Abstract
Within Asia, HIV prevalence is highest in Thailand, including thousands of children and adolescents. Care for children born with HIV [perinatal transmission of HIV (PHIV)] will need to focus on adolescents for the foreseeable future. Thai PHIV adolescents experience significant mental health and psychosocial challenges, including treatment adherence. Yet, few, if any, comprehensive interventions for them exist. CHAMP+, an evidence-based intervention adapted for Thailand, was evaluated with a pilot randomized control trial at four HIV clinics. Eighty-eight dyads of 9- to 14-year-old PHIV young adolescents/caregivers were randomized to CHAMP+ or standard of care (SOC). Eleven cartoon-based sessions were delivered over 6 months. Participants completed baseline, 6-month (postintervention), and 9-month surveys, measuring youth outcomes (e.g., mental health and adherence), contextual factors (e.g., demographics and caregiver factors), and self- and social-regulation factors (e.g., HIV knowledge and youth-caregiver communication). Multi-level modeling to account for clustering within individuals was used to assess longitudinal changes within and between groups. All families randomized to CHAMP+ completed the intervention. Although the study was not statistically powered to detect differences in treatment effects, the CHAMP+ group significantly improved at 6 months in youth mental health and adherence, HIV knowledge, youth-caregiver communication, internalized stigma, and HIV-related social support, with most improvements sustained at 9 months and significantly better improvements than the SOC group on a number of outcomes. High levels of baseline viral suppression highlight the importance of reaching these young PHIV adolescents at a period of lower risk before adherence and other challenges emerge. Designed to be delivered with limited cost/resources, CHAMP+ Thailand holds scale-up potential.
Keywords: Thailand, HIV+ adolescents, psychosocial intervention, adherence, family-based, mental health
Introduction
Globally, the pediatric HIV epidemic is evolving into an adolescent one, as expanded antiretroviral therapy (ART) access has both dramatically reduced perinatal transmission of HIV (PHIV) and increased life expectancy of babies born with HIV. Many are aging into adolescence and beyond; 2.1 million children under 15 currently living with HIV will reach adolescence, indicating an adolescent epidemic for years to come.1 Most children and adolescents with PHIV live in sub-Saharan Africa, but a sizeable understudied group resides in Asia.1 Thailand has the highest HIV prevalence in Asia at 1.1%,2 including 9700 adolescents 10–19 years old and thousands more children soon to enter adolescence.1 Mother-to-child transmission of HIV has been virtually eliminated in Thailand, such that PHIV care will need to focus on adolescents for the foreseeable future.3
PHIV youth enter adolescence with vulnerabilities beyond those experienced by other adolescents. Born with a stigmatized chronic illness when optimal treatment was often unavailable or inaccessible, many experienced multiple hospitalizations, missed normative childhood experiences, and suffered developmental delays. Moreover, in many parts of the world, PHIV youth experienced trauma and adversity related to parental/family illness/death, mental illness, and/or substance use and to living in impoverished communities with low resources for education and care.4 Among PHIV youth in both high- and low-and-middle-income countries, mental health problems are common, and some PHIV youth engage in high-risk behaviors,4–7 including substance use, sexual risk-taking, and inconsistent condom use.8–10 ART adherence rates among PHIV adolescents are also lower than among adults and younger children living with HIV,11 increasing risk to individual health and of HIV transmission to others.
Studies focused on Thai PHIV adolescents—particularly young adolescents—are sparse, but studies of older PHIV youth and young adults and studies that include both perinatally and behaviorally infected youth reveal substantial psychosocial needs, including limited knowledge about family planning, reproductive health, and sexually transmitted infections.12,13 Rates of sexual activity and drug use were relatively low in most studies, but among the sexually active, inconsistent condom use has been reported,12,14 and alcohol use is prevalent, especially among older PHIV adolescents.13,14 Other studies have found high levels of clinically significant mental health problems and psychosocial problems, including poor health attitude and self-care, life skills, communication skills, adherence, and self-value.15,16 Depression and history of double parental loss have been associated with poorer adherence in PHIV youth between 13 and 21 years old.17 Our previous qualitative work in Bangkok found older PHIV adolescents and their caregivers struggled with medication adherence, effective communication, deep societal stigma and discrimination, and HIV disclosure.18
A number of articles have called for culturally-tailored comprehensive interventions for HIV+ adolescents, as well as increased psychosocial support and counseling.19–21 However, few evidence-based interventions exist, and the very few published studies on interventions with Thai PHIV youth are largely limited to older youth/young adults and focused on changes in HIV knowledge and attitudes only.19,22 Given that early prevention of risk behavior and promotion of health behaviors are significantly more effective than efforts to intervene once behaviors are established,23 there is an urgent need for risk prevention and health promotion interventions for younger adolescents. There is increasing evidence that intervening with adult caregivers or family members is necessary to promote mental health and adherence and reduce youth sexual and substance-related risk-taking behaviors.24 Yet, we are unaware of any comprehensive psychosocial interventions for Thai PHIV young adolescents; few exist anywhere in the world.
The Collaborative HIV Prevention and Adolescent Mental Health Program
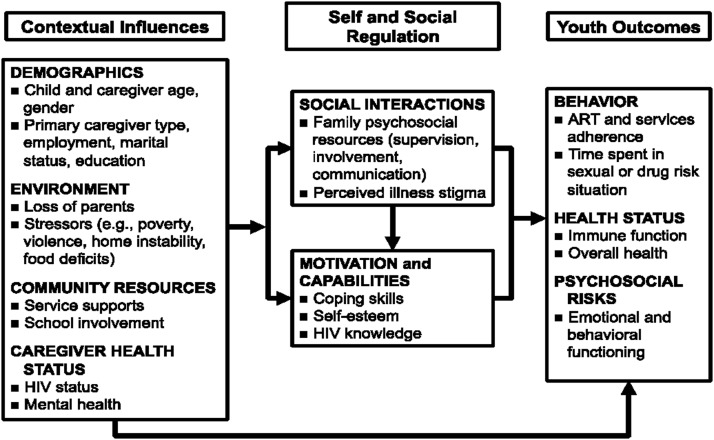
Originally developed for HIV-negative early adolescents in Chicago, United States, the Collaborative HIV Prevention and Adolescent Mental Health Program (CHAMP) is a multiple family group-based intervention to promote mental health and prevent sexual and drug use risk behavior, before risk behaviors emerge, while youth are still connected to families.25 The CHAMP model was subsequently adapted to address the many unmet needs of PHIV early adolescents and caregivers in New York in a program called CHAMP+ that retained the focus on family strengthening, but also included HIV-related content such as medication adherence, feelings about HIV and identity, disclosure, and stigma.26 Using community-based participatory research methods, the CHAMP model has since been adapted with HIV-negative and PHIV youth across multiple contexts, including New York, South Africa, Trinidad, and Argentina.26–30 All iterations of CHAMP/CHAMP+ are grounded in a modified Social Action Theory (SAT)31 (Fig. 1) which posits that youth outcomes are influenced by (1) context (e.g., stressors and resources); (2) self-regulation (motivation and capabilities); and (3) social regulation (e.g., family resources, relationships, and stigma). The programs engage youth-caregiver pairs in ∼10 sessions combining learning and discussion to strengthen parent–child communication, adolescent problem-solving and peer negotiation skills, and support within and between families.
FIG. 1.
Modified social action theory.
CHAMP+ interventions are designed to be delivered by existing staff in settings where youth receive HIV care. In South Africa, a cartoon-based curriculum was used to address literacy concerns and difficulty discussing sensitive topics.28,32 Trials of CHAMP/CHAMP+ have shown significant improvements in HIV knowledge, family communication and youth monitoring, youth mental health, and reduced risk behavior, with high levels of acceptability and improved ART adherence.26–29 To date, it has not been used in any Asian country, where cultural factors may significantly influence family-based programming.
Given the documented needs of Thai PHIV adolescents and the lack of evidence-based comprehensive interventions, a group of Thai and US care providers and researchers, in collaboration with PHIV children and caregivers, adapted CHAMP+ in Thailand using SAT, in a process that has been detailed previously.18,33 The present study has two primary aims: (1) to evaluate the short- and longer term impact of a pilot randomized control trial (RCT) of CHAMP+ Thailand on a range of behavioral (e.g., ART adherence), health (e.g., HIV viral load), psychosocial (e.g., mental health), and family (e.g., youth-caregiver communication) factors and (2) to examine the feasibility and acceptability of the intervention.
Methods
Participants
A pilot RCT of CHAMP+ was conducted from May 2015 to March 2016 with families at four HIV clinics in Thailand: HIV-NAT, Bangkok (n = 26 families; 29.6%), Khon Kaen University Hospital (n = 12 families; 13.6%), Khon Kaen Hospital (n = 21 families; 23.9%), and Phetchaburi Hospital (n = 29 families; 32.9%). Eligibility criteria included: PHIV child, 9–14 years old, aware of HIV+ status, and currently taking ART. Clinic providers recruited eligible families.
Procedures
Following informed consent by caregivers, child assent, and completion of baseline measures, families were randomized within each site using permuted block randomization with mixed block size 4 and 6 to receive the intervention or to continue in standard of care (SOC). The final sample included 88 child-caregiver dyads, with 45 randomized to CHAMP+. Study procedures were approved by the IRBs at New York University, Chulalongkorn University, Khon Kaen University, Khon Kaen Hospital, and Phetchaburi Hospital. Families received stipends for completion of assessment measures and funds to defray the cost of transport.
Intervention condition
The Thai version of CHAMP+ (called “Walking Together” or “CHAMP+ Thailand”) was delivered one weekend per month over 6 months, led by a social worker/counselor from Bangkok, using a structured facilitator's manual. CHAMP+ Thailand retains the cartoon format used in South Africa to address concerns about literacy and reluctance to discuss difficult topics (see Mellins et al.32 for description of South African curriculum). The story follows a young adolescent boy, Tam, recently diagnosed with HIV, as he navigates issues such as HIV stigma, treatment adherence, disclosure, grief/loss, puberty, social support, and communication (See Pardo et al.33 for full description of intervention and its development). Each of the curriculum's 11 sessions begins with an “ice breaker” activity then guides participants through a chapter of Tam's story and related follow-up activities and discussion questions, done both in separate youth and caregiver groups and together. Topics include concerns about growing up with HIV, communication within families, HIV stigma, HIV treatment and adherence, coping with loss/bereavement, risk behavior and responding to peer pressure, puberty and sexual relationships, future goals, and social support. With the exception of the final session, two sessions were delivered in the same day; the group ate a meal together between sessions.
SOC condition
The SOC condition consisted of routine health education and practice offered to the child and families by clinic staff at regularly scheduled medical appointments, including reviewing medications, as well as guidance regarding maintaining overall health and psychosocial support.
Measures
Youth and caregivers individually completed interviewer-administered surveys informed by SAT at three time points: baseline, 6 months later (immediately postintervention), and 9 months postbaseline; data were recorded electronically. Medical chart data, including HIV RNA viral load, were also collected for each participant pre- and postintervention. Measures are described below with baseline inter-item reliability Cronbach's α in our sample in parentheses. Unless otherwise noted below and in Table 2, all measures are coded such that higher scores indicate a more favorable outcome.
Table 2.
Multi-Level Multivariate Regression Results for Change in Outcomes for CHAMP+ Thailand Participants Over Time
| Baseline | 6 Months | 9 Months | ||||||
|---|---|---|---|---|---|---|---|---|
| CHAMP+a | SOCa | CHAMP+b | SOCb | Effectc | CHAMP+b | SOCb | Effectc | |
| Youth mental health | ||||||||
| SDQ total difficulties scored | 13.17 (10.92–15.41) | 11.94 (9.69–14.20) | −2.98 (−4.46 to −1.49)* | −0.33 (−1.85 to 1.19) | −2.65 (−4.78 to −0.53)* | −2.36 (−3.84 to −0.87)* | −0.98 (−2.50 to 0.54) | −1.38 (−3.50 to 0.75) |
| Child Depression Inventorye | 4.38 (3.37–5.40) | 4.04 (3.02–5.05) | −0.22 (−0.98 to 0.54) | −0.29 (−1.06 to 0.49) | 0.06 (−1.02 to 1.15) | −0.44 (−1.20–0.31) | −0.42 (−1.20 to 0.36) | −0.03 (−1.11 to 1.06) |
| Youth ART adherence | ||||||||
| Youth-report | ||||||||
| Number of days missed meds in past 30 days | 2.00 (1.23–2.77) | 1.50 (0.72–2.28) | −0.91 (−1.78 to −0.04)* | −0.19 (−1.08 to 0.70) | −0.73 (−1.97 to 0.52) | −0.89 (−1.76 to −0.19)* | −0.21 (−1.10 to 0.68) | −0.68 (−1.92 to 0.57) |
| When last missed meds | 2.29 (1.78–2.80) | 2.17 (1.66–2.68) | −0.03 (−0.49 to 0.44) | 0.15 (−0.33 to 0.64) | −0.18 (−0.85 to 0.50) | 0.30 (−0.17 to 0.78) | 0.29 (−0.19 to 0.77) | 0.14 (−0.66 to 0.69) |
| How well took meds past 30 days | 4.28 (3.84–4.72) | 4.33 (3.89–4.77) | 0.02 (−0.36 to 0.40) | −0.11 (−0.50 to 0.27) | 0.14 (−0.40 to 0.68) | −0.07 (−0.44 to 0.30) | −0.04 (−0.43 to 0.35) | −0.03 (−0.56 to 0.51) |
| How often took meds way supposed to in past 30 days | 4.15 (3.78–4.52) | 3.82 (3.44–4.20) | −0.01 (−0.41 to 0.40) | 0.40 (−0.02 to 0.82)** | −0.41 (−0.99 to 0.18) | 0.20 (−0.20 to 0.60) | 0.26 (−0.16 to 0.68) | −0.06 (−0.064 to 0.53) |
| Caregiver report | ||||||||
| Number of days missed meds in past 30 days | 0.32 (−0.42 to 1.06) | 0.74 (0.00–1.49) | 0.58 (−0.22 to 1.38) | −0.14 (−0.96 to 0.68) | 0.72 (−0.43 to 1.86) | −0.11 (−0.91 to 0.69) | −0.28 (−1.10 to 0.54) | 0.17 (−0.98 to 1.31) |
| When last missed meds | 2.29 (1.78–2.80) | 2.17 (1.66–2.68) | −0.03 (−0.49 to 0.44) | 0.15 (−0.33 to 0.64) | −0.18 (−0.85 to 0.50) | 0.30 (−0.17 to 0.78) | 0.29 (−0.19 to 0.77) | 0.01 (−0.66 to 0.69) |
| How well took meds past 30 days | 4.86 (4.51–5.20) | 4.60 (4.25–4.95) | 0.18 (−0.09 to 0.044) | 0.26 (−0.02 to 0.54)** | −0.08 (−0.47 to 0.30) | 0.24 (−0.02 to 0.51) | 0.40 (0.13–0.68)* | −0.16 (−0.55 to 0.22) |
| How often took meds way supposed to in past 30 days | 3.17 (2.79–3.54) | 3.50 (2.13–3.88) | 0.38 (−0.03 to 0.78)** | −0.08 (−0.50 to 0.34) | 0.46 (−0.13 to 1.04) | 0.60 (0.20–1.00)* | 0.17 (−0.25 to 0.59) | 0.43 (−0.15 to 1.02) |
| Youth HIV viral load | ||||||||
| Copies/mL of blood | 30343.34 (8817.30–51869.38) | 21344.37 (−203.89 to 42892.63) | −4626.6 (−11722.3 to 2469.1) | −685.1 (−8516.7 to 7146.4) | −3941.5 (−14509.7 to 6626.7) | (only one follow-up viral load) | ||
| HIV knowledge | ||||||||
| Youth HIV transmission knowledge | 5.25 (4.49–6.01) | 4.78 (4.02–5.55) | 2.24 (1.59–2.90)* | 0.07 (−0.60 to 0.74) | 2.17 (1.24–3.11)* | 1.27 (0.61–1.92)* | 0.30 (−0.36 to 0.97) | 0.96 (0.03–1.90)* |
| Youth HIV treatment knowledge | 4.82 (4.13–5.51) | 4.43 (3.74–5.12) | 0.78 (0.24–1.32)* | 0.12 (−0.43 to 0.67) | 0.66 (−0.11 to 1.43)** | 0.69 (0.15–1.23)* | 0.30 (−0.25 to 0.85) | 0.39 (−0.39 to 1.16) |
| Caregiver HIV transmission knowledge | 7.64 (7.15–8.13) | 7.86 (7.37–8.35) | 0.80 (0.43–1.17)* | −0.09 (−0.48 to 0.29) | 0.89 (0.36–1.43)* | 0.80 (0.43–1.17)* | 0.14 (−0.24 to 0.52) | 0.66 (0.12–1.20)* |
| Caregiver HIV treatment knowledge | 6.05 (5.48–6.62) | 6.32 (5.74–6.89) | 0.78 (0.27–1.29)* | −0.95 (−1.48 to −0.43)* | 1.73 (1.00–2.46)* | 0.47 (−0.05 to 0.98)** | −0.70 (−1.22 to −0.17)* | 1.16 (0.43–1.90)* |
| Family communication | ||||||||
| Youth-reported frequency | 1.53 (1.32–1.74) | 1.61 (1.40–1.82) | 0.33 (0.15–0.51)* | 0.04 (−0.15 to 0.22) | 0.30 (0.04–0.56)* | 0.23 (0.05–0.41)* | 0.18 (−0.01 to 0.36)** | 0.05 (−0.21 to 0.31) |
| Youth-reported comfort | 2.13 (1.78–2.47) | 2.25 (1.90–2.60) | 0.26 (0.02–0.50)* | −0.05 (−0.30 to 0.20) | 0.31 (−0.04 to 0.65)** | 0.13 (−0.11 to 0.38) | 0.07 (−0.18 to 0.31) | 0.07 (−0.28 to 0.41) |
| Caregiver-reported frequency | 2.30 (2.01–2.60) | 2.09 (1.79–2.38) | 0.13 (−0.05 to 0.31) | 0.13 (−0.06 to 0.31) | 0.01 (−0.25 to 0.26) | 0.05 (−0.13 to 0.23) | 0.20 (0.02–0.38)* | −0.15 (−0.40 to 0.11) |
| Caregiver-reported comfort | 2.85 (2.64–3.06) | 2.63 (2.42–2.85)** | 0.53 (0.30–0.75)* | 0.24 (0.01–0.46)* | 0.29 (−0.03 to 0.61)** | 0.54 (0.32–0.76)* | 0.41 (0.18–0.63)* | 0.13 (−0.19 to 0.45) |
| HIV stigma and disclosure | ||||||||
| Youth-reported internalized | 2.97 (2.78–3.16) | 2.92 (2.73–3.10) | 0.17 (−0.001 to 0.34)** | 0.15 (−0.02 to 0.33) | 0.02 (−0.22 to 0.27) | 0.01 (−0.16 to 0.19) | 0.16 (−0.02 to 0.33) | −0.14 (−0.39 to 0.10) |
| Youth-reported externalized | 2.90 (2.67–3.13) | 2.80 (2.57–3.03) | −0.00 (−0.22 to 0.22) | 0.03 (−0.20 to 0.26) | −0.03 (−0.35 to 0.29) | −0.10 (−0.33 to 0.12) | −0.01 (−0.24 to 0.22) | −0.10 (−0.41 to 0.22) |
| Caregiver-reported internalized | 2.90 (2.73–3.07) | 2.91 (2.74–3.09) | 0.26 (0.10–0.41)* | −0.02 (−0.18 to 0.14) | 0.27 (0.05–0.50)* | 0.27 (0.11–0.43)* | 0.12 (−0.04 to 0.29) | 0.15 (−0.08 to 0.37) |
| Caregiver-reported externalized | 2.67 (2.43–2.91) | 2.70 (2.46–2.94) | 0.46 (0.19–0.72)* | 0.11 (−0.16 to 0.38) | 0.35 (−0.03 to 0.73)** | 0.45 (0.19–0.72)* | 0.21 (−0.06 to 0.48) | 0.24 (−0.14 to 0.62) |
| Youth HIV disclosure comfort | 1.88 (1.65–2.11) | 2.11 (1.88–2.34)** | 0.02 (−0.17 to 0.22) | 0.02 (−0.18 to 0.22) | −0.00 (−0.28 to 0.28) | 0.11 (−0.09 to 0.30) | 0.00 (−0.20 to 0.20) | 0.11 (−0.17 to 0.39) |
| Caregiver HIV disclosure comfort | 1.84 (1.54–2.14) | 1.67 (1.38–1.97) | −0.06 (−0.30 to 0.19) | −0.01 (−0.26 to 0.25) | −0.05 (−0.40 to 0.30) | −0.16 (−0.40 to 0.09) | 0.14 (−0.011 to 0.39) | −0.30 (−0.65 to 0.06) |
| Family supervision | ||||||||
| Youth reported | 39.24 (36.94–41.54) | 38.76 (36.45–41.07) | −1.11 (−2.66 to 0.44) | 0.49 (−1.10 to 2.08) | −1.60 (−3.82 to 0.62) | −1.80 (−3.35 to −0.25)* | −0.21 (−1.80 to 1.38) | −1.59 (−3.81 to 0.63) |
| Caregiver reported | 50.56 (47.95–53.16) | 50.04 (47.43–52.65) | 0.24 (−1.52 to 2.01) | 0.28 (−1.53 to 2.09) | −0.03 (−2.56 to 2.49) | −0.04 (−1.81 to 1.72) | 1.05 (−0.76 to 2.85) | −1.09 (−3.62 to 1.44) |
| Caregiver mental health | ||||||||
| Caregiver depression (CESD) (raw score)e | 11.87 (8.48–15.27) | 14.49 (11.08–17.90) | −1.65 (−3.71 to 0.40) | −0.84 (−2.94 to 1.26) | −0.82 (−3.75 to 2.12) | −3.98 (−6.03 to −1.93)* | −2.70 (−4.80 to −0.60)* | −1.28 (−4.22 to 1.66) |
| CESD clinical depression cutoff (binary)e | 1.00 (REF.) | 1.00 (REF.) | 0.68 (0.12–3.81) | 0.58 (0.13–2.50) | 1.18 (0.12–11.16) | 0.04 (0.002–0.82)* | 0.31 (0.07–1.50) | 0.13 (0.01–3.54) |
| Social support | ||||||||
| Caregiver reported HIV-related support | 1.75 (1.45–2.05) | 1.56 (1.26–1.86) | 0.40 (0.17–0.64)* | 0.01 (−0.23 to 0.25) | 0.40 (0.06–0.73)* | 0.07 (−0.16 to 0.31) | −0.06 (−0.30 to 0.18) | 0.13 (−0.20 to 0.47) |
All measures were coded such that higher scores indicate a more favorable outcome, unless otherwise noted.
Baseline mean (95% CI)—symbols relate to p value of difference by treatment at baseline.
Change in mean score from baseline within treatment groups (95% CI).
Difference in effect by treatment group (i.e., treatment group/time point interaction) at follow-up points from multi-level linear regression model.
Higher score indicates more difficulties.
Higher score indicates more depressive symptomatology.
p < 0.05.
p < 0.10.
ART, antiretroviral therapy; CHAMP, Collaborative HIV Prevention and Adolescent Mental Health Program; CI, confidence interval; SDQ, Strengths and Difficulties Questionnaire; SOC, standard of care.
Youth outcomes
Youth mental health was assessed using two measures. First was the caregiver-reported Strengths and Difficulties Questionnaire (SDQ; Cronbach's α = 0.73),34 a widely-used brief mental health screening tool that measures behavioral difficulties and prosocial strengths in 3- to 16-year-old children. It has been translated/adapted into multiple languages, including Thai.35 Scoring results in five subscales and a total difficulties score (higher score indicates greater difficulties). Clinical cutoff “borderline” and “abnormal” scores based on a British sample indicate probable mental health problems.36 An international review of the SDQs psychometric properties found them sufficient across contexts.37 However, higher mean and median scores have been reported in some low- and middle-income countries, resulting in use of the total difficulties score as a continuous score or recommendations for different cutoffs.38–40 In a large, nationally-representative Thai sample, Woerner et al. found that the SDQ had adequate psychometric properties, but recommended higher borderline and abnormal score cutoffs.41
Second, youth self-reported depressive symptoms using the short form of the Children's Depression Inventory42 (α = 0.62). This widely-used 10-item rating of depression symptomatology in the past 2 weeks has excellent reported psychometric properties and has been effectively used as a screening tool in Thailand with general populations and with PHIV youth.43,44 Total score with higher scores indicating more depressive symptomatology was used.
Youth ART adherence was measured using widely-used individual youth- and caregiver-report items used in several large pediatric clinical trials and in the CHAMP+ pilot in South Africa.9,29,45,46 Respondents answered questions related to number of days youth missed medication in the past month, when they last missed medication, and how well they took medication overall in the past month.
Contextual influences
Demographic items included age, gender, child grade in school, caregiver relationship status and educational attainment, and caregiver HIV status. All were self-reported. Caregiver mental health was measured using the 20-item Center for Epidemiologic Studies Depression scale47 (CES-D; Cronbach's α = 0.89), which includes questions about depressive symptomatology in the past week and has shown strong psychometric properties;48 it has been used in multiple studies in Thailand over the past two decades.17,49,50 Higher scores indicate more depressive symptomatology.
Social and self-regulation
Both youth and caregiver knowledge of HIV transmission and treatment were assessed based on responses to 18 true/false questions51 [Cronbach's α = 0.68 (youth) and 0.63 (caregiver)]. Transmission-related items asked whether HIV is transmissible through routes such as mosquito bites, holding hands, or unprotected sex. Treatment-related items included statements such as, “If the viral load is ‘undetectable,’ this means there is no virus left in the body.” Response options included “true,” “false,” and “not sure.” The correct answer was coded as 1, adding to total scores, and the incorrect answer and “not sure” were coded as 0. We have also used this measure in previous CHAMP studies.26,29
Family supervision was measured using an instrument completed by both youth and caregivers that was developed and used as part of previous trials of CHAMP/CHAMP+ in the United States and South Africa26,28,29 (Cronbach's α = 0.64 for youth and 0.53 for caregivers). The caregiver and youth versions include 17 and 14 questions about rules and tracking children's whereabouts, respectively.
Youth and caregiver communication was measured with two scales focused on communication frequency and comfort, respectively (Cronbach's α = 0.77–0.88). Each includes seven topics that may be hard to discuss, including drugs/alcohol, puberty, sex, sexually transmitted diseases (STDs), and HIV/AIDS. Caregivers and youth rate their frequency of discussion and their level of comfort discussing each topic. The scales have been used in previous CHAMP and CHAMP+ programs with good psychometric properties.28,29,52
Caregivers and youth completed a measure of both externalized and internalized stigma related to HIV/AIDS that was adapted from a measure originally developed for pediatric epilepsy-related stigma53,54 and used in United States and South Africa versions of CHAMP+26,29 (Cronbach's α = 0.56–0.80). It contains eight items regarding personal feelings about HIV and perceptions about how others feel about HIV.
Comfort with HIV disclosure was measured for both youth and caregivers using a four-item scale about level of comfort discussing one's own/one's child's HIV with a variety of people such as teachers, relatives, friends, and romantic partners. Caregivers reported on social support related to their children's HIV; questions asked about the frequency of help, advice, comfort, or other support received in the past month from people in their lives.54
Analysis
We compared baseline frequencies and means of demographic variables and all outcome variables of interest by treatment group and intervention location using Pearson's chi-square testing for binary/categorical variables and two-sample t-tests for continuous variables. To compare change in scores over time between the intervention and SOC groups, we used a multi-level modeling approach. The statistical model included a random intercept to account for clustering of observations within individuals, treatment group indicator (vs. SOC), time indicator for each time point (vs. baseline), and group-by-time interaction terms. Because randomization was stratified by site, recruitment site was also included as a fixed effect covariate. The regression coefficient corresponding to the interaction term measures the difference in change over time on the outcomes and represents the effect of intervention. Continuous outcomes were modeled using the identity link function with the Stata “meglm” command, while binary outcomes were modeled using logit link function with the “meqrlogit” command. Analyses were completed using Stata/MP 15.1 (College Station, TX). We declare findings as statistically significant if their corresponding p values were no greater than 0.05.
Results
Participant baseline demographics
Baseline demographics are presented by treatment group in Table 1. Overall, 49% of youth were female, mean (SD) child age was 12.28 years (1.41), and 90% had HIV viral loads of less than 50 copies/mL. The vast majority (89%) of caregivers were female, including 45% mothers and 32% grandmothers. The proportion of families receiving grants to support their children/families was greater in the SOC group; no other significant demographic differences were found between the two groups. Demographic findings were similar across sites with one exception: caregiver HIV status. Using data from the 65% of caregivers willing to share their own HIV status, virtually all (94%) caregivers at one site reported living with HIV, compared to 61–76% at other sites. The inclusion of treatment group and site variables in our statistical models effectively controlled for all significant differences.
Table 1.
Selected Baseline Characteristics of CHAMP+ Thailand Pilot Sample by Treatment Group
| Total (N = 88) | CHAMP+ (N = 45) | SOC (N = 43) | pa | |
|---|---|---|---|---|
| N (%) | N (%) | N (%) | ||
| Study site, n (%) | ||||
| HIV-NAT, Bangkok | 26 (30) | 13 (29) | 13 (30) | 0.998 |
| Khon Kaen University Hospital | 12 (14) | 6 (13) | 6 (14) | |
| Khon Kaen Hospital | 21 (24) | 11 (24) | 10 (23) | |
| Petchaburi Hospital | 29 (33) | 15 (33) | 14 (33) | |
| Child characteristics | ||||
| Gender, n (%) | ||||
| Boy | 45 (51) | 25 (56) | 20 (47) | 0.40 |
| Girl | 43 (49) | 20 (44) | 23 (53) | |
| Age in years, mean (SD) | 12.3 (1.4) | 12.3 (1.4) | 12.3 (1.5) | 0.79b |
| Grade in school, n (%) | ||||
| Third to sixth | 38 (43) | 19 (42) | 19 (44) | 0.84 |
| Seventh to ninth | 43 (49) | 22 (49) | 21 (49) | |
| Not in school | 7 (8) | 4 (9) | 3 (7) | |
| Age of child diagnosed with HIV, n (%) | ||||
| 1–5 years old | 13 (15) | 5 (11) | 8 (19) | 0.60 |
| 6–10 years old | 33 (38) | 18 (40) | 15 (35) | |
| 11 years old+ | 42 (48) | 22 (49) | 20 (47) | |
| HIV virally suppressed (<50 copies/mL) | 78 (90) | 42 (93) | 36 (86) | 0.244 |
| Caregiver characteristics | ||||
| Gender, n (%) | ||||
| Female | 78 (89) | 41 (91) | 37 (86) | 0.45 |
| Male | 10 (11) | 4 (9) | 6 (14) | |
| Age in years, mean (SD) | 48.2 (12.5) | 47.8 (12.8) | 48.5 (12.4) | 0.67b |
| Relationship to the child, n (%) | ||||
| Mother | 40 (45) | 22 (49) | 18 (42) | 0.53 |
| Father | 4 (5) | 0 (0) | 4 (9) | |
| Stepmother | 3 (3) | 1 (2) | 2 (5) | |
| Stepfather | 1 (1) | 1 (2) | 0 (0) | |
| Grandmother | 28 (32) | 14 (31) | 14 (33) | |
| Grandfather | 2 (2) | 1 (2) | 1 (2) | |
| Aunt | 6 (7) | 3 (7) | 3 (7) | |
| Uncle | 3 (3) | 2 (4) | 1 (2) | |
| Older sibling | 1 (1) | 1 (2) | 0 (0) | |
| Education level, n (%) | ||||
| No schooling | 4 (5) | 2 (5) | 2 (5) | 0.88 |
| Some primary school | 23 (29) | 11 (26) | 12 (32) | |
| Completed primary school | 23 (29) | 12 (29) | 11 (30) | |
| Some high school | 17 (22) | 9 (21) | 8 (22) | |
| Completed high school | 12 (15) | 8 (19) | 4 (11) | |
| Employment, n (%) | ||||
| Unemployed | 21 (24) | 8 (18) | 13 (30) | 0.38 |
| Employed | 46 (52) | 25 (56) | 21 (49) | |
| Intermittently employed | 21 (24) | 12 (27) | 9 (21) | |
| Relationship status, n (%) | ||||
| Married/in relationship | 59 (67) | 34 (76) | 25 (58) | 0.20 |
| Widowed | 17 (19) | 5 (11) | 12 (28) | |
| Divorced/separated | 9 (10) | 5 (11) | 4 (9) | |
| Single (never married) | 3 (3) | 1 (2) | 2 (5) | |
| Family receipt of any grants, n (%) | 60 (68) | 26 (58) | 34 (79) | 0.032* |
| Any food insecurity in past month, n (%) | 11 (13) | 6 (13) | 5 (12) | 0.81 |
| Caregiver HIV+, n (%) | 41 (72) | 21 (66) | 20 (80) | 0.23 |
p Value associated with Pearson's chi-squared test unless otherwise noted.
p Value associated with two-sample t-test.
p < 0.05.
CHAMP, Collaborative HIV Prevention and Adolescent Mental Health Program; SOC, standard of care.
Attendance and feasibility
All enrolled participants in the CHAMP+ group completed the intervention, and the vast majority of participants (89%) attended all intervention sessions. No participants missed more than two meetings; the proportion of participants attending all sessions ranged from 64% to 100% by site. All participants completed assessments at all three time points.
Youth outcomes (ART adherence and youth mental health)
Table 2 shows baseline means (for continuous variables) or odds (for binary variables), change from baseline to 6 and 9 months within each group, and comparisons between groups in change over time for all outcomes/factors. Compared to the SOC group, the CHAMP+ group exhibited significantly greater improvement over time on a number of key outcomes. At 6 months, statistically significant improvements within the CHAMP+ group were seen in youth mental health, as measured by SDQ total difficulties scores and youth-reported number of missed days of ART in past month. At 9 months, within-group improvements were sustained. Treatment effect comparing change over time in the two groups was significantly better in the intervention group on SDQ total difficulties.
Contextual and social/self-regulation factors
Within the CHAMP+ group, at 6 months, statistically significant improvements were seen in youth and caregiver HIV knowledge, youth-caregiver communication, caregiver-reported internalized and externalized HIV stigma, and caregiver-reported HIV-related social support. At 9 months, within-group improvements were sustained in all but social support. Caregiver depressive symptoms were also significantly improved in the CHAMP+ group at 9 months. Treatment effect comparing change over time in the two groups was significantly better in the intervention group on HIV knowledge, caregiver-reported internalized stigma, and caregiver-reported HIV-related social support at 6 months. At 9 months, the treatment effect of youth and caregiver HIV knowledge remained significant.
Discussion
Our findings emphasize heightened psychosocial need in this population and offer preliminary evidence of the efficacy and feasibility of CHAMP+ as a clinic-based, culturally-tailored intervention for young PHIV adolescents in Thailand. We found improvement on key outcomes/variables within the intervention group immediately postintervention, including youth mental health, adherence, HIV knowledge, youth-caregiver communication, internalized and externalized HIV stigma, and social support, many of which were sustained through 9 months. Few significant changes were recorded in the SOC group.
Critically, CHAMP+ was effective in addressing areas of need not included in other published interventions for Thai PHIV youth, including mental health. Prior research around the world has found increased mental health problems in PHIV youth,4,16 and baseline SDQ total difficulties scores in our sample suggest that the same is true in Thailand relative to a nationally-representative Thai sample of children and adolescents also assessed using the SDQ.41 At baseline, 15% of our sample would be categorized as “abnormal” based on Thai cutoff scores, compared to 8% in the national sample, and our sample's mean SDQ total difficulties score was also slightly higher (12.09 vs. 11).41 This supports the need for programs, such as CHAMP+, that can effectively address mental health needs.
In addition, CHAMP+ is more comprehensive than interventions focused only on adherence or HIV prevention/treatment knowledge, although it incorporates and had some effect on both of those areas. Youth and caregiver knowledge about HIV transmission and treatment improved significantly within the intervention group and compared to the SOC group, although the comparison was only statistically significant for caregiver knowledge. While significant improvement in self-reported adherence was found only on a limited number of items, 90% of the sample was virally suppressed at baseline, suggesting excellent adherence among most participants at this younger age compared to some studies of older adolescents.15 Although the high level of viral suppression and overall good adherence in the sample suggest that the patient population may not be at immediate risk of adverse HIV-related health outcomes or transmission of HIV to others, prior literature suggests that as chronically ill youth and young adults transition from childhood to adulthood, treatment adherence is an ongoing challenge,55,56 foreshadowing a need to reach youth at younger ages. The older Thai PHIV youth interviewed as part of the development of CHAMP+ Thailand reported adherence as a struggle in their lives.18 CHAMP+ aims to reach young adolescents and prevent negative behaviors, such as nonadherence, before they develop. Our findings suggest that the intervention positively impacts both youth and family variables that feed into adherence, including treatment knowledge, which may serve to prevent or minimize nonadherence as they age further into adolescence. Future longitudinal studies, with larger samples, are needed to assess longer term impact of these preventive efforts.
In just six meetings, CHAMP+ had a wide-range impact on both youth and caregivers. That 100% of participants completed the program and the vast majority attended all sessions indicates a high degree of engagement, acceptance, and feasibility.
These findings should be viewed in light of some limitations. We relied on self-report for all variables other than viral load. In addition, our study included a relatively small sample of PHIV young adolescents from three different areas of Thailand, raising questions about generalizability. Although the study was not statistically powered to detect differences in treatment effects, we found statistically significant effects in youth mental health, HIV knowledge, stigma, and HIV-related social support.
The study also has some key strengths. In a RCT, random treatment assignment leads to group similarity on known and unknown prognostic factors, such that we are better able to attribute change to CHAMP+ itself. We had no missing data; all youth and caregiver participants completed surveys at all time points, allowing a complete longitudinal case analysis on the entire sample.
Despite the study's limitations, the significant effects of CHAMP+ Thailand even in a small pilot sample are very promising. Given the increasing number of PHIV adolescents, their critical psychosocial needs, the limited availability of evidence-based interventions in Thailand, and resource limitations, CHAMP+ Thailand's model may be particularly useful. Designed to be delivered by existing lay staff in medical settings where families are already engaged and implemented with limited cost/resources, CHAMP+ Thailand holds potential for scale-up.
Acknowledgments
The authors acknowledge the significant time, effort, and guidance of all of the participants in this study, including the tremendous support of the young people living with HIV and their families and care providers, as well as clinic and study staff involved in implementation and data collection at all four sites. Funding support was provided by and through a grant to amfAR, The Foundation for AIDS Research, from the US National Institutes of Health's National Institute of Allergy and Infectious Diseases, Eunice Kennedy Shriver National Institute of Child Health and Human Development, and National Cancer Institute, as part of the International Epidemiologic Databases to Evaluate AIDS (IeDEA; U01AI069907). This research was also supported by grants from the National Institute of Nursing Research (NINR Grant No. R21NR10474; Principal Investigator: C.A.M., PhD) and the National Institute of Child Health and Development (NICHD Grant No. R01 HD074052; Principal Investigator: M.M.M., PhD), as well as a center grant from the National Institute of Mental Health to the HIV Center for Clinical and Behavioral Studies at the New York State Psychiatric Institute and Columbia University (P30-MH43520; Principal Investigator: Robert Remien). J.A. and C.A.M. were partly supported by a Henry M. Jackson Foundation grant [No. R01MH102151].
Author Disclosure Statement
J.A. has received honoraria for participating in advisory meetings for ViiV Healthcare, Gilead, Merck, Roche, and AbbVie.
References
- 1. UNICEF. The state of the world's children 2017: Statistical tables. Available at: https://data.unicef.org/wp-content/uploads/2018/03/SOWC-2017-statistical-tables.pdf (Last accessed August14, 2018)
- 2. UNAIDS. Thailand. Available at: www.unaids.org/en/regionscountries/countries/thailand (Last accessed August14, 2018)
- 3. Lolekha R. Elimination of mother-to-child transmission of HIV—Thailand. MMWR Morb Mortal Wkly Rep 2016;65:562–566 [DOI] [PubMed] [Google Scholar]
- 4. Mellins CA, Malee KM. Understanding the mental health of youth living with perinatal HIV infection: Lessons learned and current challenges. J Int AIDS Soc 2013;16:18593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bomba M, Nacinovich R, Oggiano S, et al. . Poor health-related quality of life and abnormal psychosocial adjustment in Italian children with perinatal HIV infection receiving highly active antiretroviral treatment. AIDS Care 2010;22:858–865 [DOI] [PubMed] [Google Scholar]
- 6. Mellins CA, Elkington KS, Leu C-S, et al. . Prevalence and change in psychiatric disorders among perinatally HIV-infected and HIV-exposed youth. AIDS Care 2012;24:953–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Puthanakit T, Ananworanich J, Vonthanak S, et al. . Cognitive function and neurodevelopmental outcomes in HIV-infected children older than 1 year of age randomized to early versus deferred antiretroviral therapy: The PREDICT neurodevelopmental study. Pediatr Infect Dis J 2013;32:501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bauermeister JA, Elkington KS, Robbins RN, Kang E, Mellins CA. A prospective study of the onset of sexual behavior and sexual risk in youth perinatally infected with HIV. J Sex Res 2012;49:413–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mellins CA, Tassiopoulos K, Malee K, et al. . Behavioral health risks in perinatally HIV-exposed youth: Co-occurrence of sexual and drug use behavior, mental health problems, and nonadherence to antiretroviral treatment. AIDS Patient Care STDs 2011;25:413–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tassiopoulos K, Moscicki A-B, Mellins C, et al. . Sexual risk behavior among youth with perinatal HIV infection in the United States: Predictors and implications for intervention development. Clin Infect Dis 2013;56:283–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Van Dyke RB, Patel K, Kagan RM, et al. . Antiretroviral drug resistance among children and youth in the United States with perinatal HIV. Clin Infect Dis 2016;63:133–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lolekha R, Boon-Yasidhi V, Leowsrisook P, et al. . Knowledge, attitudes, and practices regarding antiretroviral management, reproductive health, sexually transmitted infections, and sexual risk behavior among perinatally HIV-infected youth in Thailand. AIDS Care 2015;27:618–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lee B, Oberdorfer P. Risk-taking behaviors among vertically HIV-infected adolescents in northern Thailand. J Int Assoc Physicians AIDS Care (Chic) 2009;8:221–228 [DOI] [PubMed] [Google Scholar]
- 14. Rongkavilit C, Naar-King S, Chuenyam T, Wang B, Wright K, Phanuphak P. Health risk behaviors among HIV-infected youth in Bangkok, Thailand. J Adolesc Health 2007;40:358..e1–e8. [DOI] [PubMed] [Google Scholar]
- 15. Manaboriboon B, Lolekha R, Chokephaibulkit K, et al. . Psychosocial needs of perinatally HIV-infected youths in Thailand: Lessons learnt from instructive counseling. AIDS Care 2016;28:1615–1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rongkavilit C, Wright K, Chen X, Naar-King S, Chuenyam T, Phanuphak P. HIV stigma, disclosure and psychosocial distress among Thai youth living with HIV. Int J STD AIDS 2010;21:126–132 [DOI] [PubMed] [Google Scholar]
- 17. Kang E, Delzell DA, Chhabra M, Oberdorfer P. Factors associated with high rates of antiretroviral medication adherence among youth living with perinatal HIV in Thailand. Int J STD AIDS 2015;26:534–541 [DOI] [PubMed] [Google Scholar]
- 18. Nestadt DF, Lakhonpon S, Pardo G, et al. . A qualitative exploration of psychosocial challenges of perinatally HIV-infected adolescents and families in Bangkok, Thailand. Vulnerable Child Youth Stud 2018;13:158–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chokephaibulkit K, Tarugsa J, Lolekha R, et al. . Outcomes of a comprehensive youth program for HIV-infected adolescents in Thailand. J Assoc Nurses AIDS Care 2015;26:758–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Udomkhamsuk W, Grimes DE, Viseskul N, Kasatpibal N. Barriers to HIV treatment adherence among Thai youth living with HIV/AIDS: A qualitative study. Pac Rim Int J Nurs Res 2014;18:203–215 [Google Scholar]
- 21. Jenkins RA, Kim B. Cultural norms and risk: Lessons learned from HIV in Thailand. J Prim Prev 2004;25:17–40 [Google Scholar]
- 22. Lolekha R, Boon‐yasidhi V, Na‐Nakorn Y, et al. . The Happy Teen programme: A holistic outpatient clinic‐based approach to prepare HIV-infected youth for the transition from paediatric to adult medical care services in Thailand. J Int AIDS Soc 2017;20:21500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Botvin GJ, Baker E, Dusenbury L, Tortu S, Botvin EM. Preventing adolescent drug abuse through a multimodal cognitive-behavioral approach: Results of a 3-year study. J Consult Clin Psychol 1990;58:437 [DOI] [PubMed] [Google Scholar]
- 24. Pequegnat W, Szapocznik J. Working with Families in the Era of HIV/AIDS. Thousand Oaks, CA: Sage, 2000. [Google Scholar]
- 25. McKay M, Paikoff RL. The Chicago HIV prevention adolescent mental health project (CHAMP) family-based intervention. Chicago: National Institute of Mental Health and the William T. Grant Foundation, 1995. [Google Scholar]
- 26. McKay M, Block M, Mellins C, et al. . Adapting a family-based HIV prevention program for HIV-infected preadolescents and their families: Youth, families and health care providers coming together to address complex needs. Soc Work Mental Health 2007;5:355–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bhana A, McKay MM, Mellins C, Petersen I, Bell C. Family-based HIV prevention and intervention services for youth living in poverty-affected contexts: The CHAMP model of collaborative, evidence-informed programme development. J Int AIDS Soc 2010;13(Suppl 2):S8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bell CC, Bhana A, Petersen I, et al. . Building protective factors to offset sexually risky behaviors among black youths: A randomized control trial. J Natl Med Assoc 2008;100:936–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bhana A, Mellins CA, Petersen I, et al. . The VUKA family program: Piloting a family-based psychosocial intervention to promote health and mental health among HIV infected early adolescents in South Africa. AIDS Care 2014;26:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Small L, Mercado M, Gopalan P, Pardo G, Mellins CA, McKay MM. Enhancing the emotional well-being of perinatally HIV-infected youth across global contexts. Glob Soc Welf 2014;1:25–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ewart CK. Social action theory for a public health psychology. Am Psychol 1991;46:931 [DOI] [PubMed] [Google Scholar]
- 32. Mellins CA, Nestadt D, Bhana A, et al. . Adapting evidence-based interventions to meet the needs of adolescents growing up with HIV in South Africa: The VUKA case example. Glob Soc Welf 2014;1:97–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pardo G, Saisaengjan C, Gopalan P, et al. . Cultural adaptation of an evidence-informed psychosocial intervention to address the needs of PHIV+ youth in Thailand. Glob Soc Welf 2017;4:209–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Goodman R. The Strengths and Difficulties Questionnaire: A research note. J Child Psychol Psychiatry 1997;38:581–586 [DOI] [PubMed] [Google Scholar]
- 35. Youth in Mind. SDQ: Information for researchers and professionals about the strengths & difficulties questionnaires. Available at: www.sdqinfo.org (Last accessed May24, 2014) [DOI] [PubMed]
- 36. Youth in Mind. Scoring the Strengths & Difficulties Questionnaire for age 4–17 or 18+. Available at: www.sdqinfo.com/py/sdqinfo/c0.py (Last accessed April24, 2018)
- 37. Stone LL, Otten R, Engels RC, Vermulst AA, Janssens JM. Psychometric properties of the parent and teacher versions of the strengths and difficulties questionnaire for 4- to 12-year-olds: A review. Clin Child Fam Psychol Rev 2010;13:254–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kashala E, Elgen I, Sommerfelt K, Tylleskar T. Teacher ratings of mental health among school children in Kinshasa, Democratic Republic of Congo. Eur Child Adolesc Psychiatry 2005;14:208–215 [DOI] [PubMed] [Google Scholar]
- 39. Du Y, Kou J, Coghill D. The validity, reliability and normative scores of the parent, teacher and self report versions of the Strengths and Difficulties Questionnaire in China. Child Adolesc Psychiatry Ment Health 2008;2:8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mellins CA, Xu Q, Nestadt DF, et al. . Screening for mental health among young South African children: The use of the Strengths and Difficulties Questionnaire (SDQ). Glob Soc Welf 2018;5:29–38 [PMC free article] [PubMed] [Google Scholar]
- 41. Woerner W, Nuanmanee S, Becker A, Wongpiromsarn Y, Mongkol A. Normative data and psychometric properties of the Thai version of the Strengths and Difficulties Questionnaire (SDQ). J Mental Health Thai 2012;19:42–57 [Google Scholar]
- 42. Kovacs M. The children's depression inventory (CDI). Psychopharmacol bull 1985;21:995–998 [PubMed] [Google Scholar]
- 43. Trangkasombat U, Likanapichitkul D. The Children's Depression Inventory as a screen for depression in Thai children. J Med Assoc Thai 1997;80:491–499 [PubMed] [Google Scholar]
- 44. Lee B, Chhabra M, Oberdorfer P. Depression among vertically HIV-infected adolescents in Northern Thailand. J Int Assoc Physicians AIDS Care 2011;10:89–96 [DOI] [PubMed] [Google Scholar]
- 45. Wilson IB, Lee Y, Michaud J, Fowler FJ, Rogers WH. Validation of a new three-item self-report measure for medication adherence. AIDS Behav 2016;20:2700–2708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Williams PL, Storm D, Montepiedra G, et al. . Predictors of adherence to antiretroviral medications in children and adolescents with HIV infection. Pediatrics 2006;118:e1745–e1757 [DOI] [PubMed] [Google Scholar]
- 47. Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Meas 1977;1:385–401 [Google Scholar]
- 48. Weissman MM, Sholomskas D, Pottenger M, Prusoff BA, Locke BZ. Assessing depressive symptoms in five psychiatric populations: A validation study. Am J Epidemiol 1977;106:203–214 [DOI] [PubMed] [Google Scholar]
- 49. Ross R, Zeller R, Srisaeng P, Yimmee S, Somchid S, Sawatphanit W. Depression, stress, emotional support, and self-esteem among baccalaureate nursing students in Thailand. Int J Nurs Educ Scholarsh 2005;2:Article25 [DOI] [PubMed] [Google Scholar]
- 50. Sutcliffe CG, German D, Sirirojn B, et al. . Patterns of methamphetamine use and symptoms of depression among young adults in northern Thailand. Drug Alcohol Depend 2009;101:146–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Levy SR, Lampman C, Handler A, Flay BR, Weeks K. Young adolescent attitudes toward sex and substance use: Implications for AIDS prevention. AIDS Educ Prev 1993;5:340–351 [PubMed] [Google Scholar]
- 52. Bhana A, Mellins CA, Small L, et al. . Resilience in perinatal HIV+ adolescents in South Africa. AIDS Care 2016;28(Suppl 2):49–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Westbrook LE, Bauman LJ, Shinnar S. Applying stigma theory to epilepsy: A test of a conceptual model. J Pediatr Psychol 1992;17:633–649 [DOI] [PubMed] [Google Scholar]
- 54. Westbrook L, Bauman L. Perceived Stigma of HIV/AIDS Scale. Bronx, NY: Albert Einstein College of Medicine, 1996. [Google Scholar]
- 55. Kim S-H, Gerver SM, Fidler S, Ward H. Adherence to antiretroviral therapy in adolescents living with HIV: Systematic review and meta-analysis. AIDS 2014;28:1945–1956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hanghøj S, Boisen KA. Self-reported barriers to medication adherence among chronically ill adolescents: A systematic review. J Adolesc Health 2014;54:121–138 [DOI] [PubMed] [Google Scholar]