Abstract
Objective
To determine whether extending initial prednisolone treatment from eight to 16 weeks in children with idiopathic steroid sensitive nephrotic syndrome improves the pattern of disease relapse.
Design
Double blind, parallel group, phase III randomised placebo controlled trial, including a cost effectiveness analysis.
Setting
125 UK National Health Service district general hospitals and tertiary paediatric nephrology centres.
Participants
237 children aged 1-14 years with a first episode of steroid sensitive nephrotic syndrome.
Interventions
Children were randomised to receive an extended 16 week course of prednisolone (total dose 3150 mg/m2) or a standard eight week course of prednisolone (total dose 2240 mg/m2). The drug was supplied as 5 mg tablets alongside matching placebo so that participants in both groups received the same number of tablets at any time point in the study. A minimisation algorithm ensured balanced treatment allocation by ethnicity (South Asian, white, or other) and age (5 years or less, 6 years or more).
Main outcome measures
The primary outcome measure was time to first relapse over a minimum follow-up of 24 months. Secondary outcome measures were relapse rate, incidence of frequently relapsing nephrotic syndrome and steroid dependent nephrotic syndrome, use of alternative immunosuppressive treatment, rates of adverse events, behavioural change using the Achenbach child behaviour checklist, quality adjusted life years, and cost effectiveness from a healthcare perspective. Analysis was by intention to treat.
Results
No significant difference was found in time to first relapse (hazard ratio 0.87, 95% confidence interval 0.65 to 1.17, log rank P=0.28) or in the incidence of frequently relapsing nephrotic syndrome (extended course 60/114 (53%) v standard course 55/109 (50%), P=0.75), steroid dependent nephrotic syndrome (48/114 (42%) v 48/109 (44%), P=0.77), or requirement for alternative immunosuppressive treatment (62/114 (54%) v 61/109 (56%), P=0.81). Total prednisolone dose after completion of the trial drug was 6674 mg for the extended course versus 5475 mg for the standard course (P=0.07). There were no statistically significant differences in serious adverse event rates (extended course 19/114 (17%) v standard course 27/109 (25%), P=0.13) or adverse event rates, with the exception of behaviour, which was poorer in the standard course group. Scores on the Achenbach child behaviour checklist did not, however, differ. Extended course treatment was associated with a mean increase in generic quality of life (0.0162 additional quality adjusted life years, 95% confidence interval −0.005 to 0.037) and cost savings (difference −£1673 ($2160; €1930), 95% confidence interval −£3455 to £109).
Conclusions
Clinical outcomes did not improve when the initial course of prednisolone treatment was extended from eight to 16 weeks in UK children with steroid sensitive nephrotic syndrome. However, evidence was found of a short term health economic benefit through reduced resource use and increased quality of life.
Trial registration
ISRCTN16645249; EudraCT 2010-022489-29.
Introduction
Idiopathic nephrotic syndrome is the commonest childhood glomerular disorder, with an annual incidence of two per 100 000 children in the United Kingdom. Children present with the disease at a median age of 2-3 years, and it is twice as common in boys and four to six times more common in people of South Asian origin.1 2 3 4
More than 90% of children who present with idiopathic nephrotic syndrome respond to a course of high dose corticosteroid treatment, and current practice is to treat most patients empirically with prednisolone.5 6 Children who respond to treatment are given a diagnostic label of steroid sensitive nephrotic syndrome and generally have a good prognosis with a low incidence of end stage renal disease.
After initial successful treatment, around 80% of children with steroid sensitive nephrotic syndrome have disease relapses requiring further courses of high dose prednisolone. About 50% develop frequently relapsing nephrotic syndrome (two or more relapses within six months of presentation or four relapses within any 12 months) or steroid dependent nephrotic syndrome (relapse while receiving prednisolone or within 14 days of stopping the drug).7 Relapses and further high dose prednisolone treatment are associated with substantial morbidity.8 When complications develop or are anticipated after repeated courses of corticosteroids, alternative immunosuppressive treatment is indicated, such as levamisole, cyclophosphamide, ciclosporin, tacrolimus, mycophenolate mofetil, or rituximab.
The best initial prednisolone regimen for children presenting with steroid sensitive nephrotic syndrome remains unknown. The eight week course first described in the 1960s by the International Study of Kidney Disease in Children (prednisolone 60 mg/m2 for four weeks then 40 mg/m2 on alternate days for four weeks) continues to be used in most UK centres and in many other countries. However, systematic review data suggest that a more intensive initial treatment course improves clinical outcomes.9 When the prednisolone in nephrotic syndrome (PREDNOS) trial started, six randomised controlled trials had compared the eight week course with a range of different prednisolone regimens of three months or longer.10 11 12 13 14 15 A 2005 Cochrane review concluded that prednisolone treatment of three months or longer statistically significantly reduced the rate of relapse at 12-24 months and the rate of frequently relapsing nephrotic syndrome.16 The Kidney Disease: Improving Global Outcomes guidelines published in 2012 supported the conclusions of this Cochrane review. These guidelines recommended daily prednisolone treatment of 60 mg/m2 or 2 mg/kg for four to six weeks followed by 40 mg/m2 or 1.5 mg/kg on alternate days and continued for two to five months, with tapering of the dose.17 Despite these recommendations, several methodological concerns relating to these six studies have resulted in the continued use of the eight week course in the UK and elsewhere.
We conducted the PREDNOS trial to compare this eight week course with a longer 16 week course in UK children. The study design was optimised to overcome the methodological concerns relating to previous studies. We first performed an external pilot study over a year that included 55 participants. This pilot study helped us to develop the design of the main trial and found that participants of different ethnicities could be recruited in district hospitals and tertiary nephrology centres. The primary objective of the main trial was to determine whether an initial 16 week extended course of prednisolone treatment increased the time to first relapse in children with steroid sensitive nephrotic syndrome compared with the eight week standard course. Secondary objectives were to determine whether the extended course reduced the relapse rate, the proportion of participants who developed frequently relapsing nephrotic syndrome or steroid dependent nephrotic syndrome, and the requirement for second and third line immunosuppressive agents. Additionally, we considered whether the extended course was associated with an increased incidence of corticosteroid related adverse events, including behavioural problems. We also performed a cost effectiveness analysis by comparing costs and quality adjusted life years for the two regimens, and the methods and results are reported in supplementary appendices 1, 3, and 4.
Methods
Participants
We performed this double blind, placebo controlled, randomised controlled trial across 125 UK National Health Service district general hospitals and tertiary paediatric nephrology centres. Children aged 1-14 years with a first episode of idiopathic nephrotic syndrome were eligible to participate if they had a first morning urine protein to creatinine ratio or albumin to creatinine ratio greater than 200 mg/mmol; had a serum or plasma albumin level less than 25 g/L; had not previously received treatment with corticosteroids, immunosuppressive or cytotoxic drugs for any form of renal disease; and had no evidence of underlying systemic disorder or use of drugs known to be associated with nephrotic syndrome. We excluded children with histological changes other than minimal change glomerulonephritis (when renal biopsy had been performed), history of poor adherence to treatment, or a known allergy to prednisolone.
Interventions and randomisation
Potential participants started prednisolone 60 mg/m2 daily for four weeks in accordance with routine clinical practice. Trial recruitment and randomisation took place when the children were thought to be corticosteroid sensitive (three consecutive days of zero or trace proteinuria on Albustix test; generally between 14 and 21 days after start of prednisolone treatment). We obtained fully informed written consent from the parents or guardians and assent from children when age appropriate. We then performed randomisation online through a secure 24 hour internet based randomisation service or by a telephone call to the Birmingham Clinical Trials Unit. Participants were randomised in a 1:1 ratio to either an extended course of treatment or a standard course of treatment by using a minimisation algorithm to ensure balanced treatment allocation by ethnicity (South Asian, white, or other) and age (5 years or less, 6 years or more). The extended treatment group received prednisolone 60 mg/m2 daily (maximum 80 mg) for four weeks followed by 12 weeks of prednisolone treatment on alternate days, starting at 60 mg/m2 (maximum 80 mg) and tapering by 10 mg/m2 every two weeks (total dose 3150 mg/m2). The standard treatment group received prednisolone 60 mg/m2 daily (maximum 80 mg) for four weeks followed by 40 mg/m2 (maximum 60 mg) on alternate days for four weeks (total dose 2240 mg/m2). In both groups, treatment in the first four weeks was open label and then it was blinded in the following 12 week phase, with matching placebo in the standard course (control) group. A central pharmacy dispensed the entire course of blinded trial drugs in crushable tablet form in blister packs, delivered by courier to the family home.
In accordance with routine clinical practice, children’s first morning urine was tested for proteinuria (Albustix). We provided parents with a diary so that they could record urine test results and the drugs administered on a daily basis. Parents also used the diary to record illnesses and consultations with healthcare professionals (such as general practitioners, nurses, or hospital emergency department clinicians), and details of drugs prescribed or purchased over the counter. Families contacted their local trial site if the child had a relapse (urine analysis showed three consecutive days of 3+ proteinuria or generalised oedema in association with 3+ proteinuria) so that treatment could be prescribed. We also instructed families to call their local site if they had any other concerns—for example, if their child had developed new adverse events or they had questions about urine analysis results.
Trial assessments and data collection
We performed trial visits at 4, 8, 12, and 16 weeks, and then at 5, 6, 8, 10, 12, 18, 24, 30, 36, 42, and 48 months after the children started treatment with open label prednisolone. They were followed up for a minimum of 24 months and up to a maximum of 48 months. The trial finished when the last participant had completed 24 months of follow-up. At each trial visit, we recorded information on relapses, adherence to trial treatment, other drug treatments, adverse events, and use of healthcare resources. A full clinical assessment was performed, including height, weight, and blood pressure measurements. Any event that resulted in death, was life threatening, required admission to hospital or prolonged an existing hospital admission, caused persistent or major disability or incapacity, or resulted in a congenital anomaly or birth defect was considered a serious adverse event. These events were reported using specific forms.
Data recorded on case report forms were generally not the source data for clinical information. However, when self reported patient information on relapses and drug changes was taken from diaries and entered onto case report forms, these forms were considered to be the source data. We did not verify source data taken from diaries because the diaries were not retained.
Parents completed three questionnaires at 4 and 16 weeks, and then at 12, 24, 36, and 48 months: the Achenbach child behaviour checklist was used to assess behavioural change in eight categories (anxious/depressed, withdrawn/depressed, somatic complaints, social problems, thought problems, attention problems, rule-breaking behaviour, and aggressive behaviour), and the paediatric quality of life inventory and the child health utility 9 dimension were used to assess quality of life.18 19 20
Sample size
The primary analysis was based on a log rank test of time to relapse. We expected a relapse rate of 60% at one year in the standard course group. To detect an absolute difference of 20% (considered a clinically meaningful difference by the paediatric nephrologists on the study team) in the relapse rate, from 60% in the standard course group to 40% in the extended course group, with 80% power and α=0.05, we needed 200 participants. Allowing for a dropout rate of 15%, the total number of participants required was 236 (118 in each group).
Statistical analysis
The primary outcome measure was the time from starting open label prednisolone treatment to first relapse. We used Kaplan-Meier survival curves to visually present the time to first relapse. We compared the primary analysis of time to first relapse between groups using a log rank test. A Cox proportional hazard model was fitted to obtain a hazard ratio and 95% confidence intervals. As a secondary analysis, we also fitted a Cox proportional hazard model, which was adjusted for the minimisation variables of ethnicity (South Asian, white, or other) and age (5 years or less, 6 years or more). It is unlikely that a child will have a relapse while taking corticosteroids; however, it is possible for children in the standard course group to experience an early relapse in weeks 9-16 when receiving placebo, which could bias the results in favour of the extended course group. Corticosteroid dependency could also differ between the groups (defined as relapsing while receiving corticosteroid treatment or within 14 days of stopping the drug). To avoid the potential for bias in these situations, we set the relapse time to 18 weeks in children who had a relapse before 18 weeks. We also performed a secondary analysis of time to first relapse using the actual relapse date. Two a priori subgroup analyses were carried out for the primary outcome for the minimisation variables of ethnicity (South Asian, white, or other) and age (5 years or less, 6 years or more). We included a treatment group by subgroup interaction parameter in the Cox proportional hazard model to assess whether there were any differences in the treatment effect across the different stratums.
Secondary outcomes were relapse rate, incidence of frequently relapsing nephrotic syndrome and of steroid dependent nephrotic syndrome, use of second line immunosuppressive drugs, rates of adverse events and serious adverse events, behavioural change using the Achenbach child behaviour checklist, and quality adjusted life years. We compared relapse rates (number for each child) using a negative binomial model to obtain an incident rate ratio. Categorical data items (eg, frequently relapsing nephrotic syndrome, steroid dependent nephrotic syndrome) were analysed using a χ2 test, and relative risks were produced. We reported adverse event data on a Likert scale (none, mild, moderate, or severe). These data were dichotomised according to whether the participant experienced the adverse event or not, and relative risks were reported. The number of children who reported a serious adverse event was reported. The Achenbach child behaviour checklist was analysed using repeated measures methods. We converted the exploratory outcomes height, weight, body mass index, and blood pressure values to standard deviation scores and presented them graphically using longitudinal plots, with no statistical analysis planned.
We included participants in the analysis according to their initial randomised treatment allocation. We excluded participants who were found to be corticosteroid resistant after randomisation. This is not expected to introduce bias because most of the dropouts occurred before the start of randomised treatment, and clinicians were unaware of the treatment assigned to their patients. We describe this analysis as intention to treat. A hazard ratio or relative risk less than 1 favoured the children who received an extended course of treatment. Estimates of treatment effects are presented with 95% confidence intervals. P values are two tailed with a P value less than 0.05 considered statistically significant. Analyses were carried out using SAS version 9.4 (Cary, NC) or Stata 14 (StataCorp, College Station, TX).
Patient and public involvement
The trial protocol was reviewed by representatives of the UK Nephrotic Syndrome Trust (NeST) and the UK Renal Patient Support Group, who provided valuable input about trial design, acceptability of trial visit frequency, and adverse event monitoring. A NeST representative participated on the trial steering committee. After publication, the trial results will be disseminated to all study collaborators. The plain English summary of the study results will be sent to the participants and/or their parents through their responsible clinician. The summary will also be available on the NeST website and the PREDNOS study website (www.birmingham.ac.uk/prednos).
Results
Participants
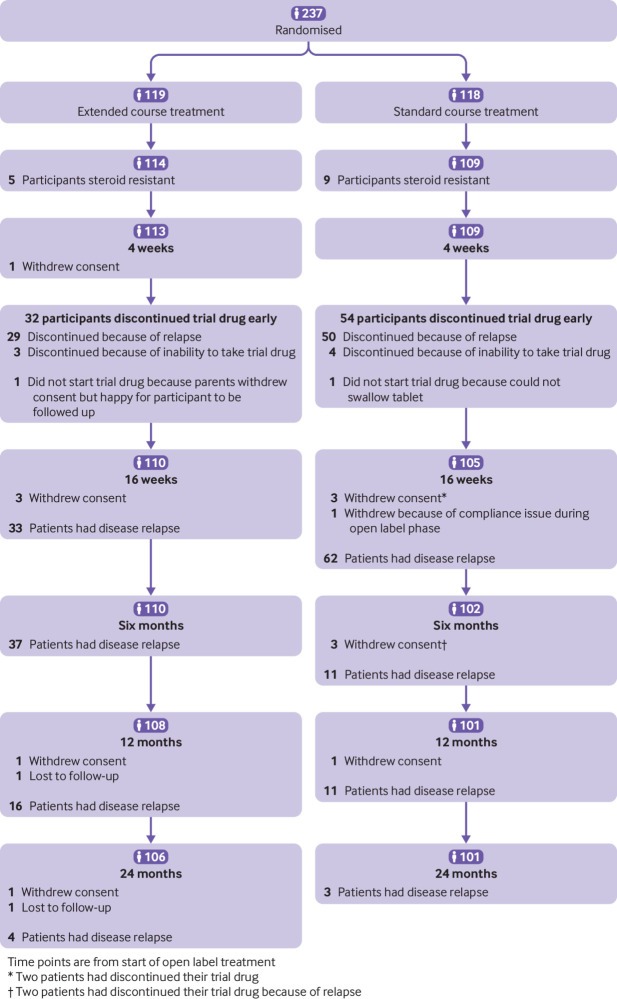
Overall, 237 participants aged 1-14 years were recruited from 86 centres between July 2011 and October 2014; 119 were randomised to an extended course of treatment and 118 to a standard course of treatment. Fourteen (five in the extended course group and nine in the standard course group) were withdrawn early after randomisation because of loss of corticosteroid sensitivity, leaving an intention to treat analysis population of 223 (114 in the extended course group and 109 in the standard course group). Figure 1 shows the patient flow through the trial. When participants withdrew consent or were lost to follow-up, data collected up until that point were included in the analysis.
Fig 1.
CONSORT diagram of participant flow through trial
Baseline characteristics did not differ between the extended course and standard course groups (table 1). Most of the intention to treat population were boys (65%, n=146), 65% (n=145) were aged less than 6 years, and 20% (n=44) were of South Asian origin. Eighty six (39%) did not complete their course of trial drugs. This was more common in the standard course group (extended course 32/114 (28%) v standard course 54/109 (50%); P=0.001) and the main reason was relapse while double blind trial drugs were being administered. Adherence to trial drugs was high; 13% (n=29) reported missed doses and most of these patients reported missing only one or two doses. Attendance rates for follow-up trial visits were high, as were submission rates of clinical data and participant questionnaires (>90% of expected data received at each time point).
Table 1.
Baseline characteristics of intention to treat population. Values are numbers (percentages) unless stated otherwise
| Characteristics | Extended course group (n=119) | Standard course group (n=118) | Total (n=237) |
|---|---|---|---|
| No of corticosteroid sensitive participants | 114 | 109 | 223 |
| Mean (SD) age (years) | 5.1 (3.2) | 4.7 (2.9) | 4.9 (3.1) |
| Age (years): | |||
| 1-2 | 28 (25) | 29 (27) | 57 (26) |
| 3-5 | 45 (39) | 43 (39) | 88 (39) |
| 6-11 | 35 (31) | 34 (31) | 69 (31) |
| 12-17* | 6 (5) | 3 (3) | 9 (4) |
| Age (years)†: | |||
| ≤5 | 73 (64) | 72 (66) | 145 (65) |
| ≥6 | 41 (36) | 37 (34) | 78 (35) |
| Boys | 68 (60) | 78 (72) | 146 (65) |
| Ethnicity†: | |||
| South Asian | 23 (20) | 21 (19) | 44 (20) |
| White | 75 (66) | 73 (67) | 148 (66) |
| Other or not stated | 16 (14) | 15 (14) | 31 (14) |
| Median (interquartile range) body mass index centile | 90.0 (69.5-97.5) | 85.3 (66.3-97.3) | 87.5 (66.6-97.3) |
| Body mass index centile: | |||
| Underweight (<5th) | 0 (0) | 2 (2) | 2 (1) |
| Healthy (5th-84th) | 48 (42) | 52 (48) | 100 (45) |
| Overweight (85th-95th) | 24 (21) | 19 (17) | 43 (19) |
| Obese (≥95th) | 42 (37) | 36 (33) | 78 (35) |
| Mean (SD) open label daily prednisolone dose (mg/m2) | 58.0 (6.8) | 58.5 (5.9) | 58.2 (6.4) |
Participants were eligible for the trial if they were aged 1-14 years at time of diagnosis.
Minimisation variable.
Primary outcome
No statistically significant difference was found in the primary outcome measure of time to first relapse between the extended course and standard course groups (fig 2; hazard ratio 0.87, 95% confidence interval 0.65 to 1.17; log rank P=0.28). The median time to first relapse was 139 (interquartile range 90-179) days for the extended course group versus 87 (64.5-134) days for the standard course group. Eighty per cent (n=179) of participants reported a relapse during trial follow-up (extended course 91/114 (80%) v standard course 88/109 (81%); difference −1%, 95% confidence interval −11% to 10%; table 2).
Fig 2.
Time to first relapse in participants receiving extended or standard course of prednisolone treatment
Table 2.
Secondary outcome measures in extended course and standard course groups. Values are numbers (percentages) unless stated otherwise
| Secondary outcome measures | Extended course group | Standard course group | Estimate (extended v short course) (95% CI) | P value |
|---|---|---|---|---|
| Relapse | n=114 | n=109 | ||
| No of relapses | 454 | 394 | — | |
| No of participants who had a relapse | 91 (80) | 88 (81) | 0.87* (0.65 to 1.17) | 0.28 |
| Mean (SD) No of relapses for each participant | 3.98 (3.30) | 3.61 (3.25) | 1.09† (0.86 to 1.39) | 0.46 |
| No of participants who had FRNS | 60 (53) | 55 (50) | 1.04‡ (0.81 to 1.35) | 0.75 |
| No of participants who had SDNS | 48 (42) | 48 (44) | 0.96‡ (0.71 to 1.29) | 0.77 |
| Second line immunosuppressive drugs | ||||
| No of participants who received immunosuppressant drugs | 62 (54) | 61 (56) | 0.97† (0.77 to 1.23) | 0.81 |
| Type of immunosuppressant drug: | ||||
| Ciclosporin | 4 (4) | 6 (6) | — | |
| Tacrolimus | 18 (16) | 8 (7) | — | |
| Levamisole | 34 (30) | 35 (32) | — | |
| Cyclophosphamide | 29 (25) | 31 (28) | — | |
| Mycophenolate mofetil | 15 (13) | 13 (12) | — | |
| Rituximab | 1 (1) | 5 (5) | — | |
| Corticosteroid dose | n=94 | n=90 | — | |
| Mean (SD) total prednisolone dose (mg)‡ | 6674.1 (4998.2) | 5474.6 (3697.3) | Mean difference=1199.5 (−83.8 to 2482.8) | 0.07 |
A ratio less than 1, and a negative mean difference, favours the extended course group.
FRNS=frequently relapsing nephrotic syndrome; SDNS=steroid dependent nephrotic syndrome.
Hazard ratio.
Incident rate ratio.
Relative risk.
Total dose received during trial (after completion of trial drug).
Prespecified subgroup analyses for the primary outcome for ethnicity (South Asian, white, or other) and age (5 years or less, 6 years or more) showed no clear evidence to suggest that the treatment effect differed between the participant subgroups. We found some evidence (P for interaction=0.08) that time to first relapse was increased in participants in the extended course group aged 5 years or less (hazard ratio 0.72, 95% confidence interval 0.50 to 1.05) compared with participants aged 6 years or more. In participants aged 6 years or more, time to first relapse was increased in those in the standard course group (1.26, 0.77 to 2.07).
Secondary outcomes
The total number of relapses in each participant ranged from zero to 15; nine participants in the extended course group and eight in the standard course group had 10 or more relapses. We found no differences in the mean number of relapses, the proportion developing frequently relapsing or steroid dependent nephrotic syndrome, or the number requiring alternative immunosuppressive treatment. The total dose of prednisolone received during the trial (after completing the course of trial drugs) was not statistically significantly different between the two treatment groups (table 2).
Adverse events and behaviour
Table 3 shows the cumulative incidence of adverse events over the 24 months of follow-up. We found no differences between the two groups for any of these adverse events except for poor behaviour reported by parents (yes or no), which was more common in the standard course group. However, when we analysed the detailed quantitative behavioural data collected using the Achenbach child behaviour checklist we found no differences in behaviour score (P=0.28) or the proportion of participants reporting normal checklist scores at any time point during the trial.
Table 3.
Cumulative incidence of adverse events over 24 months of follow-up. Values are numbers (percentages) unless stated otherwise
| Adverse event | Extended course group (n=114) | Standard course group (n=109) | Relative risk (95% CI) |
|---|---|---|---|
| Cushingoid facies | 83 (73) | 78 (72) | 1.02 (0.88 to 1.19) |
| Striae | 14 (12) | 7 (6) | 1.92 (0.81 to 4.54) |
| Hypertrichosis | 45 (39) | 41 (38) | 1.05 (0.77 to 1.45) |
| Acne | 12 (11) | 7 (6) | 1.64 (0.68 to 3.99) |
| Increased appetite | 106 (93) | 103 (94) | 1.00 (0.94 to 1.07) |
| Poor behaviour | 94 (82) | 101 (93) | 0.90 (0.82 to 0.98) |
| Glycosuria | 19 (17) | 14 (13) | 1.34 (0.72 to 2.48) |
| Cataract | 1 (1) | 1 (1) | 0.96 (0.06 to 15.00) |
| Abdominal pain | 49 (43) | 51 (47) | 0.91 (0.69 to 1.20) |
Serious adverse events
Forty six participants reported 67 serious adverse events (extended course 19/114 (17%) v standard course 27/109 (25%); P=0.13). Serious adverse events most commonly related to admission for disease relapse or bacterial infection. We considered six serious adverse events in the extended course group and five in the standard course group to be drug related, although none resulted in discontinuation of the trial drug. The one death due to unintentional injury was unrelated to the trial.
Exploratory outcomes
The mean height, weight, and body mass index z scores did not differ between the two treatment groups at any time point throughout the trial period. We found a progressive increase in height z score and decrease in body mass index z score over the trial period (supplementary figs 1 and 2). No differences were found in mean systolic and diastolic blood pressure between the two treatment groups at any time point throughout the trial, with a trend to reduction in z score with increasing time after presentation (supplementary fig 3).
Cost effectiveness analysis
Supplementary appendices 1, 3, and 4 report full details of the economic evaluation. In brief, we found the extended course of treatment to be cheaper than the standard course after allowing for all primary care, secondary care, and prescription costs over 24 months. Furthermore, the extended course produced a small incremental gain in quality of life compared with the standard course. These findings mean that the extended course was a cost effective use of healthcare resources when conventional rules of cost effectiveness were applied.
Discussion
The PREDNOS trial recruited UK children with steroid sensitive nephrotic syndrome and compared an initial extended 16 week course of prednisolone treatment with the standard eight week course described by the International Study of Kidney Disease in Children. The results did not show any clinical benefit on key primary and secondary clinical endpoints. We found no statistically significant difference between the two treatment groups in the outcome measures: time to first relapse of nephrotic syndrome; incidence of any relapse or the number of relapses experienced; the proportion of participants who went on to develop frequently relapsing or steroid dependent nephrotic syndrome; or the requirement for alternative non-corticosteroid immunosuppressive treatment. Confidence intervals for the incidence of any relapse excluded clinically important benefit. Subgroup analyses showed no clear evidence that the treatment effect differed according to ethnicity or age, although the trial was not powered to detect differences in subgroups.
Although there is no evidence that an extended course of prednisolone treatment statistically significantly reduces clinical endpoints, the direction of the effect was to delay the time to relapse. We found some evidence that an extended course of prednisolone reduced healthcare resource use in the first two years and resulted in a small improvement in quality of life. Combined in a cost effectiveness analysis, these findings provide evidence that an extended course of treatment is a cost effective use of healthcare resources.
Strengths and weaknesses of this study
The randomised, double blind, placebo controlled trial design ensured a low risk of selection, performance, detection, and selective reporting bias. The unselected trial population with broad inclusion criteria that was recruited from 86 centres across the UK included 44 (20%) participants from the South Asian community. The trial was therefore representative of UK children presenting with steroid sensitive nephrotic syndrome. The inclusion of a substantial proportion of participants of South Asian origin is of particular importance because of the increased incidence of the disease in this group. In addition, the UK South Asian population is generally under-represented in clinical trials, with recruitment posing several challenges.21
We managed to recruit around one third of all UK children who presented with steroid sensitive nephrotic syndrome over the trial period, which indicates a high level of acceptance of the trial among patients and clinicians. We used internationally recognised definitions of disease outcomes from the International Study of Kidney Disease in Children. We also chose a primary outcome measure that was believed to be of great clinical importance by clinicians and patient advisors and was consistent with the outcome measures used in many other studies. We systematically produced data on adverse effects related to corticosteroid treatment; these data are clinically relevant and of great importance to families but are often ignored. Baseline features were well balanced and the dropout rate was low and rate of completion of visits high.
Possible weaknesses include the potential exclusion of young children who were unable to take the trial drug, which was provided as a crushable tablet, rather than a suspension or in soluble or dispersible form. We found 14 participants to be steroid resistant after randomisation but before any difference in treatment regimen started, and we withdrew these children from the trial. In practice, these patients would not receive the extended or standard course of treatment, and so the intention to treat analysis was not compromised. The mean age of participants was 4.9 years, which is higher than the median age of presentation in the International Study of Kidney Disease in Children (3 years). The mean age in our trial is, however, comparable to the median ages of 4.2-6.7 years in the three most recent randomised controlled trials of corticosteroid treatment in children with steroid sensitive nephrotic syndrome.
Comparison with other studies
The Kidney Disease: Improving Global Outcomes 2012 treatment recommendations were based on six trials that were reported before the start of the PREDNOS trial, but they all had methodological deficiencies.10 11 12 13 14 15 None was adequately blinded and there were additional concerns about selection, performance, detection, and attrition biases. While we conducted the PREDNOS trial, two randomised controlled trials were reported that compared shorter and longer corticosteroid regimens. One of these trials was a high quality Japanese study that compared eight weeks of prednisolone treatment with six months of treatment.22 23 Similar to PREDNOS, the findings showed no benefit of treatment extension.
Our outcome data are similar to those reported in previous studies; in particular the overall proportion of participants who had a relapse and the rate of frequently relapsing nephrotic syndrome. Previous studies have been inconsistent in the monitoring and reporting of adverse events associated with using corticosteroids. However, meta-analyses have consistently shown no difference in risk of adverse events between an eight week course of prednisolone treatment and longer duration regimens. Similar to these findings, we found no differences in adverse events between the extended course and standard course groups in PREDNOS; one exception was poor behaviour reported by parents, which was more common in the standard course group. However, despite this difference in reported behaviour, when parents completed the Achenbach child behaviour checklist, no statistically significant differences in behaviour scores were detected that might indicate a clinical concern. Consistent with current UK clinical practice, in this trial we did not routinely perform formal slit lamp ophthalmic assessment, regular blood tests, or dual energy x ray absorptiometry scans to assess bone mineral density. These tests have been included in the protocols of other recent high quality randomised controlled trials; the overall incidence of cataract, major persistent biochemical abnormality, and bone mineral density abnormality was low.
Our data suggest a possible advantage of an extended course of prednisolone treatment in children younger than 6 years, although the trial was not powered to detect differences in subgroups. This advantage has also been identified in other randomised controlled trials23 and warrants further investigation. We suggest an individual patient data meta-analysis, particularly as several studies have shown that children aged less than 6 years generally have a higher risk of frequently relapsing nephrotic syndrome and steroid dependent nephrotic syndrome.11 23 24 25 The shift of evidence away from the use of an extended course of treatment back to a standard course of treatment raises the problem of whether further studies should investigate if corticosteroid courses can be reduced further in steroid sensitive nephrotic syndrome. This subject has been dealt with in an early randomised controlled trial, which showed the relapse rate and incidence of frequently relapsing nephrotic syndrome were higher in children who received a shorter course of treatment rather than a standard course of treatment.26 However, in common with many of the earlier studies in this disease group, the trial was at risk of several biases.
The supplementary material reports the economic evaluation, which shows the extended course of treatment to be cost effective. We found healthcare costs in the extended course group were reduced because of fewer primary care visits and hospital admissions, lower costs of some drugs, and increased quality of life measured at various time points. These economic observations are compatible with the clinical outcomes because they act in the same direction as the increase in time to relapse with the extended course of treatment. Cost effectiveness analyses focus on the combined ratio of costs and outcomes. In our analysis, the extended course was cheaper and produced a gain in quality of life, therefore it offered a cost effective use of resources.
Conclusions and policy implications
The PREDNOS trial provides evidence that an extended 16 week course of prednisolone treatment will not improve important clinical endpoints compared with the standard eight week course of treatment. However, depending on the decision making criteria, extended course treatment could offer a cost effective use of healthcare resources. Concerns about adverse events from extended prednisolone treatment were not supported.
What is already known on this topic
More than 90% of children who present with idiopathic nephrotic syndrome respond to a course of high dose oral corticosteroid, and current practice is to treat most patients with prednisolone
The Kidney Disease: Improving Global Outcomes 2012 treatment guidelines recommended that prednisolone be administered daily for four to six weeks followed by alternate daily for two to five months—this recommendation was based on six trials that were reported before the start of the PREDNOS trial, although all had methodological problems
Most UK centres and many other countries continue to use the standard eight week course of prednisolone first described by the International Study of Kidney Disease in Children in the 1960s
What this study adds
An extended initial 16 week course of prednisolone treatment in UK children with steroid sensitive nephrotic syndrome did not improve the pattern of disease relapse compared with those who received the standard eight week course
Extended course prednisolone treatment reduced healthcare resource use in the first two years and made a small improvement in quality of life
Concerns about adverse events from exposing children to extended prednisolone treatment were not supported
Acknowledgments
We thank the National Institute for Health Research (NIHR) Health Technology Assessment for funding this trial; Kidney Research UK and Kid’s Kidney Research for funding the pilot trial; the participants and their parents; the NIHR Clinical Research Network: Children for support with the trial set-up and recruitment; the clinical trials pharmacy at Birmingham Children’s Hospital for dispensing and distributing the trial drug; Elisabeth Hodson and Jonathan Craig of the Cochrane Kidney and Transplant Group; the service users group from the UK Nephrotic Syndrome Trust and the renal patient support group, in particular Wendy Cook, Samantha Davies-Abbott, and Shahid Muhammad for help with trial design and conduct and review of the English summary; the British Association for Paediatric Nephrology; and the Royal College of Paediatrics and Child Health.
See supplementary file for the detailed list of PREDNOS contributors.
The PREDNOS study will be published in full as Webb NJA, Woolley RL, Lambe T, et al. Sixteen week versus standard eight week prednisolone therapy for childhood nephrotic syndrome: the PREDNOS RCT. Health Technol Assess 2019;23 (www.birmingham.ac.uk/prednos).
Web extra.
Extra material supplied by authors
Supplementary information: appendices 1-5
Supplementary information: detailed list of PREDNOS contributors
Contributors: NJAW was the chief investigator, developed and ran the pilot study and the main study, led protocol development, recruited participants, interpreted study findings, and led the drafting of the paper. RLW undertook statistical analysis, interpreted study findings, contributed to writing the paper, and commented on the paper. TL performed cost effectiveness analyses and wrote the health economic content. EF developed the economic evaluation protocol, supervised economic analyses, contributed to writing the economic content, and commented on the paper. EAB oversaw the coordination and management of the study, had major input in developing the protocol, managed the delivery and execution of the study, contributed to writing the paper, and commented on the paper. ENB oversaw the coordination of the study from set-up to final report, had substantial input into the study set-up and management, and commented on the paper. RST was the original chief investigator, contributed to study design and management, and commented on the paper. CC was the statistical lead for the pilot study, designed the main study, helped with protocol development, interpreted study findings, and commented on the paper. JJD reviewed the statistical and economic analyses and results of the study, assisted in interpreting study findings, contributed to writing the paper, and commented on the paper. KW designed the study, interpreted study findings, and commented on the paper. NJI was responsible for the statistical aspects of the study, contributed to protocol development, supervised statistical analyses, interpreted study findings, contributed to writing the paper, and commented on the paper. NJAW is the guarantor. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: The PREDNOS study was funded by an investigator led grant from the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme (HTA grant reference No 08/53/31). This report presents independent research commissioned by the NIHR. The views and opinions expressed by the authors in this publication are those of the authors and do not necessarily reflect those of the UK National Health Service, the Medical Research Council, the NIHR Central Commissioning Facility, Evaluation, Trials and Studies Coordinating Centre, the HTA programme, or the Department of Health. The researchers in the PREDNOS study are independent of the funders. All authors had full access to all of the data (including statistical reports and tables) in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: NJAW has served on advisory boards within the past five years for Abbvie, Alexion, AMAG, Astellas, Raptor, Takeda, and UCB. These related to the design and conduct of early phase trials in childhood kidney disease. None related to the treatment of corticosteroid sensitive nephrotic syndrome.Ethical approval: This study was approved by the North West 7 Research Ethics Committee (10/H1008/122). The trial was carried out under a clinical trial authorisation in accordance with the Medicines for Human Use (Clinical Trials) Regulations (21761/0255/001-0001) and conducted in accordance with the Declaration of Helsinki.
Data sharing: Requests for access to data from the PREDNOS study should be addressed to the corresponding author at nicholas.webb@mft.nhs.uk. The individual participant data collected during the trial (including the data dictionary) will be available, after deidentification, when the article has been published with no end date. All proposals requesting data access will need to specify how the data will be used, and all proposals will need the approval of the trial coinvestigator team before data release.
Transparency: The lead author (NJAW) affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Contributor Information
Collaborators: PREDNOS Collaborative Group, Helen Bodenham-Chilton, Noreen Akhtar, Charmaine Hunt, Terry Hughes, Adam Khan, Nicholas Hilken, Neil Winkles, Adrian Wilcockson, Mark Hunter, Andrew Howman, Natalie Marchevsky, Tosin Lambe, Billingsley Kaambwa, Michelle James-Ellison, Hilary Marie Williams, Pronab Bala, Caroline Jones, Richard Holt, Henry Morgan, Elizabeth Bailey, Lauren Flanagan, Tariq Bhatti, Shailini Bahl, Lorna Walding, Ezzedin Gouta, Rajeev Gupta, Diarmuid Kerrin, Ami Parikh, Ann Duthie, Nimze Gadong, Bahadur Anjum, Bessie Crone, Hilarious De Jesus, Ivone Lancoma-Malcolm, Nicolene Plaatjies, Khorshid Khalifa, Karl McKeever, Grace McCall, Patricia McCreesh, Muriel Millar, Markus Hesseling, Madalitso Kubwalo, Annette Bolger, Lucie Hobson, David Milford, Larissa Kerecuk, Claire Norton, Narinder Rahania, Helen Williamson, Fatima Bibi, Tracy Gazeley, Julie Williams, Fiona Watson, Claire Abbot, Simon Frazer, Louise Akeroyd, Mansoor Ahmed, Dominic Muogbo, Claire Backhouse, Stephanie Boswell, Eilean Crosbie, Peter Heinz, Birgit Ulbrich, Shivaram Hegde, Pauline Jones, Rachel Lennon, Malcolm Lewis, Nicholas Plant, Mohan Shenoy, Shaila Sukthankar, Charlotte Bryant, Sarah Douglas, Helen Sumner, Jane Howell, Nour Elhadi, Irene Bishton, Oyekunle Ayonrinde, Andrea Turner, Jonathan Campbell, Aine Turner, John Gibbs, Elizabeth Newby, Satyanarayana Saladi, Alison Timmis, Caroline Burchett, Sarah De-Beger, Asha Nair, Antima Banerjee, Theo Fenton, Selwyn D’Costa, Richard Bowker, Coral Smith, Vanessa Unsworth, Anuja Natarajan, Lai Men Wong, Phil Parslow, Vijay Kandala, Salman Imran, Surendran Chandrasekaran, Sheng-Ang Ho, Ignatius Losa, Katrina Marinaki, Natalie Keenan, Jessica Nichols, Joanne Shippey, Javed Iqbal, Kirsty Watts, Angela Yannoulias, Zilla Huma, Adamu Sambo, Lyda Jadresic, Susan Beames, Detlef Bockenhauer, Daljit Hothi, Nick West, Helen Price, Manish Sinha, Emma Neal, Elisabeth Reus, Paula Sofocleous, Ian Cannings, Rajiv Sood, Verghese Mathew, Elsadeg Sharif, Harsha Bilolikar, Sonia White, Titi Ayeni, Ruth O’Connor, Michael Eisenhut, Tomasz Rajkowski, Samantha Clough, Rebecca Dixon, Karen Reep, Krishnan Balasubramanian, Hamudi Kisat, Janette Cansick, Julie Ellison, Sally Smith, Samantha Tapscott, Bridget Oates, Claire Bell, Rajendran Shyam, Evelyn Menzies, Pamela Cruickshanks, John Schulga, Craig Oxley, David Hughes, Amita Sharma, Leah Krischock, Jamie Houston, Diane Carroll, Sheryl Goddard, Elizabeth Waxman, Alan Webb, Thin Thin Saing, Colin Cunningham, Angela Brown, Victoria Causer, Sepideh Taheri, Alyson Macdonald, Mona Aslam, Imogen Norton, Dermot Dalton, Andrew Arend, Krishnakumar Thattakkat, Bukar Wobi, Bemigho Etuwewe, Tara Walker, Julie Browning, Marjorie Carling, Emma Killingbeck, Dorothy Ryan, Consolata Tsvangirai-Mahachi, Andrew Lunn, Martin Christian, Janet Craze, Catherine Derry, Robert Jones, Sarah-Jane Sharman, Sally Harwood, Steve Wadams, Heather Barham, Susan Power, Catherine Tuffrey, Simon Birch, Judith Scanlan, Andrew Gribbin, Sharon McCready, Ann Gordon, Gregory Boden, Susan Hallett, Chris Williams, Gillian Craig, Hannah Cottis, Vaughan Lewis, Christine McMillan, Nigel Osborne, Caroline Harrill, Suzanne Wilkins, Robert Kleta, Lynn Diskin, Amanda Billson, Alison Barratt, Gail Moss, Simon Rhodes, Anna Coupe, Caroline Moulds, Michael Smith, Sara Gilpin, Judith Ratcliffe, Sharryn Gardner, Lakshmi Chilukuri, Chris Cooper, Sara Bennett, Anjali Petkar, Ahsan Ul-Haq, Gill Waring, Rebecca Mann, Nicola Thorne, Zala Ibrahim, Jackie Buck, Deborah Beeby, Eric Finlay, Majorie Allen, Rebecca Mottram, Kathryn Deakin, Sally Johnson, Yincent Tse, Kathryn Bell, Denise Chisholm, Beena Padmakumar, Talaivirichan Magadevan, Mandy Hughes, Susan Rubin, Amy Frary, Sanjay Suri, Sherif El-Refee, Christine Harrison, Karen Davies, Sharon Kempson, Naeem Ayub, Sourabh Mukhopadhyay, Margaret Crawford, Mujeeb Pervez, Douglas Thomas, Gopikrishna Vemuri, Radhika Puttha, Alice Setti, Faisal Al-Zidgali, Claire Holliday, Louise Woodhead, Rodney Gilbert, Moin Saleem, Alison Kelly, Michelle Ross, Heather Smee, Nigel Coad, Angela Hall, Peter Houtman, Nayan Peepah Nardeosingh, Mireille-Yvette Formosa, Patrick Ward, Yvonne Slater, Saeeda Raja, Kishor Tewary, Shiva Shankar, Rachel Delyth Webb, Natalie Rogers, Raman Lakshman, Karine Cesar, Damien Armstrong, Julie Brown, Nicholas Brennan, Stuart Nicholls, Elizabeth Breen, Sharon Hughes, Lucy Lewis, John Scanlon, Munir Ahmed, Andrew Gallagher, Vineeta Joshi, Madhumita Mukherjee, Singara Velmurugan, Tracey McGregor, Emma Carson, Kay Riding, Nandu Thalange, Chris Upton, Louisa Fear, Glyn Jones, Vijay Tandle, Gabrielle Osborne, Simon Meyrick, Michael Fernando, Christopher Zaborowski, Alex Stannett, Udupa Venkatesh, Guy Millman, Simon Dyer, and Kerry Elliot
References
- 1. McKinney PA, Feltbower RG, Brocklebank JT, Fitzpatrick MM. Time trends and ethnic patterns of childhood nephrotic syndrome in Yorkshire, UK. Pediatr Nephrol 2001;16:1040-4. 10.1007/s004670100021 [DOI] [PubMed] [Google Scholar]
- 2. International Study of Kidney Disease in Children Nephrotic syndrome in children: prediction of histopathology from clinical and laboratory characteristics at time of diagnosis. A report of the International Study of Kidney Disease in Children. Kidney Int 1978;13:159-65. 10.1038/ki.1978.23 [DOI] [PubMed] [Google Scholar]
- 3. Feehally J, Kendell NP, Swift PG, Walls J. High incidence of minimal change nephrotic syndrome in Asians. Arch Dis Child 1985;60:1018-20. 10.1136/adc.60.11.1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sharples PM, Poulton J, White RH. Steroid responsive nephrotic syndrome is more common in Asians. Arch Dis Child 1985;60:1014-7. 10.1136/adc.60.11.1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Trompeter RS, Lloyd BW, Hicks J, White RH, Cameron JS. Long-term outcome for children with minimal-change nephrotic syndrome. Lancet 1985;1:368-70. 10.1016/S0140-6736(85)91387-X [DOI] [PubMed] [Google Scholar]
- 6.Webb NJA. Steroid sensitive nephrotic syndrome. In Pediatric Nephrology 2nd Edition. Ed Rees L, Brogan PA, Bockenhauer D, Webb NJA. Oxford University Press 2012. [Google Scholar]
- 7. Larkins N, Kim S, Craig J, Hodson E. Steroid-sensitive nephrotic syndrome: an evidence-based update of immunosuppressive treatment in children. Arch Dis Child 2016;101:404-8. 10.1136/archdischild-2015-308924 [DOI] [PubMed] [Google Scholar]
- 8. McCaffrey J, Lennon R, Webb NJ. The non-immunosuppressive management of childhood nephrotic syndrome. Pediatr Nephrol 2016;31:1383-402. 10.1007/s00467-015-3241-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Abramowicz M, Barnett HL, Edelmann CM, Jr, et al. Controlled trial of azathioprine in children with nephrotic syndrome. A report for the international study of kidney disease in children. Lancet 1970;1:959-61. 10.1016/S0140-6736(70)91093-7 [DOI] [PubMed] [Google Scholar]
- 10. Ehrich JH, Brodehl J. Long versus standard prednisone therapy for initial treatment of idiopathic nephrotic syndrome in children. Arbeitsgemeinschaft für Pädiatrische Nephrologie. Eur J Pediatr 1993;152:357-61. 10.1007/BF01956754 [DOI] [PubMed] [Google Scholar]
- 11. Bagga A, Hari P, Srivastava RN. Prolonged versus standard prednisolone therapy for initial episode of nephrotic syndrome. Pediatr Nephrol 1999;13:824-7. 10.1007/s004670050708 [DOI] [PubMed] [Google Scholar]
- 12. Jayantha UK. Comparison of ISKDC regime with a 7 months steroid regime in the first attack of nephrotic syndrome [abstract] Pediatr Nephrol 2004;19:C81. [Google Scholar]
- 13. Ksiazek J, Wyszyńska T. Short versus long initial prednisone treatment in steroid-sensitive nephrotic syndrome in children. Acta Paediatr 1995;84:889-93. 10.1111/j.1651-2227.1995.tb13787.x [DOI] [PubMed] [Google Scholar]
- 14. Norero C, Delucchi A, Lagos E, Rosati P, Chilean Co-operative Group of Study of Nephrotic Syndrome in Children [Initial therapy of primary nephrotic syndrome in children: evaluation in a period of 18 months of two prednisone treatment schedules]. Rev Med Chil 1996;124:567-72. [PubMed] [Google Scholar]
- 15. Ueda N, Chihara M, Kawaguchi S, et al. Intermittent versus long-term tapering prednisolone for initial therapy in children with idiopathic nephrotic syndrome. J Pediatr 1988;112:122-6. 10.1016/S0022-3476(88)80136-7 [DOI] [PubMed] [Google Scholar]
- 16. Hodson EM, Knight JF, Willis NS, Craig JC. Corticosteroid therapy for nephrotic syndrome in children. Cochrane Database Syst Rev 2005;(1):CD001533. [DOI] [PubMed] [Google Scholar]
- 17. Kidney Disease: Improving Global Outcomes (KDIGO) Glomerulonephritis Work Group KDIGO Clinical Practice Guideline for Glomerulonephritis. Kidney Int 2012;2:139-274. [Google Scholar]
- 18. Achenbach TM, Edelbrock CS. Manual for the child behavior checklist and revised behavior profile. University of Vermont Department of Psychiatry, 1983. [Google Scholar]
- 19. Varni JW, Burwinkle TM, Seid M, Skarr D. The PedsQL 4.0 as a pediatric population health measure: feasibility, reliability, and validity. Ambul Pediatr 2003;3:329-41. [DOI] [PubMed] [Google Scholar]
- 20. Stevens K. Valuation of the Child Health Utility 9D Index. Pharmacoeconomics 2012;30:729-47. 10.2165/11599120-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 21. Macneill V, Nwokoro C, Griffiths C, Grigg J, Seale C. Recruiting ethnic minority participants to a clinical trial: a qualitative study. BMJ Open 2013;3:e002750. 10.1136/bmjopen-2013-002750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yoshikawa N, Nakanishi K, Sako M, et al. Japanese Study Group of Kidney Disease in Children A multicenter randomized trial indicates initial prednisolone treatment for childhood nephrotic syndrome for two months is not inferior to six-month treatment. Kidney Int 2015;87:225-32. 10.1038/ki.2014.260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sinha A, Saha A, Kumar M, et al. Extending initial prednisolone treatment in a randomized control trial from 3 to 6 months did not significantly influence the course of illness in children with steroid-sensitive nephrotic syndrome. Kidney Int 2015;87:217-24. 10.1038/ki.2014.240 [DOI] [PubMed] [Google Scholar]
- 24. Yap HK, Han EJ, Heng CK, Gong WK. Risk factors for steroid dependency in children with idiopathic nephrotic syndrome. Pediatr Nephrol 2001;16:1049-52. 10.1007/s004670100024 [DOI] [PubMed] [Google Scholar]
- 25. Takeda A, Takimoto H, Mizusawa Y, Simoda M. Prediction of subsequent relapse in children with steroid-sensitive nephrotic syndrome. Pediatr Nephrol 2001;16:888-93. 10.1007/s004670100683 [DOI] [PubMed] [Google Scholar]
- 26. Anonymous Short versus standard prednisone therapy for initial treatment of idiopathic nephrotic syndrome in children. Arbeitsgemeinschaft für Pädiatrische Nephrologie. Lancet 1988;1:380-3. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information: appendices 1-5
Supplementary information: detailed list of PREDNOS contributors