Abstract
We examined the effect of repeat exposure to a non-damaging insult on central nervous system axons using the optic projection as a model. The optic projection is attractive because its axons are spatially separated from the cell bodies, it is easily accessible, it is composed of long axons, and its function can be measured. We performed closed-system ocular neurotrauma in C57Bl/6 mice using bursts of 15 or 26-psi (pounds per square inch) overpressure air that caused no gross damage. We quantified the visual evoked potential (VEP) and total and degenerative axons in the optic nerve. Repeat exposure to a 15-psi air blast caused more axon damage and vision loss than a single exposure to a 26-psi air blast. However, an increased VEP latency was detected in both groups. Exposure to three 15-psi air blasts separated by 0.5 sec caused 15% axon degeneration at 2 weeks. In contrast, no axon degeneration above sham levels was detected when the interinjury interval was increased to 10 min. Exposure to 15-psi air blasts once a day for 6 consecutive days caused 3% axon degeneration. Therefore, repeat mild trauma within an interinjury interval of 1 min or less causes synergistic axon damage, whereas mild trauma repeated at a longer interinjury interval causes additive, cumulative damage. The synergistic damage may underlie the high incidence of traumatic brain injury and traumatic optic neuropathy in blast-injured service members given that explosive blasts are multiple injury events that occur in a very short time span. This study also supports the use of the VEP as a biomarker for traumatic optic neuropathy.
Keywords: axon degeneration, blast injury, CNS, neurotrauma, interinjury interval, optic nerve, repeat injury
Introduction
Each year in the United States, approximately 1.1 million civilians visit the emergency department because of a traumatic brain injury (TBI).1 The U.S. military has confirmed 344,030 cases of TBI in active duty service members between the years 2000 and 2015.2 The first report that rapid repeat TBI could cause lasting neurodegeneration was a description of symptoms in boxers in 1928.3 Subsequently, neurological damage in boxers was confirmed by post-mortem histopathological analyses and was termed dementia pugilistica (4; for review, see another work5). In the military, blast-induced TBI is recognized as the characteristic injury of modern warfare. Most injured service members have been exposed to multiple explosive blasts during their time in service, and this repeated exposure is likely to contribute to neurological changes.6–10 Importantly, a single explosive blast typically encompasses multiple traumatic events that occur in order of seconds or less, including repeat blast exposure and blunt force trauma.11 Explosive blast waves rebound against surfaces, increasing their pressure up to 13-fold (FEMA426_Chapter 4). Thus, a single, large explosive blast generates multiple blast waves in rapid succession. In addition, the blast wind propels the individual onto a surface or propels large objects onto the individual. Military service members may also be exposed to antipersonnel land mines, which are connected in series, resulting in rapid repeat exposure to blast waves. Finally, ammunitions are also released in rapid succession and also generate primary blast waves. Recent literature suggests that cumulative concussions with longer interinjury intervals also causes lasting neuronal damage.6,12 This has been confirmed in animal studies investigating damage to axons and behavioral outcomes with inter-TBI intervals of a day or longer.13–24 In these studies, greater damage and behavioral deficits were detected in animal models and greater pathologies were detected in patients. However, in the military, the interinjury interval is much shorter than 1 day; thus, the aim of this study is to investigate the effect of shorter interinjury intervals on axons.
Considering that the neural visual system is exposed outside of the skull because of the placement of the retina and optic nerve in the back of the eye, and that around 30% of the cortex is utilized for visual processing, it is not surprising that there is a close association between TBI and vision problems. Two studies on blast-injured service members with TBI found that 65–68% also report vision problems.25,26 Over 186,000 service members were diagnosed with eye injuries in fixed U.S. Military facilities from 2000 to 2010.27 In addition, 50,000 civilians per year experience permanent vision loss as a result of trauma.28,29 And, more specifically, the incidence of indirect traumatic optic neuropathy in civilians increases from 0.5% in the general population to 2% in those with a TBI.30,31 Injuries to the retina, optic nerve, and brain occur in boxers, civilians (as a result of a variety of mechanisms), and blast-exposed military service members.30–35
The brain and neural retina are connected by the optic nerve, which is composed of the axons of the retinal ganglion cells. The axons of the optic projection are directly affected by trauma and are also a good model for other long axons, which are thought to be particularly susceptible to neurotrauma.36–40 Further, because the optic projection is spatially separated from the rest of the central nervous system (CNS), including the cell bodies of the retinal ganglion cells, it is straightforward to isolate and analyze, making it ideal for study. Therefore, in this study, we used eye-directed blast exposure as our model of closed system CNS neurotrauma. We previously reported that exposing an eye to a single 26-psi (pounds pper square inch) burst of air caused molecular changes in the neural retina 3 days after injury followed by mild axon degeneration in the optic nerve and focal neuronal cell death at 1 month after injury in the C57Bl/6 mouse strain.41,42 More recently, we have shown that these same molecular events occur after repeat exposure to a 15-psi blast.43 In this study, we investigate the effect of interinjury interval and total blast number on axon injury severity using a subthreshold blast exposure.
Methods
Mice
Adult (2- to 3-month-old) male C57Bl/6 mice were purchased from Jackson Laboratories (Bar Harbor, ME). All procedures were performed in accord with Association for Assessment and Accreditation of Laboratory Animal Care and the Association for Research in Vision and Ophthalmology guidelines and the Vanderbilt University Medical Center Institutional Animal Care and Use Committee–approved protocol. Mice were perfused with phosphate-buffered saline and 4% paraformaldehyde at collection at 2 or 4 weeks after the last blast exposure.
Trauma model
Optic nerve injury was induced as previously described with the following modifications.8 The system was modified to provide electronic control of the air release in order to precisely define the inter blast interval (Fig. 1). Briefly, mice were anesthetized with 4% isofluorane and then maintained with 2.5% isofluorane while in the housing chamber during the blast exposure. The left eye of the mouse was positioned against a hole in the housing, which was aligned with the barrel of the paintball marker. All experiments were performed in the morning. Mice were exposed to blasts of 15 psi of air as described in each experiment and in Table 1. Mice were provided gel recovery food (Clear H2O, Portland, ME) for the first 3 days post-injury. Sham blast mice were anesthetized and placed into the holder with the left eye positioned across from the barrel. The air blast was blocked from reaching the eye by a shield placed between the barrel and the eye. Therefore, mice were exposed to the anesthesia and noise of the blast, but not the air wave.
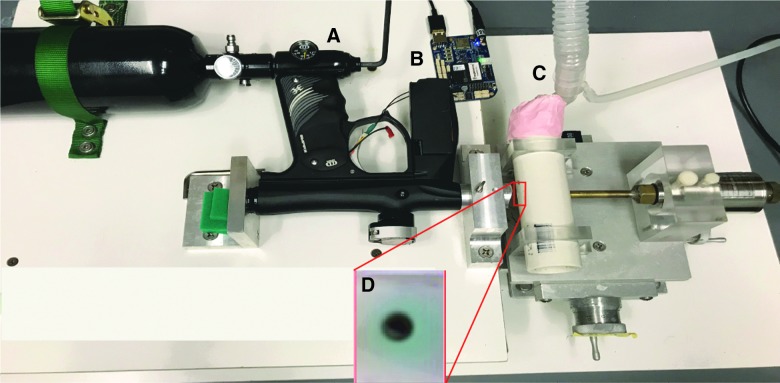
FIG. 1.
Digital temporal control of interblast interval. (A) Adjustable pressure regulator is the same as the original system. (B) Electronic bypass of paintball marker trigger to provide labview-mediated control of blast release and duration. (C) Tubing to provide low-level isofluorane during the blast exposure, thus avoiding the need for injectable anesthetics. (D) Image showing the mouse eye in the hole of the mouse housing, directly across from the machined barrel of the paintball marker. Color image is available online.
Table 1.
Blast Exposure Groups
| Pressure (psi) | No. of exposures | Time between exposures (interinjury interval) |
|---|---|---|
| 26 | 1 | N/A |
| 15 | 1 | N/A |
| 15 | 3 | 0.5 sec |
| 15 | 3 | 1 min |
| 15 | 3 | 10 min |
| 15 | 3 | 2 h |
| 15 | 3 | 24 h |
| 15 | 6 | 24 h |
| 15 | 6 | 3 × [2 × 0.5 sec] at 24-h interval |
psi, pounds per square inch; N/A, not applicable.
Visual evoked potentials
Before collection, mice were dark-adapted overnight, dilated with tropicamide for 10 min, and anesthetized with 20 mg/kg of ketamine/8 mg/kg of xylazine/8 mg/kg of urethane. Mice were placed on the heated surface of the Celeris system (Diagnosys LLC, Lowell, MA) to maintain normal body temperature. Celeris corneal electrodes with integrated stimulators were placed on the lubricated corneas, and subdermal platinum electrodes were placed in the snout and back of the head at the location of the visual cortex. Mice were exposed to 50 flashes of 1 Hz, 0.5 cd.s/m2 of white light. All mice that received visual evoked potentials (VEPs) were used for histological analysis.
Optic nerve histology
After perfusion, the proximal segment of optic nerve was collected, post-fixed in glutaraldehyde, and embedded in Resin 812 and Araldite 502 (catalog numbers 14900 and 10900, respectively; Electron Microscopy Sciences, Hatfield, PA) according to previously published protocol.41,44–46 One-micron-thick sections were collected on a Leica EM-UC7 microtome (Leica Microsystems, Wetzlar, Germany) and stained with 1% paraphenylenediamine and 1% toluidine blue. Cross-sections were imaged on a Nikon Eclipse Ni-E microscope (Nikon Instruments Inc., Melville, NY) using a 100 × oil immersion objective. Total and degenerating axons were quantified in an average of seven nerves/group using ImageJ software (National Institutes of Health, Bethesda, MD). A grid was used to count 20% of the optic nerve cross-sectional surface area to avoid bias. In addition, tissues were assigned numbers and the investigator performing the axon counts was masked to the identity of the samples.
Statistical analysis
GraphPad Prism software (GraphPad Software Inc., La Jolla, CA) was used to perform statistical analysis of all data. In all cases, a one-way analysis of variance and Dunnett's post-hoc test was performed comparing blast-exposed groups to sham.
Results
Optic nerve degeneration and vision loss after single and repeat overpressure air blasts
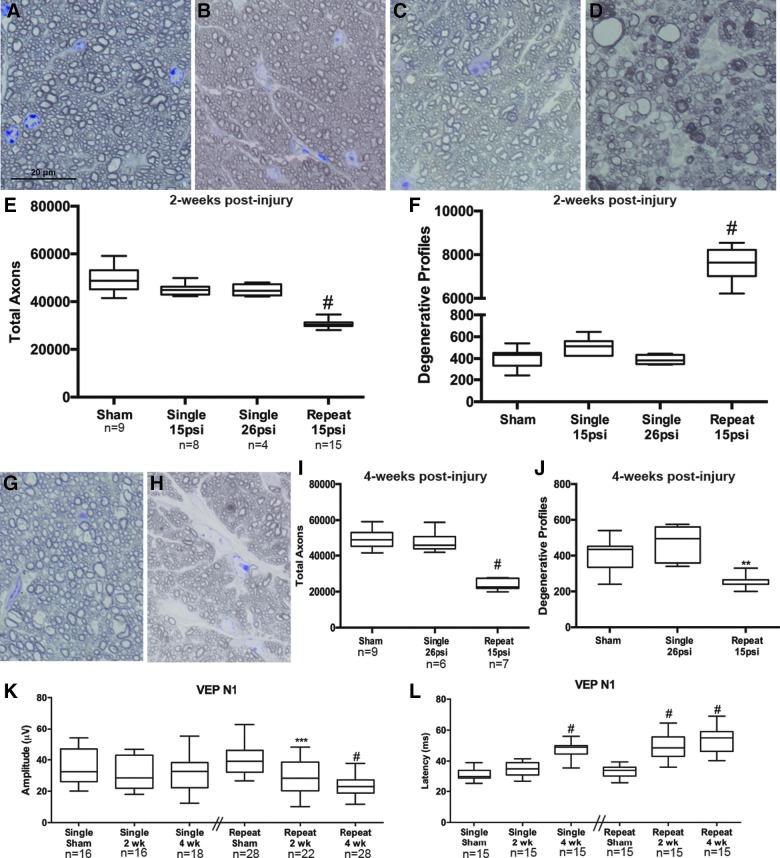
In sham mice, the optic nerves appear healthy with normal morphology of the astrocytes and axons (Fig. 2A). The optic nerves from mice exposed to a single 15- or 26-psi blast appear similar to sham at 2 weeks after blast (Fig. 2B,C). In contrast, mice that were exposed to 3 days of two 15-psi blasts given at a 0.5-sec interval (i.e., repeat 15 psi) exhibited axon degeneration in the optic nerve at 2 weeks after injury (Fig. 2D). Quantification of these data shows no statistically significant difference in the number of total or degenerative axons in the optic nerves of single 15-psi or single 26-psi blast-exposed animals at 2 weeks after exposure as compared to shams (Fig. 2E,F). In contrast, repeat 15-psi blast exposure caused a decrease in total axons (p < 0.0001 as compared to sham; Fig. 2E). At 2 weeks after repeat 15-psi blast, 32% of axons had a degenerative profile (p < 0.0001 as compared to shams; Fig. 2F).
FIG. 2.
Repeat 15-psi blast exposure causes greater and faster axon degeneration and vision loss than a single 15- or 26-psi blast. (A–D) Representative brightfield micrographs of ipsilateral optic nerve cross-sections from sham (A), 2-week single 15-psi (B), 2-week single 26-psi (C), and 2-week repeat 15-psi blast (D). (E) Quantification of total axons in each group at 2 weeks after blast. (F) Quantification of degenerative axon profiles in each group at 2 weeks after blast. (G and H) Representative brightfield micrographs of ipsilateral optic nerve cross-sections from mice 4 weeks after a single 26-psi blast (G) or a repeat 15-psi blast (H). (I) Quantification of total axons at 4 weeks after blast. (J) Quantification of degenerative axon profiles at 4 weeks after blast. (K) Quantification of the VEP N1 wave amplitude in each group. (L) Quantification of the VEP N1 wave latency in each group. psi, pounds per square inch; VEP, visual evoked potential. Color image is available online.
As we have shown previously, a single 26-psi blast to the eye of a C57Bl/6 mouse causes minimal axon degeneration at 4 weeks after injury (Fig. 2G).42 In contrast, 4 weeks after exposure to the repeat 15-psi paradigm, there is a dearth of small axon profiles and glial hypertrophy is evident (Fig. 2H). Quantification of these data shows no statistically significant difference in the number of total or degenerative axons in the optic nerves of single 26-psi blast-exposed animals at 4 weeks after exposure as compared to shams (Fig. 2I,J). In contrast, repeat 15-psi blast-exposure caused a 50% decrease in total axons (p < 0.0001 as compared to sham; Fig. 2I). Surprisingly, fewer degenerative axon profiles were detected in the repeat blast group as compared to sham optic nerves at 4 weeks after blast exposure (Fig. 2J).
The axons of the optic nerve connect to the visual pathways in the brain, ultimately reaching the visual cortex. Therefore, to quantify functional deficits, we measured the visual-evoked activity at the visual cortex. The VEP is only elicited if all previous neurons in the visual pathway respond to the light stimulus; starting with the photoreceptors, through the retina, optic nerve, and optic radiations, until finally reaching the visual cortex. The amplitude represents the number of functioning axons in the visual pathway. The latencies represent how well the surviving axons are functioning. There was no statistically significant decrease in VEP N1 amplitude in the single blast group at 2 or 4 weeks after a 26-psi air burst (Fig. 2K). VEP N1 latency was the same as sham at 2 weeks after a single 26-psi injury, but increased from 31.4 ± 4.1 to 47.5 ± 5.3 ms (standard deviation; SD) in the 4-week single 26-psi blast-exposed animals (p < 0.0001 as compared to shams; Fig. 2L). Two weeks after the repeat 15-psi air blast exposure, VEP N1 amplitude was reduced from 39.4 mV ±8.77 to 28.6 mV ±11.9 with p < 0.001 as compared to sham (Fig. 2K). VEP N1 amplitude decreased to 23.8 mV ±6.71 in the 4-week post-repeat 15-psi blast mice (p = 0.0001 as compared to shams). In the repeat 15-psi blast mice, latency was increased by 48% and 59% at 2 and 4 weeks after blast exposure, respectively, as compared to shams (p < 0.0001; Fig. 2L). The repeat blast shams had a latency of 33.5 ± 3.7, whereas the 2-week injured latency was 49.6 ± 8.7, and 4-week latency was 53.4 ± 7.7 ms.
Effect of interinjury interval of less than 2 h on axonal degeneration
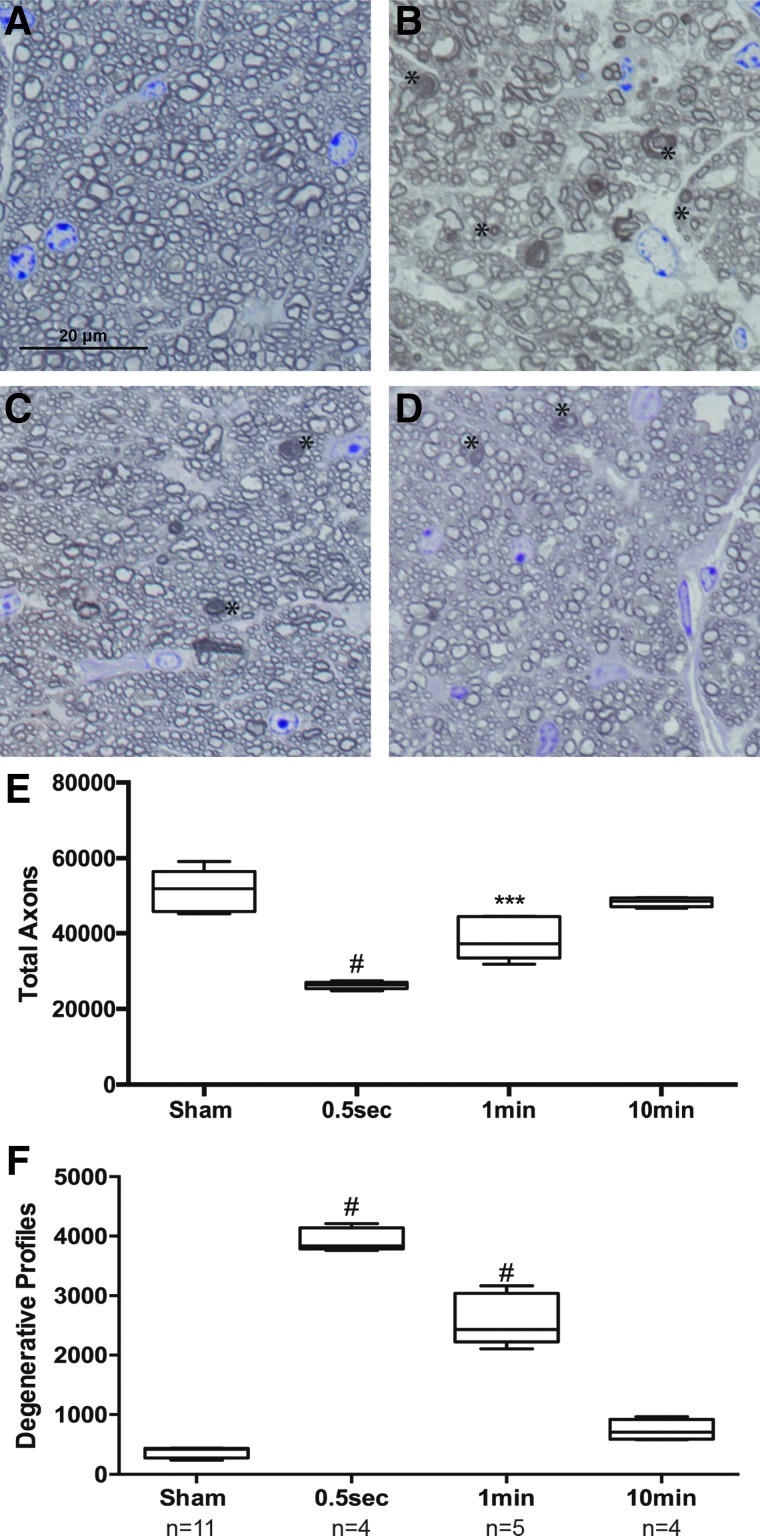
We next sought to determine the role of the interinjury interval on the increased axonal degeneration observed in the repeat 15-psi blast group as compared to the single 26-psi blast group. To do this, we held the total number of blast exposures constant at three 15-psi bursts of air. We then tested the following interinjury intervals between the three air blasts: 0.5 sec, 1 min, 10 min, or 2 h (Table 1). Optic nerves were assessed at 2 weeks post-blast (the peak of axon degeneration in our repeat blast model; Fig. 2). The optic nerves from the 0.5-sec interval group had many actively degenerative profiles, in stark contrast from the sham controls (Fig. 3A,B). The optic nerves from the 1- and 10-min interval groups contained degenerative profiles, but less so than the 0.5-sec interval group (Fig. 3C,D). The optic nerves from the 2-h interval group were comparable to shams (data not shown). Quantification of total axons showed an average of 48,905 ± 5310 axons (±SD) in sham optic nerves, 26,326 ± 1089 in 0.5-sec interval optic nerves, 38,642 ± 5656 in 1-min interval optic nerves, and 46,375 ± 4183 in 10-min interval optic nerves (Fig. 3E). This corresponds to a 46% decrease in the 0.5-sec interval group (p < 0.0001 as compared to shams). This is a 21% decrease in the 1-min interval group (p < 0.001 as compared to shams; Fig. 3E). There was no statistically significant difference in the total number of axons in the 10-min interval group as compared to shams (Fig. 3E). The detection of fewer axons at 2 weeks suggests that the blast exposure caused immediate damage to the optic nerve and the damaged axons were cleared by neighboring glia by the 2-week analysis time point.
FIG. 3.
Interinjury interval of 1 min or less is associated with increased axon degeneration. (A–D) Representative brightfield micrographs of ipsilateral optic nerve cross-sections 2 weeks after sham (A) or exposure to three 15-psi blasts separated by intervals of 0.5 sec (B), 1 min (C), or 10 min (D). (E) Quantification of total optic nerve axons at 2 weeks after blast exposure. Asterisks indicate degenerative profiles. (F) Quantification of degenerative profiles in the optic nerves at 2 weeks after blast exposure. psi, pounds per square inch. Color image is available online.
In order to determine whether there was ongoing secondary axon degeneration, we quantified degenerative profiles in the optic nerves at 2 weeks after injury. We detected an average of 405 ± 89 degenerative profiles in sham optic nerves (Fig. 3F). This compared to 3963 ± 214 in 0.5-sec interval optic nerves, 2591 ± 434 in 1-min interval optic nerves, and 739 ± 175 degenerative profiles in 10-min interval optic nerves (Fig. 3F). This corresponds to 15% ongoing axon degeneration in the 0.5-sec interval group (p < 0.0001 as compared to sham). Notably, this is half of what we detect with our repeat blast paradigm of two 15-psi blasts at 0.5-sec intervals repeated for 3 days for a total of six exposures (32% ongoing axon degeneration; Fig. 2F). There was 6.7% ongoing axon degeneration in the 1-min interval group (p < 0.0001 compared to sham). Finally, we did detect a small increase in ongoing axon degeneration in the 10-min interval group (1.6%) that was statistically significant (p < 0.05). We also performed a group with an interblast interval of 2 h. There was no difference between the 2-h group and shams (data not shown).
Effect of total number of air-blast exposures on axonal degeneration
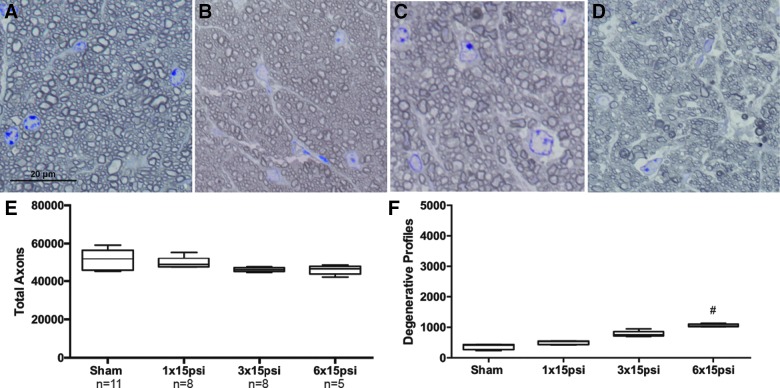
In order to determine the effect of the number of blast exposures on the injury profile, we exposed mice to one, three, or six 15-psi blasts all separated by 24 h to avoid an interblast interval effect (Table 1). The optic nerves were collected at 2 weeks after blast exposure. As also shown in Figure 2, representative micrographs show that the sham and 1 × 15 psi optic nerves look very similar (Fig. 4A,B). A few degenerative axons could be detected in the 3 × 15 psi and 6 × 15 psi optic nerves (Fig. 4C,D). The total number of axons was comparable between all groups (Fig. 4E). Quantification of degenerative axon profiles was also similar between the sham, 1 × 15 psi, and 3 × 15 psi optic nerves (Fig. 4F). There was a 4% increase in degenerative profiles in the 6 × 15 psi group (p < 0.0001) as compared to shams (Fig. 4F).
FIG. 4.
Total blast exposure number does not have a synergistic effect on axon degeneration. (A–D) Representative brightfield micrographs of ipsilateral optic nerve cross-sections 2 weeks after sham (A) or exposure to one (1 × 15 psi; B), three (3 × 15 psi; C), or six (6 × 15 psi; D) 15-psi blasts, with each blast separated by the other by 24 h. (E) Quantification of total optic nerve axons in each group. (F) Quantification of degenerative profiles in optic nerves from each group. psi, pounds per square inch. Color image is available online.
Discussion
The visual system is particularly susceptible to injuries such as those experienced by military service members and athletes, making it an ideal model for studying axonal damage after single and repeat mild neurotrauma.47–50 There are four major findings from this study using a model of repeat subthreshold neurotrauma. First, rapid repeat exposure to an overpressure air blast that is subthreshold for injury causes synergistic axon damage. Second, exposure to increasing numbers of blasts outside of the window of synergistic damage causes cumulative, additive axon damage. Third, there is a population of axons that appear to be susceptible to indirect neurotrauma. Fourth, an increased VEP latency is a sensitive marker of injury. Our study describes a useful model for studying repeat mild neurotrauma and extends previous studies by identifying a window of synergistic damage after an injury and a potential biomarker for axonal injury.
This study extends the previous literature on repeat mild neurotrauma by assessing effects of intervals less than 1 day. Our results show a synergistic effect of subthreshold injuries on axonal degeneration when the events occur at intervals of 0.5 sec or 1 min of each other, but not when 10 min or longer apart. In this study, we define synergistic as a multiplicative increase (rather than the additive effect observed with a 10 min or longer interval). An exposure to three 15-psi blasts at a 0.5-sec interval caused a 10-fold increase in degenerative profiles as compared to sham and an 8-fold increase as compared to a single 15-psi blast. An interval of 1 min caused a 7-fold increase in degenerative profiles as compared to sham and 5-fold increase as compared to a single 15-psi blast. Again, this is in contrast to a 10-min interval, which caused only a 2-fold increase as compared to sham, and a 1.5-fold increase as compared to a single blast exposure. This synergistic effect on axon damage is particularly relevant to military service members exposed to serial mines or an improvised explosive device, in which they experience multiple traumatic events within seconds to 1 min of each other. An improvised explosive device generates a primary blast wave that peaks at just a few milliseconds after detonation and rebounds as it reflects against surfaces while almost simultaneously propelling fragments and, finally, the tertiary blast wind can displace large objects or people against nearby hard surfaces.11 Thus, a blast-exposure injury is often multiple traumatic events in one that all occur within a time frame of seconds. TBI attributed to an explosive blast is considered the signature injury of modern warfare, and damage to the visual system is a frequent comorbidity in these individuals.26,51,52
The earliest molecular changes known to date after neurotrauma include decreased phosphorylation and increased proteolysis of neurofilament in axons 5 min after controlled cortical impact in mice, increased intracellular Ca2+ 1 min after optic nerve crush, and increased reactive oxygen species.53,54 Neurofilament structure can affect axon conduction velocity as well as ultimately lead to axonal degeneration attributed to inappropriate localization and transport of organelles.55 Increased intracellular Ca2+ after optic nerve crush is attributed to a mixture of entry from extracellular pools and release from intracellular stores as a result of the reactive oxygen species.54,56 Increased Ca2+ is associated with changes in neurofilament, as well as increased reactive oxygen species, which we have detected in our model.41,43 In particular, increased Ca2+ activates nitric oxide synthase, which leads to increased production of peroxynitrite, resulting in formation of nitrotyrosine and damaged proteins.57 We have detected increased nitrotyrosine in the inner retina 3 days after blast exposure.41 Future studies should explore changes in calcium flux and neurofilament at acute time points after blast exposure. It is feasible that the time course of increase and resolution of these molecular events contributes to the greater susceptibility of axons to repeated injuries occurring within an interval of 1 min or less.
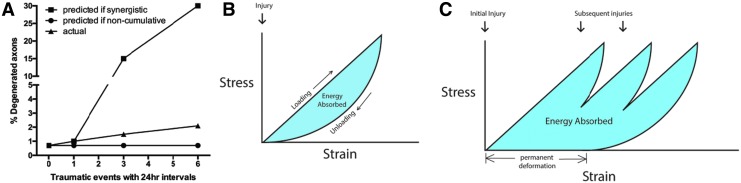
In contrast to our findings with an interval of less than 1 min, the extent of the axonal degeneration detected with a 1-day interinjury interval suggests an additive, rather than synergistic, effect as described below. A single 15-psi blast caused an increase of 133 degenerative profiles as compared to shams. The increase in degenerative profiles between the 1 × 15 psi and 3 × 15 psi groups is 277 degenerative profiles. Similarly, the increase between the 3 × 15 psi and 6 × 15 psi groups is 279 degenerative profiles. If we subtract 133 degenerative profiles from these values to account for damage from the first blast, then each additional set of blasts causes an increase in 144 (two additional blasts) or 146 (five additional blasts) degenerative profiles. Thus, the effect of each additional blast diminishes with time from the initial blast, but still accumulates over time to result in cumulative effects. These data are consistent with reports from animal models and clinical studies of TBI showing increased damage with recurring trauma at 1-day intervals, supporting the concept of a susceptibility window after even a mild injury.13–24 This result demonstrates that even subthreshold injuries cause molecular changes and/or slight axonal damage that, upon additional injuries, can accrue to the point of inducing axonal damage at statistically significant levels (Fig. 5A). Given that there was no difference in the number of degenerated profiles between single blast and sham exposed optic nerves, subsequent blasts spaced at 10 min or more would also be expected to have no effect on axon degeneration if there was not some level of cellular response each time (Fig. 5A). In our model of subthreshold blasts, the amount of axon degeneration did not reach statistical significance until animals were exposed to six blasts. In summary, these data suggest that more-sensitive measures of injury are needed to detect neuronal dysfunction before outright degeneration and to accurately assess the neuronal health of patients.
FIG. 5.
Hypothesized mechanisms for increased injury with repeat exposure to subthreshold blasts. (A) Graphical representation of predicted versus actual axon degeneration detected in the 24-h interval optic nerves. Level of actual degeneration is much less than the synergistic effect detected with a 0.5-sec or 1-min interval (predicted if synergistic). But, it is greater than if each was independently subthreshold, that is, non-cumulative (predicted if non-cumulative). Rather, we detected cumulative, additive damage from repeated subthreshold injuries occurring outside the window of synergistic damage (actual). We hypothesize that the optic nerve exhibits viscoelastic properties and is thus able to fully recover after a single insult when given sufficient time (B). However, like with viscoelastic materials, permanent damage can be caused if another stress occurs before a full recovery from the previous event(s) (C). Color image is available online.
Our study provides additional insight into the axonal damage caused by rapid repeat exposure that occurs in the time scale of injuries during explosive blasts. Although this study does not cover the bioenergetics of the orbit and optic nerve, the results suggest that this should be an area of active investigation. Our detection of increased axon degeneration when blasts occur at intervals of 0.5 sec or 1 min, but not at 10 min, suggests a biomechanical mechanism perhaps working in concert with a molecular mechansim. We present a hypothesis that the optic nerve exhibits viscoelastic properties, that is, its strain rate is time dependent (Fig. 5B,C). A viscoelastic material that is exposed to a single pressure event that results in non-damaging strain will recover its original shape and dimensions if given sufficient time (Fig. 5B). However, if repeated pressure events occur before full recovery, the material can be permanently physically imprinted and thus unable to recover its original shape and dimensions (Fig. 5C). The biomechanical injury, if it occurs, could be at the level of: 1) the optic nerve head with deformation affecting the axons; 2) the individual axons as the blast wave stretches the axons as it propagates through the optic nerve, this would likely also affect ion flux; or 3) the blood vessels. This greater physical damage could cause a greater molecular response, which, in the case of the retina and optic nerve, could result in increased axon degeneration at 2 weeks after the inciting event. This could explain why some military service members develop optic nerve atrophy and blindness over time after repeated exposure to blasts from improvised explosive devices or mines without any obvious direct injuries.30,32,58
In the repeat blast paradigm, the peak of secondary axon degeneration occurred at 2 weeks after injury. This is in general agreement with the timing of secondary optic nerve pallor and vision loss in traumatic optic neuropathy patients.59 What is perhaps more interesting is the lack of continued axon degeneration at 4 weeks after injury. This may suggest that there are axons in the optic nerve that are susceptible to injury and others that are resistant. In this case, the susceptible ones would degenerate and, in response, neighboring glia would hypertrophy and phagocytose the debris. Meanwhile, resistant axons would survive near the glial scars. This does not suggest that the remaining axons are completely healthy. In fact, the increased VEP N1 latency and detection of cumulative damage with longer injury intervals suggest otherwise. It is unclear what would cause certain axons to be more susceptible than others. We detect fewer small axon profiles after injury; however, it is unclear whether it is attributed to degeneration or swelling of the small axons. A recent study identified retinal ganglion cell subtype differences in response to optic nerve crush.60 It is feasible that there is a similar subtype difference in response to blast exposure, and that these differences in cell death also affect axon degeneration. Future studies should determine whether there is a regional response to blast in the optic nerve or whether the axon degeneration is a retina-driven response attributed to susceptibility of certain retinal ganglion cell subpopulations or retinal regions damaged by the blast injury induced by our model.
We detected an increased VEP N1 latency in mice exposed to even a single overpressure air blast. These mice did not exhibit a decrease in the VEP N1 amplitude or an increase in degenerative axon profiles. Two clinical studies have also detected an increased VEP latency in TBI patients.61,62 In addition, the VEP has been shown to be predictive of the development of traumatic optic neuropathy in trauma patients.63,64 Approximately 10–22% of these patients develop optic nerve degeneration and blindness weeks after the traumatic event.59 Unfortunately, there are no clinical predictors of who will develop secondary axonal degeneration and who will not. Identification of a biomarker for these patients would allow for earlier therapeutic intervention. Our data support the use of VEP latency as a useful diagnostic for neuronal susceptibility and/or injury. Calcium dysregulation, reactive oxygen species, inflammation, and altered synaptic connectivity have been reported in models of single and repeat neurotrauma and may underlie the visual deficits we detected.24,41,65
We previously reported that a single 26-psi air blast induces oxidative stress and caspase-1 labeling in the retina within 3 days of injury.41 More recently, we have detected activation of the inflammasome pathway in the retina after either a single 26-psi blast exposure or repeat 15-psi blast exposure using the same repeat paradigm as in this study.43 In combination with the axon degeneration reported here, these results show that molecular events are activated in cell bodies of neurons even in the absence of ongoing axonal damage. Future studies should investigate whether molecular events in the cell body underlie the increased susceptibility of axons to repeat injury.
Acknowledgments
The authors thank Dr. Robert Webster for helpful discussions. Support was provided by: DoD W81XWH-15-1-0096 and W81XWH-17-2-0055, NEI R01 EY022349, NIA R01 NS094595, Potocsnak Discovery Grant in Regenerative Medicine, Ayers Foundation Regenerative Visual Neuroscience Pilot Grant, Jones Gift, and P30EY008126, and Research Prevent Blindness, Inc.
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Corrigan J., Selassie A., and Orman J. (2010). The epidemiology of traumatic brain injury. J. Head Trauma Rehabil. 25, 72–80 [DOI] [PubMed] [Google Scholar]
- 2. Defense and Veterans Brain Injury Center. DoD worldwide numbers for TBI. http://dvbic.dcoe.mil/dod-worldwide-numbers-tbi 2017. (last accessed October10, 2017)
- 3. Martland H. (1928). Punch drunk. JAMA 91, 1103–1107 [Google Scholar]
- 4. Corsellis J., Bruton C., and Freeman-Browne D. (1973). The aftermath of boxing. Psychol. Med. 3, 270–303 [DOI] [PubMed] [Google Scholar]
- 5. Solomon G. (2018). Chronic traumatic encelopathy in sports: a historical and narrative review. Dev. Neuropsychol. 43, 279–311 [DOI] [PubMed] [Google Scholar]
- 6. Peskind E., Brody D., Cernak I., McKee A., and Ruff R. (2013). Military- and sports- related mild traumatic brain injury: clinical presentation, management, and long-term consequences. J. Clin. Psychiatry 74, 180–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Peskind E., Petrie E., Cross D., Pagulayan K., McCraw K., Hoff D., Hart K., Yu C., Raskind M., Cook D., and Minoshima S. (2011). Cerbrocerebellar hypometabolism associated with repetitive blast exposure mild traumatic brain injury in 12 Iraq war Veterans with persistent post-concussive symptoms. Neuroimage 54, Suppl. 1, S76–S82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Petrie E., Cross D., Yarnykh V., Richards T., Martin N., Pagulayan K., Hoff D., Hart K., Mayer C., Tarabochia M., Raskind M., Minoshima S., and Peskind E. (2014). Neuroimaging, behavioral, and psychological sequelae of repetitive combined blast/impact mild traumatic brain injury in Iraq and Afghanistan war veterans. J. Neurotrauma 31, 425–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Meabon J., Huber B., Cross D., Richards T., Minoshima S., Pagulayan K., Li G., Meeker K., Kraemer B., Petrie E., Raskind M., Peskind E., and Cook D. (2016). Repetitive blast exposure in mice and combat veterans causes persistent cerebellar dysfunction. Sci. Transl. Med. 8, 321ra6. [DOI] [PubMed] [Google Scholar]
- 10. Goldstein L., Fisher A., Tagge C., Zhang X., Velisek L., Sullivan J., Upreti C., Kracht J., Ericsson M., Wojnarowicz M., Goletiani C., Maglakelidze G., Casey N., Moncaster J., Minaeva O., Moir R., Nowinski C., Stern R., Cantu R., Geiling J., Blusztajn J., Wolozin B., Ikezu T., Stein T., Budson A., Kowall N., Chargin D., Sharon A., Saman S., Hall G., Moss W., Cleveland R., Tanzi R., Stanton P., and McKee A. (2012). Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Sci. Transl. Med. 4, 134ra60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cernak I., and Noble-Haeusslein L. (2010). Traumatic brain injury: an overview of pathobiology with emphasis on military populations. J. Cereb. Blood Flow Metab. 30, 255–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McKee A., Cantu R., Nowinski C., Hedley-Whyte E., Gavett B., Budson A., Santini V., Lee H., Kubilus C., and Stern R. (2009). Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J. Neuropathol. Exp. Neurol. 68, 709–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bolton A., and Saatman K. (2014). Regional neurodegeneration and gliosis are amplified by mild traumatic brain injury repeated at 24-hour intervals. J. Neuropathol. Exp. Neurol. 73, 933–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Eisenberg M., Andrea J., Meehan W., and Mannix R. (2013). Time interval between concussions and symptom duration. Pediatrics 132, 8–17 [DOI] [PubMed] [Google Scholar]
- 15. Fehily B., and Fitzgerald M. (2017). Repeated mild traumatic brain injury: potential mechanisms of damage. Cell Transplant. 26, 1131–1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gama Sosa M., De Gasperi R., Perez Garcia G., Sosa H., Searcy C., Vargas D., Janssen P., Perez G., Tschiffely A., Janssen W., McCarron R., Hof P., Haghighi F., Ahlers S., and Elder G. (2017). Lack of chronic neuroinflammation in the absence of focal hemorrhage in a rat model of low-energy blast-induced TBI. Acta Neuropathol. Commun. 5, 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jamnia N., Urban J., Stutzmann G., Chiren S., Reisenbigler E., Marr R., Peterson D., and Kozlowski D. (2017). A clinically relevant closed-head model of single and repeat concussive injury in the adult rat using a controlled cortical impact device. J. Neurotrauma 34, 1351–1363 [DOI] [PubMed] [Google Scholar]
- 18. Meehan W., Zhang J., Mannix R., and Whalen M. (2012). Increasing recovery time between injuries improves cognitive outcome after repetitive mild concussive brain injuries in mice. Neurosurgery 71, 885–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Prins M., Hales A., Reger M., Giza C., and Hovda D. (2010). Repeat traumatic brain injury in the juvenile rat is associated with increased axonal injury and cognitive impairments. Dev. Neurosci. 32, 510–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Selwyn R., Cooney S., Khayrullina G., Hockenbury N., Wilson C., Jaiswal S., Bermudez S., Armstrong R., and Byrnes K. (2016). Outcome after repetitive mild traumatic brain injury is temporally related to glucose uptake profile at time of second injury. J. Neurotrauma 33, 1479–1491 [DOI] [PubMed] [Google Scholar]
- 21. Thomsen G., Ko A., Harada M., Ma A., Wyss L., Haro P., Vit J.-P., Avalos P., Dhillon N., Cho N., Shelest O., and Ley E. (2016). Clinical correlates to assist with chronic traumatic encephalopathy diagnosis: Insights from a novel rodent repeat concussion model. J. Trauma Acute Care Surg. 82, 1039–1048 [DOI] [PubMed] [Google Scholar]
- 22. Thomsen G., Ma A., Ko A., Harada M., Wyss L., Haro P., Vit J.-P., Shelest O., Rhee P., Svendsen C., and Ley E. (2016). A model of recurrent concussion that leads to long-term motor deficits, CTE-like tauopathy and exacerbation of an ALS phenotype. J. Trauma Acute Care Surg. 81, 1070–1079 [DOI] [PubMed] [Google Scholar]
- 23. Turner R., Lucke-Wold B., Logsdon A., Robson M., Dashnaw M., Huang J., Smith K., Huber J., Rosen C., and Petraglia A. (2015). The quest to model chronic traumatic encephalopathy: a multiple model and injury paradigm experience. Front. Neurol. 6, 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Winston C., Noel A., Neustadtl A., Parsadanian M., Barton D., Chellappa D., Wilkins T., Alikhani A., Zapple D., Villapol S., Planel E., and Burns M. (2016). Dendritic spine loss and chronic white matter inflammation in a mouse model of highly repetitive head trauma. Am. J. Pathol. 186, 552–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Abbott C., Choe T., Lusardi T., Burgoyne C., Wang L., and Fortune B. (2013). Imaging axonal transport in the rat visual pathway. Biomed. Opt. Express. 4, 364–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Weichel E., Colyer M., Bautista C., Bower K., and French L. (2009). Traumatic brain injury associated with combat ocular trauma. J. Head Trauma Rehabil. 24, 41–50 [DOI] [PubMed] [Google Scholar]
- 27. Hilber D. (2011). Eye injuries, active component, U.S. Armed Forces, 2000–2010. MSMR. 18, 2–7 [PubMed] [Google Scholar]
- 28. Faul M., Xu L., Wald M., and Coronado V. (2010). Traumatic brain injury in the United States: Emergency department visits, hospitalizations, and deaths. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control: Atlanta, GA [Google Scholar]
- 29. McGwin G.J., Hall T., Xie A., and Owsley C. (2006). Trends in eye injury in the United States, 1992–2001. Invest. Ophthalmol. Vis. Sci. 47, 521–527 [DOI] [PubMed] [Google Scholar]
- 30. Philips B., Chun D., and Colyer M. (2013). Closed globe macular injuries after blasts in combat. Retina 33, 371–379 [DOI] [PubMed] [Google Scholar]
- 31. Weichel E., Colyer M., Ludlow S., Bower K., and Eiseman A. (2008). Combat ocular trauma visual outcomes during operations iraqi and enduring freedom. Ophthalmology 115, 2235–2245 [DOI] [PubMed] [Google Scholar]
- 32. Cockerham G., Rice T., Hewes E., Cockerham K., Lemke S., Wang G., Lin R., Glynn-Milley C., and Zumhagen L. (2011). Closed-eye ocular injuries in the Iraq and Afghanistan wars. N. Engl. J. Med. 364, 2172–2173 [DOI] [PubMed] [Google Scholar]
- 33. Corrales G., and Curreri A. (2009). Eye trauma in boxing. Clin. Sports Med. 28, 591–607 [DOI] [PubMed] [Google Scholar]
- 34. Leong D., Morettin C., Messner L., Steinmetz R., Pang Y., Galetta S., and Balcer L. (2018). Visual structure and function in collision sport athletes. J. Neuroophthalmol. 38, 285–291 [DOI] [PubMed] [Google Scholar]
- 35. Vlasov A., Ryan D., Ludlow S., Weichel E., and Colyer M. (2015). Causes of combat ocular trauma-related blindness from Operation Iraqi Freedom and Enduring Freedom. J. Trauma Acute Care Surg. 79, 4 Suppl. 2, S210–S215 [DOI] [PubMed] [Google Scholar]
- 36. Ryu J., Horkayne-Szakaly I., Xu L., Pletnikova O., Leri F., Eberhart C., Troncoso J., and Koliatsos V. (2014). The problem of axonal injury in the brains of veterans with histories of blast exposure. Acta Neuropathol. Commun. 2, 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Polvishock J., and Christman C. (1995). The pathobiology of traumatically induced axonal injury in animals and humans: a review of current thoughts. J. Neurotrauma 12, 555–564 [DOI] [PubMed] [Google Scholar]
- 38. Smith D., Meaney D., and Shull W. (2003). Diffuse axonal injury in head trauma. J. Head Trauma Rehabil. 18, 307–316 [DOI] [PubMed] [Google Scholar]
- 39. Johnson V., Stewart W., and Smith D. (2013). Axonal pathology in traumatic brain injury. Exp. Neurol. 246, 35–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ommaya A., and Hirsch A. (1971). Tolerances for cerebral concussion from head impact and whiplash in primates. J. Biomech. 4, 13–21 [DOI] [PubMed] [Google Scholar]
- 41. Bricker-Anthony C., Hines-Beard J., and Rex T. (2014). Molecular changes and vision loss in a mouse model of closed-globe blast trauma. Invest. Ophthalmol. Vis. Sci. 55, 4853–4862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hines-Beard J., Marchetta J., Gordon S., Chaum E., Geisert E., and Rex T. (2012). A mouse model of ocular blast injury that induces closed globe anterior and posterior pole damage. Exp. Eye Res. 99, 63–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bernardo-Colon A., Vest V., Clark A., Cooper M., Calkins D., Harrison F., and Rex T. (2018). Antioxidants prevent inflammation and preserve the optic projection and visual function in experimental neurotrauma. Cell Death Dis. 9, 1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bond W., Hines-Beard J., GoldenMerry Y., Davis M., Farooque A., Sappington R., Calkins D., and Rex T. (2016). Virus-mediated EpoR76E therapy slows optic nerve axonopathy in experimental glaucoma. Mol. Ther. 24, 230–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bricker-Anthony C., D'Surney L., Lunn B., Hines-Beard J., Jo M., Bernardo-Colon A., and Rex T. (2017). Erythropoietin either prevents or exacerbates retinal damage from eye trauma depending on treatment timing. Optom. Vis. Sci. 94, 20–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hines-Beard J., Bond W., Backstrom J., and Rex T. (2016). Virus-mediated EpoR76E gene therapy preserves vision in a glaucoma model by modulating neuroinflammation and decreasing oxidative stress. J. Neuroinflammation. 13, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Xu L., Nguyen J., Lehar M., Menon A., Rha E., Arena J., Ryu J., Marsh-Armstrong N., Marmarou C., and Koliatsos V. (2016). Repetitive mild traumatic brain injury with impact acceleration in the mouse: multifocal axonopathy, neuroinflammation, and neurodegeneration in the visual system. Exp. Neurol. 275, 436–449 [DOI] [PubMed] [Google Scholar]
- 48. Koliatsos V., Cernak I., Xu L., Song Y., Savonenko A., Crain B., Eberhart C., Frangakis C., Melnikova T., Kim H., and Lee D. (2011). A mouse model of blast injury to brain: initial pathological, neuropathological, and behavioral characterization. J. Neuropathol. Exp. Neurol. 70, 399–416 [DOI] [PubMed] [Google Scholar]
- 49. Guley N., Rogers J., Del Mar N., Deng Y., Islam R., D'Surney L., Ferrell J., Deng B., Hines-Beard J., Bu W., Ren H., Elberger A., Marchetta J., Rex T., Honig M., and Reiner A. (2016). A novel closed-head model of mild traumatic brain injury using focal primary overpressure blast to the cranium in mice. J. Neurotrauma 33, 403–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tzekov R., Quezada A., Gautier M., Biggins D., Frances C., Mouzon B., Jamison J., Mullan M., and Crawford F. (2014). Repetitive mild traumatic brain injury causes optic nerve and retinal damage in a mouse model. J. Neuropathol. Exp. Neurol. 73, 345–361 [DOI] [PubMed] [Google Scholar]
- 51. Swanson T., Isaacson B., Cyborski C., French L., Tsao J., and Pasquina P. (2017). Traumatic Brain Injury Incidence, Clinical Overview, and Policies in the US Military Health System Since 2000. Public Health Rep. 132, 251–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Urosevich T., Boscarino J., Hoffman S., Kirchner H., Figley C., Adams R., Withey C., and Boscarino J. (2018). Visual dysfunction and associated co-morbidities as predictors of mild traumatic brain injury seen among veterans in non-VA facilities: implications for clinical practice. Mil. Med. 183, e564–e570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Huh J., Laurer H., Raghupathi R., Helfaer M., and Saaatman K. (2002). Rapid loss and partial recovery of neurofilament immunostaining following focal brain injury in mice. Exp. Neurol. 175, 198–208 [DOI] [PubMed] [Google Scholar]
- 54. Knoferle J., Koch J., Ostendorf T., Michel U., Planchamp V., Vutova P., Tonges L., Stadelmann C., Bruck W., Bahr M., and Lingor P. (2010). Mechanisms of acute axonal degeneration in the optic nerve in vivo. Proc. Natl. Acad. Sci. U. S. A. 107, 6064–6069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yuan A., Rao M., Veeranna, and Nixon R. (2017). Neurofilaments and neurofilament proteins in health and disease. Cold Spring Harb. Perspect. Biol. 9, a018309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. O'Hare Doig R., Chiha W., Giacci M., Yates N., Bartlett C., Smith N., Hodgetts S., Harvey A., and Fitzgerald M. (2017). Specific ion channels contribute to key elements of pathology during secondary degeneration following neurotrauma. BMC Neurosci. 18, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Casson R. (2006). Possible role of excitotoxicity in the pathogenesis of glaucoma. Clin. Exp. Ophthalmol. 34, 54–63 [DOI] [PubMed] [Google Scholar]
- 58. Cockerham G., Goodrich G., Weichel E., Orcutt J., Rizzo J., Bower K., and Schuchard R. (2009). Eye and visual function in traumatic brain injury. J. Rehabil. Res. Dev. 46, 811–818 [DOI] [PubMed] [Google Scholar]
- 59. Levin L., Beck R., Joseph M., Seiff S., and Kraker R. (1999) The treatment of traumatic optic neuropathy: the International Optic Nerve Trauma Study. Ophthalmology 106, 1268–1277 [DOI] [PubMed] [Google Scholar]
- 60. Daniel S., Clark A., and McDowell C. (2018). Subtype-specific response of retinal ganglion cells to optic nerve crush. Cell Death Discov. 5, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gaetz M., and Weinberg H. (2000). Electrophysiological indices of persistent post-concussion symptoms. Brain Inj. 14, 815–832 [DOI] [PubMed] [Google Scholar]
- 62. Sarno S., Erasmus L., Lippert G., Frey M., Lipp B., and Schlaegel W. (2000). Electrophysiological correlates of visual impairments after traumatic brain injury. Vis. Res. 40, 3029–3038 [DOI] [PubMed] [Google Scholar]
- 63. Mahapatra A. (1991). Visual evoked potentials in optic nerve injury—does it merit to be mentioned? Indian J. Ophthalmol. 39, 20–21 [PubMed] [Google Scholar]
- 64. Tabatabaei S., Soleimani M., Alizadeh M., Movasat M., Mansoori M., Alami Z., Foroutan A., Joshaghani M., Safari S., and Goldiz A. (2011). Predictive value of visual evoked potentials, relative afferent pupillary defect, and orbital fractures in patients with traumatic optic neuropathy. Clin. Ophthalmol. 5, 1021–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Frati A., Cerretani D., Fiaschi A., Frati P., Gatto V., La Russa R., Pesce A., Pinchi E., Santurro A., Fraschetti F., and Fineschi V. (2017). Diffuse axonal injury and oxidative stress: a comprehensive review. Int. J. Mol. Sci. 18, E2600. [DOI] [PMC free article] [PubMed] [Google Scholar]