Abstract
Background
Shared decision‐making is important in child and adolescent healthcare because there is growing international recognition of children and young people's rights to be included in decisions that affect them. In order for young people to participate effectively in shared decision‐making they need to develop the skills of engagement with healthcare professionals and confidence in interacting with them. They also need to learn how to manage their condition and treatments on their own when they move into adulthood. Children and young people who participate in shared decision‐making in healthcare are likely to be more informed, feel more prepared, and experience less anxiety about the unknown. Significant improvements in cystic fibrosis (CF) survival over recent decades, due to improved therapies and better management of care, means that young people with CF are routinely transitioning to adult healthcare where increasing emphasis on self‐management brings greater complexity in decision‐making. We need to know what interventions are effective in promoting shared decision‐making for young people with CF.
Objectives
To assess the effectiveness of interventions that promote participation in shared decision‐making for children and adolescents (aged between four and 18 years) with CF.
Search methods
We searched the Cochrane Cystic Fibrosis and Genetic Disorders Group's Cystic Fibrosis Trials Register, compiled from electronic database searches and handsearches of journals and conference abstract books. We also searched the reference lists of articles and reviews addressing shared decision‐making.
Date of most recent search: 12 March 2019.
We searched PubMed, CINAHL (EBSCO), Embase (Elsevier), PsycINFO (EBSCO), WHO ICTRP, ASSIA (ProQuest), ERIC (ProQuest), ProQuest Dissertations and Theses, and ClinicalTrials.gov. We contacted study authors with published relevant research in shared decision‐making for adults to ask if they were aware of any published or ongoing studies on the promotion of the intervention for children or adolescents (or both) with CF.
Date of most recent search: 19 March 2019.
Selection criteria
We planned to include randomised controlled trials (RCTs) (but not cross‐over RCTs) of interventions promoting shared decision‐making for children and adolescents with CF aged between four and 18 years, such as information provision, booklets, two‐way interaction, checking understanding (by the participant), preparation to participate in a healthcare decision, decision‐aids, and training interventions or educational programs. We planned to include interventions aimed at children or adolescents (or both), parents or healthcare professionals or any combination of these groups provided that the focus was aimed at promoting shared decision‐making for children and adolescents with CF.
Data collection and analysis
Two authors independently reviewed papers identified in the searches.
Main results
No eligible RCTs were identified for inclusion in this systematic review.
Authors' conclusions
We were unable to identify RCTs with evidence which would support healthcare policy‐making and practice related to implementation of shared decision‐making for children and adolescents (aged between four and 18 years) with CF). We hope that having identified this gap in research, awareness will increase amongst researchers of the need to design high‐quality shared decision‐making interventions for young people with CF, perhaps adapted from existing models for adults, and to test these interventions and children's preferences in RCTs. It is also important to target health professionals with evidence‐based education programmes on shared decision‐making and a need for international consensus on addressing the variability in education programmes.
Plain language summary
Approaches for helping children and adolescents with cystic fibrosis to take part in decisions about their healthcare
Review question
We reviewed the evidence on ways to help children and adolescents with cystic fibrosis to take part in decisions about their healthcare.
Background
Cystic fibrosis is a genetic condition mainly affecting the lungs and digestive system. In the lungs there is a build‐up of sticky mucus making infection more likely and damaging the airways. Sticky secretions from the pancreas block the flow of digestive juices into the gut, so that food is not digested or absorbed properly.
Improvements in treatments mean that people with cystic fibrosis are living longer and long‐term management issues have become more relevant. In childhood there is a high burden of complicated treatments which then continues throughout life. Young people with cystic fibrosis need to be included in decisions about their healthcare, so that they can develop skills to manage their condition and assume responsibility for long‐term care. In shared decision‐making, a child or adolescent is actively included in healthcare decisions that affect them. We know from research with children that they want to be involved in healthcare decisions at a level of their choosing with the support of their parents and healthcare provider. We wanted to find out if there were any good techniques for encouraging children and young people with cystic fibrosis to take part in decisions about their healthcare and to identify any adverse effects.
This review was funded by a Cochrane Fellowship awarded to the main author.
Search date
The evidence is current to: 19 March 2019.
Study characteristics
We planned to include studies where the people taking part would be put into groups at random. We wanted to compare approaches to shared decision‐making for children and adolescents with cystic fibrosis aged between four and 18 years. Examples of these would be providing information, checking the young person understood and was prepared to participate in decision‐making about their healthcare, the use of decision‐aids and training or educational programs. We planned to include approaches aimed at children and adolescents, at parents or at healthcare professionals (or any combination of these) where the focus was to promote shared decision‐making for children and adolescents with cystic fibrosis.
Key results
We did not find any studies that were eligible to include in the review. We think future research should aim to test models of shared decision‐making for young people with cystic fibrosis, which could be developed from existing models for adults; and also work out which methods young people prefer. It is also important to train health professionals in using shared decision‐making and make sure that training is consistent across all countries.
Background
Description of the condition
Cystic fibrosis (CF) is a genetic condition with significant variations in incidence, morbidity and mortality around the world. It is the most common, life‐limiting autosomal recessively inherited condition in white populations (Williams 2010) and registry‐derived data indicate that in excess of 70,000 people are living with CF worldwide (Jackson 2018). While the condition is most common in white populations (Cystic Fibrosis Worldwide 2019), it is also found in people of other racial and ethnic groups (Cystic Fibrosis Foundation 2012). Although significantly under‐diagnosed in Asia, current evidence indicates that the prevalence of CF is low (WHO 2019). In the USA, the incidence of CF is reported to be one in every 3500 births (WHO 2019), with 30,000 people there living with CF in 2012 (Cystic Fibrosis Foundation 2012). In the European Union, one in 2000 to 3000 newborns are affected by CF (WHO 2019); and the Republic of Ireland has the highest prevalence of CF in the world at 2.98 per 10,000 head of population (Cystic Fibrosis Registry of Ireland 2019; Farrell 2008a). The Australian Cystic Fibrosis Data Registry reported holding records of 3235 people living with CF at the end of 2013 (Cystic Fibrosis Australia 2013).
The condition is caused by mutations in a gene called the cystic fibrosis transmembrane conductance regulator (CFTR) (Bronsveld 2001), which is a type of protein that regulates the transport of chloride ions across epithelial cell membranes (Brice 2007; Harris 1993). Dysfunction of this gene dehydrates secretions in the airways, the pancreatic ducts and elsewhere in the body (Cystic Fibrosis Foundation 2019) and results in the accumulation of mucus or thick sticky secretions (Brice 2007; Dyce 2015). In the lungs, mucus blocks the airways and traps organisms increasing the risk of infection; and in the pancreatic ducts mucus prevents the release of enzymes for food breakdown and absorption of essential nutrients (Cystic Fibrosis Foundation 2012). Elevated chloride output in the sweat increases the probability of dehydration and mineral imbalances.
Advances in treatment over the last three to four decades have led to marked increases in survival for people with CF, with survival now into the fourth decade (Dodge 2007). Newborn screening is increasing internationally, so the majority of individuals with CF are now diagnosed in infancy (Farrell 2017). These newborn screening programs for CF have led to improved long‐term health outcomes (Mak 2016) and consequently, both the child and the family are challenged with the condition from the early stages of their relationship. Respiratory problems predominate in children with CF followed by gastrointestinal symptoms (Aziz 2017). However, the prevalence of associated medical conditions (such as gastro‐oesophageal reflux, CF‐related diabetes (CFRD), osteoporosis or osteopenia, and lower body mass index (BMI)) increases with age and more severe impairment of lung function is found in older age groups. Daily therapies usually include nutritional supplements, pancreatic enzymes, daily chest physiotherapy, a regular exercise routine, nebulised medication to assist with airway clearance and antibiotics to treat acute exacerbations. Good adherence to recommended nutritional requirements is considered a worthwhile approach towards achieving clinically relevant outcomes such as normal growth and better lung function (Connett 2015; Matel 2009). When lung disease worsens, oxygen therapy and non‐invasive ventilation therapy may be required (Cystic Fibrosis Australia 2013). This daily treatment regimen places significant responsibility on families, children and young people with CF to ensure optimal health and to slow down disease progression. A multidisciplinary team of trained professionals is required to manage the range of complex issues that can arise in the care of a person with CF (Quon 2012); and it is now well‐established that outcomes for individuals cared for in specialist CF centres are better than for those who are not (Conway 2014).
Description of the intervention
Shared decision‐making (SDM) assists people, in partnership with their healthcare provider, to consider available treatment or management options (and the likely benefits and harms of each), to communicate their preferences and to work together to choose the course of action that best fits their preference (Drayton 2014; Polaris 2014). For children and young people SDM can be defined as decision‐making interactions between healthcare providers, the child and the parents (Lipstein 2012). It is an approach to decision‐making that integrates children's values, goals and concerns with the best available evidence about benefits, risks and uncertainties of treatment. The building of consensus and mutual agreement aims to improve the quality of children's healthcare provision (Lindley 2017). Measures of SDM generally focus on the two‐way interaction between one individual and one professional; however, SDM can also involve interactions between three or four individuals such as parents, the child and healthcare professionals, depending on the situation (Fiks 2010).
The process of SDM has been studied to a lesser extent in children's healthcare, where decision‐making usually includes key family members (Dokken 2000). Over the past decade SDM has emerged in the literature with its definition being variable. One systematic review describes SDM as an integrative model that includes essential elements such as explaining the problem, presenting options, discussion of pros and cons, individual preferences, an individual's ability or self‐efficacy, doctor's knowledge, checking patient understanding, deferral of decision and follow‐up (Makoul 2006). Elwyn built on this previous work, translating existing models into an easy to remember three‐step model for clinical settings and based on 'choice talk', 'option talk' and 'decision talk' (Elwyn 2012). More recently, Elwyn produced a decision‐aid (OPTION5) which was developed for adults; as such, this tool may not be directly applicable to children especially those as young as four (Elwyn 2013a). The (OPTION5) aid includes the following aspects:
provision of explicit explanations that decisions exist which need attention and deliberation;
provision of reassurance to patients that the provider will support the deliberation;
provision of information about treatment or management options;
elicitation of the patient's views, preferences, priorities, at a stage when the patient is better informed (preference elicitation); and
integration of the individual's preferences into the next stage of decision‐making (preference integrations).
Adult participation in healthcare decision‐making is well‐cited in contemporary publications (Elwyn 2016; Wyatt 2015). It is an accepted approach, particularly when guided by ethical principles (Elwyn 2013b; Kraus 2016). It aims to promote patient autonomy (Gulbrandsen 2016) and has demonstrated positive outcomes, including significant reductions in hospitalisation (Wennberg 2010) and individual satisfaction with healthcare (Quaschning 2013). However, SDM may also have unintended outcomes, such as decision regret (Simcox 2009); therefore, people require guidance to assess the risks and benefits of an intervention (Elwyn 2016). It is generally accepted that children and adolescents have the right to self‐determination, dignity, respect, and the right to be included in their healthcare decisions (Coyne 2008). It is important for children to be involved in SDM because adult proxy views within the healthcare setting may differ markedly from the child's view (Söderbäck 2011); however, the understanding of a child should be assessed on an individual basis in relation to the decision being made (Fennestra 2014). Also, the level of parental involvement in decision‐making may change as children grow older and become increasingly capable of participating in decision‐making (Bejarano 2015).
Interventions to promote the adoption of SDM by children and adolescents may include (but are not restricted to): building partnerships between the paediatrician, the child and the parent (Brand 2013); providing and exchanging information, verbally, on paper or online (Miller 2008); encouraging the child and parent to ask questions (Brand 2013); establishing understanding (Joffer 2016); encouraging the expression of preferences (Joffe 2003; Waligora 2016); utilising programmes that embed SDM in clinician training and continuous professional development (The Health Foundation 2012); endorsing CF self‐management programmes that advocate SDM (Savage 2007); and eliciting post‐decision reactions from the child (either satisfaction with care) or regret (Bejarano 2015). Practical examples of SDM interventions for children with CF could include:
training programmes aimed at healthcare SDM for children and young adults with CF for professionals working in children’s healthcare (Brand 2013);
education programmes for children and adolescents with CF to equip them with the skills of engagement regarding their healthcare needs;
education programmes for parents of children and young adults with CF so that they can learn the skills to support their children when taking part in healthcare decisions;
providing information to children and adolescents which is aimed at SDM for young people with CF and tailored to their needs (Hansen 2016);
providing information to children and adolescents with CF as well as their parents so that young people are better supported and informed regarding healthcare decision‐making (Homa 2015);
providing relevant information to parents of children with CF so that they are informed to support their children's healthcare decision‐making (Fixter 2017), e.g. parents suggested fact sheets, brochures and booklets should be used following a diagnosis of CF in their child (Jessup 2016); other resources aimed at SDM for children with CF might include websites to facilitate self‐management of the condition for children with CF and their families (Roehrer 2013), CD‐ROMs (Davis 2004) or video interventions targeting young persons (Kodjebacheva 2016);
decision supports aimed at healthcare SDM for children and young adults with CF, such as children’s computerised clinical decision supports (CCDS) (Stultz 2012);
counselling and dialogue for children to reduce decision conflict (Westerman 2013).
How the intervention might work
One of the key components of SDM is the provision of information to encourage an individual to have a discussion with their clinician (Elwyn 2013a). Adult studies have shown that there can be a gap in a person's knowledge about their condition and how relevant interventions work (Siklosi 2010). One study identified a CF knowledge gap in adolescents with the condition at an age when they are more likely to be involved in SDM (Chomik 2014). If left uncorrected, such gaps could impact on the progression of the condition (Chomik 2014). Research has shown that while adolescents with long‐term conditions wish to be involved in decisions about their treatment, they may also seek the involvement and assistance of their parents and doctors (Lipstein 2013). However, parents may consider decision factors differently to adolescents, with adolescents more likely to focus on immediate treatment effects and parents tending to focus on the long‐term implications (Lipstein 2016).
The building of partnerships between doctors, children and parents may encourage the exchange of healthcare information. In practice, SDM interventions for children can include parents, carers and healthcare providers, but focuses primarily on the child; it takes the child's age, condition and decision‐making ability into account (Coyne 2013). It is suggested that using SDM between healthcare professionals, individuals with CF and their families may lead to improvements in quality of care (Elf 2015), such as improved knowledge and reduced decision conflict (Wyatt 2015).
According to a recent study, five elements contribute to improved care that are co‐produced by the parent, the child, and healthcare professionals:
mental and emotional readiness to engage;
curiosity and the search for insight;
re‐framing challenges into opportunities for improvement;
listening and learning from everyone, bringing home what is relevant; and
personal participation (Sabadosa 2014).
Assisting in preparation for a decision, e.g. role play, might give a child confidence to voice his or her opinions in a real‐life situation. Interventions that encourage the asking of questions may enhance understanding, which in turn may alleviate uncertainty. Research has indicated that children's participation in SDM reduces fears and concerns, gives them a sense of competence and results in more satisfaction with healthcare provision (Angst 1996; Runeson 2002). Children with CF face practical challenges in managing their condition on a daily basis, e.g. performing physiotherapy and managing nutrition, and participation in SDM may lead to solutions tailored to their needs. As children with CF grow older they are faced with many challenges (Bregnballe 2017), age‐related CF complications may occur (Simmonds 2014) requiring increasing levels of (self) care and support (Huang 2014; Savage 2014). As such, inclusion in discussions about treatment decisions may help young persons with CF to develop self‐caring and participation skills that are necessary for long‐term self‐management. It is important that children get the opportunity to practice taking part in decisions so that they build and develop their skills of decision‐making. Furthermore, with increasing age and cognitive maturity, children better understand the purpose, risks and benefits of treatment (Fiks 2010). A study of young adults with CF transitioning to adult services showed that the acquisition of independence in everyday life was accompanied by greater autonomy in managing the condition (Duguépéroux 2008).
Programmes that embed SDM in the education of health professionals may increase awareness of SDM as essential for person‐centred care and equip professionals with the skills to engage competently with the child or young person making healthcare decisions. Self‐management programmes that advocate SDM may improve children's satisfaction with healthcare. The inclusion of a decision‐aid during a consultation with a doctor may indicate to the child that their opinions and preferences are valued. Increasingly, decision‐aids are being used to assist with the process of SDM and evidence strongly suggests that patient decision‐aids can improve the quality of decision‐making for adults (O'Connor 2007a). A Cochrane Review found that the use of decision‐aids with adults led to a significant improvement in knowledge regarding a decision, as well as significantly reducing anxiety and decision conflict (Stacey 2017). Also, a literature review concluded that patient‐centred care in adults is a key factor in increasing adherence to treatment (Robinson 2008) and a systematic review reported evidence for positive influences on patient‐centred care and self‐management (Rathert 2013).
Why it is important to do this review
Advances in treatment continue to extend the life span of young people with CF (MacKenzie 2014). As survival improves, the long‐term complications of the condition as well as depression may become increasingly evident adding to the treatment burden (Anton‐Paduraru 2014; Buntain 2004; Quon 2012). Furthermore, some adolescents with CF may transition to adult healthcare with near normal lung function (Quon 2012), but for others the transition may be associated with a decline in lung function (Duguépéroux 2008). Transition requires greater responsibility for self‐care and taking on more self‐advocacy skills (Okumura 2014).
Implementing SDM can help foster patient‐centred care and is particularly relevant given the increasing number of healthcare choices (Stacey 2010; Weiner 2014). There is currently a strong emphasis on the importance of SDM in the healthcare setting (De Boer 2013; Kiessling 2013; Klatt 2013; Légaré 2010; Muller‐Juge 2013). As healthcare providers, policymakers, and consumers explore opportunities to integrate patient‐centred concepts into standards of care, SDM is receiving increased attention (Shafir 2012). The time seems right to engage healthcare professionals and policy makers in devising policies that prioritise SDM for children and adolescents living with CF, especially given the new emerging therapies for CF. Additionally, this systematic review supports and promotes the United Nation’s Convention on the Rights of the Child (UNCRC), which places value and importance on the child's opinion with article 12 "Parties shall assure to the child who is capable of forming his or her own views the right to express those views freely in all matters affecting the child, the views of the child being given due weight in accordance with the age and maturity of the child" (United Nations 1989). Young people want be involved in their health decisions (Ahmed 2011), but some research indicates that they encounter considerable obstacles to having their views heard and respected (Coyne 2017).
An annual review is now standard practice for children with CF and consensus documents define standards for routine evaluation, monitoring and treatment (Chuang 2014; Long 2001). While one consensus document cites CF as requiring a holistic approach (Kerem 2005), others make no reference to SDM (Walters 1990). Because of additional time afforded to parents and children, the CF annual review process has the potential to promote SDM in CF care; for example, on its website, the annual review clinic in CF paediatric care at Great Ormond Street Hospital for children is described as "designed to provide more time with members of the CF team than is usually available in the CF clinic for parents and children. This allows more detailed discussion of relevant issues with children" (GOSH 2019).
Since SDM is associated with beneficial outcomes for children (Wyatt 2015), determining evidence‐based age‐appropriate SDM interventions for children and adolescents with CF is essential. Healthcare professionals need to know how to involve children and adolescents with CF in SDM and which interventions are most effective (Coyne 2014). Despite the ever‐increasing focus on decision‐aids in the literature (Abhyankar 2013; Stacey 2015), SDM is not routinely applied in clinical practice and limited use of SDM has been observed, despite previous research indicating that parents wish to collaborate in decision‐making (Lipstein 2014). Studies on children's experiences of hospitalisation report a lack of self‐determination regarding personal needs (Coyne 2006).
Objectives
To determine the effectiveness of interventions that promote SDM for children and adolescents with CF aged four to 18 years.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) and cluster RCTs. We did not include trials with a cross‐over design, since some interventions to promote SDM may have a sustained effect.
Types of participants
Children and adolescents diagnosed with CF clinically (Farrell 2008), by sweat testing (McKone 2015) or genetically (Martiniano 2014) with or without any concomitant condition and aged between four and 18 years.
Consistent with a previous Cochrane Review on SDM for children with cancer, we excluded children under four years of age as they are potentially too young to adequately engage in the intervention (Coyne 2013). Children between the age of four and 15 years have participated in discussions related to child‐parent decision‐making roles (Miller 2008). Since SDM is a collaborative process with the child, we searched for studies which included parents, carers and any healthcare professionals (e.g. doctors, nurses, allied professionals) who are involved in the care of children and adolescents with CF. However, this review was about finding out the best way to support and promote the participation of children and adolescents in SDM so that they are included in decisions that affect their healthcare and their lives.
Types of interventions
Any interventions for promoting SDM in children or adolescents which are aimed at children or adolescents, their parents or healthcare professionals (or any combinations of these groups) to usual care or to alternative SDM promotion strategies for the same group of people. We searched for SDM interventions delivered in any format including a one‐to‐one basis, a group basis, discussion sessions, role play sessions, blended learning sessions, online learning sessions and the use of hard‐copy information resources such as leaflets or workbooks. Interventions of interest were those delivered by professionals or parents or both.
Note: the term parent includes the parent or carer or guardian who is responsible for the parental role; for convenience the term parent will be applied where appropriate.
Types of outcome measures
Primary outcomes
-
Presence of shared decision‐making measured by the change in score of any validated tool
the Observing Patient Involvement 12‐item (OPTION) Scale (Elwyn 2003)
the Observer‐based Measure Observer 5‐item (OPTION) Scale (Elwyn 2013a)
decision‐making instrument facilitation antecedents (e.g. the Preparation for Decision‐making Scale (Graham 1995))
decision process (e.g. the Rochester Participatory Decision‐making Scale (Shields 2005))
Quality of life (QoL) as measured by e.g. the Cystic Fibrosis Questionnaire‐Revised (CFQ‐R) (Bryon 2005; Quittner 2005; Quittner 2009; Quittner 2012) or the CF disease‐specific (HRQoL) questionnaire (Gee 2000)
Adverse effect such as longer consultation time, increased frequency of hospital admissions, longer hospital stay, increased costs or unanticipated adverse effects as reported by study authors
Secondary outcomes
Adherence to CF medication (measured by e.g. electronic monitoring)
Anxiety (measured by e.g. the Generalised Anxiety Disorder 7‐item (GAD‐7) scale (Spitzer 2006), or the Hospital Anxiety and Depression Scale (HADS) (Zigmond 1983)
Decision conflict (as measured by the Decision Conflict Scale (O'Connor 1995) or the SURE scale (Légaré 2010))
Decision regret (as measured by the Decision Regret Scale (Brehaut 2003))
Participant satisfaction with decision
Depression (measured by e.g. Patient Health Questionnaire (PHQ) (Spitzer 1999), (PHQ‐9) scale (Kroenke 2001) or the CES‐D Scale (Eaton 2004), or the Hospital Anxiety and Depression Scale (HADS) (Zigmond 1983))
Changes in lung function (measured by forced expiratory volume in one second (FEV1))
-
Changes in nutritional indices
weight (kg)
body mass index (BMI)
height (cm)
Survival
Search methods for identification of studies
We did not restrict the searches by language, year or publication status.
Electronic searches
We searched for relevant studies from the Cochrane Cystic Fibrosis and Genetic Disorders Group's Cystic Fibrosis Trials Register using the terms: mental health in CF OR *program* OR behaviour.
The Cystic Fibrosis Trials Register is compiled from electronic searches of the Cochrane Central Register of Controlled Trials (CENTRAL) (updated each new issue of The Cochrane Library), weekly searches of MEDLINE, a search of Embase to 1995 and the prospective handsearching of two journals ‐ Pediatric Pulmonology and the Journal of Cystic Fibrosis. Unpublished work is identified by searching the abstract books of three major cystic fibrosis conferences: the International Cystic Fibrosis Conference; the European Cystic Fibrosis Conference and the North American Cystic Fibrosis Conference. For full details of all searching activities for the register, please see the relevant sections of the Cochrane Cystic Fibrosis and Genetic Disorders Group's website.
Date of most recent search: 12 March 2019.
The systematic review authors also searched the following databases (for search strategies see Appendix 1):
PubMed (www.ncbi.nlm.nih.gov/pubmed/) (1946 to 19 March 2019);
CINAHL Complete (EBSCO) (1937 to 19 March 2019);
Embase (Elsevier) (1947 to 19 March 2019);
PsycINFO (EBSCO) (1597 to 19 March 2019);
WHO International Clinical Trials Registry Platform (ICTRP) (apps.who.int/trialsearch/), searched on 19 March 2019;
ASSIA ‐ Applied Social Sciences Index and Abstracts (ProQuest) (1987 to 19 March 2019);
ERIC (ProQuest) (1966 to 19 March 2019);
ProQuest Dissertations & Theses Global (ProQuest) (1743 to 19 March 2019);
ClinicalTrials.gov (clinicaltrials.gov/), searched on 19 March 2019.
Grey literature
For search strategies, please refer to Appendix 1. We searched the following resources.
Open Grey (//opengrey.eu/): searched 17 March 2019.
Grey Matters (www.cadth.ca/resources/finding‐evidence/grey‐matters): searched 23 March 2019.
Lenus, the Irish health repository which is Ireland's leading source of health‐related research and grey literature (www.lenus.ie/hse/register): searched 14 March 2019.
RIAN, a repository which aggregates open access Irish research publications (www.rian.ie): searched 13 March 2019.
UK CF Trust Registry which collects demographic, treatment and health outcome data (UK CF Trust 2018): searched 01 February 2019.
Searching other resources
Handsearches
We searched the reference lists of the additional studies listed in this review. We also searched a hard copy of one textbook for leads toward studies with rigorous and systematic approaches to shared decision‐making (Cass 2006). Handsearches were completed on 04 April 2019.
We searched electronic copies of the journal Medical Decision Making (MDM) (February 1981 to February 2019 ) for rigorous and systematic approaches to decision‐making: searched 29 March 2019.
Reference lists
We searched the reference lists of all studies of peripheral interest for other studies relevant to this Cochrane Review.
Personal communication
We contacted (by email) researchers who developed and evaluated a decision‐aid for adults with CF for the identification of eligible studies for this Cochrane Review (Vandemheen 2009). Date contacted: 06 June 2017.
Data collection and analysis
We used Covidence software to organise and screen the unique references retrieved in the searches (Covidence 2019). If for future updates, we identify any eligible studies, we will undertake the methods outlined below:
Selection of studies
We will select studies in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We will compile a study eligibility form with clear inclusion and exclusion criteria and using this form, the authors will independently select studies for inclusion in the review from those identified by the searches. Where the eligibility of a study is not clear, we will list the study as 'Awaiting classification' and contact the investigators for clarification. Studies (not reports) will be our unit of interest (Higgins 2011a). We will use reference management software to remove duplicate records (Endnote X9) and will list all references (both full papers and abstracts) to a single study under a single identity code in Review Manager (RevMan 2014). We will list as excluded any studies that initially appear to be eligible, but which on further inspection are not, and we will record the primary reason for exclusion. We will resolve disagreements regarding the inclusion of a study by discussion leading to consensus (Higgins 2011a). In order to check agreement of consistency between the two authors (HM, IC) in selecting studies, we will generate a Kappa K co‐efficient using four categories (agree/agree), (disagree/disagree), (agree/disagree) and (disagree/agree) and will report the number of studies included in this calculation. We will apply a co‐efficient value of 0.7 (indicating a good level of agreement) (Harris 2008).
Data extraction and management
The authors will use a study selection, quality assessment and data extraction form template (developed by the Cochrane Cystic Fibrosis and Genetic Disorders Group) modified to meet the objectives of this review. We will also consider the need to develop any additional data extraction tools and will seek guidance for collecting data from the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). In order to clarify, refine and ensure consistency of data extraction, we will pilot test our data extraction form by taking a representative sample (based on the number of studies to be reviewed) to identify any problems with the tool. We will seek a consensus between authors before we modify the form.
Two authors (HM, IC) will independently extract the data. A third author will cross‐check the data collection forms. We will resolve disagreements regarding the data extraction by discussion leading to consensus (Higgins 2011a). We will extract the following details from study reports if possible and endeavour to contact study authors for clarification of any missing data.
Study characteristics: author’s name; year of publication; study title; country; ethical approval; hypothesis and alternative hypothesis; aim of study; study design; phase of RCT; span or duration of the study.
Participant characteristics: method of CF diagnosis; age; gender; ethnicity; socio‐economic group; and education.
Sample size: method used to calculate sample size; the sample size estimated prior to the trial; numbers approached to participate; numbers who agreed to participate; and numbers that actually participated; numbers in the intervention and control group; final sample size following attrition; loss to follow‐up or missing data.
Inclusion and exclusion criteria.
Intervention: content; process and comparator; whether the intervention had a training component for participants; and whether the delivery of the training was by a parent or a professional or both.
Outcome data for our listed outcomes at the following time points: one month, over one month and up to three months, over three months and up to six months (if outcome data are available for other time points, we will consider these for examination).
Data type, e.g. dichotomous data, continuous data.
Study author's conclusions and limitations.
Duration of the intervention (not the study), e.g. data for a study that has a daily intervention for one week followed by a six‐month follow‐up will be recorded as a one‐week intervention.
Additional information that may potentially affect the interpretation or applicability of results.
We will present study details in a 'Characteristics of included studies' table in the Review Manager software (RevMan 2014). One author will enter all data for meta‐analysis into the Review Manager software and a second author will independently cross‐check these for accuracy against the data extraction sheets (RevMan 2014).
Assessment of risk of bias in included studies
Two authors will independently assess the risk of bias for each included study by cross‐checking study details against the seven domains of bias set out in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). We will also use guidance on assessing study quality issued by the Cochrane Consumers and Communication Review Group (Ryan 2013). We will resolve any disagreements regarding bias by discussion to reach consensus. If we are not able to reach a consensus we will seek the input of a third person acting as arbiter. Where the characteristics of studies are inadequate to determine an assessment of bias, we will contact study authors for clarification.
We will categorise each identified element of bias, as being 'high risk of bias', 'low risk of bias' or 'unclear risk of bias' in our view according to guidance given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). We will present an overall picture of the risk of bias in graphical form (RevMan 2014). Furthermore, we will construct a summary table to report our assessment of the risk bias for each important outcome (across domains) within and across studies (Higgins 2011b).
Measures of treatment effect
Using the details in the 'Characteristics of included studies' table, we will determine whether the included studies are sufficiently similar in design, participants, interventions and outcomes to allow their data to be combined in a meta‐analysis (Schünemann 2011a). We will treat the measurement of a numerical quality as continuous data, the measurement of a categorical type as dichotomous or binary data (Deeks 2011).
We plan to report the presence or absence of SDM and survival as binary data and will present the results as a risk ratio (RR) with a 95% confidence interval (CI). We plan to present lung function values, changes in growth and changes in body weight as continuous data and will report results as a standardised mean difference (SMD) with a 95% CI.
We will categorise the outcomes measured as ordinal scales (QoL, anxiety, decision conflict, satisfaction, depression, decision regret and adherence to medication) into longer and shorter ordinal scales. We will make shorter ordinal scales into binary data by combining adjacent categories together. We will summarise longer ordinal scales using methods for continuous data (Deeks 2011).
Unit of analysis issues
For each included study, we will determine the level at which randomisation took place, i.e. by individual or by cluster. If an included study employs a cluster‐randomisation design, i.e. participants are randomised to a particular group according to clinician or particular practice, we plan to assess these studies for unit of analysis issues. If only cluster RCTs with unit of analysis issues are available for meta‐analysis we will consider carrying out 'approximate analysis' of cluster RCTs, following guidance from Donner to estimate the intracluster (or intraclass) correlation coefficient (ICC), which is required for this analysis (Donner 1980; Higgins 2011c). In light of guidance in the Cochrane Handbook for Systematic Reviews of Interventions, we will consider whether to analyse parallel RCTs and cluster RCTs separately or to combine them using the generic inverse variance method of meta‐analysis (Deeks 2011).
For studies with multiple treatment groups, we will follow guidance from the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011c).
Dealing with missing data
We will endeavour to differentiate between data which are missing at random and data which are not (e.g. due to publication bias, selective reporting bias, attrition and selection of participants) (Higgins 2011c). For data not missing at random, we will contact the study authors to establish reasons for this and to request any data we require for our analysis (e.g. means and standard deviations (SDs)). If these data are not available, we will consider imputing values either from studies in the same meta‐analysis or from studies in other meta‐analyses (Furukawa 2006). We will only impute values from studies using the same measurement scale, with the same degree of measurement error and over the same time periods (Higgins 2011c).
We will consider presenting results as an 'available‐case analysis' (where we analyse data for the participants for whom data are available). If we are able to do so without imputing values, we will present our results using an intention‐to‐treat (ITT) analysis. If we are not able to obtain data we need for our analysis, we will report the study results narratively and discuss the potential implications of their omission from a meta‐analysis (Higgins 2011c).
Assessment of heterogeneity
We plan to assess the heterogeneity (variability) between included studies. Initially, we will assess clinical heterogeneity by comparing study characteristics and participant demographics; we will assess methodological heterogeneity by comparing the different study designs and risk of bias judgements. If we consider studies to be too heterogeneous, we will not combine them in a meta‐analysis. If we are able to combine studies in a meta‐analysis we will further assess for heterogeneity by visually inspecting the forest plot to see if the CIs overlap. We also plan to assess heterogeneity using the Chi² test and the I² statistic. The I² statistic describes the percentage of total variation across studies due to heterogeneity rather than chance where an I² value of 0% indicates no observed heterogeneity and larger values show increasing heterogeneity (Higgins 2003). Since the thresholds for the interpretation of I² can be misleading, we plan to use the following guide (Deeks 2011):
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90%: may represent substantial heterogeneity;
75% to 100%: considerable heterogeneity.
We will seek advice from a statistical expert for the appraisal of our I² result.
Assessment of reporting biases
We will examine the included studies for any evidence of reporting biases (Sterne 2011a). We will compare any published protocols we identify with the final study findings to ensure that all planned outcomes are reported. If protocols are not available, we plan to compare the 'Methods' section and the 'Results' section of the final published report to identify any discrepancies. If necessary, we will contact study authors for additional clarification. Additionally, if we are able to combine at least 10 studies, we will evaluate each outcome for publication bias using a funnel plot (Sterne 2011a). We will examine these funnel plots and assess any asymmetry present, noting that this is not necessarily due to publication bias, but may have a number of other possible causes (Sterne 2011b).
Data synthesis
We will only carry out a meta‐analysis if we judge study design, participants, intervention, and outcome to be sufficiently similar to derive a clinically meaningful result. Since there is no single true effect for SDM, we will not estimate a single effect size and plan to apply a random‐effects model which makes an adjustment to the study weights according to the extent of variation or heterogeneity (Deeks 2011). Additionally, the random‐effects model will award relatively more weight to smaller studies than a fixed‐effect model in a heterogeneous set of studies (Deeks 2011). If we are not able to present the study results in a meta‐analysis, we will present them narratively by intervention type, population group and setting etc. (Schünemann 2011a).
Subgroup analysis and investigation of heterogeneity
If searches yield a sufficient number of studies which we are able to combine in a meta‐analysis we will conduct a subgroup analysis by:
age for the following ranges: 4 to 7 years; 8 to 11 years; 12 to 15 years; 16 to 18 years;
male versus female participants.
Sensitivity analysis
In order to check the robustness of the summary statistic, we plan to carry out sensitivity analyses if there are sufficient comparable trials (at least 10) included in the review:
repeating the analysis using different statistical models (fixed‐effect versus random‐effects models);
adding in and taking out trials where there is high risk of bias (rating determined using a 'Risk of Bias' graph) in relation to randomisation, allocation concealment, or blinding of the interventions from participants or trial personnel.
Summary of findings table
We will prepare a 'Summary of Findings' table to present the results of our meta‐analyses for each comparison, based on the methods described in chapter 11 of the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2011a).
We will present a separate 'Summary of findings' table for each comparison and report the outcomes listed below. Findings from interventions that promote SDM too clinically different to be summarised together will be summarised in separate comparisons.
Presence of SDM
QoL
Adverse effects (such as longer consultation time, increased frequency of hospital admissions, longer hospital stay, unanticipated adverse effects as reported by study authors)
Adherence to CF medication
Anxiety
Decision conflict
Decision regret
We will grade the quality of evidence as high, moderate or low in accordance with the specific evidence grading system described by Schünemann and developed by the Grade Working Group (Atkins 2004; Schünemann 2011b). If meta‐analysis is not possible we will present results in a narrative summary in accordance with guidance (Schünemann 2011a).
Economic issues
We did not plan to incorporate economic issues in this review since from our knowledge based on a literature review we do not envisage that studies will make explicit comparisons between alternative interventions in terms of costs (resource use).
Consumer involvement
The consumer expert for this review is Susan Biggar, Australian Health Practitioner Regulation Agency (AHPRA) Melbourne, Australia, co‐author on this review and a consumer advocate with three adult sons, two of whom have CF.
The authors also have strong links with the consumer organisation Cystic Fibrosis Ireland and we sent by e‐mail the protocol and a draft of the review to our contacts in this organisation for comment, particularly regarding the discussion and recommendations.
Results
Description of studies
No studies were identified for inclusion in the review.
Results of the search
No studies were identified for inclusion in the review. We present a study flow diagram with a summary of the results of the searches (Figure 1).
1.
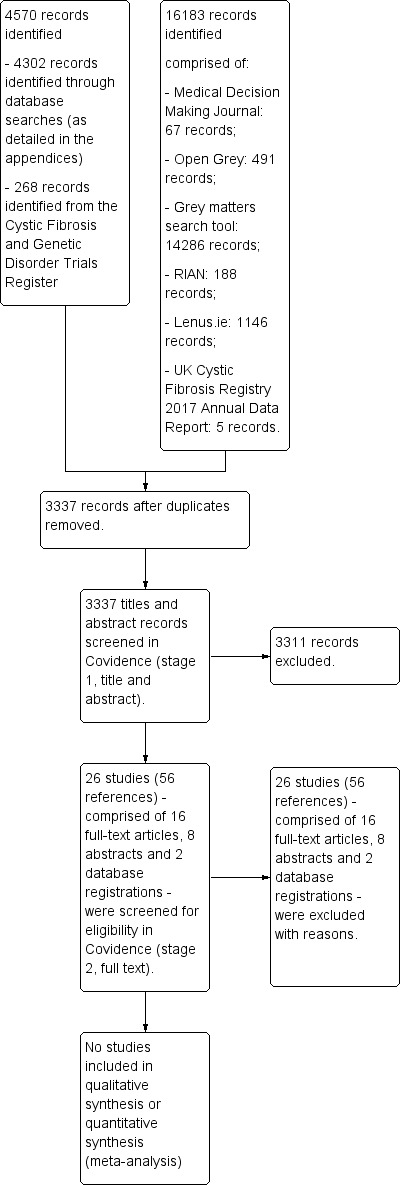
Study flow diagram showing a summary of the results of the searches
A total of 4302 records were retrieved from the searches of the electronic databases and 268 from the Cochrane Cystic Fibrosis and Genetic Disorders Group's Cystic Fibrosis Trials Register. Following the removal of duplicates, the numbers reduced to 3337. Searches of grey literature yielded 16183 items, all of which were of peripheral interest only and were immediately excluded. Therefore 3337 references were imported into the Covidence software for screening. At the first stage of screening, two review authors agreed that 3311 records did not meet our inclusion criteria and these were excluded. This left 26 studies (eight abstracts, 16 full texts and two database‐registered studies) for further screening. A total of 26 studies (56 references) were excluded. Reasons for excluding the 26 studies are shown in (Characteristics of excluded studies).
Included studies
No eligible studies were identified for inclusion in this review.
Excluded studies
A total of 26 studies were excluded. Two studies were excluded as the age range of the participants was not eligible (18 years and older) (Blumenthal 2006; Vandemheen 2009). A total of 13 RCTs were excluded as the intervention was not appropriate for inclusion (Alpern 2011; Andrade 2013; Bryon 2000; Christian 2006; Cottrell 1996; Cruz 2007; Davis 2004; DeLambo 2004; Downs 2006; Farrell 2014; Huang 2014; Blackwell 2015; Stabler 1981). Three studies were reviews (Austin 2015; Blackwell 2013; Wells 2016). Two studies with no reported randomisation were excluded as the interventions were not appropriate for inclusion ‐ one a pilot study with an experimental and control group (Belsky 1994) and one feasibility study with two groups one described as control group (Cannon 1999). One reference was a book chapter (Quittner 2000), two were qualitative studies (Happ 2013; Marciel 2010), one was a registered observational study that did not progress to publication as a journal article (Ward 2012), one was a quasi‐experimental study (Jafari 2017). One study was excluded following e‐mail communication (26 November 2017) from the main author confirming that the study did not have any hard measures of shared decision‐making (Cummings 2011).
Risk of bias in included studies
No eligible studies were identified for this review.
Effects of interventions
No eligible studies were identified for inclusion in this review.
Discussion
From birth, children and young people with CF are challenged with complex healthcare needs. There have been improvements in the survival of people with CF over recent decades due to improved therapies and better management of care. This means that young people with CF are routinely transitioning to adult healthcare where they will need to demonstrate self‐management and decision‐making skills. Studies report that young people with CF who leave home often experience many difficulties regarding the management of their condition (Bregnballe 2017). In turn, this has led to SDM for children and adolescents becoming increasingly essential so that they develop the skills of engagement in their care and treatment before transition.
Summary of main results
We limited the inclusion criteria with regards to study design to RCTs. This is because RCTs provide a high level of evidence (Polit 2008), are designed to minimise the effects of bias (Chalmers 1993) and lend themselves well to meta‐analysis (Gotink 2015). We found only one RCT which evaluated a decision‐aid for persons with CF but it was for persons aged 18 years and older (Vandemheen 2009). This age group is outside the remit of this review’s definition of children and adolescents, so it was excluded. Therefore, there were no studies identified for inclusion in this review.
Potential biases in the review process
We endeavoured to minimise potential bias in the review process by undertaking a comprehensive search strategy across a wide range of sources, with no restriction by language or publication status and independent assessment of records by two review authors.
Agreements and disagreements with other studies or reviews
The results of this Cochrane Review identified the lack of RCTs focusing on interventions for encouraging children and young people with CF to take part in decisions about their healthcare. Reviews indicate that young persons with CF place value on participation in their healthcare decisions (Jamieson 2014). As no eligible studies for inclusion in this review were identified, we are unable to compare our results with other published systematic reviews. However, a Cochrane Review assessed the effects of decision‐aids for people aged 18 years or older facing health treatment or screening and concluded that decision‐aids may improve values‐congruent choices; importantly these authors report no adverse effects on health outcomes or choice satisfaction (Stacey 2017). Additionally, the authors of this review made brief reference to the use of decision‐aids in children's healthcare setting for conditions other than CF; for example, interventions for promoting participation in SDM for children with cancer (Coyne 2013). Similar to our review, a further Cochrane Review reported an absence of high‐quality quantitative research on interventions to promote participation in SDM for children with cancer (Coyne 2013).
Authors' conclusions
Implications for practice.
No eligible studies were found for inclusion in this review. This has serious implications for practice. We were unable to identify evidence from randomised controlled trials (RCTs) with which to support healthcare policy‐making and practice related to implementation of shared decision‐making (SDM) for children and adolescents with cystic fibrosis (CF). Based on the literature examined, generalisation regarding SDM for children and adolescents is problematic as they are not a homogeneous group. Children and adolescents are likely to differ significantly in their desire and ability to participate in SDM, both across age groups and as individuals. The balance between the child's right to participate in SDM and the the risks and benefits of SDM participation needs to be assessed. Studies indicate that children’s healthcare settings involve several participants with different perspectives which also need to be considered.
A number of factors have been suggested as impacting on the likelihood of SDM being successful. The provision of appropriate information in simple jargon‐free language can help children understand and feel more involved in decision‐making. Checking a child's understanding of information provided, especially in relation to risks and benefits, and providing the child with the opportunity and time to express preferences and discuss issues may also help. Reassuring the child in terms of support from parents and professions for the decision is vital as most children prefer to share the decision and do not want full responsibility. Giving the child time to consider options, providing the opportunity to discuss any change of preference and assessing decision satisfaction or regret is essential. The use of decision‐aids has been shown to improve knowledge, assist in setting realistic expectations, and reduce indecision for adults with CF. Therefore validated decision‐aids tailored to children’s level of understanding could be useful supports for children’s participation in SDM. Providing professionals in children's CF healthcare with the opportunity to undertake SDM educational programmes may increase their awareness of children's capabilities and preferences, enhance their assessment and facilitating skills and thereby encourage inclusion of children and parents in shared healthcare decision‐making. As yet, SDM is not widely implemented in clinical practice; however, providing children and adolescents with CF and their parents with the opportunity to engage in SDM education programmes may increase young people's participation in SDM.
Implications for research.
A lack of studies for inclusion in this Cochrane Review has identified a gap in research focusing on interventions promoting participation in SDM for children and adolescents with CF aged between four and 18 years. In order to guide SDM practice in CF care there is a need for high‐quality RCT evidence and we hope that having identified this gap in research, the awareness of the need to design high‐quality SDM interventions for children and adolescents with CF and to test these interventions in RCTs will increase amongst researchers. Results of relevant RCTs could be considered for a future Cochrane Review. Initial steps to promote participation in SDM by children and adolescents with CF could be the development of valid and reliable SDM tools that meet the criteria of the International Patient Decision‐Aid Standards (IPDAS 2013; O'Connor 2007b). Since a five‐step model of SDM has been developed for adults (Elwyn 2013a), further research could examine the applicability of the framework for children and adolescents. Aspects of SDM identified in paediatric reviews could be considered (Wyatt 2015) and used to build a framework from which SDM interventions for young people with CF could be developed and tested using RCTs. It may also be important to consider evidence‐based SDM education programmes targeting professionals in CF healthcare (Lehane 2018). There is a need for international consensus on ways to address the variability in SDM training programmes (Legare 2012). Also, high‐quality RCTs focusing on triadic interactions may also be important to consider. For example, a SDM review focusing on healthcare professional's adoption of SDM only identified low‐quality evidence and was therefore unable to draw conclusions regarding the type of interventions that are effective for the increased adoption of SDM among healthcare professionals (Legare 2014). Notwithstanding this finding, investigators concluded that SDM interventions targeting patients and healthcare professionals together are more promising than targeting one or the other (Legare 2014). Since decision aids have been developed for adults (Stacey 2017; Vandemheen 2009), validation studies could be carried out on decision‐aids tailored for children and young persons with CF. Reviews focusing on health professionals' perception of barriers and facilitators to implementing SDM in clinical practice may also identify interventions that could be tested using RCTs. Qualitative studies examining children’s preferences in the delivery of information, choice of intervention type, for example role‐play, electronic or hard copy or a combination of these could also lay the groundwork for the identification of interventions that could be tested with RCTs. Qualitative studies may have an important role in the implementation of SDM for children and young persons with CF. For example, qualitative studies focusing on raising awareness of SDM among professionals in CF health care and parents of children with CF may facilitate the successful implementation of evidence‐based SDM for children with CF.
What's new
| Date | Event | Description |
|---|---|---|
| 8 June 2019 | Amended | Reference to excluded study added to Discussion section 'Summary of main results'. |
Acknowledgements
The authors would like to thank the Cochrane Cystic Fibrosis and Genetic Disorders Group, and in particular Nikki Jahnke, Managing Editor, for providing editorial assistance, recommendations, direction and support during the development of the protocol and the undertaking of the review.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Cystic Fibrosis and Genetic Disorders Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Search methods ‐ electronic searching
| Database | Date of search | Search strategy |
| PubMed (NLM) (PubMed) |
1946 to 19 March 2019 | 1. For decision making the following MeSH headings and text words were used: ("attitude of health personnel"[Mesh Terms] OR "attitude to health"[Mesh Terms] OR "choice behavior"[Mesh Terms] OR "communication"[Mesh Terms] OR "community participation"[Mesh Terms] OR "cooperative behavior"[Mesh Terms] OR "decision making"[Mesh Terms] OR "decision support techniques"[Mesh Terms] OR "decision theory"[Mesh Terms] OR "educational technology"[Mesh Terms] OR "health education"[Mesh Terms] OR "informed consent"[Mesh Terms] OR "professional‐family relations"[Mesh Terms] OR "psychology"[Subheading] OR ((affective OR cognitive OR emotional OR psychosocial OR psychosomatic) AND aspect*) OR ((choice OR compliant OR cooperative OR co‐operative OR illness) AND behavio*) OR clinical support technique* OR collaborati* OR communication* OR consensus OR consent* OR consumer* OR decision* OR disput* OR dissent* OR ((doctor OR doctors OR physician* OR provider*) AND (attitude OR patient relation*)) OR educational technology OR (health AND (attitude* OR education OR information OR literacy)) OR (informed AND (assent OR choice*)) OR misinformation OR negotiati* OR (nurs* AND role*) OR participation* OR partner* OR (patient AND (acceptance OR adherence OR attitude* OR centered OR centred OR compliance OR cooperation OR co‐operation OR education OR involvement OR nonadherence OR noncompliance OR participation OR preference* OR satisfaction)) OR (professional AND (family disagreement* OR family relation* OR patient disagreement*)) OR refusal participat* OR staff attitude* OR treatment refusal* OR uncertainty) 2. For cystic fibrosis the following MeSH headings and text words were used: (“cystic fibrosis”[Mesh Terms] OR ((cystic OR pancreas OR pancreatic) AND fibrosis) OR fibrocystic OR mucoviscidosis) 3. For RCTs/CCTs the following MeSH headings and text words were used: (((random*[tiab] OR “controlled”[tiab]) AND trial*[tiab]) OR "randomised"[tiab] OR "randomized"[tiab] OR "randomly"[tiab] OR "Randomized Controlled Trial"[Publication Type] OR "Controlled Clinical Trial"[Publication Type] OR "Randomized Controlled Trials as Topic"[Mesh Terms] OR "Placebos"[Mesh Terms] OR placebo*) The final combined search was: 1 AND 2 AND 3. Note: On the advice of the Cochrane Information Specialist, terms for Children 4‐18 years were not used to limit the search, in order to maximize recall of relevant results. [tiab = title or abstract; * = zero or more characters] |
| CINAHL Complete (EBSCO) | 1937 to 19 March 2019 | 1. For decision making the following CINAHL subject headings and text words were used: (MH "Attitude of Health Personnel+" OR MH "Attitude to Health+" OR MH "Communication+" OR MH "Consumer Participation" OR MH "Cooperative Behavior" OR MH "Decision Making+" OR MH "Decision Support Techniques+" OR MH "Educational Technology" OR MH "Health Education+" OR MH "Consent+" OR MH "Professional‐Family Relations" OR MH "Psychology+" OR MH "Nursing Role" OR ((affective OR cognitive OR emotional OR psychosocial OR psychosomatic) AND aspect*) OR ((choice OR compliant OR cooperative OR co‐operative OR illness) AND behavio*) OR clinical support technique* OR collaborati* OR communication* OR consensus OR consent* OR consumer* OR decision* OR disput* OR dissent* OR ((doctor OR doctors OR physician* OR provider*) AND (attitude OR patient relation*)) OR educational technology OR (health AND (attitude* OR education OR information OR literacy)) OR (informed AND (assent OR choice*)) OR misinformation OR negotiati* OR (nurs* AND role*) OR participat* OR partner* OR (patient AND (acceptance OR adherence OR attitude* OR centered OR centred OR compliance OR cooperation OR co‐operation OR education OR involvement OR nonadherence OR noncompliance OR preference* OR satisfaction)) OR (professional AND (family disagreement* OR family relation* OR patient disagreement*)) OR staff attitude* OR treatment refusal* OR uncertainty) 2. For cystic fibrosis the following CINAHL subject headings and text words were used: (MH "Cystic Fibrosis" OR ((cystic OR pancreas OR pancreatic) AND fibrosis) OR fibrocystic OR mucoviscidosis) 3. For RCTs/CCTs the following CINAHL subject headings and text words were used: (((random* OR controlled) AND trial*) OR MH "Placebos" OR MH "Clinical Trials" OR (TI randomized OR AB randomized) OR (TI randomly OR AB randomly) OR (TI randomised OR AB randomised) OR placebo*) The final combined search was: 1 AND 2 AND 3. Note: On the advice of the Cochrane Information Specialist, terms for Children 4‐18 years were not used to limit the search, in order to maximize recall of relevant results. [MH = CINAHL Heading; MH+ = CINAHL Heading (Exploded); TI = title; AB = abstract; * = zero or more characters] |
| Embase (Elsevier) | 1947 to 19 March 2019 | Although the presentation of the Embase search below has been changed to better fit the presentation format of the other searches, the substantive search terms are very similar 1. For decision making the following text words will be used: (((affective OR cognitive OR emotional OR psychosocial OR psychosomatic) AND aspect*) OR ((choice OR compliant OR cooperative OR co‐operative OR illness) AND behavio*) OR clinical support technique* OR collaborati* OR communication* OR consensus OR consent* OR consumer* OR decision* OR disput* OR dissent* OR ((doctor OR doctors OR physician* OR provider*) AND (attitude OR patient relation*)) OR educational technology OR (health AND (attitude* OR education OR information OR literacy)) OR (informed AND (assent OR choice*)) OR misinformation OR negotiati* OR (nurs* AND role*) OR participat* OR partner* OR (patient AND (acceptance OR adherence OR attitude* OR centered OR centred OR compliance OR cooperation OR co‐operation OR education OR involvement OR nonadherence OR noncompliance OR preference* OR satisfaction)) OR (professional AND (family disagreement* OR family relation* OR patient disagreement*)) OR staff attitude* OR treatment refusal* OR uncertainty) 2. For cystic fibrosis the following text words were used: (((cystic OR pancreas OR pancreatic) AND fibrosis) OR fibrocystic OR mucoviscidosis) 3. For RCTs/CCTs the following Embase filters were used: ([controlled clinical trial]/lim OR [randomized controlled trial]/lim) The final combined search was: 1 AND 2 AND 3. The search was limited to Embase only, using the filter [embase]/lim. Note: On the advice of the Cochrane Information Specialist, terms for Children 4‐18 years not used to limit the search, in order to maximize recall of relevant results. [/lim = Embase limit] |
| PsycINFO (EBSCO) | 1597 to 19 March 2019 | 1. For decision making the following PsycINFO Thesaurus Descriptors subject headings and text words were used: (DE "Decision Making" OR DE "Decision Support Systems " OR DE "Decision Theory " OR DE "Choice Behavior" OR DE "Group Decision Making" OR DE "Health Education" OR DE "Health Behavior" OR DE "Health Personnel Attitudes" OR DE "Health Attitudes" OR DE "Communication" OR DE "Interpersonal Communication" OR DE "Persuasive Communication" OR DE "Choice Behavior" OR DE "Informed Consent" OR ((affective OR cognitive OR emotional OR psychosocial OR psychosomatic) AND aspect*) OR ((choice OR compliant OR cooperative OR co‐operative OR illness) AND behavio*) OR clinical support technique* OR collaborati* OR communication* OR consensus OR consent* OR consumer* OR decision* OR disput* OR dissent* OR ((doctor OR doctors OR physician* OR provider*) AND (attitude OR patient relation*)) OR educational technology OR (health AND (attitude* OR education OR information OR literacy)) OR (informed AND (assent OR choice*)) OR misinformation OR negotiati* OR (nurs* AND role*) OR participat* OR partner* OR (patient AND (acceptance OR adherence OR attitude* OR centered OR centred OR compliance OR cooperation OR co‐operation OR education OR involvement OR nonadherence OR noncompliance OR preference* OR satisfaction)) OR (professional AND (family disagreement* OR family relation* OR patient disagreement*)) OR staff attitude* OR treatment refusal* OR uncertainty) 2. For cystic fibrosis the following PsycINFO Thesaurus Descriptors subject headings and text words were used: (DE "Cystic Fibrosis" OR ((cystic OR pancreas OR pancreatic) AND fibrosis) OR fibrocystic OR mucoviscidosis) 3. For RCTs and CCTs the following PsycINFO Thesaurus Descriptors subject headings and text words were used: (DE "Placebo" OR ((random* OR controlled) AND trial*) OR randomly OR randomized OR randomised OR placebo*) The final combined search was: 1 AND 2 AND 3. Note: On the advice of the Cochrane Information Specialist, terms for Children 4‐18 years were not used to limit the search, in order to maximize recall of relevant results. [DE = PsycINFO Thesaurus Descriptors; TI = title; AB = abstract; * = zero or more characters] |
| WHO International Clinical Trials Registry Platform (ICTRP) |
19 March 2019 | For cystic fibrosis the following text words were used: cystic fibrosis OR pancreatic fibrosis OR fibrocystic OR mucoviscidosis In addition, the following phrase was searched for under Condition: cystic fibrosis Searches were limited using the clinical trials in children filter, which limits to the ages 0‐18. Both recruiting and non‐recruiting trials were included. |
| ASSIA (ProQuest) | 1987 to 19 March 2019 | 1. For decision making the following ProQuest subject headings and text words were used: (SU.EXACT.EXPLODE("Decision Making" OR "Participative Decision Making") OR SU.EXACT.EXPLODE("Decision Making Skills") OR ((affective OR cognitive OR emotional OR psychosocial OR psychosomatic) AND aspect*) OR ((choice OR compliant OR cooperative OR co‐operative OR illness) AND behavio*) OR clinical support technique* OR collaborati* OR communication* OR consensus OR consent* OR consumer* OR decision* OR disput* OR dissent* OR ((doctor OR doctors OR physician* OR provider*) AND (attitude OR patient relation*)) OR educational technology OR (health AND (attitude* OR education OR information OR literacy)) OR (informed AND (assent OR choice*)) OR misinformation OR negotiati* OR (nurs* AND role*) OR participat* OR partner* OR (patient AND (acceptance OR adherence OR attitude* OR centered OR centred OR compliance OR cooperation OR co‐operation OR education OR involvement OR nonadherence OR noncompliance OR preference* OR satisfaction)) OR (professional AND (family disagreement* OR family relation* OR patient disagreement*)) OR staff attitude* OR treatment refusal* OR uncertainty) 2. For cystic fibrosis the following ProQuest subject headings and text words were used: (((cystic OR pancreas OR pancreatic) AND fibrosis) OR fibrocystic OR mucoviscidosis) 3. For RCTs and CCTs the following text words were used: (((random* OR controlled) AND trial*) OR randomly OR randomized OR randomised OR placebo*) The final combined search was: 1 AND 2 AND 3. Note: On the advice of the Cochrane Information Specialist, terms for Children 4‐18 years were not used to limit the search, in order to maximize recall of relevant results. [SU.EXACT.EXPLODE = ProQuest subject heading (exploded); * = zero or more characters] |
| ERIC (ProQuest) | 1966 to 19 March 2019 | 1. For decision making the following ProQuest subject headings and text words were used: (SU.EXACT.EXPLODE("Decision Making" OR "Participative Decision Making") OR SU.EXACT.EXPLODE("Decision Making Skills") OR ((affective OR cognitive OR emotional OR psychosocial OR psychosomatic) AND aspect*) OR ((choice OR compliant OR cooperative OR co‐operative OR illness) AND behavio*) OR clinical support technique* OR collaborati* OR communication* OR consensus OR consent* OR consumer* OR decision* OR disput* OR dissent* OR ((doctor OR doctors OR physician* OR provider*) AND (attitude OR patient relation*)) OR educational technology OR (health AND (attitude* OR education OR information OR literacy)) OR (informed AND (assent OR choice*)) OR misinformation OR negotiati* OR (nurs* AND role*) OR participat* OR partner* OR (patient AND (acceptance OR adherence OR attitude* OR centered OR centred OR compliance OR cooperation OR co‐operation OR education OR involvement OR nonadherence OR noncompliance OR preference* OR satisfaction)) OR (professional AND (family disagreement* OR family relation* OR patient disagreement*)) OR staff attitude* OR treatment refusal* OR uncertainty) 2. For cystic fibrosis the following ProQuest subject headings and text words were used: (((cystic OR pancreas OR pancreatic) AND fibrosis) OR fibrocystic OR mucoviscidosis) 3. For RCTs and CCTs the following text words were used: (((random* OR controlled) AND trial*) OR randomly OR randomized OR randomised OR placebo*) The final combined search was: 1 AND 2 AND 3. Note: On the advice of the Cochrane Information Specialist, terms for Children 4‐18 years were not used to limit the search, in order to maximize recall of relevant results. [SU.EXACT.EXPLODE = ProQuest subject heading (exploded); * = zero or more characters] |
| ClinicalTrials.gov (clinicaltrials.gov/) |
19 March 2019 | For cystic fibrosis the following text words were used: cystic fibrosis OR pancreatic fibrosis OR fibrocystic OR mucoviscidosis In addition, the following phrase was searched for under Condition: cystic fibrosis Both recruiting and non‐recruiting trials were included. As the definition for children only includes participants only up until 17, this limit was not applied and all age ranges were retrieved. |
| ProQuest Dissertations and Theses Global (ProQuest) | 1743 to 19 March 2019 | Cystic fibrosis AND (((random* OR controlled) AND trial*) OR randomized OR randomly OR randomised OR placebo*) The search was run in all indexed fields, but not within the full text of theses. Note: On the advice of the Cochrane Information Specialist, terms for Children 4‐18 years were not used to limit the search, in order to maximize recall of relevant results. |
| Open Grey: System for grey literature in Europe (//opengrey.eu/) |
17 March 2019 |
Cystic Fibrosis |
| Grey Matters: a practical tool for searching health related grey literature (www.cadth.ca/resources/finding‐evidence/grey‐matters) |
23 March 2019 | Cystic Fibrosis |
| Lenus (www.lenus.ie/hse/register) | 14 March 2019 |
Cystic Fibrosis |
| Rian ‐ Pathways to Irish Research (www.rian.ie) |
13 March 2019 |
Cystic Fibrosis |
| UK Cystic Fibrosis Registry 2017 Annual Data Report (UK CF Trust 2018) |
01 February 2019 |
Cystic Fibrosis |
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alpern 2011 | Not a relevant intervention; study measured coping efficacy in treatment adherence, HRQOL and FEV1. Received e‐mail confirmation (02 June 2017) from the main author that this abstract did not proceed to full publication as a journal article. |
| Andrade 2013 | Not a relevant intervention. Respiratory care intervention. |
| Austin 2015 | A systematic review (non‐RCT) looking at SDM for serious illness (including CF) but in adults. |
| Belsky 1994 | Not an appropriate intervention for inclusion i.e. self‐hypnosis intervention evaluating psychological and physiological functioning of children aged 7 to 18 years with CF. |
| Blackwell 2013 | A review (non‐RCT) examining of current guidelines for the development and application of PROs as a step towards paediatric collaborative medicine. |
| Blackwell 2015 | Not an appropriate intervention. Received e‐mail confirmation (19 February 2019) from the main author that the study did not have SDM as a primary or secondary outcome. The main author confirmed that the primary outcome of this study was 'treatment fidelity to an adherence intervention' |
| Blumenthal 2006 | An RCT in adults (mean age was 50 years), 37 of the 328 participants had CF. Age range not eligible for inclusion. |
| Bryon 2000 | Intervention not appropriate ‐ the home visit intervention measured self‐esteem and take‐up of clinic appointments and drugs prescribed. |
| Cannon 1999 | Pilot study. Non‐ RCT |
| Christian 2006 | Intervention not appropriate ‐ scales were applied to measure pulmonary function and psycho‐social health status i.e. perceived illness experience, self‐perception, social support, and loneliness. |
| Cottrell 1996 | Not an appropriate intervention ‐ self‐management program for CF using a manual containing three sections: (1) principles of self‐management, (2) basic anatomy and physiology and (3) stress‐management and problem‐solving strategies, e.g. with salt loss. |
| Cruz 2007 | Not an appropriate intervention ‐ the study aimed to measure the effects of behavioural‐family systems therapy on family conflict with family participants, not SDM according to our definition (Lipstein 2012). |
| Cummings 2011 | RCT testing a mentorship program designed to enhance self‐efficacy; excluded following e‐mail communication from the main author (26 November 2017) confirming that the study is no longer ongoing and did not have any hard measures of SDM. |
| Davis 2004 | Not an appropriate intervention ‐ an educational CD‐ROM intervention to increase knowledge of CF and improving coping strategies and not to promote SDM. |
| DeLambo 2004 | Not appropriate intervention. E‐mail communication with contact author (Carolyn E. Levers‐Landis) confirmed that SDM was not measured in this study. |
| Downs 2006 | Not an appropriate intervention as the outcome measures were adherence to aerosol and ACT regimens, caregiver self‐management behaviours, responsiveness of ACT performance when the child was unwell, child knowledge of ACT, child feelings about regular performance of aerosol and ACT treatment regimens, and caregiver self‐efficacy to manage aerosol and ACT treatment regimens. |
| Farrell 2014 | Not an appropriate intervention ‐ a report card aimed at improved communication for doctor counselling encounters in newborn screening for CF and not an intervention for promoting participation in SDM. |
| Happ 2013 | A qualitative descriptive study (Non‐RCT). |
| Huang 2014 | Not an appropriate intervention. Email communication with the main author confirmed that SDM was not a focus of the study which used algorithms to measure the frequency of patient‐initiated communications by cell phone texting. |
| Jafari 2017 | Not an appropriate intervention ‐ a quasi‐experimental study to assess an educational booklet aimed at reducing anxiety in mothers of children with CF and not at promoting SDM. |
| Marciel 2010 | Not an appropriate intervention ‐ qualitative study looking at the acceptability, feasibility and utility of a cell phone intervention to improve adherence. |
| Quittner 2000 | Not an appropriate study design ‐ a chapter in a book reporting an RCT design plan. |
| Stabler 1981 | Not an appropriate intervention for inclusion. The communication skills training intervention aimed at improving family functioning for families in chronic crisis coping with chronic medical problems. |
| Vandemheen 2009 | Doctoral thesis on the development and evaluation of a decision‐aid for people with CF aged 18 years or older; age range not eligible for inclusion. |
| Ward 2012 | Observational study (non‐RCT). |
| Wells 2016 | Systematic literature review (non‐RCT). |
CF: cystic fibrosis FEV1: forced expiratory volume in one second HRQOL: health‐related quality of life PROs: patient reported outcomes QOL: quality of life RCT: randomised controlled trial SDM: shared decision‐making
Contributions of authors
| Task | Who undertook task |
| Protocol stage: conceived the review | Imelda Coyne |
| Protocol/Review stage: supervisor | Imelda Coyne |
| Protocol stage: designed the protocol approach | Helen Malone, Imelda Coyne |
| Protocol stage: prepared first draft of the protocol | Helen Malone, Susan Biggar, Sheila Javadpour, Zai Edworthy, Greg Sheaf, Imelda Coyne |
| Protocol stage: co‐ordinated the protocol | Helen Malone |
| Protocol/Review stage: designing the search strategy | Greg Sheaf, Helen Malone, Imelda Coyne |
| Review stage: data collection for the review | Greg Sheaf, Helen Malone |
| Review stage: undertaking searches | Greg Sheaf, Helen Malone, Imelda Coyne, Sheila Javadpour, Zai Edworthy, Susan Biggar |
| Review stage: organising retrieval of papers | Greg Sheaf, Helen Malone |
| Review stage: Setting up Covidence tool‐kit and importing studies to Covidence | Greg Sheaf |
| Review stage: Reviewers for 1st screening (titles and abstracts) screening search results | Helen Malone, Zai Edworthy and Susan Biggar |
| Review stage: Reviewers for 2nd screening (full texts) Screening search results | Helen Malone, Zai Edworthy |
| Review stage: obtaining and screening data on unpublished studies | Greg Sheaf, Helen Malone |
| Review stage: screening retrieved papers against eligibility criteria | Helen Malone, Imelda Coyne, Zai Edworthy, Susan Biggar |
| Review stage: appraising quality of papers (assessing risk of bias) | N/A |
| Review stage: writing to authors of papers for additional information | Helen Malone |
| Review stage: providing additional data about papers | Sheila Javadpour, Zai Edworthy |
| Review stage: data management for the review | Helen Malone, Greg Sheaf |
| Review stage: Review Manager | Helen Malone |
| Review stage: analysis of data | N/A |
| Review stage: interpretation of data | N/A |
| Review stage: providing a methodological perspective | Imelda Coyne |
| Review stage: providing general advice on the review | Imelda Coyne, Susan Biggar, Sheila Javadpour, Zai Edworthy, Greg Sheaf |
| Review stage: writing the review | Helen Malone, Susan Biggar, Imelda Coyne, Greg Sheaf |
| Performing previous work that was the foundation of the current review | Imelda Coyne |
| Update stage: updating review | Helen Malone, Imelda Coyne |
N/A: not applicable to date
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research, UK.
This systematic review was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Cystic Fibrosis and Genetic Disorders Group.
-
Health Research Board, Ireland.
Helen Malone has received a CochraneTraining Fellowship from the Health Research Board Ireland
Declarations of interest
Helen Malone declares no known conflict of interest.
Imelda Coyne declares no known conflict of interest.
Greg Sheaf declares no known conflict of interest.
Sheila Javadpour declares no known conflict of interest.
Susan Biggar declares no known conflict of interest.
Zai Edworthy declares no known conflict of interest.
Edited (no change to conclusions)
References
References to studies excluded from this review
Alpern 2011 {published and unpublished data}
- Alpern AN, Mclean KA, Quitter AI. Coping efficacy moderates disease severity and health‐related quality of life in adolescents with CF. Pediatric Pulmonology 2011;46 Suppl 34:410. [Abstract no.: 542; CFGD Register: MH28b] [Google Scholar]
- Kimberg CI, Alpern AN, Armenteros EM, Quittner AL. Coping strategies demonstrated by caregivers of adolescents with CF. Pediatric Pulmonology 2010;45 Suppl 33:446. [Abstract no.: 624; CFGD Register: MH28a] [Google Scholar]
Andrade 2013 {published data only}
- Andrade T, Guitton B, Monteiro E, Deolindo V. Education for physiotherapeutic caregivers of patients with cystic fibrosis. Journal of Cystic Fibrosis 2013;12 Suppl 1:S106. [Abstract no.: 225; CFGD Register: MH34] [Google Scholar]
Austin 2015 {published data only}
- Austin CA, Mohottige D, Sudore RL, Smith AK, Hanson LC. Tools to promote shared decision making in serious illness: A systematic review. JAMA Internal Medicine 2015;175(7):1213‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Belsky 1994 {published data only}
- Belsky J, Khanna P. The effects of self‐hypnosis for children with cystic fibrosis: a pilot study. American Journal of Clinical Hypnosis 1994;36(4):282‐92. [CFGD Register: MH14] [DOI] [PubMed] [Google Scholar]
Blackwell 2013 {published data only}
- Blackwell SL, Marciel K K, Quittner AL. Utilization of patient‐reported outcomes as a step towards collaborative medicine. Paediatric Respiratory Reviews 2013;14(3):146–51. [DOI] [PubMed] [Google Scholar]
Blackwell 2015 {published data only}
- Alpern AN, McLean KA, Becker EM, Riekert KA, Quittner AL. Predictors of providers' competence delivering a problem‐solving adherence intervention. Pediatric Pulmonology 2016;51(S45):458. [CFGD Register: MH29p] [Google Scholar]
- Alpern AN, McLean KA, Marciel KK, Zhang J, Riekert KA, Quittner AL. Effects of clinical supervision on treatment fidelity in ICare. Pediatric Pulmonology 2012;47(S35):433. [Abstract no.: 572; CFGD Register: MH29g] [Google Scholar]
- Barker D, Quittner AL, Riekert KA. Relating patterns of social support from friends and family to health‐related quality of life. Pediatric Pulmonology 2013;48(Suppl 36):433. [Abstract no.: 615; CFGD Register: MH29k] [Google Scholar]
- Blackwell LS, Marciel KK, Romero CV, Quittner AL. Pain assessment in children and adolescents with cystic fibrosis: utilizing an online pain diary. Pediatric Pulmonology 2011;46(S34):411. [Abstract no.: 546; CFGD Register: MH29d] [Google Scholar]
- Blackwell LS, Quittner AL. Daily pain in adolescents with CF: Effects on adherence, psychological symptoms, and health‐related quality of life. Pediatric Pulmonology 2015;50(3):244‐51. [CFGD Register: MH29l] [DOI] [PubMed] [Google Scholar]
- Blackwell LS, Romero SL, Marciel KK, Romero CV, Quittner AL. Pain in adolescents and young adults with CF: location, severity, and predictors. Pediatric Pulmonology 2012;47(S35):430. [Abstract no.: 561; CFGD Register: MH29j] [Google Scholar]
- Eakin MN, Bilderback A, Ridge A, Kimburg C, Marciel K, Swenson A, et al. Disparities by socioeconomic status in health outcomes in pediatric CF patients. Pediatric Pulmonology 2010;45(S33):449. [Abstract no.: 449; CFGD Register: MH29r] [Google Scholar]
- Eakin MN, Bilderback A, Ridge A, Quittner AL, Zhang J, Riekert KA. Out‐of‐pocket costs for medication in the I change adherence and raise expectations (ICARE) study. Pediatric Ppulmonology 2011;46(S34):420. [Abstract no.: 568; CFGD Register: MH29c] [Google Scholar]
- Marciel KK, Hernandez C, Kimberg CI, Zhang J, Riekert KA, Quittner AL. Developmental differences in health‐related quality of life and associations between treatments and patient burden in the I change adherence and raise expectations (ICARE) study. Pediatric Pulmonology 2011;46(S34):419. [Abstract no.: 566; CFGD Register: MH29b] [Google Scholar]
- Marciel KK, Kimberg CI, Riekert KA, Swenson A, Quittner AL. Participation of multidisciplinary team members in a behavioural intervention to improve adherence in adolescents with CF. Pediatric Pulmonology 2010;45(S33):437. [Abstract no.: 599; CFGD Register: MH29a] [Google Scholar]
- McLean KA, Quittner AL, Alpern AN, Marciel KK, Riekert KA. Healthcare provider acceptability of problem‐solving in the I change adherence and raise expectations (iCARE) study. Pediatric Pulmonology 2013;48(S36):437. [Abstract no.: 626; CFGD Register: MH29i] [Google Scholar]
- Nicolais CJ, Bernstein R, Riekert KA, Quittner AL. Parent knowledge of disease management in cystic fibrosis: assessing behavioral treatment management. Pediatric Pulmonology 2018;53(2):162‐73. [CFGD Register: MH29q] [DOI] [PubMed] [Google Scholar]
- Nicolais CJ, Saez‐Flores E, Bernstein R, Riekert KA, Eakin MN, Bilderback A, et al. Frequency of self‐reported barriers in relation to medication adherence in adolescents with cystic fibrosis. Pediatric Pulmonology 2015;50(S41):365. [Abstract no.: 461; CFGD Register: MH29n] [Google Scholar]
- Quittner A, Kimberg C, Marciel K, Zhang J, Riekert K. Randomized, controlled trial of a behavioral adherence intervention for adolescents with cystic fibrosis: I change adherence and raise expectations (iCARE). Chest 2011;140(4 Suppl):908a. [CFGD Register: MH29e] [Google Scholar]
- Quittner AL, Alpern AN, McLean KA, Marciel KK, Zhang J, Riekert KA. Clinical supervision improves treatment fidelity to an adherence intervention. Journal of Cystic Fibrosis 2012;11 Suppl 1:S9. [Abstract no.: WS4.1; CFGD Register: MH29f] [Google Scholar]
- Quittner AL, Biesen J, Marciel K, Ridge A, Zhang J, Riekert KA. Health‐related quality of life in adolescents with cystic fibrosis: Associations with age, gender, nutrition and pulmonary function. American Journal of Respiratory and Critical Care Medicine 2013;187:A26. [CFGD Register: MH29o; American Thoracic Society 2013 International Conference, May 17‐22, 2013 ‐ Philadelphia Pennsylvania] [Google Scholar]
- Quittner AL, Riekert KA. I Change adherence and raise expectations: the iCARE study. Pediatric Pulmonology 2013;48(S36):136. [CFGD Register: MH29h; Symposium summary no.: S7.3] [Google Scholar]
- Saez‐Flores E, Thomas K, Riekert KA, Eakin MN, Bilderback A, Quittner AL. Associations between treatment complexity, CFQ‐R treatment burden, and adherence in adolescents with CF. Pediatric Pulmonology 2015;50(S41):439. [Abstract no.: 648; CFGD Register: MH29m] [Google Scholar]
Blumenthal 2006 {published data only}
- Blumenthal JA, Babyak MA, Carney RM, Keefe FJ, Davis RD, LaCaille RA, et al. Telephone‐based coping skills training for patients awaiting lung transplantation. Journal of Consulting and Clinical Psychology 2006;74(3):535–44. [CFGD Register: MH50a] [DOI] [PubMed] [Google Scholar]
- Hoffman BM, Stonerock GL, Smith PJ, O'Hayer CV, Palmer S, Davis RD, et al. Development and psychometric properties of the pulmonary‐specific quality‐of‐life scale in lung transplant patients. Journal of Heart and Lung Transplantation 2015;34(8):1058‐65. [CFGD Register: MH50c] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PJ, Blumenthal JA, Trulock EP, Freedland KE, Carney RM, Davis RD, et al. Psychosocial predictors of mortality following lung transplantation. American Journal of Transplantation 2016;16(1):271‐7. [CFGD Register: MH50b] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bryon 2000 {published data only}
- Bryon M, Burton J, Tostevin M, Madge S. A home visit programme to improve health status and psychosocial functioning of families with child with CF. Pediatric Pulmonology 2000;30 Supplement 20:336. [Abstract no.: 565; CFGD Register: MH9] [Google Scholar]
Cannon 1999 {published data only}
- Cannon C, Benitez J, Scarbary J, Taylor K, Guill M. In‐home videoconferencing for cystic fibrosis patient education. Pediatric Pulmonology 1999;28 Supplement 19:333. [CFGD Register: MH6] [Google Scholar]
Christian 2006 {published data only}
- *Christian BJ, D'Auria JP. Building life‐skills for children with cystic fibrosis: effectiveness of an intervention. Nursing Research 2006;55(5):300‐7. [CFGD Register: MH7f] [DOI] [PubMed] [Google Scholar]
- Christian B. Functional disability and quality of life school‐age children with cystic fibrosis. Pediatric Pulmonology 2004;38 Suppl 27:356. [Abstract no.: 475; CFGD Register: MH7c] [Google Scholar]
- Christian B. The impact of chronic illness on quality of life in children with cystic fibrosis. Pediatric pulmonology 2006;41 Suppl 29:398. [Abstract no.: 529; CFGD Register: MH7e] [Google Scholar]
- Christian B. The interface between physical activity, growth, and pulmonary function with the psychosocial impact of cystic fibrosis in children. Pediatric Pulmonology 2005;40 Suppl 28:370. [Abstract no.: 504; CFGD Register: MH7d] [Google Scholar]
- Christian BJ, D'Auria J, Belyea M. Loneliness in school age children with cystic fibrosis. Pediatric Pulmonology 2003;36 Suppl 25:366. [Abstract no.: 517; CFGD Register: MH7b] [Google Scholar]
- Christian BJ, D'Auria JP, Belyea MJ, Retsch‐Bogart GZ. Psychosocial status of school‐age children with cystic fibrosis. Pediatric Pulmonology 1999;28 Suppl 19:329. [Abstract no.: 573; CFGD Register: MH7a] [Google Scholar]
Cottrell 1996 {published and unpublished data}
- *Cottrell CK, Young GA, Creer TL, Holroyd KA, Kotses H. The development and evaluation of a self‐management program for cystic fibrosis. Pediatric Asthma, Allergy and Immunology 1996;10(3):109‐18. [CFGD Register: MH31a] [Google Scholar]
- Cottrell K. The effects of self‐management training in children/adolescents with cystic fibrosis. Thesis obtained from Ohio University 1994. [CFGD Register: MH31b]
Cruz 2007 {published data only}
- Cruz I, Snell C, Barker DH, Quittner AL, Drotar D. Effects of behavioral‐family systems therapy on family conflict and communication in adolescents with CF and their parents. Pediatric Pulmonology 2007; Vol. 42 Suppl 30:403. [CFGD Register: MH22b]
- McDonald L, Quittner AL, Grimley ME, Botteri M, Barker DH. Effects of a clinic‐based intervention on caregiving stress for parents of children with CF. Journal of Cystic Fibrosis 2007;6 Suppl 1:S75. [CFGD Register: MH22a] [Google Scholar]
Cummings 2011 {published and unpublished data}
- Cameron‐Tucker H, Cystic Fibrosis Research Team led by DW Reid. Telephone‐mentor training of health professionals to facilitate self‐management for people with cystic fibrosis. Asia Pacific Society of Respirology Conference. Gold Coast, 2007:Oral Poster: 1‐053.
- Cameron‐Tucker HL, Joseph L, Cummings E, Cystic Fibrosis Research Team led by Reid D. Outcomes of health‐mentor training to improve self‐management by people with cystic fibrosis (CF). Respirology 2009;14(s1):A58. [Poster: TP105] [Google Scholar]
- Chau S, Cummings E, Turner P. Experiences and insights from the development of a mobile self management system for cystic fibrosis sufferers in Tasmania. MedInfo. Brisbane, August 20 ‐24 2007.
- Cummings E, Hauser J, Cameron‐Tucker H, Fitzpatrick P, Jessup M, Walters EH, et al. Enhancing self‐efficacy for self‐management in people with cystic fibrosis. Studies in Health Technology and Informatics 2011;169:33‐7. [CFGD Register: MH24e] [PubMed] [Google Scholar]
- Cummings EA, Courtney‐Pratt HM, Reid DW, Robinson A, Turner P, Walters EH, et al. Issues and experiences with technology supported development of patient self‐efficacy and self‐managed of chronic disease. Australasian Journal on Ageing 2006;25(Supp 1):A14‐15 [ISSN: 1440‐6381]. [Google Scholar]
- Cummings EA, Turner P. Pathways Home: considerations for technology solutions aimed at supporting self‐management of chronic illness. Proceedings of HIC 2005 & HINZ 31 July ‐ 02 August 2005. Melbourne Convention Centre, Australia, 2005:1‐9. [ISBN: 0 9751013 5 8]
- Cummings EA, Turner P. Pathways Home: developing & deploying technology to support patient empowerment in the self‐management of chronic illness in the community. Proceedings of CollECTeR Europe 2006 9‐10 June 2006, Basel, Switzerland. 2006:261‐269.
- Cummings EA, Turner P. Pathways home project: patient self‐management and self‐efficacy through the deployment of ICTs, eValues. Proceedings of the 19th Bled eConference, 5‐7 June 2006, Bled Slovenia. 2006. [EJ ISBN: 961‐232‐191‐4]
- Cummings EA, Turner P. Patient empowerment and self‐efficacy in chronic disease management: considerations for information systems development, deployment and evaluation. Paradigms politics paradoxes. Proceedings of the 29th Information Systems Research Seminar in Scandinavia, 12‐15 August 2006, Scandinavia. 2006.
- Jessup M, Busch J, Fitzpatrick P, Turner P, Cameron‐Tucker H, Joseph L, et al. Pathways home project: a pilot study of chronic disease self‐management in cystic fibrosis (CF). Respirology 2007;12(Suppl 4):A155. [CFGD Register: MH24b; Poster: P‐1‐052] [Google Scholar]
- Jessup M, Busch J, Turner P, Cummings E, Cameron‐Tucker H, Fitzpatrick P, et al. Pathways Home Project: a pilot study of chronic disease self management in cystic fibrosis. Journal of Cystic Fibrosis 2008;7(Suppl 2):S103. [Abstract no.: 412; CFGD Register: MH24a] [Google Scholar]
- Jessup M, Cameron‐Tucker H, Cummings E, Hauser J, Joseph L, Saddington H, et al. Someone to talk to: Adolescent and adult feedback on their experience of mentoring and I. Oral presentation. In: Australian and New Zealand Cystic Fibrosis Nurses Conference, Perth. Journal of Cystic Fibrosis. 2010; Vol. 9 [Suppl 1]:s107.
- Jessup M, Hauser J, Cameron‐Tucker H, Cummings E, Reid D. Facilitating self‐management in adolescents and adults with cystic fibrosis: a pilot study. Paediatric Pulmonology 2011;46(Suppl 34):405. [Abstract no.: 530; CFGD Register: MH24d] [Google Scholar]
- Reid D. A randomised, controlled trial of behavioural modifications using an Information Technology (IT) tool to improve self‐management in adolescents and adults with Cystic Fibrosis: A pilot study. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=336025&isReview=true (accessed 8 April 2019).
- Reid D. Improving self‐management in adolescents and adults with cystic fibrosis. Fondazione Ricerca Fibrosi Cistica ‐ Onlus. (Italian Cystic Fibrosis Research Foundation).
- Turner P, Cummings E, Busch J, Reid DW, Fitzpatrick P, Cameron‐Tucker H, et al. Pathways home project: the development of an IT support tool to aid chronic disease self management in cystic fibrosis (CF). 7th Australasian Cystic Fibrosis Conference 2007, Sydney, August 11‐14. 2007.
- Turner P, Cummings E, Busch J, Reid DW, Fitzpatrick P, Cameron‐Tucker H, et al. Pathways home project: the development of an IT support tool to aid chronic disease self management in cystic fibrosis (CF). PHCRED Symposium. 2007.
- Wainwright C, Saddington H, Busch J, Blizzard L, Cameron‐Tucker H, Cheney J, et al. Improving self‐efficacy in adolescents and young adults with cystic fibrosis (CF). Journal of Cystic Fibrosis 2009;8(Suppl 2):S93. [ CFGD Register: MH24c; Abstract no.: 374] [Google Scholar]
Davis 2004 {published data only}
- Davis MA, Quittner AL, Stack C. Controlled evaluation of the STARBRIGHT CD‐ROM program for children and adolescents with cystic fibrosis. Pediatric Pulmonology 2002;Suppl 24:351. [CFGD Register: MH10a] [DOI] [PubMed] [Google Scholar]
- Davis MA, Quittner AL, Stack CM, Yang MC. Controlled evaluation of the STARBRIGHT CD‐ROM program for children and adolescents with cystic fibrosis. Journal of Pediatric Psychology 2004;29(4):259‐67. [CFGD Register: MH10b] [DOI] [PubMed] [Google Scholar]
DeLambo 2004 {published data only}
- DeLambo KE, Ievers‐Landis CE, Drotar D, Quittner AL. Association of observed family relationship quality and problem‐solving skills with treatment adherence in older children and adolescents with cystic fibrosis. Journal of Pediatric Psychology 2004;29(5):343–53. [CFGD Register: MH36a] [DOI] [PubMed] [Google Scholar]
- Romero SL, Blackwell LS, Armenteros E, Quittner AL. Patient and parent‐reported conflict: age and gender differences and associations with disease severity. Pediatric Pulmonolgy 2010;45 Suppl 33:446. [Abstract no.: 623; CFGD Register: MH36b] [Google Scholar]
Downs 2006 {published data only}
- Downs JA, Roberts CM, Blackmorel AM, Souef PN, Jenkins SC. Benefits of an education programme on the self‐management of aerosol and airway clearance treatments for children with cystic fibrosis. Chronic Respiratory Disease 2006;3(19):19‐27. [CFGD Register: MH18] [DOI] [PubMed] [Google Scholar]
Farrell 2014 {published data only}
- Farrell MH, Christopher SA, Pean Kirschner A, Roedl SJ, O'Tool FO, Ahmad NY, et al. Improving the quality of physician communication with rapid‐throughput analysis and report cards. Patient Education and Counseling 2014;97(2):248–55. [CFGD Register: MH52] [DOI] [PMC free article] [PubMed] [Google Scholar]
Happ 2013 {published data only}
- Happ MB, Hoffman LA, Higgins LW, Wolff DD, DiVirgilio D, Orenstein DM. Parent and child perceptions of a self‐regulated, home‐based exercise program for children with cystic fibrosis. Nursing Research 2013;62(5):305–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Huang 2014 {published data only}
- Huang JS, Terrones L, Tompane T, Dillon L, Pian M, Gottschalk M, et al. Preparing adolescents with chronic disease for transition to adult care: a technology program. Pediatrics 2014;133(6):e1639‐e46. [CFGD Register: MH44] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jafari 2017 {unpublished data only}
- Jafari S. Effectiveness of partnership educational method on decreasing anxiety and stress in mothers of children with cystic fibrosis. Iranian Registry of Clinical Trials 2017.
Marciel 2010 {published data only}
- *Marciel KK, Saiman L, Quittell LM, Dawkins K, Quittner AL. Cell phone intervention to improve adherence: cystic fibrosis care team, patient, and parent perspectives. Pediatric Pulmonology 2010;45(2):157–64. [CFGD Register: MH33c] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell LS, Romero SL, Romero CV, McLean KA, Dawkins K, Hoag J, et al. CFfone: a social networking site for adolescents and young adults with CF. Pediatric Pulmonology 2012;47 Suppl 35:430. [Abstract no.: 562; CFGD Register: MH33b] [Google Scholar]
- Quittner AL, Romero SL, Blackwell LS, Marciel KK, Romero CV, Dawkins K, et al. Effect of CFfone on knowledge of disease management, psychological well‐being, and health‐related quality of life in adolescents and young adults with CF. Journal of Cystic Fibrosis 2012;11 Suppl 1:S137. [Abstract no.: 313; CFGD Register: MH33a] [Google Scholar]
- Quittner AL, Romero SL, Blackwell LS, McLean KA, Marciel K, Monzon AD, et al. Preliminary data on the efficacy of an online social network for adolescents with CF. 2013 American Journal of Respiratory and Critical Care Medicine; Vol. 187. [CFGD Register: MH33f]
- Quittner AL, Romero SL, Blackwell LS, McLean KA, Monzon AD, Dawkins K. Efficacy of an online social networking site: CFFONE results. Pediatric Pulmonology 2013;48 Suppl 36:135. [CFGD Register: MH33e; Summary no.: S7.2] [Google Scholar]
- Quittner AL, Romero SL, Blackwell LS, Romero CV, Marciel KK, Dawkins K, et al. Preliminary results on the efficacy of an online social network for adolescents with CF: age and disease severity group comparisons. Pediatric Pulmonology 2012;47(Suppl 35):388. [CFGD Register: MH33d] [Google Scholar]
Quittner 2000 {published data only}
- Quittner AL, Drotar D, Levers‐Landis C, Slocum N, Seidner D, Jacobsen J. Adherence to medical treatments in adolescents with cystic fibrosis: The development and evaluation of family‐based interventions. In: Drotar D editor(s). Promoting adherence to medical treatments in chronic childhood illness: Concepts, methods and interventions. First Edition. Mahwah, New Jersey, USA: Lawrence Erlbaum Associates Publishers, 2000:383‐407. [Google Scholar]
Stabler 1981 {unpublished data only}
- Stabler B. Facilitating positive psychosocial adaptation in children with cystic fibrosis by increasing family communication and problem‐solving skills. A research report to the Cystic Fibrosis Foundation. North Carolina University, Chapel Hill. School of Medicine 1981.
Vandemheen 2009 {published and unpublished data}
- *Vandemheen K. Development and evaluation of a decision aid for cystic fibrosis patients considering referral of lung transplantation. Thesis submitted to the Faculty of Graduate and Postdoctoral Studies in part fulfilment of the requirements for the MSc degree in Nursing Health Sciences. University of Ottowa 2009.
- Arron S. Evaluation of a Decision Aid for Adult Cystic Fibrosis Patients Considering Bilateral Lung Transplantation. ClinicalTrials.gov Registry of Clinical trials USA 2006.
- Vandemheen K, Stacey D, Hennessey R, Gooyers T, Salgado J, Freitag A, et al. Translating research into practice: implementing a decision aid for adult cystic fibrosis patients. 2011 Pediatric Pulmonology;46:427. [CFGD Register: MH26c] [Google Scholar]
- Vandemheen KL, O’Connor A, Bell SC, Freitag A, Bye P, Jeanneret A, et al. Online data supplement to 'Randomized trial of a decision aid for patients with cystic fibrosis considering lung transplantation'. American Journal of Respiratory and Critical Care Medicine 2009;180(8):761‐68 Online. [CFGD Register: MH26b] [DOI] [PubMed] [Google Scholar]
- Vandemheen KL, O’Connor A, Bell SC, Freitag A, Bye P, Jeanneret A, et al. Randomized trial of a decision aid for patients with cystic fibrosis considering lung transplantation. American Journal of Respiratory and Critical Care Medicine 2009;180(8):761‐68. [CFGD Register: MH26a] [DOI] [PubMed] [Google Scholar]
Ward 2012 {unpublished data only}
- Ward N. Physiotherapy services for cystic fibrosis (CF) patients receiving home intravenous antibiotics: Australian practice. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=363303 (accessed 8 April 2019 ).
Wells 2016 {unpublished data only}
- Wells E. The role of parenting interventions in promoting treatment cystic fibrosis adherence. Thesis submitted to The University of Manchester UK for the degree of Doctor of Clinical Psychology (ClinPsyD) in the Faculty of Biology Medicine and Health. 2016.
Additional references
Abhyankar 2013
- Abhyankar P, Volk RJ, Blumenthal‐Barby J, Bravo P, Buchholz A, Ozanne E, et al. Balancing the presentation of information and options in patient decision aids: an updated review. BMC Medical Informatics and Decision Making 2013;13(Suppl 2):S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ahmed 2011
- Ahmed A, Bissell C, Charlesworth C, Shervington D, Mannan E, Panny G, et al. Children and young people's views on the 'NHS: an information revolution and local healthwatch. www.oneeastmidlands.org.uk/sites/default/files/library/NCBHealthWatchreportlowres.pdf (first posted 21 Nov 2011).
Angst 1996
- Angst DB, Deatrick JA. Involvement in health care decisions: parents and children with chronic illness. Journal of Family Nursing 1996;2(2):174‐94. [DOI: 10.1177/107484079600200205] [DOI] [Google Scholar]
Anton‐Paduraru 2014
- Anton‐Paduraru DT, Oltean C, Iliescu ML, Baciu G, Trandafir LM. Cystic‐fibrosis related diabetes. Revista Romana De Pediatrie 2014;LX111(1):7‐12. [Google Scholar]
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck‐Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Aziz 2017
- Aziz DA, Billoo AG, Qureshi A, Khalid M, Kirmani S. Clinical and laboratory profile of children with cystic fibrosis: experience of a tertiary care center in Pakistan. Pakistan Journal of Medical Sciences 2017;33(3):554‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bejarano 2015
- Bejarano C, Fuzzell L, Clay C, Leonard S, Shirley E, Wysocki T. Shared decision making in pediatrics: a pilot and feasibility project. Clinical Practice in Pediatric Psychology 2015;3(1):25–36. [DOI: 10.1037/cpp0000086] [DOI] [Google Scholar]
Brand 2013
- Brand PL, Stiggelbout AM. Effective follow‐up consultations: the importance of patient‐centered communication and shared decision making. Paediatric Respiratory Reviews 2013;14(4):224‐8. [DOI] [PubMed] [Google Scholar]
Bregnballe 2017
- Bregnballe V, Boisen KA, Schiøtz PO, Pressler T, Lomborg K. Flying the nest: a challenge for young adults with cystic fibrosis and their parents. Patient Preference and Adherence 2017;11:229–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brehaut 2003
- Brehaut JC, O'Connor AM, Wood TJ, Hack TF, Siminoff L, Gordon E, et al. Validation of a decision regret scale. Medical Decision Making 2003;23(4):281‐92. [DOI] [PubMed] [Google Scholar]
Brice 2007
- Brice P, Jarrett J, Mugford M. Genetic screening for cystic fibrosis: an overview of the science and the economics. Journal of Cystic Fibrosis 2007;6(4):255–61. [DOI] [PubMed] [Google Scholar]
Bronsveld 2001
- Bronsveld I, Mekus F, Bijman J, Ballmann M, Jonge HR, Laabs U, et al. Chloride conductance and genetic background modulate the cystic fibrosis phenotype of ΔF508 homozygous twins and siblings. Journal of Clinical Investigation 2001;108(11):1705–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bryon 2005
- Bryon M, Stramik S. (CFQ‐R) Cystic Fibrosis Questionnaire ‐ Revised. English Language Version. Forest Laboratories UK Ltd, Riverbridge House, Anchor Boulevard Crossways Business Park, Dartford, Kent, DA2 6SL.. Kent, 2005.
Buntain 2004
- Buntain HM, Greer RM, Schluter PJ, Wong JC, Batch JA, Potter JM, et al. Bone mineral density in Australian children, adolescents and adults with cystic fibrosis: a controlled cross sectional study. Thorax 2004;59(2):149‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cass 2006
- Cass H. The NHS Experience: The 'snakes and ladders' guide for patients and professionals. London & New York: Routledge, 2006. [Google Scholar]
Chalmers 1993
- Chalmers I, Enkin M, Keirse M. Preparing and updating systematic reviews of randomized controlled trials of health care. Milbank Quarterly 1993;71(3):411‐37. [PubMed] [Google Scholar]
Chomik 2014
- Chomik S, Klincewicz B, Cichy W. Disease specific knowledge about cystic fibrosis, patient education and counselling in Poland. Annals of Agricultural and Environmental Medicine 2014;21(2):420‐4. [DOI] [PubMed] [Google Scholar]
Chuang 2014
- Chuang S, Doumit M, McDonald R, Hennessy E, Katz T, Jaffe A. Annual review clinic improves care in children with cystic fibrosis. Journal of Cystic Fibrosis 2014;13(2):186‐9. [DOI] [PubMed] [Google Scholar]
Connett 2015
- Connett GJ, Pike KC. Nutritional outcomes in cystic fibrosis ‐ are we doing enough?. Respiratory Reviews 2015;16 Suppl:31‐4. [DOI: 10.1016/j.prrv.2015.07.015] [DOI] [PubMed] [Google Scholar]
Conway 2014
- Conway S, Balfour‐Lynn IM, Rijcke K, Drevinek P, Foweraker J, Havermans T, et al. European Cystic Fibrosis Society standards of care: framework for the cystic fibrosis centre. Journal of Cystic Fibrosis 2014;13 Suppl 1:S3‐22. [DOI: 10.1016/j.jcf.2014.03.009] [DOI] [PMC free article] [PubMed] [Google Scholar]
Covidence 2019 [Computer program]
- Veritas Health Innovation. Covidence systematic review software. Melbourne, Australia: Veritas Health Innovation, 2019.
Coyne 2006
- Coyne I. Children's experiences of hospitalization. Journal of Child Health Care 2006;10(4):326‐36. [DOI] [PubMed] [Google Scholar]
Coyne 2008
- Coyne I. Children’s participation in consultations and decision‐making at health service level: A review of the literature. International Journal of Nursing Studies 2008;45(11):1682–9. [DOI] [PubMed] [Google Scholar]
Coyne 2013
- Coyne I, O'Mathuna DP, Gibson R, Shields L, Sheaf G. Interventions for promoting participation in shared decision‐making for children with cancer. Cochrane Database of Systematic Reviews 2013, Issue 6. [DOI: 10.1002/14651858.CD008970.pub2] [DOI] [PubMed] [Google Scholar]
Coyne 2014
- Coyne I, Amory A, Kiernan G, Gibson F. Children's participation in shared decision‐making: Children, adolescents, parents and healthcare professionals' perspectives and experiences. European Journal of Oncology 2014;18(3):273‐80. [DOI] [PubMed] [Google Scholar]
Coyne 2017
- Coyne I, Sheehan AM, Heery E, While AE. Improving transition to adult healthcare for young people with cystic fibrosis: a systematic review. Journal of Child Health Care 2017;21(3):312‐30. [DOI] [PubMed] [Google Scholar]
Cystic Fibrosis Australia 2013
- Cystic Fibrosis Australia. Australian Cystic Fibrosis Data Registry. www.cysticfibrosis.org.au/data‐registry 2013 (accessed 14 April 2019).
Cystic Fibrosis Foundation 2012
- Cystic Fibrosis Foundation. Cystic Fibrosis Foundation Patient Registry 2012. Annual Data Report. www.cysticfibrosisdata.org/reportsUS.html (accessed 8 April 2019).
Cystic Fibrosis Foundation 2019
- Cystic Fibrosis Foundation. What is Cystic Fibrosis. https://www.cff.org/What‐is‐CF/About‐Cystic‐Fibrosis/ (accessed 9 April 2019).
Cystic Fibrosis Registry of Ireland 2019
- Cystic Fibrosis Registry of Ireland. Cystic Fibrosis Registry of Ireland. www.cfri.ie/ 2019 (accessed 14 April 2019).
Cystic Fibrosis Worldwide 2019
- Cystic Fibrosis Worldwide. What is cystic fibrosis. www.cfww.org/what‐is‐cystic‐fibrosis (accessed 14 April 2019).
De Boer 2013
- Boer P. Perceived value of CME systems in meeting the learning needs of orthopaedic surgeons in community hospitals. AMEE 2013 Conference; 2013 Aug 24 ‐ 28; Prague. 2013:363. [Abstract No.: 7C/3]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG on behalf of the Cochrane Statistical Methods Group. Chapter 9 Analysing data and undertaking meta‐analysis. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Dodge 2007
- Dodge JA, Lewis PA, Stanton M, Wilsher J. Cystic fibrosis mortality and survival in the UK:1947‐2003. European Respiratory Journal 2007;29(3):522‐6. [DOI] [PubMed] [Google Scholar]
Dokken 2000
- Dokken D, Sydnor‐Greenberg N. Exploring complementary and alternative medicine in pediatrics: parents and professionals working together for new understanding. Pediatric Nursing 2000;26(4):383‐90. [PubMed] [Google Scholar]
Donner 1980
- Donner A, Koval JJ. The estimation of intraclass correlation in the analysis of family data. Biometrics 1980;36(1):19‐25. [PubMed] [Google Scholar]
Drayton 2014
- Drayton N, Reddy D. How an active learning initiative identified shared decision making as a key influence in patient care. International Practice Development Journal 2014;4(2):1‐4. [Google Scholar]
Duguépéroux 2008
- Duguépéroux I, Tamalet A, Sermet‐Gaudelus I, Bourgeois M, Gérardin M, Desmazes‐Dufeu N, et al. Clinical changes of patients with cystic fibrosis during transition from pediatric to adult care. Journal of Adolescent Health. 2008;43(5):459‐65. [DOI] [PubMed] [Google Scholar]
Dyce 2015
- Dyce P. The diagnosis and management of cystic fibrosis‐related diabetes. Journal of Diabetes Nursing 2015;19(3):92‐6. [Google Scholar]
Eaton 2004
- Eaton WW, Muntaner C, Smith C, Tien A, Ybarra M. Center for Epidemiologic Studies Depression Scale: Review and revision (CESD and CESD‐R). In: Maruish ME, ed. The Use of Psychological Testing for Treatment Planning and Outcomes Assessment. 3rd Edition. Mahwah, New Jersey: Lawrence Erlbaum Associates, 2004:363‐377. [Google Scholar]
Elf 2015
- Elf M, Fröst P, Lindahl G, Wijk H. Shared decision making in designing new healthcare environments—time to begin improving quality. BMC Health Services Research 2015;15:114. [DOI: 10.1186/s12913-015-0782-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
Elwyn 2003
- Elwyn G, Edwards A, Wensing M, Atwell C, Grol R. Shared decision making: developing the OPTION scale for measuring patient involvement. Quality and Safety in Health Care 2003;12(2):93‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elwyn 2012
- Elwyn G, Frosch D, Thomson R, Joseph‐Williams N, Lloyd A, Kinnersley P, et al. Shared Decision Making: A Model for Clinical Practice. Journal of General Internal Medicine 2012;27(10):1361‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elwyn 2013a
- Elwyn G, Tsulukidze M, Edwards A, Légaré F, Newcombe R. Using a ‘talk’ model of shared decision making to propose an observation‐based measure: Observer OPTION 5 Item. Patient Education and Counseling 2013;93(2):265–71. [DOI] [PubMed] [Google Scholar]
Elwyn 2013b
- Elwyn G, Tilburt J, Montori VM. The ethical imperative for shared decision‐making. European Journal for Person Centered Medicine 2013;1(1):129‐31. [Google Scholar]
Elwyn 2016
- Elwyn G, Frosch DL, Kobrin S. Implementing shared decision‐making: consider all the consequences. Implementation Science 2016;11(14):1‐10. [DOI: 10.1186/s13012-016-0480-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
Endnote X9
- Clarivate Analytics. Reference management software. https://clarivate.libguides.com/endnote_training/home 2019 (accessed 8 April 2019).
Farrell 2008
- Farrell PM, Rosenstein BJ, White TB, Accurso FJ, Castellani C, Cutting GR, et al. Guidelines for diagnosis of cystic fibrosis in newborns through older adults: Cystic Fibrosis Foundation consensus report. Journal of Pediatrics 2008;153(2):S4–S14. [DOI: 10.1016/j.jpeds.2008.05.005] [DOI] [PMC free article] [PubMed] [Google Scholar]
Farrell 2008a
- Farrell PM. The prevalence of cystic fibrosis in the European Union. Journal of Cystic Fibrosis 2008;7(5):450–3. [DOI] [PubMed] [Google Scholar]
Farrell 2017
- Farrel PM, White TB, Howenstine MS, Munck A, Parad RB, Rosenfeld M, et al. Diagnosis of cystic fibrosis in screened populations. The Journal of Pediatrics 2017;181(Supplement):S33‐44. [DOI] [PubMed] [Google Scholar]
Fennestra 2014
- Fennestra B, Boland L, Lawson ML, Harrison D, Kryworuchko J, Leblanc M, et al. Interventions to support children's engagement in health‐related decisions: a systematic review. BMC Pediatrics 2014;14(109):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fiks 2010
- Fiks AG, Jimenez ME. The promise of shared decision‐making in paediatrics. Acta Paediatrica 2010;99(10):1464–6. [DOI: 10.1111/j.1651-2227.2010.01978.x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fixter 2017
- Fixter V, Butler C, Daniels J, Phillips S. A qualitative analysis of the Information needs of parents of children with cystic fibrosis prior to first admission. Journal of Pediatric Nursing 2017;34:e29–e33. [DOI: 10.1016/j.pedn.2017.01.007] [DOI] [PubMed] [Google Scholar]
Furukawa 2006
- Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta‐analyses can provide accurate results. Journal of Clinical Epidemiology 2006;59(1):7‐10. [DOI] [PubMed] [Google Scholar]
Gee 2000
- Gee L, Abbott J, Conway SP, Etherington C, Webb AK. Development of a disease specific health related quality of life measure for adults and adolescents with cystic fibrosis. Thorax 2000;55(11):946–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
GOSH 2019
- Great Ormond Street Hospital for Children. GOSH information sheet: Cystic Fibrosis Annual Reviews. www.gosh.nhs.uk/medical‐information‐0/procedures‐and‐treatments/cystic‐fibrosis‐annual‐reviews (accessed 9 April 2019).
Gotink 2015
- Gotink RA, Chu P, Jan J. V. Busschbach JJV, Benson H, Fricchione GL, Hunink MGM. Standardised mindfulness‐based interventions in healthcare: an overview of systematic reviews and meta‐analyses of RCTs. PLoS ONE 2015;10(4):e0124344. [DOI: 10.1371/journal.pone.0124344] [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Graham 1995
- Graham ID, O'Connor AM. User Manual – Preparation for Decision Making Scale [modified 2010]. Ottawa Hospital Research Institute. http://decisionaid.ohri.ca/docs/develop/User_Manuals/UM_PrepDM.pdf (accessed 14 April 2019).
Gulbrandsen 2016
- Gulbrandsen P, Clayman ML, Beach MC, Han PK, Boss EF, Ofstad EH, et al. Shared decision‐making as an existential journey: aiming for restored autonomous capacity. Patient Education and Counseling 2016;99(9):1505‐10. [DOI] [PubMed] [Google Scholar]
Hansen 2016
- Hansen R, Willy T. Patient autonomy ‐ what does it mean for clinical decision‐making in children and adolescents?. International E‐Journal of Science, Medicine & Education 2016;10(1):3‐9. [Google Scholar]
Harris 1993
- Harris A, Argent BE. The cystic fibrosis gene and its product CFTR. Seminars in Cell Biology 1993;4(1):37‐44. [DOI] [PubMed] [Google Scholar]
Harris 2008
- Harris M, Taylor G. Medical Statistics Made Easy. 2nd Edition. Bath UK: Scion publishing Ltd, 2008. [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Deeks JJ editor(s). Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JPT, Altman DG, Sterne JAC on behalf of the CSMG and the CBMG, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011c
- Higgins JPT, Deeks JJ, Altman DG on behalf of the CSMG, editor(s). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S editor(s). Cochrane Handbook of Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Homa 2015
- Homa K, Sabadosa KA, Marrow LC, Marshall BC. Experience of care from the perspective of individuals with cystic fibrosis and families: Results from 70 CFFoundation accredited programs in the USA. Journal of Cystic Fibrosis 2015;14(4):515–22. [DOI] [PubMed] [Google Scholar]
IPDAS 2013
- Led by Glyn Elwyn in the United Kingdom, Dawn Stacey in Canada. International Patient Decision Aid Standards Collaboration. http://ipdas.ohri.ca/ 2013 (accessed 8 April 2019).
Jackson 2018
- Jackson AD, Goss CH. Epidemiology of CF: How registries can be used to advance our understanding of the CF population. Journal of Cystic Fibrosis 2018;17:297–305. [DOI] [PubMed] [Google Scholar]
Jamieson 2014
- Jamieson N, Fitzgerald D, Singh‐Grewal D, Hanson CS, Craig JC, Tong A. Children’s experiences of cystic fibrosis: a systematic review of qualitative studies. Pediatrics 2014;133(6):e1683–97. [DOI: 10.1542/peds.2014-0009] [DOI] [PubMed] [Google Scholar]
Jessup 2016
- Jessup M, Douglas T, Priddis L, Branch‐Smith C, Shields L. Parental experience of information and education processes following diagnosis of their infant with cystic fibrosis via newborn screening. Journal of Pediatric Nursing 2016;31(3):e233‐41. [DOI] [PubMed] [Google Scholar]
Joffe 2003
- Joffe S. Rethink "affirmative agreement" but abandon "assent". The American Journal of Bioethics 2003;3(4):9‐11. [DOI] [PubMed] [Google Scholar]
Joffer 2016
- Joffer J, Jerden L, Ohman A, Flacking R. Exploring self‐rated health among adolescents: a think‐aloud study. BMC Public Health 2016;16(156):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kerem 2005
- Kerem E, Conway S, Elborn S, Heijermann H. Standard of care for patients with cystic fibrosis: A European Concensus. Journal of Cystic Fibrosis 2005;4(1):7‐26. [DOI] [PubMed] [Google Scholar]
Kiessling 2013
- Kiessling C. Can a situational judgement test (SJT) measure cognitive aspects of communication skills for shared decision making in medicine and teaching education?. AMEE 2013 Conference; 2013 Aug 24‐28; Prague. 2013:295. [Abstract No.: 5M/4]
Klatt 2013
- Klatt ME, Moid F, Wong S, Maniate JM. Facilitating interprofessional education and collaboration through interactive teaching. AMEE 2013 Conference; 2013 Aug 24‐28; Prague. 2013:234. [Abstract no.: 10R]
Kodjebacheva 2016
- Kodjebacheva GD, Sabo T, Xiong J. Interventions to improve child‐parent‐medical provider communication: A systematic review. Social Science & Medicine 2016;166:120e‐127. [DOI: 10.1016/j.socscimed.2016.08.003] [DOI] [PubMed] [Google Scholar]
Kraus 2016
- Kraus CK, Marco CA. Shared decision making in the ED: ethical considerations. American Journal of Emergency Medicine 2016;34(8):1668‐72. [DOI] [PubMed] [Google Scholar]
Kroenke 2001
- Kroenke K, Spitzer RL, Williams JB. The PHQ‐9: validity of a brief depression severity measure. Journal of General Internal Medicine 2001;16(9):606‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Legare 2012
- Legare F, Turcotte S, Stacey D, Ratte S, Kryworuchko J, Graham AD. Patients’ perceptions of sharing in decisions: a systematic review of interventions to enhance shared decision‐making in routine clinical practice. Patient 2012;5(1):1‐19. [DOI] [PubMed] [Google Scholar]
Legare 2014
- Légaré F, Stacey D, Turcotte S, Cossi MJ, Kryworuchko J, Graham ID, Lyddiatt A, Politi MC, Thomson R, Elwyn G, Donner‐Banzhoff N. Interventions for improving the adoption of shared decision making by healthcare professionals. Cochrane Database of Systematic Reviews 2014, Issue 9. [DOI: 10.1002/14651858.CD006732.pub3] [DOI] [PubMed] [Google Scholar]
Lehane 2018
- Lehane E, Leahy‐Warren P, O'Riordan C, Savage E, Drennan J, O'Tuathaigh C, et al. Evidence‐based practice education for healthcare professions: an expert view. BMJ Evidence‐Based Medicine 2018 Nov 15 [Epub ahead of print]. [10. 1136/ bmjebm‐ 2018‐111019] [DOI] [PMC free article] [PubMed]
Lindley 2017
- Lindly OJ, Zuckerman KE, Mistry KB. Clarifying the predictive value of family‐centered care and shared decision making for pediatric healthcare outcomes using the medical expenditure panel survey. Health Services Research Part I 2017;52(1):313‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lipstein 2012
- Lipstein EA, William B. Brinkman WB, Britto MT. What is known about parents’ treatment decisions? A narrative review of pediatric decision making. Medical Decision Making 2012;32(2):246–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lipstein 2013
- Lipstein EA, Muething KA, Dodds CM, Britto T. "I'm the One Taking It": Adolescent participation in chronic disease treatment decisions. Journal of Adolescent Health 2013;53(2):253‐9. [DOI] [PubMed] [Google Scholar]
Lipstein 2014
- Lipstein EA, Dodds CM, Britto MT. Real life clinic visits do not match the ideals of shared decision making. Journal of Pediatrics 2014;165(1):178‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lipstein 2016
- Lipstein EA, Dodds CM, Lovell DJ, Denson LA, Britto MT. Making decisions about chronic disease treatment: A comparison of parents and their adolescent children. Health Expectations 2016;19(3):716‐26. [DOI: 10.1111/hex.12210] [DOI] [PMC free article] [PubMed] [Google Scholar]
Long 2001
- Long JM, Fauset‐Jones J, Dixon MJ, Worthington‐Riley D, Sharma V, Patel L, et al. Annual review hospital visits for patients with cystic fibrosis. Journal of the Royal Society of Medicine 2001;94(Suppl 40):12‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Légaré 2010
- Légaré F, Kearing S, Clay K, Gagnon S, D’Amours D, Rousseau M, Connor A. Are you SURE? Assessing patient decisional conflict with a 4‐item screening test. Canadian Family Physician 2010;56(8):e308‐14. [PMC free article] [PubMed] [Google Scholar]
MacKenzie 2014
- MacKenzie T, Gifford AH, Sabadosa KA, Quinton HB, MS, Knapp EA, Goss CH, et al. Longevity of patients with cystic fibrosis in 2000 to 2010 and beyond: survival analysis of the Cystic Fibrosis Foundation patient registry. Annals of Internal Medicine 2014;161(4):233‐41. [DOI: 10.7326/M13-0636] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mak 2016
- Mak DYF, Sykes J, Stephenson AL, Lands LC. The benefits of newborn screening for cystic fibrosis: The Canadian experience. Journal of Cystic Fibrosis 2016;15(3):302‐8. [DOI: 10.1016/j.jcf.2016.04.001] [DOI] [PubMed] [Google Scholar]
Makoul 2006
- Makoul G, Clayman ML. An integrative model of shared decision making in medical encounters. Patient Education and Counseling. 2006;60:301–12. [DOI] [PubMed] [Google Scholar]
Martiniano 2014
- Martiniano SL, Hoppe JE, Sagel SD, Zemanick ET. Advances in the diagnosis and treatment of cystic fibrosis. Advances in Pediatrics 2014;61(1):225‐43. [DOI: 10.1016/j.yapd.2014.03.002] [DOI] [PubMed] [Google Scholar]
Matel 2009
- Matel JL, Milla CE. Nutrition in cystic fibrosis. Seminars in Respiratory and Critical care Medicine 2009;30(5):579‐86. [DOI] [PubMed] [Google Scholar]
McKone 2015
- McKone EF, Velentgas P, Swenson AJ, Goss CH. Association of sweat chloride concentration at time of diagnosis and CFTR genotype with mortality and cystic fibrosis phenotype. Journal of Cystic Fibrosis 2015;14(5):580‐6. [DOI] [PubMed] [Google Scholar]
Miller 2008
- Miller VA, Reynolds WW, Nelson RM. Parent–child roles in decision making about medical research. Ethics and Behaviour 2008;18(2‐3):161–81. [Google Scholar]
Muller‐Juge 2013
- Muller‐Juge V, Cullati S, Blondon KS, Vu NV, Salvoldelli GL, Nendaz MR. Interprofessional collaboration between residents and nurses on a simulated general internal medicine ward: behaviours enhancing teamwork quality. AMEE 2013 Conference; 2013 Aug 24‐28; Prague. 2013:234. [Abstract No.: 4AA/16]
O'Connor 1995
- O'Connor AM. Validation of a decisional conflict scale. Medical Decision Making 1995;15(1):25‐30. [DOI: 10.1177/0272989X9501500105] [DOI] [PubMed] [Google Scholar]
O'Connor 2007a
- O'Connor AM, Wennberg JE, Légaré F, Llewellyn‐Thomas HA, Moulton BW, Sepucha KR, et al. Toward the 'tipping point': decision aids and informed patient choice. Health Affairs (Project Hope) 2007;26(3):716‐25. [DOI] [PubMed] [Google Scholar]
O'Connor 2007b
- O’Connor AM, Bennett C, Stacey D, Barry MJ, Col NF, Eden KB. Do Patient Decision Aids Meet Effectiveness Criteria of the International Patient Decision Aid Standards Collaboration? A Systematic Review and Meta‐analysis. Medical Decision Making 2007;27:554–74. [DOI] [PubMed] [Google Scholar]
Okumura 2014
- Okumura MJ, Ong T, Dawson D, Neilson D, Lewis N, Richard M, et al. Improving transition from paediatric to adult cystic fibrosis care: programme implementation and evaluation. British Medical Journals, Quality and Safety 2014;23 Suppl 1:i64‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Polaris 2014
- Polaris JJ, Katz JN. "Appropriate" diagnostic testing: supporting diagnostics with evidence‐based medicine and shared decision making. BMC Research Notes 2014;16(7):922. [DOI] [PMC free article] [PubMed] [Google Scholar]
Polit 2008
- Polit DF, Beck CT. Nursing Research: generating and assessing evidence for nursing practice. 8th Edition. Tokyo: Wolters Kluwer/Lippincott Williams & Wilkins, 2008. [Google Scholar]
Quaschning 2013
- Quaschning K, Korner M, Wirtz M. Analyzing the effects of shared decision‐making, empathy and team interaction on patient satisfaction and treatment acceptance in medical rehabilitation using a structural equation modelling approach. Patient Education and Counseling 2013;91(2):167–75. [DOI] [PubMed] [Google Scholar]
Quittner 2005
- Quittner AL, Buu A, Messer MA, Modi AC, Watrous M. Development and validation of The Cystic Fibrosis Questionnaire in the United States: a health‐related quality‐of‐life measure for cystic fibrosis. Chest 2005;128(4):2347‐54. [DOI] [PubMed] [Google Scholar]
Quittner 2009
- Quittner AL, Modi AC, Wainwright C, Otto K, Kirihara J, Montgomery AB. Determination of the minimal clinically important difference scores for the Cystic Fibrosis Questionnaire‐Revised respiratory symptom scale in two populations of patients with cystic fibrosis and chronic Pseudomonas aeruginosa airway infection. Chest 2009;135(6):1610‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Quittner 2012
- Quittner AL, Sawicki GS, McMullen A, Rasouliyan L, Pasta DJ, Yegin A, Konstan MW. Psychometric evaluation of the cystic Fibrosis Questionnaire – Revised in a national survey. Quality of Life Research 2012;21:1267‐78. [DOI] [PubMed] [Google Scholar]
Quon 2012
- Quon BS, Aitken ML. Cystic fibrosis: what to expect now in the early adult years. Paediatric Respiratory Reviews 2012;13(4):206‐14. [DOI] [PubMed] [Google Scholar]
Rathert 2013
- Rathert C, Wyrwich MD, Boren SA. Patient‐centered care and outcomes: a systematic review of the literature. Medical Care Research and Review 2013;70(4):351‐79. [DOI: 10.1177/1077558712465774] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Robinson 2008
- Robinson JH, Callister LC, Berry JA, Dearing KA. Patient‐centered care and adherence: definitions and applications to improve outcomes. Journal of the American Academy of Nurse Practitioners 2008;20(12):600‐7. [DOI] [PubMed] [Google Scholar]
Roehrer 2013
- Roehrer E, Cummings E, Beggs S, Turner P, Hauser J, Micallef N, et al. Pilot evaluation of web enabled symptom monitoring in cystic fibrosis. Informatics for Health and Social Care 2013;38(4):354‐65. [DOI] [PubMed] [Google Scholar]
Runeson 2002
- Runeson I, Hallström I, Elander G, Hermerén G. Children's participation in the decision‐making process during hospitalization: an observational study. Nursing Ethics 2002;9(6):583‐98. [DOI] [PubMed] [Google Scholar]
Ryan 2013
- Ryan R, Hill S, Prictor M, McKenzie J. Cochrane Consumers and Communication Review Group Study Quality Guide. http://cccrg.cochrane.org/cochrane‐author‐resources. La Trobe University Melbourne, Published 2013. Approved (S, Hill) May 2013 (accessed 9 April 2019).
Sabadosa 2014
- Sabadosa KA, Batalden PB. The interdependent roles of patients, families and professionals in cystic fibrosis: a system for the co‐production of healthcare and its improvement. BMJ Quality and Safety 2014;23 Suppl 1:i90‐4. [DOI: 10.1136/bmjqs-2013-002782] [DOI] [PubMed] [Google Scholar]
Savage 2007
- Savage E, Callery P. Clinic consultations with children and parents on the dietary management of cystic fibrosis. Social Science & Medicine 2007;64(2):363‐74. [DOI] [PubMed] [Google Scholar]
Savage 2014
- Savage E, Beirne PV, Ni Chroinin M, Duff A, Fitzgerald T, Farrell D. Self‐management education for cystic fibrosis. Cochrane Database of Systematic Reviews 2014, Issue 9. [DOI: 10.1002/14651858.CD007641.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2011a
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and ‘Summary of findings’ tables. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Schünemann 2011b
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Shafir 2012
- Shafir A, Rosenthal J. Shared decision making: advancing patient‐centered care through state and federal implementation. Informed Medical Decisions Foundation: partnerships for quality care. Report. https://nashp.org/?s=shared+decision+making&category_name= (accessed 9 April 2019).
Shields 2005
- Shields CG, Franks P, Fiscella K, Meldrum S, Epstein RM. Rochester Participatory Decision‐Making Scale (RPAD): reliability and validity. Annals Family Medicine 2005;3(5):436‐42. [DOI: 10.1370/afm.305] [DOI] [PMC free article] [PubMed] [Google Scholar]
Siklosi 2010
- Siklosi KR, Gallagher CG, McKone EF. Development, validation, and implementation of a questionnaire assessing disease knowledge and understanding in adult cystic fibrosis patients. Journal of Cystic Fibrosis 2010;9(6):400‐5. [DOI] [PubMed] [Google Scholar]
Simcox 2009
- Simcox AM, Hewison J, Duff AJA, Morton AM, Conway SP. Decision‐making about pregnancy for women with cystic fibrosis. British Journal of Health Psychology 2009;14(Pt 2):323‐42. [DOI] [PubMed] [Google Scholar]
Simmonds 2014
- Simmonds NJ, Cullinan P, Hodson ME. Growing old with cystic fibrosis – the characteristics of long‐term survivors of cystic fibrosis. Respiratory Medicine 2009;103(4):629‐35. [DOI] [PubMed] [Google Scholar]
Spitzer 1999
- Spitzer RL, Kroenke K, Williams JBW. Validation and utility of a self‐report version of Prime MD: the PHQ study. JAMA 1999;282(18):1737‐44. [DOI] [PubMed] [Google Scholar]
Spitzer 2006
- Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD‐7. Archives of Internal Medicine 2006;166(10):1092‐7. [DOI] [PubMed] [Google Scholar]
Stacey 2010
- Stacey D, Légaré F, Pouliot S, Kryworuchko J, Dunn S. Shared decision making models to inform an interprofessional perspective on decision making: a theory analysis. Patient Education and Counseling. 2010;80(2):164‐72. [DOI] [PubMed] [Google Scholar]
Stacey 2015
- Stacey D, Vandemheen KL, Hennessey R, Gooyers T, Gaudet E, Mallick R, et al. Implementation of a cystic fibrosis lung transplant referral patient decision aid in routine clinical practice: an observational study. Implementation Science 2015;10(17):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stacey 2017
- Stacey D, Légaré F, Lewis K, Barry MJ, Bennett CL, Eden KB, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database of Systematic Reviews 2017, Issue 4. [DOI: 10.1002/14651858.CD001431.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterne 2011a
- Sterne JAC, Egger M, Moher D on behalf of the CBMG, editor(s). Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Sterne 2011b
- Sterne JAC, Sutton AJ, Ioannidis JPA, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta‐analyses of randomized controlled trials. BMJ 2011;343:d4002. [DOI: 10.1136/bmj.d4002] [DOI] [PubMed] [Google Scholar]
Stultz 2012
- Stultz JS, Nahata MC. Computerized clinical decision support for medication prescribing and utilization in pediatrics. JAMA 2012;19(6):942‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Söderbäck 2011
- Söderbäck M, Coyne I, Harder M. The importance of including both a child perspective and the child’s perspective within health care settings to provide truly child‐centred care. Journal of Child Health Care 2011;15(2):99–106. [DOI] [PubMed] [Google Scholar]
The Health Foundation 2012
- The Health Foundation. Summit Report: Leading the way to shared decision making: the critical steps for the NHS Commissioning Board to 'make no decision about me without me' a reality. www.health.org.uk/publication/leading‐way‐shared‐decision‐making (accessed 14 April 2019).
UK CF Trust 2018
- UK CF Trust. UK Cystic Fibrosis Registry 2017 Annual Data Report. www.cysticfibrosis.org.uk/news/registry‐report‐2017 2018.
United Nations 1989
- United Nations. United Nations Convention on the Rights of the Child. www.unicef.org/crc/files/Rights_overview.pdf (accessed 14 April 2019).
Waligora 2016
- Waligora M, Rozynaska J, Piaseck J. Child’s objection to non‐beneficial research: capacity and distress based models. Medicine Health Care and Philosophy 2016;19(1):65‐70. [DOI: 10.1007/s11019-015-9643-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
Walters 1990
- Walters S. Doctor‐patient relationship in cystic fibrosis ‐ a patient's perspective. Holistic Medicine 1990;5(3‐4):157‐62. [Google Scholar]
Weiner 2014
- Weiner SJ, Kelly B, Ashley N, Binns‐Calvey A, Sharma G, Schwartz A, et al. Content coding for contextualization of care: evaluating physician performance at patient‐centered decision making. Medical Decision Making 2014;34(1):98‐106. [DOI] [PubMed] [Google Scholar]
Wennberg 2010
- Wennberg DE, Marr A, Lang L, O'Malley S, Bennett G. A randomized trial of a telephone care management strategy. The New England Journal of Medicine 2010;363(13):1245‐55. [DOI] [PubMed] [Google Scholar]
Westerman 2013
- Westerman GMA, Verheij F, Winkens B, Verhulst FC, Oort FVA. Structured shared decision‐making using dialogue and visualization: a randomized controlled trial. Patient Education and Counseling 2013;90(1):74‐81. [DOI] [PubMed] [Google Scholar]
WHO 2019
- World Health Organisation. Genomic Resource Centre. Genes and Human disease: cystic fibrosis. www.who.int/genomics/public/geneticdiseases/en/index2.html (accessed 9 April 2019).
Williams 2010
- Williams R, Barker H. Cystic Fibrosis. InnovAiT: Education and Inspiration for General Practice. 2010;3(12):743‐52. [Google Scholar]
Wyatt 2015
- Wyatt KD, Betsy L, Brickman WB, Lopez GP, Asi N, Erwin P, et al. Shared decision making in pediatrics: a systematic review and meta‐analysis. Academic Pediatrics 2015;15(6):573‐83. [DOI] [PubMed] [Google Scholar]
Zigmond 1983
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica 1983;67(6):361‐70. [DOI] [PubMed] [Google Scholar]


