Recent studies showed that deletion or mutation of members of the YggS protein family causes pleiotropic effects in many organisms. Little is known about the causes, mechanisms, and consequences of these diverse phenotypes. It was previously shown that yggS mutations in E. coli result in the accumulation of PNP and some metabolites in the Ile/Val biosynthetic pathway. This work revealed that some exogenous stresses increase the aberrant accumulation of PNP in the yggS mutant. In addition, the current report provides evidence indicating that some, but not all, of the phenotypes of the yggS mutant in E. coli are due to the elevated PNP level. These results will contribute to continuing efforts to determine the molecular functions of the members of the YggS protein family.
KEYWORDS: PLPBP, PROSC, pyridoxal 5'-phosphate, pyridoxine 5'-phosphate, YggS, amino acid biosynthesis, vitamin B6
ABSTRACT
Escherichia coli YggS (COG0325) is a member of the highly conserved pyridoxal 5′-phosphate (PLP)-binding protein (PLPBP) family. Recent studies suggested a role for this protein family in the homeostasis of vitamin B6 and amino acids. The deletion or mutation of a member of this protein family causes pleiotropic effects in many organisms and is causative of vitamin B6-dependent epilepsy in humans. To date, little has been known about the mechanism by which lack of YggS results in these diverse phenotypes. In this study, we determined that the pyridoxine (PN) sensitivity observed in yggS-deficient E. coli was caused by the pyridoxine 5′-phosphate (PNP)-dependent overproduction of Val, which is toxic to E. coli. The data suggest that the yggS mutation impacts Val accumulation by perturbing the biosynthetic of Thr from homoserine (Hse). Exogenous Hse inhibited the growth of the yggS mutant, caused further accumulation of PNP, and increased the levels of some intermediates in the Thr-Ile-Val metabolic pathways. Blocking the Thr biosynthetic pathway or decreasing the intracellular PNP levels abolished the perturbations of amino acid metabolism caused by the exogenous PN and Hse. Our data showed that a high concentration of intracellular PNP is the root cause of at least some of the pleiotropic phenotypes described for a yggS mutant of E. coli.
IMPORTANCE Recent studies showed that deletion or mutation of members of the YggS protein family causes pleiotropic effects in many organisms. Little is known about the causes, mechanisms, and consequences of these diverse phenotypes. It was previously shown that yggS mutations in E. coli result in the accumulation of PNP and some metabolites in the Ile/Val biosynthetic pathway. This work revealed that some exogenous stresses increase the aberrant accumulation of PNP in the yggS mutant. In addition, the current report provides evidence indicating that some, but not all, of the phenotypes of the yggS mutant in E. coli are due to the elevated PNP level. These results will contribute to continuing efforts to determine the molecular functions of the members of the YggS protein family.
INTRODUCTION
Escherichia coli YggS (COG0325) is a member of the highly conserved pyridoxal 5′-phosphate (PLP)-binding protein (PLPBP) family whose biochemical function is unknown (1–3). The YggS protein family is present in all three domains of life, suggesting a highly conserved and important function of this protein family. The crystal structures of YggS protein family members, including E. coli YggS (PDB identifier [ID]: 1W8G), Saccharomyces cerevisiae Ybl036c (PDB ID: 1CT5), and Synechococcus elongatus PCC 7942 PipY (PDB ID: 5NM8), demonstrated that the proteins bind to PLP via Schiff base linkage with a lysine residue and exhibit structural similarity to the N-terminal domain of bacterial alanine racemase (ALR) and eukaryotic ornithine decarboxylase (ODC), both of which are classified as fold type III PLP enzymes (4, 5). Unlike most other PLP-dependent enzymes, the YggS protein family members exist as monomeric and single-domain proteins, and the re-face of PLP is solvent exposed.
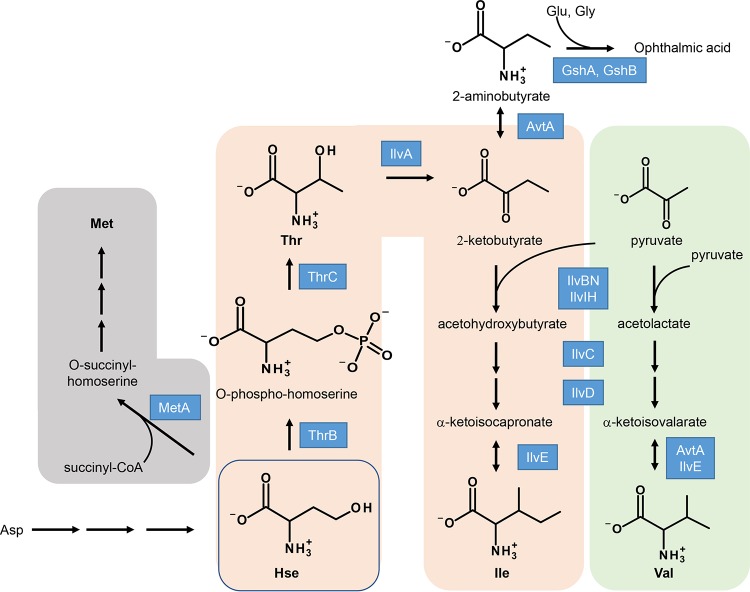
Although no biochemical function has yet been identified for this protein family, knockout or mutation of this protein family causes pleiotropic phenotypes in multiple organisms. In E. coli, a yggS mutation impacts the metabolism of Ile and Val by altering levels of 2-aminobutyrate (2-AB) and/or 2-ketobutyrate (2-KB) (1) (see Fig. 1). The yggS mutant of E. coli MG1655 accumulates certain metabolites, including 2-AB, 2-KB, γ-glutamyl-2-aminobutyryl-glycine (ophthalmic acid; OA), and Val. The same mutant has decreased coenzyme A content in the log phase and excretes a significant amount of Val in M9 culture medium at the stationary phase during growth at 30°C (1, 2). In contrast, the yggS mutant of E. coli strain BW25113 does not overproduce Val (2, 3), suggesting that the Val overproduction phenotype is a strain-dependent and/or cultivation condition-dependent phenotype. Nichols et al. showed that yggS and glyA, encoding serine hydroxymethyl transferase, are a synthetic lethal pair in E. coli (6). Impaired growth of the yggS glyA double-knockout mutant was also identified in the genome-scale cross of the glyA mutant with the entire E. coli gene deletion collection (7). Later, it was found that the double mutant can grow in a synthetic medium supplemented with Gly (3), which indicated that the gene pair is a conditional lethal mutant. Together, these data suggest that YggS plays a role(s) in the metabolism of Gly and/or C1 units. Recently, involvement of YggS in PLP homeostasis was reported. Prunetti et al. reported that the yggS mutant of E. coli BW25113 accumulates PLP precursor pyridoxine 5′-phosphate (PNP) and is sensitive to excess levels of pyridoxine (PN) (3). Related to this finding, in humans, mutations in PROSC (ortholog of YggS) were identified as the cause of vitamin B6-dependent epilepsy (8, 9). Analysis of cerebrospinal fluid samples from individuals possessing a PROSC mutation showed low PLP levels and reduced PLP-dependent enzyme activity. Those authors suggested that the YggS protein family is involved in the intracellular homeostasis regulation of PLP, while supplying PLP to apo-PLP enzymes and/or minimizing the toxic effects of PLP aldehyde (3, 8). The involvement of this protein family in the homeostasis of vitamin B6 and/or amino acids in cyanobacteria was also suggested. In Synechococcus elongatus, disruption of the YggS ortholog PipY results in sensitivity to d-cycloserine and β-chloro-d-alanine, antibiotics targeting essential PLP-dependent enzymes. In addition, the pipY gene and the putative cysteine synthase cysK gene were identified as the synthetic lethal pair (10).
FIG 1.
Metabolic pathway of Met, Thr, Ile, and Val biosynthesis in E. coli. Homoserine (Hse) is a branch-point metabolite for the synthesis of Met and Thr. 2-Ketobutyrate, precursor for 2-aminobutyrate or Ile, can be produced from Thr by IlvA. Ile is synthesized from 2-ketobutyrate and pyruvate by the action of IlvBN/IlvIH (AHAS), IlvC, IlvD, and IlvE. Val is produced from pyruvate by same enzymes in the Ile biosynthesis. Abbreviations: CoA, coenzyme A; MetA, homoserine O-succinyltransferase; ThrB, homoserine kinase; ThrC, threonine synthase; IlvA, threonine deaminase; AvtA, valine-pyruvate aminotransferase; GshA, glutamate-cysteine ligase; GshB, glutathione synthase; IlvBN, acetohydroxy acid synthase I; IlvIH, acetohydroxy acid synthase III; IlvC, ketol-acid reductoisomerase; IlvD, dihydroxy-acid dehydratase; IlvE, branched-chain-amino-acid aminotransferase.
Although mutation of the YggS family protein results in pleiotropic phenotypes, it is likely to have a highly conserved function. The Val overproduction from the E. coli yggS mutant was complemented by plasmid-borne expression of YggS and orthologs from bacilli (YlmE), yeast (Ybl036cp), and human (PROSC) but not by a YggS mutant lacking PLP-binding ability (1). In addition, the PN sensitivity of the yggS mutant was rescued with the YggS orthologs from Zea mays, Arabidopsis thaliana, S. cerevisiae, and human but not with the PROSC mutants identified in patients with vitamin B6-dependent epilepsy (8). Previous studies had shed light on the importance of YggS protein family in homeostasis of cellular metabolisms; however, little is known about the cause, relevance, and conserved mechanism of these multiple phenotypes.
This study was initiated to probe the PN sensitivity of the yggS mutant of E. coli. We show here that excess PN induces aberrant PNP accumulation in the yggS mutant, which results in the accumulation of Val, which is known to be toxic to E. coli. We further showed that a yggS mutation perturbs the Thr biosynthetic pathway and results in sensitivity to homoserine (Hse). Our data suggest that the aberrant accumulation of PNP perturbs the Thr biosynthetic pathway and then impacts amino acid homeostasis in the yggS mutant. A detailed understanding of each of the phenotypes of yggS, such as that reported here, is critical to facilitate studies to determine the molecular function of the YggS protein family.
RESULTS
Exogenous PN induces PNP accumulation and Val accumulation in the yggS mutant of E. coli.
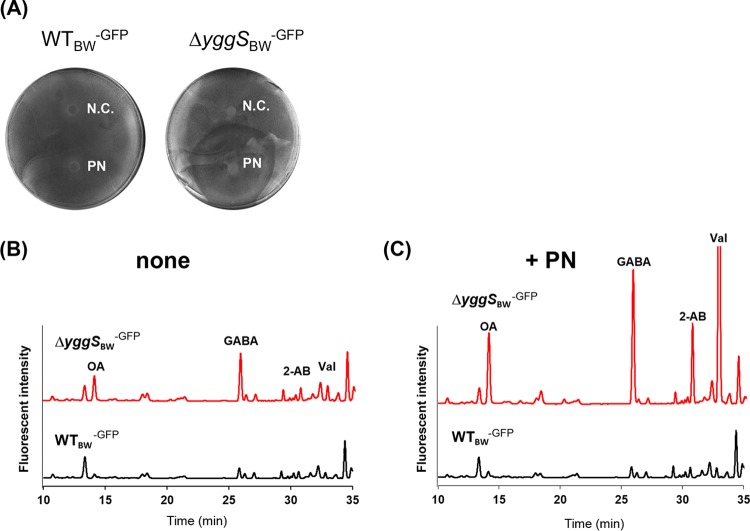
Previous investigation found that an E. coli yggS mutant was sensitive to excess of pyridoxine (PN) on M9 medium (3), but the molecular mechanism underlying the PN sensitivity phenotype has not been described. The metabolic change that occurred in an isogenic pair of strains upon PN supplementation was examined. We cultivated wild-type (WT) and yggS mutant strains of E. coli BW25113 expressing green fluorescent protein (GFP) (strains WTBW-GFP and ΔyggSBW-GFP, respectively) on M9 plates in the presence or absence of PN (1 mM) at 37°C and determined the levels of intracellular B6 vitamers and amino acids in the cells. The GFP was expressed to improve the visualization of E. coli growth on the plate. A growth assay confirmed that the excess level of PN was toxic to the ΔyggSBW-GFP strain (Fig. 2A). B6 pool analyses showed that the ΔyggSBW-GFP mutant accumulated 14 times more PNP in the cells (7.2 ± 1.2 pmol/mg cells) than the WTBW-GFP strain (0.5 ± 0.5 pmol/mg cells) when grown on M9 plates without PN (Table 1). Upon addition of PN, the levels of PNP were significantly elevated in the ΔyggSBW-GFP mutant (19 ± 3 pmol/mg cells), reaching 50 times higher than those seen with the WTBW-GFP strain (0.4 ± 0.4 pmol/mg cells). In the yggS mutant, PLP and PN pools were not influenced by the addition of PN (Table 1). Notably, exogenous PN did not affect any of the intracellular B6 vitamers in strain WTBW-GFP. Amino acid analyses found that the intracellular amino acid pools were slightly different between the two strains when grown on M9 plates without PN. The levels of OA, γ-aminobutyric acid (GABA), 2-AB, and Val were ∼3 times higher in the ΔyggSBW-GFP cells than in the WTBW-GFP cells, while the levels of other amino acids were not significantly different (Fig. 2B). Interestingly, when the ΔyggSBW-GFP strain was grown with PN, the amino acid pools were significantly altered. The ΔyggSBW-GFP strain accumulated substantial amounts of OA, GABA, 2-AB, and Val, whose concentrations were 10, 7, 40, and 10 times higher than those accumulated by the WTBW-GFP strain, respectively (Fig. 2C). These data showed that in the absence of yggS, the presence of exogenous PN significantly increased the intracellular concentrations of PNP, GABA, 2-AB, and Val in E. coli.
FIG 2.
Effect of the excess PN on the growth and intracellular amino acid pool of the yggS mutant. (A) The growth of WTBW-GFP and ΔyggSBW-GFP strains on M9 plates containing 0.1% l-arabinose at 37°C. Filter discs containing distilled water (N.C.) or 1 mM PN (PN) were placed on the plates. Exogenous PN was toxic to strain ΔyggSBW-GFP but not to strain WTBW-GFP. (B and C) Representative HPLC chromatograms of the amino acid analyses. (B) In the absence of PN, the amino acid pools of WTBW-GFP and ΔyggSBW-GFP cells were slightly different. (C) Extracellular PN significantly stimulated the production of ophthalmic acid (OA), γ-aminobutyric acid (GABA), 2-aminobutyrate (2-AB), and Val in the ΔyggSBW-GFP strain.
TABLE 1.
Effect of excess PN on intracellular B6 pool of the yggS mutanta
| Strain | pmol of indicated B6 vitamer/mg cells ± SD |
|||||
|---|---|---|---|---|---|---|
| PLP |
PNP |
PMP |
||||
| No PN | PN | No PN | PN | No PN | PN | |
| E. coli WTBW-GFP | 51 ± 3 | 71 ± 5 | 0.5 ± 0.5 | 0.4 ± 0.4 | 76 ± 2 | 111 ± 6 |
| E. coli ΔyggSBW-GFP | 48 ± 2 | 65 ± 4 | 7.2 ± 1.2 | 19 ± 3 | 188 ± 10 | 209 ± 7 |
| E. coli ΔthrBBW-GFP | 32 ± 7 | 40 ± 3 | 0.3 ± 0.2 | 0.3 ± 0.3 | 56 ± 7 | 89 ± 7 |
| E. coli ΔthrB ΔyggSBW-GFP | 31 ± 2 | 42 ± 6 | 3.9 ± 0.5 | 15 ± 3 | 64 ± 3 | 99 ± 14 |
| E. coli ΔthrCBW-GFP | 24 ± 4 | 32 ± 3 | 0.5 ± 0.01 | 1.1 ± 0.3 | 50 ± 5 | 76 ± 7 |
| E. coli ΔthrC ΔyggSBW-GFP | 23 ± 1 | 30 ± 4 | 3.5 ± 0.3 | 13 ± 2.4 | 56 ± 3 | 86 ± 13 |
E. coli strains were grown on the M9 plate containing 0.1% l-arabinose at 37°C for 16 h in the absence or presence of excess PN (1 mM). Note that the ΔthrBBW-GFP and ΔthrB ΔyggSBW-GFP strains were grown in the presence of 0.4 mM Thr. The ΔthrCBW-GFP and ΔthrC ΔyggSBW-GFP strains were grown with 0.4 mM Thr. The cells were collected by scraping, and the total intracellular B6 vitamers were quantified as described in Materials and Methods. The yggS mutants accumulated PNP, and exogenous PN further elevated the PNP level in the yggS mutant. Data represent means ± standard deviations of results from at least three independent experiments.
Excess PN induces PNP and causes Val toxicity in the yggS mutant.
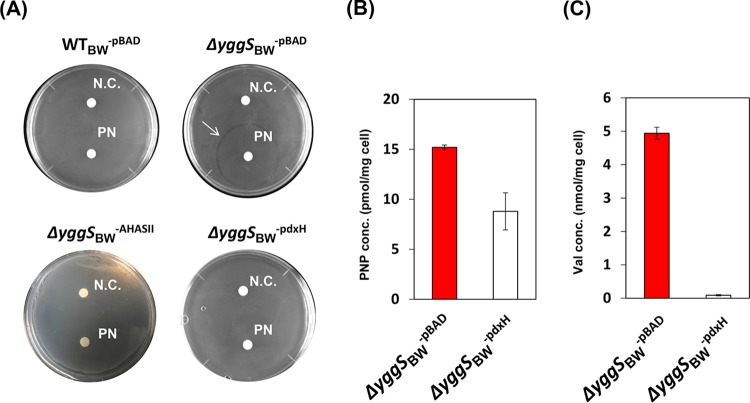
The high concentration of Val in the yggS mutant grown with the excess of PN suggested an explanation for the resulting growth inhibition. The E. coli K-12 strain is sensitive to Val (11–13). Excess Val inhibits the two acetohydroxy acid synthases (AHASs) active in E. coli K-12 strain, which are encoded by ilvBN (AHAS I) and ilvIH (AHAS III). Acetohydroxy acid synthase activity is required for branched-chain amino acid biosynthesis, and inhibition of such activity can lead to the accumulation of the toxic metabolite 2-ketobutyrate (2-KB) (11–13). When a Val-insensitive AHAS (encoded by ilvGM) from Salmonella enterica was expressed in the yggS mutant (strain ΔyggSBW-AHASII), growth on the M9 plate in the presence of PN was restored (Fig. 3A). These data allowed the conclusion that the toxicity of PN to a yggS mutant was due to the increased level of Val.
FIG 3.
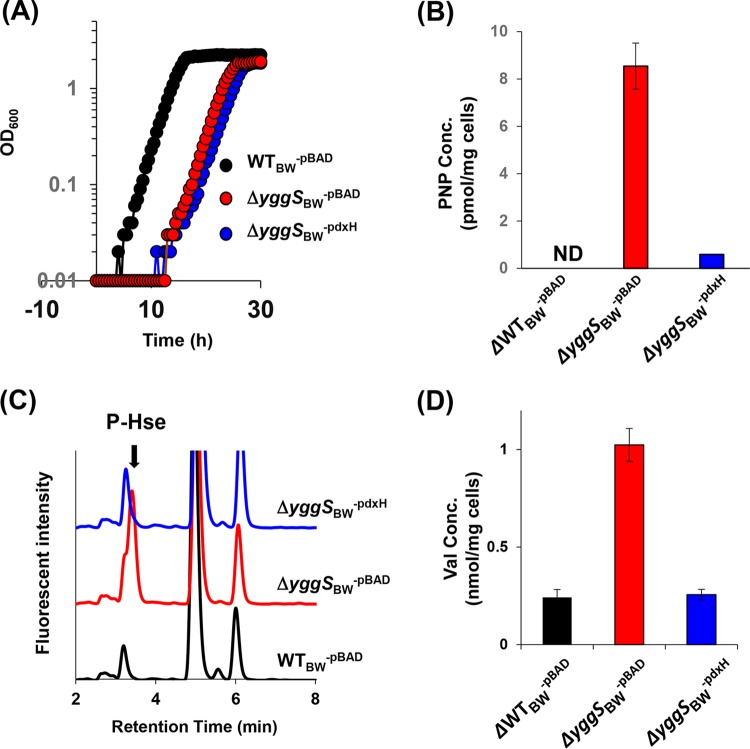
Effect of overexpression of PdxH or AHASII on the PN sensitivity or Val productivity of the yggS mutant. (A) Strain WTBW-pBAD, ΔyggSBW−pBAD, ΔyggSBW-PdxH, or ΔyggSBW-AHASII was grown on M9 plates at 37°C. Filter discs containing distilled water (N.C.) or 1 mM PN (PN) were placed on the plates. The expression of either of AHASII or PdxH in the yggS mutant eliminated the PN sensitivity. (B and C) Effect of pdxH expression on the PNP levels (B) and Val concentrations (C) in the yggS mutant. The ΔyggSBW−pBAD and ΔyggSBW-PdxH strains were cultivated on the M9 plate in the presence of PN at 37°C. Intracellular concentrations of PNP and Val were quantified as described in Materials and Methods. The expression of PdxH significantly decreased the PNP and Val levels in the yggS mutant. Error bars show standard deviations of results from at least three independent experiments.
We considered whether the increased PNP accumulation in a yggS mutant could be responsible for the Val overproduction. Consistently, it was reported previously that an E. coli strain lacking pyridoxine/pyridoxamine 5′-phosphate oxidase (PdxH) excretes Glu and unknown metabolites that trigger Val inhibition (14). PdxH catalyzes the oxidation of PNP (and pyridoxamine phosphate [PMP]) to PLP in the final step of vitamin B6 biosynthesis (15), and therefore the pdxH mutant might accumulate PNP and PMP. We expressed pdxH in the yggS mutant (strain ΔyggSBW-pdxH) in an effort to lower the intracellular PNP concentration and examined the effect on the PN sensitivity and thus Val accumulation. As shown in Fig. 3A, the expression of pdxH eliminated the inhibitory effect of PN on the yggS mutant. As expected, B6 pool analysis showed that expression of pdxH significantly decreased the intracellular PNP levels in the yggS mutant (Fig. 3B). Significantly, expression of pdxH in the yggS mutant resulted in a reduction in the intracellular concentration of Val to the level present in the wild-type strain (Fig. 3C). These data support the conclusion that elevation of the level of intracellular PNP in a yggS mutant causes an increase in Val by an uncharacterized mechanism. The increased Val prevents growth by inhibition of AHAS and the resulting accumulation of 2-KB.
Homoserine (Hse) metabolism is altered in a yggS mutant.
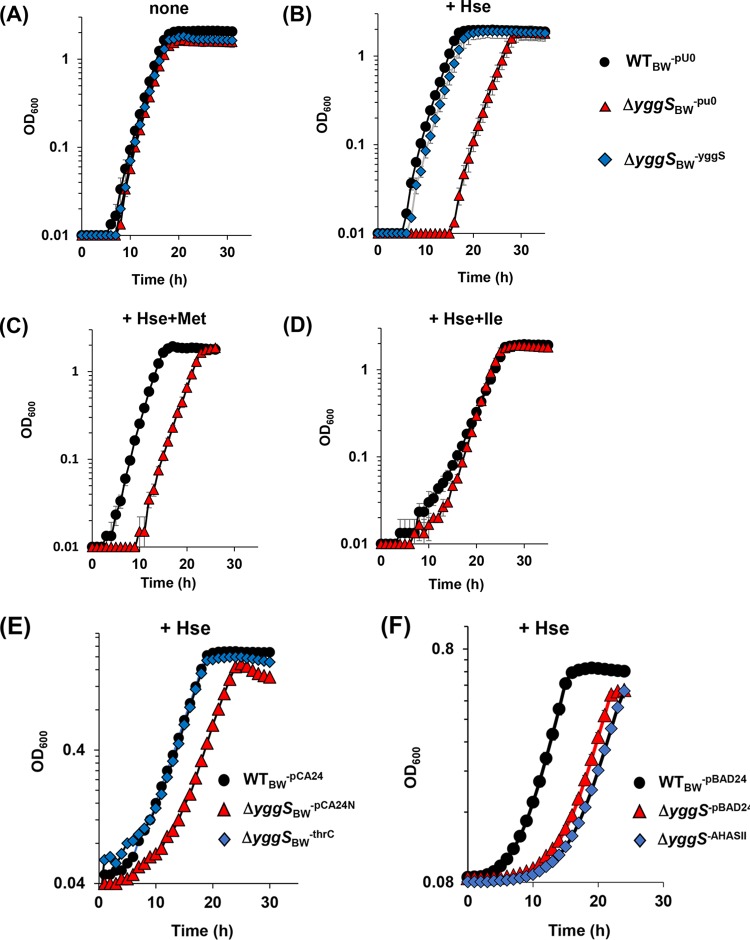
A yggS mutation was shown previously to affect the 2-AB/2-KB metabolic pathway(s) and to result in overproduction of Val in the E. coli cells (1, 2). Those and other observations prompted us to query growth of the yggS mutant in response to several amino acids or keto acids involving in the branched-chain amino acid biosynthesis pathway and related pathways (Thr, 2-AB, 2-KB, L-homoserine [Hse], or Met). The wild-type strain (WTBW-pU0) and the yggS-deficient strain (mutant ΔyggSBW-pU0) had indistinguishable growth rates in M9 medium (Fig. 4A) and in the presence of Thr, 2-AB, 2-KB, or Met (data not shown). In contrast, when 1 mM Hse was added to the M9 medium at 30°C, the ΔyggSBW-pU0 mutant exhibited a nearly 10-h lag before achieving a growth rate similar to that of the WTBW-pU0 strain (Fig. 4B). The duration of the lag was prolonged with an increased Hse concentration in the medium. Plasmid-borne expression of the yggS strain completely eliminated the growth defect caused by Hse, confirming that the phenotype was due to the yggS deletion (Fig. 4B, diamond). Further growth assays showed that the Hse sensitivity of the ΔyggSBW-pU0 mutant was overcome in the presence of exogenous Ile but not in the presence of Met (Fig. 4C and D). Supplementation with 1 mM Thr or 1 mM 2-AB, each of which can be converted to Ile in vivo, also effectively eliminated the Hse sensitivity (data not shown). Surprisingly, when grown at 37°C, the WTBW-pU0 strain and the ΔyggSBW-pU0 mutant were inhibited by exogenous Hse to the same extent (data not shown). These data suggested that the effect of YggS with respect to its role in Hse metabolism is affected by temperature and thus is likely to be an indirect effect. We sought to investigate this phenotype further to better understand the molecular function of YggS.
FIG 4.
Inhibition of the yggS mutant by Hse and its alleviation by thrC overexpression. (A to D) Growth curves of the WTBW-pU0, ΔyggSBW-pU0, and ΔyggSBW-YggS strains in liquid M9 medium without additive (A) or with 1 mM Hse (B), 0.1 mM Met and 1 mM Hse (C), or 0.1 mM Ile and 1 mM Hse (D) at 30°C are represented. (E) Growth of strains WTBW-pCA24N, ΔyggSBW-pCA24N, and ΔyggSBW-ThrC in M9 medium containing 1 mM Hse at 30°. (F) Comparison of the growth levels of strains WTBW-pBAD24, ΔyggSBW-pBAD24, and ΔyggSBW-AHASII in M9 medium containing 1 mM Hse at 30°C. Error bars show standard deviations of results from at least three independent experiments.
Exogenous Hse induces accumulation of metabolites in the Thr/Ile/Val biosynthetic pathway in the yggS mutant.
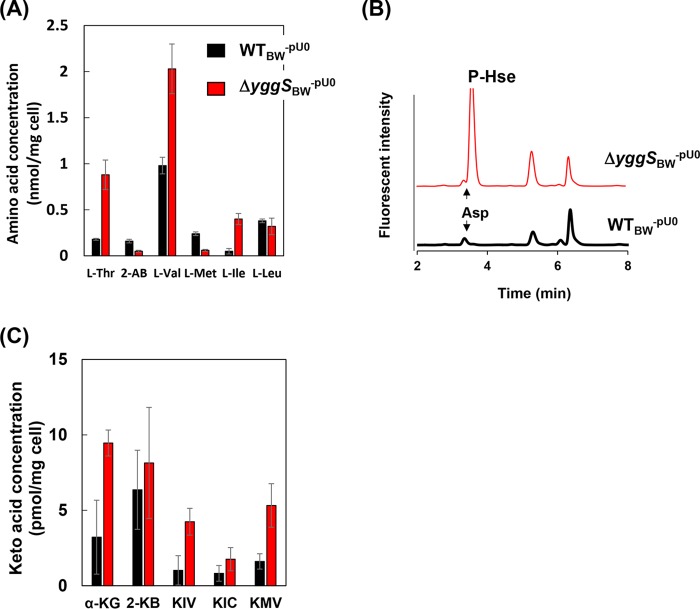
Intracellular concentrations of amino and keto acids were analyzed in the WTBW-pU0 strain and in the ΔyggSBW-pU0 mutant in the presence and absence of Hse. In the absence of Hse, the intracellular amino acid composition of the WTBW-pU0 strain was indistinguishable from that of the ΔyggSBW-pU0 mutant, with the exception of ophthalmic acid (OA), which accumulated to levels approximately 10 times higher in the ΔyggSBW-pU0 mutant than in the WTBW strain (2). In the presence of Hse, the ΔyggSBW-pU0 strain accumulated a significantly more Thr, Val, and Ile in the cells than the wild-type strain, with 5-fold-, 2-fold-, and 8-fold-higher concentrations, respectively (Fig. 5A). In addition, the ΔyggSBW-pU0 strain accumulated a significant amount of l-O-phosphohomoserine (P-Hse) in the cells (Fig. 5B). A significant concentration (∼50 μM) of P-Hse was also detected in the stationary-phase medium with the ΔyggSBW-pU0 strain but not in the cells or in the medium with the WTBW-pU0 strain. In contrast, the levels of 2-AB and Met were slightly lower in the ΔyggSBW-pU0 strain than in the WTBW-pU0 strain (Fig. 5A). The levels of other proteinogenic amino acids were not significantly influenced by the yggS mutation. Results of the keto acid analysis are shown in Fig. 5C. Consistent with the results of the amino acid analyses, when grown in M9 medium supplemented with Hse, the ΔyggSBW-pU0 cells accumulated more α-ketoisovalerate (KIV; precursor of Val), α-ketoisocapronate (KIC; precursor of Ile), α-keto-β-methylvalerate (KMV; precursor of Leu), and α-ketoglutarate than the WTBW-pU0 cells. The results suggest there is increased flux through the biosynthetic pathways for branched-chain amino acids in the yggS mutant.
FIG 5.
Effect of Hse with respect to the metabolites of the Thr, Ile, and Val biosynthetic pathways of the yggS mutant. The intracellular concentrations of amino acids and keto acids of the WTBW-pU0 and ΔyggSBW-pU0 strains grown in M9 medium supplemented with 1 mM Hse at 30°C are shown in panels A and C, respectively. HPLC chromatograms of the amino acid analyses showing P-Hse accumulation in the ΔyggSBW-pU0 strain are shown in panel B. Error bars show standard deviations of results from at least three independent experiments.
The accumulation of P-Hse in the yggS mutant raised the formal possibility that P-Hse was a substrate for YggS. Many PLP-dependent enzymes exhibit a shift of the peak at around 420 nm derived from Schiff base linkage between PLP and the Lys residue of the protein upon substrate binding. Addition of P-Hse to purified YggS did not induce any spectrum changes (Fig. S1). Further, incubation of YggS and P-Hse in the presence or absence of keto acid (pyruvate or α-ketoglutarate) did not cause any changes in P-Hse concentrations in the reaction mixture that would suggest that a catalytic reaction had taken place. These experiments failed to detect any reactivity of YggS with P-Hse, although as negative results they are not definitive. The P-Hse accumulation could suggest that threonine synthase (ThrC) activity is the rate-limiting step in the catabolism of Hse in the yggS mutant. The ThrC activity in the ΔyggSBW strain (5.1 ± 0.2 nmol/min/mg protein) was ∼1.3 times higher than that in the WTBW strain (4.0 ± 0.1 nmol/min/mg protein). These data did not support the idea that the P-Hse accumulation was due to low activity of ThrC in the yggS mutant.
Sensitivity to Hse correlates with P-Hse accumulation.
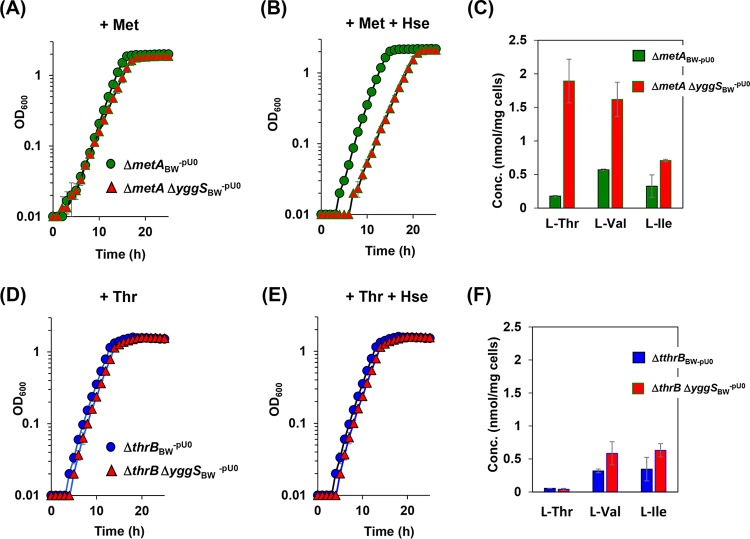
Hse is the branch point intermediate in the pathway for Thr and Met biosynthesis in E. coli (Fig. 1). Homoserine O-succinyltransferase (encoded by metA) and homoserine kinase (encoded by thrB) catalyze the initial step for Met and Thr biosynthesis from Hse (16, 17). Deletion of metA or thrB eliminates the metabolic flux into Met or Thr from Hse. A yggS-deficient strain was constructed in both a metA background and a thrB background, and the strains were assessed for growth in the presence or absence of Hse when the required supplements (Met and Thr, respectively) were provided. In the absence of Hse, the ΔmetA ΔyggSBW-pU0 strains and ΔthrB ΔyggSBW-pU0 strains showed growth indistinguishable from that of the parental control strains (mutants ΔmetABW-pU0 and ΔthrBBW-pU0) (Fig. 6A and D). In the presence of Hse, the thrB mutation, but not the metA mutation, suppressed the Hse sensitivity of the yggS mutant (Fig. 6B and E). These data resulted in the conclusion that the flux through the Thr biosynthetic pathway was required to generate Hse sensitivity of an yggS mutant of E. coli. It is formally possible that P-Hse negatively impacts the growth of E. coli. To test this possibility, we overexpressed ThrC, catalyzing conversion of P-Hse to Thr in the yggS mutant, which is expected to decrease the intracellular concentration of P-Hse. The yggS mutant harboring the plasmid carrying thrC was not sensitive to Hse (Fig. 4E). These data are consistent with the accumulation of P-Hse being the primary cause of the Hse sensitivity of the yggS mutant. Because the yggS mutant grown with Hse also accumulated Val and KIV in the cells, it was formally possible that the Hse sensitivity was indirectly due to Val toxicity. This possibility was eliminated as a consequence of the fact that expression of Val-insensitive AHAS (AHASII; ilvGM) in the yggS mutant failed to eliminate the growth defect caused by Hse (Fig. 4F).
FIG 6.
Effect of yggS mutation in the metA or thrB background on the growth or amino acid pools of the strains examined. (A and B) The ΔmetABW-pU0 (●) and ΔmetA ΔyggSBW-pU0 (△) strains were grown in M9 medium supplemented with 0.1 mM Met in the absence (A) or presence (B) of 1 mM Hse. (C) Intracellular amino acid concentrations of the ΔmetABW-pU0 and ΔmetA ΔyggSBW-pU0 strains grown in the presence of 0.1 mM Met and 1 mM Hse. (D and E) The growth of the ΔthrBBW-pU0 (●) and ΔthrB ΔyggSBW-pU0 (△) strains in the M9 medium supplemented with 0.5 mM Thr (D) or with 0.5 mM Thr and 1 mM Hse (E). (F) Intracellular amino acid concentrations of the ΔthrBBW-pU0 and ΔthrB ΔyggSBW-pU0 strains grown in M9 medium supplemented with 0.5 mM Thr and 1 mM Hse. The yggS thrB double mutant did not show Hse sensitivity or perturbation of the amino acid pool. Data are presented as means ± standard deviations of results from at least three independent experiments.
Hse perturbs amino acid metabolism in the yggS mutant via PNP accumulation.
In the absence of Hse, a ΔyggSBW-pU0 strain accumulated a 10-times-higher concentration of PNP than was seen with the WTBW-pU0 strain (3.3 ± 0.2 and 0.3 ± 0.3 pmol/mg cells, respectively), while the other B6 vitamers were similar in the two strains (Table 2). When Hse was provided in the medium, the level of PNP content was further elevated in the yggS mutant but not in the wild-type strain. In the presence of Hse, the ΔyggSBW-pU0 mutant accumulated PNP at 8.0 ± 0.2 pmol/mg cells, which was a level >80 times higher than that seen with the WTBW strain and 2.4 times higher than that seen with the ΔyggSBW-pU0 mutant grown in the absence of Hse (Table 2). In contrast, the levels of PLP were not significantly different between the two strains.
TABLE 2.
Effect of exogenous Hse on intracellular B6 pool of the yggS mutanta
| Strain | pmol of indicated B6 vitamer/mg cells ± SD |
|||||
|---|---|---|---|---|---|---|
| PLP |
PNP |
PMP |
||||
| No Hse | Hse | No Hse | Hse | No Hse | Hse | |
| E. coli WTBW-pU0 | 14 ± 2 | 15 ± 1 | 0.3 ± 0.3 | ND | 24 ± 4 | 26 ± 3 |
| E. coli ΔyggSBW-pU0 | 9.0 ± 2 | 17 ± 2 | 3.3 ± 0.2 | 8.0 ± 0.2 | 19 ± 2 | 29 ± 3 |
Strains WTBW-pU0 and ΔyggSBW-pU0 were grown in the M9 medium in the presence or absence of 1 mM Hse at 30°C. Log-phase cells were collected, and total intracellular B6 vitamers were quantified as described in Materials and Methods. The yggS mutants accumulated PNP, and exogenous Hse further elevated the PNP level in the yggS mutant. Data represent means ± standard deviations of results from at least three independent experiments. ND, not detected.
The elevation of PNP levels in the yggS mutant suggested that, like the PN sensitivity, increased PNP levels represent a primary cause of Hse sensitivity. Growth was conducted with the ΔyggSBW-PdxH strain, which overexpresses PdxH. The expression of pdxH did not eliminate the Hse sensitivity of the yggS mutant (Fig. 7A), despite the finding that there was a significant decrease in the level of PNP in the ΔyggSBW-PdxH mutant (Fig. 7B). Further increases in the expression of pdxH, aiming at complete elimination of PNP (by increasing the concentration of arabinose to up to 0.2%), instead inhibited the growth of both the wild-type strain and the yggS mutant. Expression of pdxH in the yggS mutant significantly reduced the intracellular concentrations of P-Hse and Val (Fig. 7C and D). There is no obvious explanation for the slow growth of the ΔyggSBW-PdxH mutant in the presence of Hse at present. In total, our data showed that PNP accumulation correlates with the P-Hse (and Val) overproduction found in the yggS mutant but neither metabolite was directly linked to the growth defect that is caused by the presence of Hse.
FIG 7.
Effect of pdxH expression on Hse sensitivity. (A) Growth of strains WTBW-pBAD, ΔyggSBW-pBAD, and ΔyggSBW-pdxH in the M9 medium supplemented with 1 mM Hse at 30°C. (B) Intracellular concentrations of PNP in the three strains grown in the M9 medium supplemented with 1 mM Hse. ND, not detected. (C) HPLC chromatograms representative of the amino acid analyses, showing a decreased P-Hse concentration in strain ΔyggSBW-pdxH. (D) Intracellular concentrations of Val in the three strains grown in the M9 medium supplemented with 1 mM Hse. Error bars show means ± standard deviations of results from at least three independent experiments.
Flux through the Thr biosynthetic pathway is required for the amino acid pool perturbation but for not PNP accumulation in the yggS mutant.
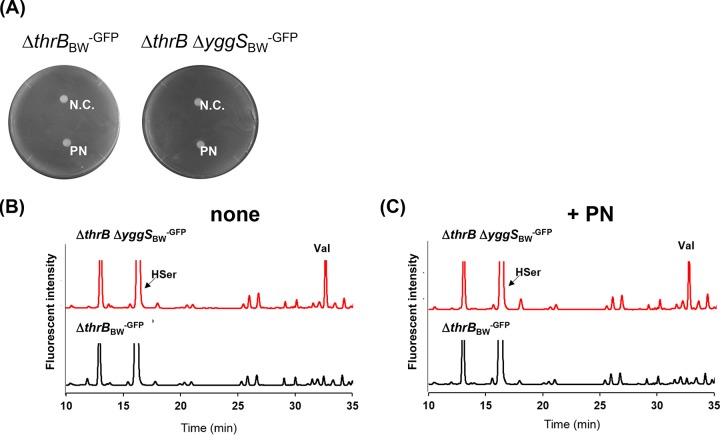
We found that the ΔthrB-pU0 and ΔthrB ΔyggSBW-pU0 mutants grown in the presence of Hse exhibited almost identical intracellular amino acid compositions, including Thr, Ile, and Val concentrations (Fig. 6E). This result suggests that the Thr biosynthetic pathway flux is involved in the perturbation of the intracellular amino acid pools in the yggS mutant. Importantly, the same was true in the yggS mutant grown with excess PN on the M9 plate, since exogenous PN did not compromise the growth of the ΔthrB ΔyggSBW-GFP mutant (Fig. 8A). No further alteration of the intracellular amino acid pool that included OA, GABA, 2-AB, and Val was detected in the ΔthrB ΔyggSBW-GFP mutant by PN (Fig. 8B and C). Furthermore, we found that the exogenous PN did not compromise the growth of the ΔthrC ΔyggSBW-GFP mutant. No amino acid pool alternation was induced in the ΔthrC ΔyggSBW-GFP mutants by the excess of PN (Fig. S2). These observations indicated that the alteration of the Thr biosynthetic pathway affected the disturbance of the intracellular amino acid pools in the yggS mutant.
FIG 8.
Effect of ThrB mutation with respect to PN sensitivity and amino acid pool disturbance in the yggS mutant. (A) The growth of the thrBBW-GFP and ΔthrB ΔyggSBW-GFP strains on M9 plates containing 0.4 mM Thr and 0.1% l-arabinose. Filter discs containing 20 μl of distilled water (N.C.) or 1 mM pyridoxine (PN) were placed on the plates. The ΔthrB ΔyggSBW-GFP strain did not show PN sensitivity. (B and C) HPLC chromatograms representing the amino acid analyses performed in the absence (B) or presence (C) of PN. Excess PN did not induce perturbation of the amino acid pool in the yggS mutant.
Excess 2-AB/2-KB induces Val overproduction in E. coli cells (1). Therefore, Val overproduction can be explained by the increasing supply of 2-AB/2-KB mediated by the increased production of the precursor Thr. In contrast, the P-Hse accumulation can be explained by the relative increase or decrease of ThrB or ThrC enzyme activity in the yggS mutant. We first examined whether PNP directly influences the two enzymes. However, our in vitro experiments failed to detect any activation or inhibition effects of PNP (at up to 100 μM PNP) on purified ThrB or ThrC enzymes under the conditions examined. In contrast, we found that the levels of ThrC in the cell extract of yggS mutant (23.4 ± 0.2 nmol/min/mg protein) were 2 times higher than those seen with the wild-type strain (11.4 ± 0.4 nmol/min/mg protein) under conditions of growth in the M9 medium.
Importantly, B6 pool analyses revealed that mutation of thrB or thrC does not influence the extent of the PNP accumulation in the yggS mutant (Table 1). In the absence of PN, the ΔthrB ΔyggSBW-GFP mutant accumulated PNP at 3.9 ± 0.5 pmol/mg cells, which was 15 times higher than the level seen with the ΔthrBBW-GFP mutant (0.3 ± 0.2 pmol/mg cells). When PN was provided, ΔthrB ΔyggSBW-GFP cells accumulated considerably more PNP (15 ± 3 pmol/mg cells), and the level was 45 times higher than the accumulation in the parental strain (Table 1) and comparable to that observed with the ΔyggSBW-GFP cells. Similar results were obtained with the thrC mutants (Table 1). Our data thus suggest that the perturbation of the Thr biosynthetic pathway flux plays a minor role in regulating PNP levels in the yggS mutant.
DISCUSSION
Previous studies showed that the disruption of yggS in E. coli causes pleiotropic phenotypes, especially with respect to related to amino acid and vitamin B6 metabolisms. However, little had been known about the cause, mechanism, and relevance of the phenotypes for the yggS mutant. The current study showed that a high concentration of intracellular PNP is the root cause for the two apparently unrelated phenotypes previously reported in a yggS mutant, the PN sensitivity and Val overproduction. In the absence of yggS, the E. coli cell accumulates PNP, which is further induced by certain stresses, including those represented by excess PN or Hse levels. Elevated levels of PNP itself do not prevent E. coli growth but influence flux through Thr biosynthesis and then perturb the Ile/Val biosynthetic pathway to accumulate some metabolite in the pathway under certain conditions. Accumulation of Val is likely to prevent growth of the yggS mutant.
The mechanism of the PNP-dependent perturbation of the Thr biosynthetic pathway is YggS independent, and its nature is currently unclear. We observed increasing ThrC activity in the cell extract of yggS mutant, which suggests increasing expression of the thr operon consisting of the thrL, thrA, thrB, and thrC genes (18). The thr operon is controlled by levels of certain amino acids, including Thr and Ile (19). PLP mostly exists in a protein-bound form, while free-form PNP may exist in the cells. The concentration of PNP in the yggS mutant was equal to or greater than that of PLP, as judged by our B6 pool analyses (Table 1; see also Table 2). Therefore, it is possible that PNP inhibits some PLP-dependent enzymes and/or transcriptional regulators and then alters the amino acid pool and expression of the thr operon. Such an indirect mode of action could explain the cultivation condition-dependent and/or strain-dependent pleiotropic phenotypes of the yggS mutant.
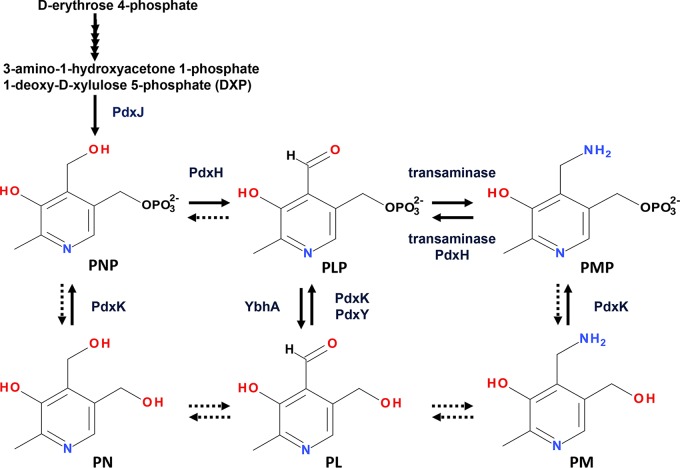
Our next challenge will be identification of the cause and mechanism of the PNP accumulation. We found that the yggS mutant accumulated PNP in all genetic backgrounds (thrB, thrC, metA, and E. coli MG1655) and under all cultivation conditions (LB and M9 medium containing Casamino Acids at both 30°C and 37°C) examined, indicating the essential role of YggS in low-level maintenance of PNP. A previous study (3) and our in vitro investigation failed to detect any in vitro reactivity of YggS toward PNP, which suggested indirect involvement in the homeostasis of PNP. In E. coli, PNP is synthesized by the deoxyxylulose 5-phosphate (DXP)-dependent pathway through the action of 4-hydroxythreonine-4-phosphate dehydrogenase (PdxA) and PNP synthase (PdxJ) (20–24). It is subsequently oxidized by PdxH to produce PLP (15). Once PLP is produced, it is recycled through a salvage pathway involving pyridoxal kinase (PdxK and PdxY) (25, 26), pyridoxal phosphatase (YbhA) (27), and PNP/PMP oxidase (PdxH) (Fig. 9). Either or both of increasing supply or decreasing metabolism of PNP can explain the PNP accumulation in the yggS mutant. One plausible model for the PNP accumulation is involvement of YggS in the maintenance of PdxH activity in vivo. Elevation of PMP levels in addition to PNP in the yggS mutant (Table 2) could support this hypothesis. Interestingly, the distribution of the YggS protein family is different from that of PdxH; many organisms that utilize a DXP-independent pathway to synthesize PLP lack PdxH. YggS orthologs, including those of human and yeast exhibiting 20% to 40% sequence identity, are capable of complementation of the phenotypes of yggS mutant (1, 3). These facts suggest that YggS is indirectly involved in the maintenance of PdxH activity. It is possible that the YggS protein family is involved in the low-level maintenance of free PLP by storing PLP in the protein. The YggS protein family is abundant in eukaryotic cells. In S. cerevisiae, the copy numbers for 90% of the yeast proteins are within the vicinity of 2,000, while there are 17,500 copies of YBL036c in one cell. In HeLa cells, the median level of protein expression is 21,000 copies per cell, but approximately 200,000 copies of PROSC protein are expressed in a cell (28). Therefore, the disruption of this protein family could increase the level of the free PLP pool in the cells. The elevation of cellular PLP levels can increase the levels of holo-PLP-dependent enzymes, as well as those of the unwanted PLP adducts formed with proteins and small metabolites. The elevation of free PLP levels could cause feedback inhibition of PdxH and the accumulation of PNP in the cells (15). Further studies will be required to examine these hypotheses and to identify the molecular function of YggS protein family. We think that the data presented here provide an important starting point for further study of this protein family.
FIG 9.
Biosynthetic pathway of PNP to the coenzyme PLP and the salvage pathway of PLP in E. coli. PNP is synthesized by the deoxyxylulose 5-phosphate (DXP)-dependent pathway. It is subsequently oxidized by PNP/PMP oxidase (PdxH) to produce PLP. PLP is recycled through a salvage pathway involving pyridoxal kinase (PdxK and PdxY), pyridoxal phosphatase (YbhA), and PdxH. The putative reactions in this pathway are represented by broken arrows.
MATERIALS AND METHODS
Bacterial strains, media, and chemicals.
The bacterial strains used in this study were generated from E. coli BW25113 (Table 3). M9-glucose and LB media were prepared as described previously (1). The E. coli wild-type strain, i.e., strain BW25113, and its derivatives (Keio collection) and pCA24N and the derivatives (ASKA collection) were obtained from the National Bioresource Project (NBRP; Keio collection) (29, 30). Antibiotics were added as needed to the M9 and LB media at the following concentrations: ampicillin, 100 μg/ml; kanamycin, 30 μg/ml; chloramphenicol, 30 μg/ml. l-Arabinose and isopropyl-β-d-thiogalactoside (IPTG) were added to the medium to reach final concentrations of 0.1 mM and 0.02%, respectively. Amino acids were purchased from Wako Pure Chemical Corporation (Osaka, Japan) or Sigma-Aldrich (St. Louis, MO, USA). PLP, PL, and PN were purchased from Nacalai Tesque (Kyoto, Japan). PNP was prepared by the reduction of PLP with NaBH4 in aqueous solution.
TABLE 3.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Genotype or description | Source or reference |
|---|---|---|
| Strains | ||
| E. coli WTBW | E. coli K-12 BW25113 wild type; Δ(araD-araB)567 ΔlacZ4787(::rrnB-3) rph-1 Δ(rhaD-rhaB)568 hsdR514 | Keio collection |
| E. coli ΔyggSBW | BW25113 ΔyggS::kan (JW2918-KC) | Keio collection |
| E. coli ΔmetABW | BW25113 ΔmetA::kan (JW3973-KC | Keio collection |
| E. coli ΔthrBBW | BW25113 ΔthrB::kan (JW0002-KC) | Keio collection |
| E. coli ΔthrCBW | BW25113 ΔthrC::kan (JW0003-KC) | Keio collection |
| E. coli JW3973-KCkan- | BW25113 ΔmetA | This study |
| E. coli JW0002-KCkan- | BW25113 ΔthrB | This study |
| E. coli JW0003-KCkan- | BW25113 ΔthrC | This study |
| E. coli ΔmetA ΔyggSBW | BW25113 ΔmetA ΔyggS::kan | This study |
| E. coli ΔthrB ΔyggSBW | BW25113 ΔthrB ΔyggS::kan | This study |
| E. coli ΔthrC ΔyggSBW | BW25113 ΔthrC ΔyggS::kan | This study |
| E. coli WTBW-pu0 | WTBW/pU0 | 1 |
| E. coli WTBW-GFP | WTBW/pBAD18-GFP | This study |
| E. coli WTBW-pBAD24 | WTBW/pBAD24 | This study |
| E. coli WTBW-pBAD | WTBW/pBAD-Myc-HisA | This study |
| E. coli WTBW-pCA24N | WTBW/pCA24N | This study |
| E. coli ΔyggSBW-pu0 | ΔyggSBW/pU0 | 1 |
| E. coli ΔyggSBW-yggS | ΔyggSBW/pUS | 1 |
| E. coli ΔyggSBW-GFP | ΔyggSBW/pBAD-GFP | This study |
| E. coli ΔyggSBW-pBAD24 | ΔyggSBW/pBAD24 | This study |
| E. coli ΔyggSBW-pBAD | ΔyggSBW/pBAD-Myc-HisA | This study |
| E. coli ΔyggSBW-AHASII | ΔyggSBW/pBAD-AHASII | This study |
| E. coli ΔyggSBW-pdxH | ΔyggSBW/pBAD-PdxH | This study |
| E. coli ΔyggSBW-pCA24N | ΔyggSBW/pCA24N | This study |
| E. coli ΔyggSBW-thrC | ΔyggSBW/pCA24N-thrC | This study |
| E. coli ΔmetABW-pu0 | ΔmetABW/pU0 | This study |
| E. coli ΔmetA ΔyggSBW | ΔmetA ΔyggSBW/pU0 | This study |
| E. coli ΔthrBBW-pu0 | ΔthrBBW/pU0 | This study |
| E. coli ΔthrB ΔyggSBW-pu0 | ΔthrB ΔyggSBW/pU0 | This study |
| E. coli ΔthrCBW-pu0 | ΔthrCBW/pU0 | This study |
| E. coli ΔthrC ΔyggSBW-pu0 | ΔthrC ΔyggSBW/pU0 | This study |
| E. coli ΔthrBBW-GFP+ | ΔthrBBW/pBAD-GFP | This study |
| E. coli ΔthrB ΔyggSBW-GFP+ | ΔthrB ΔyggSBW/pBAD-GFP | This study |
| E. coli ΔthrCBW-GFP | ΔthrCBW/pBAD-GFP | This study |
| E. coli ΔthrC ΔyggSBW-GFP | ΔthrC ΔyggSBW/pBAD-GFP | This study |
| Plasmids | ||
| pU0 | pUC19 containing partial sequence of yggS | 1 |
| pUS | pUC19 expressing YggS-His | 1 |
| pCP20 | Expresses yeast Flp recombinase gene | 36 |
| pKD46 | Express lambda red recombinase | 31 |
| pKD13 | Template plasmid | 31 |
| pBAD-GFP | pBAD18/GFP | Laboratory collection |
| pBAD24 | pBAD24 empty vector | Laboratory collection |
| pBAD24-AHASII | pBAD24 containing S. enterica ilvGM (pDM1599) | Laboratory collection |
| pBAD-PdxH | pBAD-Myc-HisC expressing E. coli PdxH | This study |
| pCA24N | pCA24N empty vector | ASKA clone |
| pCA24N-thrC | pCA24N expressing ThrC | ASKA clone |
Growth analyses.
E. coli strains were precultured overnight in LB at 30°C. The cells (200 μl) were washed twice with 1 ml PBS (10 mM Na2HPO4, 1.8 mM KH2PO4, 140 mM NaCl, 2.7 mM KCl, pH 7.5) and resuspended in 1 ml of PBS. For the liquid culturing, the cells were inoculated into M9 medium at an initial optical density at 600 nm (OD600) of 0.01 to 0.05. The cells were grown to full density at 30°C with shaking. Amino acids were added at the indicated concentrations. Cells growth was recorded using an OD-Monitor C&T apparatus (Taitec Co., Ltd., Koshigaya, Japan) and glass test tubes (16.5 mm in diameter by 165 mm in height) or an ELx808 absorbance microplate reader (BioTek, Winooski, VT, USA) and 96-well plates. For the growth on the M9 plate, the E. coli cells in the PBS were further diluted 100-fold and spread (200 μl) onto an M9 plate. After gentle drying, a paper disc (6 mm in diameter) was placed on the plate, and 20 μl of 1 mM PN was spotted on the disc. The plate was incubated at 37°C and was photographed with an LuminoGraph II imaging system (ATTO Corp., Tokyo, Japan).
Genetic techniques.
Deletion of the yggS genes was performed using the bacteriophage λ-Red recombinase system described previously by Datsenko and Wanner (31). The ΔmetA ΔyggSBW strain was constructed as follows. The E. coli JW3973-KC strain possessing pCP20 was streaked on an LB plate and grown at 42°C, forming strain JW3973-KCkan-. A PCR product was generated with primers yggS-H1 and yggS-H2 (32) using Tks Gflex DNA polymerase (TaKaRa) and pKD13 as a template. The PCR product was purified from agarose gel and electroporated into the JW3973-KCkan- strain harboring pKD46. The resultant transformants that appeared on an LB plate containing 30 μg/ml kanamycin were screened by PCR for the appropriate insertion of the kanamycin resistance gene with primers yggS-200up and yggS-300dwn (32). Construction of the ΔthrB ΔyggSBW mutant or the ΔthrC ΔyggSBW mutant was performed in a similar way using JW0002-KC or JW0003-KC as the parental strain.
Construction of pBAD-PdxH was performed as follows. The E. coli pdxH gene was amplified by PCR with KOD-plus2 DNA polymerase (Toyobo). The primers used were pdxH-pBAD-fw (GGCCGCCATGGCAATGTCTGATAACGACGAATTGCAGCAAA) and pdxH-pBAD-rv (GGCCGGAATTCTCAGGGTGCAAGACGATCAATCTTCCA). The resultant PCR product was purified by agarose gel electrophoresis, digested by NcoI and EcoRI (NEB Japan), and ligated into pBAD-Myc/HisC vector (Invitrogen) digested with the same restriction enzymes. Constructs were transformed into E. coli XL10-Gold (Stratagene). The sequence of the pdxH insertion was confirmed by sequencing. Expression of active PdxH was confirmed by complementation of a pdxH-deficient E. coli strain.
Amino acid analysis.
Cells were grown in the M9 medium at 30°C to a final OD600 of 0.5 or on M9 plates at 37°C for 24 h. The cell pellet was washed once with ice-cold PBS and resuspended in 5% (wt/vol) trichloroacetic acid (TCA) solution (100 μl for 10 mg wet cells) as described previously (33). After vortex mixing and incubation at 4°C for 30 min, cell debris was removed by centrifugation at 20,000 × g for 20 min at 4°C. Amino acids were derivatized as described previously (33). Briefly, 25 μl of the diluted amino acid extract was mixed with the same amount of o-phthalaldehyde (OPA)–N-acetyl-l-cysteine (NAC)–borate reagent and incubated for 15 min at 4°C. After the addition of 200 μl of distilled water and centrifugation at 20,000 × g for 15 min at 4°C, the derivatives were analyzed by high-performance chromatography (HPLC) using an Hitachi HPLC system equipped with a Chromaster 5110 pump, a model 5210 auto sampler, a model 5310 column oven (Hitachi, Tokyo, Japan), and Shimadzu fluorescent detector RF-10AXL (Shimadzu Corp., Kyoto, Japan). The excitation and emission wavelengths were 350 nm and 450 nm, respectively. The column oven was maintained at 28°C, and the total flow was 0.8 ml/min. A C18 column (Mightysil RP-18 GP II column [4.6 by 150 mm, 3-μm particle size]) equipped with a guard column (Kanto Chemical, Tokyo, Japan [4.6 by 5 mm, 5 μm]) was used for analyses. Linear gradients of buffer B (10 mM Na2HPO4 [pH 6.5], 60% methanol) and buffer A (10 mM Na2HPO4 [pH 6.5], 10% [vol/vol] methanol) were used for separation of derivatives of amino acid (0 to 20 min, 0% to 23%; 20 to 25 min, 23% to 57%; 25 to 40 min, 57% to 100%).
B6 vitamer analysis.
B6 vitamers (PLP, PNP, PMP, PL, PN, and PM) were quantified according to previously published protocols (3, 34) with slight modifications. Briefly, B6 vitamers were extracted from the cells with 10 volumes (vol/wt) of 0.8 M HClO4 containing 50 μM deoxypyridoxine (100 μl of the HClO4 solution for 10 mg E. coli wet cells). The suspension was subjected to extensive vortex mixing, and a total of 5 volumes (vol/wt) of 0.8 M K2CO3 solution (50 μl for 10 mg E. coli cells) was added. The mixture was centrifuged, and the resultant supernatant (25 μl) was used for the HPLC analysis employing fluorescence detection. The excitation and emission wavelengths were 328 nm and 393 nm, respectively. The B6 vitamers were separated by the use of an octadecylsilyl (ODS) column (Cosmosil AR-II; Nacalai Tesque) (250 by 4.6 mm, 5-μm particle size) using a gradient program. Mobile phase A (33 mM phosphoric acid and 8 mM 1-octanesulfonic acid, adjusted to pH 2.2 with KOH) and mobile phase B (80% acetonitrile [vol/vol]) were used for separation. The total flow rate was 0.8 ml/min. The gradient program (linear gradient) used was as follows: 0% mobile phase B to 1% B for 5 min, 1% B to 19% B for 5 min, 19% B to 28% B for 10 min, and 28% B to 63% B for 5 min. A postcolumn reagent (1 g/liter sodium bisulfite–1.0 M potassium phosphate buffer [adjusted to pH 7.5 with KOH], 0.3 ml/min) was used to enhance PLP fluorescence.
ThrC assay.
Cell-free extracts were prepared using mid-log-phase cells grown in the M9 medium at 30°C. The cells (50 mg) were resuspended in 10 volumes of a disruption buffer (500 μl) containing 100 mM potassium phosphate, 0.5 mM dithiothreitol, and 20% glycerol (pH 7.5). The cell suspension was sonicated and centrifuged (20,000 × g, 20 min, 4°C), and the resultant clear lysate was dialyzed. The protein concentration in the sample was determined by the procedure of Bradford with a Bio-Rad protein assay kit (Bio-Rad) using bovine serum albumin as the standard. ThrC activity was measured as previously described (35). Briefly, the cell extract was added to a solution containing 50 mM HEPES-NaOH (pH 8.0), 100 mM (NH4)2SO4, 5 μM PLP, and 1 mM P-Hse in a total volume of 100 μl. After 30 min of incubation at 37°C, the reaction was terminated by the addition of malachite green reagent solution and the inorganic phosphate liberated from P-Hse was quantified.
Supplementary Material
ACKNOWLEDGMENTS
We thank Huong Vu for constructing plasmid pDM1599.
This work was supported by grants from the JSPS KAKENHI (grants 16K18686 and 17KK0153 to T.I.) and the Ito Foundation (to T.I.). The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
T.I. designed the research, performed the experiments, and wrote the manuscript. K.Y., R.H., and A.Y. performed the experiments. D.M.D. analyzed data and wrote the manuscript, and H.H. and T.Y. analyzed the data. We declare that we have no conflict of interest.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.00430-19.
REFERENCES
- 1.Ito T, Iimori J, Takayama S, Moriyama A, Yamauchi A, Hemmi H, Yoshimura T. 2013. Conserved pyridoxal protein that regulates Ile and Val metabolism. J Bacteriol 195:5439–5449. doi: 10.1128/JB.00593-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ito T, Yamauchi A, Hemmi H, Yoshimura T. 2016. Ophthalmic acid accumulation in an Escherichia coli mutant lacking the conserved pyridoxal 5’-phosphate-binding protein YggS. J Biosci Bioeng 122:689–693. doi: 10.1016/j.jbiosc.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 3.Prunetti L, El Yacoubi B, Schiavon CR, Kirkpatrick E, Huang L, Bailly M, El Badawi-Sidhu M, Harrison K, Gregory JF, Fiehn O, Hanson AD, de Crécy-Lagard V. 2016. Evidence that COG0325 proteins are involved in PLP homeostasis. Microbiology 162:694–706. doi: 10.1099/mic.0.000255. [DOI] [PubMed] [Google Scholar]
- 4.Eswaramoorthy S, Gerchman S, Graziano V, Kycia H, Studier FW, Swaminathan S. 2003. Structure of a yeast hypothetical protein selected by a structural genomics approach. Acta Crystallogr D Biol Crystallogr 59:127–135. doi: 10.1107/S0907444902018012. [DOI] [PubMed] [Google Scholar]
- 5.Tremiño L, Forcada-Nadal A, Contreras A, Rubio V. 2017. Studies on cyanobacterial protein PipY shed light on structure, potential functions, and vitamin B6-dependent epilepsy. FEBS Lett 591:3431–3442. doi: 10.1002/1873-3468.12841. [DOI] [PubMed] [Google Scholar]
- 6.Nichols RJ, Sen S, Choo YJ, Beltrao P, Zietek M, Chaba R, Lee S, Kazmierczak KM, Lee KJ, Wong A, Shales M, Lovett S, Winkler ME, Krogan NJ, Typas A, Gross CA. 2011. Phenotypic landscape of a bacterial cell. Cell 144:143–156. doi: 10.1016/j.cell.2010.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Côté JP, French S, Gehrke SS, MacNair CR, Mangat CS, Bharat A, Brown ED. 2016. The genome-wide interaction network of nutrient stress genes in Escherichia coli. mBio 7:e01714-16. doi: 10.1128/mBio.01714-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Darin N, Reid E, Prunetti L, Samuelsson L, Husain RA, Wilson M, El Yacoubi B, Footitt E, Chong WK, Wilson LC, Prunty H, Pope S, Heales S, Lascelles K, Champion M, Wassmer E, Veggiotti P, de Crécy-Lagard V, Mills PB, Clayton PT. 2016. Mutations in PROSC disrupt cellular pyridoxal phosphate homeostasis and cause vitamin-B6-dependent epilepsy. Am J Hum Genet 99:1325–1337. doi: 10.1016/j.ajhg.2016.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Plecko B, Zweier M, Begemann A, Mathis D, Schmitt B, Striano P, Baethmann M, Vari MS, Beccaria F, Zara F, Crowther LM, Joset P, Sticht H, Papuc SM, Rauch A. 2017. Confirmation of mutations in PROSC as a novel cause of vitamin B6-dependent epilepsy. J Med Genet 54:809–814. doi: 10.1136/jmedgenet-2017-104521. [DOI] [PubMed] [Google Scholar]
- 10.Labella JI, Cantos R, Espinosa J, Forcada-Nadal A, Rubio V, Contreras A. 2017. PipY, a member of the conserved COG0325 family of PLP-binding proteins, expands the cyanobacterial nitrogen regulatory network. Front Microbiol 8:1244. doi: 10.3389/fmicb.2017.01244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Felice M, Squires C, Levinthal M, Guardiola J, Lamberti A, Iaccarino M. 1977. Growth inhibition of Escherichia coli K-12 by L-valine: a consequence of a regulatory pattern. Mol Gen Genet 156:1–7. doi: 10.1007/BF00272245. [DOI] [PubMed] [Google Scholar]
- 12.De Felice M, Squires C, Levinthal M. 1978. A comparative study of the acetohydroxy acid synthase isoenzymes of Escherichia coli K-12. Biochim Biophys Acta 541:9–17. doi: 10.1016/0304-4165(78)90263-5. [DOI] [Google Scholar]
- 13.Jackson JH, Herring PA, Patterson EB, Blatt JM. 1993. A mechanism for valine-resistant growth of Escherichia coli K-12 supported by the valine-sensitive acetohydroxy acid synthase IV activity from ilvJ662. Biochimie 75:759–765. doi: 10.1016/0300-9084(93)90125-C. [DOI] [PubMed] [Google Scholar]
- 14.Lam HM, Winkler ME. 1992. Characterization of the complex pdxH-tyrS operon of Escherichia coli K-12 and pleiotropic phenotypes caused by pdxH insertion mutations. J Bacteriol 174:6033–6045. doi: 10.1128/jb.174.19.6033-6045.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao G, Winkler ME. 1995. Kinetic limitation and cellular amount of pyridoxine (pyridoxamine) 5'-phosphate oxidase of Escherichia coli K-12. J Bacteriol 177:883–891. doi: 10.1128/jb.177.4.883-891.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burr B, Walker J, Truffa-Bachi P, Cohen GN. 1976. Homoserine kinase from Escherichia coli K-12. Eur J Biochem 62:519–526. doi: 10.1111/j.1432-1033.1976.tb10186.x. [DOI] [PubMed] [Google Scholar]
- 17.Born TL, Blanchard JS. 1999. Enzyme-catalyzed acylation of homoserine: mechanistic characterization of the Escherichia coli metA-encoded homoserine transsuccinylase. Biochemistry 38:14416–14423. doi: 10.1021/bi991710o. [DOI] [PubMed] [Google Scholar]
- 18.Thèze J, Saint-Girons I. 1974. Threonine locus of Escherichia coli K-12: genetic structure and evidence for an operon. J Bacteriol 118:990–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lynn SP, Gardner JF, Reznikoff WS. 1982. Attenuation regulation in the thr operon of Escherichia coli K-12: molecular cloning and transcription of the controlling region. J Bacteriol 152:363–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao G, Winkler ME. 1996. 4-Phospho-hydroxy-L-threonine is an obligatory intermediate in pyridoxal 5′-phosphate coenzyme biosynthesis in Escherichia coli K-12. FEMS Microbiol Lett 135:275–280. doi: 10.1111/j.1574-6968.1996.tb08001.x. [DOI] [PubMed] [Google Scholar]
- 21.Kim J, Kershner JP, Novikov Y, Shoemaker RK, Copley SD. 2010. Three serendipitous pathways in E. coli can bypass a block in pyridoxal-5′-phosphate synthesis. Mol Syst Biol 6:436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim J, Copley SD. 2012. Inhibitory cross-talk upon introduction of a new metabolic pathway into an existing metabolic network. Proc Natl Acad Sci U S A 109:E2856–E2864. doi: 10.1073/pnas.1208509109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cane DE, Hsiung Y, Cornish JA, Robinson JK, Spenser ID. 1998. Biosynthesis of vitamin B6: the oxidation of 4-(phosphohydroxy)-L-threonine by PdxA. J Am Chem Soc 120:1936–1937. doi: 10.1021/ja9742085. [DOI] [Google Scholar]
- 24.Laber B, Maurer W, Scharf S, Stepusin K, Schmidt FS. 1999. Vitamin B6 biosynthesis: formation of pyridoxine 5’-phosphate from 4-(phosphohydroxy)-l-threonine and l-deoxy-d-xylulose-5-phosphate by PdxA and PdxJ protein. FEBS Lett 449:45–48. doi: 10.1016/S0014-5793(99)00393-2. [DOI] [PubMed] [Google Scholar]
- 25.Yang Y, Zhao G, Winkler ME. 1996. Identification of the pdxK gene that encodes pyridoxine (vitamin B6) kinase in Escherichia coli K-12. FEMS Microbiol Lett 141:89–95. doi: 10.1111/j.1574-6968.1996.tb08368.x. [DOI] [PubMed] [Google Scholar]
- 26.Yang Y, Tsui HC, Man TK, Winkler ME. 1998. Identification and function of the pdxY gene, which encodes a novel pyridoxal kinase involved in the salvage pathway of pyridoxal 5'-phosphate biosynthesis in Escherichia coli K-12. J Bacteriol 180:1814–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sugimoto R, Saito N, Shimada T, Tanaka K. 2018. Identification of YbhA as the pyridoxal 5'-phosphate (PLP) phosphatase in Escherichia coli: importance of PLP homeostasis on the bacterial growth. J Gen Appl Microbiol 63:362–368. doi: 10.2323/jgam.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 28.Kulak NA, Pichler G, Paron I, Nagaraj N, Mann M. 2014. Minimal, encapsulated proteomic-sample processing applied to copy-number estimation in eukaryotic cells. Nat Methods 11:319–324. doi: 10.1038/nmeth.2834. [DOI] [PubMed] [Google Scholar]
- 29.Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H. 2006. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol 2:2006.0008. doi: 10.1038/msb4100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kitagawa M, Ara T, Arifuzzaman M, Ioka-Nakamichi T, Inamoto E, Toyonaga H, Mori H. 2006. Complete set of ORF clones of Escherichia coli ASKA library (a complete set of E. coli K-12 ORF archive): unique resources for biological research. DNA Res 12:291–299. doi: 10.1093/dnares/dsi012. [DOI] [PubMed] [Google Scholar]
- 31.Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ito T, Uozumi N, Nakamura T, Takayama S, Matsuda N, Aiba H, Hemmi H, Yoshimura T. 2009. The implication of YggT of Escherichia coli in osmotic regulation. Biosci Biotechnol Biochem 73:2698–2704. doi: 10.1271/bbb.90558. [DOI] [PubMed] [Google Scholar]
- 33.Ito T, Tokoro M, Hori R, Hemmi H, Yoshimura T. 2018. Production of ophthalmic acid using engineered Escherichia coli. Appl Environ Microbiol 84:e02806-17. doi: 10.1128/AEM.02806-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sampson DA, O'Connor DK. 1989. Analysis of B-6 vitamers and pyridoxic acid in plasma, tissues and urine using high performance liquid chromatography. Nutr Res 9:259–272. doi: 10.1016/S0271-5317(89)80069-7. [DOI] [Google Scholar]
- 35.Laber B, Gerbling KP, Harde C, Neff KH, Nordhoff E, Pohlenz HD. 1994. Mechanisms of interaction of Escherichia coli threonine synthase with substrates and inhibitors. Biochemistry 33:3413–3423. doi: 10.1021/bi00177a035. [DOI] [PubMed] [Google Scholar]
- 36.Cherepanov PP, Wackernagel W. 1995. Gene disruption in Escherichia coli: TcR and KmR cassettes with the option of Flp-catalyzed excision of the antibiotic-resistance determinant. Gene 158:9–14. doi: 10.1016/0378-1119(95)00193-A. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.