The MLSA scheme based on six housekeeping genes (HKGs) (gyrA, gyrB, infB, recN, rpoA, and topA) is well established as a reliable tool for taxonomic, evolutionary, and population diversity analyses of the genus Shewanella in this study. The standard MLSA method allows researchers to make rapid, economical, and precise identification of Shewanella strains. The robust phylogenetic network of MLSA provides profound insight into the evolutionary structure of the genus Shewanella. The population genetics of Shewanella species determined by the MLSA approach plays a pivotal role in clinical diagnosis and routine monitoring. Further studies on remaining species and genomic analysis will enhance a more comprehensive understanding of the microbial systematics, phylogenetic relationships, and ecological status of the genus Shewanella.
KEYWORDS: Shewanella, evolutionary relationship, identification, multilocus sequence analysis, population biology, taxonomic classification
ABSTRACT
The genus Shewanella comprises a group of marine-dwelling species with worldwide distribution. Several species are regarded as causative agents of food spoilage and opportunistic pathogens of human diseases. In this study, a standard multilocus sequence analysis (MLSA) based on six protein-coding genes (gyrA, gyrB, infB, recN, rpoA, and topA) was established as a rapid and accurate identification tool in 59 Shewanella type strains. This method yielded sufficient resolving power in regard to enough informative sites, adequate sequence divergences, and distinct interspecies branches. The stability of phylogenetic topology was supported by high bootstrap values and concordance with different methods. The reliability of the MLSA scheme was further validated by identical phylogenies and high correlations of genomes. The MLSA approach provided a robust system to exhibit evolutionary relationships in the Shewanella genus. The split network tree proposed twelve distinct monophyletic clades with identical G+C contents and high genetic similarities. A total of 86 tested strains were investigated to explore the population biology of the Shewanella genus in China. The most prevalent Shewanella species was Shewanella algae, followed by Shewanella xiamenensis, Shewanella chilikensis, Shewanella indica, Shewanella seohaensis, and Shewanella carassii. The strains frequently isolated from clinical and food samples highlighted the importance of increasing the surveillance of Shewanella species. Based on the combined genetic, genomic, and phenotypic analyses, Shewanella upenei should be considered a synonym of S. algae, and Shewanella pacifica should be reclassified as a synonym of Shewanella japonica.
IMPORTANCE The MLSA scheme based on six housekeeping genes (HKGs) (gyrA, gyrB, infB, recN, rpoA, and topA) is well established as a reliable tool for taxonomic, evolutionary, and population diversity analyses of the genus Shewanella in this study. The standard MLSA method allows researchers to make rapid, economical, and precise identification of Shewanella strains. The robust phylogenetic network of MLSA provides profound insight into the evolutionary structure of the genus Shewanella. The population genetics of Shewanella species determined by the MLSA approach plays a pivotal role in clinical diagnosis and routine monitoring. Further studies on remaining species and genomic analysis will enhance a more comprehensive understanding of the microbial systematics, phylogenetic relationships, and ecological status of the genus Shewanella.
INTRODUCTION
The genus Shewanella, first described by MacDonell and Colwell, belongs to the family Shewanellaceae (1). The members of this genus are Gram-negative, facultatively anaerobic, oxidase-positive, and motile bacteria (2–4). At the time of writing, there are 60 to 70 recognized species in the genus of Shewanella (http://www.bacterio.net/shewanella.html) (5–7). The majority of Shewanella species inhabit a wide range of environments, including free-living Shewanella species in oceans (8–11). Multiple Shewanella species are frequently yielded from consumable products as spoilage bacteria and from clinical specimens as opportunistic pathogens (12–14). In addition, the genus Shewanella plays a critical role in bioremediation (15), and certain strains have been used in bioelectrical systems (16, 17).
To date, polyphasic approaches are performed to assign the phylogenetic placement and taxonomic classification of Shewanella species. Commercial biochemical systems, such as Vitek and API, are available for species identification in clinical laboratories. However, only two species, namely, Shewanella algae and Shewanella putrefaciens, have been recorded in the database (12, 13). Phylogenetic analysis based on the 16S rRNA gene as a molecular marker was utilized to yield an evolutionary relationship for taxa (18). The disadvantage of the application of the 16S rRNA gene was the low resolving power to discriminate closely related species due to their high sequence similarities (19). Recently, a more rapidly evolving housekeeping gene (HKG) of gyrB was selected as an alternative phylogenetic indicator for Shewanella species classification (7, 20–22). Nevertheless, the quality of sequences submitted in public databases is poor (22–24). The genome-wide parameters, consisting of in silico DNA-DNA hybridization (isDDH) (25) and average nucleotide identity (ANI) (26), take the place of the wet-lab DNA-DNA hybridization (DDH) to unravel bacterial systematics. However, the process of genome sequencing is expensive and time-consuming; meanwhile, limited genomes of Shewanella type strains are available in public databases. These conditions make this approach impractical in clinical and daily investigations for rapid and efficient identification.
The effective multilocus sequence analysis (MLSA) scheme has been used in the phylogenetic and taxonomic analyses of several bacterial taxa (19, 27). Nevertheless, rare information is delineated among the genus Shewanella. Hence, in this study, we established a reliable MLSA method to classify Shewanella species by assessing the nucleotide sequences and phylogenies of six individual and concatenated HKGs (gyrA, gyrB, infB, recN, rpoA, and topA) in almost 60 Shewanella type strains. The phylogenetic framework of concatenated sequences provided a significant understanding of the evolutionary relationship in the genus Shewanella on the basis of multiple distinct taxonomic clades. The MLSA scheme was further utilized to determine the population biology of 86 tested strains collected in China.
RESULTS
Individual gene analysis.
In this study, fragments of 16S rRNA gene and six HKGs (gyrA, gyrB, infB, recN, rpoA, and topA) were amplified successfully for all Shewanella strains. Sequence diversity and phylogenetic analysis of fifty-nine type strains (see Table S2 in the supplemental material) were performed to assess the interspecies taxonomy among the genus Shewanella. The results of sequence diversity for the 16S rRNA gene are shown in Table 1 . The high occurrences of >98.65% interspecies similarity in the 16S rRNA gene implied the low resolution to distinguish Shewanella species. The low bootstrap values indicated the unstable topology in the phylogenetic tree, and close evolutionary branches were discovered (Fig. S1). Among the six HKG analyses, greater values of parsimony informative sites and nucleotide diversity were obtained (Table 1). In addition, the phylogenetic trees of all HKGs demonstrated more distinct branches and greater bootstrap values than the phylogenetic tree of the 16S rRNA gene (Fig. S1). However, this was not sufficient to differentiate all members of the genus Shewanella. Lower bootstrap values for the outer branches and discordance in the partial topology of six HKGs were still observed.
TABLE 1.
Nucleotide sequence diversity of 59 Shewanella type strains
| Locus | Length (bp) | Parsimony informative sites |
Nucleotide diversity (Pi) | Similarity (%) |
Ka/Ks | ||
|---|---|---|---|---|---|---|---|
| No. | % | Range | Mean | ||||
| 16S rRNA | 1,434 | 148 | 10.3 | 0.043 | 89.8–100 | 95.0 | NAa |
| gyrA | 498 | 229 | 46.0 | 0.223 | 68.3–100 | 77.7 | 0.117 |
| gyrB | 1,110–1,119 | 492 | 44.0 | 0.194 | 73.2–99.9 | 80.8 | 0.089 |
| infB | 663 | 289 | 43.6 | 0.193 | 73.5–100 | 80.7 | 0.105 |
| recN | 633–636 | 457 | 71.9 | 0.360 | 52.2–99.8 | 64.0 | 0.275 |
| rpoA | 615 | 221 | 35.9 | 0.125 | 79.7–100 | 87.5 | 0.052 |
| topA | 657–660 | 358 | 54.2 | 0.264 | 65.9–100 | 73.5 | 0.168 |
| MLSA | 4,176–4,191 | 2,046 | 48.8 | 0.223 | 71.1–99.9 | 77.7 | 0.143 |
NA, not applicable.
MLSA.
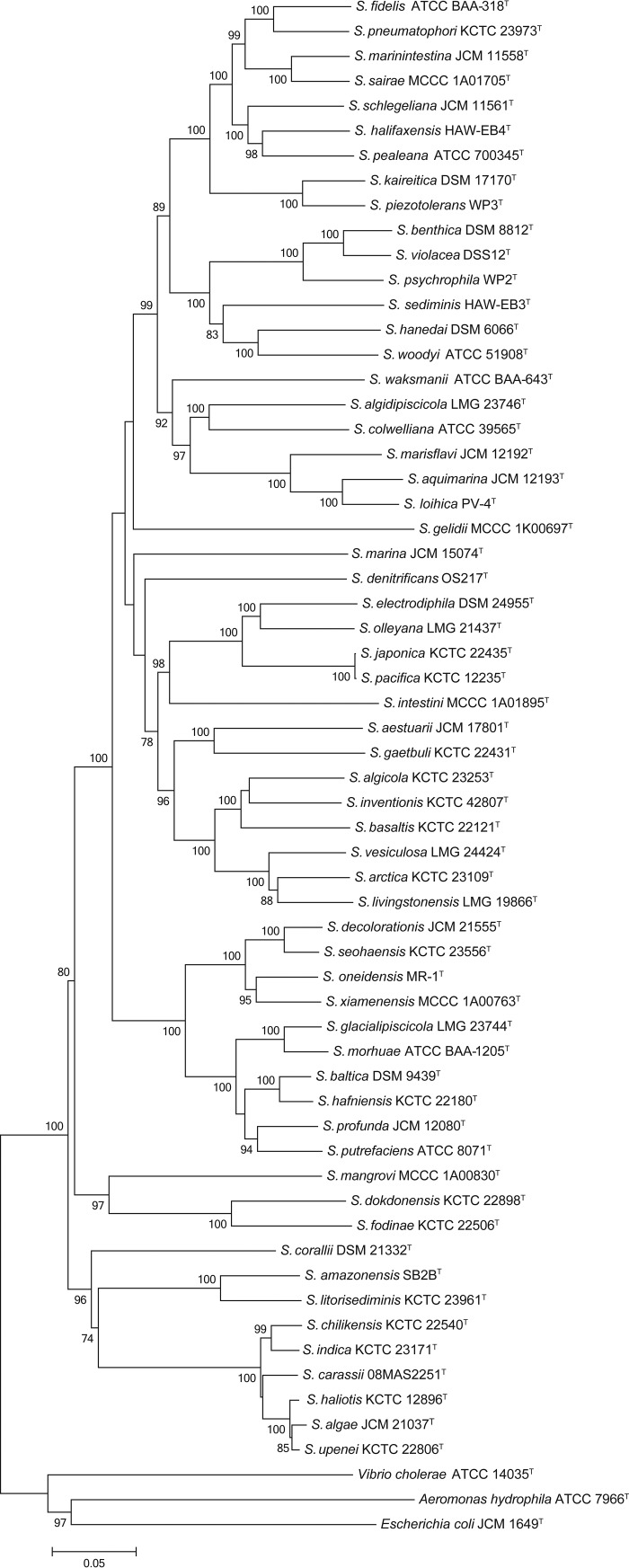
The concatenated sequences of protein-coding genes for 59 Shewanella type strains comprised 2,046 (48.8%) parsimony informative sites, with a nucleotide diversity value of 0.223 (Table 1). The analysis of sequences indicated that the multilocus sequence analysis (MLSA) scheme possessed an appropriate resolution and balanced the divergent evolutionary rates of six HKGs. The neighbor-joining phylogenetic tree based on concatenated alignment showed independent branches for interspecies, except for two sets of species, S. algae-S. haliotis-S. upenei (28) and S. japonica-S. pacifica (Fig. 1). These five species were likely to be misclassified, and more approaches were needed to perform the identification. The branches to discriminate Shewanella species were supported by high bootstrap values, except for S. algicola-S. inventionis and S. carassii. Bootstrap results indicated that the taxonomic groups involving those three species shared close evolutionary relationships. The phylogenetic tree of concatenated sequences was also reconstructed by the maximum-likelihood algorithm (Fig. S2). Almost the same topology was obtained, and the only exception was the location of S. carassii, which was supported with relatively low bootstrap values as described above.
FIG 1.
Phylogenetic tree reconstructed by the neighbor-joining method based on six concatenated gene sequences (gyrA, gyrB, infB, recN, rpoA, and topA [4,191 bp]) of 59 Shewanella type strains. The robustness of tree topologies was evaluated with 1,000 bootstrap replications, and values of >70% are shown at the nodes of the branches. The scale bar indicates substitutions per site. The strains Aeromonas hydrophila ATCC 7966T, Escherichia coli JCM 1649T, and Vibrio cholerae ATCC 14035T served as outgroups.
Comparative analysis between MLSA and genomes.
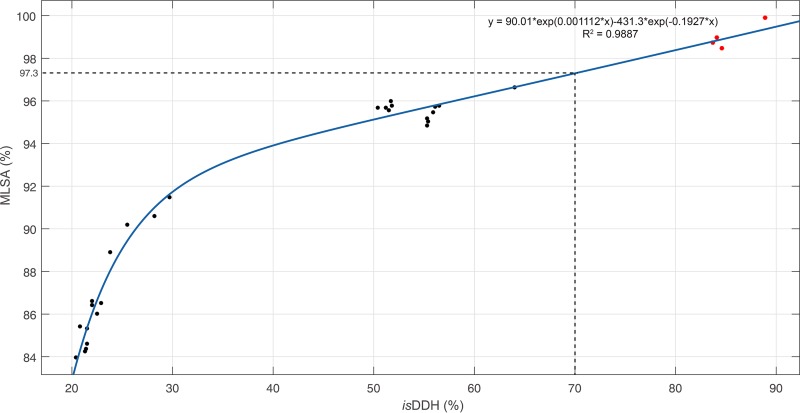
To further validate the reliability of MLSA, a whole-genome-based phylogenetic tree was constructed, and correlation analysis was performed among 28 type strains whose genomes were publicly available. The phylogeny of MLSA yielded a topology similar to that of core genes, and only a slight difference was observed in the position of S. carassii (Fig. S3). Similarities between the MLSA and isDDH analyses were calculated and are shown in Table S3. The isDDH values among distant species were concentrated at 20%. The isDDH values were highly correlated with the MLSA similarities (R2 = 0.9887) in closely related Shewanella species (Fig. 2). Based on the simulative equation y = 90.01 × exp(0.001112 × x) – 431.3 × exp(−0.1927 × x), the 70% isDDH value was equivalent to the 97.3% MLSA similarity, which could serve as a species boundary in the genus Shewanella.
FIG 2.
Correlation analysis between similarities of isDDH and MLSA for the genus Shewanella. The vertical line indicates a 70% isDDH threshold, and the horizontal line indicates the corresponding 97.3% MLSA similarity. The four points greater than the species boundary are marked in red.
Nevertheless, >97.3% concatenated sequence similarities were observed among two sets of species, i.e., S. algae-S. haliotis-S. upenei (28) and S. japonica-S. pacifica. The corresponding isDDH results between those groups of species were in the range of 83.7 to 88.9%, which exceeded the 70% species threshold (Fig. 2). The further pairwise ANI results between type strains of S. algae-S. haliotis, S. algae-S. upenei, and S. haliotis-S. upenei were 98.2, 98.1, and 98.2%, respectively, and the value of that between S. japonica and S. pacifica was 98.8%. All ANI values were greater than the boundary of 95% for species delineation. The genomic analysis based on isDDH and ANI provided compelling evidence for correct taxonomic position, indicating that S. algae, S. haliotis, and S. upenei were the same species (28) and that S. pacifica belonged to S. japonica. Additional phenotypic characteristics were detected among these five strains (Table 2). Minor differences in biochemical results were obtained between S. algae, S. haliotis, and S. upenei. The phenotypic discrepancies between S. japonica and S. pacifica were discovered for various growth conditions and the assimilation of N-acetylglucosamine. These results confirmed the conclusion of a recent report that identified S. haliotis as a synonym of S. algae according to whole-genome sequencing (28). Considering the genetic, genomic, and phenotypic characteristics, the S. upenei reported by Kim et al. (29) should be regarded as a later heterotypic synonym of S. algae proposed by Simidu et al. (30); meanwhile, the S. pacifica identified by Ivanova et al. (31) should be reclassified as a later heterotypic synonym of S. japonica described by Ivanova et al. (32).
TABLE 2.
Distinctive phenotypic characteristics among five Shewanella strainsa
| Characteristic | Strain 1 | Strain 2 | Strain 3 | Strain 4 | Strain 5 |
|---|---|---|---|---|---|
| Growth at/in: | |||||
| 4°C | – | – | – | – | + |
| 35°C | + | + | + | + | – |
| 0% (wt/vol) NaCl | + | + | + | + | – |
| 6% (wt/vol) NaCl | + | + | + | – | + |
| Ornithine decarboxylase | + | + | + | – | – |
| Utilization of: | |||||
| d-Glucose | + | – | – | + | + |
| d-Maltose | – | – | – | + | + |
| N-Acetylglucosamine | + | + | + | + | – |
| DNA G+C content (mol%) | 53.1 | 52.9 | 53.1 | 40.8 | 40.7 |
Strains: 1, S. algae JCM 21037T; 2, S. haliotis KCTC 12896T; 3, S. upenei KCTC 22806T; 4, S. japonica KMM 3299T; 5, S. pacifica KMM 3597T. +, Positive; –, negative.
Distinct taxonomic clades.
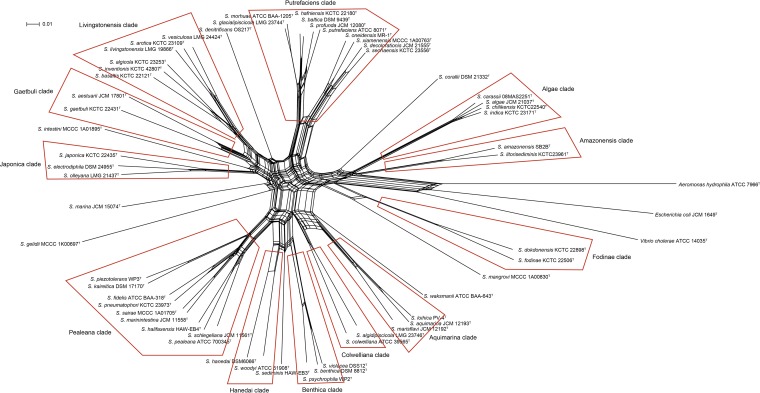
Given the results of sequence diversity, topological stability, and concordance with genomes, the MLSA scheme of six protein-coding genes (gyrA, gyrB, infB, recN, rpoA, and topA) was validated for taxonomic and evolutionary analysis among members of the Shewanella genus. The concatenated sequences for 56 species after emendation were subjected to construct the split network tree to explore evolutionary relationships among taxa (Fig. 3). Twelve distinct monophyletic clades were identified, i.e., the Algae, Amazonensis, Aquimarina, Benthica, Colwelliana, Fodinae, Gaetbuli, Hanedai, Japonica, Livingstonensis, Pealeana, and Putrefaciens clades (Table 3). The Shewanella species within the same clade shared <4 mol% GC variation and >84% MLSA concatenated similarity. There are eight orphan Shewanella species, namely, S. corallii, S. denitrificans, S. gelidii, S. intestini, S. mangrovi, S. marina, S. sediminis, and S. waksmanii, which form a distinct branch clearly separated from all taxonomic clades in the phylogenetic network, except for S. sediminis. S. sediminis harbored a far evolutionary distance similar to both Hanedai and Benthica clades and was located on the boundary of clade differentiation. Combined with the ambiguous relationships between S. sediminis and clades Hanedai and Benthica in a single HKG phylogenetic tree, S. sediminis was considered an orphan species. Twelve evolutionary clades were always maintained in phylogenetic trees of individual and concatenated HKGs. There were only slight differences observed, i.e., S. woodyi-S. hanedai in gyrB, S. colwelliana-S. algidipiscicola and S. gaetbuli-S. aestuarii in infB, and S. algidipiscicola-S. colwelliana in topA, which were positioned closely but did not group within one clade in phylogenies.
FIG 3.
Concatenated split network tree based on six gene loci. The gyrA, gyrB, infB, recN, rpoA, and topA gene sequences from 56 validated Shewanella species were concatenated and reconstructed using the SplitsTree 4 program. Twelve distinct clades were identified and are indicated in the figure by red outlines.
TABLE 3.
G+C content and MLSA concatenated similarity of clades in Shewanella species
| Clade | Described species | No. of species | G+C content (mol%)a | MLSA concatenated similarity (%) |
|---|---|---|---|---|
| Algae | S. algae, S. carassii, S. chilikensis, and S. indica | 4 | 53–54 | 94.8–96.6 |
| Amazonensis | S. amazonensis and S. litorisediminis | 2 | 54 | 91.2 |
| Aquimarina | S. aquimarina, S. loihica, and S. marisflavi | 3 | 50–53 | 89.0–93.4 |
| Benthica | S. benthica, S. psychrophila, and S. violacea | 3 | 47–49 | 90.6–94.6 |
| Colwelliana | S. colwelliana and S. algidipiscicola | 2 | 46–47 | 85.4 |
| Fodinae | S. fodinae and S. dokdonensis | 2 | 50–51 | 87.4 |
| Gaetbuli | S. gaetbuli and S. aestuarii | 2 | 43 | 84.3 |
| Hanedai | S. hanedai and S. woodyi | 2 | 44–46 | 87.0 |
| Japonica | S. japonica, S. electrodiphila, and S. olleyana | 3 | 43 | 87.7–89.6 |
| Livingstonensis | S. livingstonensis, S. algicola, S. arctica, S. basaltis, S. inventionis, and S. vesiculosa | 6 | 43–44 | 85.1–91.8 |
| Pealeana | S. pealeana, S. fidelis, S. halifaxensis, S. kaireitica, S. marinintestina, S. piezotolerans, S. pneumatophori, S. sairae, and S. schlegeliana | 9 | 44–46 | 84.0–93.5 |
| Putrefaciens | S. putrefaciens, S. baltica, S. decolorationis, S. glacialipiscicola, S. hafniensis, S. morhuae, S. oneidensis, S. profunda, S. seohaensis, and S. xiamenensis | 10 | 46–50 | 84.6–96.3 |
Calculated based on the concatenated sequences of six HKGs.
Population genetics of Shewanella species in China.
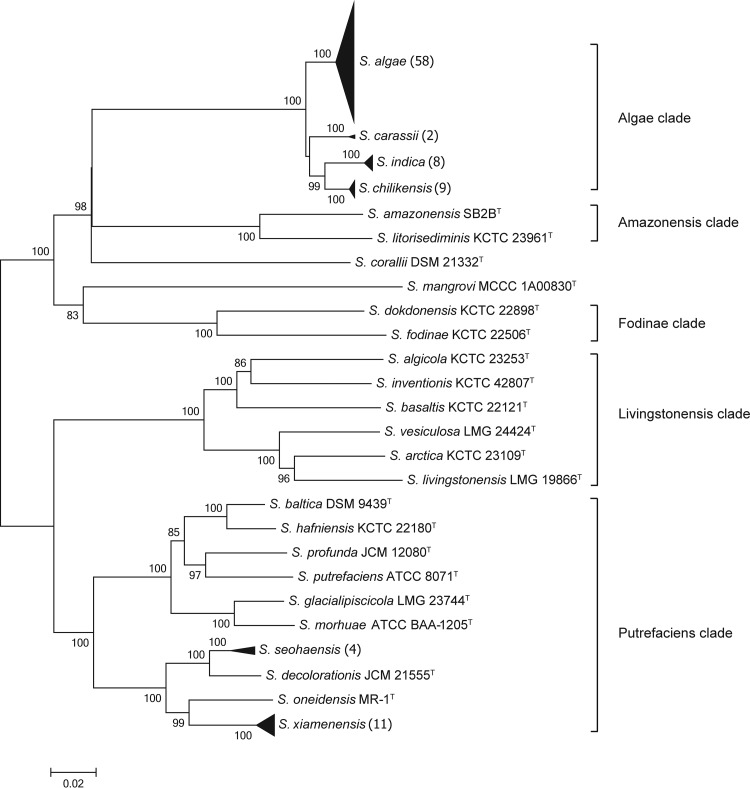
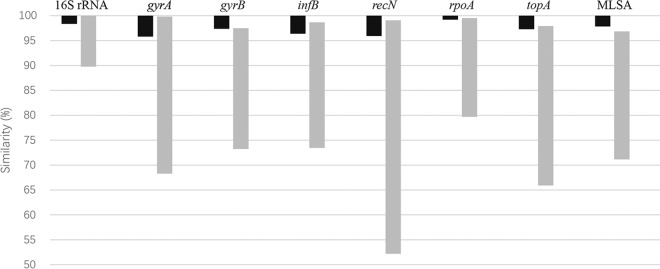
Eighty-six Shewanella strains isolated from diverse samples were involved in the analysis of sequences and phylogeny to evaluate the intraspecies relationships and investigate the distribution of Shewanella species in China. As shown in the concatenated phylogenetic tree (Fig. 4), 86 strains were divided into six compact clusters with high bootstrap support of 100%. Each cluster was represented by a unique Shewanella type strain situated in the Algae and Putrefaciens clades. In comparison with the concatenated phylogenetic tree, several unexpected locations were observed in the single HKG tree: strain 08MAS2647 in the S. algae cluster fell into the S. chilikensis cluster in gyrA; strains 08MAS2647, 11MAS2711, 11MAS2745, and 11MAS2746 in the S. algae cluster formed a subcluster next to the S. carassii cluster in infB; and strains in the S. algae, S. carassii, and S. chilikensis clusters exhibited a close affiliation that could not be separated from each other in recN. Although some strains could be grouped into clusters properly in individual phylogenetic trees, clusters were supported with low bootstrap values, such as S. algae cluster in the 16S rRNA, gyrA, and infB genes, as well as S. seohaensis cluster in the gyrA and gyrB genes (Fig. S4). Hence, concatenated sequences derived from six HKGs exhibited good performance and robustness in identifying Shewanella strains. Since strains were defined as corresponding species in the concatenated phylogenetic tree, ranges of intraspecies and interspecies similarities for genes among the 56 validated Shewanella species were measured and are shown in Fig. 5. Overlaps between the intraspecies and interspecies similarities were observed among the genes 16S rRNA, gyrA, infB, recN, rpoA, and topA. A small interval was detected in the gyrB gene, with only 0.1% variance. A notable gap was discovered in concatenated sequences. A minimum intraspecies similarity was found among S. seohaensis strains (97.8%), and the maximum interspecies similarity existed between S. chilikensis and S. indica (96.8%), which differed by 1% variation, corresponding to approximately 40-bp divergences.
FIG 4.
Phylogenetic tree reconstructed by the neighbor-joining method based on six concatenated gene sequences (gyrA, gyrB, infB, recN, rpoA, and topA, 4191 bp) of 86 Shewanella tested strains and 26 related type strains. The number of tested strains for each compact cluster (black triangle) is shown in parentheses (each of these clusters also contained one type strain). The robustness of tree topologies was evaluated with 1,000 bootstrap replications, and values of >70% are shown at the nodes of the branches. The scale bar indicates the substitutions per site.
FIG 5.
Intraspecies and interspecies similarities of 16S rRNA, six HKGs, and MLSA for 56 validated Shewanella species. The ranges of similarity are displayed in black (intraspecies) and gray (interspecies).
A total of 86 Shewanella strains collected from China were assessed to define species via the MLSA approach. The most dominant Shewanella species was identified as S. algae (66.3%), followed by S. xiamenensis (11.6%), S. chilikensis (9.3%), S. indica (8.1%), S. seohaensis (3.5%), and S. carassii (1.2%). Except for S. seohaensis, which was only isolated from the environment, the remaining five species were relevant to clinical patients. It is noteworthy that S. algae, S. xiamenensis, S. chilikensis, and S. indica were also discovered in food samples consisting of both marine products and cooked food for sale. Consequently, MLSA as a proper discrimination for Shewanella species played a significant role in public health and regular surveillance.
DISCUSSION
In this study, the MLSA scheme, based on six HKGs (gyrA, gyrB, infB, recN, rpoA, and topA), was established to carry out efficient classification, reflect evolutionary relationships, and delineate population biology in the genus Shewanella. Totals of 59 recognized type strains and 86 Chinese strains were investigated to explore the interspecies and intraspecies sequence diversity and phylogenetic topology in Shewanella species.
Previously, the 16S rRNA gene was applied as a traditional genetic marker among the genus Shewanella (7, 33, 34). However, the resolving power of the 16S rRNA gene was restricted, with fewer parsimony informative sites and lower nucleotide diversity values. A narrow range of sequence variation was observed, and multiple pairs of Shewanella species shared >99% similarity. The latest proposed threshold of 98.65% for 16S rRNA was insufficient to differentiate species in the genus Shewanella (35). In addition, the existence of sequence variation among rrn operons would perplex the species definition and evolutionary analysis for taxa (36). Hence, protein-coding genes with a greater genetic resolution were utilized to determine the taxonomic positions of Shewanella species.
Comparable analysis was performed among six HKGs (gyrA, gyrB, infB, recN, rpoA, and topA). Misclassification of some tested strains was discerned in the gyrA and infB genes for the high biological diversity among S. algae strains. High interspecies similarities of these HKGs were generated, making it difficult to discern closely related species. The gyrB gene has always been used as a basic detection for novel Shewanella species identification (7, 20–22). However, the criterion for gyrB analysis was not well established, and the boundary between interspecies and intraspecies similarities was inconspicuous. The recN gene was the most variable HKG, with the greatest rates of parsimony informative sites and the widest spectrum of interspecies similarity. Although the recN gene was unsuccessful in making a distinction in the Algae clade, the effective discrimination was proven by high sequence substitution rates in the majority of species. The rpoA gene was more conserved than other HKGs, with limited variable sites. None of the tested strains were phylogenetically located at unexpected positions, and only a slight overlap was detected between the intraspecies and interspecies ranges. The topA gene possessed a high genetic divergence next to the recN gene. The unstable taxonomic subtree with a low bootstrap value was discovered in the Colwelliana clade. The various evolutionary rates and inconsistencies of phylogenetic topology were discovered in these six loci. Therefore, concatenated sequences with integrated and sufficient information should be taken into account to obtain the exact Shewanella species classification.
The concatenation of six HKGs demonstrated enough resolution power to discern Shewanella species in regard to variable sites, sequence divergences, and independent branches. A notable gap between the ranges of interspecies and intraspecies similarities was favorable for defining the strains unambiguously at the species level, and 97.3% MLSA similarity was proposed as a species threshold in the genus Shewanella. The neighbor-joining phylogenetic tree indicated that all validated species positioned at a distinct branch were clearly separated from closely related taxa. The stability of the phylogenetic tree was proven by bootstrap and topology analysis. The concatenated sequence phylogeny was supported by high bootstrap values among interspecies having a significant advantage over all individual genes. The phylogenetic tree grouped Shewanella strains into intraspecies clusters and taxonomic clades with almost 100% bootstrap support. The use of the maximum-likelihood method had a slight impact on the tree topology. The reliability of the MLSA scheme was validated by comparison with genomic sequences. Identical phylogenies were constructed by concatenated sequences of six HKGs and core genes. A high correlation between the similarities of the MLSA and isDDH was discovered. Combined with the analysis of the resolution, stability, and reliability for nucleotide sequences and phylogenies, the MLSA approach of six HKGs (gyrA, gyrB, infB, recN, rpoA, and topA) showed a significant performance for the precise classification of Shewanella species.
Under comprehensive analysis, exceptional cases were only observed among two sets of recognized species, i.e., S. algae-S. haliotis-S. upenei (28) and S. japonica-S. pacifica. Based on molecular, genomic, and phenotypic analyses, these five species were reclassified correctly, and the taxonomic structure of the Shewanella genus was refined. It is noteworthy that previous studies proposing these five novel species depended largely on the individual sequence analysis of 16S rRNA, experimental DDH, and biochemical tests (10, 29–32). The high sequence similarities of 16S rRNA genes between phylogenetic neighbors have already been observed, and the results of wet-lab DDH below 70% were regarded as the gold standard for species classification (37). However, the experimental DDH was hard to reproduce completely by different laboratories; thus, the digital DDH based on the bacterial genomes was recommended in microbial systematics (25, 26). The phenotypic traits are inclined to be conservative among the Shewanella genus, and limited characteristics are suitable to discriminate Shewanella species. The deviation of the biochemical results could be attributed to the different manual procedures and bacterial growth statuses. The phenotypic discrepancies in growth conditions and the carbon source utilization observed among S. japonica and S. pacifica were also reported in the reclassification of S. affinis and S. colwelliana (38). Therefore, the accurate molecular method of MLSA is considered a promising alternative tool for species identification and is superior to genomic analysis in terms of high efficiency and low cost.
In addition, the MLSA scheme provided a portable and robust system to reflect evolutionary relationships for the genus Shewanella. Twelve distinct phylogenetic clades were proposed with identical G+C contents and greater nucleotide similarity in concatenated sequences. The Chinese strains collected from clinical specimens and routine monitoring were located on Algae and Putrefaciens clades. These results indicated that species in monophyletic clades have a tendency to share a close genetic relationship, tracing back to common ancestry, and occupy similar geographical positions. These clades could be almost retrieved from individual HKG phylogenies, further elucidating the accurate and stable evolutionary structure in the Shewanella taxon. Eight orphan species separated from all phylogenetic clades were defined. Attempts to involve the remaining species and identify the novel Shewanella species were conducive to exploring taxonomic positions for these species. In summary, the concatenated phylogeny provided significant insight into the evolutionary structure of the Shewanella genus.
Furthermore, it has been verified that Shewanella species, as marine pathogens, are associated with human diseases (12). Misidentifications to the species level were fairly common in clinical diagnoses due to the poor discernment system (39). In this study, 86 Shewanella strains collected from environmental, food, and clinical samples in China were mainly defined as S. algae, followed by S. xiamenensis, S. chilikensis, S. indica, S. seohaensis, and S. carassii, via the MLSA scheme. Five Shewanella species were verified to have clinical connections: S. algae, S. carassii, S. chilikensis, S. indica, and S. xiamenensis. It was likely that some Shewanella pathogens identified as S. algae in previous studies belonged to S. carassii, S. chilikensis, and S. indica for their high 16S rRNA similarities. Apart from S. carassii, four species were also frequently collected from marine products, as well as from cooked food for sale. It was reported that a common mechanism causing Shewanella infections was ascribed to the consumption of seafood or raw fish (12). Therefore, more attention is needed to reinforce continuous surveillance for the genus Shewanella by the MLSA approach in the processes of clinical diagnosis and food sales.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
A total of 145 Shewanella strains were involved in this study. Forty-two type strains were collected from the China General Microbiological Culture Collection Center (CGMCC), the German Collection of Microorganisms and Cell Cultures (Deutsche Sammlung von Mikroorganismen und Zellkulturen [DSMZ]), the Japan Collection of Microorganisms (JCM), the Korean Collection for Type Cultures (KCTC), the Belgian Coordinated Collections of Microorganisms (BCCM/LMG Bacteria Collection), and the Marine Culture Collection of China (MCCC). Detailed information of type strains is listed in Table S1. Eighty-six tested strains were isolated from patients (n = 44), food (n = 35), and the environment (n = 7) in four provinces (Anhui, Hainan, Liaoning, and Shandong) of China from 2007 to 2016. The 42 Shewanella type strains were incubated at suitable conditions according to the protocols of culture collection. The tested strains were isolated and characterized according to procedure described previously (40). The pure colonies were cultured on Marine Agar 2216 (BD, Difco) at 35°C for 18 h.
DNA extraction, gene selection, and primer design.
Genomic DNA from Shewanella strains was extracted with a genomic DNA extraction kit (TaKaRa, Dalian, China) according to the manufacturer’s instructions. The 16S rRNA gene of tested strains was amplified and sequenced with two universal primers (27F and 1492R) described previously (41). Six HKGs (gyrA, gyrB, infB, recN, rpoA, and topA) were chosen for the MLSA scheme. The degenerate primers of HKGs for PCR amplification, except the gyrB gene (42), were designed from genome sequences of Shewanella type strains in the GenBank database (Table S1) to accommodate a wide taxonomic scope. The nondegenerate primers in the 5′ region for sequencing are underlined in Table 4.
TABLE 4.
Primers used in this study
| Locus | Primer | Sequence (5′–3′)a | Positionb | Amplicon size (bp) | Annealing temp (°C) | Source or reference |
|---|---|---|---|---|---|---|
| 16S rRNA | 16S-27F | AGAGTTTGATCCTGGCTCAG | 8–27 | 1,503 | 52 | 45 |
| 16S-1492R | GGTTACCTTGTTACGACTT | 1492–1510 | ||||
| gyrA | gyrA-164F | TGAAGAACGATTGGAACAARCCNTAYAARAARTC | 164–197 | 664 | 56 | This study |
| gyrA-827R | TTTTCAATCAAACGAGCTTTGTTHACYTGRTAHGG | 793–827 | ||||
| gyrB | UP-1 | GAAGTCATCATGACCGTTCTGCAYGCNGGNGGNAARTTYGA | 274–314 | 1,256 | 58 | 42 |
| UP-2r | AGCAGGGTACGGATGTGCGAGCCRTCNACRTCNGCRTCNGTCAT | 1486–1529 | ||||
| infB | infB-1426F | ATGCCACAGACTATTGAAGCDATYCARCAYGC | 1426–1457 | 830 | 56 | This study |
| infB-2255R | GCATCAGCACGAACGTTAAARCCNAYMAKRATNGC | 2221–2255 | ||||
| recN | recN-415F | AGTGAGCATCAACTGACCYTRYTNGAYAGYTAYGC | 415–449 | 863 | 54 | This study |
| recN-1277R | GGTTGTAAAGGTTGCCCTGGGTTDGTNSWNAC | 1246–1277 | ||||
| rpoA | rpoA-83F | TGGAGCCGCTTGAGCGTGGTTTYGGHCAYAC | 83–113 | 751 | 56 | This study |
| rpoA-833R | ATGTAATGAATCGCTTCGGCYTTYARRCAGTT | 802–833 | ||||
| topA | topA-70F | GAATTCATCGTTAAGTCGAGYGTDGGBCAYRT | 70–101 | 860 | 60 | This study |
| topA-929R | CGCTGGGCCATCATCATGGTYTTYTTNACNCC | 898–929 |
The nondegenerate primers in the 5′ region for sequencing are underlined.
Position numbering is based on the complete genome of Escherichia coli K-12 (NC_000913.3).
PCR amplification and sequencing.
Amplification reactions for six HKGs were performed in a total volume of 25 μl, containing 12.5 μl of 2× EsTaq MasterMix (Cwbiotech, China), 2 μl of each forward and reverse primer (10 μM), 1.5 μl of template DNA (10 to 30 ng/μl), and 7 μl of ultrapure water, using a SensoQuest LabCycler. The PCR mixture was subjected to denaturation at 94°C for 10 min, followed by 35 cycles of denaturation at 94°C for 30 s, annealing at 54 to 60°C for 30 s, and extension at 72°C for 60 s, with a final extension step at 72°C for 10 min. More detailed information on annealing temperatures is given in Table 4. PCR amplicons were verified by electrophoresis on 1% agarose. The amplified products were purified and sequenced using the ABI 3730xl platform.
Analysis of nucleotide diversity.
The sequences of 16S rRNA, gyrA, gyrB, infB, recN, rpoA, and topA genes used for MLSA were trimmed to positions 56 to 1455, 247 to 744, 337 to 1446, 1519 to 2181, 565 to 1200, 139 to 756, and 106 to 768, respectively, corresponding to Escherichia coli numbering (43). The evolutionary distances and sequence similarities of the 16S rRNA gene and of individual and concatenated HKGs were calculated using MEGA X v10.05 with Kimura’s two-parameter model. The parsimony informative sites and Ka/Ks ratios (Ka, the number of nonsynonymous substitutions per nonsynonymous site; Ks, the number of synonymous substitutions per synonymous site) were analyzed using DnaSP 6.0 (44).
Phylogenetic analysis.
The nucleotide sequences were aligned using MEGA X v10.05. The phylogenetic trees of the 16S rRNA gene and the individual and concatenated sequences of six HKGs (gyrA, gyrB, infB, recN, rpoA, and topA) were constructed by neighbor-joining and maximum-likelihood methods with MEGA X v10.05. The model selected was Kimura’s two-parameter with the pairwise-deletion option. The parameter used for maximum-likelihood inference was the nearest-neighbor interchange. The robustness of tree topologies was evaluated with 1,000 bootstrap replications, and values of >70% are shown at the nodes of the branches. The split network tree of MLSA was performed by SplitsTree 4.14.4 using the Jukes-Cantor correlation.
Genomic relatedness.
Twenty-eight Shewanella type strains with complete genomes available in GenBank (Table S1) were utilized to investigate the concordance and correlation between MLSA and genomes. Core genes of genomic sequences identified by OrthoMCL 2.0.9 were concatenated to construct the phylogenetic tree. The isDDH results were measured by the Genome-to-Genome Distance Calculator (GGDC) (http://ggdc.dsmz.de/). The ANI values were estimated by using the web-based platform EZBioCloud (http://www.ezbiocloud.net/tools/ani) with the OrthoANIu algorithm. The correlation between the isDDH results and MLSA similarities was simulated using MATLAB R2016a (MathWorks, Inc.) with nonlinear interpolation analysis.
Phenotypic characteristics.
Further phenotypic tests were performed using the five strains of Shewanella species whose isDDH values were greater than the species threshold. The type strains of species were examined in parallel under suitable conditions. Physiological and biochemical traits were determined by using commercial strips, including API 20E and API 20NE (bioMérieux, France), in accordance with standard manufacturer’s instructions.
Data availability.
The nucleotide sequences of the six HKGs have been deposited in the GenBank nucleotide sequence database under the following accession numbers: MH090144 to MH090185 (gyrA), MH090186 to MH090202 (gyrB), MH090203 to MH090244 (infB), MH090245 to MH090286 (recN), MH090287 to MH090328 (rpoA), and MH090329 to MH090370 (topA).
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by grants from the National Natural Science Foundation of China (31570134) and the National Sci-Tech Key Project (2018ZX10102001, 2018ZX10734404, 2018ZX10713001-002, and 2018ZX10713003-002) from the Ministry of Health, China.
The authors have declared that no competing interests exist.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.03126-18.
REFERENCES
- 1.MacDonell MT, Colwell RR. 1985. Phylogeny of the Vibrionaceae and recommendation for two new genera, Listonella and Shewanella. Syst Appl Microbiol 6:171–182. doi: 10.1016/S0723-2020(85)80051-5. [DOI] [Google Scholar]
- 2.Bowman JP. 2005. Genus XIII. Shewanella MacDonell and Colwell 1986, 355VP (effective publication: MacDonell and Colwell 1985,180), p 480–491. In Brenner DJ, Krieg NR, Staley JT, Garrity GM (ed), Bergey’s manual of systematic bacteriology, 2nd ed Springer, New York, NY. [Google Scholar]
- 3.Venkateswaran K, Moser DP, Dollhopf ME, Lies DP, Saffarini DA, MacGregor BJ, Ringelberg DB, White DC, Nishijima M, Sano H, Burghardt J, Stackebrandt E, Nealson KH. 1999. Polyphasic taxonomy of the genus Shewanella and description of Shewanella oneidensis sp. nov. Int J Syst Bacteriol 49:705–724. doi: 10.1099/00207713-49-2-705. [DOI] [PubMed] [Google Scholar]
- 4.Gauthier G, Gauthier M, Christen R. 1995. Phylogenetic analysis of the genera Alteromonas, Shewanella, and Moritella using genes coding for small-subunit rRNA sequences and division of the genus Alteromonas into two genera, Alteromonas (emended) and Pseudoalteromonas gen. nov., and proposal of twelve new species combinations. Int J Syst Bacteriol 45:755–761. doi: 10.1099/00207713-45-4-755. [DOI] [PubMed] [Google Scholar]
- 5.Sudha Rani P, Saini MK, Pinnaka AK, Sampath Kumar G, Kumar S, Vemuluri VR, Tanuku N. 2019. Shewanella submarina sp. nov., a gammaproteobacterium isolated from marine water. Int J Syst Evol Microbiol 69:39–45. doi: 10.1099/ijsem.0.003059. [DOI] [PubMed] [Google Scholar]
- 6.Yun BR, Park S, Kim MK, Park J, Kim SB. 2018. Shewanella saliphila sp. nov., Shewanella ulleungensis sp. nov., and Shewanella litoralis sp. nov., isolated from coastal seawater. Int J Syst Evol Microbiol 68:2960–2966. doi: 10.1099/ijsem.0.002929. [DOI] [PubMed] [Google Scholar]
- 7.Fang Y, Wang Y, Liu Z, Lu B, Dai H, Kan B, Wang D. 2017. Shewanella carassii sp. nov., isolated from surface swabs of crucian carp and faeces of a diarrhoea patient. Int J Syst Evol Microbiol 67:5284–5289. doi: 10.1099/ijsem.0.002511. [DOI] [PubMed] [Google Scholar]
- 8.Huang J, Sun B, Zhang X. 2010. Shewanella xiamenensis sp. nov., isolated from coastal sea sediment. Int J Syst Evol Microbiol 60:1585–1589. doi: 10.1099/ijs.0.013300-0. [DOI] [PubMed] [Google Scholar]
- 9.Park SC, Baik KS, Kim MS, Kim D, Seong CN. 2009. Shewanella marina sp. nov., isolated from seawater. Int J Syst Evol Microbiol 59:1888–1894. doi: 10.1099/ijs.0.005470-0. [DOI] [PubMed] [Google Scholar]
- 10.Kim D, Baik KS, Kim MS, Jung BM, Shin TS, Chung GH, Rhee MS, Seong CN. 2007. Shewanella haliotis sp. nov., isolated from the gut microflora of abalone, Haliotis discus hannai. Int J Syst Evol Microbiol 57:2926–2931. doi: 10.1099/ijs.0.65257-0. [DOI] [PubMed] [Google Scholar]
- 11.Hirota K, Nodasaka Y, Orikasa Y, Okuyama H, Yumoto I. 2005. Shewanella pneumatophori sp. nov., an eicosapentaenoic acid-producing marine bacterium isolated from the intestines of Pacific mackerel (Pneumatophorus japonicus). Int J Syst Evol Microbiol 55:2355–2359. doi: 10.1099/ijs.0.63804-0. [DOI] [PubMed] [Google Scholar]
- 12.Janda JM, Abbott SL. 2014. The genus Shewanella: from the briny depths below to human pathogen. Crit Rev Microbiol 40:293–312. doi: 10.3109/1040841X.2012.726209. [DOI] [PubMed] [Google Scholar]
- 13.Liu PY, Lin CF, Tung KC, Shyu CL, Wu MJ, Liu JW, Chang CS, Chan KW, Huang JA, Shi ZY. 2013. Clinical and microbiological features of Shewanella bacteremia in patients with hepatobiliary disease. Intern Med 52:431–438. doi: 10.2169/internalmedicine.52.8152. [DOI] [PubMed] [Google Scholar]
- 14.Pagani L, Lang A, Vedovelli C, Moling O, Rimenti G, Pristera R, Mian P. 2003. Soft tissue infection and bacteremia caused by Shewanella putrefaciens. J Clin Microbiol 41:2240–2241. doi: 10.1128/JCM.41.5.2240-2241.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boukhalfa H, Icopini GA, Reilly SD, Neu MP. 2007. Plutonium(IV) reduction by the metal-reducing bacteria Geobacter metallireducens GS15 and Shewanella oneidensis MR1. Appl Environ Microbiol 73:5897–5903. doi: 10.1128/AEM.00747-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kouzuma A, Kasai T, Hirose A, Watanabe K. 2015. Catabolic and regulatory systems in Shewanella oneidensis MR-1 involved in electricity generation in microbial fuel cells. Front Microbiol 6:609. doi: 10.3389/fmicb.2015.00609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nimje VR, Chen CY, Chen HR, Chen CC, Huang YM, Tseng MJ, Cheng KC, Chang YF. 2012. Comparative bioelectricity production from various wastewaters in microbial fuel cells using mixed cultures and a pure strain of Shewanella oneidensis. Bioresour Technol 104:315–323. doi: 10.1016/j.biortech.2011.09.129. [DOI] [PubMed] [Google Scholar]
- 18.Yarza P, Yilmaz P, Pruesse E, Glöckner FO, Ludwig W, Schleifer K-H, Whitman WB, Euzéby J, Amann R, Rosselló-Móra R. 2014. Uniting the classification of cultured and uncultured bacteria and archaea using 16S rRNA gene sequences. Nat Rev Microbiol 12:635–645. doi: 10.1038/nrmicro3330. [DOI] [PubMed] [Google Scholar]
- 19.Glaeser SP, Kampfer P. 2015. Multilocus sequence analysis (MLSA) in prokaryotic taxonomy. Syst Appl Microbiol 38:237–245. doi: 10.1016/j.syapm.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 20.Kim JY, Yoo HS, Lee DH, Park SH, Kim YJ, Oh DC. 2016. Shewanella algicola sp. nov., a marine bacterium isolated from brown algae. Int J Syst Evol Microbiol 66:2218–2224. doi: 10.1099/ijsem.0.001014. [DOI] [PubMed] [Google Scholar]
- 21.Park HY, Jeon CO. 2013. Shewanella aestuarii sp. nov., a marine bacterium isolated from a tidal flat. Int J Syst Evol Microbiol 63:4683–4690. doi: 10.1099/ijs.0.055178-0. [DOI] [PubMed] [Google Scholar]
- 22.Sung HR, Yoon JH, Ghim SY. 2012. Shewanella dokdonensis sp. nov., isolated from seawater. Int J Syst Evol Microbiol 62:1636–1643. doi: 10.1099/ijs.0.032995-0. [DOI] [PubMed] [Google Scholar]
- 23.Bozal N, Montes MJ, Tudela E, Jimenez F, Guinea J. 2002. Shewanella frigidimarina and Shewanella livingstonensis sp. nov. isolated from Antarctic coastal areas. Int J Syst Evol Microbiol 52:195–205. doi: 10.1099/00207713-52-1-195. [DOI] [PubMed] [Google Scholar]
- 24.Miyazaki M, Nogi Y, Usami R, Horikoshi K. 2006. Shewanella surugensis sp. nov., Shewanella kaireitica sp. nov. and Shewanella abyssi sp. nov., isolated from deep-sea sediments of Suruga Bay, Japan. Int J Syst Evol Microbiol 56:1607–1613. doi: 10.1099/ijs.0.64173-0. [DOI] [PubMed] [Google Scholar]
- 25.Auch AF, von Jan M, Klenk H-P, Göker M. 2010. Digital DNA-DNA hybridization for microbial species delineation by means of genome-to-genome sequence comparison. Stand Genomic Sci 2:117–134. doi: 10.4056/sigs.531120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee I, Kim YO, Park SC, Chun J. 2015. OrthoANI: an improved algorithm and software for calculating average nucleotide identity. Int J Syst Evol Microbiol 66:1100–1103. doi: 10.1099/ijsem.0.000760. [DOI] [PubMed] [Google Scholar]
- 27.Ochoa-Díaz MM, Daza-Giovannetty S, Gómez-Camargo D. 2018. Bacterial genotyping methods: from the basics to modern. Methods Mol Biol 1734:13–20. doi: 10.1007/978-1-4939-7604-1_2. [DOI] [PubMed] [Google Scholar]
- 28.Szeinbaum N, Kellum CE, Glass JB, Janda JM, DiChristina TJ. 2018. Whole-genome sequencing reveals that Shewanella haliotis Kim et al. 2007 can be considered a later heterotypic synonym of Shewanella algae Simidu et al. 1990. Int J Syst Evol Microbiol 68:1356–1360. doi: 10.1099/ijsem.0.002678. [DOI] [PubMed] [Google Scholar]
- 29.Kim KK, Kim YO, Park S, Kang SJ, Nam BH, Kim DN, Oh TK, Yoon JH. 2011. Shewanella upenei sp. nov., a lipolytic bacterium isolated from bensasi goatfish Upeneus bensasi. J Microbiol 49:381–386. doi: 10.1007/s12275-011-0175-5. [DOI] [PubMed] [Google Scholar]
- 30.Simidu U, Kita-Tsukamoto K, Yasumoto T, Yotsu M. 1990. Taxonomy of four marine bacterial strains that produce tetrodotoxin. Int J Syst Bacteriol 40:331–336. doi: 10.1099/00207713-40-4-331. [DOI] [PubMed] [Google Scholar]
- 31.Ivanova EP, Gorshkova NM, Bowman JP, Lysenko AM, Zhukova NV, Sergeev AF, Mikhailov VV, Nicolau DV. 2004. Shewanella pacifica sp. nov., a polyunsaturated fatty acid-producing bacterium isolated from sea water. Int J Syst Evol Microbiol 54:1083–1087. doi: 10.1099/ijs.0.02993-0. [DOI] [PubMed] [Google Scholar]
- 32.Ivanova EP, Sawabe T, Gorshkova NM, Svetashev VI, Mikhailov VV, Nicolau DV, Christen R. 2001. Shewanella japonica sp. nov. Int J Syst Evol Microbiol 51:1027–1033. doi: 10.1099/00207713-51-3-1027. [DOI] [PubMed] [Google Scholar]
- 33.Sravan Kumar R, Sasi Jyothsna TS, Sasikala C, Seong CN, Lim CH, Park SC, Ramana CV. 2010. Shewanella fodinae sp. nov., isolated from a coal mine and from a marine lagoon. Int J Syst Evol Microbiol 60:1649–1654. doi: 10.1099/ijs.0.017046-0. [DOI] [PubMed] [Google Scholar]
- 34.Ivanova EP, Nedashkovskaya OI, Sawabe T, Zhukova NV, Frolova GM, Nicolau DV, Mikhailov VV, Bowman JP. 2004. Shewanella affinis sp. nov., isolated from marine invertebrates. Int J Syst Evol Microbiol 54:1089–1093. doi: 10.1099/ijs.0.02992-0. [DOI] [PubMed] [Google Scholar]
- 35.Kim M, Oh HS, Park SC, Chun J. 2014. Towards a taxonomic coherence between average nucleotide identity and 16S rRNA gene sequence similarity for species demarcation of prokaryotes. Int J Syst Evol Microbiol 64:346–351. doi: 10.1099/ijs.0.059774-0. [DOI] [PubMed] [Google Scholar]
- 36.Sun DL, Jiang X, Wu QL, Zhou NY. 2013. Intragenomic heterogeneity of 16S rRNA genes causes overestimation of prokaryotic diversity. Appl Environ Microbiol 79:5962–5969. doi: 10.1128/AEM.01282-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moore WEC, Stackebrandt E, Kandler O, Colwell RR, Krichevsky MI, Truper HG, Murray RGE, Wayne LG, Grimont PAD, Brenner DJ, Starr MP, Moore LH. 1987. International Committee on Systematic Bacteriology: report of the Ad Hoc Committee on Reconciliation of Approaches to Bacterial Systematics. Int J Syst Bacteriol 37:463–464. doi: 10.1099/00207713-37-4-463. [DOI] [Google Scholar]
- 38.Satomi M, Vogel BF, Venkateswaran K, Gram L. 2007. Description of Shewanella glacialipiscicola sp. nov. and Shewanella algidipiscicola sp. nov., isolated from marine fish of the Danish Baltic Sea, and proposal that Shewanella affinis is a later heterotypic synonym of Shewanella colwelliana. Int J Syst Evol Microbiol 57:347–352. doi: 10.1099/ijs.0.64708-0. [DOI] [PubMed] [Google Scholar]
- 39.Sumathi BG, Kumarswamy SR, Amritam U, Arjunan R. 2014. Shewanella algae: first case report of the fast emerging marine pathogen from squamous cell carcinoma patient in India. South Asian J Cancer 3:188–189. doi: 10.4103/2278-330X.136819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang YL, Wang DC, Zhan SW, Zheng JX, Liu Y, Tao Y, Shi ZF, Hao M, Yu L, Kan B. 2009. Isolation and characterization of Shewanella spp. from patients of food poisoning. Zhonghua Liu Xing Bing Xue Za Zhi 30:836–840. (In Chinese.) doi: 10.3760/cma.j.issn.0254-6450.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 41.Weisburg WG, Barns SM, Pelletier DA, Lane DJ. 1991. 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol 173:697–703. doi: 10.1128/jb.173.2.697-703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamamoto S, Harayama S. 1995. PCR amplification and direct sequencing of gyrB genes with universal primers and their application to the detection and taxonomic analysis of Pseudomonas putida strains. Appl Environ Microbiol 61:1104–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brosius J, Palmer ML, Kennedy PJ, Noller HF. 1978. Complete nucleotide sequence of a 16S ribosomal RNA gene from Escherichia coli. Proc Natl Acad Sci U S A 75:4801–4805. doi: 10.1073/pnas.75.10.4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Librado P, Rozas J. 2009. DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25:1451–1452. doi: 10.1093/bioinformatics/btp187. [DOI] [PubMed] [Google Scholar]
- 45.Lane DJ. 1991. 16S/23S rRNA sequencing, p 115–175. In Stackebrandt E, Goodfellow M (ed), Nucleic acid techniques in bacterial systematics. Wiley, New York, NY. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The nucleotide sequences of the six HKGs have been deposited in the GenBank nucleotide sequence database under the following accession numbers: MH090144 to MH090185 (gyrA), MH090186 to MH090202 (gyrB), MH090203 to MH090244 (infB), MH090245 to MH090286 (recN), MH090287 to MH090328 (rpoA), and MH090329 to MH090370 (topA).