Abstract
Objectives
Aging increases the risk for development of adipose tissue dysfunction, insulin resistance, dyslipidemia, and liver steatosis. Lipocalin 2 (Lcn2) deficient mice are more prone to diet-induced obesity and metabolic dysfunction, indicating a protective role for Lcn2 in younger mice. In this study, we determined whether overexpressing Lcn2 in adipose tissue can protect against age-related metabolic deterioration.
Methods
We developed ap2-promoter-driven Lcn2 transgenic (Tg) mice and aged Lcn2 Tg mice for the metabolic assessments.
Results
We found decreased adipocyte size in inguinal white adipose tissue (iWAT) from 10-month-old Lcn2 Tg mice relative to WT. This was accompanied by increased markers of adipogenesis in iWAT and attenuation of the age-related decline in AMP-activated protein kinase (AMPK) phosphorylation in adipose tissue depots. In addition to improvements in adipose tissue function, whole-body metabolic homeostasis was maintained in aged Lcn2 Tg mice. This included improved glucose tolerance and reduced serum triglycerides in older Lcn2 Tg mice relative to WT mice. Further, liver morphology and liver lipid levels were improved in older Lcn2 Tg mice, alongside a decrease in markers of liver inflammation and fibrosis.
Conclusions
We demonstrate that overexpression of Lcn2 in adipose tissue not only preserves adipose tissue function during aging but also promotes maintenance of glucose tolerance, decreases dyslipidemia, and prevents liver lipid accumulation and steatosis.
Keywords: Lipocalin 2, Adipose tissue, Adipose beiging, Healthspan
Highlights
-
•
Lcn2 overexpression in adipose tissue promotes beiging of iWAT and BAT mitochondrial oxidation.
-
•
Lcn2 overexpression in adipose tissue improves thermogenic adaptation to cold in younger mice.
-
•
Adipose Lcn2 overexpression prevents age-related decline in thermogenic gene expression in brown and beige fat tissue.
-
•
Lcn2 overexpression in adipose tissue preserves adipose tissue function during aging.
-
•
Adipose Lcn2 overexpression promotes metabolic homeostasis and prevents age-related liver lipid accumulation.
1. Introduction
Aging is the greatest risk factor for perturbations in metabolism including insulin resistance, dyslipidemia, and inflammation [1], [2], [3], [4]. Increasing adiposity and ectopic fat accumulation along with decreased lean mass contribute to the development of inflammation and insulin resistance in older humans [3], [5], [6]. Unlike white adipose tissue (WAT), the presence and activity of brown adipose tissue (BAT) and beige adipocytes in WAT is decreased in aging [7], [8], [9], [10], [11]. As brown and beige adipocytes play an important antidiabetic role in controlling glucose and lipid homeostasis through thermogenesis-dependent and -independent mechanisms such as endocrine functions of brown and beige fat [12], [13], [14], [15], [16], [17], [18], [19], this decline may contribute to age-related metabolic deterioration.
Brown adipose tissue (BAT), a major tissue responsible for adaptive thermogenesis, functions in burning fat to generate heat via a UCP1-mediated non-shivering thermogenic mechanism [20]. Increased fat oxidation to support this process leads to increases in whole body energy expenditure. Brown-like adipocytes or “beige” adipocytes in WAT depots have higher mitochondrial number, increased UCP1 expression, and are thermogenically competent [21], [22]. Beige adipose tissue activation also contributes to increased energy expenditure and oxidation of fatty acids and glucose during non-shivering thermogenesis [22]. In addition to cold, several circulating factors have been identified as inducing thermogenesis in adipose tissue. For example, fibroblast growth factor 21 (FGF21) secreted from adipose tissue and cardiac natriuretic peptides both promote expression of UCP1 in WAT [23], [24]. BAT has also been identified in the supraclavicular and neck region of humans, and presence of these depots is associated with a lower body mass index (BMI) [7], [11]. Further, humans exposed to cold have increased blood flow and glucose uptake in BAT together with increased energy expenditure [25]. Interestingly, cells from human BAT depots express a genetic profile more similar to rodent beige adipocytes as opposed to brown adipocytes [26]. This suggests that increasing beiging of WAT is a potential therapeutic target for increasing energy expenditure and preventing obesity.
Lipocalin 2 (Lcn2) is a circulating factor secreted from adipose tissue in response to obesity, inflammation, and nutrient/growth signals [27]. Our group has found that mice lacking Lcn2 gain more weight on a high-fat diet (HFD), develop more severe insulin resistance, and have impaired thermogenesis [28], [29]. Law et al. found an opposite phenotype, wherein mice lacking Lcn2 are protected from developing insulin resistance in response to high fat diet [30]. An additional study found only slight differences in insulin sensitivity between wild-type (WT) and Lcn2 knock-out mice [31]. More recently, bone-derived Lcn2 has been shown to improve insulin sensitivity and regulate food intake by suppressing appetite [32]. This study supports a role for Lcn2 in the prevention of obesity and metabolic complications.
Previous studies utilized whole-body knock-out of Lcn2, so the exact contribution adipose tissue-derived Lcn2 makes to this phenotype is unknown. Further, there are no studies examining the role of Lcn2 overexpression, specifically in adipose tissue, on regulating whole-body metabolism, which is important for fully understanding the metabolic role of Lcn2. Additionally, Lipocalin 2 (Lcn2) protein levels are increased in visceral adipose tissue of aged mice and in plasma of older humans, suggesting a role for Lcn2 in aging [33], [34]. However, its role in metabolic healthspan and whether Lcn2 protects against age-related metabolic diseases has not been well-studied. Therefore, the objective of this study is to determine whether Lcn2 overexpression in adipose tissue can have beneficial effects and prevent the deleterious changes in whole-body metabolism that are associated with aging. We developed ap2-driven Lcn2 transgenic mice and utilized male mice to analyze markers of metabolic healthspan.
2. Materials and methods
2.1. Generation of adipocyte fatty acid-binding protein (aP2) gene promoter-driven Lcn2 transgenic mice
To generate aP2-promoter-driven Lcn2 transgenic mice, a full length Lcn2 cDNA fragment was cloned to a pCI expression vector containing the 5.5 kb mouse aP2 promoter/enhancer by XhoI and Not I sites. The final plasmid is designated paP2–Lcn2. The integrity of the plasmid was confirmed by DNA sequencing of the ligation junctions.
The ap2-Lcn2 transgenic construct was digested with PvuI, after which the cloning vector and transgenic fragment were separated by agarose gel electrophoresis. The transgene was purified using a Qiagen plasmid purification kit (Qiagen) and then microinjected into the pronuclei of fertilized eggs collected from C57BL/6N mice (Charles River) at 2 μg/ml in 10 mM Tris–HCl (pH7.5), 0.1 mM EDTA, and 100 mM NaCl. After culturing overnight in M16 culture medium (Millipore), those embryos reaching 2-cell stage of development were implanted into the oviducts of pseudopregnant foster mothers. Offspring born to the foster mothers were genotyped by PCR. Two transgenic founder mice were identified by PCR genotyping (Supplemental Fig. 1A). The transgenic strain with aP2-Lcn2 transgene was maintained by mating a hemizygous mouse (Tg/0) to C57BL/6J wild-type (+/+) mice, purchased from Jackson Laboratory (Bar Harbor, ME). This generated hemizygous (Tg/0) mice and non-carriers (0/0). Every mouse was genotyped to distinguish the hemizygotes and non-carriers. The age- and sex-matched non-carriers served as the controls of transgenic mice in the experiments.
2.2. Animals
Animals were housed at 22 °C with free access to water in a specific pathogen-free facility at the University of Minnesota. Animal studies were conducted with the approval of the University of Minnesota Animal Care and Use Committee and conformed to the National Institute of Health guidelines for laboratory animal care. For cold exposure, mice were fasted overnight prior to being placed in individual cages at 4 °C with free access to water. Rectal temperature was recorded every 30 min for 4 h. For the aging study, mice were fed a regular chow diet for the duration of the study and sacrificed at 10 or 18 months of age following an overnight fast. For the study with LPS stimulation, 3 month- and 10 month-old WT male mice were given 0.3 mg/kg of LPS via intraperitoneal injection. After 6 h of treatment, mice were sacrificed for blood and tissue collection. The age-matched mice with i.p. injection of saline served as controls.
2.3. Glucose tolerance test (GTT) and insulin tolerance test (ITT)
Mice were fasted overnight (18 h) prior to the GTT or fasted for 6 h prior to the ITT. Mice were given intraperitoneal glucose (0.75 g/kg) or insulin (0.75 units/kg) injections at time 0. Blood glucose levels were monitored using an Ascensia Contour glucometer (Ascensia Diabetes Care, Parsippany, NJ) at 0-, 15-, 30-, 60-, 90-, and 120-minutes following glucose or insulin injection.
2.4. Metabolic phenotyping
Male WT and Lcn2 Tg mice were fed a RCD. At 16 weeks of age, mice were individually caged and food intake was measured over a 5-day period using a Biodaq food intake monitoring system (Research Diets Inc., New Brunswick, NJ). The final 2 days of food intake monitoring were averaged to determine total food intake in grams/day. Indirect calorimetry was measured over a 3-day period using the Oxymax Comprehensive Lab Monitoring System (Columbus Instruments, Columbus, OH). The final light and dark cycle were used to statistically determine differences in energy expenditure between genotypes.
2.5. Histology and immunohistochemistry of UCP1
Tissues were fixed in 10% neutral buffered formalin (Thermo Scientific, Rockford, IL) and embedded in paraffin. After deparaffinization and rehydration, tissues were sectioned and stained with hematoxylin and eosin (H&E) using a standard protocol. For immunohistochemistry of UCP1in inguinal adipose tissue, sections were incubated with 0.3% H2O2 and then blocked with 3% BSA in Tris-buffered saline buffer (TBS; pH 7.4), followed by incubation with primary antibody against UCP1 (Abcam, ab10983) overnight at 4 °C. The bound antibodies were detected using the R.T.U. Vectastain universal ABC kit (Vector Laboratories, Burlingame, CA) and ImmPACT DAB peroxidase substrate kit (Vector Laboratories).
2.6. Serum assays
Serum insulin, Lcn2, and Leptin were measured by the Mouse Insulin ELISA kit (Thermo Scientific, Frederick, MD), Mouse Lcn2 ELISA kit (Biolegend, San Diego, CA), and a Quantikine ELISA kit for mouse leptin (R&D Systems, Minneapolis, MN), respectively. Serum triglycerides and β-hydroxybutyrate were measured using the Stanbio LiquiColor® Triglyceride and β-hydroxybutyrate kit (Stanbio, Boerne, Texas), respectively. All assays were performed following the manufacturer's instruction.
2.7. Triglycerides and Cholesterol in liver
Lipids were extracted from frozen liver (100 mg) using the Bligh-Dyer method. Triglyceride and Cholesterol levels were measured using the Stanbio LiquiColor® Triglycerides and Cholesterol kit (Stanbio, Boerne, Texas), respectively.
2.8. Quantitative real-time PCR
Total RNA was isolated from tissues using TRIZOL reagent (Invitro, Carlsbad, CA). RNA was DNAase-treated prior to the synthesis of cDNA using Superscript II reverse transcription kit (Invitrogen, Carlsbad, CA). Real-time quantitative PCR was conducted using SYBR Green qPCR Master Mix (SABiosciences, Frederick, MD) with a StepOne Real-time PCR System (Applied Biosystem, Foster City, CA). The ΔΔCt method was used to calculate mRNA expression and Tbp or Cyclophillin served as an internal control. The primer sequences for amplifying the target genes are summarized in Table S1.
2.9. Western blot analysis
Equivalent amounts of protein were run on an SDS-PAGE gel and transferred to a nitrocellulose membrane prior to incubation with primary and secondary antibodies. The sources of primary antibodies are as follows: Lcn2 and UCP1 (R&D systems, Minneapolis, MN); β-actin, adiponectin, PPARγ, AMPKα, and phosphorylated AMPKα (Thr172) (Cell Signaling Technology, Danvers, MA). Secondary antibodies are from R&D Systems (Minneapolis, MN). ECL western blotting substrate (Pierce, Rockford, IL) was used to detect reactivity. Densitometry results were quantified by Image J software and presented as a ratio of targeted protein to the corresponding β-actin.
2.10. Statistical analysis
Values are reported as mean +/− standard error of the mean (SEM). Statistical significance was determined by two-tailed Student's t test, where a P value less than 0.05 was considered significant. For statistical analysis of the indirect calorimetry data, repeated-measure ANCOVA was used. The analysis included the absolute values with the associated body weight used as a covariate and the data presented as least-square means.
3. Results
3.1. Overexpression of Lcn2 in adipose tissue improves thermogenic adaptation to cold
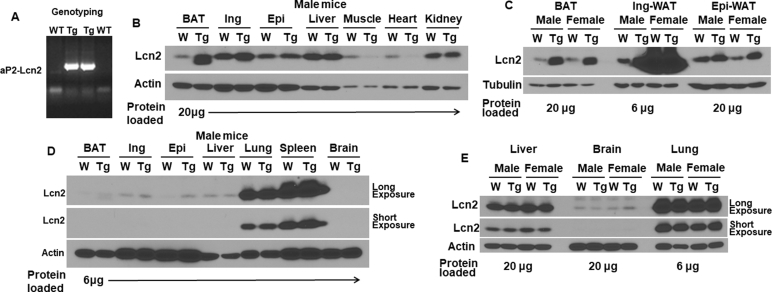
As indicated in Supplemental Fig. 1A, two transgenic founder mice were identified by PCR genotyping. When compared to WT mice protein levels of Lcn2 were increased in adipose tissue depots from male Lcn2 transgenic (Tg) mice (Supplemental Fig. 1B). Lcn2 overexpression was more substantial in BAT from both male and female Tg mice when compared to the iWAT and eWAT (Supplemental Fig. 1B, C). There was no difference in Lcn2 protein levels in liver, kidney, lung, spleen, or brain when comparing Tg mice to WT controls (Supplemental Fig. 1B, D). This indicates the ap2-driven Lcn2 transgene successfully overexpresses Lcn2 selectively in adipose tissue depots. Levels of Lcn2 overexpression in BAT and eWAT appear to be similar between male and female Lcn2 transgenic mice (Supplemental Fig. 1C). However, there were markedly higher levels of Lcn2 in iWAT from female mice versus male mice (Supplemental Fig. 1C).
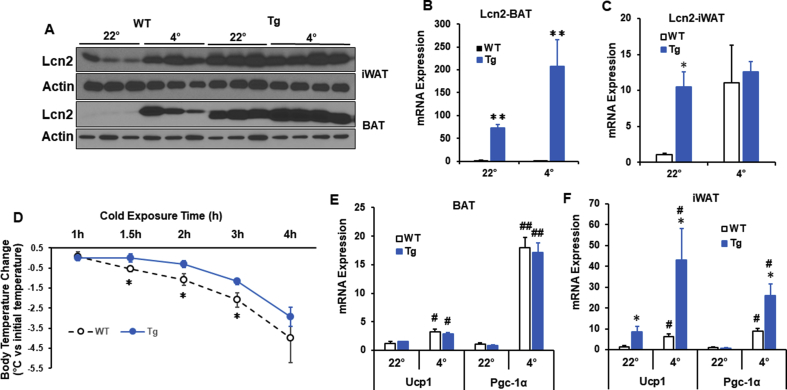
To determine the effect of overexpressing Lcn2 in adipose tissue on adaptive thermogenesis, we placed regular chow diet (RCD)-fed WT and Lcn2 Tg male mice at 4 °C and measured body temperature every 30 min for 4 h. After 4 h of exposure to 4 °C, Lcn2 levels in BAT and iWAT were increased in WT mice (Figure 1A). When compared to WT mice, Tg mice expressed higher levels of both Lcn2 protein (Figure 1A) and Lcn2 gene expression (Figure 1C) in BAT at room temperature and in response to cold. In iWAT, Tg mice had increased Lcn2 protein (Figure 1A) and Lcn2 gene expression (Figure 1C) versus WT at room temperature (Figure 1C). At 1.5 h, 2 h, and 3 h, Tg mice maintained a significantly higher body temperature when compared to WT mice (Figure 1D). Interestingly, thermogenic genes were not increased in BAT from Tg mice (Figure 1E), but Ucp1 and Pgc-1α gene expression was significantly higher in Lcn2 Tg iWAT (Figure 1F).
Figure 1.
Lcn2 overexpression in adipose tissue and adaptive thermogenesis in young Lcn2 Tg mice at the age of 16 weeks. Lcn2 protein (A) and gene expression (B–C) levels in brown adipose tissue (BAT) and inguinal white adipose tissue (iWAT) of WT and Lcn2 Tg mice during cold exposure. (D) Body temperature of WT (n = 8) and Lcn2 Tg mice (n = 10) during acute cold exposure. Mice in an ambient temperature of 22 °C serve as controls (n = 9 for Tg and n = 11 for WT mice). (E–F) Thermogenic gene expression in BAT and iWAT during cold exposure, n = 3–5 mice/group. #p < 0.05 or ##p < 0.05 vs 22 °C; *p < 0.05 or **p < 0.01 vs WT of same treatment.
3.2. Overexpression of Lcn2 in adipose tissue promotes beiging of iWAT and alters energy metabolism under non-stressed conditions
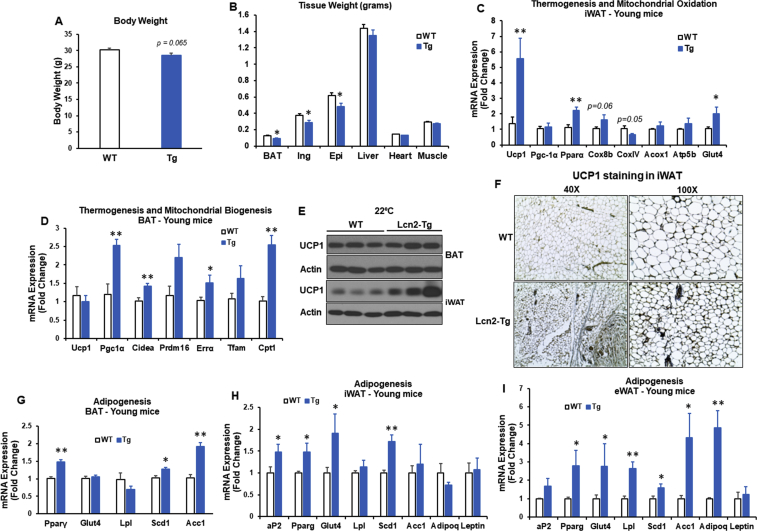
Increased Ucp1 expression in Lcn2 Tg iWAT under room temperature conditions (Figure 1F) suggests that Lcn2 has a beiging effect. Overexpression of UCP1 driven by aP2 promoter in adipose tissue has previously been reported to prevent obesity [35]. Next, we examined the metabolic effect of adipose Lcn2 overexpression in young mice under non-stressed conditions. Male mice at the age of 16 weeks were fed RCD and under the fed state before sacrifice. We found Lcn2 Tg mice had a trend towards a decrease in body weight (p = 0.065) (Figure 2A). Tissue weight of all three fat depots (BAT, iWAT, and eWAT) were significantly decreased in Lcn2 Tg mice when compared to WT mice (Figure 2B).
Figure 2.
Body weight, thermogenesis, and adipogenesis in young Lcn2 Tg mice on a RCD and exposed to an ambient temperature of 22°C. Body weight (A) and tissue weight (B) of WT and Lcn2 Tg male mice on a RCD at 16 weeks of age, n = 8–10 mice/group. Expression of thermogenic and mitochondrial genes in iWAT (C) and BAT (D) from 16-week-old WT and Lcn2 Tg male mice. UCP1 protein expression (E) in BAT and iWAT and UCP1 staining by immunohistochemistry (F) in iWAT of WT and Lcn2 Tg mice at 16 weeks of age. Adipogenic gene expression in BAT (G), iWAT (H), and epididymal WAT (eWAT) (I) from 16-week-old WT and Lcn2 Tg male mice (n = 8–10 mice/group).
Interestingly, Lcn2 Tg mice had a significant increase in expression of the thermogenic gene Ucp1 and genes involved in fatty acid oxidation, including Pparα and Cox8b, as well as a trend increase in Acox1 and Atp5b in iWAT (Figure 2C). In BAT from Tg mice, while Ucp1 gene expression was unchanged (Figure 2D), expression of Pgc-1α, Cidea, Errα, Cpt1, and Cox8b genes were significantly increased under room temperature conditions. Further, changes in Ucp1 gene expression were confirmed with UCP1 protein levels in iWAT and BAT in WT and Lcn2-Tg mice (Figure 2E). Immunohistochemistry of UCP1 showed that Lcn2 Tg mice have more UCP1-positive adipocytes and smaller adipocytes in iWAT (Figure 2F). These results indicate that overexpression of Lcn2 in adipose tissue itself can promote beiging and stimulate fat oxidation in iWAT and BAT without additional beiging stimulation such as cold. To further test if Lcn2 Tg mice have increased adipogenic capability and healthy adipocytes, we examined the expression of genes involved in adipogenesis and glucose/lipid metabolism. Interestingly, we found the expression of Pparg, aP2, Glut4, Lpl, and Scd1 was significantly increased in iWAT and eWAT of Lcn2 Tg mice compared to WT mice (Figure 2G–I); the expression of adiponectin and Acc1 was also markedly upregulated in Lcn2 Tg eWAT (Figure 2I).
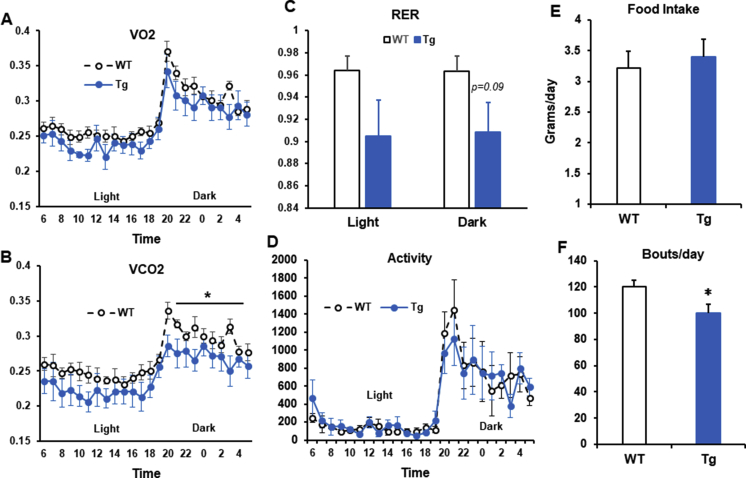
To determine the effect of Lcn2 overexpression in adipose tissue on energy expenditure, RCD-fed, 16-week-old, WT and Lcn2 Tg male mice were placed in metabolic chambers for three days to analyze energy expenditure via indirect calorimetry. While whole body oxygen consumption (VO2) was unchanged in Lcn2 Tg mice (Supplemental Fig. 2A), carbon dioxide output (VCO2) was significantly decreased during the dark phase (Supplemental Fig. 2B). A similar decrease in VCO2 output compared to VO2 consumption was previously reported in mice fed a ketogenic diet (36), pointing towards potential alterations in energy utilization preference. In line with decreased VCO2 production, a trend towards a decrease in respiratory exchange ratio (RER) was also seen in Lcn2 Tg mice during both the light and dark phase (Supplemental Fig. 2C), indicating a possible increase in fat utilization when compared to WT mice. There was no difference in activity level or total food intake between WT and Tg mice (Supplemental Fig. 2D, E). Although WT and Tg mice had no difference in total food intake, bouts/day were significantly decreased in Tg mice, indicating Tg mice eat less frequently than WT mice (Supplemental Fig. 2F). This could potentially signify a difference in satiety or behaviors surrounding food intake in response to overexpression of Lcn2 in adipose tissue.
To determine the protective effect of Lcn2 overexpression in adipose tissue on high fat diet (HFD)-induced obesity, we fed WT and Lcn2 Tg mice a HFD (Bioserv, 60% of kilocalories from lard-derived fat) from 4 to 5 weeks of age until 20–21 weeks of age. Despite Lcn2 Tg mice having increased expression of Ucp1 in iWAT, we found no differences in body weight, adiposity or insulin sensitivity between young WT and Lcn2 Tg male mice following HFD-feeding for 10 weeks (data not shown). As HFD has previously been found to significantly increase Lcn2 levels in WT mice [27], it is possible that Lcn2 levels become similar between WT and Lcn2 Tg mice in response to HFD and the effect of overexpressing Lcn2 is diminished under this condition.
3.3. Overexpression of adipose Lcn2 attenuates age-related decline in beiging of iWAT
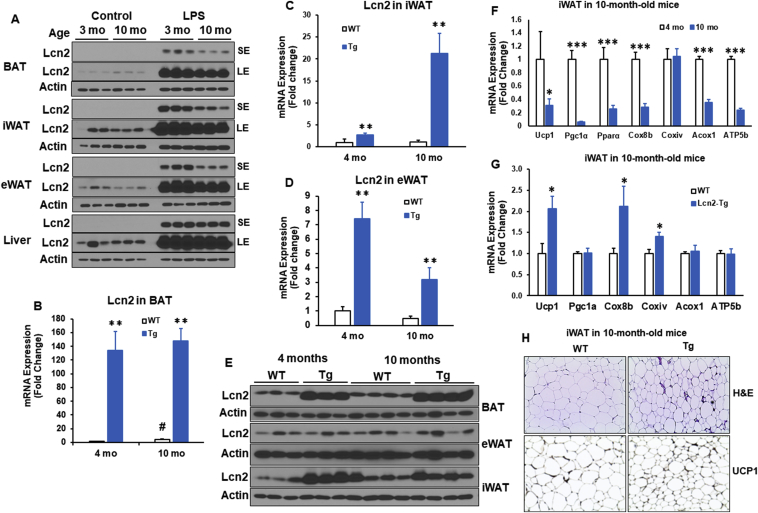
Lcn2 has previously been reported to increase in response to aging in mice and humans [33], [34]. We measured Lcn2 protein in adipose tissue depots and liver from young, 3-month-old mice and older, 10-month-old mice to determine changes in Lcn2 with aging. In the steady state, Lcn2 protein levels were very low in three adipose tissue depots; age didn't seem to significantly change the Lcn2 protein levels in adipose tissue (Figure 3A). As Lcn2 is highly inducible in response to inflammation [27], we also examined Lcn2 induction by lipopolysaccharide (LPS) in younger and older mice. Surprisingly, Lcn2 levels in all three adipose tissue depots but not liver from older mice had a significantly attenuated response to LPS when compared to the younger mice (Figure 3A). This raises the question of whether attenuation of Lcn2 in response to aging and inflammatory stimuli contributes to the progression of age-related diseases and whether overexpression of Lcn2 can prevent this.
Figure 3.
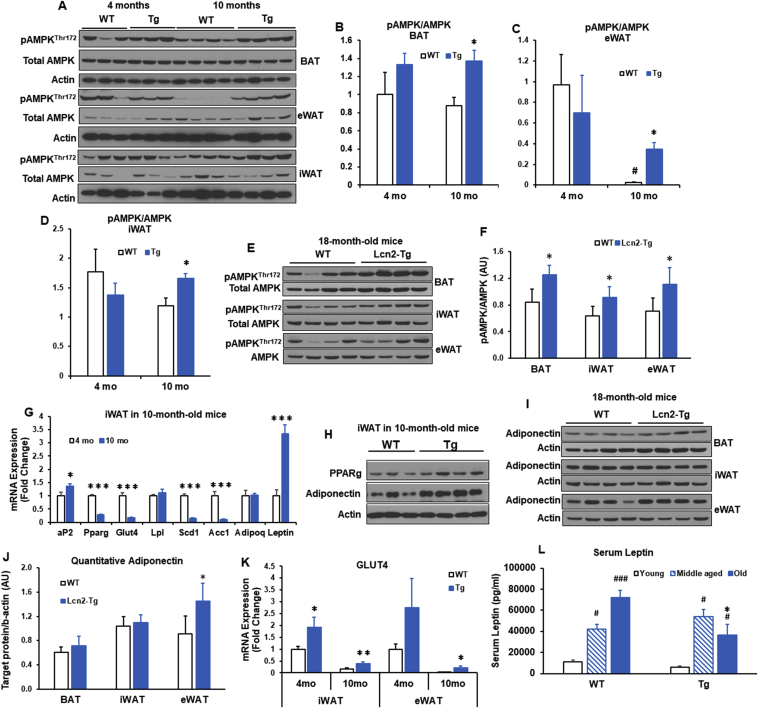
Effect of Lcn2 overexpression in adipose tissue on thermogenesis in middle-aged mice. (A) Lcn2 protein expression in BAT, iWAT, eWAT, and liver from 3-month- and 10-month-old male mice treated with LPS (0.3 mg/kg, i.p.) (n = 3 mice/group). Age-matched mice without LPS treatment serve as controls (n = 3 mice/group). Lcn2 gene expression in BAT (B), iWAT (C), and eWAT (D) as well as Lcn2 protein expression (E) in BAT, iWAT, and eWAT from male mice at 4 months and 10 months of age (n = 6–8 mice/group). (F) Thermogenic gene expression in iWAT from 4-month- and 10-month-old WT male mice (n = 6–8 mice/group). (G) Thermogenic gene expression in iWAT from 10-month-old WT and Lcn2 Tg male mice. (H) H&E staining and immunohistochemistry for UCP1 of iWAT sections from 10-month-old WT and Lcn2 Tg male mice.*p < 0.05; **p < 0.01 vs WT; mo, month; i.p., intraperitoneal.
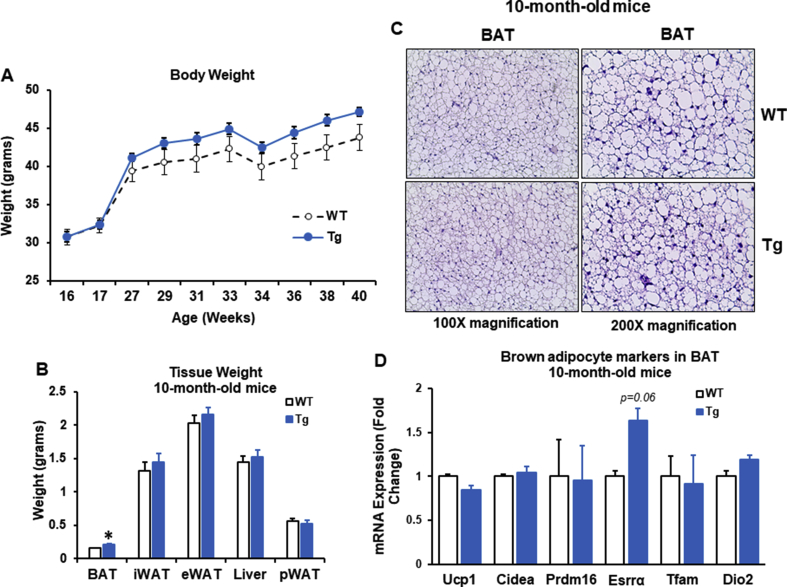
To that end, we aged male WT and Lcn2 Tg mice until 10-months, generally equivalent to middle-age in humans, and 18 months, equivalent to old-age in humans. Lcn2 protein and gene expression levels were confirmed to be higher in BAT, iWAT, and eWAT from both 4-month and 10-month old Tg mice when compared to WT mice of the same age (Figure 3B–E). WT and Lcn2 Tg mice gained similar amounts of body weight with age under the RCD condition (Supplementary Fig. 3A). In Lcn2 Tg mice, there was no difference in iWAT, eWAT, or liver weight following aging (Supplemental Fig. 3B). There was a slight, but significant increase in BAT weight in Lcn2 Tg mice when compared to WT (Supplemental Fig. 3B). Histology of BAT demonstrated that brown adipocyte size was similar to WT in Lcn2 Tg mice (Supplemental Fig. 3C), indicating the increase in BAT mass in Lcn2 Tg mice is not due to adipocyte hypertrophy or whitening of brown fat. While thermogenic genes were unchanged in BAT from older Lcn2 Tg mice, there was a trend towards increased expression of estrogen-related receptor alpha (Errα), an important regulator of oxidative metabolism (Supplemental Fig. 3D). This indicates increased BAT mass in older Tg mice may be beneficial.
With aging, the expression of thermogenic and mitochondrial genes including Ucp1, Pgc-1α, Pparα, Cox8b, Acox1, and Atp5b was markedly down-regulated in iWAT (Figure 3F). Interestingly, Lcn2 Tg mice were able to maintain significantly higher expression levels of Ucp1, Cox8b, and Coxiv in iWAT than WT mice at 10 months old of age (Figure 3G). While there was no change in iWAT weight (data not shown), adipocyte size was markedly smaller following aging in male Lcn2 Tg mice when compared to WT mice at 10 months old of age (Figure 3H). Additionally, there was a relatively increased UCP1-positive cells in iWAT of Lcn2 Tg mice versus WT mice (Figure 3H). Decreased adipocyte hypertrophy and increased UCP1 and mitochondrial gene expression suggest that Lcn2 Tg iWAT is metabolically heathier in middle age.
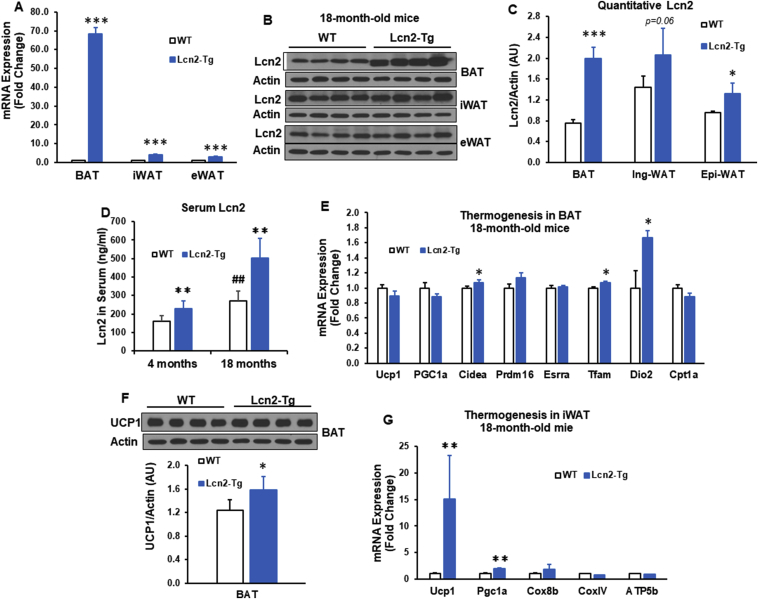
To determine if Lcn2 overexpression can continue to have a beneficial effect on adipose tissue metabolism in old age, mice were aged until 18 months. Overexpression of Lcn2 gene and protein in adipose depots was confirmed by qPCR and western-blotting (Figure 4A–C). Moreover, serum levels of Lcn2 were also significantly higher in Lcn2 Tg mice compared to WT mice at both 4 months and 18 months of age (Figure 4D). Compared to WT mice, Lcn2 Tg mice at 18 months of age had significantly higher expression levels of thermogenic and mitochondrial genes (Cidea, Tfam, and Dio2) (Figure 4E) and UCP1 protein in BAT (Figure 4F). There was also a significant increase in gene expression of Ucp1 and Pgc-1α as well as a trend increase in Cox8b expression in Lcn2 Tg iWAT (Figure 4G).
Figure 4.
Effect of overexpression in adipose tissue on thermogenesis in old mice. (A) Lcn2 gene expression (A) as well as Lcn2 protein expression and quantification (B–C) in BAT, iWAT, and eWAT from 18-month-old WT and Lcn2 Tg male mice (n = 5–7/group). (D) Serum levels of Lcn2 in 4-month-old (n = 6–8 mice/group) and 18-month-old (n = 5–7 mice/group) WT and Lcn2 Tg male mice. Thermogenic gene expression (E) and UCP1 protein expression (F) in BAT from 18-month-old WT and Lcn2 Tg male mice (n = 5–7 mice/group). (G) Thermogenic gene expression in iWAT from 18-month-old WT and Lcn2 Tg male mice (n = 5–7 mice/group). *p < 0.05, **p < 0.01, ***p < 0.001 vs WT; ##p < 0.01, ###p < 0.001 vs 4 months.
3.4. Overexpression of adipose Lcn2 improves metabolic function of adipose tissue in aged mice
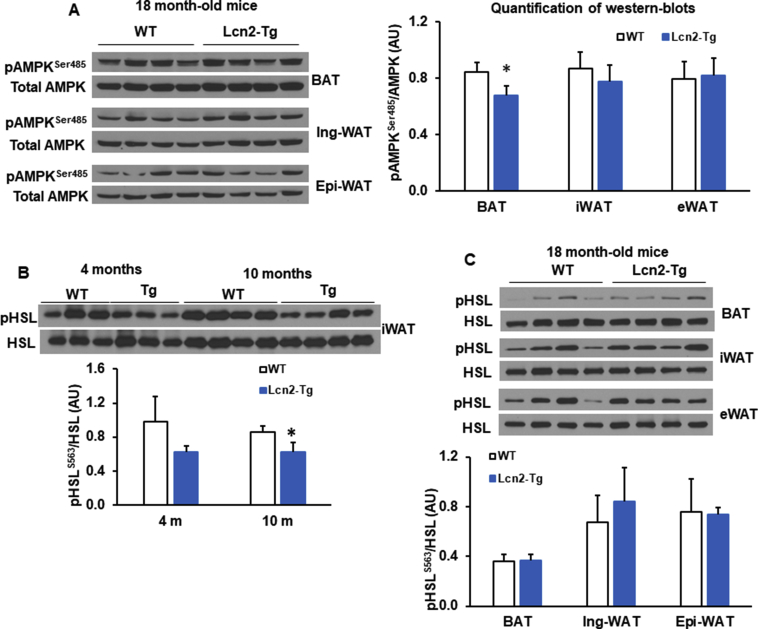
Several metabolic pathways have been implicated in aging [3]. Activating AMPK has been reported to promote healthspan and longevity [3], [36]. In younger mice, there was no significant difference in phosphorylation of AMPK at Thr 172 in BAT, eWAT or iWAT when comparing genotypes (Figure 5A–D). Following aging, levels of AMPK phosphorylation at Thr 172 decline in WT mice. However, Lcn2 Tg mice at 10 months of age maintained significantly higher levels of AMPK phosphorylation at Thr 172 in BAT, eWAT, and iWAT compared to WT mice (Figure 5A–D). Similarly, levels of AMPK phosphorylation at Thr 172 were also higher in three adipose tissue depots of 18 month-old Lcn2 Tg mice (Figure 5E, F). However, AMPK phosphorylation at Ser485 remained unchanged in iWAT and eWAT, but slightly decreased in BAT of 18-month-old Lcn2 Tg mice compared to WT mice (Supplemental Fig. 4A).
Figure 5.
AMPK phosphorylation and adipogenic markers in adipose tissue from middle-aged and old mice. (A–D) AMPKThr172 phosphorylation levels and quantification in BAT, eWAT, and iWAT from 4-month- and 10-momth-old WT and Lcn2 Tg male mice (n = 6–8 mice/group). (E and F) AMPK Thr172 phosphorylation levels and quantification (fold change) in BAT, eWAT, and iWAT from 18-month-old WT and Lcn2 Tg male mice (n = 5–7 mice/group). (G) Adipogenic gene expression in iWAT from 4-month- and 10-month-old WT male mice (n = 6–8 mice/group). (H) Protein levels of PPARγ and adiponectin in iWAT from 10-month-old WT and Lcn2 Tg male mice. (I and J) Adiponectin protein expression and quantification in BAT, iWAT, and eWAT from 18-month-old WT and Lcn2 Tg male mice (n = 5–7 mice/group. (K) Glut 4 gene expression in iWAT and eWAT from 4-month- and 10-month-old WT and Lcn2 Tg male mice (n = 6–8 mice/group). (L) Serum levels of leptin at 4 months (young), 10 months (middle aged), and 18 months (old) of age from WT and Lcn2 Tg male mice. *p < 0.05 vs WT of same age; **p < 0.01 vs WT of same age; #p < 0.05, ##p < 0.01, ###p < 0.001 vs 4 m (young).
Consistent with the literature [4], we found the expression of genes involved in adipogenesis and glucose/lipid metabolism, including Pparg, Glut4, Acc1, and Scd1 was markedly down-regulated in iWAT and eWAT of older WT mice (10 months of age) compared to young WT mice (4 months of age) (Figure 5G), while leptin expression was significantly upregulated following age (Figure 5G). In iWAT from older Lcn2 Tg mice, we found increased protein levels of PPARγ (Figure 5H) and adiponectin relative to WT mice (Figure 5H, I). This could be suggestive of improved adipogenesis in adipose tissue in older Lcn2 Tg mice. Consistent with improved adipogenesis, the levels of Glut4 expression were significantly higher in iWAT and eWAT from Lcn2 Tg mice (Figure 5K). Moreover, serum leptin levels were increased with aging in WT mice (Figure 5L); this age-related increase in leptin was significantly attenuated in Lcn2 Tg mice at the 18 months of age (Figure 5L). This suggests that Lcn2 Tg mice have improved age-related leptin resistance. Together these data indicate improved adipose tissue function in older Lcn2 Tg mice, which may have implications for whole-body metabolic homeostasis.
In the previous publications [28], [29], [37], our results from global Lcn2 KO mouse model have indicated that Lcn2 regulates BAT and beige fat thermogenesis through a non-adrenergic pathway. For example, in Lcn2 KO adipocytes, NE-induced Ucp1 expression, PKA phosphorylation, and HSL phosphorylation was not affected. Consistent with the results from Lcn2 KO mice, overexpression of Lcn2 had no significant effect on the phosphorylated levels of HSL in all three fat depots from young, middle-aged, and old mice (Supplemental Fig. 4B, C). These data indicate that Lcn2-induced upregulation of Ucp1 and thermogenesis are not through the adrenergic signaling pathway.
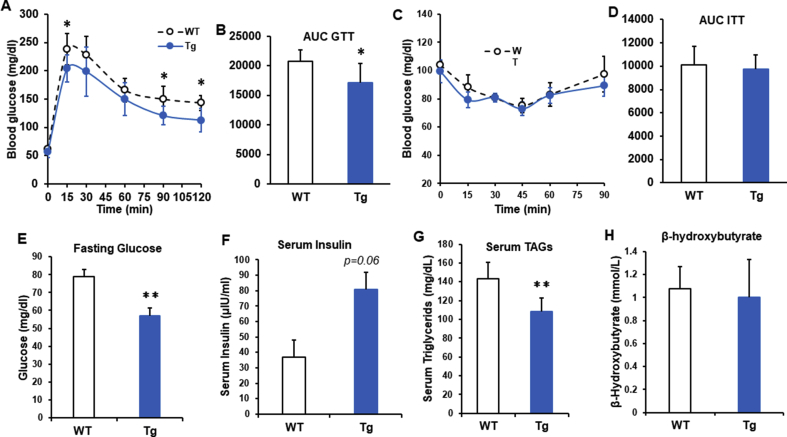
Glucose and insulin tolerance tests (GTT and ITT) were conducted at 3 months and 10 months of age in WT and Lcn2 Tg male mice fed a RCD. In younger male Lcn2 Tg mice, there was no difference in glucose tolerance or insulin sensitivity when compared to WT mice (data not shown). However, in older Lcn2 Tg mice, area under the curve following glucose administration was significantly lower than WT mice, indicating improved glucose tolerance (Figure 6A, B). This suggests that overexpression of Lcn2 in adipose tissue mitigates the development of age-related glucose intolerance. Despite improved GTT, there was no difference in insulin sensitivity between WT and Lcn2 Tg mice at 10 months of age (Figure 6C, D).
Figure 6.
Glucose homeostasis in middle-aged Lcn2 Tg mice. (A, B) Glucose tolerance test (GTT) and area under the curve (AUC) in 10-month-old WT and Lcn2 Tg male mice (n = 6–8 mice/group). (C, D) Insulin tolerance test (ITT) and AUC in 10-month-old WT and Lcn2 Tg male mice (n = 6–8 mice/group). Blood glucose (E), serum levels of insulin (F), triglycerides (TAGs) (G), and β-hydroxybutyrate (H) from 10-month-old WT and Lcn2 Tg male mice following overnight fasting (n = 6–8 mice/group). *p < 0.05, **p < 0.01 vs WT.
Consistent with improved glucose tolerance, Lcn2 Tg mice had decreased fasting blood glucose levels at 10 months of age (Figure 6E). This was accompanied by a trend towards increased serum insulin levels (Figure 6F). The decrease in fasting glucose and improved glucose tolerance could potentially be due to the increase in serum insulin in Lcn2 Tg mice, leading to increased insulin-stimulated glucose uptake despite unchanged insulin sensitivity. Additionally, Serum triglycerides were significantly lower in 10-month-old Lcn2 Tg mice relative to WT mice (Figure 6G), whereas serum β-hydroxybutyrate was not different between WT and Lcn2 Tg mice (Figure 6H).
3.5. Overexpression of Lcn2 expression in adipose tissue protects against age-related liver lipid accumulation and improves liver metabolic health
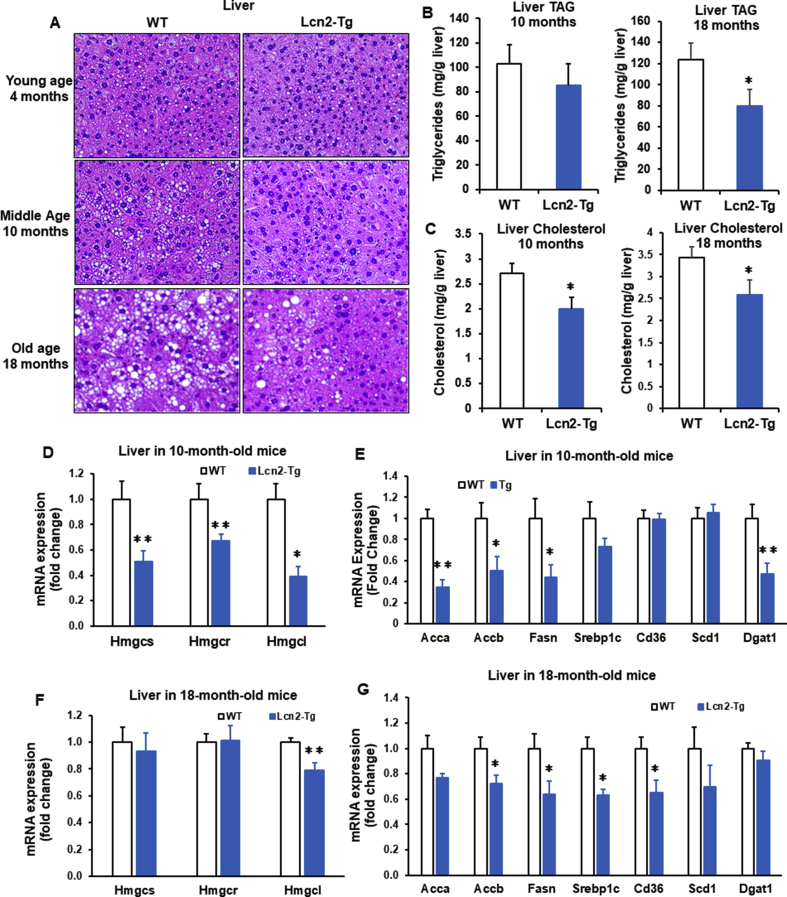
We next determined if improved adipose tissue function by Lcn2 overexpression can have an impact on liver lipid accumulation and metabolism in aged mice. Morphologically, liver lipid accumulation was increased following aging in WT mice; this age-related increase in liver lipids was significantly attenuated in 10-month-old, particularly 18-month-old Lcn2 Tg mice (Figure 7A). In line with morphologic changes, liver triglyceride levels were significantly decreased in 18-month-old Lcn2 Tg mice and showed a decreasing trend in 10-month-old Lcn2 Tg mice (Figure 7B). Moreover, liver cholesterol levels were also significantly decreased in both 10-month-old and 18-month-old Lcn2 Tg mice compared to WT controls (Figure 7C). Consistent with decreased liver lipid levels, genes involved in lipogenesis and cholesterol synthesis were also significantly decreased in Lcn2 Tg liver relative to WT at 10 months of age (Figure 7D, E) as well as at 18 months of age (Figure 7F, G).
Figure 7.
Liver lipid accumulation in middle-aged and old Lcn2 Tg mice. (A) H&E staining of liver sections from 4-month-, 10-month-, and 18-month-old WT and Lcn2 transgenic (Tg) male mice (n = 5–8 mice/group). (B) Triglycerides (TAG) and (C) cholesterol levels in liver from 10-month- and 18 month-old WT and Lcn2 Tg male mice (n = 5–8 mice/group). Expression of genes involved in cholesterol synthesis (D) and lipogenesis (E) in liver from 10-month-old WT and Lcn2 Tg male mice (n = 6–8 mice/group). Expression of genes involved in cholesterol synthesis (F) and lipogenesis (G) expression in liver from 18-month-old WT and Lcn2 Tg male mice (n = 5–7 mice/group). *p < 0.05, **p < 0.01 vs WT.
Decreases in lipid accumulation in liver suggest improved liver function. Indeed, we saw decreases in the expression of genes involved in oxidative stress, inflammation, liver fibrosis, and gluconeogenesis in Lcn2 Tg liver (Supplemental Fig. 5A–F). Together, this suggests that overexpression of Lcn2 in adipose tissue can promote liver health, which may further contribute to the improvements in whole-body glucose and lipid homeostasis seen in old Lcn2 Tg mice.
4. Discussion
Increased amount and activity of brown and beige adipose tissue is associated with energy expenditure and a decrease in BMI [7], [25], [38]. Aging is associated with decreased BAT activity and beiging of WAT. We have previously found that Lcn2 KO mice are cold intolerant and have impaired thermogenic programming and beiging of iWAT [28], [29], [37]. Lcn2 KO mice are also more susceptible to dyslipidemia, weight gain, and liver lipid accumulation during HFD feeding [28]. In this study, we developed an ap2-promoter-driven Lcn2 transgenic mouse model to test the beneficial effects of overexpressing Lcn2 in adipose tissue on energy metabolism and health span. We found Lcn2 Tg mice had improved thermogenesis and mitochondrial oxidation in BAT and beige adipose tissue. Overexpression of Lcn2 in adipose tissue improves age-related glucose intolerance and liver lipid accumulation and metabolism.
Interestingly, we saw significant upregulation of Ucp1 and genes for thermogenesis and mitochondrial oxidation in BAT and iWAT of Lcn2 Tg mice at the young age under non-stressed conditions. This suggests that overexpression of Lcn2 in adipose tissue is sufficient to activate BAT and beiging of iWAT. Further, Lcn2 Tg mice had a trend toward decreased body weight and a significant decrease in adipose tissue weight and adipocyte size. A trend toward decreased RER suggests that Lcn2 Tg mice are oxidizing more fat, which could explain the decrease in adiposity. Indeed, we saw an increase in the expression of genes involved in mitochondrial biogenesis and fat oxidation in BAT and iWAT from Lcn2 Tg mice. These results are consistent with our previous research showing Lcn2 KO mice have impaired mitochondrial function and fat oxidation in brown and beige adipocytes and increased adiposity [28], [29], [37].
From measurement of whole body energy expenditure in young WT and Lcn2 Tg mice, we found Lcn2 Tg mice have decreased CO2 output, with no change in O2 consumption during the dark, or active, cycle. A similar phenotype was seen during both the light and dark cycle in animals fed a ketogenic diet [39], leading us to examine ketone body levels in Lcn2 Tg mice. However, serum levels of β-hydroxybutyrate in Lcn2 Tg mice were largely unchanged under both room temperature and cold conditions. This suggests that most of fatty acids are promoted to be oxidized in BAT and beige adipocytes but not for ketogenesis in liver in Tg mice.
Similar to obesity, aging also increases the risk for glucose intolerance, insulin resistance, dyslipidemia, impaired nutrient signaling, and liver dysfunction [2], [3], [6]. We therefore hypothesized that Lcn2 may play a role in preventing age-related metabolic deterioration. In Lcn2 Tg mice, we found improvements in key metabolic factors that decline with age including adipose tissue function, whole-body glucose and lipid homeostasis, and liver lipid accumulation. Increasing AMPK activity through genetic or pharmacological means has a beneficial effect on glucose and lipid metabolism, leading to improved healthspan and longevity [3], [36], [40]. Further, AMPK regulates PGC-1α to induce mitochondrial biogenesis and fat oxidation in adipose tissue [41]. AMPK activation in adipocytes is required for cold-induced thermogenic activation of BAT and beiging of WAT; deleting AMPK in adipocytes aggravates HFD-inudced insulin resistance and liver lipid accumulation [42]. In WT mice, we saw a decline in AMPK phosphorylation in adipose tissue depots of older mice when compared to younger mice. Older Tg mice have significantly higher levels of AMPK phosphorylation, suggesting Tg adipose tissue has improved metabolic function.
There is an age-related decline in PPARγ in adipose tissue, leading to reduced adipogenic capacity [4], [43]. Impairment of adipogenesis in subcutaneous adipose tissue during aging is a major contributor to lipid spillover into circulation and non-adipose tissues [4]. In older Lcn2 Tg mice, there was an increase in adipogenic markers, including increased PPARγ and adiponectin levels in iWAT. This was further reflected in an increase in the PPARγ target gene, Glut4, in iWAT from Lcn2 Tg mice. In rodent studies, treatment with the PPARγ-agonist troglitazone resulted in smaller adipocyte size and reduced plasma triglyceride level, with no change in overall adipose tissue mass [44]. Consistent with this, we saw smaller adipocyte size in iWAT and decreased serum triglyceride levels in older Lcn2 Tg mice, despite no difference in WAT weight. This suggests that increased PPARγ and improved adipose tissue function in older Lcn2 Tg mice may have implications for whole-body energy homeostasis.
Lcn2 Tg mice had significantly improved GTT when compared to WT mice, indicating overexpression of Lcn2 in adipose tissue attenuates the age-related decline in glucose tolerance. At the same time, there was no difference in insulin sensitivity in Lcn2 Tg mice relative to WT. Enhanced glucose tolerance could therefore be a result of the increase in serum insulin levels in Tg mice. We also saw changes in insulin-responsive genes in Tg mice, including modulation of the gluconeogenic gene Pck1 in liver and increased Glut4 expression in WAT. In agreement with our data, a recent study by Mosialou et al. demonstrated increased insulin levels in mice treated chronically with Lcn2 and enhanced insulin secretion from pancreatic islets treated with Lcn2 [32]. Together, this suggests that Lcn2 from adipose tissue may potentiate insulin secretion, which suppresses hepatic gluconeogenesis and increases glucose transport, to prevent a decline in glucose tolerance during aging.
Advancing age is a risk factor for the development of non-alcoholic fatty liver disease (NAFLD), marked by liver lipid accumulation leading to fibrosis and inflammation [45]. During aging, decreased capacity for lipid storage in subcutaneous adipose tissue and oxidation in thermogenic adipose tissue results in lipid accumulation in liver [4]. Further, circulating factors secreted from adipose tissue play a role in the development of NAFLD [46], [47]. In middle- and old-aged Lcn2 Tg mice, we found less apparent lipid droplets in liver relative to WT mice. Further, cholesterol/TG levels and genes involved in cholesterol synthesis and lipogenesis were decreased in liver from Lcn2 Tg mice. Consistent with a decrease in lipid accumulation in the liver, we saw a decrease in gene markers of oxidative stress, inflammation, and fibrosis. This suggests that overexpression of Lcn2 in adipose tissue protects from liver lipid accumulation and further liver injury and inflammation. Since we saw Lcn2 in serum is increased in Tg mice, the improvement of liver health could be directly due to increased serum Lcn2 or indirectly through improving adipose tissue function.
A cell-surface receptor for Lcn2 binding and endocytosis of iron-bacterial siderophore complex has been identified [48]. However, the Lcn2 signaling pathway, particularly mediating AMPK activation is completely unknown and warrants further investigation. We speculate that AMPK activation may be stimulated by increased ROS production as a result of increased mitochondrial oxidation in Lcn2 Tg. Additionally, the mechanism for the role of Lcn2 in thermogenic activation through activating immune cells in adipose tissue need further investigation.
5. Conclusion
In summary, we found that overexpression of Lcn2 in adipose tissue led to improved cold adaptation with increased Ucp1 gene expression in iWAT, altered whole-body energy expenditure with a trend towards fat oxidation, increased oxidative gene expression, decreased adipose tissue weight and adipocyte size, and decreased glucose/TG levels. Together, these data suggest that Lcn2 may improve mitochondrial function of BAT and increase beiging in iWAT, leading to increased lipid oxidation and a decrease in adiposity. Our results also suggest that overexpression of Lcn2 preserves adipose tissue function and prevents age-related glucose intolerance and liver lipid accumulation.
Acknowledgements
This work was supported by a NIDDK Grant (R56DK080743) awarded to X.C., NIDDK-funded (T32DK083250) traineeship to J.D., and the General Mills Foundation Chair in Genomics for Healthful Foods to X.C.
We would also like to thank Dr. Alessandro Bartolomucci and Dr. Maria Razzoli at the UMN Integrative Biology and Physiology Phenotyping Core for their expertise and assistance with food intake and indirect calorimetry.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.molmet.2019.03.007.
Conflict of interest
None declared.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
figs1.
figs2.
figs3.
figs4.
figs5.
References
- 1.Anderson G.F., Hussey P.S. 2000. Population Aging : A. [DOI] [PubMed] [Google Scholar]
- 2.Liu H.H., Li J.J. Aging and dyslipidemia: a review of potential mechanisms. Ageing Research Reviews. 2015;19(2015):43–52. doi: 10.1016/j.arr.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Barzilai N., Huffman D.M., Muzumdar R.H., Bartke A. The critical role of metabolic pathways in aging. Diabetes. 2012;61(6):1315–1322. doi: 10.2337/db11-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palmer A.K., Kirkland J.L. Aging and adipose tissue: potential interventions for diabetes and regenerative medicine. Experimental Gerontology. 2016;86:97–105. doi: 10.1016/j.exger.2016.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuk J.L., Saunders T.J., Davidson L.E., Ross R. Age-related changes in total and regional fat distribution. Ageing Research Reviews. 2009;8(4):339–348. doi: 10.1016/j.arr.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 6.Jura M., Kozak L.P. Obesity and related consequences to ageing. Age. 2016;38(1) doi: 10.1007/s11357-016-9884-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Marken Lichtenbelt W.D., Vanhommerig J.W., Smulders N.M., Drossaerts J.M.A.F.L., Kemerink G.J., Bouvy N.D. Cold-activated Brown adipose tissue in healthy men. New England Journal of Medicine. 2009;360(15):1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 8.Saito M., Okamatsu-ogura Y., Matsushita M., Watanabe K., Yoneshiro T., Nio-kobayashi J. 2009. High incidence of metabolically active Brown adipose effects of cold exposure and adiposity. October 58; pp. 1526–1531. July. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoneshiro T., Aita S., Matsushita M., Okamatsu-Ogura Y., Kameya T., Kawai Y. Age-related decrease in cold-activated brown adipose tissue and accumulation of body fat in healthy humans. Obesity. 2011;19(9):1755–1760. doi: 10.1038/oby.2011.125. [DOI] [PubMed] [Google Scholar]
- 10.Rogers N.H., Landa A., Park S., Smith R.G. Aging leads to a programmed loss of brown adipocytes in murine subcutaneous white adipose tissue. Aging Cell. 2012;11(6):1074–1083. doi: 10.1111/acel.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cypess A.M., Lehman S., Williams G., Tal I., Goldfine A.B., Kuo F.C. Identification and importance of Brown adipose tissue in adult humans a BS TR AC T. New England Journal of Medicine. 2009;360:1509–1526. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sidossis L., Kajimura S. Brown and beige fat in humans: thermogenic adipocytes that control energy and glucose homeostasis. Journal of Clinical Investigation. 2015;125(2):478–486. doi: 10.1172/JCI78362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poher A.L., Veyrat-Durebex C., Altirriba J., Montet X., Colin D.J., Caillon A. Ectopic UCP1 overexpression in white adipose tissue improves insulin sensitivity in Lou/C rats, a model of obesity resistance. Diabetes. 2015;64(11):3700–3712. doi: 10.2337/db15-0210. [DOI] [PubMed] [Google Scholar]
- 14.Chondronikola M., Volpi E., Børsheim E., Porter C., Annamalai P., Enerbäck S. Brown adipose tissue improves whole-body glucose homeostasis and insulin sensitivity in humans. Diabetes. 2014;63(12):4089–4099. doi: 10.2337/db14-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee P., Smith S., Linderman J., Courville A.B., Brychta R.J., Dieckmann W. Temperature-acclimated brown adipose tissue modulates insulin sensitivity in humans. Diabetes. 2014;63(11):3686–3698. doi: 10.2337/db14-0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boutant M., Joffraud M., Kulkarni S.S., García-Casarrubios E., García-Roves P.M., Ratajczak J. SIRT1 enhances glucose tolerance by potentiating brown adipose tissue function. Molecular Metabolism. 2015;4(2):118–131. doi: 10.1016/j.molmet.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bartelt A., Bruns O.T., Reimer R., Hohenberg H., Ittrich H., Peldschus K. Brown adipose tissue activity controls triglyceride clearance. Nature Medicine. 2011;17(2):200–206. doi: 10.1038/nm.2297. [DOI] [PubMed] [Google Scholar]
- 18.Kazak L., Chouchani E.T., Jedrychowski M.P., Erickson B.K., Shinoda K., Cohen P. A creatine-driven substrate cycle enhances energy expenditure and thermogenesis in beige fat. Cell. 2015;163(3):643–655. doi: 10.1016/j.cell.2015.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ikeda K., Kang Q., Yoneshiro T., Camporez J.P., Maki H., Homma M. UCP1-independent signaling involving SERCA2bmediated calcium cycling regulates beige fat thermogenesis and systemic glucose homeostasis. Nature Medicine. 2017;23(12):1454–1465. doi: 10.1038/nm.4429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.CANNON B. Brown adipose tissue: function and physiological significance. Physiological Reviews. 2004;84(1):277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 21.Shabalina I.G., Petrovic N., deJong J.M.A., Kalinovich A.V., Cannon B., Nedergaard J. UCP1 in Brite/Beige adipose tissue mitochondria is functionally thermogenic. Cell Reports. 2013;5(5):1196–1203. doi: 10.1016/j.celrep.2013.10.044. [DOI] [PubMed] [Google Scholar]
- 22.Seale P., Conroe H.M., Estall J., Kajimura S., Frontini A., Ishibashi J. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. Journal of Clinical Investigation. 2011;121(1):96–105. doi: 10.1172/JCI44271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fisher F.F., Kleiner S., Douris N., Fox E.C., Mepani R.J., Verdeguer F. FGF21 regulates PGC-1α and browning of white adipose tissues in adaptive thermogenesis. Genes & Development. 2012;26(3):271–281. doi: 10.1101/gad.177857.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bordicchia M., Liu D., Amri E.Z., Ailhaud G., Dessì-Fulgheri P., Zhang C. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. Journal of Clinical Investigation. 2012;122(3):1022–1036. doi: 10.1172/JCI59701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Orava J., Nuutila P., Lidell M.E., Oikonen V., Noponen T., Viljanen T. Different metabolic responses of human brown adipose tissue to activation by cold and insulin. Cell Metabolism. 2011;14(2):272–279. doi: 10.1016/j.cmet.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 26.Wu J., Bostrom P., Sparks L.M., Ye L., Choi J.H., Giang A.H. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012 doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y., Foncea R., Deis J.A., Guo H., Bernlohr D.A., Chen X. Lipocalin 2 expression and secretion is highly regulated by metabolic stress, cytokines, and nutrients in adipocytes. PLoS One. 2014;9(5):1–9. doi: 10.1371/journal.pone.0096997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo H., Jin D., Zhang Y., Wright W., Bazuine M., Brockman D.A. Lipocalin-2 deficiency impairs thermogenesis and potentiates diet-induced insulin resistance in mice. Diabetes. 2010;59(6):1376–1385. doi: 10.2337/db09-1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Y., Guo H., Deis J.A., Mashek M.G., Zhao M., Ariyakumar D. Lipocalin 2 regulates brown fat activation via a nonadrenergic activation mechanism. Journal of Biological Chemistry. 2014;289(32):22063–22077. doi: 10.1074/jbc.M114.559104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Law I.K.M., Xu A., Lam K.S.L., Berger T., Mak T.W., Vanhoutte P.M. Lipocalin-2 deficiency attenuates insulin resistance associated with aging and obesity. Diabetes. 2010;59(4):872–882. doi: 10.2337/db09-1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jun L.S., Siddall C.P., Rosen E.D. A minor role for lipocalin 2 in high-fat diet-induced glucose intolerance. The Australian Journal of Pharmacy: Endocrinology and Metabolism. 2011;301(5):E825–E835. doi: 10.1152/ajpendo.00147.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mosialou I., Shikhel S., Liu J.M., Maurizi A., Luo N., He Z. MC4R-dependent suppression of appetite by bone-derived lipocalin 2. Nature. 2017;543(7645):385–390. doi: 10.1038/nature21697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo H., Zhang Y., Brockman D.A., Hahn W., Bernlohr D.A., Chen X. Lipocalin 2 deficiency alters estradiol production and estrogen receptor signaling in female mice. Endocrinology. 2012;153(3):1183–1193. doi: 10.1210/en.2011-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Naudé P.J.W., Eisel U.L.M., Comijs H.C., Groenewold N.A., De Deyn P.P., Bosker F.J. Neutrophil gelatinase-associated lipocalin: a novel inflammatory marker associated with late-life depression. Journal of Psychosomatic Research. 2013:444–450. doi: 10.1016/j.jpsychores.2013.08.023. [DOI] [PubMed] [Google Scholar]
- 35.Kopecky J., Clarke G., Enerbäck S., Spiegelman B., Kozak L.P. Expression of the mitochondrial uncoupling protein gene from the aP2 gene promoter prevents genetic obesity. Journal of Clinical Investigation. 1995;96(6):2914–2923. doi: 10.1172/JCI118363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burkewitz K., Zhang Y., Mair W.B. Cell metabolism AMPK at the nexus of energetics and aging. Cell Metabolism. 2014;20:10–25. doi: 10.1016/j.cmet.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deis J.A., Guo H., Wu Y., Liu C., Bernlohr D.A., Chen X. Lipocalin 2 regulates retinoic acid-induced activation of beige adipocytes. Journal of Molecular Endocrinology. 2018;61(3):115–126. doi: 10.1530/JME-18-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harms M., Seale P. Brown and beige fat: development, function and therapeutic potential. Nature Medicine. 2013;19(10):1252–1263. doi: 10.1038/nm.3361. [DOI] [PubMed] [Google Scholar]
- 39.Prince A., Zhang Y., Croniger C., Puchowicz M. Oxygen Transport to Tissue. 2014;812:323–328. XXXVI. [Google Scholar]
- 40.Bijland S., Mancini S.J., Salt I.P. Role of AMP-activated protein kinase in adipose tissue metabolism and inflammation. Clinical Science. 2013;124(8):491–507. doi: 10.1042/CS20120536. [DOI] [PubMed] [Google Scholar]
- 41.Wan Z., Root-Mccaig J., Castellani L., Kemp B.E., Steinberg G.R., Wright D.C. Evidence for the role of AMPK in regulating PGC-1 alpha expression and mitochondrial proteins in mouse epididymal adipose tissue. Obesity. 2014;22(3):730–738. doi: 10.1002/oby.20605. [DOI] [PubMed] [Google Scholar]
- 42.Mottillo E.P., Desjardins E.M., Crane J.D., Smith B.K., Green A.E., Ducommun S. Lack of adipocyte AMPK exacerbates insulin resistance and hepatic steatosis through Brown and beige adipose tissue function. Cell Metabolism. 2016;24(1):118–129. doi: 10.1016/j.cmet.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ye P., Zhang X.J., Wang Z.J., Zhang C. Effect of aging on the expression of peroxisome proliferator-activated receptor γ and the possible relation to insulin resistance. Gerontology. 2006;52(2):69–75. doi: 10.1159/000090951. [DOI] [PubMed] [Google Scholar]
- 44.Okuno A., Tamemoto H., Tobe K., Ueki K., Mori Y., Iwamoto K. Troglitazone increases the number of small adipocytes without the change of white adipose tissue mass in obese Zucker rats. Journal of Clinical Investigation. 1998;101(6):1354–1361. doi: 10.1172/JCI1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frith J., Newton J.L. Liver disease in older people. CME Journal Geriatric Medicine. 2009;11(3):110–114. [Google Scholar]
- 46.Polyzos S.A., Kountouras J., Mantzoros C.S. Adipokines in nonalcoholic fatty liver disease. Metabolism - Clinical and Experimental. 2016;65(8):1062–1079. doi: 10.1016/j.metabol.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 47.Wang G.X., Zhao X.Y., Meng Z.X., Kern M., Dietrich A., Chen Z. The brown fat-enriched secreted factor Nrg4 preserves metabolic homeostasis through attenuation of hepatic lipogenesis. Nature Medicine. 2014;20(12):1436–1443. doi: 10.1038/nm.3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Devireddy L.R., Gazin C., Zhu X., Green M.R. A cell-surface receptor for lipocalin 24p3 selectively mediates apoptosis and iron uptake. Cell. 2005 doi: 10.1016/j.cell.2005.10.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.