Abstract
Background
One-stop clinics provide comprehensive diagnostic testing in one outpatient appointment. They could benefit patients with conditions, such as cancer, whose outcomes are improved by early diagnosis, and bring efficiency savings for health systems.
Objective
To assess the use and outcomes of one-stop clinics for symptoms where cancer is a possible diagnosis.
Design and setting
Systematic review of studies reporting use and outcomes of one-stop clinics in primary care patients.
Method
We searched MEDLINE, Embase, and Cochrane Library for studies assessing one-stop clinics for adults referred after presenting to primary care with any symptom that could be indicative of cancer. Study selection was carried out independently in duplicate with disagreements resolved through discussion.
Results
Twenty-nine studies were identified, most were conducted in the UK and observational in design. Few included a comparison arm. A pooled comparison of the cancer conversion rate of one-stop and multi-stop clinics was only possible for breast symptoms, and we found no significant difference. One-stop clinics were associated with significant reductions in the interval from referral to testing (15 versus 75 days) and more patients diagnosed on the same day (79% versus 25%) compared to multi-stop pathways. The majority of patients and GPs found one-stop clinics to be acceptable.
Conclusion
This review found one-stop clinics were associated with reduced time from referral to testing, increased same day diagnoses, and were acceptable to patients and GPs. Our conclusions are limited by high levels of heterogeneity, scarcity of comparator groups, and the overwhelmingly observational nature of included studies.
Keywords: Cancer, diagnostic tests, primary care, review
Introduction
General Practitioners (GPs) see fewer than eight new cases of cancer each year, yet many more patients consult their GP with symptoms that could be indicative of cancer (1). In some circumstances, GPs may refer patients to ‘one-stop clinics’ to investigate the cause of the symptoms during a single outpatient appointment. One-stop clinics are equipped and staffed so that patients may undergo a range of tests carried out at the same location, have their results reviewed by specialists, receive a diagnosis and, in some cases, have treatment on the same day without referral back to the GP (2). One-stop clinics are often arranged by symptom and specialty, e.g. one-stop breast lump clinic or urology clinic.
The rationale behind a one-stop service is that the efficiency of carrying out all tests in a single appointment and having the results reviewed immediately by a consultant could speed up the diagnostic process, potentially benefitting patients and creating cost savings for the health system (2). This reasoning appears sound as it has been reported that cancer patients can undergo multiple investigations at the request of multiple clinicians before receiving treatment, adding complexity, time, and cost to the diagnostic process (3). Furthermore this model of care could have a positive impact on patient outcomes as when patients are able to access treatment more quickly their satisfaction with the diagnostic process increases and distress is reduced (4,5). Despite this, some authors have cautioned that the time saved may not benefit all patients, particularly if further tests or imaging are required to confirm a diagnosis (6).
Objectives
To our knowledge, there has been no published systematic review to summarize the evidence for one-stop clinics for the investigation of symptoms that could be indicative of cancer, and so we set out to review the evidence for the use of one-stop clinics. We report the proportion of patients diagnosed with cancer or other conditions (the conversion rate) and where possible indications for referral, time to testing, appropriateness, other conditions diagnosed, and acceptability to GPs and patients. We include direct comparisons with outcomes in patients in the same population referred to multi-stop pathways where available.
Methods
Search
We registered the systematic review protocol with PROSPERO (7) and searched the following electronic databases: MEDLINE, Embase, and the Cochrane Library. The search strategy as reported in Supplementary Table 1 was adapted for each individual database. Our search strategy was purposefully kept broad and included terms describing other diagnostic strategies such as direct access and fast track testing. This was done to avoid missing relevant papers because of differences in terminology, and with a view to summarising the literature for the other diagnostic strategies which has been reported elsewhere (8).
We included studies describing adults (aged ≥18 years) attending primary care and undergoing one-stop testing for symptoms where cancer diagnosis was a possibility. One-stop clinics were defined as clinics accepting referrals from a GP without first consulting a specialist, where all the necessary tests for a diagnosis were available on the same day. Some studies included ‘multi-stop’ pathway patients as a comparison group. These patients were referred for tests that required multiple clinic appointments. Restrictions were not placed on the location of the clinics, nor were clinics excluded if their stated aim was something other than cancer detection provided cancer diagnosis was a possibility from the symptoms investigated. In addition, reference lists of identified reviews and all included studies were checked for studies meeting our inclusion criteria. Finally a ‘related article’ search was performed in PubMed on all included studies. No time or language limits were placed on the searches.
Study selection and data extraction
Retrieved articles were title and abstract screened independently by two reviewers (CFS, AT) and any disagreements were resolved through discussion with a third reviewer (BN). The included papers were independently read in full by the same two reviewers and disagreements regarding the inclusion of papers were resolved through discussion. Four initial screening questions were used for each paper, which had to be answered in the affirmative for the paper to be included: (1) One-stop testing confirmed? (2) No specialist triage of referrals? (3) GP one-stop referral outcome data reported separately? (4) Cancer diagnosis possible? Data from retained studies was extracted by two independent reviewers (CFS, GH) into a pre-prepared Excel spreadsheet. The data extraction of the two reviewers was compared, and if necessary, any disagreements were resolved by a third reviewer (BN).
Quality assessment
The risk of bias and quality of the studies was assessed using the Newcastle-Ottawa tool to review cohort studies and a modified version for cross-sectional studies, and the Cochrane Risk of Bias tool to assess the risk of bias in randomised controlled trials (9–11). The results of the quality assessment are used for descriptive purposes, to provide an overall assessment of the quality of the included studies and no studies were excluded based on quality alone.
Analysis
We had two primary outcomes of interest. Firstly, the number of cancers diagnosed at a one-stop clinic or multi-stop pathway, recorded as an absolute number and expressed as a cancer conversion rate (CR). The CR is the number of patients receiving a cancer diagnosis expressed as a proportion of all patients attending the clinic. Secondly, the non-cancer diagnoses (with corresponding CR). Secondary outcomes of interest were indications for referral, time to diagnosis, the appropriateness of referrals, and measures of patient and clinician acceptability.
CRs were calculated for cancer and non-cancer diagnoses for each study, grouped by indication/symptom type, and pooled to give a CR for each indication. Pooled estimates were calculated for the CR for each indication; investigated at one-stop clinics or multi-stop pathways where appropriate, using the metaprop command in Stata weighted using the Freeman–Tukey double arcsine transformation to allow for variation in sample sizes (12,13). A Wilcoxon–Mann–Whitney test was used to investigate whether one-stop clinics reduced the interval from referral to testing, from referral to diagnosis, and whether more patients referred to one-stop clinics received their diagnosis on the same day. In addition, a narrative review of GP and patient acceptability was conducted.
Results
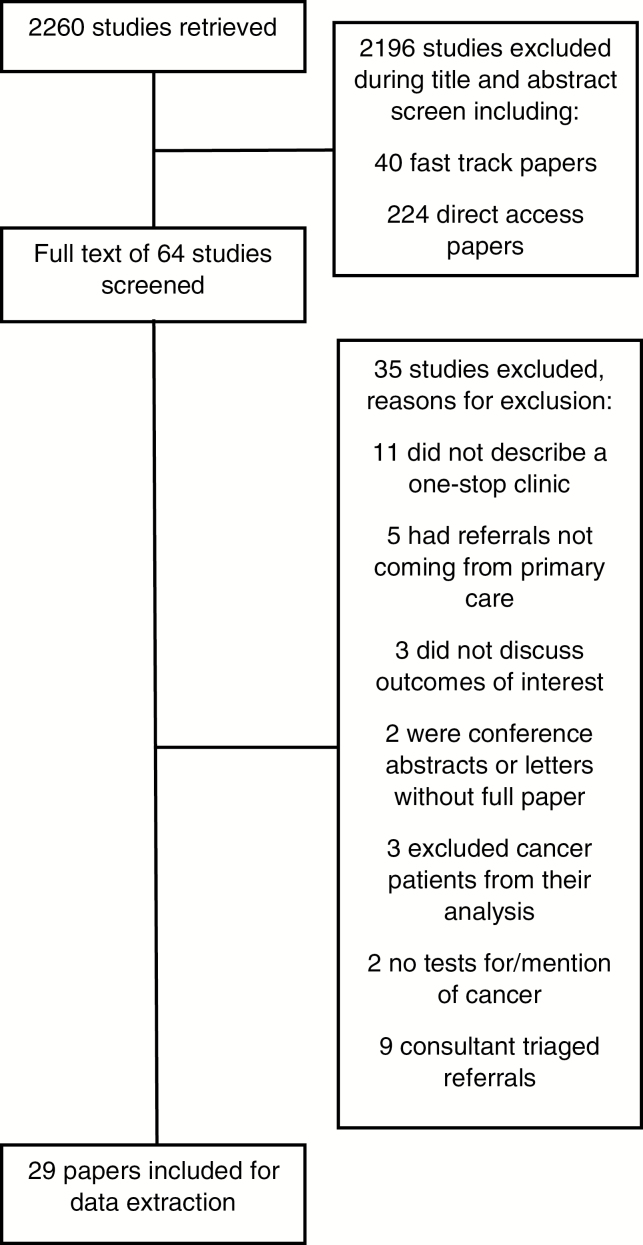
The PRISMA flow diagram in Figure 1 outlines the selection of the 29 papers included in the review. Table 1 presents a list of the included papers.
Figure 1.
PRISMA diagram—studies included and excluded.
Table 1.
Characteristics of the papers included in systematic review of GP referrals to one-stop clinics (1998–2016): first author and reference, country, study design, participant selection, indication(s) for referral, total patients, number of patients in one-stop group, number of patients in comparator group.
| First author and reference | Country study conducted | Study design | Participant selection | Indication(s) for one-stop clinic referral | Number patients included (Total N | One-stop group (n) | Comparator group (n) |
|---|---|---|---|---|---|---|---|
| Abu et al. (26) | UK | Cohort | Consecutive | Abnormal vaginal bleeding | 239 | 113 | 126 |
| Agaba et al. (31) | UK | Cross-sectional | Consecutive | Lower GI symptoms | 250 | 250 | — |
| Al Hamarneh et al. (6) | UK | Cohort | Consecutive | Neck lump | 333 | 333 | — |
| Barrass and Wood (37) | UK | Cross-sectional | Consecutive | Urological | 200 | 100 | 100 |
| Britton et al. (27) | UK | Cross-sectional | Consecutive | Breast symptoms | 7613 | 7613 | — |
| Coull et al. (38) | UK | Cross-sectional | Consecutive | Urological | 257 | 257 | — |
| Dey et al. (14) | UK | Randomised controlled trial | All patients in the time period | Breast symptoms | 478 | 267 | 211 |
| Eltahir et al. (28) | UK | Cohort | Consecutive | Breast symptoms | 1110 | 1110 | — |
| Forde et al. (18) | Ireland | Cohort | Consecutive | Elevated PSA | 215 | 215 | — |
| Gregory et al. (41) | UK | Cross-sectional | Consecutive | Enlarged lymph node | 82 | 82 | — |
| Harcourt et al. (15) | UK | Randomised controlled trial | Consecutive | Breast symptoms | 791 | 416 | 375 |
| Jones and Bourne (20) | UK | Cross-sectional | Consecutive | Abnormal vaginal bleeding | 93 | 93 | — |
| Lee et al. (16) | Australia | Cross-sectional | Consecutive | Lower GI symptoms | 1529 | 1529 | — |
| Lotfallah et al. (21) | UK | Cross-sectional | Consecutive | Abnormal vaginal bleeding | 308 | 308 | — |
| McCombie et al. (17) | Australia | Cohort | Consecutive | Elevated PSA | 200 | 200 | — |
| Mehrotra et al. (29) | UK | Cross-sectional | All patients with breast cancer | Breast symptoms | 4400 | 4400 | — |
| Melleney and Willoughby (39) | UK | Cross-sectional | Consecutive | Dyspepsia | 100 | 100 | — |
| Mohamed and Nair (22) | UK | Cross-sectional | Consecutive | Abnormal vaginal bleeding | 80 | 80 | — |
| Moore et al. (34) | UK | Cross-sectional | Consecutive | Testicular symptoms | 845 | 845 | — |
| Muthuveloe et al. (35) | UK | Cross-sectional | Consecutive | Testicular symptoms | 1757 | 1757 | — |
| Panda (23) | UK | Cohort | Consecutive | Abnormal vaginal bleeding | 522 | 522 | — |
| Patel et al. (30) | UK | Cohort | Consecutive | Breast symptoms | 321 | 321 | — |
| Rochester et al. (36) | UK | Cohort | Consecutive | Testicular symptoms | 1017 | 1017 | — |
| Rutter et al. (40) | UK | Cross-sectional | Consecutive | Dyspepsia | 362 | 362 | — |
| Shah (33) | UK | Cohort | Consecutive | Elevated PSA | 729 | 729 | — |
| Sulaiman et al. (24) | UK | Cohort | Consecutive | Abnormal vaginal bleeding | 146 | 95 | 51 |
| Tan et al. (19) | Singapore | Cross-sectional | Consecutive | Haematuria | 113 | 113 | — |
| Toomey et al. (32) | UK | Cohort | Consecutive | Lower GI symptoms | 344 | 344 | — |
| Yakasai et al. (25) | UK | Cross-sectional | Consecutive | Abnormal vaginal bleeding | 753 | 753 | — |
GI: gastrointestinal; PSA: prostate specific antigen.
The included articles were published between 1998 and 2016, and all were observational studies with the exception of two randomised-controlled trials (14,15). Twenty-five of the studies were carried out in clinics based in the UK, two in Australia (16,17), and one each from Ireland (18) and Singapore (19). All studies were focussed on a specific set of symptoms or specialty, i.e. there were no clinics that accepted referrals where there was a general suspicion of cancer. The indications for referral included: seven studies on post-menopausal or abnormal vaginal bleeding (20–26), six studies on breast symptoms (14,15,27–30), three for lower gastrointestinal (GI) symptoms (16,31,32), three for elevated prostate specific antigen (PSA) (17,18,33), three for testicular symptoms (34–36), two for urological symptoms (37,38), two for dyspepsia (39,40), one for haematuria (19), one for unexplained lymphadenopathy (41), and one for neck lumps (6). When reported, one-stop clinics were held in a hospital setting.
Quality assessment
The majority of studies (90%) included a representative sample of consecutive patients. The overall study quality was poor: most (90%) assessed patient outcomes through retrospective review of clinical record data and 90% presented a descriptive analysis only without including a comparator group. Only the two randomised controlled trials justified their sample sizes, but neither was blinded. One of the randomised controlled trials was judged to be at low risk of bias (14), while the other at moderate risk of bias due to missing detail in the paper, suboptimal methods of allocation concealment, and attrition of participants with characteristics that may have introduced bias (15). See Supplementary Tables 2–4 for full details.
Diagnoses by symptom type
The pooled cancer CR ranged from 1.7% (95% confidence interval (CI) 0.5–3.5%) for lower GI symptoms (3 studies) to 42.8% (CI 28.5–57.7%) for elevated PSA (3 studies) (Table 2). Only three studies presented comparator data from patients referred to multi-stop pathways: one for abnormal vaginal bleeding and two for breast symptoms (14,15,24). No significant difference was found in the cancer CR between one-stop and multi-stop pathways for breast symptoms (P = 0.28) (Table 2).
Table 2.
Cancer and non-cancer conversion rates by indication for referral in adult patients referred to a one-stop clinic with symptoms that could be indicative of cancer
| Indication for one-stop referral | Conversion rates | ||||||
|---|---|---|---|---|---|---|---|
| Cancer | Non-cancer diagnoses | ||||||
| Studies (N) | Range (%) | Pooled % (95% CI) | Studies (N) | Range % | Pooled % (95% CI) | ||
| Abnormal vaginal bleeding | One-stop | 6 | 1.3–5.2 | 3.7 (2.7 – 4.9) | 5 | 4.2 – 58.1 | 30.3 (17.0 – 45.5) |
| Multi-stop | 1 | 5.9 | — | 1 | 17.7 | — | |
| Breast symptoms | One-stop | 6 | 5.3–16.1 | 8.5 (6.8–10.4) | 2 | 68.9–90.0 | 81.5 (78.3–84.6) |
| Multi-stop | 2 | 9.1–12.3 | 10.2 (7.8–12.8)* | 1 | 64.5 | — | |
| Lower gastrointestinal symptoms | 3 | 0.9–3.2 | 1.7 (0.5–3.5) | 3 | 59.2–96.8 | 82.1 (61.8–95.8) | |
| Elevated PSA | 3 | 31.1–55.5 | 42.8 (28.5–57.7) | 1 | 24 | — | |
| Haematuria | 1 | 14.2 | — | 1 | 28.3 | — | |
| Enlarged lymph node | 1 | 19.5 | — | 1 | 80.5 | — | |
| Neck lump | 1 | 16.2 | — | 1 | 64.3 | — | |
| Testicular symptoms | 3 | 1.0–5.6 | 3.2 (0.9–6.7) | 3 | 15.0–75.7 | 49.5 (14.0–85.4) | |
| Upper gastrointestinal symptoms | 2 | 3.3–7 | 3.9 (2.3–5.9) | 2 | 41.2–72.0 | 48.2 (43.6–52.7) | |
*P = 0.28.
Sixty-six percent of the included studies reported non-cancer diagnoses: the pooled non-cancer CR in one-stop clinics ranged from 30.3% (CI 17.0–45.5%) for abnormal vaginal bleeding (5 studies) to 82.1% (CI 61.8–95.8%) for lower GI symptoms (3 studies). Two studies reported the number of non-cancer diagnoses made in comparator arms: one investigating abnormal vaginal bleeding and the other breast symptoms (14,24). In both cases the non-cancer CR was higher in the one-stop clinic, however these differences arise from comparing the pooled analysis of one-stop clinics to single studies describing the outcomes of a multi-stop comparator clinic and so should be viewed with caution.
For a full breakdown of all cancer and non-cancer diagnoses CRs see Supplementary Tables 5–12.
Time to testing and diagnosis
One-stop clinics were associated with a significant reduction in the waiting time between referral and clinic appointment from a mean of 75 days (standard deviation (SD) 47 days) in multi-stop pathways (4 studies) to 15 days (SD 7 days) in one-stop clinics (9 studies) (r = 2.78, P = 0.005). Only one study reported the change in diagnostic interval and found a significant decrease from usual care clinics (28 days) associated with the one-stop clinic (15 days, P = 0.004) (37). Significantly more patients referred to one-stop clinics (79.2%) compared to multi-stop clinics (24.7%) were also diagnosed on the same day (r = −1.95, P = 0.05) (14 studies).
Appropriateness of referral to one-stop clinic
Three studies reported the eligibility of patients and the appropriateness of referrals to one-stop clinics (14,30,39). Two reported that 3% of the patients referred were excluded because they were deemed to be ineligible based on clinician judgement (14,39) without giving further detail, and the third reported that 34% of patients attending the one-stop breast clinic were referred inappropriately according to UK NHS guidelines (30). None of the included studies examined the appropriateness of referrals to one-stop clinics relative to usual care clinics.
Acceptability of one-stop clinics
Acceptability to patients was assessed in nine studies. Over 87% of patients were either satisfied or very satisfied with the service provided by one-stop clinics (22,32,33,41), or felt that it had been a ‘helpful’ (40) experience. In a qualitative evaluation, a one-stop menstrual clinic was rated higher than usual care on provision of information, continuity of care, waiting time for an appointment, clinic organisation, explanation of tests, and provision of results (26). Eighty-three percent of patients in another study felt that having all of their tests on one day was ‘of benefit’ (39), however this study also reported that fewer patients regarded their management in the one-stop dyspepsia clinic as satisfactory compared to those seen in the usual care clinic.
One-stop breast clinics were also found to significantly reduce patient anxiety in the period immediately after their appointment, but this disappeared in the longer term (14,15). One study reported that women who had received a quicker (one-stop) diagnosis of breast cancer reported higher levels of anxiety than those who had been diagnosed over two appointments, but this difference was not statistically significant (15). A significant difference was found 8 weeks later, when women diagnosed with cancer in the one-stop clinic reported higher levels of depression than those diagnosed in a two-stop clinic.
Three papers assessed GPs views on the acceptability of one-stop clinics. In one study, GPs rated the one-stop urology clinic as a 9/10 in terms of the quality of service and information provided, and the resolution of their patients’ presenting symptoms, and 7/10 in terms of accessibility (33). In another study, 88% of GPs said they were satisfied and would use the one-stop lymph node clinic again (41). In the third study, 91% of GPs rated the clinic as ‘good’ or ‘very good’, and 84% said they preferred the one-stop clinic for the management of patients with dyspepsia while only 11% said they preferred direct access endoscopy (40).
Finally, one study reported that when patients were reassured by all tests being negative in the usual care multi-stop clinic, they were significantly more likely to have further clinic attendance than those reassured in the one-stop clinic (54.4% versus 44.4%, P = 0.0005) (15).
Discussion
In this systematic review and meta-analysis, we aimed to summarise the current evidence for one-stop clinics for the investigation of patients from primary care with symptoms that could be indicative of cancer. The pooled cancer CR ranged from 1.7 to 42.8% and the pooled non-cancer CR ranged from 30.3 to 82.1%, depending on indication(s) for referral. The studies comparing one-stop and multi-stop pathways reported similar CRs with significantly shorter intervals from referral to testing associated with one-stop clinics. Significantly more patients received a diagnosis on the same day and significantly fewer patients required additional clinic appointments after attending a one-stop clinic. Patient and GP satisfaction was found to be generally high, and the reported adverse effects of one-stop clinics were either statistically non-significant or of little clinical significance (42).
Strengths and weaknesses
To our knowledge, this is the first systematic review to summarise the evidence for one-stop clinics. We performed a comprehensive search and applied strict selection criteria to ensure that we only included studies examining the outcomes of one-stop clinics in patients referred from primary care. The majority of studies were, however, judged to be of poor quality: most were descriptive retrospective audits limiting external validity, and very few studies included a comparator group. As a result, we felt that making firm conclusions about the diagnostic performance of one-stop pathways in relation to multi-stop pathways would be inappropriate. Furthermore, all of the included one-stop clinics focused on individual symptoms or specialties, e.g. lower GI symptoms or urology clinic, but all patients attending these clinics may not have had the same symptoms. For example, some clinics which investigated abnormal vaginal bleeding included women with post-menopausal bleeding only, while others included a range of symptoms from irregular menstruation to post-coital bleeding: symptoms which carry different positive predictive values for cancer and indicate heterogeneity within the study populations.
Comparison with the literature
Although high quality studies examining one-stop clinics are rare, this is just one of a number of diagnostic testing pathways that are being explored. As well as one-stop clinics, direct access testing is being studied as a route where GPs may refer a patient for a specific diagnostic test, e.g. CT scan or MRI, without the need to refer to or consult with a specialist with the aim of speeding up access to testing (43). A recent systematic review reported that like one-stop clinics, direct access clinics were associated with a reduction in time from referral to testing (8). Unlike one-stop clinics, however, there was no significant reduction in time from referral to diagnosis. This review also found that the cancer and non-cancer CRs for direct access clinics were similar to those of usual care clinics, and GPs and patients rated their performance highly.
Another route to testing where there is a small but growing body of literature is multi-disciplinary diagnostic centres (MDCs). MDCs are similar to one-stop clinics in that the patient is provided with all the necessary tests and consultations for a diagnosis at the same centre. Unlike one-stop clinics, however, MDCs tend not to be focussed on specific specialties, instead investigating a range of, often non-specific, symptoms. MDCs may also require the GP to have ordered certain tests, e.g. blood tests or even some diagnostic imaging, before referral to the MDC is accepted. Despite these differences, the aim of both one-stop clinics and MDCs to provide a comprehensive range of tests in one centre warrants comparisons between their outcomes. The Danish non-specific symptoms and signs of cancer patient pathway (NSSC-CPP) and the pathways under investigation by Cancer Research UK’s Accelerate Coordinate and Evaluate (ACE) program are examples of MDCs at different stages of implementation and assessment (44,45). The NSSC-CPP has reported a cancer CR of 16% in 1278 patients referred to the pathway which is within the range of cancer CRs reported by one-stop clinic studies included in this review even without the preliminary GP ordered tests (46). Furthermore, the introduction of cancer pathways such as the NSSC-CPP have resulted in a significant reduction in waiting times for patients across a range of cancer types (47). We too found that one-stop clinics could significantly reduce the time from GP referral to clinic appointment, with an average waiting time of 15 days, just outside the 14 day target set by the Independent Cancer Taskforce (1).
Implications for research and practice
The decrease in time from GP referral to testing, and the comparable cancer CRs between one-stop and multi-stop clinics, provide some evidence in support of the use of one-stop clinics. This evidence, however, needs to be strengthened so that appropriate comparisons and evidence-based conclusions can be reached about the relative effectiveness of one-stop and multi-stop clinics. Approaches which remove the need for patients to attend multiple appointments in order to receive a diagnosis have recently been championed in recommendations for cancer research (1). One-stop clinics investigating specific, often ‘red flag’ symptoms and MDCs investigating non-specific symptoms both fulfil these recommendations (44,48). Despite the savings that may occur from a reduced number of consultations, there have been reports that the staff requirements of one-stop clinics can result in higher costs (14). Currently, due to the paucity of studies that compare the costs and outcomes of one-stop clinics to a multi-stop diagnostic process, it is not possible to determine whether these costs outweigh the benefits.
In addition, although we have shown that one-stop clinics are associated with reduced time from GP referral to testing and diagnosis, it is still unknown whether one-stop clinics are associated with earlier cancer stage at diagnosis or increased survival. None of the studies included in this review discussed the clinics’ impact on the stage at which cancer diagnoses were made. NHS England has recently allocated £200 million to achieving faster and earlier cancer diagnosis (49) as part of its strategy to meet the Independent Cancer Taskforce’s goal of 57% of cancer patients surviving 10 years and 75% surviving 1 year by 2020 (1). One-stop clinics could be a valuable tool in achieving this goal, but with the lack of studies examining stage of diagnosis we only have part of the evidence needed to determine whether they could have a role to play.
The gaps in the evidence should be addressed by studies heeding the shortcomings of previous research in this field. The studies that we assessed to be of higher quality were those that described a randomised trial rather than observational design (14), provided a full account of the patients that entered the study (14,17,23,30,36), and justified the statistical methods used (37). These are only a small proportion of the studies included in this review and this highlights the need for more, high quality studies that make use of prospective designs, randomisation of patients to one-stop and comparator groups, and rely less on clinical records data not collected for the purposes of the study.
The overwhelming majority of studies concluded that one-stop clinics were a patient-centred, efficient route to diagnosis. There was less agreement about how one-stop clinics should be delivered. Some authors suggested cost savings could be made by using existing resources rather than investing in new facilities (37). Contrary to this, others stated that the difficulties which arose from patients having to navigate the hospital to attend different investigations meant that restructuring was necessary so that investigation, consultation, and administration could be carried out side by side (38). While a third group of authors felt that diagnostic tests should be performed away from the consultation to prevent the test being seen as inevitable and to emphasise the importance of the consultation (40). Initial difficulties running one-stop clinics settled over time and clinicians were able to see as many patients as they had previously in the multi-stop clinic (15). As one-stop clinics produced similar diagnosis rates to multi-stop clinics or screening programmes, without increasing demands on resources or increasing diagnostic errors (15,29), it seems unlikely that one-stop clinics will place extra demands on the clinical teams who will treat the detected cancers but rather that they may ease demand if it can be shown that cancers are detected at an earlier, more treatable stage. This is a hypothesis that should be addressed in future research.
If one stop clinics encourage referral of patients who would not have been referred to standard multi-step pathways, then there is a potential risk of overdiagnosis of cancer—diagnosis of a cancer that would not have caused harm during a patient’s lifetime. Overdiagnosis of cancer is most commonly described in the context of screening but it is possible to diagnose cancer in patients with symptoms from another cause, especially when lower risk patients are tested (50). Based on the findings of this review, we are unable to comment on the risk of overdiagnosis in one-stop clinics: only one study reported patients diagnosed with asymptomatic breast cancer and most also had symptomatic breast cancer (29). Some authors considered the CR’s for cancer and serious disease to be too low (30,39) whilst others concluded that testing more patients to reduce the risk of under-diagnosis was important (19,37). GPs will play a vital role in minimising inappropriate referrals to achieve a balance between under- and overdiagnosis.
Conclusion
In conclusion, this study provides a first review of the literature on one-stop clinics to investigate symptoms that could be indicative of cancer. Streamlining services in the NHS, which is beset with financial difficulties and ever-growing demands on resources, is an intuitive strategy to begin tackling these issues. This is especially pertinent for conditions such as cancer with patients benefitting from expedited diagnosis. The shortened time from referral to test and higher numbers of patients receiving their diagnosis on the same day are efficiency savings that we have shown to be possible with one-stop clinics and these provide evidence in support of their continued use and increased rigorous assessment.
Supplementary Material
Acknowledgements
BDN, AT and CB designed the protocol for this review. CFS, AT and BDN carried out the screening of the papers, CFS and GAH extracted the data from the included papers. CFS carried out the statistical analyses. BDN, DL and FG provided clinical expertise and interpretation. CFS wrote the first draft of the paper and BDN edited it. All authors reviewed, edited, and approved the final manuscript.
Declaration
Funding: C.F.S. is funded through a grant from Cancer Research UK.
Ethical approval: As this paper is a systematic review of published literature, no ethical approval is required.
Conflict of interest: none.
References
- 1. Independent Cancer Taskforce. Achieving World-Class Cancer Outcomes: A strategy for England 2015–2020. 2015. [Google Scholar]
- 2. NHS England. Demand Management Good Practice Guide. NHS England, 2016. https://www.england.nhs.uk/wp-content/uploads/2016/12/demand-mgnt-good-practice-guid.pdf [Google Scholar]
- 3. Ellis PM, Vandermeer R. Delays in the diagnosis of lung cancer. J Thorac Dis 2011; 3: 183–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Robinson KM, Christensen KB, Ottesen B, Krasnik A. Diagnostic delay, quality of life and patient satisfaction among women diagnosed with endometrial or ovarian cancer: a nationwide Danish study. Qual Life Res 2012; 21: 1519–25. [DOI] [PubMed] [Google Scholar]
- 5. Risberg T, Sørbye SW, Norum J, Wist EA. Diagnostic delay causes more psychological distress in female than in male cancer patients. Anticancer Res 1996; 16: 995–9. [PubMed] [Google Scholar]
- 6. Al Hamarneh O, Liew L, Shortridge RJ. Diagnostic yield of a one-stop neck lump clinic. Eur Arch Otorhinolaryngol 2013; 270: 1711–4. [DOI] [PubMed] [Google Scholar]
- 7. Brian Nicholson AT, Bankhead C. General practitioner referrals to one-stop clinics: use and clinical outcomes. PROSPERO 2017. CRD42017056386. 2017. http://www.crd.york.ac.uk/PROSPERO/ display_record.php?ID=CRD42017056386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Friedemann Smith C, Tompson A, Jones N et al. . Direct access cancer testing in primary care: a systematic review of use and clinical outcomes. BJGP 2018; in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wells GA SB, O’Connell D, Peterson J et al. . The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on June 2017).
- 10. Higgins JPT, Altman DG, Gøtzsche PC et al. . The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Herzog R, Álvarez-Pasquin MJ, Díaz C, Del Barrio JL, Estrada JM, Gil Á. Are healthcare workers’ intentions to vaccinate related to their knowledge, beliefs and attitudes? A systematic review. BMC Public Health 2013; 13: 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stata Statistical Software: Release 14 [computer program]. College Station, TX: StataCorp LP, 2015. https://www.stata.com/support/faqs/resources/citing-software-documentation-faqs/ [Google Scholar]
- 13. Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health 2014; 72: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dey P, Bundred N, Gibbs A et al. . Costs and benefits of a one stop clinic compared with a dedicated breast clinic: randomised controlled trial. BMJ 2002; 324: 507. [PMC free article] [PubMed] [Google Scholar]
- 15. Harcourt D, Ambler N, Rumsey N, Cawthorn SJ. Evaluation of a one-stop breast lump clinic: a randomized controlled trial. Breast 1998; 7: 314–9. [Google Scholar]
- 16. Lee JS, Rieger NA, Stephens JH, Hewett PJ, Rodda DJ, Lawrence MJ. Six-year prospective analysis of the rectal bleeding clinic at the Queen Elizabeth Hospital, Adelaide, South Australia. ANZ J Surg 2007; 77: 553–6. [DOI] [PubMed] [Google Scholar]
- 17. McCombie SP, Hawks C, Emery JD, Hayne D. A ‘One Stop’ Prostate Clinic for rural and remote men: a report on the first 200 patients. BJU Int 2015; 116 (Suppl 3): 11–7. [DOI] [PubMed] [Google Scholar]
- 18. Forde JC, O’Connor KM, Casey L et al. . A rapid access diagnostic clinic for prostate cancer: the experience after one year. Ir J Med Sci 2011; 180: 505–8. [DOI] [PubMed] [Google Scholar]
- 19. Tan PK, Chang HC, Sitoh YY. Haematuria clinic–a preliminary audit and considerations for a one-stop assessment centre. Singapore Med J 1998; 39: 501–3. [PubMed] [Google Scholar]
- 20. Jones K, Bourne T. The feasibility of a ‘one stop’ ultrasound-based clinic for the diagnosis and management of abnormal uterine bleeding. Ultrasound Obstet Gynecol 2001; 17: 517–21. [DOI] [PubMed] [Google Scholar]
- 21. Lotfallah H, Farag K, Hassan I, Watson R. One-stop hysteroscopy clinic for postmenopausal bleeding. J Reprod Med 2005; 50: 101–7. [PubMed] [Google Scholar]
- 22. Mohamed H, Nair P. One-stop clinic for postmenopausal bleeding at district general hospital: does it have a role?J Obstet Gynaecol 2003; 23: 182–4. [DOI] [PubMed] [Google Scholar]
- 23. Panda JK. One-stop clinic for postmenopausal bleeding. J Reprod Med 2002; 47: 761–6. [PubMed] [Google Scholar]
- 24. Sulaiman S, Chong KW, Gaudoin M. One-stop postmenopausal bleeding clinics reduce patient waiting times and theatre costs. Scott Med J 2004; 49: 152–4. [DOI] [PubMed] [Google Scholar]
- 25. Yakasai A, Allam M, Thompson AJ. Incidence of bladder cancer in a one-stop clinic. Ann Afr Med 2011; 10: 112–4. [DOI] [PubMed] [Google Scholar]
- 26. Abu JI, Habiba MA, Baker R et al. . Quantitative and qualitative assessment of women’s experience of a one-stop menstrual clinic in comparison with traditional gynaecology clinics. BJOG 2001; 108: 993–9. [DOI] [PubMed] [Google Scholar]
- 27. Britton P, Duffy SW, Sinnatamby R et al. . One-stop diagnostic breast clinics: how often are breast cancers missed?Br J Cancer 2009; 100: 1873–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Eltahir A, Jibril JA, Squair J et al. . The accuracy of “one-stop” diagnosis for 1,110 patients presenting to a symptomatic breast clinic. J R Coll Surg Edinb 1999; 44: 226–30. [PubMed] [Google Scholar]
- 29. Mehrotra P, Townend A, Lunt L. Incidental breast cancers identified in the one-stop symptomatic breast clinic. J Breast Cancer 2011; 14: 28–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Patel RS, Smith DC, Reid I. One stop breast clinics–victims of their own success? A prospective audit of referrals to a specialist breast clinic. Eur J Surg Oncol 2000; 26: 452–4. [DOI] [PubMed] [Google Scholar]
- 31. Agaba AE, Berry N, Agaba PO, Charaklias N, Wong LS. One stop rectal bleeding clinic: the coventry experience. Int Surg 2006; 91: 288–90. [PubMed] [Google Scholar]
- 32. Toomey P, Asimakopoulos G, Zbar A, Kmiot W. ‘One-stop’ rectal bleeding clinics without routine flexible sigmoidoscopy are unsafe. Ann R Coll Surg Engl 1998; 80: 131–3. [PMC free article] [PubMed] [Google Scholar]
- 33. Shah J. Assessment of activity and outcome from a one-stop clinic for men with suspected prostate cancer: five years’ experience. J Clin Urol 2016;9:5–10. [Google Scholar]
- 34. Moore JA, O’Neil C, Fawcett D. A one-stop clinic for men with testicular anxiety. Ann R Coll Surg Engl 2009; 91: 23–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Muthuveloe D, Nkwam N, Hutton P, Wallace DM, Viney R, Patel P. Urologist led one-stop testicular clinic: the UK ‘gold standard’. Springerplus 2016; 5: 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rochester M, Scurrell S, Parry JR. Prospective evaluation of a novel one-stop testicular clinic. Ann R Coll Surg Engl 2008; 90: 565–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Barrass BJR, Wood SJ. The new standard of care in urology outpatients? a one-stop clinic improves efficiency and quality. J Clin Urol. 2013; 6: 408–13. [Google Scholar]
- 38. Coull N, Rottenberg G, Rankin S et al. . Assessing the feasibility of a one-stop approach to diagnosis for urological patients. Ann R Coll Surg Engl 2009; 91: 305–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Melleney EM, Willoughby CP. Audit of a nurse endoscopist based one stop dyspepsia clinic. Postgrad Med J 2002; 78: 161–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rutter MD, Michie AF, Trewby PN. The one-stop dyspepsia clinic–an alternative to open-access endoscopy for patients with dyspepsia. J R Soc Med 1998; 91: 524–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gregory RK, Cunningham D, Fisher TA et al. . Investigating lymphadenopathy–report on the first 12 months of the lymph node diagnostic clinic at the Royal Marsden Hospital. Postgrad Med J 2000; 76: 566–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Brocken P, Prins JB, Dekhuijzen PN, van der Heijden HF. The faster the better?—A systematic review on distress in the diagnostic phase of suspected cancer, and the influence of rapid diagnostic pathways. Psychooncology 2012; 21: 1–10. [DOI] [PubMed] [Google Scholar]
- 43. Sibbald B. Direct access to diagnostic services. Br J Gen Pract 2009; 59: e144–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nicholson BD, Oke J, Friedemann Smith C et al. . The Suspected CANcer (SCAN) pathway: protocol for evaluating a new standard of care for patients with non-specific symptoms of cancer. BMJ Open 2018; 8: e018168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Vedsted P, Olesen F. A differentiated approach to referrals from general practice to support early cancer diagnosis - the Danish three-legged strategy. Br J Cancer 2015; 112 (Suppl 1): S65–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ingeman ML, Christensen MB, Bro F, Knudsen ST, Vedsted P. The Danish cancer pathway for patients with serious non-specific symptoms and signs of cancer-a cross-sectional study of patient characteristics and cancer probability. BMC Cancer 2015; 15: 421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Probst HB, Hussain ZB, Andersen O. Cancer patient pathways in Denmark as a joint effort between bureaucrats, health professionals and politicians–a national Danish project. Health Policy 2012; 105: 65–70. [DOI] [PubMed] [Google Scholar]
- 48. Cancer Research UK. ACE Programme Projects 2016. http://www.cancerresearchuk.org/health-professional/diagnosis/ace-programme/ace-programme-projects#ACE_projects_wave23 (accessed on 1 November 2017).
- 49. NHS England. Diagnosing cancer earlier and faster https://www.england.nhs.uk/cancer/early-diagnosis/ (accessed on 18 April 2018).
- 50. Nicholson BD. Detecting cancer in primary care: Where does early diagnosis stop and overdiagnosis begin?Eur J Cancer Care 2017; 26: e12692. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.