices, rehabilitation, rehabilitation devices, step activity monitor, underfoot load monitoring
Introduction
The tibia is the most commonly fractured long bone.1 Tibia fractures can be caused by both low impact (i.e. walking, fall from standing) and high impact mechanisms of injury (i.e. sports injury, motor vehicle accident).2 Complications such as delayed healing or non-union occur in 13–60%3,4 of cases and place additional burden on the patient because they prolong the recovery period and are associated with significant pain.5 It is well known that fracture healing is highly dependent on the mechanical environment,6 which is the magnitude and type of load experienced by the fractured bone. Under-loading has been shown to result in impeded bone formation and over-loading conditions have resulted in dislocation of the bone fragments.7–9 In order to promote bone healing, the standard of care instructs patients to gradually increase load applied to the injured limb from no-load to full-load conditions,10 during recovery which typically lasts 6–12 weeks or more.11 Moreover, the ability of a patient to bear weight on the injured limb is a commonly used clinical factor to assess fracture healing.12
One method to analyze the ability of a patient to bear weight is through the assessment of gait.13 In general, ambulation is a basic component of various daily activities and quantification of ambulation can provide important information regarding an individual's functional capacity. In 2012, Macri et al.13 used video assessment to grade the gait of tibia fracture patients on a scale of 1–4 and found that a patient's ability to ambulate was correlated to the healing stage of the fractured bone. Patients with a higher score were able to ambulate normally, while patients with a lower score had difficulties ambulating. However, this qualitative measure of ambulation is time consuming, limited by subjectivity, and requires clinical visits for data points. An objective, out-of-clinic method to quantify ambulation may provide clinicians with a more practical tool to monitor fracture healing. However, monitoring progress in fracture healing outside of the clinical environment has been difficult due to unknown patient behaviors such as the number of steps taken,14 the actual amount of loading15 and time spent wearing a rehabilitative boot. The lack of a reliable method to continuously monitor the type and magnitude of loading is one of the main reasons why quantifying the mechanical environment experienced by lower extremity fractures has been difficult.
In an attempt to quantify the mechanical environment experienced by an injured lower extremity, several technologies have been developed. One set of devices is underfoot load monitoring systems which measure force underneath the foot. These systems have been limited by inadequate sensor performance including hysteresis, non-linearity, drift, short recording time ( < 24 h), and/or high cost.16–19 These limitations have led to a paucity of continuous, out-of-clinic underfoot load data from lower extremity fracture patients. Apart from underfoot load monitoring systems, several specialized research tools such as implantable microelectromechanical sensors and instrumented internal fixators have been developed.20–22 However, these tools are limited to certain fracture types, are invasive due to the need for surgical implantation and retrieval and some require clinical visits for data acquisition.
While the aforementioned load monitoring devices can measure the magnitude of loading, these systems are generally not designed to measure ambulation. An exception is the OpenGo insole which measures the amount of active time spent by ankle fracture patients for a duration of six weeks.23 It is unclear, however, if the amount of time spent active, refers to dynamic or static activities. Activity monitors that are designed for consumer fitness and wellness tracking have also been used to quantify ambulation with varying results. The most popular wearable device, the Fitbit, has been shown to underestimate the number of steps taken at a slow, moderate, and brisk walking speeds on a treadmill.24 Diaz et al. reported underestimations in step counts of up to 3% for hip worn FitBit models and up to 23% for wrist worn FitBit models. In another study with healthy volunteers walking both indoors and outdoors, devices such as the Nike+ Fuel Band, Jawbone Up, and Tractivity showed poor accuracy at slow walking speeds by underestimating the number of footsteps by 35.39 ± 21.17%, 10.08 ± 8.04%, and 10.92 ± 16.26% respectively.25 Due to their varying accuracies, these devices may not be appropriate to use to accurately quantify ambulation in lower extremity fracture patients. Quantifying ambulation requires a reliable and accurate methodology to detect footsteps—including footsteps during the early recovery period where the underfoot loads are low. Therefore, there is a need for a reliable, robust and affordable tool to measure ambulation during recovery which may allow for new insights into tibia fracture healing and equip care providers with data to guide improved healing outcomes.
In previous studies by our group, a robust underfoot load measuring device, the Ambulatory Tibia Load Analysis System (ATLAS), was developed and shown to overcome many of the current technological limitations in long-term limb load monitoring such as sensor linearity, drift, and duration of recording time outside of the clinic.26 The ATLAS consists of three separate piezoresistive pressure sensors with two sensors positioned under the medial and lateral metatarsal heads, and one sensor under the heel.27 The sensors are integrated into a controlled ankle movement (CAM) walker, which is worn by the patient.
The goal of this study was to develop an algorithm to count steps from underfoot load data from the ATLAS in order to assess ambulation in tibia fracture patients as they are recovering, both during the initial healing phase as well as late stage healing. In this study, we developed an automated step counting algorithm to accurately and reliably determine the number of steps from ATLAS underfoot loading data. Verification of the step counting algorithm was performed in the laboratory using a cyclic loading device and software generated loading curves. Validation was performed with healthy volunteers wearing an ATLAS instrumented CAM walker boot during a 2-min walking test (2MWT) on a Noraxon Scifit treadmill. The accuracy of the step counting algorithm was compared to a visual step count and treadmill generated step count. Subsequently, the step counting algorithm was used to assess ambulation in 10 tibia fracture patients.
Materials and methods
The study was conducted at the University Orthopedic Center and the Department of Bioengineering at the University of Utah, according to the protocol reviewed and approved by the University of Utah IRB. Healthy volunteers (ref IRB 81249) and tibia fracture patients (ref IRB 61719) provided informed written consent to participate in these studies. Unless otherwise specified, all software programs were run with default or recommended settings from a 2.66 GHz Intel Core i7 Windows OS desktop machine equipped with 16 GB of RAM.
Algorithm description
All of the data analyzed by the algorithm consisted of the sum of the three sensors of the ATLAS. The ATLAS continuously records underfoot loading, and therefore the data contain information about both ambulation and other activities, such as static loading and momentary loading (e.g. required to maintain balance, “shuffling” at the early stage of recovery, etc.). Thus, an algorithm is necessary to filter data associated with non-ambulatory events in order to count steps. The algorithm was developed and informed by analyzing partial and full weight-bearing waveforms from the first three patients in this study. The initial analysis of this data revealed that steps taken by tibia fracture patients in a walking boot often deviated from the healthy gait, which has been previously reported in healthy volunteers simulating partial weight bearing.28 In order to overcome the challenge of counting steps based on irregular loading patterns, we developed an algorithm with three conditions. The desired function of the algorithm was to first detect the heel or forefoot contact with the ground by looking for a maximum load value and then detect when the foot discontinued contact with the ground by looking for a minimum load value. In addition, the algorithm was designed to disregard quickly occurring maxima that may have occurred due to a variety of events such as balancing or shuffling during the numerous weeks of recording data. Underfoot load data that met all three conditions of the algorithm were counted as footsteps.
The first condition of the algorithm was to detect a heel or forefoot strike by identifying local maxima based on a threshold. From observations with our initial data, it was quickly determined that a fixed threshold system was unable to compensate for the dynamic nature of weight-bearing progression over the many weeks of using the CAM Walker. In order to determine a dynamic threshold, we sampled data from the first three patients in the study to determine the relationship regarding the threshold (differences between local maxima and minima) and the corresponding peak loads (maxima). These variables were sampled at time points during the early, middle, and late periods of recovery. Linear regression analysis revealed a slope value of 40% which indicated that differences between maxima and minima were approximately 0.4 times the peak load value. Thus, we set our threshold to be at least 40% of the daily average peak load. Because the average load increases over time, thresholding with respect to individual days allows for the capture of steps taken from partial weight-bearing to full weight-bearing conditions. Peak load values (foot strike) greater than or equivalent to the threshold were passed on to the second condition of the algorithm for further filtering.
The second condition of the algorithm was to disregard peak loads that occurred too quickly to be associated with ambulation. The fast gait for humans ages 20–49 is approximately 2.48 ± .12 steps/s.29 It is unlikely that a lower limb fracture patient would exceed this rate. Therefore, the condition was set that if two maxima occurred at a frequency faster than 1.3 steps/s, the lower amplitude maximum would be disregarded. This condition was designed to remove peaklets that occurred as the recovering patients attempted to ambulate, balance, shuffle and perform other daily activities. The remaining peak loads were then passed on to the third condition of the algorithm to determine the final step count.
Finally, the third condition of the algorithm was to detect the swing phase of gait by detecting a local minimum. During normal gait, the foot strike with the ground is preceded and followed by foot swing. This scenario is represented in the underfoot load data by a maximum value surrounded on both sides by two minima values. In order to account for the irregular gait of tibia fracture patients, we also needed to detect abnormal steps such as when the foot begins in the air, strikes the ground and then the patient balances on that foot. In this case, the underfoot load data would by represented by a minimum value followed by a maximum value. In order to capture both of these types of steps, the algorithm was designed to disregard maxima that were not preceded or followed by a minimum of less than 20% of patient body weight within 1 s. Using the ALTAS system in the laboratory, we found that the baseline value of the system could shift up to 10% based on how tightly the walking boot straps were fastened. To account for the variability in strap tightness due to patients donning and doffing the boot each day, we doubled the observed 10% value as a safety factor.
Laboratory hardware and software verification
In order to simulate steps from patients, a cyclic loading system was designed. The system consists of three pneumatic cylinders controlled using a National Instruments LabView (Labview 2013, http://www.ni.com/labview/) system programmed for step simulation. The Labview software allows for customization of the number of loading cycles, time loaded or unloaded, and input waveform. In this simplified system, each strike of the disk piston on the ATLAS represents a step.
Additionally, a test waveform was created to represent underfoot load data. A piecewise sine wave consisting of four segments was programmed using MATLAB. Each segment contained varying frequency, amplitude, and/or offset values in order to simulate different walking conditions. The segments were designed to determine whether or not the algorithm could indeed filter miscellaneous activities in the loading data. The equation used to construct the waveform is presented in equation (1)
| (1) |
Study procedure
A total of 14 healthy volunteers (6 males, 8 females, age range 21–63 years) participated in the treadmill walking validation study. Each subject was fitted with the ATLAS instrumented CAM walker boot (right leg). Each trial consisted of a 2MWT on a Noraxon Scifit treadmill at a speed of 1.12 m/s. This speed was similar to speeds used in previous treadmill studies30,31 but slightly decreased to minimize potential difficulties in ambulation with the walking boot. While each patient walked, treadmill and ATLAS data were recorded. Each test was video recorded in order to collect a manual step count for each trial. Step counts recorded by the ATLAS and treadmill were compared to the number of steps determined by manually counting steps captured on the video, which was used as the reference for each trial. Steps determined by ATLAS were calculated by the ATLAS step counting algorithm and step counts from the treadmill were calculated by the Noraxon treadmill software. Each participant performed the test twice.
Data sets from tibia fracture patients were obtained from a study at the University of Utah Orthopaedic Center. Ten tibia fracture patients (6 males 4 females, age range 20–55 years) were recruited from this institution. Each patient was fitted with an ATLAS system that integrates with the clinically prescribed MaxTrax CAM walking boot. Each patient was instructed in the study procedures by a member of the clinical team.
ATLAS data
Data analysis was performed in MATLAB 2014a (Mathworks, www.mathworks.com). The objective of data analysis was to generate a loading curve, to identify individual steps and to determine step statistics from ATLAS-recorded underfoot load data. All of the ATLAS data analyzed by the algorithm consisted of the sum of the underfoot load from the three sensors (1 underneath the heel and 2 underneath the forefoot). Loading values obtained from patients were normalized for the patient's body weight from the beginning of the study. Step count data from the treadmill, ATLAS and video records were tabulated for each trial and average and standard deviation values were computed. The data were plotted, and correlation coefficients (linear regression) were calculated.
Results
Laboratory hardware verification
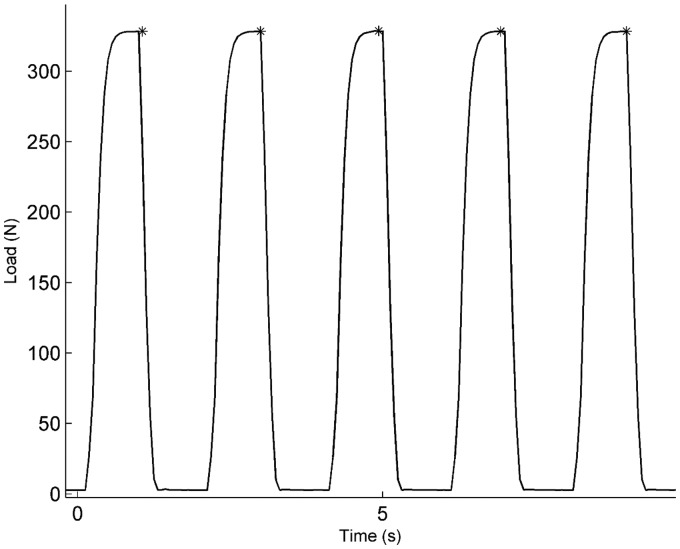
The total load recorded by ATLAS during a 10-s interval from the cyclic load test is shown in Figure 1. Five peak loads are observed with a baseline value between each maximum. The five asterisks indicate the five maxima values that were detected by the algorithm and interpreted as steps. The step counting algorithm detected 2000 out of 2000 cycles that were programmed for this experiment.
Figure 1.
Representative 10-s interval of ATLAS data during the cyclic loading test. Asterisks indicate maxima detected by the step counting algorithm. The number of cycles during this interval, 5, matches the number of “steps” detected by the ATLAS algorithm.
Laboratory verification using simulated loading curves
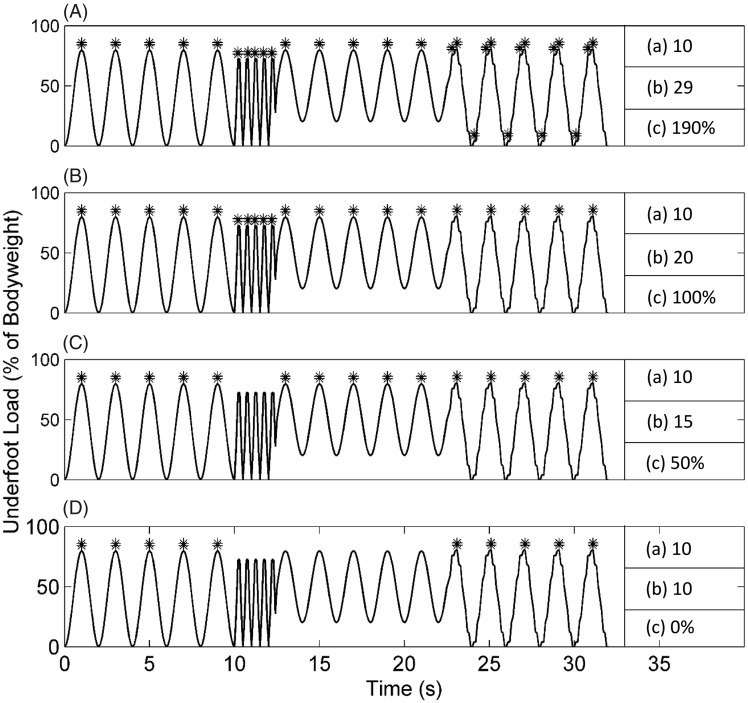
A sine wave with varying amplitudes and frequencies was created to simulate various walking conditions is shown in Figure 2. The number of steps detected by different conditions of the algorithm as well as the number of steps detected by the algorithm as a whole is also shown. When individual conditions of the algorithm were used, percent errors of 50% or greater were observed. The testing demonstrated that all three components of the step counting algorithm are necessary in order to obtain the correct number of steps.
Figure 2.
Simulated steps detected from an artificial waveform representing ambulation. The four subplots used different peak detection conditions: (A) constant small threshold, (B) first condition of the algorithm, (C) first and second conditions of the algorithm, (D) all three conditions of the algorithm. Asterisks at the waveform peaks indicate the detected simulated steps. (a) Indicates number of maxima expected, (b) maxima detected, and (c) percent error. Only the combination of all three conditions of the algorithm resulted in the correct step count.
Validation of step counting software using treadmill
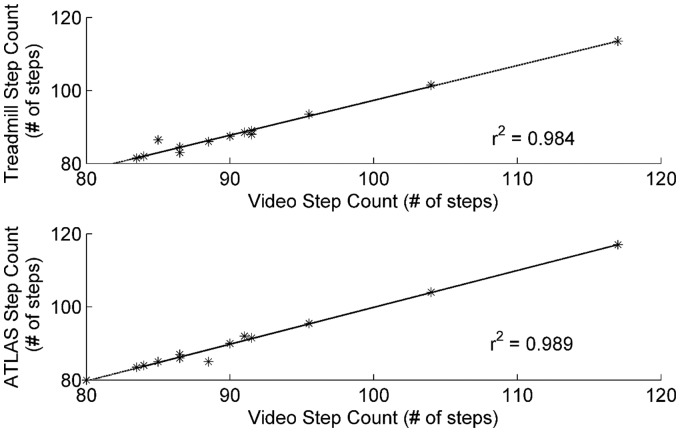
During the 2MWT, the treadmill, ATLAS, and video count detected footsteps of 89 ± 9 steps, 91 ± 10 steps, and 91 ± 10 steps, respectively. Figure 3 shows the correlation between the calculated number of steps from each device with respect to the actual number of steps derived from video analysis. The number of steps as detected by the ATLAS had the highest correlation with the actual number of steps taken with an r2 value of 0.989. The number of steps as calculated by treadmill was also highly correlated to the actual number of steps with an r2 value of 0.984.
Figure 3.
ATLAS step count is highly correlated to the actual step count. The highest correlation with the reference values was recorded for the ATLAS (r2 = 0.989), with the treadmill following closely (r2 = 0.984).
Continuous recovery data
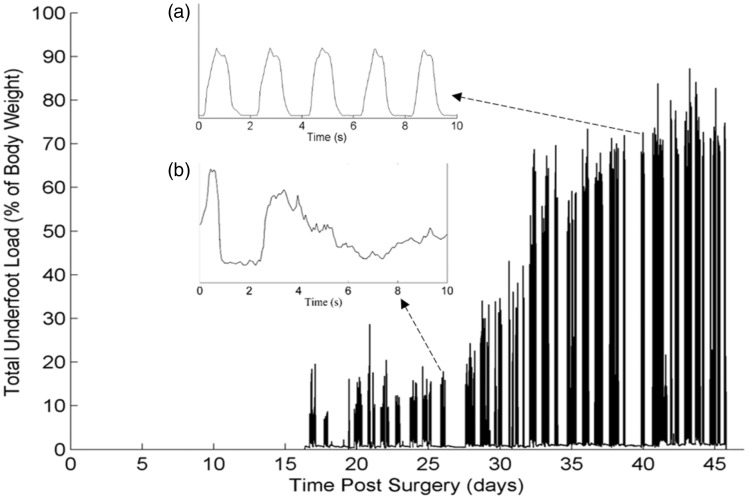
In this section, examples of the application of the step counting algorithm in two patients are discussed. Figure 4 shows 28 days of continuous loading data obtained in tibia fracture Patient 1. The load values for this patient begin 17 days after surgery when the patient was first instructed to wear the ATLAS. During the first two weeks of ATLAS use, loading values never exceeded 50% of the patient's bodyweight. However, after additional weeks of recovery, this patient was weight bearing at nearly 90% of their bodyweight. Inset graphs (A) and (B) represent the differences in ambulation that occur over time. The underfoot load while ambulating is irregular and variable 9 days after surgery, but becomes more patterned and consistent 42 days after surgery.
Figure 4.
Progressive nature of weight bearing during the rehabilitation period of a tibia fracture patient. The patient began wearing the boot 17 days after surgery. Insert (a) shows a 10-s segment of data from day 26, and insert (b) shows a 10 s segment of data from day 40.
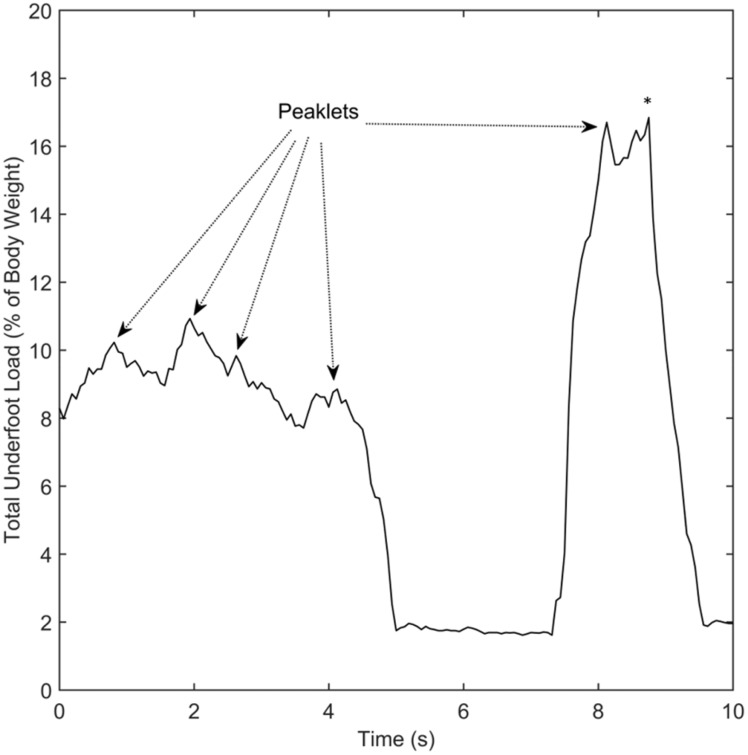
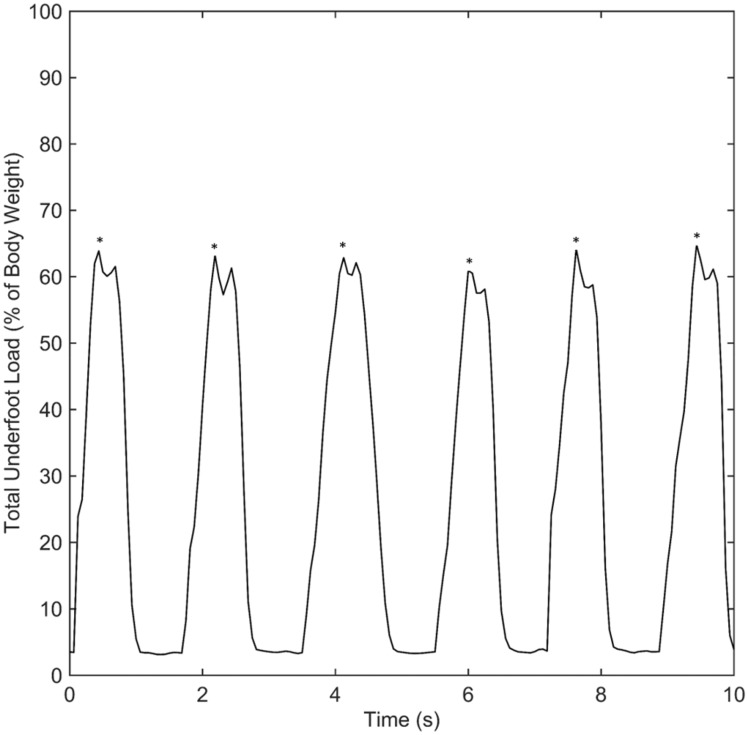
Practical application of the step counting algorithm to patient data is shown in Figures 5 and 6. In Figures 5 and 6, asterisks indicate steps detected by the algorithm. Figure 5 shows the total underfoot load during a 10-s period from the early stage of healing. The data appear disordered and only one step was detected during this period. During the first 5 s of this data, the total underfoot load varies irregularly from 8% of body weight to approximately 11% of body weight. This results in numerous peaklets (local maxima) which lack the loading profile typically seen during ambulation in a walking boot. In contrast, the 10 s of data from the late stage of healing shown in Figure 6 depict loading values which relatively consistently varied from 6% of body weight to 66%. In this case, six steps were detected from the dynamically varying data and the overall profile of loading during each step is similar in magnitude and duration. The six steps in Figure 6 all contain the same pattern of two peaklets, representing loading of the heel and forefoot sensors during each step, while the single step detected in Figure 5 has several peaklets during the step. Overall, there are qualitative differences in the nature of ambulation and loading values between the early and late stages of healing.
Figure 5.
Example of irregular loading pattern at relatively low percentage of bodyweight during the early stage of rehabilitation. This graph shows the percentage of loading normalized to body weight over a 10-s interval. Examples of peaklets resulting from behavior that does not appear to be ambulation are denoted by dashed arrows. The algorithm disregarded the peaklets and detected a single step, denoted by the (*), in this portion of the data.
Figure 6.
Example of regular load pattern at a relatively high percentage of bodyweight during the late stage of rehabilitation. This 10-s interval of data shows the relatively consistent and patterned waveform seen in most patients during the late stages of healing. Overall, the features of the waveform are similar to that normal ambulation in a walking boot. The (*) above the peak indicates the six steps detected by the algorithm in this portion of the data.
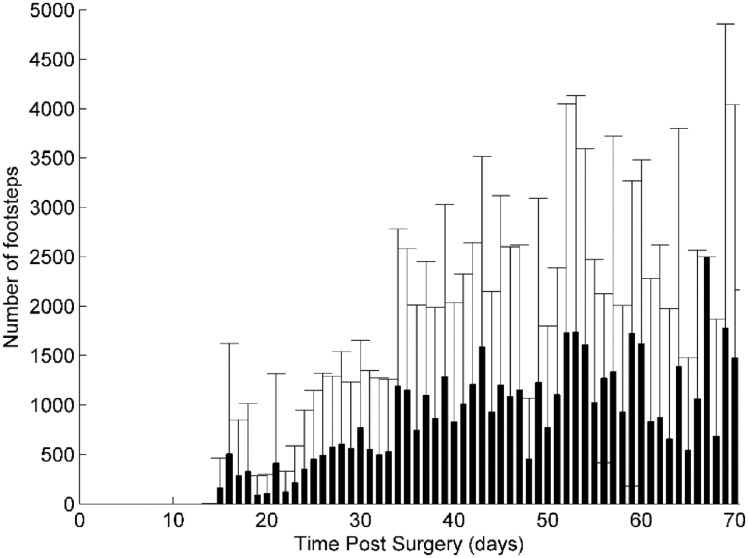
An overview of the number of steps taken per day by 10 patients (mean, SD) is shown in Figure 7. The large standard deviation values (up to ∼3000 steps/day) indicate the diversity of patient behavior during partial weight-bearing rehabilitation. Overall, there appears to be an increase in the mean number of steps taken per day during the first five to six weeks after surgery. After this time period, the mean number of steps taken per day intermittently increases and decreases. Despite the lack of a continuing trend, the mean number of steps per day is generally greater than 1000 steps per day after two weeks of recovery as compared to the first two weeks.
Figure 7.
Average number of footsteps taken per day by 10 patients. Large standard deviations indicate a wide variety of patient ambulatory behavior. The number of steps taken per day tends to increase during the first five weeks and is followed by intermittent increases and decreases.
Discussion
Ambulation may be an influential factor in the healing of lower extremity fractures and could also be used as a monitoring tool to measure the progression of fracture healing.13,32 However, the ambulatory behavior of tibia fracture patients remains unknown due to an inability to continuously and reliably quantify ambulation outside of a clinical setting. Our goal was to develop an algorithm to assess ambulation in tibia fracture patients, outside of the clinic, during recovery in a CAM walker. Our results indicated that a reliable algorithm to count steps from ATLAS underfoot load data was developed and validated. To the best of our knowledge, this is the first report on the out-of-clinic ambulatory behavior of tibia fracture patients from the first day of recovery in a CAM walker to the day a clinician determined that the fracture was healed and the CAM boot was no longer needed.
The accuracy and reliability of the algorithm were verified using hardware and software in the laboratory to simulate two simplified models of ambulation. When a cyclic loading system was used to simulate ambulation, the algorithm correctly identified all of the simulated steps (Figure 1). Similarly, when a loading waveform containing data representing both footsteps and miscellaneous activities was input into the algorithm, the algorithm filtered out extraneous activities and correctly identified the simulated steps (Figure 2). These experiments indicated that the algorithm could reliably identify footsteps in simplified models of ambulation. In addition, validation testing was performed on healthy volunteers. Our results showed that the algorithm derived step count had a high correlation (r2 = 0.989) with the actual number of steps taken as determined by a manual video count (Figure 3), indicating that the algorithm could accurately detect steps taken in a CAM walker boot during walking. Comparing the accuracy of our device to common commercial devices, the accuracy of our step counting algorithm is greater than that of the wrist worn FitBit Flex, but similar to the hip worn FitBit One. Previous validation and reliability studies performed on a treadmill have shown that step counts from the FitBit Flex and Fitbit One are moderately correlated (0.77 ≤ r2 ≤ 0.85)24 and highly correlated (0.97 ≤ r2 ≤ 0.99),33,34 respectively, to the manual step count.
The collection of continuous, out-of-clinic, underfoot load data from tibia fracture patients revealed specific patient behavior and provided insight into the progressive nature of weight bearing over time (Figure 4). While previous studies have captured discrete changes in weight bearing,35,36 this study provided the first continuous picture of weight bearing during a period of 28 days. From day 17 to day 45, there was an overall nonlinear increase in weight bearing. Within that period, there were consecutive days where the increase in weight bearing was followed by a sudden decrease as seen in days 21 to 22 and days 34 to 35. One explanation for this behavior may be that the patient over-loaded the injured limb leading to pain which is reflected by the subsequent decrease in weight bearing. When the weight-bearing data were analyzed with a time scale in seconds, ambulatory behavior was revealed (Figure 4(a) and (b)).
Application of the step counting algorithm to data obtained continuously by the ATLAS system qualitatively showed that ambulation during the initial stages of recovery (Figure 5) differed from ambulation in later stages of recovery (Figure 6). While the loading curve in Figure 5 contained varying maxima values, the loading curve in Figure 6 appeared to be regular with consistent maxima values. The variance in initial loading (Figure 5) may be due to the patient continuously adjusting the amount of load on the injured limb due to pain (such as shuffling) or attempting to partially weight bear while using crutches. Previous studies have also found a difference in gait in the initial stages of recovery compared to the later stages of recovery.13,37 However, the aforementioned studies were performed on an animal model or assessed gait at discrete time points, in a clinical setting. Thus, results from these studies may not be representative of patient community ambulatory behavior. Our results may be more representative of actual patient ambulation since they were based on measurements obtained continuously, outside of a clinical setting. Since new literature has pointed towards a correlation between the quality of gait and fracture healing,13 clinicians may be able to utilize the step counting algorithm to gain objective insight into continuous, personalized fracture healing.
Our overall assessment of ambulatory behavior revealed much variance in the number of steps taken between patients during recovery. We believe this is the first report of a continuous step count for tibia fracture patients obtained outside of a clinical setting. A general increase in the daily step count was observed (Figure 7) for the first five weeks of use followed by intermittent increases and decreases during the following three weeks. One possible explanation for this behavior is that a subset of patients may have been non-compliant and wore the ATLAS instrumented CAM walker less consistently once they were able to ambulate comfortably without the CAM walker (around week 5). In addition, there was a large standard deviation for each data point in this group of 10 patients. This variance between patients underscores the fact that patients have different behaviors and may respond differently to prescribed rehabilitative protocols. Instead of a standardized protocol for given fracture types,10 individualized rehabilitative protocols based on ambulatory data obtained by the algorithm may optimize healing outcomes. Despite a paucity of data on patient ambulation during recovery outside of a clinical setting, our findings are similar to the most current results in the literature. While Braun et al.23 only performed measurements for a total of six weeks, they also observed that the average time spent active by a group of 10 ankle fracture patients increased overtime and that there was a relatively large standard deviation in the time spent active. Time spent active may include ambulation by both the healthy limb and the injured limb. However, literature has shown that loading and ambulation on the injured limb promote healing36,38 and therefore time spent active may be an imprecise measure of ambulation experienced by the injured limb. Further studies with an increased number of patients may reveal how ambulation affects healing and allows for scientifically derived activity suggestions to promote fracture healing.
One of the limitations of this study was that validation of the step counting algorithm was only performed with healthy subjects for a trial duration of 2 min. The 2MWT is commonly used to determine ambulatory capacity of unhealthy individuals, due to the practicality and efficiency of the test.39,40 While there is no established protocol for validation of a step counting system, previous studies have used walking durations of 1–3 min.41–43 As a result, the authors decided the 2MWT was a feasible protocol to validate the step counting algorithm. Another limitation is our inability to report on ambulation when patients are non-compliant and do not wear the ATLAS. It is possible that a recovering patient can take numerous steps without wearing ATLAS, which would not be detected by the algorithm. While we cannot control the compliance of patients, we can continuously monitor the underfoot load and speculate when patients do not wear the ATLAS based on long periods of no loading. In other words, the ATLAS system could also be used as a tool to measure patient compliance. Lastly, we did not give wearable sensors to the tibia fracture patients during the recovery period. However, it is important to note that no activity monitor has been validated in a tibia fracture patient model and as a result we would have been unable to make objective comparisons between our device and one that is not intended for this purpose. In addition, prescribing activity monitors to tibia fracture patients is not part of the current standard of care, whereas the CAM walker boot is commonly prescribed by orthopaedic clinicians. While prescribing the use of an activity monitor may have been a major change to established protocols, integration of the ATLAS into the sole of the CAM Walker was a minimal modification to the current post-surgical standards.
In summary, the algorithm reported here provided a means to assess patient ambulation and may be used in the future to connect patients and care providers to guide therapy and monitor rehabilitation. We have shown that our algorithm can be applied to ATLAS underfoot load data to continuously count the number of steps taken and provided an assessment of the ambulatory behavior of tibia fracture patients. The algorithm revealed a qualitative difference in gait during the initial stages of recovery compared to the later stages of recovery. Additionally, a relatively large variance in daily ambulation within a group of 10 patients over a six-week period was observed, suggesting differences in ambulatory behavior. In the future, the ambulatory behavior of patients, as derived by our algorithm, may be used by clinicians to develop personalized healing regimens, which could allow patients to resume their normal activities more quickly and lower surgical revision rates. Future studies correlating radiographic measures of healing, such as the RUST score,44 to ambulation may also provide additional evidence for the use of ambulation as a measure for fracture healing.
Acknowledgements
We would like to thank Mikayla Lyman and Andrea Gurule their assistance in recruiting patients. The authors also thank Johanna de Gennaro and Alex Singleton for their assistance in manufacturing the ATLAS sensors.
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The only conflict of interest is that the coauthors EK, TP, and RH have patents related to the load monitoring technology described in this manuscript.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the U.S. Department of Defense (DOD) CDMRP under Award No. W81XWH1220089 and Award No.W81XWH1220087. The opinions, interpretations, conclusions, and recommendations of this manuscript are those of the authors and are not necessarily endorsed by the Department of Defense.
Guarantor
RH
Contributorship
ALK contributed to study initiation, data collection, monitoring study progress, choice of analytic strategy, manuscript preparation, critical revisions for intellectual content and final manuscript approval.
BT contributed to monitoring study progress, choice of analytic strategy, manuscript preparation, critical revisions for intellectual content and final manuscript approval.
AS contributed to study conception and design, study initiation, data collection, monitoring study progress, critical revisions for intellectual content and final manuscript approval.
SS contributed to study conception and design, study initiation,, critical revisions for intellectual content and final manuscript approval.
MA contributed to data collection, monitoring study progress, critical revisions for intellectual content and final manuscript approval.
EK contributed to study conception and design, study initiation, monitoring study progress, critical revisions for intellectual content and final manuscript approval.
TP contributed to study conception and design, study initiation, monitoring study progress, critical revisions for intellectual content and final manuscript approval.
BH contributed to study conception and design, study initiation, monitoring study progress, critical revisions for intellectual content and final manuscript approval.
References
- 1.Miller NC, Askew AE. Tibia fractures. An overview of evaluation and treatment. Orthop Nurs 2007; 26: 216–223. quiz 224–215. [DOI] [PubMed] [Google Scholar]
- 2.Anandasivam NS, Russo GS, Swallow MS, et al. Tibial shaft fracture: a large-scale study defining the injured population and associated injuries. J Clin Orthopaed Trauma 2017; 8: 225–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Audige L, Griffin D, Bhandari M, et al. Path analysis of factors for delayed healing and nonunion in 416 operatively treated tibial shaft fractures. Clin Orthop Relat Res 2005; 438: 221–232. [DOI] [PubMed] [Google Scholar]
- 4.Riemer BL, DiChristina DG, Cooper A, et al. Nonreamed nailing of tibial diaphyseal fractures in blunt polytrauma patients. J Orthop Trauma 1995; 9: 66–75. [DOI] [PubMed] [Google Scholar]
- 5.Antonova E, Le TK, Burge R, et al. Tibia shaft fractures: costly burden of nonunions. BMC Musculoskeletal Disord 2013; 14: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghiasi MS, Chen J, Vaziri A, et al. Bone fracture healing in mechanobiological modeling: a review of principles and methods. Bone Rep 2017; 6: 87–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buckwalter JA, Grodzinsky AJ. Loading of healing bone, fibrous tissue, and muscle: implications for orthopaedic practice. J Am Acad Orthop Surg 1999; 7: 291–299. [DOI] [PubMed] [Google Scholar]
- 8.Cornell CN, Lane JM. Newest factors in fracture healing. Clin Orthop Relat Res 1992, pp. 297–311. [PubMed] [Google Scholar]
- 9.Marsh DR, Li G. The biology of fracture healing: optimising outcome. Br Med Bull 1999; 55: 856–869. [DOI] [PubMed] [Google Scholar]
- 10.Kubiak EN, Beebe MJ, North K, et al. Early weight bearing after lower extremity fractures in adults. J Am Acad Orthopaed Surgeons 2013; 21: 727–738. [DOI] [PubMed] [Google Scholar]
- 11.Green DP, Rockwood CA, Bucholz RW, et al. Rockwood and green's fractures in adults, Philadelphia, PA: Lippincott Williams & Wilkins, 2010. [Google Scholar]
- 12.Morshed S. Current options for determining fracture union. Adv Med 2014; 2014: 708574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Macri F, Marques L, Backer R, et al. Validation of a standardised gait score to predict the healing of tibial fractures. J Bone Joint Surg Br 2012; 94: 544–548. [DOI] [PubMed] [Google Scholar]
- 14.Kwasnicki R, Hettiaratchy S, Okogbaa J, et al. Return of functional mobility after an open tibial fracture. Bone Joint J 2015; 97: 1118–1125. [DOI] [PubMed] [Google Scholar]
- 15.Vasarhelyi A, Baumert T, Fritsch C, et al. Partial weight bearing after surgery for fractures of the lower extremity – is it achievable?. Gait Posture 2006; 23: 99–105. [DOI] [PubMed] [Google Scholar]
- 16.Hsiao H, Guan J, Weatherly M. Accuracy and precision of two in-shoe pressure measurement systems. Ergonomics 2002; 45: 537–555. [DOI] [PubMed] [Google Scholar]
- 17.Hurkmans HL, Bussmann JB, Benda E, et al. Accuracy and repeatability of the Pedar Mobile system in long-term vertical force measurements. Gait Posture 2006; 23: 118–125. [DOI] [PubMed] [Google Scholar]
- 18.Saito M, Nakajima K, Takano C, et al. An in-shoe device to measure plantar pressure during daily human activity. Med Eng Phys 2011; 33: 638–645. [DOI] [PubMed] [Google Scholar]
- 19.Crea S, Donati M, De Rossi SMM, et al. A wireless flexible sensorized insole for gait analysis. Sensors 2014; 14: 1073–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klosterhoff BS, Ong KG, Krishnan L, et al. Wireless implantable sensor for non-invasive, longitudinal quantification of axial strain across rodent long bone defects. J Biochem Eng 2017; 139: 142778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGilvray KC, Unal E, Troyer KL, et al. Implantable microelectromechanical sensors for diagnostic monitoring and post-surgical prediction of bone fracture healing. J Orthopaed Res 2015; 33: 1439–1446. [DOI] [PubMed] [Google Scholar]
- 22.Seide K, Aljudaibi M, Weinrich N, et al. Telemetric assessment of bone healing with an instrumented internal fixator. J Bone Joint Surg Br 2012; 94: 398–404. [DOI] [PubMed] [Google Scholar]
- 23.Braun BJ, Bushuven E, Hell R, et al. A novel tool for continuous fracture aftercare – clinical feasibility and first results of a new telemetric gait analysis insole. Injury 2016; 47: 490–494. [DOI] [PubMed] [Google Scholar]
- 24.Diaz KM, Krupka DJ, Chang MJ, et al. Fitbit®: an accurate and reliable device for wireless physical activity tracking. Int J Cardiol 2015; 185: 138–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Storm FA, Heller BW, Mazza C. Step detection and activity recognition accuracy of seven physical activity monitors. PLoS One 2015; 10: e0118723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.North K, Kubiak EN, Hitchcock RW. Sensor packaging design for continuous underfoot load monitoring. Biomed Microdev 2012; 14: 217–224. [DOI] [PubMed] [Google Scholar]
- 27.North K, Kubiak EN, Hitchcock RW, et al. Load monitoring system for partial weight bearing therapy for rehabilitation of lower extremity fractures. Conf Proc IEEE Eng Med Biol Soc 2013; 2013: 152–155. [DOI] [PubMed] [Google Scholar]
- 28.North K, Potter MQ, Kubiak EN, et al. The effect of partial weight bearing in a walking boot on plantar pressure distribution and center of pressure. Gait Posture 2012; 36: 646–649. [DOI] [PubMed] [Google Scholar]
- 29.Oberg T, Karsznia A, Oberg K. Basic gait parameters: reference data for normal subjects, 10-79 years of age. J Rehabil Res Dev 1993; 30: 210–223. [PubMed] [Google Scholar]
- 30.Case MA, Burwick HA, Volpp KG, et al. Accuracy of smartphone applications and wearable devices for tracking physical activity data. JAMA 2015; 313: 625–626. [DOI] [PubMed] [Google Scholar]
- 31.Kooiman TJ, Dontje ML, Sprenger SR, et al. Reliability and validity of ten consumer activity trackers. BMC Sports Sci Med Rehab 2015; 7: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosenbaum D, Macri F, Lupselo FS, et al. Gait and function as tools for the assessment of fracture repair – the role of movement analysis for the assessment of fracture healing. Injury 2014; 45: S39–S43. [DOI] [PubMed] [Google Scholar]
- 33.Takacs J, Pollock CL, Guenther JR, et al. Validation of the Fitbit One activity monitor device during treadmill walking. J Sci Med Sport 2014; 17: 496–500. [DOI] [PubMed] [Google Scholar]
- 34.Lauritzen J, Muñoz A, Sevillano L, et al. The usefulness of activity trackers in elderly with reduced mobility: a case study. Stud Health Technol Inform 2013; 192: 759–762. [PubMed] [Google Scholar]
- 35.Joslin CC, Eastaugh-Waring SJ, Hardy JRW, et al. Weight bearing after tibial fracture as a guide to healing. Clin Biomech 2008; 23: 329–333. [DOI] [PubMed] [Google Scholar]
- 36.Aranzulla P, Muckle D, Cunningham J. A portable monitoring system for measuring weight-bearing during tibial fracture healing. Med Eng Phys 1998; 20: 543–548. [DOI] [PubMed] [Google Scholar]
- 37.Seebeck P, Thompson M, Parwani A, et al. Gait evaluation: a tool to monitor bone healing?. Clin Biomech 2005; 20: 883–891. [DOI] [PubMed] [Google Scholar]
- 38.Epari D, Duda G, Thompson M. Mechanobiology of bone healing and regeneration: in vivo models. Proc Inst Mech Eng Part H 2010; 224: 1543–1553. [DOI] [PubMed] [Google Scholar]
- 39.Brooks D, Parsons J, Hunter JP, et al. The 2-minute walk test as a measure of functional improvement in persons with lower limb amputation. Arch Phys Med Rehab 2001; 82: 1478–1483. [DOI] [PubMed] [Google Scholar]
- 40.Parker K, Kirby RL, Adderson J, et al. Ambulation of people with lower-limb amputations: relationship between capacity and performance measures. Arch Phys Med Rehab 2010; 91: 543–549. [DOI] [PubMed] [Google Scholar]
- 41.Braun BJ, Veith NT, Hell R, et al. Validation and reliability testing of a new, fully integrated gait analysis insole. J Foot Ankle Res 2015; 8: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Foster RC, Lanningham-Foster LM, Manohar C, et al. Precision and accuracy of an ankle-worn accelerometer-based pedometer in step counting and energy expenditure. Prevent Med 2005; 41: 778–783. [DOI] [PubMed] [Google Scholar]
- 43.Shepherd EF, Toloza E, McClung CD, et al. Step activity monitor: increased accuracy in quantifying ambulatory activity. J Orthopaed Res 1999; 17: 703–708. [DOI] [PubMed] [Google Scholar]
- 44.Whelan DB, Bhandari M, Stephen D, et al. Development of the radiographic union score for tibial fractures for the assessment of tibial fracture healing after intramedullary fixation. J Trauma Acute Care Surg 2010; 68: 629–632. [DOI] [PubMed] [Google Scholar]