Abstract
BACKGROUND
Recently, the use of intraoperative magnetic resonance imaging (ioMRI) has evolved in neurosurgery. Challenges related to ioMRI-augmented procedures are significant, since the magnetic field creates a potentially hazardous environment. Strict safety guidelines in the operating room (OR) are necessary. Checklists can minimize errors while increasing efficiency and improving workflow.
OBJECTIVE
To describe the Zurich checklists for safety in the ioMRI environment.
METHODS
We summarize the checklist protocol and the experience gained from over 300 surgical procedures performed over a 4-yr period using this new system for transcranial or transsphenoidal surgery in a 2-room high-field 3 Tesla ioMRI suite.
RESULTS
Particularities of the 2-room setting used at our institution can be summarized as (1) patient transfer from a sterile to a nonsterile environment and (2) patient transfer from a zone without to a zone with a high-strength magnetic field. Steps on the checklist have been introduced for reasons of efficient workflow, safety pertaining to the strength of the magnetic field, or sterility concerns. Each step in the checklist corresponds to a specific phase and particular actions taken during the workflow in the ioMRI suite. Most steps are relevant to any 2-room ioMRI-OR suite.
CONCLUSION
The use of an ioMRI-checklist promotes a zero-tolerance attitude for errors, can lower complications, and can help create an environment that is both efficient and safe for the patient and the OR personnel. We highly recommend the use of a surgical checklist when applying ioMRI.
Keywords: Complications, Infection, intraoperative magnetic resonance imaging, Surgical checklist, Transsphenoidal surgery, 2-room concept, Safety
ABBREVIATIONS
- ioMRI
intraoperative magnetic resonance imaging
- MR
magnetic resonance
- MRI
magnetic resonance imaging
- OR
operating room
- PEEK
polyether ether ketone
- T
tesla
- WHO
World Health Organization
Modern neurosurgical patient care is characterized by significant technological advances. In particular, the use of intraoperative magnetic resonance imaging (ioMRI) has recently increased.1-3 The reasons for this include its greater precision and positive influence on both the extent of resection and the safety of neuro-oncological procedures.4,5 The drawbacks include high acquisition, installation, and operation costs, as well as prolonged anesthesia times. The ioMRI-augmented procedures have a certain degree of complexity, and while it generally has a good safety profile, the strong magnetic field creates a potentially hazardous environment,6 requiring strict safety guidelines in the operating room (OR).
The introduction of any new technology into the OR inevitably exposes the patient to an unknown set of risks. Given the complexity and potential hazard of applying the MRI technique,6,7 we developed a standard operating procedure (=“checklist”) for the use of ioMRI, similar to the World Health Organization (WHO) surgical safety checklist. The particularities of the 2-room setting used in our clinic can be summarized as (1) patient transfer from a sterile to a nonsterile environment and (2) patient transfer from a zone without to a zone with a high-strength magnetic field.
Visitors to our department have frequently requested permission to copy this checklist. As the checklist seems to be of interest to the neurosurgical community, the objective of this work is to make it widely available and describe our experience with its utilization. In addition, the article sums up current knowledge about the dangers of ioMRI use, safety considerations in the ioMRI-OR suite and equipment considerations. Illustrations and Video, Supplemental Digital Content 1 provide further insight into the organization of the Zurich ioMRI-OR suite and the use of the checklist.
METHODS
The Zurich ioMRI-OR Suite
In 1995, our neurosurgical department acquired the first General Electric open intraoperative low-field magnetic resonance imaging machine (GE healthcare, Boston, Massachusetts) in Switzerland.8,9 In 2013, the magnetic resonance imaging (MRI) setting was updated with a high-field 3 Tesla (T) ioMRI suite (Siemens 3T Skyra VD13 [Siemens Healthineers, Erlangen, Germany]) and a NORAS MRI Products intraoperative 8-channel head coil (Noras MRI products, Höchberg, Germany). Our experience with the checklist is derived from over 300 procedures conducted using the new system.4,5,8,10,11
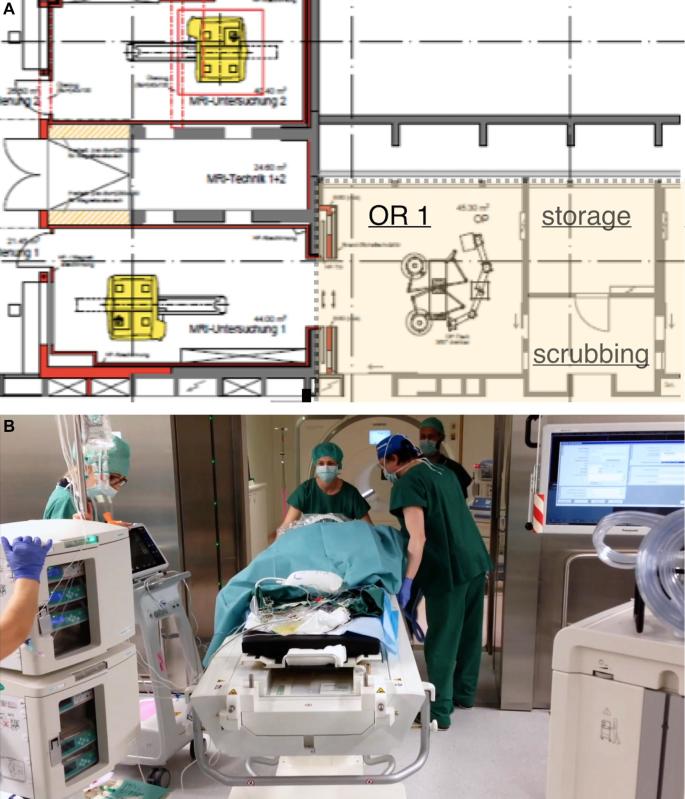
Our 3T-ioMRI suite consists of a 2-room concept in which the patient is moved and the magntic resonance (MR) scanner remains fixed (Figure 1A). The operating theater is connected to the MRI suite by a sliding double-door (Figure 1B). The MRI is available for regular in- and out-patient use by the radiology department, but its schedule can be freed in a timely fashion whenever ioMRI image acquisition is requested. The zones in the ioMRI-OR suite are clearly labeled (Table, Supplemental Digital Content 2).
FIGURE 1.
The setting of the ioMRI-OR is outlined A, where the OR is separated from the ioMRI suite by a sliding double-doorB.
For each elective neurosurgical procedure that involves the use of ioMRI, the surgery date is booked simultaneously with the estimated ioMRI time slot for the same day. The patient attends a preoperative appointment in the department of neuroradiology, during which the patient is checked for general contraindications for MRI scanning by MRI technicians, according to a standardized protocol (Table, Supplemental Digital Content 3).
Indication and Timing of ioMRI
We book the ioMRI for adult and pediatric brain tumor surgeries, particularly for the following indications: pituitary adenoma/suprasellar mass that is resected via a transsphenoidal approach. In adults, we use the ioMRI mostly for large and/or difficult to reach intra-axial tumors (low- and high-grade gliomas), where the extent of resection is crucial. This is often combined with intraoperative neuromonitoring and/or awake surgery. We have also increasingly employed ioMRI for pediatric patients with infra- and supratentorial brain tumors.
The time of image acquisition is usually the moment when the surgeon determines that as much of the tumor as possible has been resected. Given that the extent of tumor resection is positively correlated with surgical experience, our senior resident and junior faculty staff appreciates the ioMRI technique as an instrument to reduce the learning curve within the context of surgical training, while making sure that patient care is excellent and no residual resectable tumor is left behind. In cases with particularly large masses, the surgeon may choose to acquire the ioMR images at an earlier point, especially in situations where orientation is difficult and/or anatomy has shifted, leading to loss of navigation reliability.
Anesthesiological Setup
Prior to the preoperative assessment, the neuroanesthesiology team is informed that surgery is planned in the ioMRI suite. We use particular anesthesia machines that have been certified for use in the MRI environment (the types used can be provided upon request). The physiological monitor allows the monitoring of central venous, intra-arterial and intracranial pressures. We do not use wireless electrocardiography and pulse oximetry monitoring, as they may interfere with image quality or disturb the transfer of the patient to the MRI suite.6 Syringe pumps for total intravenous anesthesia and end-tidal gas analyzers are MRI-compatible.
General Surgical Safety Checklist
Before each surgical procedure, a modified WHO “Safe Surgery Checklist” is used at various time points. The checklist is run through by the neurosurgeon, neuroanesthesiologist and the (OR) nursing staff, and addresses patient identification, informed consent, surgical site and side, patient positioning, instrument counts, specimens, and medical safety.
Equipment Nomenclature
All implantable devices and medical equipment in the ioMRI suite have been labeled according to their safety indications (Figure, Supplemental Digital Content 4). MRI-safe items pose no risk in such an environment and are certified for use in zone IV (Table, Supplemental Digital Content 2). MRI-unsafe items pose a hazard in all MRI environments and are thus forbidden within zone IV (removed outside the 5-Gauss [0.5 T] line). MRI-conditional items have been demonstrated to be hazardous only in certain MRI environments (eg, an MRI-conditional item may be safe up to 100 Gauss [10 T] in a 1.5 T magnet, but may be unsafe beyond that field strength).12
Ethical Considerations
No institutional review board approval was needed for this work.
RESULTS
Slightly different checklists for transsphenoidal and open microsurgical cranial procedures were developed and are reported in Tables 1 and 2. The listed steps have been introduced for reasons of efficient workflow (marked *), safety pertaining to the magnetic field strength (marked **), or sterility issues (marked ***). Most steps are relevant to any 2-room ioMRI-OR suite, although some are specifically relevant for the Siemens magnet (Siemens Healthineers) with NORAS (Noras MRI products) head coil (marked with °). Each step in the checklist corresponds to a specific phase and particular actions taken during the workflow in the ioMRI suite (see also Video, Supplemental Digital Content 1).
TABLE 1.
Checklist for Open Microsurgical Tumor Resections Using the ioMRI
| Before sterile draping | |
|---|---|
| Questions to OR/circulation nurse | Response |
| Lower head coil installed?* | Water-tightly packed (cover: 80 × 80 cm). |
| Sticky cloth (75 × 90 cm)a?* | Attached to the shoulder. |
| 2 × Moltexb?* | Placed before and behind pins. |
| PEEK pins installedc?** | Quantity: |
| Earplugs?** | Applied bilaterally. |
| Electrocautery plate installed?* | Location: |
| Hands, Feet?* | Positioned straight. |
| Phantom?* | Fits well. |
| Questions to anesthesiology team | Response |
| Electrical cables?** | Not in contact with the patient. |
| Tracheal tube?* | Positioned flat. |
| Hose?* | Not fixed with towel clamps. |
| Patient positioned on a dry cover (after insertion of the urinary catheter)?* | Double-checked. |
| Questions to neuromonitoring team | Response |
| Skin-skin contact?** | Isolation material installed. |
| Electrical cables?** | Fixed over transfer table. |
| After sterile draping | |
| Questions to OR/circulation nurse | Response |
| Sterile towel clamps?** | Quantity: |
| Before transfer covering | |
| Questions to OR/circulation nurse | Response |
| Scar?*** | Covered hermetic with sterile film. |
| Collection bag fluid below head?* | Removed. |
| Sterile towel clamps?** | Removed. Quantity: |
| Navigation star?** | Removed. |
| Leyla adapter?** | Removed. |
| Electrocautery plate and cable?** | Removed. |
| Neuromonitoring device?** | Removed from the table. |
| After transfer covering | |
| Questions to OR/circulation nurse | Response |
| Upper head coil installed?* | Installed. Mind the “little man”! (°) |
| Cover 125cmd?* | Overlaid. |
| Covering sheets?* | Shortened. |
| White transfer plate? (°)* | Visible. |
| OR table, docking?* | Brought into transfer position. |
| Sliding door MRI?* | Free of obstacles. |
| Coils? Connectors?* | Exposed. |
| Date: __________________ | |
| Signature: ___________________ | Patient etiquette: |
The * indicate the main rationale: * = organizational; ** = magnetic field strength-related; *** = sterility. Most steps are relevant to any 2-room ioMRI-OR suite, whereas some are specifically relevant for the Siemens magnet (Siemens Healthineers, Erlangen, Germany) with NORAS (Noras MRI products, Höchberg, Germany) head coil (marked with °).
aProduct ID 2626, Mundus Medici GmbH, Düsseldorf, Germany; bProduct ID 161008, MoliNea Plus, Hartmann, Neuhausen, Switzerland; cPEEK pins reusable, NORAS MRI products, Höchberg, Germany; dProduct ID 401008125, Schlieren, Switzerland.
TABLE 2.
Checklist for Transsphenoidal Pituitary Tumor Resections Using the ioMRI Suite
| Before sterile draping | |
|---|---|
| Questions to OR/circulation nurse | Response |
| Lower head coil installed?* | Water-tightly packed (cover: 80 × 80 cm). |
| Sticky cloth (75 × 90 cm)a?* | Attached to the shoulder. |
| 2 ×Moltexb?* | Placed before and behind pins. |
| PEEK pins installedc?** | Quantity: |
| Earplugs?** | Applied bilaterally. |
| Electrocautery plate installed?* | Location: |
| Hands, Feet?* | Positioned straight. |
| Phantom?* | Fits well. |
| Questions to anesthesiology team | Response |
| Electrical cables?** | Not in contact with the patient. |
| Neck?* | Tamponated with fabric. |
| Tracheal tube?* | Positioned flat. |
| Hose?* | Not fixed with towel clamps. |
| Patient positioned on a dry cover (after insertion of the urinary catheter)?* | Double-checked. |
| After sterile draping | |
| Questions to OR/circulation nurse | Response |
| Sterile towel clamps?** | Quantity: |
| “Spitztupfer” installedd?* | Quantity: |
| Before transfer covering | |
| Questions to OR/circulation nurse | Response |
| Scar?*** | “Netzel” implantede |
| Collection bag fluid below head?* | Removed. |
| Sterile towel clamps?** | Removed. Quantity: |
| Navigation star?** | Removed. |
| Electrocautery plate and cable?** | Removed. |
| After transfer covering | |
| Questions to OR/circulation nurse | Response |
| Upper head coil installed?* | Installed. Mind the “little man”! (°) |
| Cover 125cmf?* | Overlaid. |
| Covering sheets?* | Shortened. |
| White transfer plate? (°)* | Visible. |
| OR table, docking?* | Brought into transfer position. |
| Sliding door MRI?* | Free of obstacles. |
| Coils? Connectors?* | Exposed. |
| Date: __________________ | |
| Signature: ___________________ | Patient etiquette: |
The ** indicate the main rationale: * = organizational; ** = magnetic field strength-related; *** = sterility. Most steps are relevant to any 2-room ioMRI-OR suite, whereas some are specifically relevant for the Siemens magnet (Siemens Healthineers, Erlangen, Germany) with NORAS (Noras MRI products, Höchberg, Germany) head coil (marked with °)
aProduct ID 2626, Mundus Medici GmbH, Düsseldorf, Germany; bProduct ID 161008, MoliNea Plus, Hartmann, Neuhausen, Switzerland; cPEEK pins reusable, NORAS MRI products, Höchberg, Germany; dProduct ID 35003, Novimed Medizintechnik, Dietikon, Switzerland; e Product ID 35004, Novimed Medizintechnik, Dietikon, Switzerland; f Product ID 401008125, Schlieren, Switzerland.
Time-Point 1: Before Sterile Draping
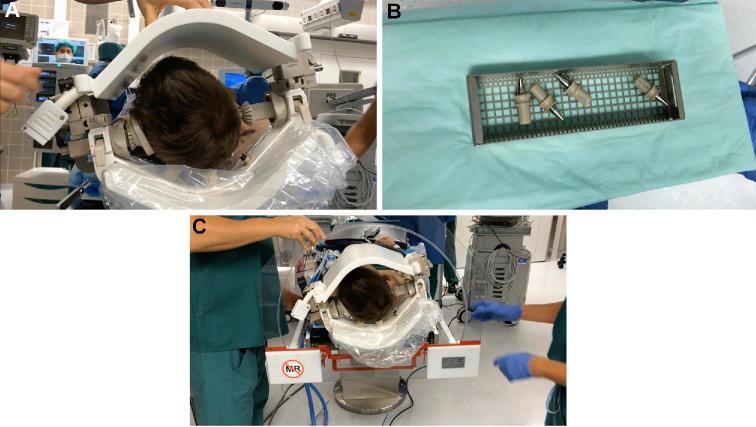
Before sterile draping, the setup consisting of the lower and upper head coil must be tested (Figure 2A). MRI-compatible head-pinning systems are employed. We use Polyether ether ketone (PEEK) pins for the head clamp (Figure 2B). Earplugs are used to protect the patient from potentially harmful noise in the MRI. The location of the electrocautery plate is noted for later removal. In order to fit into the tight MRI channel, hands and feet need to be positioned straight, the tracheal tube must be positioned flat and the phantom for the MRI channel must fit over the upper head coil (Figure 2C). The neuronavigation system is coregistered as needed. The anesthesiology and neuromonitoring teams ensure that electrical cables are not in contact with the patient and nonmagnetic towel clamps are used to fix hoses or cables. Patients should be positioned on a dry cover.
FIGURE 2.
Critical steps are taken before draping. A, It needs to be ensured that the setup consisting of the lower and upper head coil is complete.B, MRI-compatible PEEK pins for the head clamp are used.C, We make sure the phantom for the MRI channel fits well over the upper head coil.
Time-Point 2: After Sterile Draping
After sterile draping and before the start of the surgery, we record the number of magnetic sterile clamps. The surgery proceeds to the point when the surgeon decides to acquire the ioMR image.
Time-Point 3: Before Transfer Covering
Before placing the cover for patient transfer to the MRI, we check that the skin (usually quickly readapted by the surgeon after hemostasis; Figure 3A) is hermetically covered with a sterile film (Figure 3B). The bag collecting fluid below the head is removed, along with all sterile towel clamps, navigation star, Leyla adapter, electrocautery plate, and neuromonitoring devices (Figure 3B).
FIGURE 3.
Critical steps are taken before transfer. A, The skin is only provisionally readapted by the surgeon after exact hemostasis.B, The skin is then covered hermetically with a sterile film and the bag collecting fluid below the head is removed, along with all sterile towel clamps, navigation star, Leyla adapter, electrocautery plate, and neuromonitoring devices. The cover for the transfer is then placed(C, for transcranial;D, for transsphenoidal surgery). E, Prior to moving the patient, the upper head coil is installed.F, Covers are placed and shortened to avoid getting stuck during transfer.
Time-Point 4: After Transfer Covering
After placing the cover for the transfer (Figure 3C for transcranial and Figure 3D for transsphenoidal surgery) and prior to moving the patient, the upper head coil is firmly installed (Figure 3E). Covers are shortened so as to avoid getting stuck during transfer (Figure 3F). It is essential to have good view of the white transfer plate and to move the table in the correct docking position. The sliding door to the MRI room must be free of obstacles and all coils and connectors should be exposed.
Completing the full checklist takes about 2 min per patient. Currently, there are no “hard stops”, or segments of the checklist that the entire team must complete before moving onto the other sections. All of the points listed on the checklist are essential for each case. If errors/omissions are detected, these are to be taken care of before the surgeon advances to the next step of the procedure. No additional “emergency procedure” checklists have been created to deal with complications and adverse events that may occur (eg, metal objects, fixation device movement, etc). These events need to be handled on a case-by-case basis.
DISCUSSION
The modern neurosurgical OR contains an increasing amount of personnel and complex equipment. Standardized protocols ensure the complete and effective communication of safety concerns and are key to creating a safe environment for the patient. The present work demonstrates the Zurich ioMRI-OR suite checklist that was developed with the aim of harmonizing the workflow and ensuring patient safety. As MRI-unsafe and MRI-conditional equipment is routinely used during nonimaging portions of the surgery, the checklist provides a systematic method of accounting for such devices. It is our experience that using the checklist is conducive to smooth and efficient procedures and reduces ambiguities in the workflow.
Initiation of Checklist
Since its initiation, the new 3T-ioMRI-OR suite has been used for over 300 procedures in both adult and pediatric patients, mainly for transsphenoidal endoscopic tumor resections, open microsurgical large tumor resections, and epilepsy surgery. Most of the aspects covered in the checklist were added by meticulous planning before performing our first patient transfer, but the checklist was modified over time in response to difficulties encountered and incidents experienced. For example, the earplugs were added because OR technicians remaining in the MRI with the patient complained about the heavy noise. We realized that although patients were anesthetized, adequate ear protection needed to be considered for them as well. In general, a variety of neuromonitoring devices, such as needle electrodes used in motor and sensory evoked potential monitoring, can present difficulties as they create potential hazards if they are not removed prior to imaging.12 Thermal injury due to neuromonitoring wires coming into contact with the patient was noticed early on in the use of the suite, but was prevented by adding the “no touch” policy to the checklist.11 In a similar manner, infection rates declined over time as the protocol for a hermetical cover using sterile film was applied.10 All electric material, as well material that is not approved for use inside the MRI zone, was easily identified before the first transfer (eg, electrocautery plate, metal inside the fluid collection bag, navigation star, etc). One incident occurred in which a metallic towel clamp was not removed before transfer to the MRI zone. It was noticed in time and removed before the patient entered the MRI, but resulted in an extension of the checklist. Since this incident, both the surgical and anesthesia checklists include listing the number of clamps used for the surgical cover. In each phase of the procedure, the checklist now helps the surgical team confirm that every critical step is completed before proceeding with the operation and the transfer to the MRI.
In order to be of use, a checklist must be efficient and not too lengthy, therefore other ideas that were considered less relevant were not added. There are some additional aspects that must be considered however, such as a patient's weight, that are not part of our checklist. We once encountered a problem with a 150 kg (about 330 lb) patient, when the OR table bent down at the tip so that docking with the transfer table was not possible. Simply applying a counterweight by pressing on the other side of the table resolved the issue, but at first, we were puzzled as to how to handle this problem. Therefore, it is important to note that even when applying a checklist that covers a broad range of possible errors and complications, it is essential to remain flexible and consider additional issues that may apply to individual situations.
Safety is a key concept in surgery. Initiated by the Committee on Quality of Health Care publication in 2000,13 awareness of patient safety in hospital care pathways has continued to increase. In 2008, the WHO thus launched its surgical safety checklist, which has become a fundamental component of surgical patient care and safety. In brief, any perioperative event that could negatively influence patient safety becomes a potential target for checklist interception.14 Applying the checklist in 8 hospitals over 1 yr significantly lowered the rates of complications and death.15 As a consequence today, more than 300 organizations worldwide have adopted this checklist, and this number is continually rising.16 It is believed that checklists mainly improve 2 aspects of care: (1) communication between team members, in order to reduce errors and (2) awareness of the technical challenges inherent to the procedure, in order to properly prepare for possible emergencies.17 Reviews of current reports on the use of checklists in neurosurgery and the evidence supporting their use have recently been published.14,18
To make mistakes is human, and neurosurgeons are not immune.13 Adverse events are estimated to occur in about 14.6% of in surgical patients,19 a third of which lead to significant disability or death, and about half of which are believed to be preventable.20 Particularly in neurosurgery, where minor errors may have devastating consequences, the need for standardized protocols is of paramount importance.14 Errors can be avoided if the proper tools are available. Process manuals and checklists are some of the tools that have been successfully applied in other high-risk industries such as aviation, nuclear power, and logistics/management. Likewise in the medical enterprise, standardized checklists may help overcome weaknesses inherent to humans, such as memory deficiencies, but also psychological factors including anxiety, impatience, distraction, and stress.3
Safety in ioMRI
As each type of ioMRI suite presents its own unique challenges to patient safety, the currently available literature regarding safety protocols is briefly summarized here.
In 2011, Matsumae et al3 from Tokai, Japan described their protocol, consisting of a specially trained on-duty safety nurse together with a surgical safety manual and a surgical safety checklist (covering preoperative inspection, preincision, preparation for ioMRI, post-ioMRI, postsurgery, and non-normal/emergency situations).3 The key person is the safety nurse, who functions independently, focuses on safety issues, and reads the safety checklist aloud, followed by oral confirmation by all other crewmembers in the ioMRI suite. The ioMRI system in Tokai corresponds to ours, as the patient is moved to the scanner on a specific transfer system (transfer is part of the checklist). Using this protocol, the authors have had no dangerous incidents or accidents in the 3 yr of experience on which their report was based.3
In 2012, the Cleveland group reported on a protocol implemented for their mobile MRI magnet, after having gained 2 yr of experience.17 In contrast to ours, the Cleveland 1.5-T magnet is docked outside the OR when not in use, and ceiling-mounted rails enable its transfer to the stationary patient. Accordingly, significant movement of the patient is not required. Their system involves the separation of the ioMRI-OR into units or zones, depending on the level of physical risk and distance from the magnet. For any MRI performed, a safety screening form is filled out by the patient and verified by the nursing staff and physicians. In addition, prior to transferring a patient to the ioMRI-OR, a preoperative MRI nursing checklist is filled out. Finally, a modified WHO-conforming general safe surgery checklist is used.
In 2014, a group from Singapore analyzed the rate and type of incidents experienced over a 19-mo period after the introduction of a 1.5T-ioMRI suite.6 In this system, patients are installed on a rotating operating table that also serves as an MRI tray and can be rotated 180° for scanning purposes. All personnel entering the ioMRI suite undergo a compulsory video training program and safety checklists are completed at various stages, ensuring that patients entering the ioMRI environment have no contraindications and carry no objects that must be avoided. In a total number of n = 271 cases, the group recorded n = 43 incidents, of which 14 could be attributed to human error (32.6%). Nine of these incidents were related to checklist issues, and the checklist was improved throughout the study. None of the incidents resulted in harm to the patient.6
In 2016, a group from St. Petersburg, Russia, published their concept of a modified WHO surgical safety checklist for MRI-related safety in a hybrid OR.21,22 As in our system, the patient is moved to the MRI located in a separate room for scanning purposes. The authors stress the importance of the clear color marking of magnetic field lines on the floor, the labeling of equipment and devices according to their degree of MR safety, and the appointment of a “Procedure Safety Officer”, besides general aspects such as the thorough planning of the complete setup, the development of standard operating procedures and regular staff education.22
Potential Dangers of the ioMRI Technique
The MRI unit generates 3 physical forces that may cause danger to the patient or OR staff: the static magnetic field, the gradient or time-varying magnetic field, and the radiofrequency field.17
The static magnetic field is created by the superconducting coils. It is the main and constant field of the MR unit and is measured in tesla (T). With the earth's gravitational pull of about 0.05 mT, a 3 T magnet corresponds to 60 000 times the force of gravity.23 Even though no direct adverse effects to the human body have been established, mechanical effects can be dangerous if ferromagnetic objects are accelerated like projectiles across this gradient.24 In the field of neurosurgery, there have been reports of ferromagnetic aneurysm clip migration with subsequent fatal hemorrhage during image acquisition.25 To avoid the dangers of the static field, patients undergoing an ioMRI procedure should be carefully screened for contraindications (Table, Supplemental Digital Content Table 3).17
The gradient, or time-varying, magnetic field is a smaller and intermittent field, created at the moment of image slice acquisition of specific body segments. It is this field that produces the characteristic, strength-dependent noise of an MRI, which can lead to hearing loss and thus makes hearing protection essential.26 The application of earplugs to anesthetized patients is required in all of our ioMRI checklists (Tables 1 and 2),17,27 and OR staff accompanying the patient during scanning should also wear ear protection devices.
Between 1990 and 2006, the UK Medicines and Healthcare Products Regulatory Agency received 163 user incidence reports and 58 vigilance reports concerning MRI, covering mainly radiofrequency induced burns, as well as projectile, cryogen-related, foreign metal object and noise-related incidents, besides injuries related to equipment or device malfunction and physiological effects.7 In their 2008 report, the Joint Commission on Accreditation of Healthcare Organizations pointed out similar safety issues and gave recommendations for preventing accidents and injuries in MRI suites.28 These recommendations include the restriction of access to the MRI environment to trained personnel only, patient screening for safe entry (Table, Supplemental Digital Content 2), detailed checklists administered by trained personnel, and regular continued education about the MRI environment for all medical and ancillary staff.
The challenges for the anesthesiology team when caring for patients in the neurosurgical ioMRI suite have recently been summarized29 and the American Society of Anesthesiologists provides recommendations on anesthetic care during magnetic resonance imaging.30 Further articles with specific checklists for anesthetic care in the ioMRI environment have also been published,2,31 including a comprehensive list of equipment to be located outside the 5-Gauss (0.5 T) line.31
Advantages and Disadvantages of a Checklist
A variety of personnel from different backgrounds work collaboratively in the modern ioMRI-OR environment. The use of a checklist can foster effective communication and team building, guide the education of new staff, and maintain a smooth workflow. Our checklist purposely contains instructions in simple and standardized words. It has previously been acknowledged that this is especially important when the speaker and listener are physically separated.3,32 The checklist promotes monitoring, challenges OR team members and defines responsibilities, all of which were identified as crucial for preventing errors and supporting teamwork.33 By empowering surgeons, anesthesiologists, and other OR team members alike to speak up if a safety issue arises, the successful implementation of the checklist creates a culture of open communication and an egalitarian relationship between surgical team members, where hierarchical rivalries become subordinate to the higher goal of achieving patient safety.14 In addition to checklists, staff activities such as aviation training courses can help to further build a safety and teamwork culture in OR environments.
Applying a checklist requires time, which may appear to be a drawback in already lengthy interventions. Completing our checklist does not take more time than 2 min however, as long as no errors/omissions are detected. In case the checklist identifies an error/omission, additional time may be needed to solve the problem (eg, applying hearing protection into the auditory canal, installing an electrocautery plate, etc). Nevertheless, only noticing these issues at a later point in time would lead to greater delay of the procedure. As our team is now familiar with the checklists, possible errors/omissions are anticipated even before the checklists are applied. Those 2 additional minutes therefore result in a more efficient workflow, fewer errors, and saved net time overall.
CONCLUSION
Although the use of ioMRI is challenging, safe care is achieved through an appropriate understanding of the factors unique to the ioMRI environment, as well as good communication, a collaborative approach and proper procedural planning. The use of an ioMRI-checklist promotes a zero-tolerance attitude for errors, can lower complications and helps create an environment that is both efficient and safe for the patient and the OR personnel. We highly recommend the use of a surgical checklist when applying ioMRI-augmented neurosurgical procedures.
Disclosure
The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Supplementary Material
Acknowledgments
The authors thank all neuroradiological, neurosurgical, and anesthesiological colleagues, as well as the neurosurgery operating room nursing staff (in particular Monika Grimm) from the University Hospital Zurich, Switzerland who were involved in the generation and development of the checklist presented in this article.
Supplemental Digital Content 1. Video. Video demonstrating the use of the Zurich ioMRI suite and the application of the checklist.
Supplemental Digital Content 2. Table. MRI safety zones, defined by the American College of Radiology.
Supplemental Digital Content 3. Table. Before a MRI scan, patients attend a preoperative appointment at the department of neuroradiology, during which the patient is checked by technical assistants for general contraindications for MRI, according to this standardized protocol. Patients are asked to sign below.
Supplemental Digital Content 4. Figure. MRI safety symbols, as defined by the American Society for Testing and Materials (ASTM) International. MR-safe items are labeled by a white square with green border and green lettering. MR-unsafe items are labeled by a white circle with a red edge, a red slash, and black lettering. MR-conditional items are labeled by a yellow triangle with a black border and black lettering.
REFERENCES
- 1. Black PM, Moriarty T, Alexander E 3rd et al.. Development and implementation of intraoperative magnetic resonance imaging and its neurosurgical applications. Neurosurgery. 1997;41(4):831–845; discussion 842-835. [DOI] [PubMed] [Google Scholar]
- 2. Henrichs B, Walsh RP. Intraoperative MRI for neurosurgical and general surgical interventions. Curr Opin Anaesthesiol. 2014;27(4):448–452. [DOI] [PubMed] [Google Scholar]
- 3. Matsumae M, Nakajima Y, Morikawa E et al.. Improving patient safety in the intra-operative MRI suite using an on-duty safety nurse, safety manual and checklist. Acta neurochir Suppl. 2011;109:219–222. [DOI] [PubMed] [Google Scholar]
- 4. Neidert MC, Hostettler IC, Burkhardt JK et al.. The influence of intraoperative resection control modalities on survival following gross total resection of glioblastoma. Neurosurg Rev. 2016;39(3):401–409. [DOI] [PubMed] [Google Scholar]
- 5. Serra C, Burkhardt JK, Esposito G et al.. Pituitary surgery and volumetric assessment of extent of resection: a paradigm shift in the use of intraoperative magnetic resonance imaging. Neurosurg Focus. 2016;40(3):E1–E9. [DOI] [PubMed] [Google Scholar]
- 6. Tan JKT, Tan TK, Goh JPS, Ghadiali NF. Prospective review of safety incidents reported in the iMRI OT (Intraoperative Magnetic Resonance Imaging Operating Theatre). Proceedings of Singapore Healthcare. 2014;23(4):273–281. [Google Scholar]
- 7. De Wilde JP, Grainger D, Price DL, Renaud C. Magnetic resonance imaging safety issues including an analysis of recorded incidents within the UK. Prog Nucl Magn Reson Spectrosc. 2007;51(1):37–48. [Google Scholar]
- 8. Bernays RL, Kollias SS, Khan N, Romanowski B, Yonekawa Y. A new artifact-free device for frameless, magnetic resonance imaging-guided stereotactic procedures. Neurosurgery. 2000;46(1):112–117; discussion 116–117. [PubMed] [Google Scholar]
- 9. Stienen MN, Serra C, Stieglitz LH, Krayenbühl N, Bozinov O, Regli L. UniversitätsSpital Zürich: 80 years of neurosurgical patient care in Switzerland. Acta Neurochir. 2018;160(1):3–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dinevski N, Sarnthein J, Vasella F et al.. Postoperative neurosurgical infection rates after shared-resource intraoperative magnetic resonance imaging: A single-center experience with 195 cases. World Neurosurg. 2017;103:275–282. [DOI] [PubMed] [Google Scholar]
- 11. Sarnthein J, Luchinger R, Piccirelli M, Regli L, Bozinov O. Prevalence of complications in intraoperative magnetic resonance imaging combined with neurophysiologic monitoring. World Neurosurg. 2016;93:168–174. [DOI] [PubMed] [Google Scholar]
- 12. Shellock FG, Woods TO, Crues JV 3rd. MR labeling information for implants and devices: explanation of terminology. Radiology. 2009;253(1):26–30. [DOI] [PubMed] [Google Scholar]
- 13. Kohn LT, Corrigan J, Donaldson MS. To Err is Human: Building a Safer Health System. Vol 6 Washington, DC: National Academy Press; 2000. [PubMed] [Google Scholar]
- 14. Zuckerman SL, Green CS, Carr KR, Dewan MC, Morone PJ, Mocco J. Neurosurgical checklists: a review. Neurosurg Focus. 2012;33(5):E1–E11. [DOI] [PubMed] [Google Scholar]
- 15. Haynes AB, Weiser TG, Berry WR et al.. A surgical safety checklist to reduce morbidity and mortality in a global population. N Engl J Med. 2009;360(5):491–499. [DOI] [PubMed] [Google Scholar]
- 16. Wilson I, Walker I. The WHO surgical safety checklist: the evidence. J Perioper Pract. 2009;19(10):362–364. [DOI] [PubMed] [Google Scholar]
- 17. Rahmathulla G, Recinos PF, Traul DE et al.. Surgical briefings, checklists, and the creation of an environment of safety in the neurosurgical intraoperative magnetic resonance imaging suite. Neurosurg Focus. 2012;33(5):E1–E10. [DOI] [PubMed] [Google Scholar]
- 18. Enchev Y. Checklists in neurosurgery to decrease preventable medical errors: A review. Balkan Med J. 2015;32(4):337–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Griffin FA, Classen DC. Detection of adverse events in surgical patients using the trigger tool approach. Qual Saf Health Care. 2008;17(4):253–258. [DOI] [PubMed] [Google Scholar]
- 20. Vincent C, Neale G, Woloshynowych M. Adverse events in British hospitals: preliminary retrospective record review. Bmj. 2001;322(7285):517–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cherkashin M, Berezina N, Kuplevatsky V. Modified WHO surgical safety check-lists for MRI-related safety in hybrid operating room. Annual Meeting of the European Society of Radiology. Vienna, Austria: ECR (Poster C-0014); 2016. [Google Scholar]
- 22. Cherkashin M, Berezina N, Serov A, Fedorov A, Andreev G, Kuplevatsky V. Safety management for MR-Guided Interventions. iMRI. 2016;20(3):152–157. [Google Scholar]
- 23. Menon DK, Peden CJ, Hall AS, Sargentoni J, Whitwam JG. Magnetic resonance for the anaesthetist. Anaesthesia. 1992;47(3):240–255. [DOI] [PubMed] [Google Scholar]
- 24. Chaljub G, Kramer LA, Johnson RF 3rd, Johnson RF Jr, Singh H, Crow WN. Projectile cylinder accidents resulting from the presence of ferromagnetic nitrous oxide or oxygen tanks in the MR suite. Am J Roentgenol. 2001;177(1):27–30. [DOI] [PubMed] [Google Scholar]
- 25. Klucznik RP, Carrier DA, Pyka R, Haid RW. Placement of a ferromagnetic intracerebral aneurysm clip in a magnetic field with a fatal outcome. Radiology. 1993;187(3):855–856. [DOI] [PubMed] [Google Scholar]
- 26. Kanal E, Shellock FG, Talagala L. Safety considerations in MR imaging. Radiology. 1990;176(3):593–606. [DOI] [PubMed] [Google Scholar]
- 27. Smith JA. Hazards, safety, and anesthetic considerations for magnetic resonance imaging. Top Companion Anim Med. 2010;25(2):98–106. [DOI] [PubMed] [Google Scholar]
- 28. Joint Commission on Accreditation of Healthcare Organizations USA Preventing accidents and injuries in the MRI suite. Sentinel Event Alert. 2008;8(38):1–3. [PubMed] [Google Scholar]
- 29. McClain CD, Chimbira WT. Anaesthetic concerns for patients undergoing neurosurgical procedures utilising intra-operative magnetic resonance imaging. Eur Neurol Rev. 2013;8(2):164–169. [Google Scholar]
- 30. Practice advisory on anesthetic care for magnetic resonance imaging. Anesthesiology. 2015;122(3):495–520. [DOI] [PubMed] [Google Scholar]
- 31. Henrichs B, Walsh RP. Intraoperative magnetic resonance imaging for neurosurgical procedures: anesthetic implications. AANA J. 2011;79(1):71–77. [PubMed] [Google Scholar]
- 32. Pronovost P, Berenholtz S, Dorman T, Lipsett PA, Simmonds T, Haraden C. Improving communication in the ICU using daily goals. J Crit Care. 2003;18(2):71–75. [DOI] [PubMed] [Google Scholar]
- 33. Helmreich RL, Musson DM. Surgery as team endeavour. Can J Anesth. 2000;47(5):391–392. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.