Abstract
During normal participation in football, players are exposed to repetitive subconcussive head impacts, or impacts that do not result in signs and symptoms of concussion. To better understand the effects of repetitive subconcussive impacts, the biomechanics of on-field head impacts and resulting brain deformation need to be well characterized. The current study evaluates local brain response to typical youth football head impacts using the atlas-based brain model (ABM), an anatomically accurate brain finite element (FE) model. Head impact kinematic data were collected from three local youth football teams using the Head Impact Telemetry (HIT) System. The azimuth and elevation angles were used to identify impacts near six locations of interest, and low, moderate, and high acceleration magnitudes (5th, 50th, and 95th percentiles, respectively) were calculated from the grouped impacts for FE simulation. Strain response in the brain was evaluated by examining the range and peak maximum principal strain (MPS) values in each element. A total of 40,538 impacts from 119 individual athletes were analyzed. Impacts to the facemask resulted in 0.18 MPS for the high magnitude impact category. This was 1.5 times greater than the oblique impact location, which resulted in the lowest strain value of 0.12 for high magnitude impacts. Overall, higher strains resulted from a 95th percentile lateral impact (41.0g, 2556 rad/sec2) with two predominant axes of rotation than from a 95th percentile frontal impact (67.6g, 2641 rad/sec2) with a single predominant axis of rotation. These findings highlight the importance of accounting for directional dependence and relative contribution of axes of rotation when evaluating head impact response.
Keywords: brain injury, FE model, HITS, strain, youth football
Introduction
Approximately 3,500,000 athletes participate in organized football at the youth level in the United States. It is estimated that 1,100,000–1,900,000 sports-related concussions occur each year in the youth population, with the majority occurring during football.1,2 Although football has a high rate of concussion, exposure to repetitive subconcussive head impacts, which occur as part of normal participation in the sport, and associated changes in the brain related to neurodegenerative diseases is of increasing concern.3–7 Although head impact exposure in football has been extensively studied, the tissue-level response of the brain to subconcussive head impacts is not well understood.8–12 Biomechanical factors of head impact, such as impact severity, location, and direction, and their relationship to tissue-level strain response can be characterized using finite element (FE) models to better understand the effects of repetitive subconcussive impacts. To characterize brain response to head impact exposure, FE studies quantify the strain response of the brain to conditions representative of typical football impacts. In 2014, Ji and coworkers used the Dartmouth Head Injury Model (DHIM) and the Simulated Injury Monitor (SIMon) to investigate brain-strain-related responses in a range of loading conditions representative of football impacts experienced at the youth, high school, and collegiate levels.13 Brain deformation was measured using deformation metrics proposed to have a correlation to brain injury, such as maximum principal strain (MPS) and von Mises stress.14,15 This study also investigated the relative contributions of linear and angular acceleration to the strain response, and found that isolated linear acceleration generates negligible strain. Smith and coworkers (2015) used the University College Dublin Brain Trauma Model (UCDBTM) to evaluate strain response and establish MPS thresholds for indirect (0.15), direct (0.14), and combined loading (0.24) scenarios.16 Darling and coworkers (2016) used the head model from the Global Human Body Models Consortium (GHBMC) full body model to evaluate the strain response to two typical loading conditions experienced in football: frontal and crown impacts.17 The maximum strains occurred in the brainstem for both conditions and had MPS values of 0.088 and 0.045 for the frontal and crown impacts, respectively. Additionally, multiple studies have examined strain response in various injurious scenarios.14,15,18,19 The objective of these studies was to evaluate possible brain injury predictors and establish strain threshold levels corresponding to concussion. Threshold injury analysis also examined strain levels and thresholds in brain volumes of interest, specifically the corpus callosum. Proposed strain thresholds corresponding to 50% chance of mild traumatic brain injury (mTBI) include 0.19 using the Wayne State University Brain Injury Model (WSUBIM), 0.26 using the Kungliga Tekniska Högskolan (KTH) model, and 0.28 using the Dartmouth Subject-Specific Head Model (SSM).18
Directional dependence in brain response has been reported throughout the literature by researchers using animal models as well as FE models.20 Gennarelli and coworkers (1982) subjected groups of monkeys to rotation about the X, Y, or Z axis and observed that coronal head motions produced the most serious neurological disturbances. Additionally, coronal plane rotation was the only motion that commonly produced axonal damage in the brainstem.21 Using the WSUBIM, Zhang and coworkers (2001) compared the brain responses between frontal and lateral impacts and observed decreased brain tolerance to lateral impact.20 Kleiven (2006) used the KTH model to study the influence of impact direction on MPS, which was chosen as a predictor of central nervous system (CNS) injuries based on its association with diffuse axonal injury (DAI).22,23 It was found that the largest strains occur for lateral and axial rotational impulses and that substantially smaller strains occur for translational impulses. Load curve shape and loading direction were investigated by Post and coworkers (2012) using the UCDBTM by evaluating MPS and von Mises stress.24 The study found that the regions of highest stress and strain varied with impact direction, and that the shape of the load curve influenced the peak value, time to peak, and location of maximum stress and strain. Weaver and coworkers (2012) examined the influence of impact direction using the SIMon model.25 The findings demonstrated differences in strain response with directions of rotation, even when input magnitude was controlled. As the magnitude was held constant, there were substantial variations in the location and volume of the higher strain elements with changes in the direction of rotation. Additionally, large variations in injury risk were observed for Abbreviated Injury Scale (AIS) 1 injuries, suggesting that direction of rotation may be particularly important for prediction of less severe brain injuries.
There is currently no consensus on a universal kinematic-based injury metric predicting concussion or corresponding injury tolerance threshold. One possible reason is that impact kinematics are not directly correlated to the brain deformation response that is thought to contribute to injury. Finite element models (FEMs) allow estimation of tissue-level brain response, which has shown promise in correlating regional brain response to local brain injury and tissue changes, which suggests this may be the way forward to identify a superior metric for brain injury discrimination.18 Anatomic specificity is crucial when evaluating brain deformation in detail. The strength of a metric derived using an FEM with high anatomic accuracy will be an improvement over metrics derived using more simplified models. The objective of the current study is to evaluate local and regional brain response to typical youth football head impacts using an anatomically accurate brain FEM. This study employed real impacts collected over the course of four youth football seasons to characterize the response of the brain at six impact locations using scaled experimental acceleration curves. Representative low, moderate, and high acceleration magnitudes at each impact location were created experimentally and simulated with the atlas-based brain model (ABM). Brain response was evaluated by analyzing the range of MPS and by calculating various strain-related metrics by impact location and direction.
Methods
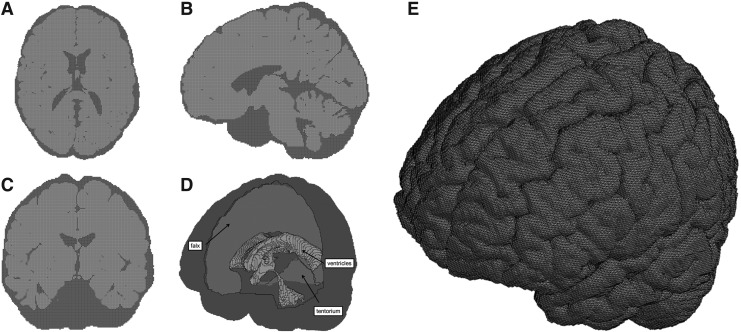
In the current study, a high-resolution, anatomically accurate brain FEM, the ABM, was used to simulate various head impact scenarios at six locations of interest at three magnitudes per location.26 The ABM (Fig. 1) has been validated against brain displacement measurements in five cadaver impact experiments originally conducted by Hardy and coworkers (2001).27,28
FIG. 1.
Axial (A), sagittal (B), and coronal (C) cross sections; an isometric view showing the falx, tentorium, and ventricles (D); and an isometric view of the outer surface of the brain (E) showing sulci and gyri of the atlas-based brain model (ABM).
Impact locations
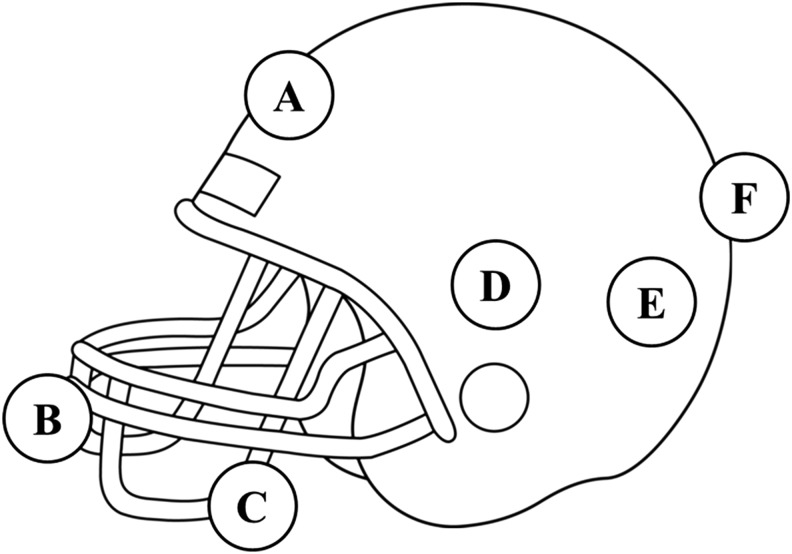
Six impact locations, adapted from Rowson and coworkers (2011) and Beckwith and coworkers (2012), were investigated in the current study (Fig. 2).29,30 Experimental impact tests were conducted on a helmeted Hybrid III (HIII) 50th percentile anthropomorphic test device (ATD) using a pneumatic linear impactor at each of the six impact sites. The kinematic data describing motion of the linear and angular motion of the headform from each test were used to define the boundary conditions of the ABM impact simulations.
FIG. 2.
Data collection
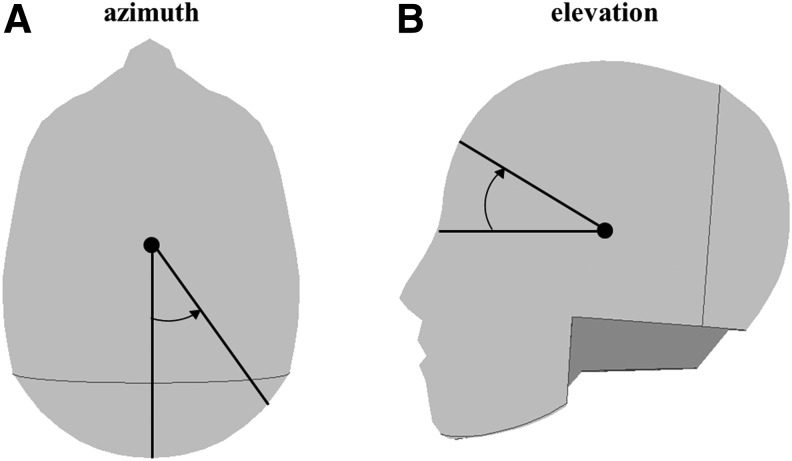
As part of an ongoing study, real-time head impact data were collected during all practices and games for three youth football teams over the course of four seasons. Data collection, previously reported by Kelley and coworkers (2017), was conducted with the Head Impact Telemetry (HIT) System.11 For each impact, the impact location using two angles: azimuth (θ) and elevation (α) was computed. The azimuth angle is measured from the back of the head (θ = 0 degrees) about the z-axis to the front of the head (θ = 180 degrees), and the elevation angle as measured from the nose (α = 0 degrees) to the top of the head (α = 90 degrees) (Fig. 3).
FIG. 3.
Azimuth (A) and elevation (B) angles.
The youth head impact data were analyzed by impact location according to the azimuth and elevation angles for each impact. If the azimuth and elevation angles for a given impact were within 15 degrees of the azimuth and elevation angles of one of the six impact locations of interest, that impact was extracted and included in the distribution of impacts at the corresponding impact site.31 From the distribution of extracted impacts at each site, 5th, 50th, and 95th percentile linear and rotational acceleration values were calculated.
FE simulations
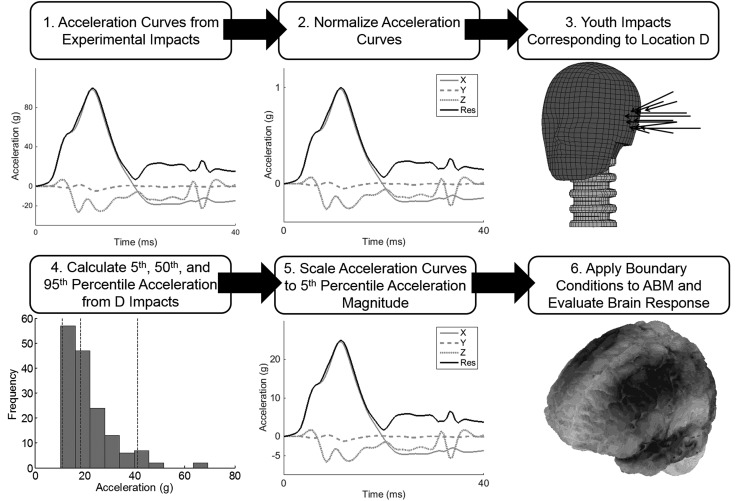
The boundary conditions for each impact were derived from linear impact tests performed with a helmeted HIII ATD head phantom and neck at the six impact locations of interest (Fig. 2).29,30 For each impact location, the linear and rotational acceleration curves output by the HIII ATD were scaled to the corresponding magnitudes to represent the 5th, 50th, and 95th percentile impacts. This resulted in three simulations per impact location, heretofore referred to as “low,” “moderate,” and “high” magnitude. The acceleration curves were integrated to obtain velocity curves, which were used as boundary conditions to drive the ABM simulations.
An example of the workflow for simulating the 5th percentile impact at location D is shown in Figure 4.
FIG. 4.
Example of work flow for simulating the low magnitude impact at location D.
Evaluating brain response
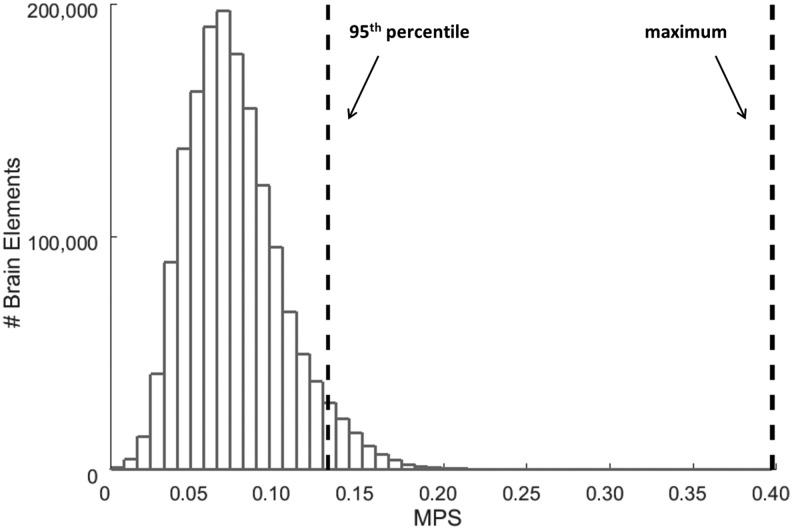
MPS was used to quantify the strain response in the brain using two different response metrics: 95th percentile MPS and cumulative strain damage measure (CSDM).32 The 95th percentile MPS was calculated at each time point by analyzing the distribution of MPS values in all brain elements and calculating the 95th percentile. This resulted in a curve with a value corresponding to each time point in the simulation. The peak of this curve was taken as the 95th percentile MPS metric. The 95th percentile MPS value was used instead of the maximum MPS value, because the 95th percentile is less sensitive to the extremely large strain values sometimes encountered by a small minority of elements/brain volume in these simulations. It therefore better represents the overall deformation patterns of the brain.33 From Figure 5, which shows the distribution of MPS values for the 1,600,000 brain elements at a single time point, we see how much the maximum value and 95th percentile value can differ from each other.
FIG. 5.
Single time point maximum principal strain (MPS) distribution for high magnitude simulation at location A.
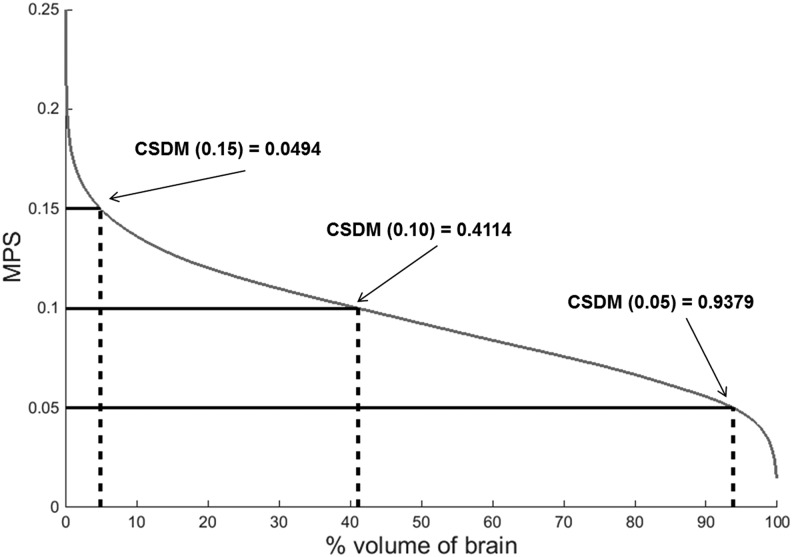
CSDM was calculated using the relationship between MPS for every element and the percentage of brain volume exceeding a given MPS value. This curve was generated by ranking the MPS for each brain element from high to low, and evaluating this versus the percentage of total brain volume (Fig. 6). Specific CSDM values, such as CSDM (0.05), CSDM (0.10), and CSDM (0.15), were calculated from this curve as shown in Figure 6. An additional metric was calculated as the area under the CSDM curve (Fig. 6), called CSDM area.
FIG. 6.
Maximum principal strain (MPS) versus percent volume of brain exceeding given MPS threshold, or cumulative strain damage measure (CSDM) curve. Specific CSDM values are calculated from this curve as shown for CSDM (0.05), CSDM (0.10), and CSDM (0.15).
Finally, the relationships between strain metrics and various kinematic values and brain injury risk measures were investigated. The kinematic values examined were linear acceleration, rotational acceleration, and rotational velocity. The brain injury metrics examined were the concussion correlate (CC) and the head injury criterion (HIC). The CC was derived from a risk curve for concussion as developed by Rowson and coworkers (2013) that accounted for both linear acceleration and rotational acceleration.34,35 CC is calculated using the following function:
 |
where, a is peak linear acceleration (in g), and α is peak rotational acceleration (in rad/sec2); the coefficients were from Rowson and coworkers (2013) was: β0 = −10.2, β1 = 4.33e-02, β2 = 8.73e-4, and β3 = −9.20e-7.34 HIC was calculated according to:
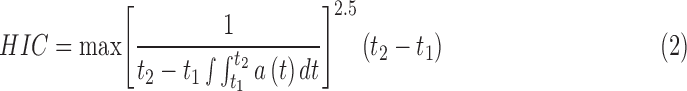 |
where t1 and t2 are any two arbitrary times during the resultant linear acceleration of the head CG and the limit on the HIC time interval (t2 – t1) was 15 ms.36
Statistical analysis
Finally, generalized linear models were used to evaluate the effect of impact characteristics (kinematic values and impact location) on computed strain metrics using SAS software version 9.4 (SAS Institute Inc., Cary, NC).
Results
A total of 40,538 impacts from 119 individual athletes (ages 9–13) were recorded; 6,706 of these were determined to be within 15 degrees of one of the six locations considered in the current study.11 Table 1 displays the linear and rotational accelerations for the low, moderate, and high magnitude impacts by impact location.
Table 1.
Linear Acceleration, Rotational Acceleration, and Rotational Velocity Magnitudes for Simulated Impacts by Location
| Linear acceleration (g) | Rotational acceleration (rad/sec2) | Rotational velocity (rad/sec) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| L | M | H | L | M | H | L | M | H | ||
| A | Front | 17.1 | 31.2 | 67.6 | 657 | 1269 | 2641 | 4.5 | 8.7 | 18.1 |
| B | Facemask | 10.7 | 17.3 | 43.5 | 611 | 1009 | 2432 | 4.9 | 8.1 | 19.6 |
| C | Front boss | 10.3 | 14.8 | 32.1 | 530 | 765 | 1672 | 3.5 | 5.1 | 11.1 |
| D | Side | 11.0 | 18.4 | 41.0 | 671 | 1116 | 2556 | 5.3 | 8.8 | 20.1 |
| E | Oblique | 10.6 | 17.0 | 36.5 | 650 | 1050 | 2188 | 4.7 | 7.7 | 15.9 |
| F | Rear | 11.0 | 18.1 | 51.7 | 668 | 1078 | 3072 | 4.3 | 6.9 | 19.6 |
L, low; M, moderate; H, high.
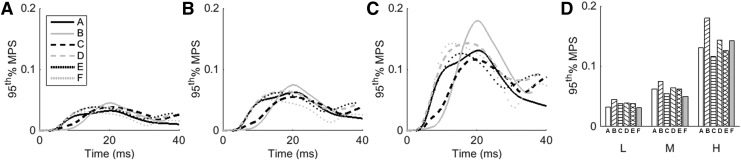
The 95th percentile MPS curves over time are displayed inFigure 7 for each of the six impact locations at the three simulated magnitudes. The 95th percentile MPS values were calculated from the peaks of these curves (Fig. 7D). The 95th percentile MPS values corresponding to the low magnitude impacts ranged from 0.031 to 0.045. Similarly, the 95th percentile MPS ranges for the moderate and high magnitude impacts were 0.050–0.075 and 0.116–0.180. On average, the 95th percentile MPS value in the moderate impact was 64.8% larger than the corresponding value for the low impact, and the 95th percentile MPS value for the high magnitude impact was 128.8% larger than the corresponding moderate value.Figure 8 shows the strain contours at the time of maximum MPS for the high (95th percentile) magnitude impacts at each location.
FIG. 7.
Ninety-fifth percentile maximum principal strain (MPS) values over time at each impact location for low (A), moderate (B), and high (C) magnitude impacts, and the maximum values from these curves (D).
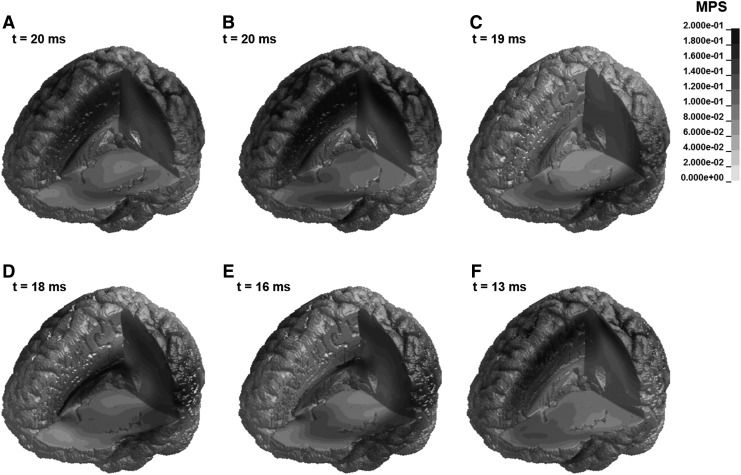
FIG. 8.
Maximum principal strain (MPS) contours within the brain at the time of maximum MPS for the high magnitude impacts.
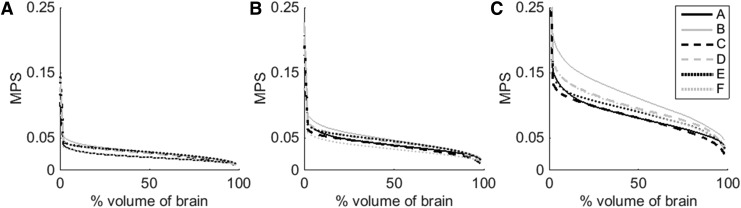
At all six impact locations and for each of the magnitudes, CSDM values were computed from the relationship between MPS and the percent volume of brain exceeding the corresponding MPS value (Fig. 9). CSDM values indicating the volume of brain exceeding MPS thresholds of 0.05, 0.10, and 0.15 were calculated and are referred to as CSDM (0.05), CSDM (0.10), and CSDM (0.15), respectively (Table 2).
FIG. 9.
Maximum principal strain (MPS) versus percent volume of brain exceeding given MPS value for low (A), moderate (B), and high (C) magnitude impacts.
Table 2.
Cumulative Strain Damage Measure (CSDM) Values
| CSDM (0.05) | CSDM (0.10) | CSDM (0.15) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| L | M | H | L | M | H | L | M | H | |
| A | 0.158 | 19.333 | 91.239 | 0.000 | 0.111 | 23.375 | 0.000 | 0.002 | 2.273 |
| B | 2.604 | 40.715 | 97.161 | 0.002 | 0.366 | 59.965 | 0.000 | 0.004 | 17.136 |
| C | 0.556 | 16.896 | 86.458 | 0.003 | 0.052 | 23.170 | 0.000 | 0.002 | 0.617 |
| D | 1.038 | 31.182 | 93.755 | 0.011 | 0.323 | 43.061 | 0.000 | 0.026 | 4.845 |
| E | 0.519 | 33.882 | 92.536 | 0.002 | 0.114 | 34.699 | 0.000 | 0.004 | 1.477 |
| F | 0.066 | 7.267 | 93.789 | 0.000 | 0.004 | 41.142 | 0.000 | 0.000 | 4.945 |
CSDM values correspond to % volume of brain >0.05, 0.10, or 0.15 maximum principal strain (MPS).
L, low; M, moderate; H, high.
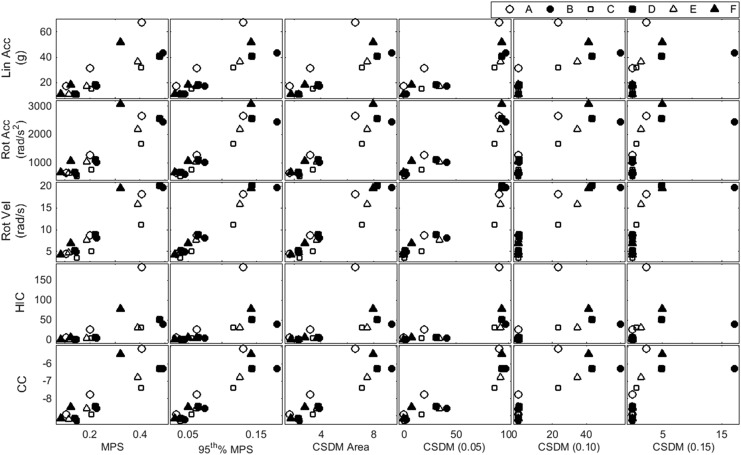
To further investigate the relationship among impact location, kinematics, and brain response, the relationships between kinematic measures and various tissue-level strain metrics were investigated (Fig. 10). The kinematic metrics considered are linear acceleration, rotational acceleration, rotational velocity, HIC, and CC, and the strain metrics are max MPS, 95th percentile MPS, CSDM area, CSDM (0.05), CSDM (0.10), and CSDM (0.15). Statistical analysis indicated that rotational velocity (p = 0.007) and impact location (p = 0.005) both had a significant effect on 95th percentile MPS, with location (η2 = 0.101) explaining slightly more of the variance than rotational velocity (η2 = 0.033). Linear and rotational acceleration both did not have a significant effect on MPS (p = 0.370 and p = 0.086, respectively).
FIG. 10.
Scatter matrix showing relationships between kinematic and tissue-level strain metrics. The five kinematic metrics considered are linear acceleration, rotational acceleration, rotational velocity, head injury criterion (HIC), and concussion correlate (CC); the six strain metrics are max maximum principal strain (MPS), 95th percentile MPS, cumulative strain damage measure (CSDM) area, CSDM (0.05), CSDM (0.10), and CSDM (0.15).
Discussion
In this study, youth football head impacts representing the 5th, 50th, and 95th percentile impacts at six impact locations were reconstructed in the laboratory and simulated using a high resolution FEM. A total of 18 impacts were simulated with the ABM (three magnitudes at six locations). The acceleration magnitudes were calculated using a data set of real-time head impacts measured from three youth football teams over the course of four seasons. Acceleration curves corresponding to each impact location were obtained from experimental linear impact testing and scaled to the corresponding 5th, 50th, and 95th percentile acceleration values. Brain response was evaluated by impact location using MPS based metrics.
The number of impacts corresponding to a single impact location ranged from 158 (location D) to 4084 (location A) (Table 1). The range in frequency indicates that some locations considered in the current study (A, B, F) occur at a higher frequency than others (C, D, E). The locations with the highest impact frequency were located on the front/top of the helmet (A and B). This finding agrees with reported results for this age range.10–12 Other studies that use the HIT System use four classifications for impact location: front, side, rear, and top.10–12 In this classification, the top region is defined as elevation angles >65 degrees. For all elevation angles <65 degrees, the head is divided into four equally spaced bins centered on the midsagittal and coronal planes.37 Impacts to the right and left side are grouped together as side impacts. All of the impact locations considered in the current study do not fall into a single classification as defined by the HIT System. For example, the elevation angles for location A range from 42 degrees to 72 degrees, which means that some impacts associated with location A would be classified as “top” and some as “front.” Although the four HIT System classifications do not correspond directly to the impact locations considered in the current study, some comparisons can still be made. In the current study, the largest linear acceleration impacts occurred at location A for all three impact magnitudes. The largest rotational acceleration impacts occurred at location F for the low and high magnitude simulations, and at location A for the moderate condition. The location experiencing the highest linear acceleration is consistently reported as “top,” whereas the largest rotational accelerations occur at the front in some youth impact studies and on the side in others.11,12,38
Figure 7 shows that both the peak 95th percentile MPS and the timing of the peak value vary by impact location. Location B, a facemask impact, consistently results in the largest 95th percentile MPS value, whereas the minimum value occurs at location F (rear) in the low and moderate simulations and at location C (front boss) in the high magnitude simulation. The peak 95th percentile MPS values range from 0.031 to 0.045 for the low magnitude impacts, from 0.050 to 0.075 for intermediate magnitude impacts, and from 0.116 to 0.180 for the high magnitude impacts. Similarly, the MPS contours shown in Figure 8 indicate that the location of maximum MPS varies by impact location. The location resulting in the largest volume of high strain is location B (facemask), which agrees with the results in Table 2. For impacts with primarily anterior-posterior motion (A, B, and F), the maximum strains occur in the frontoparietal region of the brain. For locations D and E, which are side impacts with lateral components, the peak strains occur closer to the ventricles, inferior to the location of peak strain for impacts at locations A, B, and F.
Previous studies have reported MPS values similar to those found in the current study. Darling and coworkers (2016) reported maximum principal strains ranging from 0.023 to 0.088 for two typical football head impacts, which is similar to the results found in the current study at the low and moderate impacts.17 One of the concussion impact cases evaluated by McAllister and coworkers (2011) had similar peak impact kinematics to the high magnitude impact at location D (side). The corresponding maximum principal strain reported by McAllister and coworkers was 0.144, which agrees with the value of 0.143 found in the current study for a similar impact.18 Similarly, the high magnitude impact at location C (front boss) had kinematics similar to one of the conditions studied by Zanetti and coworkers (2013), who reported a peak MPS value of 0.084.19 This is comparable to the strain response (peak MPS value of 0.1161) observed in the current study for the location C impact.
Figure 9 shows that location B (facemask) results in the largest CSDM values. CSDM values using strain thresholds of 0.05, 0.10, and 0.15 MPS were determined from the curves shown in Figure 9 (Table 2). A limitation of threshold-based CSDM is that it tends to censor information about impacts below the chosen threshold, which may or may not be important in subconcussive impacts. This was the case for CSDM in the current study, as demonstrated by 0.05 being the only strain threshold to yield values greater than zero for low magnitude impacts. Therefore, this threshold (0.05) was used to further analyze the relationship to impact characteristics. Although CSDM (0.05) is not typically associated with brain injury, it is still a valuable tool for comparing response and severity of impacts and for looking at subconcussive brain deformations.
The relationship between brain response and impact characteristics, as well as overall trends by impact location, can be further examined using the strain and CSDM metrics reported in Figure 7 and Table 2, respectively (Fig. 10) At a given impact location, increasing acceleration or velocity magnitude corresponds to a higher strain metric, but relative magnitudes are not consistent among different impact locations. For example, lower kinematic values at the facemask are associated with higher CSDM (0.05) values relative to frontal and rear impacts.
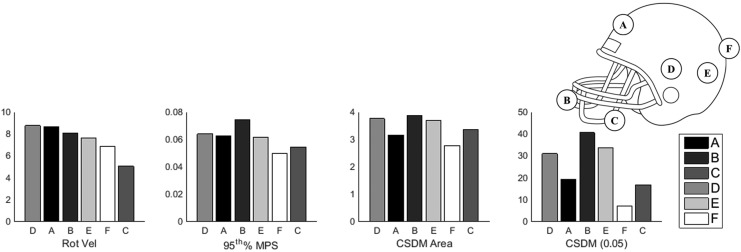
This study highlights the importance of accounting for directional dependence when evaluating head impact response. Figure 11 shows selected impact characteristics – rotational velocity, 95th percentile MPS, CSDM area, and CSDM (0.05) – for all impact locations at the moderate impact magnitude. The impact characteristics shown in Figure 11 are all plotted in order of decreasing rotational velocity. If these impacts were evaluated without considering impact direction, it would likely be concluded that impact D, a lateral impact, was the most severe, because the impact at that location has the highest peak rotational velocity magnitude. Looking at the brain deformation metrics, which account for variation in primary axis of rotation among impacts at different locations, we see that the response at location B, at the facemask, resulted in the highest strains.
FIG. 11.
Selected impact characteristics for the high magnitude impact, sorted by decreasing rotational velocity.
Limitations of the current study include errors associated with experimental data collection methods as well as estimation of boundary conditions (impact direction and magnitude). There is error associated with calculating impact characteristics using the HIT System. Beckwith and coworkers tested the HIT System with the head of a 50th percentile HIII ATD, and found that the HIT System overestimated peak linear acceleration by 0.9% and underestimated peak rotational acceleration by 6.1%.30 The estimation of the boundary conditions is another limitation of this study, as the linear and angular acceleration curve shapes were presumed from experimental impact results. The authors have determined, however, that resulting strain uncertainty caused by uncertainty of the impact direction and magnitude of loading is low. Linear and rotational acceleration values associated with the 5th, 50th, and 95th percentile impacts remain relatively consistent when varying impact locations by ±5 degrees, ±10 degrees, ±15 degrees, and ±20 degrees. For the low magnitude impacts, there is a maximum difference in linear/rotational acceleration of 0.88g/71.3 rad/sec2. Similarly, there is very little variation for the medium impacts, with maximum difference in linear/rotational acceleration of 1.80g/86.4 rad/sec2. This variation is much smaller than the difference between low and medium magnitudes, which has an average difference in linear and rotational acceleration of 6.2g and 361.5 rad/sec2, respectively. For the high magnitude impacts there is slightly more variation, with a maximum difference in linear/rotational acceleration of 9.35g/558.2 rad/sec2. Even though there is more variation associated with the high magnitude impacts, the variation is still much smaller than the difference between medium and high magnitudes, which has an average difference in linear and rotational acceleration of 24.0g and 1396.5 rad/sec2, respectively. Although we would expect strain results to vary with the different acceleration magnitudes associated with varying impact locations (± 5 degrees, ±10 degrees, ±15 degrees, and ±20 degrees), the variation in strains would be much smaller than those associated with changes among the low, medium, and high magnitude cases. For example, the change in strain associated with varying the linear acceleration magnitude by 3.8g is 0.0001 and that associated with varying the rotational acceleration magnitude by 266.2 rad/sec2 is 0.0002. We can therefore conclude that impact location plays a greater role in the strain variability than impact magnitude at the same percentile impact.
A challenge in brain injury prediction is that there is no consensus on a kinematic-based injury metric or a corresponding concussion tolerance threshold. This may be because impact kinematics are not directly correlated to the response that causes injury. Another possibility is that the current kinematic-based metrics do not account for region-specific mechanical responses and deformations. FEMs allow estimation of tissue-level brain response, which has shown promise in correlating regional brain response to local brain injury and tissue changes, suggesting that this may be the way forward to identify more useful brain injury metrics.39 The current study evaluates local and regional brain response to typical youth football head impacts using the ABM, an anatomically accurate brain FEM. This study demonstrates that MPS varies by impact location and highlights the importance of accounting for directional dependence when evaluating head impact response. Accounting for impact direction by evaluating strain response, the location that resulted in the largest strains was location B, a frontal impact through the facemask. The locations that resulted in the lowest strains were location C, a side impact, and F, a rear impact. Higher strains resulted from a 40g lateral impact with two predominant axes of rotation than from a 70g frontal impact with one predominant axis of rotation. This finding highlights the importance of accounting for directional dependence and relative contribution of axes of rotation when evaluating head impact response. Quantifying the relationship among impact location, impact magnitude, and brain deformation will aid researchers, clinicians, and equipment manufacturers in brain injury prevention.
Acknowledgments
Research reported in this article was supported by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under Award Numbers R01NS094410 and R01NS082453. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors give special thanks to the Childress Institute for Pediatric Trauma at Wake Forest Baptist Medical Center for providing support for this study. The authors also thank the youth football league's coordinators, coaches, parents, athletes, and athletic trainer whose support made this study possible. Finally, the authors thank Amanda Dunn, Matt Bennett, Eliza Szuch, Danielle Rocheleau, Joeline Kane, Katie Fabian, Ana Katsafanas, Megan Anderson, and Leslie Hoyt for their valuable assistance in data collection.
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Bryan M.A., Rowhani-Rahbar A., Comstock R.D., and Rivara F. (2016). Sports-and recreation-related concussions in US youth. Pediatrics 138, pii: [DOI] [PubMed] [Google Scholar]
- 2. Rosenthal J.A., Foraker R.E., Collins C.L., and Comstock R.D. (2014). National high school athlete concussion rates from 2005–2006 to 2011–2012. Am. J. Sports Med. 42, 1710–1715 [DOI] [PubMed] [Google Scholar]
- 3. Stamm J.M., Koerte I.K., Muehlmann M., Pasternak O., Bourlas A.P., Baugh C.M., Giwerc M.Y., Zhu A., Coleman M.J., and Bouix S. (2015). Age at first exposure to football is associated with altered corpus callosum white matter microstructure in former professional football players. J. Neurotrauma 32, 1768–1776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stamm J.M., Bourlas A.P., Baugh C.M., Fritts N.G., Daneshvar D.H., Martin B.M., McClean M.D., Tripodis Y., and Stern R.A. (2015). Age of first exposure to football and later-life cognitive impairment in former NFL players. Neurology 84, 1114–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stern R.A., Riley D.O., Daneshvar D.H., Nowinski C.J., Cantu R.C., and McKee A.C. (2011). Long-term consequences of repetitive brain trauma: chronic traumatic encephalopathy. PM R 3, S460–S467 [DOI] [PubMed] [Google Scholar]
- 6. Montenigro P.H., Alosco M.L., Martin B., Daneshvar D.H., Mez J., Chaisson C., Nowinski C.J., Au R., McKee A.C., and Cantu R.C. (2016). Cumulative head impact exposure predicts later-life depression, apathy, executive dysfunction, and cognitive impairment in former high school and college football players. J. Neurotrauma 34, 328–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alosco M.L., Tripodis Y., Jarnagin J., Baugh C.M., Martin B., Chaisson C.E., Estochen N., Song L., Cantu R.C., and Jeromin A. (2017). Repetitive head impact exposure and later-life plasma total tau in former National Football League players. Alzheimers Dement. Diagn. Assess. Dis. Monit. 7, 33–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Broglio S.P., Sosnoff J.J., Shin S., He X., Alcaraz C., and Zimmerman J. (2009). Head impacts during high school football: a biomechanical assessment. J. Athl. Train. 44, 342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Duma S.M., Manoogian S.J., Bussone W.R., Brolinson P.G., Goforth M.W., Donnenwerth J.J., Greenwald R.M., Chu J.J., and Crisco J.J. (2005). Analysis of real-time head accelerations in collegiate football players. Clin. J. Sport Med. 15, 3–8 [DOI] [PubMed] [Google Scholar]
- 10. Urban J.E., Davenport E.M., Golman A.J., Maldjian J.A., Whitlow C.T., Powers A.K., and Stitzel J.D. (2013). Head impact exposure in youth football: high school ages 14 to 18 years and cumulative impact analysis. Ann. Biomed. Eng. 41, 2474–2487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kelley M., Urban J., Miller L., Jones D., Espeland M., Davenport E., Whitlow C., Maldjian J., and Stitzel J. (2017). Head impact exposure in youth football: comparing age and weight based levels of play. J Neurotrauma 34, 1939–1947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cobb B.R., Urban J.E., Davenport E.M., Rowson S., Duma S.M., Maldjian J.A., Whitlow C.T., Powers A.K., and Stitzel J.D. (2013). Head impact exposure in youth football: elementary school ages 9–12 years and the effect of practice structure. Ann. Biomed. Eng. 41, 2463–2473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ji S., Zhao W., Li Z., and McAllister T.W. (2014). Head impact accelerations for brain strain-related responses in contact sports: a model-based investigation. Biomech. Model. Mechanobiol. 13, 1121–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang L., Yang K.H., and King A.I. (2004). A proposed injury threshold for mild traumatic brain injury. J. Biomech. Eng. 126, 226–236 [DOI] [PubMed] [Google Scholar]
- 15. Kleiven S. (2007). Predictors for traumatic brain injuries evaluated through accident reconstructions. Stapp Car Crash J. 51, 81–114 [DOI] [PubMed] [Google Scholar]
- 16. Smith T.A., Halstead P.D., McCalley E., Kebschull S.A., Halstead S., and Killeffer J. (2015). Angular head motion with and without head contact: implications for brain injury. Sports Eng. 18, 165–175 [Google Scholar]
- 17. Darling T., Muthuswamy J., and Rajan S. (2016). Finite element modeling of human brain response to football helmet impacts. Comput. Methods Biomech. Biomed. Engin. 19, 1432–1442 [DOI] [PubMed] [Google Scholar]
- 18. McAllister T.W., Ford J.C., Ji S., Beckwith J.G., Flashman L.A., Paulsen K., and Greenwald R.M. (2012). Maximum principal strain and strain rate associated with concussion diagnosis correlates with changes in corpus callosum white matter indices. Ann. Biomed. Eng. 40, 127–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zanetti K., Post A., Karton C., Kendall M., Hoshizaki T.B., and Gilchrist M.D. (2013). Identifying injury characteristics for three player positions in American football using 8>physical and finite element modeling reconstructions. Int. Res. Counc. Biomech. Inj. IRCOBI, 525–535 [Google Scholar]
- 20. Zhang L., Yang K.H., and King A.I. (2001). Comparison of brain responses between frontal and lateral impacts by finite element modeling. J. Neurotrauma 18, 21–30 [DOI] [PubMed] [Google Scholar]
- 21. Gennarelli T.A., Thibault L.E., Adams J.H., Graham D.I., Thompson C.J., and Marcincin R.P. (1982). Diffuse axonal injury and traumatic coma in the primate. Ann. Neurol. 12, 564–574 [DOI] [PubMed] [Google Scholar]
- 22. Kleiven. (2006). Evaluation of head injury criteria using a finite element model validated against experiments on localized brain motion, intracerebral acceleration, and intracranial pressure. Int. J. Crashworthiness 11, 65–79 [Google Scholar]
- 23. Margulies S.S., and Thibault L.E. (1992). A proposed tolerance criterion for diffuse axonal injury in man. J. Biomech. 25, 917–923 [DOI] [PubMed] [Google Scholar]
- 24. Post A., Hoshizaki B., and Gilchrist M.D. (2012). Finite element analysis of the effect of loading curve shape on brain injury predictors. J. Biomech. 45, 679–683 [DOI] [PubMed] [Google Scholar]
- 25. Weaver A.A., Danelson K.A., and Stitzel J.D. (2012). Modeling brain injury response for rotational velocities of varying directions and magnitudes. Ann. Biomed. Eng. 40, 2005–2018 [DOI] [PubMed] [Google Scholar]
- 26. Miller L.E., Urban J.E., and Stitzel J.D. (2016). Development and validation of an atlas-based finite element brain model. Biomech. Model Mechan. 15, 1201–1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Miller L., Urban J., and Stitzel J. (2017). Validation performance comparison for finite element models of the human brain. Comput. Methods Biomech. Biomed. Engin. 20, 1273–1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hardy W.N., Foster C.D., Mason M.J., Yang K.H., King A.I., and Tashman S. (2001). Investigation of head injury mechanisms using neutral density technology and high-speed biplanar x-ray. Stapp Car Crash J. 45, 337–368 [DOI] [PubMed] [Google Scholar]
- 29. Rowson S., Beckwith J.G., Chu J.J., Leonard D.S., Greenwald R.M., and Duma S.M. (2011). A six degree of freedom head acceleration measurement device for use in football. J. Appl. Biomech. 27, 8–14 [DOI] [PubMed] [Google Scholar]
- 30. Beckwith J.G., Greenwald R.M., and Chu J.J. (2012). Measuring head kinematics in football: correlation between the head impact telemetry system and Hybrid III headform. Ann. Biomed. Eng. 40, 237–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sproule D.W., Campolettano E.T., and Rowson S. (2017). Football helmet impact standards in relation to on-field impacts. Proc. Inst. Mech. Eng. P J. Sports Eng. Technol. 231, 317–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Takhounts E.G., Eppinger R.H., Campbell J.Q., Tannous R.E., Power E.D., and Shook L.S. (2003). On the development of the SIMon finite element head model. Stapp Car Crash J. 47, 107–133 [DOI] [PubMed] [Google Scholar]
- 33. Panzer M.B., Myers B.S., Capehart B.P., and Bass C.R. (2012). Development of a finite element model for blast brain injury and the effects of CSF cavitation. Ann. Biomed. Eng. 40, 1530–1544 [DOI] [PubMed] [Google Scholar]
- 34. Rowson S., and Duma S.M. (2013). Brain injury prediction: assessing the combined probability of concussion using linear and rotational head acceleration. Ann. Biomed. Eng. 41, 873–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Laituri T.R., El-Jawahri R.E., Henry S., and Sullivan K. (2015). Field-based assessments of various AIS2+ head risk curves for frontal impact. SAE Technical Paper
- 36. Eppinger R., Sun E., Bandak F., Haffner M., Khaewpong N., Maltese M., Kuppa S., Nguyen T., Takhounts E., and Tannous R. (1999). Development of improved injury criteria for the assessment of advanced automotive restraint systems–II. Natl. Highw. Traffic Saf. Adm. 1–70 [Google Scholar]
- 37. Greenwald R.M., Gwin J.T., Chu J.J., and Crisco J.J. (2008). Head impact severity measures for evaluating mild traumatic brain injury risk exposure. Neurosurgery 62, 789–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Daniel R.W., Rowson S., and Duma S.M. (2012). Head impact exposure in youth football. Ann. Biomed. Eng. 40, 976–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ji S., and Zhao W. (2014). A pre-computed brain response atlas for instantaneous strain estimation in contact sports. Ann. Biomed. Eng. 43, 1877–1895 [DOI] [PMC free article] [PubMed] [Google Scholar]