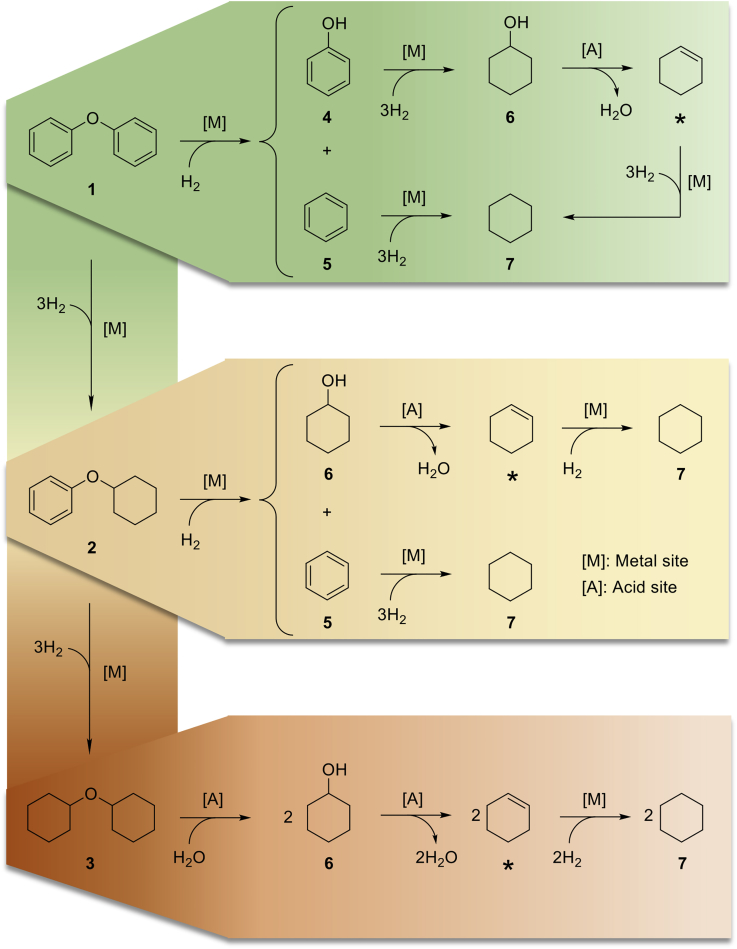
Figure 5.
Reaction Pathways Proposed for the Hydrotreating of Diphenyl Ether into Cyclohexane in the Presence of a Bifunctional Catalyst ([M] and [A] Stand for Metal and Acid Sites, Respectively)
In the reaction network, three ether linkages, with distinct reactivities toward hydrogenolysis catalyzed by Ni sites, are formed. The progressive saturation of diphenyl ether reduces the reactivity of the ether linkage toward hydrogenolysis over Ni sites (He et al., 2012). Noteworthy, Ni catalysts are typically inactive for the cleavage of dialkyl ethers. Thereby, the hypothetical pathway for the HDO of dicyclohexyl ether will require acid sites to start and proceed via hydrolysis → dehydration → hydrogenation to yield cyclohexane. This pathway is, however, not followed under the reaction conditions of this study. In this manner, dicyclohexyl ether accumulated in the reaction mixtures. For clarity, cyclohexene was not numbered but indicated by an asterisk, as this intermediate is consumed upon its formation so that it is not detected in the reaction mixtures.